Abstract
In daylight, noise generated by cones determines the fidelity with which visual signals are initially encoded. Subsequent stages of visual processing require synapses from bipolar cells to ganglion cells, but whether these synapses generate a significant amount of noise was unknown. To characterize noise generated by these synapses, we recorded excitatory postsynaptic currents from mammalian retinal ganglion cells and subjected them to a computational noise analysis. The release of transmitter quanta at bipolar cell synapses contributed substantially to the noise variance found in the ganglion cell, causing a significant loss of fidelity from bipolar cell array to postsynaptic ganglion cell. Virtually all the remaining noise variance originated in the presynaptic circuit. Circuit noise had a frequency content similar to noise shared by ganglion cells but a very different frequency content from noise from bipolar cell synapses, indicating that these synapses constitute a source of independent noise not shared by ganglion cells. These findings contribute a picture of daylight retinal circuits where noise from cones and noise generated by synaptic transmission of cone signals significantly limit visual fidelity.
Introduction
In daylight, the visual system is remarkably sensitive, able to discriminate distances smaller than a cone outer segment and detect contrasts a hundred times weaker than the background (Campbell & Robson, 1968; Shapley & Victor, 1986). One limit to cone vision is the stochastic nature of light: if an object provides only a few more or a few less photons than background illumination then it is not statistically different from noise. Other limiting factors are the transduction molecules, synaptic vesicles, ionic channels and spikes that convey information about light to the brain. All of these neural elements are stochastic, generate noise, and might reduce sensitivity. This raises the question: where are the dominant noise sources in the visual system that have the greatest effects on sensitivity?
Cones are a significant source of noise at the first stage of visual processing. It has been suggested that thermally generated isomerizations of cone photopigments limit daylight sensitivity, just as thermal noise in rods limit night-time sensitivity (Donner, 1992). Yet voltage noise recorded from cones has more power at high frequencies than would be expected from thermal noise alone, indicating that additional noise originates from random fluctuation in the components of the visual transduction cascade and from cGMP-gated channels in the cone's outer segment (Schneeweis & Schnapf, 1999; Angueyra & Rieke, 2013). Calculations based on the statistical properties of vesicular neurotransmitter release indicate that transmission across the cone ribbon synapse may generate more noise than sources inside the cone (Choi et al. 2005; Borghuis et al. 2009).
There is evidence for additional sources of noise in stages of visual processing after the cones. Ala-Laurila et al. (2011) concluded that as much as one-half of the noise that reaches the ganglion cell might come from sources downstream of the cones but did not identify these noise sources. Borghuis et al. (2009) estimated visual thresholds from an ideal model of photon absorptions in photoreceptor outer segments and from recordings of horizontal and ganglion cells. Threshold increased from outer segments to inner nuclear layer and then again to ganglion cells, indicating that besides cones, an important but unidentified noise source lay in the inner plexiform layer.
Here we provide evidence that cone bipolar cell synapses on the dendrites of retinal ganglion cells are a major source of noise in the inner retina that causes a significant transmission loss. Virtually all the remaining noise enters the ganglion cell from the presynaptic circuit. The noise generated by synapses on the ganglion cell leads to a critical reduction in signal-to-noise ratio from bipolar cell array to postsynaptic ganglion cell. Thus our results indicate that noise sources that reduce visual sensitivity are not concentrated near the photoreceptors, but also lie close to the ganglion cells. Presumably, neural circuitry must be designed to limit the effect of noise on information transmission. By showing where and how much noise is generated in the retinal circuit, our results have important implications for how the retina is designed for daylight vision.
Methods
Ethical approval
All procedures conformed to the University of Pennsylvania and National Institutes of Health guidelines for the use and care of animals in research, and were reviewed by the University of Pennsylvania Committee for the Care and Use of Animals.
Electrophysiology and imaging
An adult male Hartley guinea pig (400–600 g, >8 weeks old) that had been housed in a normal light/dark cycle with one cage-mate was anaesthetized with ketamine (133 mg kg−1 i.m.), xylazine (13 mg kg−1 i.m.), and pentobarbital (100 mg kg−1 i.p.). An eye was removed and the animal was killed by an overdose of pentobarbital (0.1 ml i.c.). All procedures were performed according to the University of Pennsylvania and National Institutes of Health guidelines. A piece of retina about 1 cm × 1 cm square, attached to pigment epithelium, choroid and sclera, was taken from that portion of the visual streak that lay in the dorsal retina. The piece of retina was mounted in a chamber on an upright microscope and superfused with Ames’ medium (Sigma-Aldrich, St. Louis, MO, USA) that was saturated with 5% CO2–95% O2, adjusted with glucose to ∼300 mOsm kg−1, and contained (in mm): 120 NaCl, 3.1 KCl, 0.5 KH2PO4, 23 Na2HCO3, 1.2 MgSO4, 1.15 CaCl2, plus amino acids and vitamins (pH 7.4, 34°C). To substitute Mg2+ for Ca2+ without altering any other ionic concentration, we omitted amino acids and vitamins from the control solution and then substituted MgCl2 for CaCl2. In some experiments, isradipine, 1,2,3,4-Tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide disodium salt (NBQX), 3-((R)-2-Carboxypiperazin-4-yl)-pro-pyl-1-phosphonic acid (CPP), or (2S)-2-amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid (LY341495) was added to the superfusate (Tocris, Bristol, UK).
A glass patch pipette (6–10 MΩ) was formed with a Sutter P-87 puller (Sutter, Novato, CA, USA) and was filled with a solution containing (in mm): 110 CsOH, 120 gluconate, 10 NaOH, 1 EGTA, 2.5 Na+, 10 Hepes, 10 lidocaine N-ethyl chloride (QX-314 Cl), 6 Lucifer Yellow. The pipette solution was adjusted to 310 mOsm kg−1 with glucose and to pH 7.2 with gluconate. Given this pipette solution, the calculated reversal potential for glutamate channels (Eglut) with equal permeability to Cs+, Na+ and K+ was ∼4 mV and the calculated reversal potential for GABA and glycine receptors (ECl) was ∼ −67 mV.
Recordings were acquired with a Multiclamp 700B patch clamp amplifier, and digitized at 5 kHz using pCLAMP 10 (Molecular Devices, Sunnyvale, CA, USA) (4-pole Bessel filter, fc = 2 kHz). The access resistance was 45 ± 3 MΩ and the holding current was −89 ± 40 pA, which resulted in a small voltage error of −4 ± 2 mV. Thus all voltages were corrected for a calculated junction potential of −13 mV but not for the voltage error.
To visualize ganglion cells for recording, the retina with attached pigment and sclera was transilluminated with infrared light and examined with differential interference contrast optics (IR-DIC, 800–840 nm, × 60 water immersion lens, 0.9 NA). During recording Lucifer Yellow diffused from the pipette into the cell. After recording, and often while the patch pipette was still attached, cells were photographed with epifluorescent optics and a cooled-CCD camera (Hamamatsu, Hamamatsu City, Shizuoka, Japan) (×20 water immersion lens, 0.5 NA). To measure the stratification depth of ganglion cell dendrites in the inner plexiform layer (IPL), a stack of about 10 optical sections was captured that combined IR-DIC images of cell somas at both edges of the IPL and fluorescent dendrites within the IPL. To quantify stratification, we counted the number of sections from the fluorescent dendrites to somas at either edge of the IPL.
We targeted ganglion cells with medium-sized somas (<14 μm) for whole-cell voltage clamp recording and selected those with clearly separable asynchronous EPSCs that occurred after light stimulation (aEPSCs) (Fig. 2A). Among analysed cells we found local edge cells, ON and OFF parasol cells, and some we could not identify (Freed & Liang, 2010). Local edge cells have narrow dendritic fields (200–250 μm diameter), many short (<10 μm) secondary dendritic branches and stratify narrowly near the middle of the IPL (40–60% of the depth) (Rockhill et al. 2002). OFF parasol cells (200–230 μm) have primary dendrites that fan out from the soma, have secondary dendrites that evenly fill the spaces between the primary dendrites, and stratify narrowly above the middle of the IPL (Roska et al. 2006). ON parasol cells (180–220 μm) are directionally selective, have a branching pattern similar to the local edge cells, and stratify narrowly below the middle of the IPL (Roska et al. 2006; Hoshi et al. 2009).
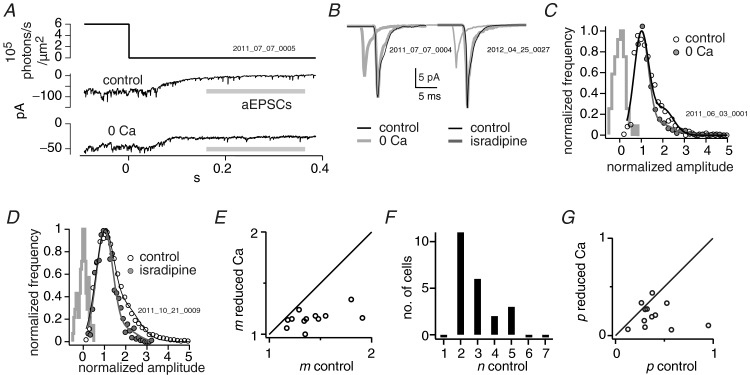
Figure 2.
A, asynchronous EPSCs (aEPSCs) recorded from an ON parasol cell when the visual stimulator was stepped to zero intensity. B, the average aEPSC during zero Ca2+ or isradipine (grey), when scaled up, matches the average aEPSC in control (black). C, zero Ca2+ eliminated large multiquantal aESPCs, reducing the skew of the aEPSC amplitude distribution. Distributions are fitted with the sum of Gaussians expected from aEPSCs composed of 1, 2 and 3 quanta. Amplitudes were normalized by dividing them by the amplitude of the quantal current. The grey histogram shows baseline noise between aEPSCs. D, like zero Ca2+, isradipine eliminated large multiquantal aEPSCs from the amplitude distribution. E, scatter plot showing effect of zero Ca2+ or isradipine on m, the average quantal content of an aEPSC. Each data point is from a different cell: all cells are below the diagonal, thus m was always reduced. F, histogram of the number of release sites in the control condition. The most common number of release sites among cells was n = 2. G, scatter plot of the effect of zero Ca2+ or isradipine on the probability of release p. Most cells are below the diagonal, thus p was reduced.
Visual stimulus
The stimulus was provided by a 556 nm light-emitting diode that projected diffusely over the entire ∼1 cm2 piece of retina. The circuitry driving the diode enabled a stimulus time constant of 140 μs. The flickering stimulus was randomly sampled at 1000 Hz from a Gaussian distribution but limited to 30 Hz by low-pass filtering. The average intensity of the stimulus was 3 × 105 photons μm−2 s−1 resulting in a photoisomerization rate of 4.6 × 104 s−1 for a rod and 3.3 × 104 s−1 for an M cone ( nm,
nm,  nm, rod outer segment: 16.2 μm × 3 μm2, cone outer segment: 8 μm × 3 μm2; Yin et al. 2006). At these photoisomerization rates, the non-colour opponent ganglion cells we recorded from the dorsal retina have responses that are approximately equally divided between rods and M cone signals (Yin et al. 2006). Also at these photoisomerization rates, rod signals enter ganglion cells predominantly via cones and cone bipolar cells (Manookin et al. 2008).
nm, rod outer segment: 16.2 μm × 3 μm2, cone outer segment: 8 μm × 3 μm2; Yin et al. 2006). At these photoisomerization rates, the non-colour opponent ganglion cells we recorded from the dorsal retina have responses that are approximately equally divided between rods and M cone signals (Yin et al. 2006). Also at these photoisomerization rates, rod signals enter ganglion cells predominantly via cones and cone bipolar cells (Manookin et al. 2008).
Data analysis
Each trial of the visual stimulus consisted of 6 s of flicker, then a 2 s step to maximal intensity, then a 2 s step to minimum intensity. aEPSCs were detected during the steps using a ‘peak location’ algorithm that smoothed the recording (1 ms) and found a local maximum (the leading edge), followed by a local minimum (the peak), followed by an exponential decay (criterion coefficient of determination for fit to exponential R2 = 0.5) (Tian et al. 1998). Threshold aEPSC amplitude was set at 3 pA to exceed the amplitude of noise between aEPSCs (2.8 ± 0.4 pA). To average aEPSCs, their leading edges of aEPSC were normalized to zero and their peaks placed in register; the time to peak was then measured from 10% to 90% of the peak value, and the decay time constant by fitting with an exponential function.
Signal and noise spectra (PSDs) were constructed from the Fourier transform of currents x(t) and y(t),
| (1) |
where * denotes the complex conjugate and <…> denotes averaging currents across a series of overlapping 0.8192 s windows (4096 samples; one-seventh overlap). For signal spectra, x(t) and y(t) were the currents averaged across stimulus trials; for noise spectra, x(t) and y(t) were residuals after subtracting the average from the currents. For power spectra, x(t) and y(t) were identical but for cross spectra, x(t) and y(t) were from different cells (a cross spectrum is the Fourier transform of the cross-correlation function). Data analysis was performed using IGOR (Wavemetrics, Lake Oswego, OR, USA), JMP (SAS Institute Cary, NC, USA), and Excel (Microsoft Corp., Redmond, WA, USA). All error bars and error estimates are the standard error of the mean (SEM). The criterion for statistical significance was α = 0.05.
Binomial model of asynchronous release
In control superfusate, the distribution of aEPSC amplitudes was skewed, had an excess of large events, and thus departed markedly from a normal distribution. In zero Ca2+ or with isradipine, the disparity between the amplitude distribution and the normal distribution was markedly reduced, and in some cells the distribution matched a normal distribution almost exactly, evidence that the distribution of quantal amplitudes was normal (see Supplemental Fig. S1 available online). Thus we fitted the distribution of aEPSC amplitudes with the sum of Gaussians G(),
| (2) |
where μ is the amplitude of a quantal current, σ is the standard deviation of this amplitude, and ck is the frequency of aEPSCs that are composed of k quanta. To implement a model of multiquantal release with n sites with a release probability p, the parameter ck in eqn 2 was constrained to a binomial distribution. When eqn 2 was unconstrained, the average quantal content was calculated as  . When eqn 2 was constrained to a binomial distribution, because failures to release quanta were undetectable in our experiments, the average quantal content of aEPSCs was calculated as
. When eqn 2 was constrained to a binomial distribution, because failures to release quanta were undetectable in our experiments, the average quantal content of aEPSCs was calculated as  (Singer et al. 2004).
(Singer et al. 2004).
Probability of a multiquantal aEPSC occurring by chance
If quanta occur independently and at random, then the probability that a quantum occurs within an interval Δt of the next quantum, and thus close enough in time to contribute to the same aEPSC, would be Δt × r, where r is the time-averaged rate at which quanta occur (Fatt & Katz, 1952). The interval Δt was approximated as the smallest interval between detected aEPSCs, which is the interval of confusion within which two quanta cannot be detected as separate (1.7 ± 0.1 ms). Quantal rate r was derived from the rate of multiquantal aEPSCs R by the equation  where m is the average quantal content of an aEPSC.
where m is the average quantal content of an aEPSC.
To calculate the actual frequency with which quanta contribute to the same aEPSC we fitted the binomial model to the distribution of aEPSC amplitudes. The frequency of uniquantal aEPSCs  was derived from the binomial distribution using k = 1 and experimental values for n and p. The frequency with which quanta contributed to a uniquantal aEPSC, Puni, was calculated as
was derived from the binomial distribution using k = 1 and experimental values for n and p. The frequency with which quanta contributed to a uniquantal aEPSC, Puni, was calculated as 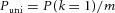 . Finally, the probability that a quantum contributed to an aEPSC composed of multiple quanta (k > 1) was calculated as
. Finally, the probability that a quantum contributed to an aEPSC composed of multiple quanta (k > 1) was calculated as  .
.
Deconvolution of postsynaptic currents with quantal current
We chose a computational method of deconvolution because direct numerical deconvolution produces high frequency noise and negative values that require subsequent smoothing (Borges et al. 1995; Diamond & Jahr, 1995). Deconvolution was accomplished by using a linear–non-linear model that included a linear filter and a static non-linearity (Fig. 1; Chichilnisky, 2001). The flickering stimulus s(t) was passed through the filter f(t), a static non-linearity n(l) and then a Poisson noise generator which produced a different time-varying quantal rate ri(t) for each trial i of the model. To produce the final output of the model, the quantal rate was convolved with the quantal current q(t) to generate postsynaptic currents.
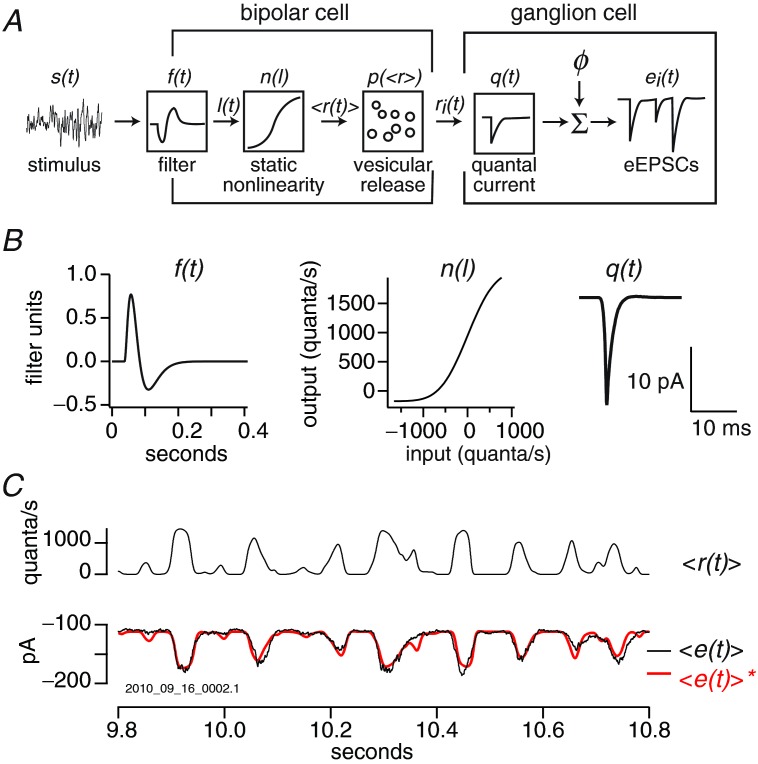
Figure 1.
A, schematic diagram of the model. From left to right, a white-noise stimulus is transformed by a linear filter f(t) and then a static non-linearity n(l), producing an average quantal rate <r(t)>, which is the input to a Poisson noise generator p(<r(t)>). Here <…> denotes averaging across trials. For each trial i of the model, the Poisson noise generator produces a different instantaneous quanta rate ri(t), which is convolved with the quantal current q(t), then added to an offset  , to generate a current ei(t). B, components of the model for an OFF parasol cell. C, the model has seven parameters, specifying the filter f(t), the non-linearity n(l), and the offset
, to generate a current ei(t). B, components of the model for an OFF parasol cell. C, the model has seven parameters, specifying the filter f(t), the non-linearity n(l), and the offset  . These parameters are iteratively adjusted until the average generated current <e(t)>* fits the average recorded current <e(t)>. After this fitting procedure, the average quantal rate <r(t)> estimates the deconvolution of the average recorded current <e(t)> with the quantal current q(t).
. These parameters are iteratively adjusted until the average generated current <e(t)>* fits the average recorded current <e(t)>. After this fitting procedure, the average quantal rate <r(t)> estimates the deconvolution of the average recorded current <e(t)> with the quantal current q(t).
The filter component of the model was constructed from the equation
 |
(3) |
We found that in practice h = 1 and n = 4 generated a biphasic filter and provided the best fit between the model's output and the recorded currents (Fig. 1B). So we held h and n to these values, leaving only two free parameters for the filter, the time constants: τ1 and τ2. The static non-linearity was constructed from the cumulative normal distribution C(l),  where: β and γ were a scaling factor and an offset, respectively, for the linear input l; α and δ were a scaling factor and an offset, respectively, for the non-linear output. The model had an additional parameter ϕ, an offset added to the postsynaptic currents that specified a baseline current corresponding to zero release rate. Thus in total the model had seven free parameters.
where: β and γ were a scaling factor and an offset, respectively, for the linear input l; α and δ were a scaling factor and an offset, respectively, for the non-linear output. The model had an additional parameter ϕ, an offset added to the postsynaptic currents that specified a baseline current corresponding to zero release rate. Thus in total the model had seven free parameters.
We used the correlation function between visual stimulus and currents to generate an initial approximation of the linear filter, and then fitted this function with eqn 3 to provide the initial parameters for the filter. Then, the Poisson noise generator was removed from the model, and all the model parameters were adjusted iteratively to minimize χ2 error between generated and recorded average postsynaptic currents (Levenberg–Marquardt). Next, to generate synaptic noise, the Poisson noise generator was added back to the model and the model was run over as many trials as had been recorded. To account for the contribution of variability in quantal amplitude to noise, the spectrum for synaptic noise Nsyn(f) was multiplied by the factor  , where
, where  is the coefficient of variation for the amplitude of the quantal current, derived by fitting eqn 2 to the distribution of aEPSC amplitudes (Katz & Miledi, 1972). For 38 cells, the coefficient of variation averaged 0.48 ± 0.02.
is the coefficient of variation for the amplitude of the quantal current, derived by fitting eqn 2 to the distribution of aEPSC amplitudes (Katz & Miledi, 1972). For 38 cells, the coefficient of variation averaged 0.48 ± 0.02.
Linear–non-linear models have proven to accurately replicate synaptic currents in retinal ganglion cells by capturing their strong rectification (Kim & Rieke, 2001; Zaghloul et al. 2003). This rectification leads to a clear baseline current that corresponds to zero quantal rate, from which the currents make inward excursions (Liang & Freed, 2010). For the model to fit this baseline required that the parameters δ and ϕ had unambiguous values: after fitting, δ ensured that the basal quantal rate was zero and ϕ ensured that the baseline current corresponded to zero quantal rate. However, for a few cells in LY341495, there was no clear baseline current, which rendered the values for δ and ϕ non-unique. In these few cases, we constrained δ to a value that ensured a high-frequency match between the noise spectrum generated by the model Nsyn(f) and the recorded postsynaptic noise Npost(f), and left ϕ as a free parameter that was determined by the iterative fitting process.
The ability of the model to predict the recorded currents was accessed by calculating the correlation coefficient between the average current generated by the model and the recorded currents: rPearson = 0.70 ± 0.02. The predictive ability of the model was then compared with that of the current averaged over stimulus repetitions, which is the maximum likelihood prediction. The correlation between the average current and the recorded currents was rPearson = 0.88 ± 0.02 (18 cells). Thus the model was 81 ± 0.02% as accurate as the maximum likelihood prediction.
The validity of using the model to estimate synaptic noise depended on how well it: (1) predicted the recorded currents, (2) represented the statistics of quantal release that sets the relationship between mean and variance, and (3) represented the kinetics of the quantal current. First, the model predicted the recorded currents about 80% as well as the maximum likelihood prediction, a typical figure of merit for linear–non-linear models (Kim & Rieke, 2001; Zaghloul et al. 2003; Beaudoin et al. 2008; Liang & Freed, 2010). Second, the model incorporated Poisson statistics for quantal release, which predicts that mean current is proportional to its variance, and that the ratio of signal power to noise power (SNR) is proportional to quantal rate. Both of these predictions have been extensively confirmed (Freed, 2000, 2005; Freed & Liang, 2010). Third, the kinetics of the quantal current, i.e. its amplitude and time course, were not parameterized in the model but empirically derived from an independent analysis of asynchronous quantal release. Given an experimentally derived quantal current and the average recorded current, the model generated synaptic noise that consistently matched recorded postsynaptic noise at high frequencies (Fig. 3G and H). The model provided an estimate of the portion of postsynaptic noise due to synaptic noise that was consistent across multiple cells of various types (SEM/mean = 8%; Fig. 4A).
Figure 3.
A, postsynaptic currents recorded from an OFF parasol cell during two trials of a flicker stimulus. B, current averaged over 24 trials (black) and average currents generated by convolution of the quantal current with quantal rate (red). C, residuals calculated by subtracting average current in B from individual currents in A. D, average quantal rate and quantal current q(t) (scale bar for q(t): 10 pA × 10 ms). E, currents generated by convolving quantal rate with the quantal current q(t). F, residuals from the generated currents. G, power spectra of the residuals from recordings and generated currents, representing total postsynaptic noise, Npost(f) (black), and noise from quantal release at bipolar cell synapses, Nsyn(f) (red). The lower red trace was generated using q(t); the higher red trace was generated by substituting the average aEPSC for q(t). H, power spectra from a local edge cell with the same conventions as panel G. I, analysis of the elementary event underlying synaptic noise for an OFF parasol cell. Fitting error was the differences between the logarithms of Npost(f) and Nsyn(f), summed from 50 to 200 Hz. The best fit was obtained when the quantal current was scaled about 1.05 times. J, diagonal graph of the best fitting amplitude against the measured amplitude of the quantal current. Data points (18 cells) follow the diagonal, indicating an equivalence between elementary event and measured quantal current.
Figure 4.
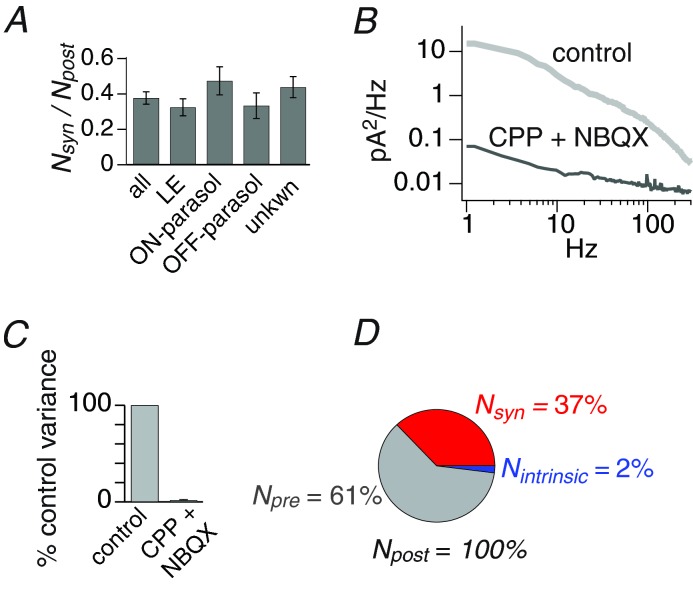
A, the ratio of quantal noise Nsyn to total postsynaptic noise Npost. Error bar indicates SEM. Results from different cell types were not statistically different. B, spectrum of total noise Npost(f) in the control condition and when glutamate receptors were blocked with CPP + NBQX. C, summary of intrinsic noise variance remaining after blocking glutamate receptors in 5 cells. D, the contributions of quantal release at bipolar cell synapses Nsyn, the presynaptic circuit Npre, and sources intrinsic to the ganglion cell Nintrinsic, to total noise variance in the postsynaptic currents Npost.
Results
Recording EPSCs resulting from asynchronous release of transmitter quanta
The goal of our study was to characterize the noise that bipolar cell synapses contribute to postsynaptic currents in a ganglion cell during dynamic visual stimulation. Our basic approach was to deconvolve the average measured postsynaptic current with a quantal current to derive a quantal rate. This rate was used to generate currents whose variability represents noise resulting from the quantal release of transmitter. We then compared this noise with the total noise in the postsynaptic current to estimate how much of the total noise was due to quantal release of transmitter.
The first step in our analysis was to measure the postsynaptic effect of a quantum of transmitter, the quantal current. To reduce quantal rate, we recorded in the whole-cell mode from the smallest types of ganglion cell in the guinea pig retina (<250 μm dendritic field diameter, 11 local edge, 10 ON parasol, 5 OFF parasol, 6 of unknown type). We found in practice that these small cell types had the lowest quantal rates, presumably because the number of synapses is proportional to dendritic length (Xu et al. 2008). We further reduced quantal rate by transiently hyperpolarizing the presynaptic bipolar cell: this was accomplished by stepping light intensity from minimum to maximum for OFF ganglion cells and to the minimum light intensity for ON ganglion cells. After a short delay following the step, during which the presynaptic bipolar cell hyperpolarized (<0.2 s), there occurred a series of small inward currents. These currents had an average peak amplitude of ∼14 pA, implying a peak conductance of ∼200 pS. The average event had a time-to-peak of 1.3 ± 0.4 ms and a decay time constant of 2.7 ± 0.2 ms. Therefore, these events were similar to previous descriptions of spontaneous EPSCs (Taylor et al. 1995; Tian et al. 1998). Because these currents were evoked by a light step, but occurred at random after the step, we called them asynchronous EPSCs (aEPSCs) to distinguish them from spontaneous activity during constant illumination and from synchronized activity during visual stimulation (Fig. 2A; Singer et al. 2004).
Distribution of aEPSC amplitudes indicates multiquantal release
To measure the quantal current we considered that both rod and cone bipolar cells can release quanta in coordinated bursts, causing ESPCs composed of 1, 2, or rarely more quanta (Singer et al. 2004; Sagdullaev et al. 2006; Jarsky et al. 2011). This coordination occurs in the absence of a synchronizing stimulus, indicating that coordinated release is an intrinsic property of the synapse. Such ‘multiquantal’ release may be widespread in the nervous system, and has been demonstrated for conventional synapses in the cerebellum and in the hippocampus, as well as at ribbon synapses in the cochlea (Tong & Jahr, 1994; Auger et al. 1998; Wadiche & Jahr, 2001; Glowatzki & Fuchs, 2002; Oertner et al. 2002; Raghavachari & Lisman, 2004). Therefore to derive the quantal current from aEPSCs, we considered the possibility that they were composed of multiple quanta.
To probe for multiple quanta contributing to aEPSCs, we reduced Ca2+ influx into the presynaptic terminal. This strategy, when applied to the synapses of rod and cone bipolar cells, reduces the probability of vesicular fusion at a release site, thus reducing the probability that multiple coordinated quanta are released at multiple sites; the result is smaller EPSCs with reduced quantal content (Singer et al. 2004; Sagdullaev et al. 2006; Jarsky et al. 2010). Also, because multiple transmitter quanta are released simultaneously and their postsynaptic currents sum in register, reducing the number of quanta reduces the EPSC's amplitude but does not greatly alter its shape.
Therefore, to confirm multiquantal release in our preparation, we substituted Mg2+ for all the Ca2+ in the superfusate or partially blocked L-type channels with 3 μm isradipine. Nominally zero Ca2+ in the superfusate will reduce calcium in the extracellular space, but will not entirely remove it; as a result, synaptic release was diminished in frequency but continued for longer than the typical duration of our recordings (∼30 min) (Dmitriev et al. 1999). We combined the two treatments (7 cells zero Ca2+, 9 cells isradipine; one-tailed Wilcoxon signed rank statistical test that does not assume normality or equal variance). Consistent with a reduction in the number of quanta, peak amplitude was reduced from 14 ± 2 to 9 ± 1 pA (P = 0.0001). Consistent with near-simultaneous quanta, there was no significant effect on the rise time (control 1.3 ± 0.1 ms; treatment 1.4 ± 0.1 ms; P = 0.10) or on the decay time constant (control 2.4 ± 0.2 ms; treatment 2.5 ± 0.3 ms; P = 0.64) (Fig. 2B).
The distribution of aEPSC amplitudes was positively skewed with an excess of large aEPSCs (Fig. 2C and D). Zero Ca2+ or isradipine reduced this skew, consistent with a reduction in the frequency of multiquantal aEPSCs. To estimate the average quantal content of an aEPSC, we fitted the distribution of amplitudes with the sum of multiple Gaussian distributions, where the integrals of these Gaussians represented the frequencies of aEPSCs that contain 1, 2, 3 quanta, etc. (see Methods; Stern et al. 1992; Jonas et al. 1993; Paulsen & Heggelund, 1996). In the control condition, an aEPSC contained on average 1.4 ± 0.06 quanta. Zero Ca2+ or isradipine reduced content significantly to 1.1 ± 0.02 quanta. The average quantal content of an aEPSC was reduced in all cells (12/12) but predictably, because an aEPSC cannot contain less than one quantum, those cells with a quantal content close to one in control showed the smallest reductions (Fig. 2E).
During multiquantal release, a precipitating event, possibly the opening of one or a few calcium channels, coordinates the fusion of vesicles with the outer membrane at multiple release sites (Singer et al. 2004; Jarsky et al. 2010; Bartoletti et al. 2011). Such coordinated release should produce aEPSCs that follow binomial statistics, and to test for this we fitted the distribution of aEPSC amplitudes with the sum of Gaussians as before, but constrained the integrals of these Gaussians to a binomial distribution. The parameters of this distribution were psite, which is the probability of release at a single site during the generation of an aEPSC, and n, which is the number of sites. The binomially constrained equation fitted the aEPSC amplitude distribution as well as when it was unconstrained (binomial: χ2 = (1.2 ± 0.3) × 10−6; unconstrained: χ2 = (1.3 ± 0.3) × 10−6; 26 cells) and provided essentially the same quantal content (binomial: m = 1.5 ± 0.05; unconstrained: m = 1.5 ± 0.06). The best binomial fits were obtained when the number of release sites n was 2–6, with 2 being the most common value among cells (Fig. 2F). This result is consistent with previous studies: two release sites have been indicated by a binomial model of quantal release at the rod bipolar cell synapse (Singer et al. 2004). This suggests that most often two vesicles release in a coordinated fashion, perhaps because the vesicles are across from each other on opposite sides of the synaptic ribbon. Also consistent with a binomial model for multiquantal release, the release probability at a site was psite = 0.30 ± 0.04 in the control condition but was significantly reduced to psite = 0.16 ± 0.03 when calcium influx was reduced (Fig. 2G).
We considered an alternative explanation, that multiquantal aEPSCs were due to the chance coincidence of quanta that occur without coordination. Yet given the low quantal rate (r = 36 ± 1 s−1) the frequency with which a quantum would coincide by chance with one or more others would be corresponding low, PPoisson = 0.05 ± 0.002 (30 cells). The observed frequency with which quanta coincided, estimated from the average quantal content of an aEPSC, was much higher, Pobs = 0.73 ± 0.003 (see Methods). Thus only about 7% of the quanta that contribute to a multiquantal aEPSC could be predicted by random coincidence, and so almost all multiquantal aEPSCs were due to coordinated release.
Noise analysis of light-evoked currents indicates uniquantal release
To estimate the amount of noise generated by quantal release at the bipolar cell synapse, we presented a full-field flickering stimulus to the retina (SD/mean = 0.3), repeating the same flicker pattern typically over 50 trials. This stimulus had an average intensity of 3 × 105 photons μm−2 s−1 and caused visual signals to enter the retinal circuit predominantly through cones (see Methods). We recorded excitatory postsynaptic currents from the same individual ganglion cells from which we recorded aESPCs (Fig. 3A). We constructed residuals by subtracting the current averaged over trials from the currents from individual trials (Trong & Rieke, 2008; Fig. 3B and C). To derive a quantal current, we scaled the average aEPSC to match the quantal amplitude derived from fitting the aEPSC amplitude distribution with the sum of Gaussians. Next we convolved the quantal current with the quantal rate to generate postsynaptic currents (Fig. 3D and E) and constructed their residuals as we had for recorded currents (Fig. 3F).
From the residuals of recorded currents we constructed a spectrum to show the frequency content of total postsynaptic noise Npost(f). From the residuals of the generated currents we constructed another spectrum to show the frequency content of noise from quantal release at the bipolar cell synapse Nsyn(f) (Fig. 3G and H). The spectrum Nsyn(f) was adjusted to account for noise added by variability in quantal current amplitude (Freed, 2005). When synaptic noise Nsyn(f) and total postsynaptic noise Npost(f) were compared, they matched very well at high frequencies (Fig. 3G and H). A high frequency match was expected because stochastic quantal release adds the high frequency component of postsynaptic noise (Ashmore & Copenhagen, 1983).
It might be objected that, during flickering stimulation, quanta could be coordinated by multiquantal release, which would generate more noise than if quanta occurred independently. To test for this, instead of using a quantal current in our analysis, we substituted the average aEPSC, which is multiquantal. In this case, Nsyn(f) always exceeded Npost(f) at high frequencies (Fig. 3G and H). Using a sub-quantal amplitude brought Nsyn(f) well below Npost(f) at high frequencies. Any elementary current that was larger than a quantum caused the synaptic noise to exceed postsynaptic noise at high frequencies – an inconsistency because postsynaptic noise includes noise generated by synapses. Therefore, the elementary current underlying synaptic noise could not be composed of multiple quanta.
The amplitude that produced the best high-frequency match was 104 ± 3% of the amplitude of the quantal current (18 cells) (Fig. 3I). In other words, the quantal current underlying the aEPSC was within 4% of the elementary current that generated a high-frequency match between synaptic and postsynaptic noise. This match was consistent across cells of different type and across experimental preparations, and was unlikely to be due to coincidence (Fig. 3J), indicating that the quantal current was the elementary current underlying the generation of synaptic noise.
Dynamic visual stimulation reduces release probability per site
We tested the idea that for synchronous light-evoked release, the probability of vesicular release at a single site is reduced, which reduces the probability of coordinated release from multiple sites. We considered that during flicker stimulation, postsynaptic currents consisted of a series of transient inward EPSCs (eEPSCs), due to the synchronous release of tens of quanta (Freed, 2005; Supplemental Fig. S2). Restricting our analysis to local edge cells, we estimated the quantal content of each eEPSC by dividing its charge transfer (222 ± 46 ms pA) by the charge transfer of a single quantum (37 ± 2 ms pA), resulting in 60 ± 11 quanta per eEPSC (Freed, 2005). The number of synapses on a local edge ganglion cell, estimated from confocal microscopic imaging of synaptic ribbon on dendrites, varies from 1000 to 2000 (Xu et al. 2008; Freed & Liang, 2010). Dividing the average number of quanta by the minimum number of synapses gave a release probability for an entire synapse of psyn = 0.03; the probability of release at a site is even less because a synapse has multiple sites. This probability is much less than the probability of release during an aEPSC recorded from a local edge cell (psite = 0.22 ± 0.01). Apparently then, eEPSCs are associated with a reduced probability of release at a single site, which reduces the probability of coordinated release at multiple sites. Consistent with this, the eEPSCs were much longer than the aEPSCs (decay time constant: 320 ± 50 vs. 2.0 ± 0.1 ms), indicating that quanta were better coordinated for aEPSCs (Singer et al. 2004). eEPSCs are larger than aEPSCs despite being associated with a reduced release probability because they are generated by a synchronous release at many synapses across the ganglion cell's dendritic arbor, while the aEPSCs are generated by asynchronous release at fewer synapses scattered across this arbor. aEPSCs and eEPSCs were recorded within seconds of one another from the same cells, implying that release probability can be reduced on short time scales by dynamic stimulation. A reduction in release probability has been demonstrated for the rod bipolar synapse by subjecting the rod bipolar cell to dynamic (flickering) voltages, and has been attributed to a reduction in the number of vesicles available for release and to Ca2+ channel inactivation (Branco & Staras, 2009; Jarsky et al. 2011).
Synapses on the ganglion cell add a substantial amount of noise to its postsynaptic currents
The preceding analysis indicated that release during flickering stimulation was uncoordinated and thus uniquantal. Therefore, we used a quantal current to generate the spectrum of noise from transmitter release at the cone bipolar cell synapse Nsyn(f). We then integrated this spectrum between 1 and 300 Hz to give variance Nsyn, which averaged 111 ± 18 pA2. Similarly, the spectrum of total postsynaptic noise Npost(f) was integrated to give Npost, which averaged 336 ± 69 pA2. Dividing Nsyn by Npost indicated that synapses on the ganglion cell generated 37 ± 3% of the noise in the postsynaptic currents. This percentage did not vary significantly across the four cell groups from which we recorded (Kruskal–Wallis test does not assume normality or equal variance; 9 local edge, 3 ON parasol, 3 OFF parasol; 2 of unknown type; P = 0.45) (Fig. 4A).
Besides cone bipolar cell synapses, there are two other sources that might contribute noise to the ganglion cell's postsynaptic excitatory currents: (1) noise entering the ganglion cell from the circuit; (2) noise intrinsic to the ganglion cell. To measure intrinsic noise we recorded flicker-evoked excitatory currents as before, then blocked the excitatory synapses with the glutamate antagonists CPP and NBQX. We found that this treatment blocked 98 ± 0.4% of the noise, indicating that about 2% of noise is intrinsic to the ganglion cell (5 cells) (Fig. 4B and C). Intrinsic noise results from stochastic processes associated with the cell membrane: channel gating, ionic currents and thermal motion of charge carriers (White et al. 2000; van Rossum et al. 2003; Faisal et al. 2008). Voltage-gated channels amplify noise from sources intrinsic and extrinsic to the ganglion cell, but under the voltage clamp conditions of our experiments, such noise amplification would be suppressed (van Rossum et al. 2003; Dhingra & Smith, 2004; Dhingra et al. 2005). Therefore, we subtracted intrinsic noise (2% of total noise) and synaptic noise from total noise to give circuit noise Npre, which averaged 217 ± 57 pA2 across 17 cells and contributed 61 ± 3% of total noise. To summarize, bipolar cell synapses on the ganglion cell contribute a substantial portion (∼40%) of the noise in excitatory currents, with virtually all the remaining noise (∼60%) coming from the retinal circuit (Fig. 4D).
Some ganglion cells form gap junctions with amacrine cells, although this has not been demonstrated for the ganglion cells recorded here. If amacrine cells were to generate or transmit substantial noise, then some circuit noise might enter the ganglion cell through gap junctions. Noise coming through gap junctions would be blocked by glutamate antagonists, and thus would not be accounted for in our estimate of intrinsic noise (Murphy & Rieke, 2011; Ala-Laurila et al. 2011). Thus our estimate of circuit noise, which requires subtracting synaptic and intrinsic noise from total noise, would include noise entering through both chemical and electrical synapses.
Partially blocking the cone synapse increases the contribution of bipolar cell synapses to noise
If bipolar synapses contribute noise to currents in ganglion cells, then we should be able to change the relative contributions of circuit and bipolar synapses by interfering with synaptic transmission from cones to bipolar cells. Antagonizing the metabotropic glutamate receptor 6 (mGluR6) at the cone synapse on the ON bipolar cell should reduce the amount of noise contributed by the cones. Additionally, antagonizing this receptor should depolarize the ON bipolar cell, increase the rate of release from its synapses on the ganglion cell, and thus increase synaptic noise (Fig. 5A).
Figure 5.
A, schematic diagram of the circuit that generates noise in the ON ganglion cell showing the cone synapse  and the bipolar cell synapse
and the bipolar cell synapse  . B, top graph: average postsynaptic current is a series of transient eEPSCs (black). The instantaneous quantal current was convolved with the average quantal rate to generate an average current (red). Bottom graph: the instantaneous quantal rate is a series of transient increases. The currents descend and quantal rate ascends from a baseline (dashed black lines). C, antagonizing the cone synapse with LY341495 brought postsynaptic currents and quantal rate away from their baselines, and reduced their modulation by light. D, spectrum of total postsynaptic noise Npost(f) (black) decreases more than spectrum of synaptic noise Nsyn(f) (red). Thus synaptic noise becomes a larger portion of total noise. E, aEPSCs in control (dashed) and LY341495 (grey). F, scatter plot showing the effect of LY341495 on the ratio of circuit noise to total postsynaptic noise Npre/Npost. Data points (7 cells) are below the diagonal, indicating that antagonizing the cone synapse decreased circuit noise in the ganglion cell. G, test for saturation of currents. Graph of maximum (black) and minimum (red) currents in control and LY341495. The black dashed lines ascend from control to LY3 and the red lines descend, indicating that the currents in LY341495 stay within the limits of the control currents.
. B, top graph: average postsynaptic current is a series of transient eEPSCs (black). The instantaneous quantal current was convolved with the average quantal rate to generate an average current (red). Bottom graph: the instantaneous quantal rate is a series of transient increases. The currents descend and quantal rate ascends from a baseline (dashed black lines). C, antagonizing the cone synapse with LY341495 brought postsynaptic currents and quantal rate away from their baselines, and reduced their modulation by light. D, spectrum of total postsynaptic noise Npost(f) (black) decreases more than spectrum of synaptic noise Nsyn(f) (red). Thus synaptic noise becomes a larger portion of total noise. E, aEPSCs in control (dashed) and LY341495 (grey). F, scatter plot showing the effect of LY341495 on the ratio of circuit noise to total postsynaptic noise Npre/Npost. Data points (7 cells) are below the diagonal, indicating that antagonizing the cone synapse decreased circuit noise in the ganglion cell. G, test for saturation of currents. Graph of maximum (black) and minimum (red) currents in control and LY341495. The black dashed lines ascend from control to LY3 and the red lines descend, indicating that the currents in LY341495 stay within the limits of the control currents.
We antagonized the mGluR6 receptor with LY341495, and then recorded from seven ON parasol cells. LY341495 reduced the light-induced modulation of the postsynaptic currents and quantal rates, consistent with a partial blockage of the cone synapse (the signal power of currents was reduced by 54 ± 11%; Fig. 5B and C). LY341495 also brought the currents and the quantal rates away from their baselines, consistent with a depolarization of the ON bipolar cell (Fig. 5B and C). The amplitude of the aESPC, however, was not significantly affected (control 14 ± 2 pA; LY341495 16 ± 3 pA; P = 0.63), nor was its time-to-peak (control 1.3 ± 0.1 ms; LY341495 1.3 ± 0.1 ms; P = 0.62) or decay time constant (control 2.7 ± 0.1; LY341495 2.7 ± 0.1 ms; P = 0.63; Fig. 5E). LY341495 reduced total postsynaptic noise Npost by 68 ± 6%.
The portion of noise that synapses on the ganglion cell contributed to total postsynaptic noise, Nsyn/Npost, increased significantly from 42 ± 6% to 67 ± 7%, with a concomitant significant reduction in the circuit contribution, Npre/Npost, from 55 ± 6 to 31 ± 7% (Fig. 5D and F). Thus as expected, blocking the cone synapse on the ON bipolar cell reduced circuit noise and increased synaptic noise.
The relationship between presynaptic membrane voltage and transmitter release rate is a sigmoidal function which sets two limits for transmitter release: a threshold at hyperpolarized potentials and a saturation at depolarized potentials. Normally the bipolar cell membrane voltage is close to threshold and as a result, postsynaptic currents show a clear baseline corresponding to zero release rate (dashed lines in Fig. 5B and C). We were concerned, however, that LY341495 might depolarize the ON bipolar cell to saturation, effectively throttling its synapse on the ganglion cell and preventing cone noise from reaching the ganglion cell. Yet the currents in LY341495 were always within the range of currents found in the control condition, indicating that the relationship between voltage and transmitter release was no more thresholded or more saturated in LY341495 than it was in the control condition (Fig. 5G). We were also concerned that LY341495 might depolarize the ON bipolar cell, depleting releasable vesicles (Burrone & Lagnado, 2000; Singer & Diamond, 2006), but vesicle depletion would reduce synaptic noise, inconsistent with the increase in synaptic noise we observed. The depolarizing effect of LY341495 on ON bipolar cells has been shown to desensitize over seconds by a Ca2+-dependent mechanism, which may explain how LY341495 blocked the cone synapse without throttling the cone bipolar cell synapse (Berntson et al. 2004; Kaur & Nawy, 2012).
Evidence that ganglion cells do not share bipolar cell synapses as common noise sources
Electron micrographs of the primate retina show that postsynaptic to each bipolar cell synapse there are two profiles. These profiles are either from an amacrine cell and a ganglion cell, or two amacrine cells, but rarely two ganglion cells (Dowling & Boycott, 1966). This dyadic organization implies that ganglion cells rarely share bipolar cell synapses. Assuming guinea pig retina has the same organization, its ganglion cells should share noise from bipolar cells (and from all neural elements that feed noise into bipolar cells) but not from transmitter release at bipolar cell synapses.
To test this idea, we recorded excitatory currents from pairs of ganglion cells of like polarity, either both ON or both OFF, so that the noise they shared would reflect noise from either ON or OFF circuits (Fig. 6A and B). We constructed the cross spectrum between the residuals of the two cells to show the frequency content of shared noise Nshared(f). We then constructed the difference spectrum Npost(f) – Nsyn(f) for each cell to show the frequency content of presynaptic noise (Fig. 6C). When shared and difference spectra were compared, they matched well, consistent with the idea that shared noise and presynaptic noise have the same source, somewhere in the circuit upstream of the bipolar cell synapse (Fig. 6D and E). The difference spectrum would include a small amount (∼2%) of noise intrinsic to the ganglion cell, but apparently this did not disrupt the match. Shared noise, Nshared(f), and noise from bipolar cell synapses, Nsyn(f), were a poor match, consistent with the idea that ganglion cells do not share noise from bipolar cell synapses (Fig. 6D and E).
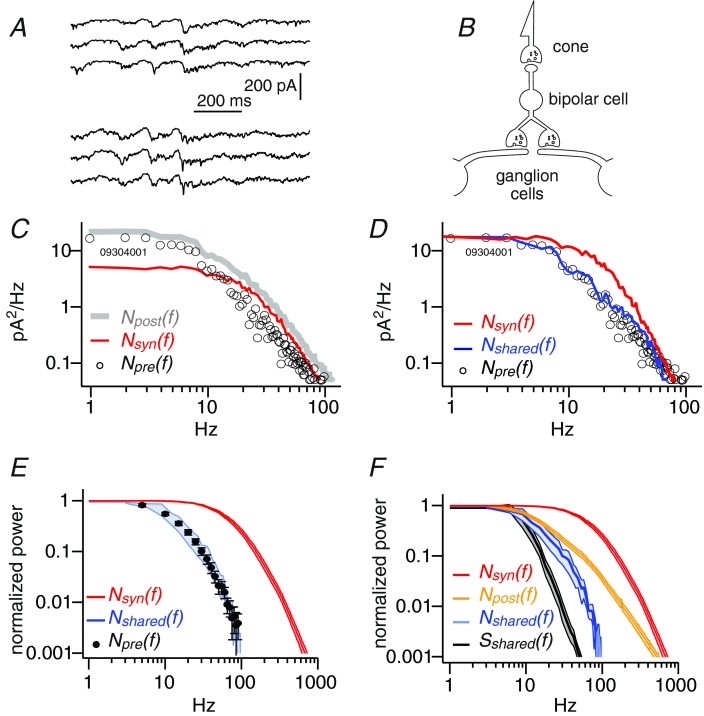
Figure 6.
A, excitatory postsynaptic currents recorded simultaneously from a pair of local edge ganglion cells. B, ganglion cells share circuitry (including common cones and bipolar cells) but rarely share bipolar cell synapses. C, the power spectra of total postsynaptic noise, Npost(f) (grey), noise from quantal release at bipolar cell synapses, Nsyn(f) (red), and from the presynaptic circuit calculated as Npre(f) = Npost(f) – Nsyn(f) (circles). D, the cross-spectrum of shared noise Nshared(f) (blue), when appropriately scaled, matches presynaptic circuit noise Npre(f) (circles), but not noise from bipolar cell synapses Nsyn(f) (red). E, summary of results for 13 pairs of cells: shared noise (blue) matches presynaptic circuit noise (circles) but not synaptic noise (red). F, cross spectrum of shared noise (blue) is attenuated at a lower frequency than total postsynaptic noise (yellow) or noise from bipolar synapses (red). Cross spectrum of shared signal (black) is attenuated at a lower frequency than any noise source. For E and F, all spectra were normalized to their value at 1 Hz before averaging. Shaded regions show mean ± SEM.
Noise from the circuit was attenuated by tenfold at moderate frequencies (32 ± 7 Hz) but noise from bipolar cell synapses extended to much higher frequencies (151 ± 11 Hz; Fig. 6F). Voltage noise recorded in bipolar cells from vesicular release at the cone terminal attenuates tenfold at 40 Hz (Ashmore & Copenhagen, 1983). Voltage noise in primate cones has a somewhat higher attenuation frequency (60 Hz) (Schneeweis & Schnapf, 1999). Thus within the accuracy allowed by species differences and variation in recording methods, circuit noise resembles noise from synaptic release at the cone terminal, suggesting that this is where it originates. An alternative idea is that circuit noise contains high frequencies at its source but is low-pass filtered by passage through bipolar cells. This seems unlikely because a bipolar cell's passive electrotonic properties can transmit frequencies over 100 Hz (Oltedal et al. 2009). Regenerative currents or amacrine–bipolar cell feedback would not low-pass filter, but would have the opposite effect, boosting high frequencies (Euler & Masland, 2000; Saszik & DeVries, 2012). Thus it is likely that circuit noise is restricted to moderate frequencies at its source.
To examine the frequency content of signals shared by ganglion cells, we constructed the cross spectrum of their signals. Shared signal was attenuated by tenfold at 16 ± 5 Hz, somewhat lower than the 32 Hz attenuation frequency for circuit noise (Fig. 6F). This was consistent with idea that the signals shared by ganglion cells originate from photoreceptor responses to photons, which are confined to low frequencies (Schneeweis & Schnapf, 1999).
Transmission efficiency at synapses on the ganglion cell is constant across moderate contrasts
As a metric for transmission efficiency across bipolar cell synapses on the ganglion cell we used the ratio of post-and presynaptic SNRs, SNRpost/SNRpre. Here SNR denotes the ratio of signal power to noise power. To estimate transmission efficiency, we reasoned that both signal and noise power in the bipolar cell array are amplified and filtered by synapses to produce signal Spost and noise Npre in the ganglion cell's postsynaptic currents. Thus the ratio Spost/Npre is equal to the SNR in the presynaptic bipolar cell array. By definition, the ratio Spost/Npost is the SNR of currents in the postsynaptic ganglion cell. By this reasoning, the transmission efficiency across the bipolar cell synapses simplifies to the ratio Npre/Npost (see ‘Transmission efficiency across the cone bipolar cell synapse’ in the Appendix). We have already estimated Npre/Npost to be about 60%, and therefore transmission efficiency at bipolar cell synapses is about 0.6.
This estimate of transmission efficiency was for a contrast of SD/mean = 0.3, which is the upper limit to contrast before the Gaussian distribution of intensities is truncated. We wondered if transmission efficiency would hold for lower contrasts, and so we varied the contrast of the flickering stimulus from 0.1 to 0.3 (Fig. 7A and B). We found that postsynaptic SNR increased linearly with contrast (Fig 7C) but transmission efficiency across the synapse remained virtually unchanged (Fig. 7D). Indeed the slope of transmission efficiency and contrast was only –0.07, statistically indistinguishable from zero (one-tailed t test for Pearson's r; rPearson = 0.34; 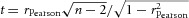 , P = 0.63). In the Appendix, we show how this experimental finding can be explained by assuming quantal release at a synapse is a Poisson process, and that quantal rates at the cone and bipolar cell synapses increase in proportion with increasing contrast.
, P = 0.63). In the Appendix, we show how this experimental finding can be explained by assuming quantal release at a synapse is a Poisson process, and that quantal rates at the cone and bipolar cell synapses increase in proportion with increasing contrast.
Figure 7.
A, power spectra of noise from quantal release at bipolar cell synapses Nsyn(f) (red) and total postsynaptic noise Npost(f) (grey). Darker grey indicates higher temporal contrast (SD/mean ≈ 0.1, 0.15, 0.2 and 0.3). B, SNR spectra for different contrasts. C, SNRpost, the integral of the SNR spectra, plotted against contrast for 5 ganglion cells, with the average and SEM indicated in red. On average, SNRpost increased linearly with contrast. D, transmission efficiency across bipolar cell synapses, calculated as the ratio of postsynaptic SNR to presynaptic SNR, is virtually unaffected by contrast.
Discussion
Here we provide evidence that during dynamic photopic stimulation, synapses on the ganglion cell generate a substantial portion of the noise in its postsynaptic currents. Noise intrinsic to the ganglion cell contributes negligibly to postsynaptic currents. Thus after synaptic noise is accounted for, the remaining noise in the postsynaptic currents enters from the presynaptic circuit. This circuit noise has a frequency content similar to noise shared by ganglion cells but a very different frequency content from noise from bipolar cell synapses, indicating that these synapses constitute a source of independent noise not shared by ganglion cells. Noise from bipolar cell synapses causes a loss of SNR from the bipolar cell array to the ganglion cell. Yet when the efficiency of synaptic transmission is expressed as a ratio of postsynaptic to presynaptic SNRs, this ratio remains constant as contrast increases.
Borghuis et al. (2009) estimated the visual threshold set by the stochastic properties of photons falling on the outer segments and compared it to the thresholds of horizontal and ganglion cells they recorded from a retinal preparation. They found an approximately 4-fold increase from the outer segment to the outer plexiform layer and another approximately 3.5-fold increase from the outer plexiform layer to the spike output of the retina. Here we add that bipolar cell synapses on the ganglion cell are a significant noise source that would contribute to this second threshold increase.
To compare our results quantitatively to those of Borghuis et al. we note that their threshold estimate was for a 500 μm diameter spot that would fill the small receptive fields of the ganglion cells from which we recorded. Threshold is approximated by the inverse of the square root of SNR, and therefore our transmission efficiency of 0.6 translates to a  -fold increase in threshold across the bipolar cell synapse. Multiplying this by another 2.5-fold increase due to spike generation estimated by Dhingra & Smith (2004) results in a 3.25-fold increase in threshold from bipolar cell to ganglion cell spike train, fairly close to the 3.5-fold estimated by Borghuis et al.
-fold increase in threshold across the bipolar cell synapse. Multiplying this by another 2.5-fold increase due to spike generation estimated by Dhingra & Smith (2004) results in a 3.25-fold increase in threshold from bipolar cell to ganglion cell spike train, fairly close to the 3.5-fold estimated by Borghuis et al.
Our results add new information to what had been known about noise sources in a ganglion cell's excitatory postsynaptic currents. Ala-Laurila et al. (2011) found that cones are a noise source that is shared by ganglion cells but did not identify other sources of noise in the postsynaptic currents. Here we identify one component of noise in the ganglion cell's postsynaptic current as originating in the presynaptic circuit, which agrees with Ala-Laurilla et al.'s finding because this circuit include cones. We discover that the only other substantial component of noise is contributed by bipolar cell synapses on the ganglion cell. We provide evidence that, unlike cones, these synapses are not a significant source of shared noise in the ganglion cell's excitatory currents by showing that synaptic noise and shared noise have a very different frequency content and are therefore different components of noise in postsynaptic currents. This agrees with electron micrographs which indicate that ganglion cells do not share bipolar cell synapses (Dowling & Boycott, 1966).
Implications of our results to the design of the retina for daylight
Our results contribute to an overall picture of how the retina is designed for daylight. Retinal information processing is divided into at least two stages, corresponding roughly to the outer and inner plexiform layers, and both stages add noise to the signal, reducing visual sensitivity. This picture of daylight processing resembles our current understanding of how circuits for darkness are constructed. Behavioural studies of humans and electrophysiological studies of ganglion cells in the intact eye indicated that single photons are detectable (Barlow et al. 1971; Sakitt, 1972; Mastronarde, 1983). Yet sometimes photons are detected where there are none. Such ‘dark events’, according to calculations based on behavioural studies, occur at a rate that matches the rate of thermal isomerizations in a rod (∼0.01 s−1; Baylor et al. 1984; Donner, 1992), implying that transduction is the source of dark noise and that subsequent visual processing is virtually noiseless. Yet, as reviewed by Field et al. (2005), uncertainty about where photons are lost – either before transduction or afterward within the circuit – weakens the behavioural evidence for noiseless visual processing. Indeed recordings of flash-evoked noise from rods, rod bipolar cells and AII amacrine cells in vitro indicate greater amounts of dark noise than would be expected from thermal noise alone (Nelson, 1982; Schneeweis & Schnapf, 1999; Dunn et al. 2006; Pang et al. 2007). The implication is that fluctuations in the transduction cascade (continuous noise), and quantal transmitter release from rods and rod bipolar cells are additional sources of noise. Thus direct recordings indicate that in darkness, the retina adds noise at two stages, in both inner and outer plexiform layers.
Remarkably, in the dark, noise levels in the AII amacrine and ON ganglion cells are essentially the same when normalized for the number of converging rods, indicating that the intermediary electrical synapses and cone bipolar cell synapses are relatively noiseless (Dunn et al. 2006). Apparently then, there is a distinction between rod and cone circuits: the same array of cone bipolar synapses is noiseless and therefore without loss when it processes single photon signals in the dark but does lose information – as we show – when it processes multiple photon signals in the light.
How can an array of synapses be without loss in the dark but lose information in the light? We suggest that the answer lies in different requirements for information encoding by light and dark circuits. The dark circuit can encode in a binary fashion – photon or no photon – and can use high gain. To ensure against noise being encoded as a photon, i.e. false positives, the rod synapse thresholds out noise (Field & Rieke, 2002; Taylor & Smith, 2004). To amplify signals that pass this threshold, the rod bipolar cell has a steep relationship between presynaptic voltage and transmitter release rate (Oesch & Diamond, 2011) and the AII amacrine cell has regenerative currents (Smith & Vardi, 1995; Tian et al. 2010). Multiquantal release at the rod bipolar cell synapse increases amplification of single photon signals, and may have a similar advantage for the cone bipolar cell synapse (Jarsky et al. 2011). These multiple stages of amplification increase quantal rate at the bipolar cell synapse and this, as we show in the Appendix, increases transmission efficiency across this synapse. The daylight circuit, however, cannot take advantage of this amplification or the resulting increase in transmission efficiency because it has very different encoding requirements from the dark circuit. The daylight circuit must encode a gradient of contrasts and other visual features and must therefore drive the cone bipolar cell synapses through a range of quantal rates. Therefore for the daylight circuit, amplification must be moderate, with the tradeoff that transmission efficiency is reduced.
Key points
At chemical synapses, vesicles fuse with the presynaptic membrane at random, generating noise in postsynaptic currents.
To determine how much noise synapses generate, we recorded excitatory postsynaptic currents from ganglion cells in an in vitro preparation of the mammalian retina during flickering visual stimulation.
Postsynaptic currents received noise from three sources: substantial noise from bipolar cell synapses, somewhat more from the presynaptic retinal circuitry, but little from sources intrinsic to the ganglion cell.
Presynaptic circuit elements but not bipolar cell synapses were significant sources of noise shared by pairs of ganglion cells.
Signal-to-noise ratio was substantially reduced from the presynaptic bipolar cell array to the postsynaptic ganglion cell, indicating that synaptic noise can reduce the amount of information transmitted to a neuron.
Acknowledgments
We thank Drs Robert G. Smith and Noga Vardi for productive discussions.
Abbreviations
- aEPSCs
excitatory postsynaptic currents that occur asynchronously after light stimulation
- eEPSCs
excitatory postsynaptic currents evoked during light stimulation
- IPL
inner plexiform layer
- mGluR6
metabotropic glutamate receptor 6
- Nsyn
noise variance in a ganglion cell's postsynaptic currents due to vesicular release from bipolar cells
- Npre
noise variance in a ganglion cell's postsynaptic currents that comes from the presynaptic circuit
- Npost
total noise variance in a ganglion cell's postsynaptic currents
- Nshared
spectrum of noise shared by the postsynaptic currents of two ganglion cells
- SNR
ratio of signal power to noise power
Appendix
Transmission efficiency across the cone bipolar cell synapse
We found that a ganglion cell's excitatory postsynaptic currents, Npost, had two significant noise components: bipolar cell synapses, Nsyn, and the presynaptic circuit, Npre. Here, we show that transmission efficiency across the bipolar cell synapse can be estimated using these components. Consider that the synapse amplifies signal and noise in the bipolar cell by a gain factor g to produce signal Spost and noise Npre in the postsynaptic currents. So the presynaptic signal-to-noise ratio SNRpre is equal to
| (A1) |
and the ratio of post-to presynaptic SNRs, which is our measure of transmission efficiency, is
| (A2) |
Thus transmission efficiency at the synapse can be estimated from our computational method of noise analysis.
Next, we make sense of our finding that transmission efficiency across the synapse is approximately constant in the range of moderate contrasts. From our decomposition of postsynaptic noise into two significant components, the postsynaptic SNR is
| (A3) |
Quantal release at the bipolar cell synapse produces signal Spost and noise Nsyn and thus its SNR is 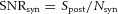 . Therefore, we can express the postsynaptic SNR in terms of the presynaptic SNR and the SNR of the bipolar cell synapse:
. Therefore, we can express the postsynaptic SNR in terms of the presynaptic SNR and the SNR of the bipolar cell synapse:
| (A4) |
Our recordings from ganglion cells indicate that the SNR of quantal release from bipolar cells is proportional to quantal rate: this result is consistent with Poisson statistics (Freed, 2000, 2005; Freed & Liang, 2010). We assume that the SNR of the presynaptic circuit is the result of Poisson statistics at the cone synapse (Choi et al. 2005). If so, the SNRs of the presynaptic circuit and of bipolar cell synapses have proportionality constants kpre and ksyn such that
| (A5) |
Here rpre is the quantal rate at the cone synapse, hrpre the quantal rate at the bipolar cell synapse, and h is a factor that sets the gain between quantal rates at cone and bipolar cell synapses. Factor h would be set by several relationships, some of them non-linear: presynaptic voltage and calcium influx; calcium influx and vesicle fusion rate; glutamate per vesicle; postsynaptic channels per synapse; conductance per channel. Yet for small changes in presynaptic voltage occasioned by moderate contrasts, presumably these relationships are piecewise linear. Substituting eqn 8 into eqn 7 results in
| (A6) |
Dividing eqn 9 by the equality  results in an expression for the transmission efficiency across the bipolar cell synapse:
results in an expression for the transmission efficiency across the bipolar cell synapse:
| (A7) |
Equation 10 shows that transmission efficiency is maximized if SNR for the bipolar cell synapse grows quickly with quantal rate (high ksyn), as would occur for high release probability or many synapses. Also, efficiency is maximized if quantal release at the first synapse drives release at the second synapse briskly (high h). Yet loss does not depend on quantal rate at cone or bipolar cell synapses per se, and thus would not be altered by changes in quantal rate occasioned by changes in contrast.
Our reasoning assumes that other noise sources in the presynaptic circuit besides the cone synapse (Angueyra & Rieke, 2013) would not disrupt the relationship between contrast and the SNR of this circuit. Our reasoning also assumes that the SNR for a synapse is proportional to quantal rate, an assumption that would be violated if there were significant spontaneous release that generates additive noise. The bipolar cell meets this assumption because, without temporal contrast, it is hyperpolarized close to the threshold for transmitter release, and its spontaneous release rate is minimal (Freed & Liang, 2010). The cone synapse also meets this requirement, but only in the high photopic conditions of our study, when continuous release from the cone is curtailed (Choi et al. 2005). With spontaneous release curtailed, synapses become rectifying and thus experience higher rates with higher contrasts of our flickering stimulus, despite its lack of a DC component (Liang & Freed, 2010).
Additional information
Competing interests
None declared.
Author contributions
The experiments were carried out at the Department of Neuroscience, University of Pennsylvania. M.A.F. conceived, designed, performed and analysed the experiments, and drafted the article. Z.L. performed experiments and edited the article.
Funding
This work was supported by the National Eye Institute, USA (grant R01 EY013333 to M.A.F.).
References
- Ala-Laurila P, Greschner M, Chichilnisky EJ, Rieke F. Cone photoreceptor contributions to noise and correlations in the retinal output. Nat Neurosci. 2011;14:1309–1316. doi: 10.1038/nn.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angueyra JM, Rieke F. Origin and effect of phototransduction noise in primate cone photoreceptors. Nat Neurosci. 2013;16:1692–700. doi: 10.1038/nn.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore JF, Copenhagen DR. An analysis of transmission from cones to hyperpolarizing bipolar cells in the retina of the turtle. J Physiol. 1983;340:569–597. doi: 10.1113/jphysiol.1983.sp014781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger C, Kondo S, Marty A. Multivesicular release at single functional synaptic sites in cerebellar stellate and basket cells. J Neurosci. 1998;18:4532–4547. doi: 10.1523/JNEUROSCI.18-12-04532.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB, Levick WR, Yoon M. Responses to single quanta of light in retinal ganglion cells of the cat. Vis Res. 1971;Suppl. 3:87–101. doi: 10.1016/0042-6989(71)90033-2. [DOI] [PubMed] [Google Scholar]
- Bartoletti TM, Jackman SL, Babai N, Mercer AJ, Kramer RH, Thoreson WB. Release from the cone ribbon synapse under bright light conditions can be controlled by the opening of only a few Ca2+ channels. J Neurophysiol. 2011;106:2922–2935. doi: 10.1152/jn.00634.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, Nunn BJ, Schnapf JL. The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. J Physiol. 1984;357:575–607. doi: 10.1113/jphysiol.1984.sp015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudoin DL, Manookin MB, Demb JB. Distinct expressions of contrast gain control in parallel synaptic pathways converging on a retinal ganglion cell. J Physiol. 2008;586:5487–5502. doi: 10.1113/jphysiol.2008.156224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson A, Smith RG, Taylor WR. Postsynaptic calcium feedback between rods and rod bipolar cells in the mouse retina. Vis Neurosci. 2004;21:913–924. doi: 10.1017/S095252380421611X. [DOI] [PubMed] [Google Scholar]
- Borges S, Gleason E, Turelli M, Wilson M. The kinetics of quantal transmitter release from retinal amacrine cells. Proc Natl Acad Sci U S A. 1995;92:6896–6900. doi: 10.1073/pnas.92.15.6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghuis BG, Sterling P, Smith RG. Loss of sensitivity in an analog neural circuit. J Neurosci. 2009;29:3045–3058. doi: 10.1523/JNEUROSCI.5071-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco T, Staras K. The probability of neurotransmitter release: variability and feedback control at single synapses. Nat Rev Neurosci. 2009;10:373–383. doi: 10.1038/nrn2634. [DOI] [PubMed] [Google Scholar]
- Burrone J, Lagnado L. Synaptic depression and the kinetics of exocytosis in retinal bipolar cells. J Neurosci. 2000;20:568–578. doi: 10.1523/JNEUROSCI.20-02-00568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell FW, Robson JG. Application of Fourier analysis to the visibility of gratings. J Physiol. 1968;197:551–566. doi: 10.1113/jphysiol.1968.sp008574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichilnisky EJ. A simple white noise analysis of neuronal light responses. Network. 2001;12:199–213. [PubMed] [Google Scholar]
- Choi SY, Borghuis BG, Rea R, Levitan ES, Sterling P, Kramer RH. Encoding light intensity by the cone photoreceptor synapse. Neuron. 2005;48:555–562. doi: 10.1016/j.neuron.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Dhingra NK, Freed MA, Smith RG. Voltage-gated sodium channels improve contrast sensitivity of a retinal ganglion cell. J Neurosci. 2005;25:8097–8103. doi: 10.1523/JNEUROSCI.1962-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra NK, Smith RG. Spike generator limits efficiency of information transfer in a retinal ganglion cell. J Neurosci. 2004;24:2914–2922. doi: 10.1523/JNEUROSCI.5346-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond JS, Jahr CE. Asynchronous release of synaptic vesicles determines the time course of the AMPA receptor-mediated EPSC. Neuron. 1995;15:1097–1107. doi: 10.1016/0896-6273(95)90098-5. [DOI] [PubMed] [Google Scholar]
- Dmitriev A, Pignatelli A, Piccolino M. Resistance of retinal extracellular space to Ca2+ level decrease: implications for the synaptic effects of divalent cations. J Neurophysiol. 1999;82:283–289. doi: 10.1152/jn.1999.82.1.283. [DOI] [PubMed] [Google Scholar]
- Donner K. Noise and the absolute thresholds of cone and rod vision. Vision Res. 1992;32:853–866. doi: 10.1016/0042-6989(92)90028-h. [DOI] [PubMed] [Google Scholar]
- Dowling JE, Boycott BB. Organization of the primate retina: Electron microscopy. Proc R Soc Lond B Biol Sci. 1966;166:80–111. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- Dunn FA, Doan T, Sampath AP, Rieke F. Controlling the gain of rod-mediated signals in the mammalian retina. J Neurosci. 2006;26:3959–3970. doi: 10.1523/JNEUROSCI.5148-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Masland RH. Light-evoked responses of bipolar cells in a mammalian retina. J Neurophysiol. 2000;83:1817–1829. doi: 10.1152/jn.2000.83.4.1817. [DOI] [PubMed] [Google Scholar]
- Faisal AA, Selen LPJ, Wolpert DM. Noise in the nervous system. Nat Rev Neurosci. 2008;9:292–303. doi: 10.1038/nrn2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatt P, Katz B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952;117:109–128. [PMC free article] [PubMed] [Google Scholar]
- Field GD, Rieke F. Mechanisms regulating variability of the single photon responses of mammalian rod photoreceptors. Neuron. 2002;35:733–747. doi: 10.1016/s0896-6273(02)00822-x. [DOI] [PubMed] [Google Scholar]
- Field GD, Sampath AP, Rieke F. Retinal processing near absolute threshold: from behavior to mechanism. Annu Rev Physiol. 2005;67:491–514. doi: 10.1146/annurev.physiol.67.031103.151256. [DOI] [PubMed] [Google Scholar]
- Freed MA. Rate of quantal excitation to a retinal ganglion cell evoked by sensory input. J Neurophysiol. 2000;83:2956–2966. doi: 10.1152/jn.2000.83.5.2956. [DOI] [PubMed] [Google Scholar]
- Freed MA. Quantal encoding of information in a retinal ganglion cell. J Neurophysiol. 2005;94:1048–1056. doi: 10.1152/jn.01276.2004. [DOI] [PubMed] [Google Scholar]
- Freed MA, Liang Z. Reliability and frequency response of excitatory signals transmitted to different types of retinal ganglion cell. J Neurophysiol. 2010;103:1508–1517. doi: 10.1152/jn.00871.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA. Transmitter release at the hair cell ribbon synapse. Nat Neurosci. 2002;5:147–154. doi: 10.1038/nn796. [DOI] [PubMed] [Google Scholar]
- Hoshi H, Liu WL, Massey SC, Mills SL. ON inputs to the OFF layer: bipolar cells that break the stratification rules of the retina. J Neurosci. 2009;29:8875–8883. doi: 10.1523/JNEUROSCI.0912-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarsky T, Cembrowski M, Logan SM, Kath WL, Riecke H, Demb JB, Singer JH. A synaptic mechanism for retinal adaptation to luminance and contrast. J Neurosci. 2011;31:11003–11015. doi: 10.1523/JNEUROSCI.2631-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarsky T, Tian M, Singer JH. Nanodomain control of exocytosis is responsible for the signaling capability of a retinal ribbon synapse. J Neurosci. 2010;30:11885–11895. doi: 10.1523/JNEUROSCI.1415-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P, Major G, Sakmann B. Quantal components of unitary EPSCs at the mossy fibre synapse on CA3 pyramidal cells of rat hippocampus. J Physiol. 1993;472:615–663. doi: 10.1113/jphysiol.1993.sp019965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972;224:665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur T, Nawy S. Characterization of Trpm1 desensitization in ON bipolar cells and its role in downstream signalling. J Physiol. 2012;590:179–192. doi: 10.1113/jphysiol.2011.218974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KJ, Rieke F. Temporal contrast adaptation in the input and output signals of salamander retinal ganglion cells. J Neurosci. 2001;21:287–299. doi: 10.1523/JNEUROSCI.21-01-00287.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Freed MA. The On pathway rectifies the Off pathway of the mammalian retina. J Neurosci. 2010;30:5533–5543. doi: 10.1523/JNEUROSCI.4733-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manookin MB, Beaudoin DL, Ernst ZR, Flagel LJ, Demb JB. Disinhibition combines with excitation to extend the operating range of the OFF visual pathway in daylight. J Neurosci. 2008;28:4136–4150. doi: 10.1523/JNEUROSCI.4274-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronarde DN. Correlated firing of cat retinal ganglion cells. I. Spontaneously active inputs to X-and Y-cells. J Neurophysiol. 1983;49:303–324. doi: 10.1152/jn.1983.49.2.303. [DOI] [PubMed] [Google Scholar]
- Murphy GJ, Rieke F. Electrical synaptic input to ganglion cells underlies differences in the output and absolute sensitivity of parallel retinal circuits. J Neurosci. 2011;31:12218–12228. doi: 10.1523/JNEUROSCI.3241-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. AII amacrine cells quicken time course of rod signals in the cat retina. J Neurophysiol. 1982;47:928–947. doi: 10.1152/jn.1982.47.5.928. [DOI] [PubMed] [Google Scholar]
- Oertner TG, Sabatini BL, Nimchinsky EA, Svoboda K. Facilitation at single synapses probed with optical quantal analysis. Nat Neurosci. 2002;5:657–664. doi: 10.1038/nn867. [DOI] [PubMed] [Google Scholar]
- Oesch NW, Diamond JS. Ribbon synapses compute temporal contrast and encode luminance in retinal rod bipolar cells. Nat Neurosci. 2011;14:1555–1561. doi: 10.1038/nn.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltedal L, Veruki ML, Hartveit E. Passive membrane properties and electrotonic signal processing in retinal rod bipolar cells. J Physiol. 2009;587:829–849. doi: 10.1113/jphysiol.2008.165415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Abd-El-Barr MM, Gao F, Bramblett DE, Paul DL, Wu SM. Relative contributions of rod and cone bipolar cell inputs to AII amacrine cell light responses in the mouse retina. J Physiol. 2007;580:397–410. doi: 10.1113/jphysiol.2006.120790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen O, Heggelund P. Quantal properties of spontaneous EPSCs in neurones of the guinea-pig dorsal lateral geniculate nucleus. J Physiol. 1996;496:759–772. doi: 10.1113/jphysiol.1996.sp021725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavachari S, Lisman JE. Properties of quantal transmission at CA1 synapses. J Neurophysiol. 2004;92:2456–2467. doi: 10.1152/jn.00258.2004. [DOI] [PubMed] [Google Scholar]
- Rockhill RL, Daly FJ, MacNeil MA, Brown SP, Masland RH. The diversity of ganglion cells in a mammalian retina. J Neurosci. 2002;22:3831–3843. doi: 10.1523/JNEUROSCI.22-09-03831.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roska B, Molnar A, Werblin FS. Parallel processing in retinal ganglion cells: how integration of space-time patterns of excitation and inhibition form the spiking output. J Neurophysiol. 2006;95:3810–3822. doi: 10.1152/jn.00113.2006. [DOI] [PubMed] [Google Scholar]
- Sagdullaev BT, McCall MA, Lukasiewicz PD. Presynaptic inhibition modulates spillover, creating distinct dynamic response ranges of sensory output. Neuron. 2006;50:923–935. doi: 10.1016/j.neuron.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Sakitt B. Counting every quantum. J Physiol. 1972;223:131–150. doi: 10.1113/jphysiol.1972.sp009838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saszik S, DeVries SH. A mammalian retinal bipolar cell uses both graded changes in membrane voltage and all-or-nothing Na+ spikes to encode light. J Neurosci. 2012;32:297–307. doi: 10.1523/JNEUROSCI.2739-08.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeweis DM, Schnapf JL. The photovoltage of macaque cone photoreceptors: adaptation, noise, and kinetics. J Neurosci. 1999;19:1203–1216. doi: 10.1523/JNEUROSCI.19-04-01203.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapley R, Victor J. Hyperacuity in cat retinal ganglion cells. Science. 1986;231:999–1002. doi: 10.1126/science.3945816. [DOI] [PubMed] [Google Scholar]
- Singer JH, Diamond JS. Vesicle depletion and synaptic depression at a mammalian ribbon synapse. J Neurophysiol. 2006;95:3191–3198. doi: 10.1152/jn.01309.2005. [DOI] [PubMed] [Google Scholar]
- Singer JH, Lassova L, Vardi N, Diamond JS. Coordinated multivesicular release at a mammalian ribbon synapse. Nat Neurosci. 2004;7:826–833. doi: 10.1038/nn1280. [DOI] [PubMed] [Google Scholar]
- Smith RG, Vardi N. Simulation of the AII amacrine cell of mammalian retina: functional consequences of electrical coupling and regenerative membrane-properties. Vis Neurosci. 1995;12:851–860. doi: 10.1017/s095252380000941x. [DOI] [PubMed] [Google Scholar]
- Stern P, Edwards FA, Sakmann B. Fast and slow components of unitary EPSCs on stellate cells elicited by focal stimulation in slices of rat visual cortex. J Physiol. 1992;449:247–278. doi: 10.1113/jphysiol.1992.sp019085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WR, Chen E, Copenhagen DR. Characterization of spontaneous excitatory synaptic currents in salamander retinal ganglion cells. J Physiol. 1995;486:207–221. doi: 10.1113/jphysiol.1995.sp020803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WR, Smith RG. Transmission of scotopic signals from the rod to rod-bipolar cell in the mammalian retina. Vision Res. 2004;44:3269–3276. doi: 10.1016/j.visres.2004.07.043. [DOI] [PubMed] [Google Scholar]
- Tian M, Jarsky T, Murphy GJ, Rieke F, Singer JH. Voltage-gated Na channels in AII amacrine cells accelerate scotopic light responses mediated by the rod bipolar cell pathway. J Neurosci. 2010;30:4650–4659. doi: 10.1523/JNEUROSCI.4212-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian N, Hwang TN, Copenhagen DR. Analysis of excitatory and inhibitory spontaneous synaptic activity in mouse retinal ganglion cells. J Neurophysiol. 1998;80:1327–1340. doi: 10.1152/jn.1998.80.3.1327. [DOI] [PubMed] [Google Scholar]
- Tong G, Jahr CE. Multivesicular release from excitatory synapses of cultured hippocampal neurons. Neuron. 1994;12:51–59. doi: 10.1016/0896-6273(94)90151-1. [DOI] [PubMed] [Google Scholar]
- Trong PK, Rieke F. Origin of correlated activity between parasol retinal ganglion cells. Nat Neurosci. 2008;26:1343–1351. doi: 10.1038/nn.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rossum MC, O'Brien BJ, Smith RG. Effects of noise on the spike timing precision of retinal ganglion cells. J Neurophysiol. 2003;89:2406–2419. doi: 10.1152/jn.01106.2002. [DOI] [PubMed] [Google Scholar]
- Wadiche JI, Jahr CE. Multivesicular release at climbing fiber-Purkinje cell synapses. Neuron. 2001;32:301–313. doi: 10.1016/s0896-6273(01)00488-3. [DOI] [PubMed] [Google Scholar]
- White JA, Rubinstein JT, Kay AR. Channel noise in neurons. Trends Neurosci. 2000;23:131–137. doi: 10.1016/s0166-2236(99)01521-0. [DOI] [PubMed] [Google Scholar]
- Xu Y, Vasudeva V, Vardi N, Sterling P, Freed MA. Different types of ganglion cell share a synaptic pattern. J Comp Neurol. 2008;507:1871–1878. doi: 10.1002/cne.21644. [DOI] [PubMed] [Google Scholar]
- Yin L, Smith RG, Sterling P, Brainard DH. Chromatic properties of horizontal and ganglion cell responses follow a dual gradient in cone opsin expression. J Neurosci. 2006;26:12351–12361. doi: 10.1523/JNEUROSCI.1071-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghloul KA, Boahen K, Demb JB. Different circuits for ON and OFF retinal ganglion cells cause different contrast sensitivities. J Neurosci. 2003;23:2645–2654. doi: 10.1523/JNEUROSCI.23-07-02645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.