Abstract
Submucosal neurons are vital regulators of water and electrolyte secretion and local blood flow in the gut. Due to the availability of transgenic models for enteric neuropathies, the mouse has emerged as the research model of choice, but much is still unknown about the murine submucosal plexus. The progeny of choline acetyltransferase (ChAT)-Cre × ROSA26YFP reporter mice, ChAT-Cre;R26R-yellow fluorescent protein (YFP) mice, express YFP in every neuron that has ever expressed ChAT. With the aid of the robust YFP staining in these mice, we correlated the neurochemistry, morphology and electrophysiology of submucosal neurons in distal colon. We also examined whether there are differences in neurochemistry along the colon and in neurally mediated vectorial ion transport between the proximal and distal colon. All YFP+ submucosal neurons also contained ChAT. Two main neurochemical but not electrophysiological groups of neurons were identified: cholinergic (containing ChAT) or non-cholinergic. The vast majority of neurons in the middle and distal colon were non-cholinergic but contained vasoactive intestinal peptide. In the distal colon, non-cholinergic neurons had one or two axons, whereas the cholinergic neurons examined had only one axon. All submucosal neurons exhibited S-type electrophysiology, shown by the lack of long after-hyperpolarizing potentials following their action potentials and fast excitatory postsynaptic potentials (EPSPs). Fast EPSPs were predominantly nicotinic, and somatic action potentials were mediated by tetrodotoxin-resistant voltage-gated channels. The size of submucosal ganglia decreased but the proportion of cholinergic neurons increased distally along the colon. The distal colon had a significantly larger nicotinic ion transport response than the proximal colon. This work shows that the properties of murine submucosal neurons and their control of epithelial ion transport differ between colonic regions. There are several key differences between the murine submucous plexus and that of other animals, including a lack of conventional intrinsic sensory neurons, which suggests there is an incomplete neuronal circuitry within the murine submucous plexus.
Introduction
The enteric nervous system (ENS) is a complex network of neurons and glia contained within the walls of the gastrointestinal tract that regulates many functions of the gut. The submucous plexus of the ENS is crucial for maintaining body fluid homeostasis and contains secretomotor and vasodilator neurons that control water and electrolyte secretion and local blood flow (Vanner & Surprenant, 1996; Vanner & MacNaughton, 2004). Furthermore, defects of the submucous plexus, particularly at the level of excitability of its neurons, are associated with many bowel disorders that produce diarrhoea. This dysfunction includes, but is not limited to, hypersecretion induced by bacterial toxins and inflammation (Xia et al. 2000; Neunlist et al. 2003; Lomax et al. 2005; Poole et al. 2007; Gwynne et al. 2009; Avula et al. 2013).
In recent years, however, the mouse has emerged as the research model of choice due to the availability of transgenic mouse models, especially models for enteric pathologies. Particular emphasis has been placed on examining the functional roles of the submucous plexus of the mouse colon (Buresi et al. 2005; Hyland & Cox, 2005; Sorensen et al. 2010; Okamoto et al. 2012). This focus is possibly due to the relative ease of dissection of the murine colon compared to its small intestine, and considerable interest in examining colonic inflammation (MacNaughton et al. 1998; Klompus et al. 2010; Hock et al. 2011; Juric et al. 2013). However, the colonic regions examined in different studies often differ, which makes direct comparison between studies difficult, especially as there may be regional differences in the properties of submucosal neurons and in the epithelial responses between the proximal and distal colon of rodents (Cunningham & Lees, 1995; Cunningham et al. 1997; Klompus et al. 2010). Moreover, a significant lack in knowledge of the murine submucous plexus has hampered our understanding of the neural reflex pathways involved in epithelial ion transport. Owing to over three decades of research, the guinea-pig ileum remains the best studied model of the ENS and it is often used as a reference for research on other gut regions and animal models (Porter et al. 1996, 1999; Mann et al. 1999; Hens et al. 2000; Lomax & Furness, 2000; Gwynne & Bornstein, 2007; Mongardi Fantaguzzi et al. 2009). Despite evidence of clear interspecies differences, the interpretation of mouse data to date still relies on assumptions drawn predominantly from work on guinea-pig intestine.
Current understanding of murine submucous neurons is mainly based on two studies that examined the neurochemistry of submucosal neurons in the ileum (Mongardi Fantaguzzi et al. 2009), and the electrophysiology and morphology of submucosal neurons in the colon (Wong et al. 2008). It appears that the two broad categories of secretomotor neurons, cholinergic (containing choline acetyltransferase, ChAT) and non-cholinergic (expressing vasoactive intestinal peptide, VIP) are conserved between the guinea-pig and mouse ileum (Gwynne & Bornstein, 2007; Mongardi Fantaguzzi et al. 2009). There is immunohistochemical data showing cholinergic submucosal neurons in mouse colon (Sang & Young, 1998), but their functional roles are unclear. The guinea-pig submucous plexus includes a subpopulation of intrinsic sensory neurons that are identified by their Dogiel type II (DII) morphology and AH-type (prolonged after-hyperpolarizing potentials following action potentials) electrophysiology (Lomax et al. 2001; Gwynne & Bornstein, 2007). In contrast, no ileal submucosal neurons in mouse display DII morphology (Mongardi Fantaguzzi et al. 2009) and, while putative intrinsic sensory neurons were reported in the colon, none of the neurons examined displayed AH-type electrophysiology (Wong et al. 2008). Furthermore, it is not currently known how the properties of the murine submucosal neurons correlate with each other and how this differs in different gut regions.
In this study, we used ChAT-Cre;R26R-yellow fluorescent protein (YFP) mice, which express YFP in cholinergic neurons, to examine the neurochemistry of colonic submucosal neurons and found that there was a gradient along the colon in total number of neurons per ganglion and in the proportion of cholinergic YFP+ neurons. We combined conventional immunohistochemistry and intracellular recording to correlate the neurochemistry, morphology and electrophysiology of submucosal neurons in the distal colon and revealed key differences in murine submucosal neurons from those in the guinea-pig. We also showed that there were differences in neurally mediated ion transport between the proximal and distal colon mucosa.
Methods
Experimental animals
Experiments were performed on the colon of adult mice of either sex weighing 20–30 g. Two strains were used: C57Bl/6 (n = 17) and ChAT-Cre;R26R-YFP mice (n = 35). ChAT-Cre;R26R-YFP mice were bred in house and were the offspring of homozygous ChAT-Cre and homozygous ROSA26YFP reporter mice, both lines originally obtained from the Jackson Laboratory (Bar Harbor, ME, USA). Mice were killed by cervical dislocation, a method approved by the University of Melbourne Animal Experimentation Ethics Committee. All experiments conformed with The Journal of Physiology policy on animal experimentation (Drummond, 2009). The whole colon (from the base of the caecum to the anus) was removed and immediately placed in physiological saline (composition in mm: NaCl 118, NaHCO3 25, d-glucose 11, KCl 4.8, CaCl2 2.5, MgSO4 1.2, NaH2PO4 1.0) bubbled with carbogen gas (95% O2/5% CO2). The colonic segments were then cut along the mesenteric border, stretched and pinned flat mucosal side up in a Petri dish lined with a silicone elastomer (Sylgard 184; Dow Corning, North Ryde, NSW, Australia).
Examining the chemical coding of submucosal neurons along the colon
The full length of a stretched colon was 7.6–8.5 cm. Three colonic regions were examined: proximal (just below the base of the caecum), middle (about 2.5 cm from the base of the caecum, an area after the transverse folds of the proximal colon mucosa) and distal (1–2 cm above the anus). To generate whole mount submucous plexus preparations (area of about 1 × 1 cm), the mucosa first was removed from the other layers by microdissection from the three regions of fresh colon. The entire colon from C57/Bl6 (n = 7) and ChAT-Cre;R26R-YFP (n = 7) mice was then fixed overnight in 4% formaldehyde in 0.1 m phosphate buffer, pH 7.2, at 4 °C. Subsequently, the tissue was given three washes with phosphate-buffered saline (PBS), and submucosal plexus preparations from the exposed proximal, middle and distal regions were dissected away from the muscle layers. These preparations were incubated for 30 min with 1% Triton X-100 (ProSchiTech, Thuringowa, QLD, Australia). The tissue was then given three washes with PBS, followed by overnight incubation with primary antibodies (Table 1) at 4°C. After three washes with PBS, the tissue was incubated with secondary antibodies (Table 2) for about 2.5 h. The tissue was given another three washes with PBS, and then mounted on a slide. Images of submucosal plexuses were taken using a Zeiss Pascal confocal laser scanning microscope.
Table 1.
Primary antisera used
| Antigen | Species | Source | Concentration |
|---|---|---|---|
| VIP | Rabbit | Millipore | 1:1000 |
| ChAT | Goat | Chemicon | 1:100 |
| Hu | Human | Gift from Dr V. Lennon | 1:5000 |
| Calretinin | Goat | Swant | 1:1000 |
| Green fluorescent protein (GFP) | Goat | Rockland | 1:400 |
| Rabbit | Molecular Probes | 1:500 | |
| Calcitonin gene-related peptide (CGRP) | Goat | Biogenesis | 1:1000 |
| Nitric oxide synthase (NOS) | Sheep | Gift from Dr P. Emson | 1:1000 |
| Tyrosine hydroxylase (TH) | Sheep | Chemicon | 1:160 |
Table 2.
Secondary antisera used
| Species in which primary antisera were raised | Secondary antisera | Source | Concentration |
|---|---|---|---|
| Rabbit | Donkey anti-rabbit Alexa 488 | Molecular Probes | 1:400 |
| Donkey anti-rabbit Alexa 647 | Molecular Probes | 1:400 | |
| Donkey anti-rabbit CY5 | Jackson ImmunoResearch | 1:100 | |
| Sheep or goat | Donkey anti-sheep Alexa 647 | Molecular Probes | 1:500 |
| Donkey anti-sheep Alexa 488 | Molecular Probes | 1:400 | |
| Human | Donkey anti-human Texas Red | Jackson ImmunoResearch | 1:100 |
| Donkey anti-human Alexa 594 | Jackson ImmunoResearch | 1:800 |
Submucosal preparations from proximal, middle and distal colonic regions of C57Bl/6 (n = 4) and ChAT-Cre;R26R-YFP mice (n = 4) were imaged using a 40× objective lens. The intensities of staining for neurochemical markers (ChAT and calcitonin gene-related peptide (CGRP)) were variable but unambiguous. A neuron was considered to be immunoreactive for a neurochemical marker if the staining of the cytoplasm was higher than background such that an unstained nucleus could be seen. At least 200 Hu+ (pan-neuronal marker) neurons were counted in each proximal colonic preparation, and 100 Hu+ neurons counted in each middle and distal colonic preparation. From these populations, the number of YFP+/ChAT+ neurons and/or VIP+ neurons was counted in each region. The number of Hu+ neurons per ganglion was also examined in each region. A ganglion was defined as a group of neurons that were at least 20 μm apart from neighbouring ganglia. The chemical coding of submucosal neurons in the distal colon was further analysed by counting at least 100 Hu+ neurons and examining co-expression of YFP or VIP with other subtype-specific markers (three animals were examined for each marker unless otherwise indicated).
To examine projections of submucosal neuronal subtypes to the mucosa, the colon was fixed with 4% formaldehyde and frozen sections were obtained from all three colonic regions using previously described methods (see supplementary methods, Gwynne et al. 2009). Briefly, 4 or 6 μm sections were cut on a cryostat (Microm HM 525, Fronine Laboratory Supplies, Riverstone, NSW, Australia), and mounted on positively charged slides (SuperFrostPlus, Menzel-Glaser, Braunschweig, Germany). Sections were left to dry for 1 h, and then immunostained (4°C) with antibodies against Hu, YFP and VIP. After mounting with coverslips, images were taken using confocal microscopy.
Correlating electrophysiology, morphology and neurochemistry of submucosal neurons in the distal colon
The mucosal layer was removed, and submucosal plexus preparations were dissected away from the muscle layers of distal colonic regions (about 2 cm oral to the anus). Submucosal ganglia were viewed at ×200 magnification using an Olympus inverted light microscope (Olympus IX70). Conventional intracellular recording techniques were applied to impale and record from submucosal neurons. Microelectrodes (100–200 MΩ) containing 1 m KCl and 2% biocytin (Sigma Aldrich, Castle Hill, NSW, Australia) were used. The excitability and synaptic potential profile for submucosal neurons were examined as previously described (Foong et al. 2012).
Following electrophysiological studies, preparations were fixed overnight in 4% formaldehyde at 4°C. The preparations were then processed to reveal biocytin-filled neurons as previously described (Foong et al. 2012). To determine whether the impaled neurons were YFP+ and to examine their dendritic morphologies, cell bodies were imaged with a ×40 objective lens on a confocal microscope. Neurons were also imaged with lower power objectives (×10 and ×20) to allow examination of their axonal projection patterns.
Data measurements and statistics
Electrophysiological data were recorded and analysed using Axoscope 10.2.0.14 software (Axon Instruments, Union City, CA, USA). The excitability of neurons was investigated by measuring the action potential threshold, counting the maximum number of action potentials evoked by 500 ms depolarizing current pulses and measuring the total duration of their firing. The input resistance of neurons was determined from line of best fit (linear regression) to an I–V plot.
Ussing chamber studies
Submucosal–mucosal sheets were prepared from four C57Bl/6 mice as described previously (Hyland and Cox, 2005). Each animal provided four preparations: two from the proximal colon (beneath the caecum) and another pair from the distal colon (no more than 2 cm oral to the anus). Preparations were then mounted in Ussing chambers (exposed area of 0.14 cm2) continuously bathing both sides in 5 ml carbogenated physiological saline maintained at 37°C. The preparations were voltage clamped at 0 mV (DVC1000; WPI, Sarasota, FL, USA) as previously described and the resulting short-circuit current (Isc) was recorded continuously. After a stable basal Isc was achieved (within 20 min), veratridine, the nicotinic antagonist hexamethonium (200 μm), nicotine (10 μm) and tetrodotoxin (TTX, 100 nm) were applied to the serosal hemichamber. Adjacent colonic preparations received veratridine at either 1 or 30 μm, and peak changes in Isc responses were converted to units of μA cm−2.
Statistics
All results are presented as mean ± SEM. A one-way analysis of variance (ANOVA) with Tukey Kramer post hoc test was performed to compare neurochemical data between different colonic regions. A two-tailed Student's paired t-test was performed for electrophysiological experiments. For comparison between animals and Ussing chamber data, a Student's unpaired t-test or one-way ANOVA with Bonferroni's post-test was conducted. P values of less than 0.05 were considered to be significant.
Drugs used
Hexamethonium bromide, nicotine, veratridine, pyridoxal phosphate-6-azo(benzene-2,4-disulfonic acid) (PPADS) (all from Sigma-Aldrich), TTX (Sigma-Aldrich or Alomone Labs, Jerusalem, Israel) and veratridine were prepared as stock solutions and diluted in physiological saline before addition to the superfusing solution or the reservoir supplying the serosal hemichamber.
Results
YFP-immunoreactive neurons also express ChAT and CGRP
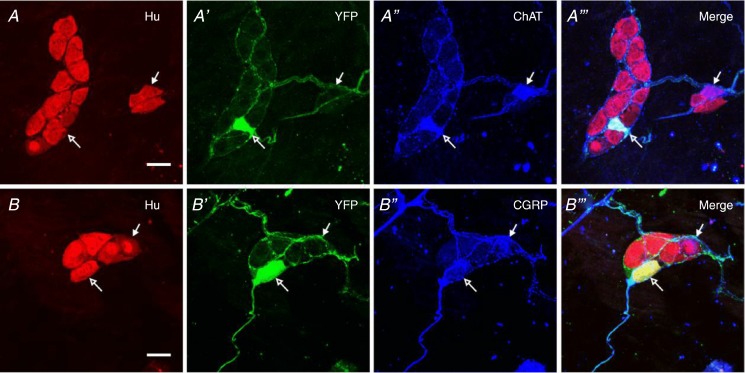
To validate ChAT-Cre;R26R-YFP mice as models to study cholinergic submucosal neurons, we examined whether YFP+ submucosal neurons from distal colon exhibited ChAT-immunoreactivity. Almost all ChAT-immunoreactive (IR) neurons were YFP+ (90 ± 3.7% of ChAT-IR neurons, total of 45 ChAT-IR neurons from three mice); importantly, all YFP+ neurons were ChAT-IR (n = 3; Fig. 1A). Furthermore, all YFP+ neurons were CGRP-IR, while 90.9 ± 6.4% of all CGRP-IR neurons were YFP+ (29 YFP+ neurons from three mice; Fig. 1B).
Figure 1.
Whole-mount preparations of the submucous plexus of the mouse distal colon immunostained for Hu (red), YFP (green) and ChAT or CGRP (blue). Some Hu+ neurons were immunoreactive for YFP (open arrows, A and B). YFP immunoreactivity was observed in the cell bodies of neurons, in fibres running within interganglionic fibre tracts, and in fibres and terminals surrounding neurons in ganglia. A, all YFP+ neurons contained ChAT (open arrows), while a minority of ChAT+ neurons were not YFP+ (9.4 ± 3.7% ChAT+ neurons, n = 3 animals; filled arrows). B, all YFP+ neurons also contained CGRP (open arrows), but some CGRP+ neurons were not YFP+ (9.1 ± 6.4% CGRP+ neurons, n = 3 animals; filed arrows). Scale bars = 20 μm.
The number of neurons per ganglion decreases, while the proportion of ChAT/YFP+ neurons increases distally along the colon
The number of neurons per ganglion in the proximal colon was significantly greater than in the middle and distal colon (Fig. 2A–E; number of ganglia examined: proximal 127, middle 85, distal 95; P < 0.001, data from four ChAT-Cre;R26R-YFP mice). The smallest ganglia of the three regions examined were in the distal colon (5 ± 1 neurons per ganglion, n = 4; Fig. 2D and E). There was no significant difference between the two mouse species (data from C57Bl6 mice not shown).
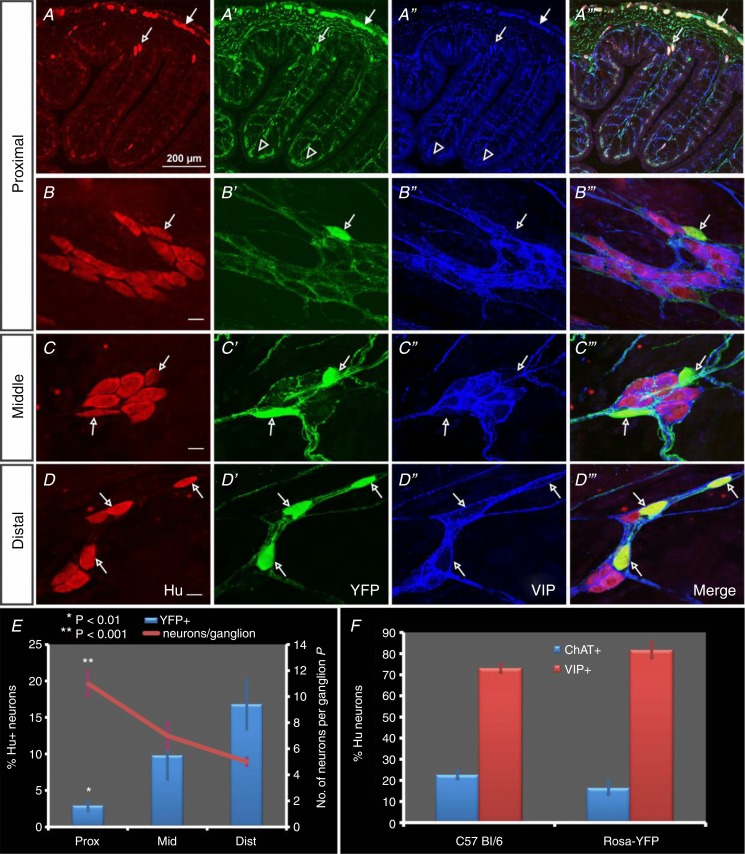
Figure 2.
Cryostat sections of the mouse proximal colon (A), and whole-mount preparations of the submucous plexus of proximal (B), middle (C) and distal (D) colon immunostained for Hu (red), YFP (green) and VIP (blue). A, YFP+ and VIP+ neuronal somata were present in the myenteric plexus (filled arrows) and submucous plexus (open arrows), and the fibres of these neurons were found in the circular muscle layers and mucosa (open arrowheads). B–D, the majority of Hu+ neurons in all colonic regions were either immunoreactive for YFP (open arrows) or VIP, which did not appear throughout the cytoplasm, but was concentrated in a crescent around the nucleus, presumably in the Golgi apparatus. There were many YFP+ and VIP+ fibres and terminals located within submucosal ganglia and interganglionic fibre tracts. E, the number of neurons per ganglion decreased while the proportion of YFP+ neurons increased distally along the colon (n = 4 ChAT-Cre;R26R-YFP mice). The vast majority of submucosal neurons in the distal colon were non-cholinergic but contained VIP. F, there was no significant difference in the proportions of cholinergic and non-cholinergic/VIP+ neurons in the distal colon of C57Bl/6 and ChAT-Cre;R26R-YFP mice. Scale bars = 20 μm unless stated otherwise.
The proportion of cholinergic neurons was significantly lower in the proximal colon (2.9 ± 0.7% of all Hu+ neurons were YFP+) than in the distal colon (16.8 ± 3.4% of all Hu+ neurons were YFP+; P < 0.01, n = 4 ChAT-Cre;R26R-YFP mice; Fig. 2E; data from C57Bl/6 mice not shown). However, the vast majority of Hu+ submucosal neurons of the middle and distal colonic regions were non-cholinergic (did not contain YFP or ChAT) and expressed VIP (Fig. 2F). The VIP+ neurons in the proximal colon could not be adequately quantified as somatic VIP staining in these neurons was obscured by brightly stained, and abundant, VIP+ nerve terminals surrounding the ganglia. In all three colonic regions YFP+ and VIP+ fibres were observed in the mucosa and smooth muscle layers (proximal colon data shown in Fig. 2A) as well as within the submucous plexus itself (Fig. 2B–D). In the middle and distal colon, neurons that were cholinergic and VIP+ were rare (<2% of all Hu+ neurons), and a small proportion of Hu+ neurons did not contain YFP/ChAT or VIP (<4% of all Hu+ neurons). There was no significant difference in the proportion of cholinergic/YFP+ or non-cholinergic (YFP+/VIP+) neurons in the submucous plexus along the colon of C57Bl/6 or ChAT-Cre;R26R-YFP mice (distal colon data only are shown in Fig. 2F).
Calretinin, nitric oxide synthase (NOS) and tyrosine hydroxylase (TH) immunoreactivities in submucosal neurons in the colon were also examined. In the distal colon of C57Bl/6 mice, most Hu+ neurons were calretinin-IR (87.8 ± 6.6%, n = 2). A subset of neurons were NOS-IR (26.9 ± 2.5% Hu+ neurons, n = 3) or TH-IR (38.8 ± 13.6% Hu+ neurons, n = 3). Almost all (>95%) NOS-IR and TH-IR neurons were VIP+ (n = 3).
Correlating electrophysiology, morphology and neurochemistry of submucosal neurons in the distal colon
Intracellular recordings were made from submucosal neurons from the distal colon of C57Bl/6 (n = 12) and ChAT-Cre;R26R-YFP mice (n = 35). This region was chosen to allow comparison with the only previous study of electrophysiological properties of mouse submucosal neurons (Wong et al. 2008). In C57Bl/6 mice, 35 neurons were impaled, with successful recordings from 28 neurons; the morphology of 12/28 neurons was revealed by post hoc localization of biocytin. Of the 35 neurons, five provided morphological data only. On the other hand, 85 neurons were impaled from ChAT-Cre;R26R-YFP mice and electrophysiological recordings were obtained from 76 neurons; 39/76 were further characterized morphologically. Eight neurons supplied morphological data only.
All submucosal neurons in the distal colon display S-type electrophysiology
The electrophysiological properties of submucosal neurons recorded from the distal colon of C57Bl/6 and ChAT-Cre;R26R-YFP mice are summarized in Table 3. There were no obvious differences between the two mouse strains. The resting membrane potentials and input resistances were very similar to S-type submucosal neurons of the guinea-pig distal colon (Lomax et al. 2001). In comparison with a previous report on a similar preparation (Wong et al. 2008), we found that neurons had a lower threshold for action potential discharge, and could be classified into two groups according to their firing patterns: (1) fired 2–28 action potentials over the full duration of the current pulse (tonic firing, Fig. 3A'); or (2) fired 1–3 action potentials at the beginning of the depolarizing current pulse (phasic firing, Fig. 3B'). However, in agreement with the previous study (Wong et al. 2008), a prominent shoulder was observed on the repolarizing phase of the voltage response to a hyperpolarizing current pulse (A-type potassium conductance, IA) in some neurons, while characteristics common to neurons that exhibit AH-type electrophysiology, such as a sag in the voltage response to a hyperpolarizing current (Ih) and anode break action potentials, were rarely observed. Only 2/13 neurons that exhibited IA current also had an Ih and/or anode break action potentials. Prolonged after-hyperpolarizing potentials (AHPs) following action potentials were never observed (n = 46).
Table 3.
Electrophysiological properties of submucosal neurons in the distal colon
| AP threshold (pA) |
Max. no. of APs |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RMP (mV) | Rin (MΩ) | Phasic | Tonic | Phasic | Tonic | Pres ab-APs | Pres fEPSPs | Pres Ih | Pres IA | Pres sEPSPs | Pres IPSPs |
| −51 ± 1 | 230 ± 12 | 92 ± 12 | 56 ± 4 | 1–14 | 2–28 | 0.02% | 99% | 13% | 33% | 38% | 57% |
| (n = 23) | (n = 40) | (n = 26) | (n = 20) | (n = 26) | (n = 20) | n = 1/41 | n = 98/99 | n = 5/40 | n = 13/40 | n = 16/42 | n = 26/46 |
ab, anode break; AP, action potential; fEPSPs, fast excitatory postsynaptic potentials; IPSPs, inhibitory postsynaptic potentials; Pres, presence; sEPSPs, slow excitatory postsynaptic potentials.
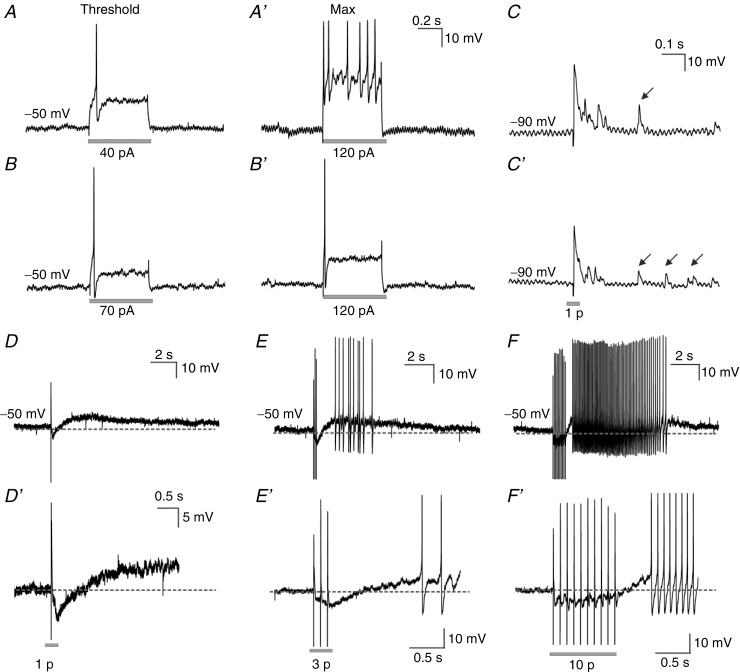
Figure 3.
In response to a 500 ms long depolarizing step current, neurons fired action potentials over the full duration of the current pulse (A, threshold response; A′, maximum response reflecting tonic firing), or only at the beginning of the current pulse (B, threshold response; B′, maximum response reflecting phasic firing). Almost all neurons examined displayed fast EPSPs in response to a single pulse stimulus. In addition to the stimulus locked fast EPSPs, some neurons also exhibited a burst of fast EPSPs after the stimulus (arrows) that varied between trials in the same neuron (C, trial 1; C′, trial 2). Some neurons also exhibited slow EPSPs and IPSPs in response to a single stimulus (D) or a train of stimuli (E, three pulses 20 Hz; F, 10 pulses 20 Hz). Although the slow EPSPs were small in amplitude, they often evoked action potentials (E and F). D′ and F′, traces D and F were expanded and cropped to reveal the IPSPs evoked at the beginning of the stimulus.
Electrical stimulation of interganglionic fibre tracts revealed the synaptic potential profile of submucosal neurons. Almost all neurons (99%) exhibited fast excitatory postsynaptic potentials (EPSPs) spontaneously and/or in response to single pulse stimulation (Fig. 3C). The stimulus-locked fast EPSPs evoked were typically followed by a volley, lasting up to 500 ms, of fast EPSPs with latencies that varied from trial to trial (Fig. 3C and C'). Fast EPSPs are common to S-type but not AH-type neurons in the ENS (Lomax et al. 2001; Nurgali et al. 2004). Some neurons exhibited slow EPSPs (38%) and/or inhibitory postsynaptic potentials (IPSPs, 57%) that were small in amplitude (<5 mV). These were evoked in response to trains of stimuli (3 or 10 pulses, 20 Hz) as reported previously (Wong et al. 2008), but also in response to single pulse stimulation (Fig. 3D–F).
Non-cholinergic submucosal neurons in the distal colon have either one or two axons
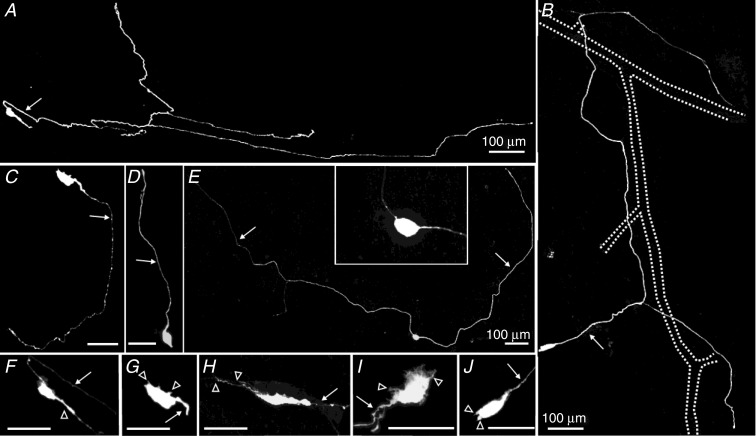
Morphologies of 64 neurons were examined (Fig. 4). The majority had only one axon (59/64, Fig. 4A–D) and the remaining five neurons had two axons (multiaxonal, Fig. 4E). Neurons with two axons had smooth cell bodies (Fig. 4E). Four of these neurons projected their axons circumferentially, while one neuron projected an axon orally and the other anally alongside blood vessels. Uniaxonal neurons displayed a variety of dendritic morphologies (Fig. 4F–J). Their axons projected orally, anally and/or circumferentially (Fig. 4A–D). The projection patterns of uniaxonal neurons were quite variable as their axons were commonly observed to loop around and change direction. These neurons also had a wide range of projection lengths (150–2410 μm). Twenty-three (three multiaxonal) of the 64 neurons had axons that branched (up to six branches) and ran closely alongside blood vessels (Fig. 4B).
Figure 4.
Neurons in the submucous plexus had one (A–D) or two (E) axons (arrows). Uniaxonal neurons project their axons circumferentially (A), orally (C) or anally (D). The projection patterns of some of these neurons are quite variable as their axons are commonly observed to loop around and change direction. The axons of neurons that project circumferentially can have several branches (A and B), and most of these branching neurons run closely alongside blood vessels (B, dotted lines = blood vessels). Uniaxonal neurons display a wide range of dendritic morphologies (F–J, open arrowheads = dendrites). E, neurons with two axons have smooth cell bodies (inset) and typically project circumferentially. Scale bars = 20 μm unless indicated otherwise.
It was reported previously in guinea-pigs that intracellular recording from neurons weakens their immunoreactivity for certain neurochemical markers (Bornstein et al. 1986, 1987, 1989). In mice, given that submucosal neuronal cell bodies stain only weakly for ChAT or VIP, it was previously impossible to examine the neurochemistry of impaled neurons. In this study, the advantageous bright and robust staining of YFP in ChAT-Cre;R26R-YFP mice was used to determine whether impaled biocytin-filled neurons were cholinergic or not. Forty-seven of the 64 neurons were obtained from ChAT-Cre;R26R-YFP mice. Of these neurons, four had two axons and were not immunoreactive for YFP. Only 4/47 neurons were immunoreactive for YFP; these neurons were uniaxonal and did not have a common dendritic morphology or projection pattern.
Somatic action potentials are TTX-resistant, and fast transmission is mainly nicotinic
Previous electrophysiological studies of myenteric and submucosal neurons in mice have shown that action potentials are mainly mediated by TTX-sensitive voltage-gated Na+ channels (VGSCs) (Wong et al. 2008; Hao et al. 2012). However, TTX-resistant VGSC mRNA (NaV1.5 and 1.9) is present in the myenteric plexus (Hao et al. 2012), and TTX-resistant prepotentials are displayed by submucosal neurons in the murine ENS (Wong et al. 2008). In the present study, TTX (1 μm) was applied to examine the VGSCs involved in action potentials exhibited by submucosal neurons of the mouse distal colon. TTX did not affect the current threshold for somatic action potential firing (control: 64 ± 10 pA; TTX: 70 ± 12 pA), the maximum number of action potentials fired (control: 4 ± 1; TTX: 3 ± 2) or the duration of firing (control: 364 ± 76 ms; TTX: 228 ± 94 ms) (all n = 7 neurons; P > 0.05). However, synaptic potentials and antidromic action potentials evoked by stimulation of interganglionic fibre tracts were completely abolished by TTX in all neurons examined (n = 6).
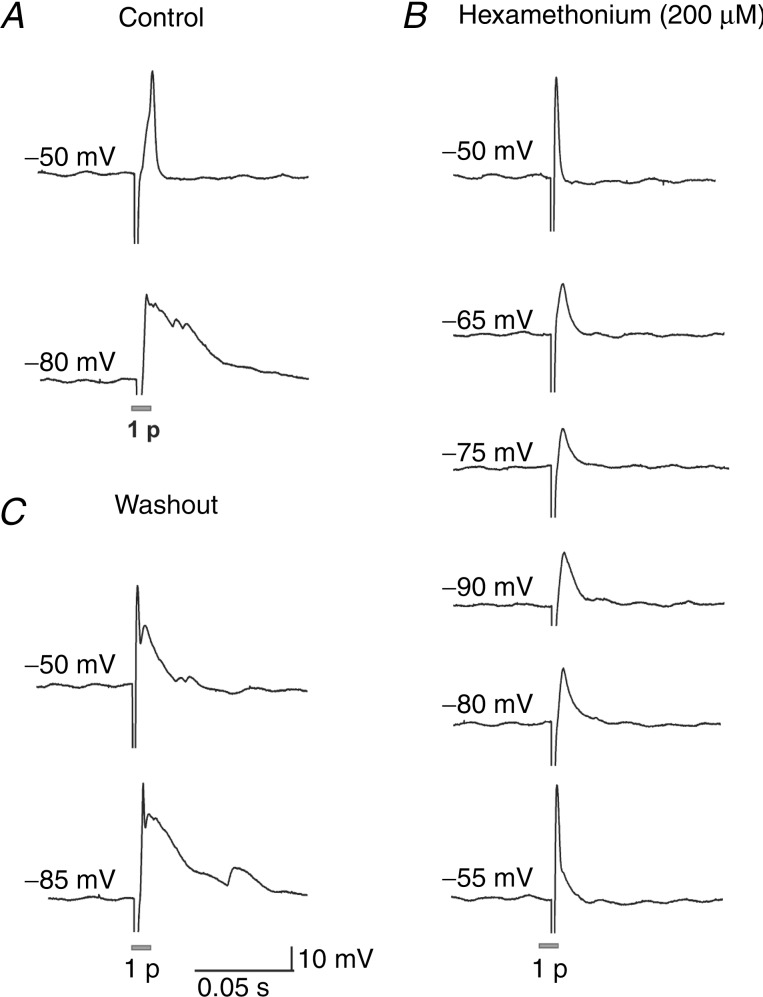
There are three known fast excitatory neurotransmitters in the ENS: acetylcholine (ACh), ATP and serotonin (Monro et al. 2004; Nurgali et al. 2004). ACh and ATP mediate fast EPSPs in the murine myenteric plexus (Nurgali et al. 2004). The previous study of the mouse submucous plexus showed that fast transmission is mainly mediated by nicotinic ACh receptors, but the presence of a nicotinic-insensitive component suggested involvement of other fast neurotransmitters (Wong et al. 2008). We applied hexamethonium (a nicotinic receptor blocker, 200 μm), and measured the amplitudes of any residual responses as the membrane potential was progressively hyperpolarized from −50 to −90 mV (in −10 mV steps). If the residual responses were fast EPSPs then they should have been enhanced by hyperpolarization. Hexamethonium significantly reduced the amplitude (control: 33 ± 2 mV; hexamethonium: 10 ± 4 mV; P <0.0001, n = 9) and duration (control: 98 ± 14 ms; hexamethonium: 17 ± 4 ms; P = 0.0005, n = 9) of the electrically stimulated response (membrane potential held at −80 to −90 mV; Figs 5 and 6E–G). A small hexamethonium-resistant component was observed in 7/9 neurons. On one occasion where the impalement was held long enough, PPADS (30 μm, a P2 receptor antagonist) was applied to the residual component but had no effect. Furthermore, the hexamethonium-resistant component was not enhanced by hyperpolarizing the membrane in all neurons examined (n = 7; Fig 5B). Therefore, hexamethonium completely abolished fast EPSPs in all neurons examined (n = 9) and the residual component was probably a proximal process potential.
Figure 5.
A–C, electrophysiological recordings from a single submucosal neuron in the distal colon. The responses were evoked by applying single pulse stimulation to an interganglionic fibre tract. A, in control, the neuron exhibits an action potential at resting membrane potential (–50 mV). Fast EPSPs are typically enhanced by hyperpolarization, and hence were revealed by holding the membrane potential of the neuron at a hyperpolarized state (−80 mV). B, hexamethonium (200 μm) significantly reduced the electrically evoked response. The amplitude of the residual component was not affected by progressive hyperpolarization of the neuron's membrane potential (−50 to −90 mV). Therefore, all fast EPSPs were abolished by hexamethonium and the residual component is probably a proximal process potential. C, fast EPSPs were recovered by washout of the drug.
Figure 6.
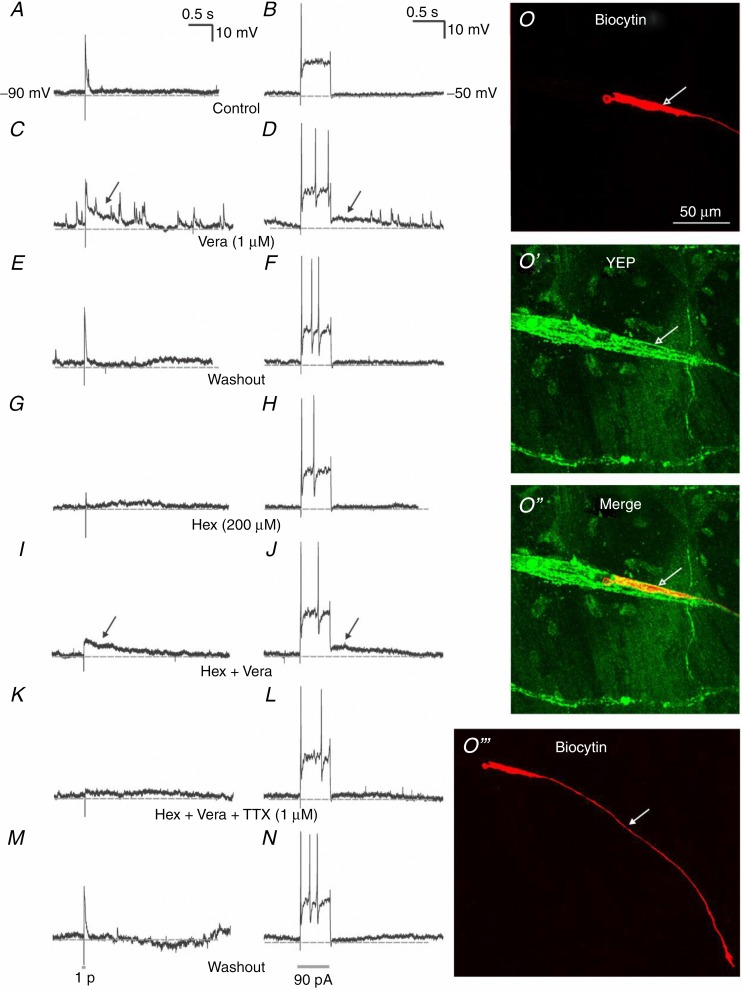
A–N, electrophysiological recordings of a sequential pharmacology experiment from a single submucosal neuron from a ChAT-Cre;R26R-YFP mouse. A, fast EPSPs in response to a single stimulus applied to an interganglionic fibre tract. B, a long depolarizing step current evokes a single action potential. C and D, veratridine (1 μm) evoked a long depolarizing potential (black arrow) and an increase in spontaneous fast EPSPs that was reversible with washout (E and F). Hexamethonium (200 μm) abolished fast EPSPs (G) but had no effect on action potential firing (H). I and J, hexamethonium also blocked spontaneous fast EPSPs evoked by veratridine without affecting veratridine-induced long depolarizing potentials. K and L, TTX abolished the veratridine-induced long depolarizing potentials, but had no effect on somatic action potential firing (L). M and N, the effects of all three antagonists were reversible with washout. O′′′, the recorded neuron was uniaxonal (white arrow = axon). O and O′′, it was not cholinergic (YFP–), but its cell body (white open arrow) was surrounded by many YFP+ terminals.
Veratridine is an alkaloid toxin found in Liliaceae plants, known to enhance the channel activity of most TTX-sensitive VGSCs resulting in neurotransmitter release (Catterall et al. 2005). It is commonly used to excite neurons, particularly in Ussing chamber experiments to examine neurally mediated ion transport (Fichna et al. 2009; Hyland & Cox, 2005; Hyland et al. 2010; O'Malley et al. 2012). We have previously shown in guinea-pig small intestine that application of veratridine induced a concentration-dependent increase in Isc, and produced a robust, half-maximal response at 1 μm (Foong et al. 2010). In the present study, we added veratridine (1 μm) to the superfusate to examine its effects on the activity of murine submucosal neurons. Veratridine did not affect the resting membrane potential of neurons, threshold for firing of somatic action potentials (control: 74 ± 27 pA; veratridine: 87 ± 27 pA), maximum number of action potentials fired (control: 4 ± 1; veratridine: 2 ± 1) or duration of firing (control: 237 ± 101 ms; veratridine: 242 ± 99 ms) (all n = 7; P > 0.05). However, in the presence of veratridine, action potentials were followed by long depolarizations (7 ± 2 mV, 0.4–11.5 s; n = 9) that were abolished by TTX (n = 2). It also induced a continuous burst of fast EPSPs that were enhanced by hyperpolarizing the membrane potential. Prior stimulation of interganglionic fibres was required in 5/10 neurons to trigger these bursts of fast EPSPs. Veratridine-induced bursts of fast EPSPs were abolished by hexamethonium (n = 2) (Fig 6A–N).
There is a larger nicotinic component in the distal colon Isc response than in proximal colon
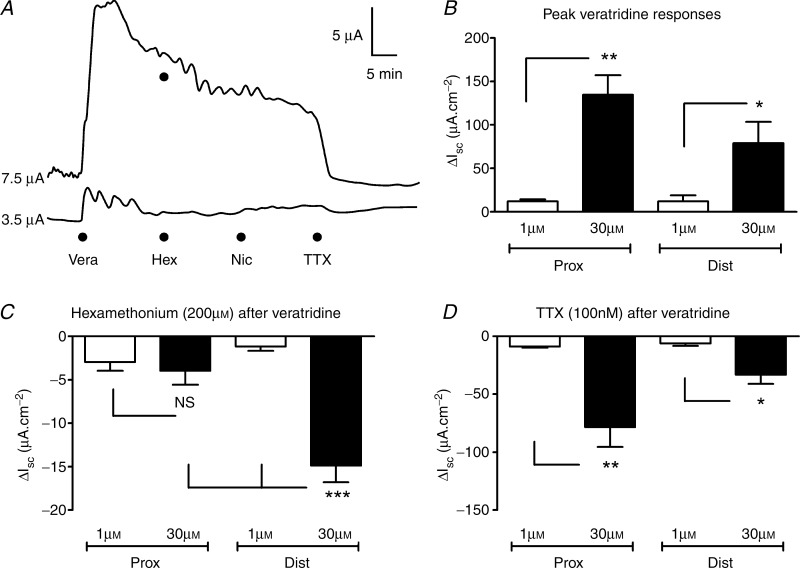
Veratridine (1 or 30 μm) was applied basolaterally to submucosal–mucosal preparations of the proximal and distal colon (n = 4 each). After the veratridine-induced Isc response had achieved its peak, hexamethonium (200 μm) was added to determine the contribution of nicotinic receptors to this long-lasting neurogenic response. Subsequently, nicotine (10 μm, data not shown) and finally TTX (100 nm) were added to confirm specific actions of hexamethonium and veratridine, respectively (Fig. 7A).
Figure 7.
A, representative distal colon responses to basolateral veratridine (Vera, 1 μm lower trace; 30 μm upper trace) followed in both preparations by addition of hexamethonium (Hex, 200 μm) nicotine (Nic, 10 μm) and tetrodotoxin (TTX, 100 nm) simultaneously. B, pooled peak responses to 1 and 30 μm veratridine in proximal (Prox) and distal colon (Dist). C, hexamethonium induced reductions in Isc after veratridine addition to the serosal surface of the proximal or distal colon preparations. D, pooled responses showing TTX sensitivity of the remaining veratridine-elevated Isc in proximal or distal colon mucosa. Each bar is the mean ± 1 SEM (n = 4 throughout) and statistical significance was determined using either Student's unpaired test (in B and D) or one-way ANOVA with Bonferroni's post-test (comparing all groups in C). NS, non-significant difference; *P < 0.05, **P < 0.01, ***P < 0.001.
In both proximal and distal colonic preparations, veratridine induced a concentration-dependent increase in Isc, with the higher concentration (30 μm) producing a significantly larger rise in Isc (Fig. 7B). Hexamethonium inhibited a small proportion of the veratridine response in both preparations, significantly so in the distal colon mucosa after the higher veratridine concentration (Fig. 7C). In the presence of hexamethonium, nicotine had no effect (Fig. 7A, pooled data not shown) confirming that the antagonist had blocked nicotinic receptors. Most of the remaining response to veratridine was abolished by TTX (Fig. 7A and D), further demonstrating that the elevated Isc evoked by veratridine was neurally mediated.
Discussion
Our results show that submucosal neurons in the mouse colon can be segregated into two main classes by their neurochemistry: cholinergic and non-cholinergic/VIP+. While the proportion of cholinergic neurons increases distally, the VIP+ population comprises the vast majority along the entire length of the colon. However, the two neurochemical classes in the distal colon do not possess distinctive electrophysiological properties or morphologies; all submucosal neurons examined exhibited S-type electrophysiology, regardless of their morphology. Furthermore, somatic action potentials of these neurons were mediated by TTX-resistant channels. We have also confirmed that these neurons exhibit fast EPSPs mediated by nicotinic acetylcholine receptors, inhibitory postsynaptic potentials and slow EPSPs (Wong et al. 2008). However, our data indicate that there are notable differences in the neural circuitry along the length of the mouse colon, with a significant increase in the involvement of cholinergic transmission via nicotinic receptors between the proximal and distal colon. It also appears that in the distal colon there may be recurrent neural circuits involving cholinergic neurons that produce nicotinic fast EPSPs in VIP+ secretomotor neurons.
ChAT-Cre;R26R-YFP mice as a model for studying cholinergic neurons in the submucous plexus
In the colon of ChAT-Cre;R26R-YFP mice, almost all ChAT-IR submucosal neurons expressed YFP, while all YFP+ neurons were ChAT-IR. Furthermore, the neurochemistry, morphology and electrophysiology of submucosal neurons in ChAT-Cre;R26R-YFP mice did not differ from those of the commonly used research mouse model, C57Bl/6. These findings validate the use of ChAT-Cre;R26R-YFP mice as a model that can be used to examine cholinergic and non-cholinergic submucosal neurons. The strong YFP staining overcomes previous limitations in the study of cholinergic neurons due to poor sensitivity and staining using antibodies targeting ChAT. Indeed, ChAT-Cre;R26R-YFP mice have been used recently to study the development of cholinergic neurons in the myenteric plexus of the ENS, which was previously impossible using conventional immunohistochemistry (Hao et al. 2013).
Several studies have reported that neurons transiently express certain neurochemical phenotypes early in development of the murine ENS. These neurons include transiently catecholaminergic enteric neurons (Baetge & Gershon, 1989; Obermayr et al. 2013) and neuronal NOS (nNOS) neurons in the submucous plexus (Young & Ciampoli, 1998). A previous study, using ChAT-Cre;R26R-YFP mice, identified a small number of YFP+ myenteric neurons that did not express ChAT (Hao et al. 2013). As these mice were the progeny of ChAT-Cre and ROSA26YFP reporter mice, YFP staining would be preserved in every cell that ever expressed ChAT, and thus it is likely that these adult YFP+/ChAT– myenteric neurons transiently expressed ChAT during development. In contrast, we found that all adult YFP+ submucosal neurons also expressed ChAT. Thus, submucosal neurons do not transiently express ChAT during development. Nonetheless, we identified a small number of submucosal neurons in these mice that were ChAT+/YFP– in accordance with the myenteric plexus (Hao et al. 2013). This discrepancy could be due to incomplete expression of the Cre recombinase enzyme and/or recombination in these neurons.
Species and regional issues
Our finding that there is virtually no colocalization of ChAT and VIP in submucosal neurons of the mouse colon is similar to previous reports on the neurochemistry of submucosal neurons in mouse ileum and guinea-pig ileum and colon (Bornstein & Furness, 1988; Brookes, 2001; Lomax et al. 2001; Mongardi Fantaguzzi et al. 2009). Our results are consistent with the repeated conclusions of pharmacological studies that there are both cholinergic and non-cholinergic secretomotor neurons in the submucosal plexus of most, if not all, mammals (Keast et al. 1985; Hubel et al. 1987; Kuwahara et al. 1989; Biagi et al. 1990). These conclusions are supported for mouse colon by our observation that there are both VIP+ and ChAT (seen as YFP-labelled) terminals in the colonic mucosa (Fig. 2A). However, studies of both human and pig tissues indicate that there is substantial overlap between ChAT (or the alternative marker for cholinergic neurons, vesicular acetylcholine transporter) and VIP in submucosal neurons of these two species (Poonyachoti et al. 2002; Bornstein et al. 2012), despite pharmacological evidence that they have both cholinergic and non-cholinergic secretomotor neurons (Hayden & Carey, 2000; Banks et al. 2004; Burleigh & Banks, 2007).
There are also significant species differences seen with other neurochemical markers of submucosal neurons. In the present study, we found that calretinin was predominantly localized to VIP+ neurons, something that has also been reported for submucosal neurons in the mouse ileum. This finding contrasts with guinea-pig submucous plexus, where calretinin is found exclusively in a subpopulation of cholinergic neurons. Similarly, some VIP+ neurons in mouse colon co-express nNOS, something also seen in the rat (Chino et al. 2002), but nNOS is not seen in submucosal neurons of guinea-pig ileum (Bornstein & Furness, 1988; Brookes, 2001), although it is present in all VIP neurons of guinea-pig colon (Lomax & Furness, 2000). These observations highlight the neurochemical diversity of submucosal neurons across species and regions within species.
This present study is also consistent with guinea-pig studies indicating that there are significant regional differences within the submucosal plexus (Cunningham & Lees, 1995; Cunningham et al. 1997; Lomax and Furness, 2000; Lomax et al. 2001; Gwynne & Bornstein, 2007). We found that the proportion of cholinergic neurons increased from proximal to distal colon, but in all cases was much less than is seen in the mouse ileum where about 40% of submucosal neurons are cholinergic (Mongardi Fantaguzzi et al. 2009). In the ileum about half the cholinergic neurons are immunoreactive for CGRP (Mongardi Fantaguzzi et al. 2009), while in the colon all cholinergic neurons coexpress CGRP. This suggests that there are two different populations of cholinergic submucosal neurons in the mouse ileum, but only one in the colon.
Species and regional diversity is also seen in the functional properties of the submucosal neurons. In the guinea pig, these neurons can be classified into two main groups by their electrophysiology and corresponding morphology: S-type/uniaxonal and AH-type/multiaxonal. Non-cholinergic/VIP+ neurons make up about half of all the submucosal neurons in guinea-pig ileum and they are exclusively uniaxonal. Some cholinergic neurons have prolonged AHPs following their action potentials and have smooth bodies and multiple axons (DII morphology); these AH-type/DII neurons are probably intrinsic sensory neurons (Lomax et al. 2001; Gwynne & Bornstein, 2007). In contrast, about 80% of all submucosal neurons in the mouse colon are non-cholinergic/VIP+ and all neurons were classed electrophysiologically as S-type. Most have only one axon, and those that have two axons are non-cholinergic. Further, all neurons examined exhibited S-type electrophysiology, shown by lack of prolonged AHPs following action potentials and the presence of fast EPSPs. Thus, AH/multiaxonal submucosal neurons appear to be absent from mouse colon, as has previously been suggested for the ileum of this species (Mongardi Fantaguzzi et al. 2009). In accordance with this observation, stretch-activation of colonic migrating complexes in the mouse colon is not dependent on the activation of sensory inputs originating from the submucous plexus (Zagorodnyuk & Spencer, 2011). While AH-type/multiaxonal neurons were not observed, we cannot exclude the possibility of sensory neurons in this preparation, especially as uniaxonal neurons in the guinea-pig can have sensory functions (Mazzuoli & Schemann, 2009; Spencer & Smith, 2004).
In the guinea-pig, TTX-resistant action potentials are uniquely associated with neurons exhibiting AH-type electrophysiology (Lomax et al. 2001). However, we found that somatic, but not antidromic, action potentials exhibited by all S-type submucosal neurons in the mouse colon were TTX-resistant. Thus, it is likely that the subtypes of VGSCs at the somata and along the axon or terminals of these neurons are different. Fast EPSPs in the guinea-pig ENS and mouse myenteric plexus are mediated by at least two neurotransmitters (Monro et al. 2004; Nurgali et al. 2004). However, fast EPSPs exhibited by submucosal neurons in the murine distal colon were mediated by nicotinic receptors, with no detectable involvement from other neurotransmitters.
Functional implications
The gradient in proportion of cholinergic submucosal neurons is replicated by a similar trend in veratridine-initiated secretomotor responses. The nicotinic antagonist hexamethonium depressed neurogenic Isc responses in submucosa/mucosal preparations from distal colon, but not proximal colon, despite the veratridine response being slightly greater in the latter. In each case, the Isc response was effectively abolished by TTX, indicating that it was mediated by neural activity in the submucosal plexus, although it appears that this activity incorporates firing of cholinergic interneurons in the distal colon, but not in the proximal colon. These results are consistent with the observation that veratridine evokes ongoing fast EPSPs in distal colon neurons, which suggests that this sodium channel activator can excite recurrent excitatory networks of submucosal neurons in the mouse distal colon and that at least some of the neurons in these networks are cholinergic interneurons. This is also consistent with our finding that single electrical stimuli applied to internodal strands can evoke bursts of fast EPSPs that are not tightly stimulus locked, but occur over durations of up to 500 ms. The long duration of these bursts precludes the fast EPSPs being the direct result of activation of terminals of myenteric neurons and, as they are blocked by hexamethonium, they probably result from the activation of cholinergic neurons within the submucosal plexus. However, the number of cholinergic submucosal neurons in mouse colon is small even in the distal colon and our anatomical data do not provide direct support for the presence of cholinergic interneurons. In guinea-pig ileum, similar electrophysiological data indicating the presence of submucosal cholinergic interneurons may be explained by the responses being due to activation of intrinsic sensory neurons located within this plexus (Bornstein & Furness, 1988). However, our data indicate that this is unlikely to be an adequate explanation in the mouse.
The failure to identify intrinsic sensory neurons in the mouse submucosal plexus in the present and previous studies (Wong et al. 2008; Mongardi Fantaguzzi et al. 2009) suggests that the conventional complete secretomotor reflex circuits described in the guinea-pig or rat (Christofi et al. 2004; Cooke et al. 2004; Frieling et al. 1999) are not found in mouse colonic submucosa. This observation is important for interpretation of Ussing chamber and other studies of water and electrolyte transport in preparations lacking the myenteric plexus or consideration of other neurons with sensory function (Mazzuoli & Schemann, 2009). Similarly, the difference in contribution of cholinergic interneurons to Isc responses in proximal and distal colon suggests that region of colonic tissue investigated may profoundly affect the results of studies concerning inflammation and the microbiome.
Conclusions
Our study is the first to correlate several properties of submucosal neurons of the mouse colon, and demonstrates how the differential properties between colonic regions could affect the mucosal secretory response. Although the submucous plexus of the mouse has some similarities with that of the guinea-pig, we have highlighted significant interspecies differences that should be considered in the interpretation of data. Furthermore, the presence of significant regional differences emphasizes the necessity for future studies to examine the submucous plexus of the murine small intestine specifically.
Key points
Submucosal neurons are crucial regulators of gut secretion. Despite significant interest in using mouse models for enteric neuropathies, much is still unknown about their submucous innervation.
We examined properties of submucosal neurons in the mouse distal colon using immunohistochemical and intracellular recording techniques, and investigated colonic regional differences in neurochemistry and neurally mediated ion transport responses.
Two main neurochemical but not electrophysiological classes of neurons were identified: cholinergic (containing choline acetyltransferase) and non-cholinergic. Non-cholinergic neurons had one or two axons; the cholinergic neurons examined were uniaxonal. Neurons exhibited predominantly nicotinic fast excitatory postsynaptic potentials and somatic action potentials mediated by tetrodotoxin-resistant voltage-gated channels.
The distal colon had smaller ganglia, a higher proportion of cholinergic neurons (they remain a minority) and a larger nicotinic secretory component than the proximal colon.
Properties of submucosal neurons in the mouse distal colon differ from other colonic regions, and from submucosal neurons in other species.
Acknowledgments
We thank Heather Young for her invaluable suggestions and for reviewing the manuscript, Annette Bergner and Colin Anderson for maintaining the ChAT-Cre;R26R-YFP mice, and Dr Lennon and Dr Emson for their gifts of the human anti-Hu and sheep anti-nNOS primary antibodies, respectively.
Glossary
- ACh
acetylcholine
- AHPs
after-hyperpolarizing potentials
- AH-type
prolonged after-hyperpolarizing potentials following action potentials
- CGRP
calcitonin gene-related peptide
- ChAT
choline acetyltransferase
- DII
Dogiel type II
- ENS
enteric nervous system
- EPSPs
excitatory postsynaptic potentials
- IA
A-type potassium conductance
- Ih
hyperpolarizing current
- IPSPs
inhibitory postsynaptic potentials
- IR
immunoreactive
- Isc
short-circuit current
- (n)NOS
(neuronal) nitric oxide synthase
- PPADS
pyridoxal phosphate-6-azo(benzene-2,4-disulfonic acid
- TH
tyrosine hydroxylase
- TTX
tetrodotoxin
- VGSCs
voltage-gated Na+ channels
- VIP
vasoactive intestinal peptide
- YFP
yellow fluorescent protein
Additional information
Competing interests
None.
Author contributions
This work wasconducted in the laboratories headed by J.C.B. and H.M.C. J.P.P.F.: conception and design, collection, analysis and interpretation of data, manuscript writing; I.R.T.: design of experiments, collection, analysis and interpretation of data; H.M.C.: conception and design, analysis and interpretation of data, manuscript revision; J.C.B.: conception and design, analysis and interpretation of data, manuscript preparation and revision. All authors approved the final version of the manuscript.
Funding
This work was supported by the NHMRC Project Grant 1006453 (J.C.B.).
References
- Avula LR, Buckinx R, Favoreel H, Cox E, Adriaensen D, Van Nassauw L, Timmermans JP. Expression and distribution patterns of Mas-related gene receptor subtypes A–H in the mouse intestine: inflammation-induced changes. Histochem Cell Biol. 2013;139:639–658. doi: 10.1007/s00418-013-1086-9. [DOI] [PubMed] [Google Scholar]
- Baetge G, Gershon MD. Transient catecholaminergic (TC) cells in the vagus nerves and bowel of fetal mice: relationship to the development of enteric neurons. Dev Biol. 1989;132:189–211. doi: 10.1016/0012-1606(89)90217-0. [DOI] [PubMed] [Google Scholar]
- Banks MR, Golder M, Farthing MJ, Burleigh DE. Intracellular potentiation between two second messenger systems may contribute to cholera toxin induced intestinal secretion in humans. Gut. 2004;53:50–57. doi: 10.1136/gut.53.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagi B, Wang YZ, Cooke HJ. Effects of tetrodotoxin on chloride secretion in rabbit distal colon: tissue and cellular studies. Am J Physiol. 1990;258:G223–230. doi: 10.1152/ajpgi.1990.258.2.G223. [DOI] [PubMed] [Google Scholar]
- Bornstein JC, Costa M, Furness JB. Synaptic inputs to immunohistochemically identified neurones in the submucous plexus of the guinea-pig small intestine. J Physiol. 1986;381:465–482. doi: 10.1113/jphysiol.1986.sp016339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein JC, Furness JB. Correlated electrophysiological and histochemical studies of submucous neurons and their contribution to understanding enteric neural circuits. J Auton Nerv Syst. 1988;25:1–13. doi: 10.1016/0165-1838(88)90002-1. [DOI] [PubMed] [Google Scholar]
- Bornstein JC, Furness JB, Costa M. Sources of excitatory synaptic inputs to neurochemically identified submucous neurons of guinea-pig small intestine. J Auton Nerv Syst. 1987;18:83–91. doi: 10.1016/0165-1838(87)90137-8. [DOI] [PubMed] [Google Scholar]
- Bornstein JC, Furness JB, Costa M. An electrophysiological comparison of substance P-immunoreactive neurons with other neurons in the guinea-pig submucous plexus. J Auton Nerv Syst. 1989;26:113–120. doi: 10.1016/0165-1838(89)90159-8. [DOI] [PubMed] [Google Scholar]
- Bornstein JC, Gwynne RM, Sjovall H. Enteric neural regulation of mucosal secretion. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. Oxford: Academic Press; 2012. pp. 769–790. [Google Scholar]
- Brookes SJ. Classes of enteric nerve cells in the guinea-pig small intestine. Anat Rec. 2001;262:58–70. doi: 10.1002/1097-0185(20010101)262:1<58::AID-AR1011>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Buresi MC, Vergnolle N, Sharkey KA, Keenan CM, Andrade- Gordon P, Cirino G, Cirillo D, Hollenberg MD, MacNaughton WK. Activation of proteinase-activated receptor-1 inhibits neurally evoked chloride secretion in the mouse colon in vitro. Am J Physiol Gastrointest Liver Physiol. 2005;288:G337–345. doi: 10.1152/ajpgi.00112.2004. [DOI] [PubMed] [Google Scholar]
- Burleigh DE, Banks MR. Stimulation of intestinal secretion by vasoactive intestinal peptide and cholera toxin. Auton Neurosci. 2007;133:64–75. doi: 10.1016/j.autneu.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII. Nomenclature and structure–function relationships of voltage-gated sodium channels. Pharmacol Rev. 2005;57:397–409. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- Chino Y, Fujimura M, Kitahama K, Fujimiya M. Colocalization of NO and VIP in neurons of the submucous plexus in the rat intestine. Peptides. 2002;23:2245–2250. doi: 10.1016/s0196-9781(02)00264-4. [DOI] [PubMed] [Google Scholar]
- Christofi FL, Wunderlich J, Yu JG, Wang YZ, Xue J, Guzman J, Javed N, Cooke H. Mechanically evoked reflex electrogenic chloride secretion in rat distal colon is triggered by endogenous nucleotides acting at P2Y1, P2Y2, and P2Y4 receptors. J Comp Neurol. 2004;469:16–36. doi: 10.1002/cne.10961. [DOI] [PubMed] [Google Scholar]
- Cooke HJ, Xue J, Yu JG, Wunderlich J, Wang YZ, Guzman J, Javed N, Christofi FL. Mechanical stimulation releases nucleotides that activate P2Y1 receptors to trigger neural reflex chloride secretion in guinea pig distal colon. J Comp Neurol. 2004;469:1–15. doi: 10.1002/cne.10960. [DOI] [PubMed] [Google Scholar]
- Cunningham SM, Hirai K, Mihara S, Lees GM. Electrophysiological characteristics of submucosal neurones in the proximal colon of guinea-pigs: comparisons with caecum and descending colon. Exp Physiol. 1997;82:859–870. doi: 10.1113/expphysiol.1997.sp004069. [DOI] [PubMed] [Google Scholar]
- Cunningham SM, Lees GM. Neuropeptide Y in submucosal ganglia: regional differences in the innervation of guinea-pig large intestine. J Auton Nerv Syst. 1995;55:135–145. doi: 10.1016/0165-1838(95)00035-v. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in the Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichna J, Schicho R, Andrews CN, Bashashati M, Klompus M, McKay DM, Sharkey KA, Zjawiony JK, Janecka A, Storr MA. Salvinorin A inhibits colonic transit and neurogenic ion transport in mice by activating κ-opioid and cannabinoid receptors. Neurogastroenterol Motil. 2009;21:1326–e128. doi: 10.1111/j.1365-2982.2009.01369.x. [DOI] [PubMed] [Google Scholar]
- Foong JP, Nguyen TV, Furness JB, Bornstein JC, Young HM. Myenteric neurons of the mouse small intestine undergo significant electrophysiological and morphological changes during postnatal development. J Physiol. 2012;590:2375–2390. doi: 10.1113/jphysiol.2011.225938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foong JP, Parry LJ, Bornstein JC. Activation of neuronal SST1 and SST2 receptors decreases neurogenic secretion in the guinea-pig jejunum. Neurogastroenterol Motil. 2010;22:1209–1216. doi: 10.1111/j.1365-2982.2010.01566.x. [DOI] [PubMed] [Google Scholar]
- Frieling T, Dobreva G, Weber E, Becker K, Rupprecht C, Neunlist M, Schemann M. Different tachykinin receptors mediate chloride secretion in the distal colon through activation of submucosal neurones. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:71–79. doi: 10.1007/pl00005327. [DOI] [PubMed] [Google Scholar]
- Gwynne RM, Bornstein JC. Synaptic transmission at functionally identified synapses in the enteric nervous system: roles for both ionotropic and metabotropic receptors. Curr Neuropharmacol. 2007;5:1–17. doi: 10.2174/157015907780077141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwynne RM, Ellis M, Sjovall H, Bornstein JC. Cholera toxin induces sustained hyperexcitability in submucosal secretomotor neurons in guinea pig jejunum. Gastroenterology. 2009;136:299–308. doi: 10.1053/j.gastro.2008.09.071. [DOI] [PubMed] [Google Scholar]
- Hao MM, Bornstein JC, Young HM. Development of myenteric cholinergic neurons in ChAT-Cre;R26R-YFP mice. J Comp Neurol. 2013;521:3358–3370. doi: 10.1002/cne.23354. [DOI] [PubMed] [Google Scholar]
- Hao MM, Lomax AE, McKeown SJ, Reid CA, Young HM, Bornstein JC. Early development of electrical excitability in the mouse enteric nervous system. J Neurosci. 2012;32:10949–10960. doi: 10.1523/JNEUROSCI.1426-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden UL, Carey HV. Neural control of intestinal ion transport and paracellular permeability is altered by nutritional status. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1589–1594. doi: 10.1152/ajpregu.2000.278.6.R1589. [DOI] [PubMed] [Google Scholar]
- Hens J, Schrodl F, Brehmer A, Adriaensen D, Neuhuber W, Scheuermann DW, Schemann M, Timmermans JP. Mucosal projections of enteric neurons in the porcine small intestine. J Comp Neurol. 2000;421:429–436. [PubMed] [Google Scholar]
- Hock M, Sotak M, Kment M, Pacha J. The early effect of dextran sodium sulfate administration on carbachol-induced short-circuit current in distal and proximal colon during colitis development. Physiol Res. 2011;60:921–931. doi: 10.33549/physiolres.932222. [DOI] [PubMed] [Google Scholar]
- Hubel KA, Renquist K, Shirazi S. Ion transport in human cecum, transverse colon, and sigmoid colon in vitro. Baseline and response to electrical stimulation of intrinsic nerves. Gastroenterology. 1987;92:501–507. doi: 10.1016/0016-5085(87)90148-x. [DOI] [PubMed] [Google Scholar]
- Hyland NP, Cox HM. The regulation of veratridine-stimulated electrogenic ion transport in mouse colon by neuropeptide Y (NPY), Y1 and Y2 receptors. Br J Pharmacol. 2005;146:712–722. doi: 10.1038/sj.bjp.0706368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland NP, Rybicka JM, Ho W, Pittman QJ, Macnaughton WK, Sharkey KA. Adaptation of intestinal secretomotor function and nutrient absorption in response to diet-induced obesity. Neurogastroenterol Motil. 2010;22:602–e171. doi: 10.1111/j.1365-2982.2010.01504.x. [DOI] [PubMed] [Google Scholar]
- Juric M, Xiao F, Amasheh S, May O, Wahl K, Bantel H, Manns MP, Seidler U, Bachmann O. Increased epithelial permeability is the primary cause for bicarbonate loss in inflamed murine colon. Inflamm Bowel Dis. 2013;19:904–911. doi: 10.1097/MIB.0b013e3182813322. [DOI] [PubMed] [Google Scholar]
- Keast JR, Furness JB, Costa M. Investigations of nerve populations influencing ion transport that can be stimulated electrically, by serotonin and by a nicotinic agonist. Naunyn Schmiedebergs Arch Pharmacol. 1985;331:260–266. doi: 10.1007/BF00634247. [DOI] [PubMed] [Google Scholar]
- Klompus M, Ho W, Sharkey KA, McKay DM. Antisecretory effects of neuropeptide Y in the mouse colon are region-specific and are lost in DSS-induced colitis. Regul Peptides. 2010;165:138–145. doi: 10.1016/j.regpep.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Kuwahara A, Cooke HJ, Carey HV, Mekhjian H, Ellison EC, McGregor B. Effects of enteric neural stimulation on chloride transport in human left colon in vitro. Digest Dis Sci. 1989;34:206–213. doi: 10.1007/BF01536052. [DOI] [PubMed] [Google Scholar]
- Lomax AE, Bertrand PP, Furness JB. Electrophysiological characteristics distinguish three classes of neuron in submucosal ganglia of the guinea-pig distal colon. Neuroscience. 2001;103:245–255. doi: 10.1016/s0306-4522(00)00545-5. [DOI] [PubMed] [Google Scholar]
- Lomax AE, Furness JB. Neurochemical classification of enteric neurons in the guinea-pig distal colon. Cell Tissue Res. 2000;302:59–72. doi: 10.1007/s004410000260. [DOI] [PubMed] [Google Scholar]
- Lomax AE, Mawe GM, Sharkey KA. Synaptic facilitation and enhanced neuronal excitability in the submucosal plexus during experimental colitis in guinea-pig. J Physiol. 2005;564:863–875. doi: 10.1113/jphysiol.2005.084285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNaughton WK, Lowe SS, Cushing K. Role of nitric oxide in inflammation-induced suppression of secretion in a mouse model of acute colitis. Am J Physiol. 1998;275:G1353–1360. doi: 10.1152/ajpgi.1998.275.6.G1353. [DOI] [PubMed] [Google Scholar]
- Mann PT, Furness JB, Southwell BR. Choline acetyltransferase immunoreactivity of putative intrinsic primary afferent neurons in the rat ileum. Cell Tissue Res. 1999;297:241–248. doi: 10.1007/s004410051352. [DOI] [PubMed] [Google Scholar]
- Mazzuoli G, Schemann M. Multifunctional rapidly adapting mechanosensitive enteric neurons (RAMEN) in the myenteric plexus of the guinea pig ileum. J Physiol. 2009;587:4681–4694. doi: 10.1113/jphysiol.2009.177105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongardi Fantaguzzi C, Thacker M, Chiocchetti R, Furness JB. Identification of neuron types in the submucosal ganglia of the mouse ileum. Cell Tissue Res. 2009;336:179–189. doi: 10.1007/s00441-009-0773-2. [DOI] [PubMed] [Google Scholar]
- Monro RL, Bertrand PP, Bornstein JC. ATP participates in three excitatory postsynaptic potentials in the submucous plexus of the guinea pig ileum. J Physiol. 2004;556:571–584. doi: 10.1113/jphysiol.2004.060848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunlist M, Barouk J, Michel K, Just I, Oreshkova T, Schemann M, Galmiche JP. Toxin B of Clostridium difficile activates human VIP submucosal neurons, in part via an IL-1β-dependent pathway. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1049–1055. doi: 10.1152/ajpgi.00487.2002. [DOI] [PubMed] [Google Scholar]
- Nurgali K, Stebbing MJ, Furness JB. Correlation of electrophysiological and morphological characteristics of enteric neurons in the mouse colon. J Comp Neurol. 2004;468:112–124. doi: 10.1002/cne.10948. [DOI] [PubMed] [Google Scholar]
- O'Malley D, Dinan TG, Cryan JF. Interleukin-6 modulates colonic transepithelial ion transport in the stress-sensitive Wistar Kyoto rat. Front Pharmacol. 2012;3:190. doi: 10.3389/fphar.2012.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermayr F, Stamp LA, Anderson CR, Young HM. Genetic fate-mapping of tyrosine hydroxylase-expressing cells in the enteric nervous system. Neurogastroenterol Motil. 2013;25:e283–291. doi: 10.1111/nmo.12105. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Bayguinov PO, Broadhead MJ, Smith TK. Ca2+ transients in submucous neurons during the colonic migrating motor complex in the isolated murine large intestine. Neurogastroenterol Motil. 2012;24:769–778. doi: 10.1111/j.1365-2982.2012.01934.x. [DOI] [PubMed] [Google Scholar]
- Poole DP, Matsuyama H, Nguyen TV, Eriksson EM, Fowler CJ, Furness JB. Inflammation and inflammatory agents activate protein kinase C epsilon translocation and excite guinea-pig submucosal neurons. Gastroenterology. 2007;133:1229–1239. doi: 10.1053/j.gastro.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Poonyachoti S, Kulkarni-Narla A, Brown DR. Chemical coding of neurons expressing δ- and κ-opioid receptor and type I vanilloid receptor immunoreactivities in the porcine ileum. Cell Tissue Res. 2002;307:23–33. doi: 10.1007/s00441-001-0480-0. [DOI] [PubMed] [Google Scholar]
- Porter AJ, Wattchow DA, Brookes SJ, Costa M. Projections of nitric oxide synthase and vasoactive intestinal polypeptide-reactive submucosal neurons in the human colon. J Gastroenterol Hepatol. 1999;14:1180–1187. doi: 10.1046/j.1440-1746.1999.02026.x. [DOI] [PubMed] [Google Scholar]
- Porter AJ, Wattchow DA, Brookes SJ, Schemann M, Costa M. Choline acetyltransferase immunoreactivity in the human small and large intestine. Gastroenterology. 1996;111:401–408. doi: 10.1053/gast.1996.v111.pm8690205. [DOI] [PubMed] [Google Scholar]
- Sang Q, Young HM. The identification and chemical coding of cholinergic neurons in the small and large intestine of the mouse. Anat Rec. 1998;251:185–199. doi: 10.1002/(SICI)1097-0185(199806)251:2<185::AID-AR6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Sorensen MV, Sausbier M, Ruth P, Seidler U, Riederer B, Praetorius HA, Leipziger J. Adrenaline-induced colonic K+ secretion is mediated by KCa1.1 (BK) channels. J Physiol. 2010;588:1763–1777. doi: 10.1113/jphysiol.2009.181933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Smith TK. Mechanosensory S-neurons rather than AH-neurons appear to generate a rhythmic motor pattern in guinea-pig distal colon. J Physiol. 2004;558:577–596. doi: 10.1113/jphysiol.2004.063586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanner S, MacNaughton WK. Submucosal secretomotor and vasodilator reflexes. Neurogastroenterol Motil. 2004;16(1):39–43. doi: 10.1111/j.1743-3150.2004.00473.x. [DOI] [PubMed] [Google Scholar]
- Vanner S, Surprenant A. Neural reflexes controlling intestinal microcirculation. Am J Physiol. 1996;271:G223–230. doi: 10.1152/ajpgi.1996.271.2.G223. [DOI] [PubMed] [Google Scholar]
- Wong V, Blennerhassett M, Vanner S. Electrophysiological and morphological properties of submucosal neurons in the mouse distal colon. Neurogastroenterol Motil. 2008;20:725–734. doi: 10.1111/j.1365-2982.2008.01117.x. [DOI] [PubMed] [Google Scholar]
- Xia Y, Hu HZ, Liu S, Pothoulakis C, Wood JD. Clostridium difficile toxin A excites enteric neurones and suppresses sympathetic neurotransmission in the guinea pig. Gut. 2000;46:481–486. doi: 10.1136/gut.46.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young HM, Ciampoli D. Transient expression of neuronal nitric oxide synthase by neurons of the submucous plexus of the mouse small intestine. Cell Tissue Res. 1998;291:395–401. doi: 10.1007/s004410051009. [DOI] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Spencer NJ. Localization of the sensory neurons and mechanoreceptors required for stretch-evoked colonic migrating motor complexes in mouse colon. Front Physiol. 2011;2:98. doi: 10.3389/fphys.2011.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]