Abstract
In contrast to other fat-soluble vitamins, dietary vitamin K is rapidly lost to the body resulting in comparatively low tissue stores. Deficiency is kept at bay by the ubiquity of vitamin K in the diet, synthesis by gut microflora in some species, and relatively low vitamin K cofactor requirements for γ-glutamyl carboxylation. However, as shown by fatal neonatal bleeding in mice that lack vitamin K epoxide reductase (VKOR), the low requirements are dependent on the ability of animals to regenerate vitamin K from its epoxide metabolite via the vitamin K cycle. The identification of the genes encoding VKOR and its paralog VKOR-like 1 (VKORL1) has accelerated understanding of the enzymology of this salvage pathway. In parallel, a novel human enzyme that participates in the cellular conversion of phylloquinone to menaquinone (MK)-4 was identified as UbiA prenyltransferase-containing domain 1 (UBIAD1). Recent studies suggest that side-chain cleavage of oral phylloquinone occurs in the intestine, and that menadione is a circulating precursor of tissue MK-4. The mechanisms and functions of vitamin K recycling and MK-4 synthesis have dominated advances made in vitamin K biochemistry over the last five years and, after a brief overview of general metabolism, are the main focuses of this review.
Keywords: phylloquinone, menaquinones, vitamin K epoxide, γ-carboxyglutamate proteins, vitamin K antagonists, anticoagulants, warfarin, warfarin resistance, vitamin K epoxide reductase, vitamin K dehydrogenase, UbiA prenyltransferase-containing domain 1
Vitamin K differs from other fat-soluble vitamins (A, D, and E) in that it functions as a cofactor for a single microsomal enzyme, namely γ-carboxyglutamyl carboxylase (GGCX). GGCX is needed to catalyze a reaction in which specific peptide-bound glutamate residues found in certain specialized proteins are converted to γ-carboxyglutamate (Gla). This posttranslational protein modification discovered in 1974 (1, 2) is the only firmly established biochemical function of vitamin K. The resultant vitamin K-dependent (VKD) proteins, or Gla proteins, are diverse in both structure and function and are found in many cell and tissue types. It is this very diversity of Gla proteins that makes vitamin K a truly multi-functional vitamin. The central role that vitamin K plays in hemostasis is the only health role for vitamin K that is supported by incontrovertible evidence. The functions of many Gla proteins remain uncertain, but are suspected to play roles in processes as diverse as bone and cardiovascular mineralization, vascular hemostasis, energy metabolism, immune response, brain metabolism, and in cellular growth, survival, and signaling (3–10). For the most part the health roles of Gla proteins beyond their roles in coagulation are unclear or fuzzy. Apart from its cofactor function, there is evidence for biochemical functions of vitamin K that are independent of γ-glutamyl carboxylation (3, 11).
Vitamin K also differs from other fat-soluble vitamins in that there are naturally occurring and synthetic antagonists that block the synthesis of fully γ-carboxylated Gla proteins. Apart from their well-known use as clinical anticoagulants and rodenticides, coumarin vitamin K antagonists (VKAs) offer a powerful laboratory tool for investigating the metabolism of vitamin K, and the roles of Gla proteins in putative physiological processes. They also pose interesting questions as to whether they impair the noncoagulation functions of vitamin K and hence the health of people taking them.
The metabolic processes that lead from the intestinal absorption of dietary vitamin K to its entry into cells, its subsequent storage, intermediary metabolism, and catabolism was reviewed by us in depth in 2008 (11) and in the context of vitamin K requirements in 2012 (12). The main goal of this present review is to focus on the biochemical aspects not covered in these reviews and on advances made in the last decade, particularly in the last 5 years. Two main advances in the last decade stand out; the first was the identification in 2004 of the gene encoding vitamin K epoxide reductase (VKOR) (13, 14) and the second was the identification in 2010 of the gene encoding the menaquinone (MK)-4 biosynthetic enzyme, UbiA prenyltransferase-containing domain 1 (UBIAD1) (15). Both these discoveries have opened up their respective fields to facilitate studies of the mechanisms and importance of cellular vitamin K recycling and MK-4 biosynthesis in mammals. We also highlight the importance of side-chain structure to the way the body metabolizes and utilizes vitamin K not only for its well-known cofactor function but also for other putative functions.
DIVERSITY OF CHEMICAL STRUCTURES AND SOURCES OF VITAMIN K
Naturally occurring forms of vitamin K
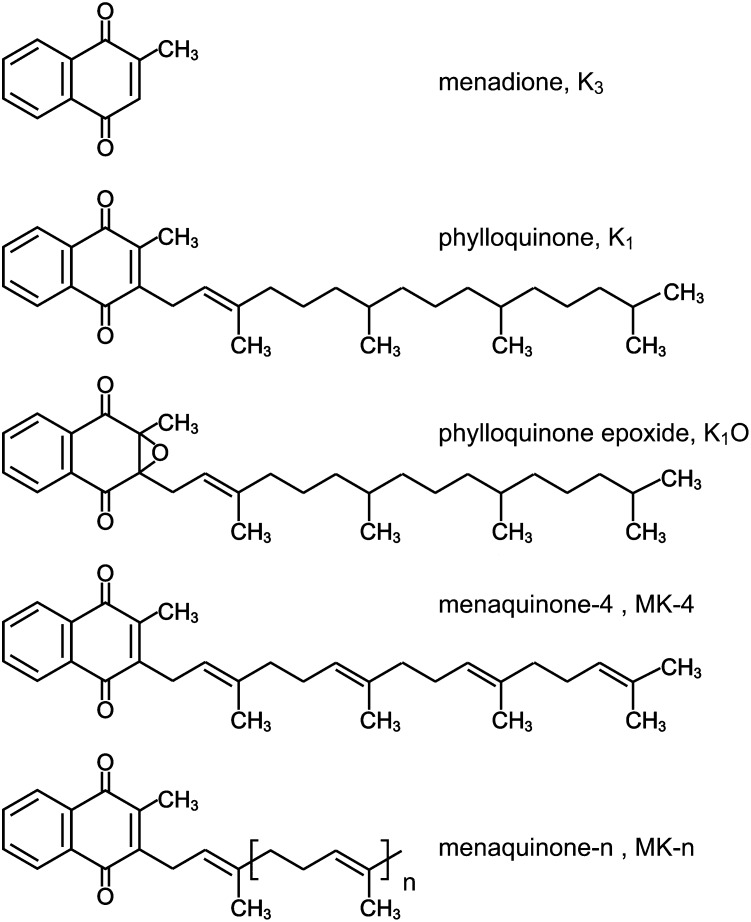
Vitamin K compounds are naphthoquinones that belong to the wider group of isoprenoid quinones and which occur and function in membranes of living organisms. They are characterized by a common 2-methyl-1,4-naphthoquinone ring structure (menadione) and a hydrophobic polyisoprenoid side chain attached at the 3-carbon position of the nucleus. This side chain can vary in both length and degree of saturation (Fig. 1).
Fig. 1.
Chemical structures of vitamin K compounds.
Phylloquinone (vitamin K1).
Phylloquinone (K1) is ubiquitous in the plant kingdom and is structurally distinguished by possessing the same phytyl (hexahydrotetraprenyl) side chain as found in the plant pigment chlorophyll. Although chemically designated a “phylloquinone series” (16), with members that theoretically have longer or shorter isoprenoid side chains of this saturated type, K1 is the only biologically important member of the series and the major form of vitamin K in Western diets (17–19). As befits its photosynthetic function, the highest K1 contents (normally in the range 400–700 μg/100 g) are found in green-leafy vegetables (17). The next best sources are certain vegetable oils (e.g., soybean, rapeseed, and olive oils), which contain 50–200 μg/100 g and are also important contributors to dietary vitamin K intakes (17). Lower but nutritionally useful amounts of K1 are found in other vegetable oils, fruits, cereals, meat, and dairy products (17, 18).
MK family (vitamin K2 family).
MKs are evolutionarily the most ancient type of isoprenoid quinones and the most widespread bacterial respiratory quinones (20). They are synthesized by a limited number of obligate and facultative anaerobic bacteria (21, 22), some of which occupy a niche in the microflora of the human gut (22, 23). The family contains a wide spectrum of isoprenologs in which the side chain comprises a polymer of repeating prenyl units. They are named according to the number of these prenyl units (thus MK-n). The major bacterial MKs present in the human large intestine are MK-10 and MK-11 synthesized by Bacteroides, MK-8 by Enterobacteria, MK-7 by Veillonella, and MK-6 by Eubacterium lentum (22, 23).
MKs account for only about 10–25% of the vitamin K content of Western diets (24, 25). Of the individual MKs in Western diets, MK-4 accounts for about 30–40% and the remainder comprise mainly MK-9, MK-8, and MK-7, in that order (24, 25). The highest food sources of long-chain MKs are animal livers and foods prepared with a bacterial fermentation stage such as cheeses (mainly MK-8 and MK-9) and natto (MK-7). The Japanese food natto (made by fermenting cooked soybeans with Bacillus subtilis natto) has a very high content of MK-7 of about 1,000 μg/100 g in a highly bioavailable form (26). Yeasts do not synthesize MKs.
Many bacterial strains synthesize MKs in which one or more of the prenyl units is saturated. The additional hydrogen atoms are indicated by a prefix (dihydro-, tetrahydro-, etc.) and are abbreviated MK-n(H2), MK-n(H4), etc. The contribution of partially saturated MKs to vitamin K nutrition has been largely ignored, but they are synthesized by members of the gut microflora (22) and by bacteria used in food fermentation processes. An example of the latter is tetrahydromenaquinone-9 [MK-9(H4)] which is the major respiratory MK in propionibacterium species used in the manufacture of certain cheeses. Nutritionally relevant amounts of MK-9(H4) are found in Norwegian Jarlsberg and Swiss emmental cheeses (27).
MK-4 is an atypical MK that is not commonly synthesized by bacteria (21), but can be synthesized in vivo by invertebrates and vertebrates given a suitable naphthoquinone precursor such as menadione or K1 (11, 15, 28).
Issues of bioavailability of K1 and MKs from different foods are complex and are considered in previous reviews (12, 29). In general, dietary forms of vitamin K that are membrane bound (K1 in plants and MKs in bacteria) have a poor bioavailability, whereas vitamin K contained in oil-based and processed foods is absorbed more efficiently. Stable-isotope studies have shown that the absorption of K1 is dependent both on the food matrix and the type of accompanying meal (30). There have been no comparable stable-isotope studies with any MKs and robust data on their bioavailability is presently unavailable (29).
Importance of using correct nomenclature
Although traditional nomenclature distinguishes the plant form K1 from the family of bacterial MKs (K2), this nomenclature is somewhat artificial because the synthesis of K1 by chloroplasts of eukaryotic algae and plants probably evolved from an endosymbiotic relationship with cyanobacteria. That K1 biosynthesis originated from prokaryotic endosymbionts is supported by genetic studies of the biosynthetic enzymes responsible for the synthesis of K1 and MKs (20). In this sense K1 and MKs are not only evolutionally closely related, but K1 is simply a partially saturated species of MK-4, namely II-, III-, and IV-hexahydro MK-4.
As pointed out by Suttie (31), inappropriate nomenclature of the different forms of vitamin K in the scientific literature is common and has led to highly misleading claims relating to vitamin K function and requirements. The most common error is to refer to “vitamin K2” as though it were a single chemical entity with the implication that all MKs possess identical metabolic profiles and activities. This trend originated in Japan from where numerous scientific articles on the cell biology and health effects refer to vitamin K2 in the title and text (including several cited in this review) for studies carried out with a specific MK, usually with MK-4 but increasingly with MK-7. This stemmed from the commercial synthesis and medical use within Japan of MK-4, trade names menatetrenone and Glakay (Eisai Co., Ltd., Tokyo, Japan) but also widely promoted as vitamin K2. The main clinical indication for MK-4 in Japan is for the prevention and treatment of osteoporosis, although it is also used for the prevention of vitamin K deficiency bleeding in neonates; nearly all other countries use K1 for this indication. Confusion as to which chemical structure is being described by vitamin K2 is exacerbated by the increasing availability of dietary supplements of MK-7. The increasing promotion of MK-7 for bone and cardiovascular health had its origins from the widespread consumption in Japan of natto, which contains high amounts of MK-7 (26).
If obfuscation of vitamin K nomenclature is common in the scientific literature, it is rife on the Internet. For example manufacturers and traders of vitamin K2 health supplements often do not reveal whether the product contains MK-4 or MK-7. Why is this important? Apart from the obvious need to fully inform the public, there may be unintended health consequences of ignorance. One important medical issue is that vitamin K supplements may interfere with the stability of anticoagulant therapy in patients taking VKAs such as warfarin. It is known that the agonist effect of vitamin K on VKA anticoagulant therapy is strongly dependent on the chemical form of vitamin K. For example, dose-response studies have revealed that the antidotal potency of MK-7 is much greater than that of K1 (32–34). As a consequence, whereas daily supplements of K1, up to 100 μg, do not normally compromise anticoagulant stability in subjects taking VKAs (32), daily supplements of MK-7 as low as 10–20 μg may rapidly destabilize therapeutic anticoagulant control (34). Given that in some countries about 1% of the population is taking VKAs for the treatment and prophylaxis of thromboembolic disease, there is clearly a need to inform professionals and the public of the different potencies of available forms of vitamin K.
OVERVIEW OF VITAMIN K METABOLISM
This section will provide a brief overview of the metabolism of vitamin K congeners with emphasis on human metabolism and the effect of side-chain structure on metabolism. For greater depth of detail of some aspects, the reader is referred to previous reviews (11, 12).
Intestinal absorption, transport, and cellular uptake
The pathways and general principles of intestinal absorption of dietary vitamin K into the enterocytes, packaging into chylomicrons, secretion into lymph lacteals, and entry into the blood via the thoracic duct are common for all fat-soluble vitamins (35, 36). Within the vitamin K family, much more is known about the absorption and metabolic fate of K1 than for MKs. This is because K1 predominates in the diet (19) and is the only vitamer routinely measured in blood (37). Concentrations of individual MKs in plasma, such as MK-4, are very low or undetectable (38). An exception is that MK-7 is often detectable in people who regularly eat natto, mainly because MK-7 has a long plasma half-life and at the same equimolar daily intake produces plasma concentrations that are ∼5-fold higher than those for K1 (33).
No specific transport proteins are known for vitamin K and, with the exception of menadione, the highly lipophilic naturally occurring forms are all transported in plasma by lipoproteins. In the fasting state and during the postprandial phase, 50–90% of K1 is carried by triacylglycerol-rich lipoproteins (TRLs) comprising chylomicron remnants (CRs) and VLDLs (39–42). The remainder is approximately equally distributed between LDLs and HDLs, although after a meal there is a progressive increase with time in both the absolute and relative amounts of K1 in both LDLs and HDLs (40–42). Although there is a paucity of studies directly comparing postprandial plasma kinetics of different molecular forms of vitamin K, there is clear evidence that the length and degree of saturation of the isoprene side chain significantly influences the kinetics of appearance, and clearance from the circulation (33, 41). Plasma clearance of radiolabeled (43) or stable isotope-labeled (44) K1 over the first 8–10 h fits two exponential components with half-lives of ∼0.3 h and ∼2.5 h, respectively. The clearance of unlabeled MK-4 is similarly rapid (41). After oral administration of an equimolar mixture of K1, MK-4, and MK-9, peak concentrations of MK-4 and MK-9 were 39 and 12%, respectively, of the peak concentration for K1, and MK-4 peaked significantly earlier than K1 (41). During the first 4 h after intake, all molecular forms were predominately associated with TRLs. Thereafter, there was divergence in lipoprotein distribution. MK-4 appeared very early in LDLs and HDLs and by 8 h 80% of MK-4 was carried by LDLs. In contrast, MK-9 was not found in HDLs at any time point and did not appear in LDLs until after 8 h; thereafter the proportion of MK-9 in LDLs increased as its proportion in TRLs declined. By 24 h about 50% of MK-9 was present in the LDL fraction and this increased to >90% at 48 h (41). The high proportion of MK-9 in LDLs accounts for its slow rate of clearance with a terminal half-life estimated to be ∼60 h (41). In a separate study, the clearance of MK-7 was also shown to be very slow with a terminal plasma half-life of around 3 days (33). Although lipoprotein analysis was not carried out, it is likely that MK-7 is also transferred to LDLs (33). A major consequence of this delayed clearance of MK-7 is that it was shown to have a much greater efficacy for γ-carboxylating osteocalcin in bone than equimolar doses of K1 (33).
There are few studies of the mechanisms of cellular uptake of vitamin K and the only direct investigations have been carried out with cultured bone cells (45–47). Newman et al. (45) showed the dependence for the human osteoblastic uptake of K1 on heparan sulfate proteoglycans on the cell surface and apoE in lipoprotein particles. Importantly, these investigations in cell culture attempted to replicate the physiological situation by presenting the cells with labeled K1 that had been incorporated into different lipoprotein fractions (45). The results were consistent with known mechanisms of the hepatic uptake of CRs (48). Later studies by Niemeier and coworkers set out to identify the receptors and mechanism of uptake of CRs and vitamin K by bone cells (46, 47). In their first study, they showed that human osteoblast cell lines expressed the LDL receptor and the LDL receptor-related protein 1 (LRP1) with the latter also being strongly expressed by osteoblasts and marrow stromal cells of normal human bone (46). By using specific inhibitors of LRP1, they found strong evidence that LRP1 played a predominant role in vitamin K uptake by cultured osteoblasts when the latter were presented with K1-enriched human CRs isolated from a subject who had taken 10 mg K1 with a meal (46). Specific uptake of K1 was demonstrated from both physical uptake of K1 and by increased γ-carboxylation of osteocalcin (46). In a second study using an in vivo murine model, Niemeier et al. (47) showed that fluorescently-labeled CRs localized with the sinusoidal endothelial cells in bone, which they hypothesized, served as a docking site to concentrate CRs in the bone marrow. Taking account of the requirements for apoE and heparan sulfate proteoglycans, they further hypothesized that CRs travel through the endothelial fenestrae to the subendothelial space where CRs are enriched with osteoblast-derived apoE, interact with osteoblast receptors, and are internalized (47). When mice were injected with K1-loaded CRs, there was a significant increase in the γ-carboxylated fraction of osteocalcin in the circulation within 4 h of the CRs. This represented the first in vivo evidence that K1 carried by CRs has direct access to osteoblasts during the postprandial phase of absorption and that the K1 taken up is very soon utilized for the synthesis and posttranslational carboxylation of osteocalcin (47).
Storage and catabolism
Plasma concentrations of K1 in healthy fasting people are of the order of 0.5 nM, which is one, three, and four orders of magnitude lower than circulating concentrations of 25-hydroxyvitamin D, retinol, and α-tocopherol, respectively. The low plasma concentrations of K1 are mirrored by low tissue reserves. Adult liver concentrations of K1 are approximately 10 pmol/g wet tissue (49, 50). The median total liver pool of K1 in British adults was estimated at about 20 nmol, but including MKs this rises to 200–300 nmol (11, 49). In fact, the liver is the only organ or tissue in which the majority of vitamin K reserves comprise long-chain forms, MK-7 through to MK-13, with only low concentrations of MK-4 (50). Extrahepatic tissues in rats (51, 52) and humans (50) contain mainly K1 and/or MK-4. In humans, K1 concentrations comparable to the liver (10 pmol/g) are present in heart and pancreas, but low K1 concentrations (<2 pmol/g) are present in brain, kidney, and lung (50). The tissue distribution of MK-4 varies quite widely. High MK-4 concentrations (6 pmol/g) were found in human brain and kidney and even higher concentrations (22 pmol/g) in the pancreas (50). The ratio of MK-4: K1 ranged from ≤0.3 in liver and heart to ≥6 in brain and kidney (50). The human pancreas contained approximately equal concentrations of K1 and MK-4 and the total content of >50 pmol/g was the highest of all tissues examined (50).
Tracer studies with labeled K1 have shown that a sizable fraction of a single oral dose is rapidly catabolized in the liver and excreted predominantly in the bile, but also in urine (43). This extensive catabolism of K1 by the liver explains the rapid turnover and depletion of hepatic reserves in patients on a low K1 diet (53). It has been estimated that ∼60–70% of the amounts of K1 absorbed from each meal will ultimately be lost to the body by excretion (54). This alone suggests that the body stores of K1 are being constantly replenished.
Both K1 and members of the MK series are catabolized in the liver by a common degradative pathway in which the polyisoprenoid side chain is shortened to two major carboxylic acid aglycones with 7- and 5-carbon side chains, respectively, and which are excreted in bile and urine mainly as glucuronides (11, 43, 55). Although fine details are lacking, the chemical structures of isolated metabolites are consistent with their formation by an oxidative degradation of the isoprenoid side chain by an initial ω-hydroxylation followed by a progressive side-chain shortening via the β-oxidation pathway (11, 43, 55, 56). It is well known that the same pathway operates to shorten the side chains of other isoprenoid quinones, such as ubiquinones and phytylplastoquinone (55), as well as for vitamin E compounds as discussed in more detail in a previous article in this theme series (36). In an early study, Thierry and Suttie (57) noticed that in studies in which rats had been injected with radiolabeled K1, the specific activity in the mitochondria increased with the total amount of K1 administered while that in microsomes stayed relatively constant. This suggests that mitochondria take up excess K1, and is consistent with a mitochondrial role in β-oxidation of vitamin K.
One uniting feature of vitamin K and vitamin E metabolism is the finding that the same cytochrome P450 enzyme, CYP4F2, carries out ω-hydroxylation of both vitamins (36, 58). A possible connection of the in vivo activity of this enzyme to the ability to metabolize vitamin K comes from the observation that patients taking warfarin who possess the V433M polymorphism of CYP4F2 have an increase in warfarin dose requirements (59). Furthermore, human liver microsomes obtained from carriers of this V433M variant had lower protein concentrations of CYPF2 and a reduced ability to hydroxylate K1 (58). This raises the question of whether carriers of this variant have decreased dietary requirements for vitamin K compared with individuals without it.
THE VITAMIN K-EPOXIDE CYCLE
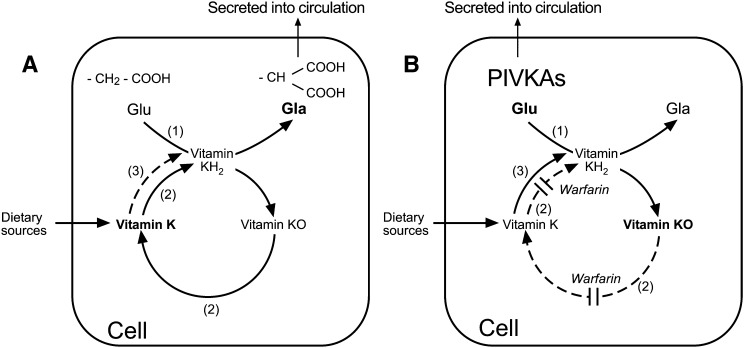
The term “vitamin K-epoxide cycle” or simply “vitamin K cycle” denotes a metabolic pathway that describes the cellular recycling of the metabolite vitamin K 2,3-epoxide (K>O) that is produced as a by-product during the synthesis of VKD proteins in the endoplasmic reticulum (ER). The vitamin K cycle is intimately linked to the GGCX and appears to be present in all cells that synthesize Gla-containing proteins. The cycle comprises two major enzymic activities: a VKOR activity that converts the epoxide metabolite to the native quinone form and a second vitamin K reductase activity that reduces K quinone to the K hydroquinone (KH2) (Fig. 2). The second vitamin K reductase activity is necessary because only KH2 can serve as a cofactor for the GGCX. Vitamin K reductase activity is needed both to reduce recycled vitamin K molecules that have participated in γ-glutamyl carboxylation in the ER as well as to reduce “new” molecules of vitamin K that are introduced into the cycle. Vitamin K is present in foods in its stable oxidized quinone form, so that before dietary vitamin K can function it must undergo reduction to KH2 either before or after entry to the vitamin K cycle. Recent evidence, especially from studies in VKOR knockout mice (60), indicates that VKOR is the main, and probably only, enzyme that can reduce KO to K in vivo, at least for the hepatic synthesis of VKD coagulation proteins at physiologically relevant tissue concentrations of vitamin K. However, the question of which enzymes are responsible for the physiological two-electron reduction of K to KH2 is the subject of ongoing research and debate.
Fig. 2.
Metabolism of vitamin K via the vitamin K-epoxide cycle in the absence (A) and presence (B) of warfarin. A: In the absence of warfarin, peptide-bound glutamic acid (Glu) residues are transformed to γ-carboxy glutamic acid (Gla) residues by the enzyme γ-glutamyl carboxylase (GGCX) shown as enzyme activity (1). The active cofactor form of vitamin K required by the GGCX is the reduced form vitamin K quinol (KH2). During γ-glutamyl carboxylation, the carboxylated substrates (Gla proteins) are secreted into the circulation and KH2 becomes oxidized to vitamin K epoxide (K>O). This epoxide metabolite is reduced to vitamin K quinone by the enzyme VKOR, shown as enzyme activity (2). Vitamin K quinone is then reduced to KH2 by a vitamin K reductase activity to complete the cycle. The reduction of vitamin K quinone to KH2 may be achieved by VKOR or by a NAD(P)H-dependent activity shown as enzyme activity (3). There are several candidate quinone dehydrogenases for activity (3). B: In the presence of a VKA such as warfarin, the activity of the VKOR (2) is inhibited leading to an accumulation of K>O in the cell, and release into the circulation. As a consequence of VKOR inhibition there is a reduced capacity of the cell to generate sufficient active KH2 cofactor to enable the GGCX to carboxylate VKD peptide substrates. This results in the cellular synthesis of inactive species of undercarboxylated proteins called proteins induced by vitamin K absence or antagonism (PIVKAs), which for coagulation and bone proteins are secreted into the circulation. Given a sufficient supply of vitamin K (e.g., from the diet) the alternative quinone reductase activity (3) can bypass the warfarin inhibition of the VKOR to provide the KH2 substrate for the GGCX and hence overcome the inhibitory action of warfarin, even under extreme blockade.
Discovery and identification of VKOR
It is of interest to reflect that the discovery of both the vitamin K-epoxide cycle and VKOR arose out of studies of the metabolism of vitamin K in the late 1960s in an attempt to understand why VKAs such as warfarin prevented the synthesis of VKD coagulation proteins. At that time the only VKD proteins known were factors II, VII, IX, and X and the cofactor function of vitamin K was yet to be discovered. One line of reasoning was that warfarin interfered with the normal metabolism of vitamin K. In an attempt to test this hypothesis, Matschiner et al. (61) administered tracer doses of 3H- and 14C-labeled K1 to rats and analyzed the metabolite pattern in the livers of warfarin-treated and control rats. They found that a new metabolite that was chemically closely related to K1 accumulated in the liver of warfarin-treated, but not control, rats. This metabolite could only be resolved from K1 by the application of reversed-phase partition TLC, and after further spectral analysis and chemical synthesis its structure was shown to be K1>O (already known to chemists as vitamin K oxide) (61).
Although Bell and Matschiner (62) initially thought that K>O was a competitive inhibitor of vitamin K action, by 1974 it was accepted that warfarin was inhibiting an enzyme activity (now known to be VKOR) that was essential for maintaining a supply of KH2 to the GGCX, and that K>O was itself a coproduct of Gla formation (31). It was not until 2004 that two groups led by Oldenburg (13) and Stafford (14) independently identified the gene encoding VKOR, thus paving the way for studies of the enzyme at the molecular level. Reassuringly, an identical gene sequence was revealed using different methodologies. Oldenburg's group used a classical cloning approach in patients identified with hereditary combined deficiency of VKD clotting factors in humans (with raised plasma K>O concentrations), or with hereditary warfarin resistance (63). Stafford's group made use of siRNA to inhibit VKOR activity in a cell line expressing sufficient VKOR to be measurable. Both groups drew on the known genetic mapping of warfarin resistance in rats (64). In 2006, Stafford's group described the purification of VKOR as a single peptide (65). The two groups who identified VKOR have reviewed the state of knowledge as at 2008 (66, 67), while a very recent review provides an excellent independent assessment of structural and functional controversies of the human enzyme (68).
Drugs that inhibit the vitamin K cycle and function of vitamin K
A major feature of the vitamin K cycle is that drugs of several molecular categories, including naturally occurring compounds, have been discovered that prevent the regeneration of the KH2 cofactor. The target of most vitamin K inhibitors is VKOR, although some coumarins may also inhibit vitamin K reductases (see below). Inhibitors of VKOR are readily identified in vivo by the tissue accumulation and release into plasma of measurable concentrations of K>O. The principal drug classes known to inhibit VKOR are 3-substituted 4-hydroxycoumarins and 1,3-indandiones (31). The pro-hemorrhagic properties of these compounds were discovered long before VKOR was recognized as the target enzyme in the 1970s and their use as effective clinical anticoagulants and rodenticides continues to this day. Their discovery owed much to a hemorrhagic disease in cattle (sweet clover disease), first described in the 1920s and which proved the catalyst for the isolation by Karl Link's Wisconsin group of the hemorrhagic agent, a bis-coumarin that they named dicoumarol (69). By 1941 Link's group had shown that dicoumarol had been produced by a coupling of two 4-hydroxycoumarin molecules generated by bacterial action on clover coumarin (69). Although briefly used as a clinical anticoagulant, dicoumarol was soon superseded by other coumarins (e.g., warfarin, phenprocoumon, and acenocoumarol) and indandiones (e.g., phenindione). Other unrelated compounds also inhibit VKOR. The most clinically relevant are antibiotics containing an N-methyl-thiotetrazole side chain (e.g., moxalactam, cefamandole, etc.) which in the 1980s were found to be responsible for a bleeding risk in patients taking them. These antibiotics are weak inhibitors of VKOR as shown by findings that they only caused bleeding in patients with low serum phylloquinone concentrations and/or other indications of malnourishment (70). Another naturally occurring and highly potent inhibitor of VKOR is ferulenol, derived from Ferula communis (giant fennel), which is a 4-hydroxy coumarin in which the 3-substituent comprises three isoprenyl units (71). Ferulenol was reported to be >20 times more potent as an inhibitor of the VKOR than warfarin (71). The degree of inhibition was dependent on side-chain length with increasing potency up to, but not beyond, three isoprenyl units (71).
A paradigm of the nature of the antagonism by coumarin anticoagulants in humans and animals is that even when VKOR is completely inhibited in vivo, the coagulation function (but not necessarily other functions in extrahepatic tissues) can be rescued by providing high doses of vitamin K (72–74). Although physicians were well aware of the antidotal properties of vitamin K, it was the pioneering and extensive investigations by Wallin and colleagues that provided the first insights into the existence and properties of a vitamin K dehydrogenase that was insensitive to coumarin antagonists (75–78). In brief, Wallin proposed that vitamin K reduction could be achieved by two independent pathways: a “physiological” disulfhydryl-dependent reductase pathway that was highly sensitive to warfarin (pathway I) and a NAD(P)H-dependent reductase pathway (pathway II) that was less sensitive to warfarin and was responsible for the antidotal effect of vitamin K (73, 78). Furthermore, in rat liver, pathway II comprised at least two dehydrogenases (73, 78), both present in microsomes (76). Recent and informative functional studies of the vitamin K cycle in mammalian cells are described in more detail below.
VKOR-knockout mice
Significant insights into the in vivo role of VKOR were recently obtained by investigations of the phenotype of mice in which the VKOR protein had been abrogated by knockout technologies (60). Homozygous Vkor−/− mice appeared to be normal at birth but all died within 2 to 20 days of birth due to extensive bleeding, mainly in the brain, caused by severe depletion of carboxylated VKD coagulation proteins. However, newborn Vkor−/− mice could be rescued by oral supplementation with K1 at a daily dose of 30–50 μg K1/day. Even at this supra-physiological dosage, VKD clotting factor activities in Vkor−/− mice remained significantly reduced compared with wild-type animals and heterozygous littermates. Interestingly, the strategy of maternal supplementation with 100 μg K1/day (maximum dose 3.5 mg/kg body mass) did not lead to sufficient transfer of K1 via breast milk to rescue the neonates. In addition, the absence of VKOR affected normal skeletal development with Vkor-deficient animals having a reduced length of the calcified regions of long bones. This skeletal phenotype is consistent with the same or similar abnormalities that have been attributed to putative disturbances of the normal functions of bone Gla proteins caused by use of VKAs during pregnancy (79), mutations of VKOR (80), maternal vitamin K deficiency (81), and mutations of matrix Gla protein (MGP) (82).
The lethality of the Vkor-knockout at habitual dietary vitamin K intakes rules out a redundancy in the existence of a substitute enzyme in liver that can support γ-glutamyl carboxylation of VKD coagulation proteins and shows the evolutionary importance of VKOR in maintaining hemostasis. In one sense, the ability to rescue the bleeding phenotype of Vkor-deficient mice mirrors that of poisoning with VKAs in that in both conditions supplementing with higher than physiological amounts of vitamin K reverses the coagulopathy. At a simplified level, affected individuals have higher dietary requirements for vitamin K. Precise details of the rescue mechanism have yet to be addressed and although the Vkor-knockout informs us that VKOR is the only liver enzyme that can reduce K>O, it does not tell us if VKOR is normally responsible for reducing K to KH2. These issues are further addressed in the sections that follow.
Probing the function of the vitamin K-epoxide cycle using cell-based assays
A major problem with carrying out in vitro studies of VKOR is that topology modeling predicts that the majority of the amino acid residues are either located inside the ER membrane or in a hydrophobic region (83). As a consequence, purified recombinant VKOR requires an intact membrane for its function (65). Recombinant VKOR alone is sufficient to catalyze the conversion of both K>O to K and K to KH2 in vitro (65), but it remains to be established whether VKOR carries out both reactions in vivo. It is also known that both reductions require the same cysteine residues (132 and 135) at the CXXC active-site motif of VKOR (84).
In order to circumvent the problems of studying the vitamin K-epoxide cycle in isolated cell fractions, Stafford and colleagues recently developed a cell-based reporter assay system (85). The reporter protein in this assay was a chimeric VKD protein in which the Gla domain of protein C (PC) had been exchanged with the Gla domain of factor IX (85). This replacement enabled the ready detection of the factor IXgla-PC fusion protein using a monoclonal antibody that was specific for the carboxylated Gla domain of factor IX (85). The chimeric reporter protein was then stably expressed in HEK293 cells and in AV12 cells. The reason for choosing HEK293 cells is that they are commonly used for the biosynthesis of VKD proteins and recombinant PC produced by HEK293 cells is fully γ-carboxylated. On the other hand, under similar growth conditions, PC produced by AV12 cells is partially carboxylated with 20% anticoagulant activity compared with plasma-derived PC. This suggested that HEK293 and AV12 cells might have different vitamin K pathways for synthesizing VKD proteins. The results showed that both cell lines secreted the carboxylated reporter protein when fed K1 or K1>O, although the AV1 cells did this much less efficiently. As expected, both cell lines were sensitive to warfarin with K1>O as substrate (50% inhibition of reporter protein carboxylation at 0.1 μM), consistent with the inhibition of VKOR in the cells. However, if the cells were fed increasing doses of K1 instead of K1O, the inhibition of carboxylation by warfarin could be overcome in HEK293 cells but not in AV12 cells. In warfarin-treated HEK293 cells, the requirements for K1 to restore maximal carboxylation were greatly increased from 1 μM to 22 μM, whereas the highest 22 μM concentration of K1 had a minimal effect on carboxylation in AV12 cells. These experiments confirmed that HEK293 cells (but not AV12 cells) possess significant warfarin resistant “antidotal vitamin K reductase activity” that is able to maintain sufficient KH2 production to sustain γ-glutamyl carboxylation when the VKOR is completely inhibited. Another implication of these results is that AV12 cells contain a warfarin-sensitive enzyme other than VKOR that converts K to KH2 (85).
In a refinement to their cell-based model, transcription activator-like effector nucleases were used to knockout the endogenous VKOR activity in HEK293 cells (86). This enabled the study of the reduction and possible recycling of vitamin K in VKOR-deficient cells without using warfarin. As expected, knocking out VKOR led to a loss of K>O reductase activity but did not affect vitamin K dehydrogenase activity. Unexpectedly, after culturing VKOR-knockout cells for several generations, HEK293 cells almost completely regained their ability to utilize K>O for γ-carboxylation of factor IXgla-PC, even though VKOR was not being expressed. However, GGCX activity was again abolished in HEK293 cells in which both VKOR and its paralog VKOR-like 1 (VKORL1) had been knocked out. The data suggested that VKORL1 had been responsible for the regained K>O reductase activity of the VKOR-knockout cells, probably by induction, and that VKORL1 could reduce K>O as efficiently as VKOR to support γ-glutamyl carboxylation (86). Furthermore, VKORL1 showed the same sensitivity to warfarin inhibition as VKOR (86). Such studies demonstrate the promise of cell-based assays together with genome editing for functional studies of the vitamin K cycle.
Probing the mechanism of warfarin resistance
The first report in 1964 of the hereditary transmission of a resistance to the action of coumarin anticoagulants was in a family from which the propositus required a daily warfarin dose that was approximately 15-fold higher than normal (145 mg) to achieve stable anticoagulation (87). At around the same time, reports of the spread of resistance to first generation rodenticides were also being reported (88, 89) and after the introduction of second-generation rodenticides (so called superwarfarins) to these compounds as well (90, 91). The discovery of VKOR in 2004 as the target protein for VKAs (13, 14) has resulted in the identification of ∼30 mutations in humans (92) and ∼10 in rodents (93) associated with VKA resistance. Attempts to investigate the molecular basis of warfarin resistance displayed by rodents and humans possessing naturally occurring mutations in VKOR have yielded mixed and often paradoxical results (13, 92, 93). Until very recently, most studies of the resistance of naturally occurring VKOR mutants to VKAs have been carried out using an in vitro VKOR assay in the presence of detergent and using the nonphysiological reductant DTT to provide the reducing equivalents to substitute for the presently unknown physiological reductant(s). Using such VKOR assays, only about one-third of the missense variants defined as resistant to first generation VKAs have shown the expected degree of resistance in humans (13, 92) or rodents (93–95). Paradoxically, compared with wild-type VKOR, many rodent and human mutations with a resistant genotype showed an increased sensitivity to warfarin inhibition in the DTT-driven assay or very low enzymic activity in the absence of warfarin (92–95). Up to the year 2012, a total of 28 spontaneous single mutations in the VKOR coding sequence had been reported in patients defined from dose criteria as being warfarin resistant (92). When 25 of these rare human VKOR mutations were expressed as membrane-bound proteins in Pichia pastoris, only 6 mutations showed significant resistance to warfarin and 10 showed almost complete (>98%) loss of activity for converting K1>O to K1, even in the absence of warfarin (92). One amino acid site of mutation in VKOR that has consistently shown both resistance to warfarin and normal activity for at least four different substitutions in rodents (93) and humans (92) is tyrosine139. This mutation hotspot is part of the hydrophobic sequence Thr-Tyr-Ala, which is also found in the NAD(P)H-dependent oxidoreductase (NQO1), another enzyme that is sensitive to coumarin VKAs (see section on this enzyme below). In NQO1, tyrosine139 has been identified as the dicoumarol binding site (96), and based on the consequences of mutations in rodents (93) and humans (97), tyrosine139 has also been proposed as being important for the binding site of VKAs to VKOR. At the time of these studies, the lack of apparent activity of many other VKOR mutations was difficult to explain. Clearly if reproduced in vivo, it is difficult to conceive how affected animals and humans with such an inactive VKOR could generate sufficient vitamin KH2 for synthesis of coagulation Gla proteins, particularly given the new knowledge that Vkor-knockout mice die of fatal bleeding shortly after birth (60). For resistant rodents, it has been suggested that evolutionary pressure might have given rise to upregulation of other pathways for the generation of vitamin KH2 for which there is some evidence (93), but it was difficult to explain inactivity of VKOR variants in humans with clinical resistance to VKAs (92). One possible explanation is that these data reflect the artificiality of the DTT-driven VKOR assay.
Recently, more physiologically representative methods for assessing structure-function relationships of VKOR variants in humans or animals with a warfarin-resistant phenotype were independently introduced by the groups of Oldenburg (98) and Stafford (86). Both groups expressed putative resistant variants of VKOR in HEK293 cells in which either full-length factor IX (98) or factor IXgla-PC (86) had been coexpressed as the reporter for VKOR activity. One difference was that Stafford's group knocked out both VKOR and VKORL1 in HEK293 cells to eliminate endogenous K>O reductase activity (86). In contrast to the in vitro assay data described above, these cell systems showed much better correlations of IC50 values with warfarin dosage requirements in patients (86, 98). However, 5 out of 10 variants that previously had been found to be associated with a resistant phenotype in humans (92) did not show resistance when expressed in cells with the double-gene knockout (86). This suggested that other factors apart from VKOR activity were responsible for warfarin resistance. Again, in marked contrast to previous in vitro data (92), all variants had a basal activity (i.e., in the absence of warfarin) that was similar to wild-type cells (86, 98). Importantly, all 10 VKOR variants previously reported to have no basal activity in the DTT-driven in vitro assay (92) were found to be fully active in a cell-based assay (86). This is consistent with findings in patients or relatives harboring warfarin-resistant heterozygous VKOR mutations who, when not taking warfarin, showed no evidence of defective VKOR function as judged by the absence of detectable undercarboxylated factor II or K1>O (99).
Targeting which dehydrogenase(s) reduce vitamin K to vitamin KH2 in vivo
VKOR.
Given that several vitamin K dehydrogenases have been implicated in the reduction of vitamin K, the identity of the enzyme(s) carrying out this reduction either in the presence or absence of warfarin remains to be established. One candidate is VKOR itself. VKOR is known to be capable of the reduction of vitamin K (65, 66), although a study with purified VKOR indicated that the rate of conversion of K1>O to K1 was approximately 50-times faster than the rate for K1 to K1H2 (65). To investigate the role of VKOR in their cell-based assay system, Tie et al. (85) expressed (transiently and/or stably) a warfarin-resistant VKOR mutant (Tyr139Phe) in HEK293 and AV12 cells that also stably expressed the factor IXgla-PC reporter. VKOR function was tested by feeding the transfected cell lines with K1>O in the presence of an inhibitory concentration of warfarin. As expected, the mutant VKOR in HEK293 cells readily overcame warfarin inhibition and produced significant amounts of reporter protein, although this did not distinguish whether HEK293 cells were utilizing the warfarin-resistant VKOR mutant for this reaction or the antidotal enzyme. In contrast, the transfected AV12 cells failed to produce significant reporter protein when fed K1>O and warfarin K despite the fact that the Tyr139Phe mutant VKOR was shown to be active in AV12 cells in the absence of warfarin (85). Tie et al. (85) reasoned that if the VKOR were the major physiological contributor to the conversion of K1 to KH2, then the AV12 cells should have produced significant carboxylated reporter protein as observed in HEK293 cells. The results supported the hypothesis that the in vivo function of VKOR is to convert K>O to K and that a second enzyme is required to convert K to KH2, the latter conclusion being in agreement with the much lower in vitro activity of VKOR for vitamin K reduction (65). Another unexpected implication of the behavior of the AV12 cell line is that the enzyme that converts K to KH2 must be warfarin sensitive.
NQO1.
Another long-known candidate for vitamin K reduction is NQO1 (EC 1.6.5.2), also known as DT-diaphorase. NQO1 is a flavoprotein that catalyzes the two-electron reduction of several quinones including vitamin K (100–103). It is ubiquitously present in tissues with high concentrations in the livers of most animals and can utilize both NADH and NADPH as electron donors. The enzyme is located mainly in the cytosol (>90%), but some is associated with mitochondria and microsomes (101). The discovery in the 1960s that NQO1 was strongly inhibited by dicoumarol (100), proved a useful tool in studies of the enzyme, and was probably the catalyst for the idea that NQO1 may be involved in the in vivo reduction of vitamin K. NQO1 is also inhibited by coumarin and indandione derivatives, but dicoumarol remains the strongest known inhibitor of NQO1 (101, 104), and interestingly far stronger an inhibitor than warfarin (78, 105) or even the second-generation VKA (superwarfarin) difenacoum (78), which is 10-fold more potent as an anticoagulant than warfarin in vivo (106).
Quinones lacking a side chain, of which menadione is a good example, are the most active electron acceptors for NQO1, and the activity decreases with increasing length of the side chain (101). Early studies by Martius, Ganser, and Viviani (107) showed that detergent (Tween)-solubilized K1 and MK-3, -4, -6, and -9 reacted with the enzyme slowly but incorporating the vitamin into liposomes greatly enhanced the rate of reduction.
Wallin's group showed that selective immunoadsorption of NQO1 from solubilized rat microsomes completely abolished the warfarin-sensitive dehydrogenase activity (75), but neutralized only 50% of GGCX activity in a system driven by addition of K1 + NADH (75, 76, 78). Adding back purified NQO1 or K1H2 restored GGCX activity. These experiments provided evidence that in rats, NQO1 contributed ∼50% of the total NADH-supported dehydrogenase activity of pathway II; the remaining activity that was immunologically distinct to NQO1 was attributed to unknown microsomal dehydrogenase(s) (78). Because NQO1 is known to be sensitive to coumarin drugs, Wallin reasoned that the unknown microsomal dehydrogenase(s) was responsible for the coumarin-resistant dehydrogenase activity that could counteract the effects of coumarin drugs (78). The finding that the specific activity of NQO1 in human liver represents <1% of the activity in rat liver (77) suggests that the coumarin-sensitive NQO1 plays only a minor role in the dehydrogenase pathway II for vitamin K reduction in humans, certainly in the presence of warfarin but probably also in its absence (74, 77, 78). Recent evidence that NQO1 is not the antidotal enzyme of pathway II in HEK293 cells was obtained by Stafford's group who showed that dicoumarol strongly inhibited reporter protein γ-carboxylation when K>O was the vitamin K source, but not when vitamin K was fed to the cells (85).
It has been reported that Nqo1-null mice do have bleeding problems suggesting the nonessentiality of NQO1 for supporting γ-glutamyl carboxylation (103). Very recently, Nqo1-null mice were utilized to assess the contribution of NQO1 to the reduction of vitamin K in greater depth (108). First it was shown that mice given a lethal dose of warfarin could be rescued by concomitant administration of supraphysiological doses of K1 with 100% survival rates for both Nqo1-deficient and wild-type mice (108). Second, in vitro measurements of the conversion of K1 to KH2 by Nqo1-deficient mouse microsomes demonstrated a warfarin-resistant dehydrogenase activity that was distinct from VKOR and required NAD(P)H and manganese as cofactors. The identity of this microsomal enzyme(s) is unknown, but the fact that (like NQO1) it requires pyridine nucleotides argues against NQO2 as a candidate, while its lack of warfarin sensitivity argues against VKORL1 (108).
VKORL1.
During the identification of VKOR, Oldenburg and colleagues reported that the vertebrate genome contains a second gene that encodes for a paralogous VKORL1 (13). VKORL1 is more highly conserved among vertebrates than VKOR (109) and, like its counterpart, is localized to the ER (110). In a study of the properties of VKORL1 in crude HEK293 cell membranes, Westhofen et al. (110) showed that VKORL1 was capable of reducing epoxides of both K1 and MK-4 to their respective quinones. However, the affinity of VKORL1 for K1>O and MK-4>O substrates was about 2- and 7-fold lower than their affinities for VKOR (110). Their attempt (110) to compare turnover number (kcat) for K1>O reduction by VKORL1 with the turnover number for VKOR previously determined by Chu et al. (65) appears to be flawed because the VKORL1 calculation is based on the amount of total protein rather than the amount of VKORL1 enzyme. On this basis, their conclusion that the reduction of K1>O by VKORL1 is >4 orders of magnitude less efficient than for VKOR (110) has been questioned (86). Nevertheless, the reasoning that VKOR has evolved a greater efficiency for reducing vitamin K epoxides than VKORL1 (110) is certainly supported by the failure of VKORL1 activity to substitute for the loss of VKOR activity in Vkor−/− mice, at least for hepatic coagulation factor synthesis (60). On the other hand, experiments in cultured HEK293 cells showed that VKORL1 reduces K>O as efficiently as VKOR to support γ-glutamyl carboxylation (86).
Westhofen et al. (110) also showed that VKORL1 was capable of reducing K1 to K1H2 and hypothesized that the primary role of this paralog enzyme was to carry out the reduction of vitamin K quinones. This hypothesis was not based on measurements of reductase activity per se, but on experiments that suggested that KH2 produced by VKORL1 serves an intracellular antioxidant function. A fuller consideration of the possible antioxidant functions of vitamin K is given in the section below entitled “Other putative roles of vitamin K dehydrogenases and epoxide reductases.”
Evidence that VKORL1 may indeed be important for recycling vitamin K in some extrahepatic tissues has recently emerged (111). To assess the functional properties of VKORL1, human and rat VKOR and VKORL1 were overexpressed in Pichia pastoris. This showed that in the DTT-driven VKOR assay, human VKORL1 was more effective in reducing K>O than human VKOR, although the reverse was found for the rat. However, a major finding was that the rat and human VKORL1 proteins were 50- and 30-fold more resistant to warfarin than their respective VKOR proteins. Real-time PCR showed that Vkorl1 mRNA in rats was expressed in all tissues analyzed, but the ratio to Vkor mRNA varied by tissue. Liver tissue showed the highest Vkor mRNA with a relative expression ∼10-fold higher than that for Vkorl1. In extrahepatic tissues, the expression of Vkor mRNA was much lower than found in the liver (4- to 8-fold lower in kidney, lung, and testis, and 30-fold lower in the brain) and in some extrahepatic tissues such as brain and testis, Vkorl1 mRNA exceeded that for Vkor. A similar expression pattern was seen for mouse tissues. Notably, the expression of Vkorl1 transcripts in liver, lung, and testis of wild-type mice was the same as in highly vitamin K-supplemented Vkor−/− mice. The significance of the different tissue expression of Vkorl1 and Vkor mRNA was further investigated by measuring K1>O reductase activity in the livers and testis of wild-type and Vkor−/− mice. The results showed that the K1>O reductase activity of liver microsomes in Vkor−/− mice was almost undetectable and represented only 2.9% of K1>O reductase activity of wild-type mice. On the other hand, testis microsomes from Vkor−/− mice retained 35% of the original total K1>O reductase activity of wild-type animals, an activity that was attributed to VKORL1 and which, like VKOR, was blocked by high dose (100 μM) warfarin. Similar measurements of K1>O reductase activities were carried out in rat tissues with increasing concentrations of warfarin. This showed that the susceptibility to warfarin varied between tissues. Based on the warfarin data, the authors developed a mathematical model to predict the proportion of K1>O reductase activity that was catalyzed in each rat tissue by VKOR or VKORL1 during anticoagulation with warfarin. The model predicted that whereas VKOR supported >90% of K1>O reductase activity in liver, kidney, and brain, VKORL1 supported >50% of the activity in testis and rat osteosarcoma cells, and >20% in lung (111). The authors hypothesized that this different tissue susceptibility to VKAs and rescue by VKORL1 may partly explain why it has been difficult to find serious side effects of warfarin that are attributable to lack of VKD functions in extrahepatic tissues. Examples given by the authors were possible effects of VKAs on bone and cardiovascular health mediated by inadequate carboxylation of osteocalcin and MGP. Such a hypothesis is very difficult to verify. It needs to be remembered that therapeutic anticoagulation does not block, but only lowers, the γ-glutamyl carboxylation status and hemostatic potential of hepatic Gla proteins and the same is equally true for extrahepatic Gla proteins. Finally, Vkor−/− mice did show evidence of skeletal abnormalities characteristic of vitamin K deficiency or VKA antagonism, which was obviously not prevented by VKORL1.
It is worth mentioning that the relative expression of Vkorl1 mRNA was higher than for Vkor mRNA in brain, testis, and osteoblast-like cells (111), while previous data for MK-4 distribution shows that brain and bone (testis not measured) also have higher ratios of MK-4 to K1 (51). Also, the lowest ratio by far of Vkorl1 to Vkor expression was found in the liver (111), which is also the organ with the lowest ratio of MK-4 to K1 (51). It might be worth investigating whether MK-4 is the favored substrate for VKORL1, although the relative in vitro affinity data for K1>O and MK-4>O substrates does not support this (110).
Other putative roles of vitamin K dehydrogenases and epoxide reductases
Detoxification role of NQO1.
As already mentioned, NQO1 catalyzes the two-electron reduction of quinones. In the case of vitamin K compounds, apart from supporting physiological redox functions, NQO1 may promote their detoxification by forming relatively stable hydroquinones and facilitating conjugation reactions (101, 103). Whereas extra-dietary supplements or pharmacological doses of naturally occurring K1 and MK are not associated with any known harmful effects in humans, high doses of menadione are associated with well-described dose-dependent toxic reactions, especially in newborn infants (112, 113). Toxic reactions to menadione commonly include hemolytic symptoms that may lead to severe hemolytic anemia and are explained by the well-described cytotoxic properties of menadione (112, 114, 115). The cytotoxicity of menadione (and other quinones) is attributed to two principal mechanisms: production of reactive oxygen species (ROS) through redox cycling and arylation of nucleophilic compounds such as thiols (114–116). The toxic arylation properties of menadione are explained by the high reactivity of the unsubstituted 3-position enabling reaction with free and protein bound thiols to form thio ethers (116, 117). Because of this strong cytotoxicity, menadione has been widely used as a model compound to study oxidative stress in mammalian systems (114–116, 118).
Menadione is one of the most avid electron acceptors for NQO1 ever tested (100, 101), and its two-electron reduction to the hydroquinone has been proposed as a detoxifying pathway for menadione (101, 114, 115). Importantly, the two-electron reduction of menadione does not generate unstable semiquinone and ROS, and thus affords protection against cellular damage induced by oxidative stress (101, 103, 114). Support for the importance of NQO1 to the detoxification of menadione comes from studies in Nqo1-null mice, which demonstrate increased menadione toxicity as compared with wild-type mice (119). In addition, the inhibitor of NQO1 dicoumarol potentiates menadione and other quinone toxicity in vivo and in cell cultures (101, 114).
Added credence for a possible role of NQO1 in physiological vitamin K metabolism comes from new evidence that menadione is a naturally occurring intermediate in the cellular conversion of phylloquinone to MK-4 (15, 120–122). The extent of possible natural menadione production in the body is shown by the findings that 1–5% of an administered dose of K1 or MK-4 was excreted in human urine as menadione metabolites within 24 h (for more details see section below on menadione as an intermediate of MK-4 biosynthesis).
Possible antioxidant role of VKORL1.
Vitamin K in its reduced form, KH2, is known to be a potent radical-scavenging species (123). Based on the suppression of lipid peroxidation in rat liver microsomes, Thijssen and coworkers had suggested in 1997 that the vitamin K epoxide cycle could provide a localized defense against free radicals in its ER membrane environment (124). This suggestion was based on experiments that showed that hydroquinones of K1 and MK-4, but not their quinones, effectively suppressed lipid peroxidation reactions induced by pro-oxidants in rat liver microsomes (124). However, the antioxidant response was completely abolished by 2-chloro-phylloquinone (chloro-K1) and by warfarin, which are inhibitors of GGCX and VKOR, respectively. These findings demonstrated the need for a functional vitamin K-epoxide cycle and that the radical-scavenging vitamin K species was being continually regenerated (124).
In our previous review (11), we described the interesting later findings of Li et al. (125) that both K1 and MK-4 could protect developing oligodendrocytes and neurons against oxidative injury and ROS generation that had been induced by glutathione depletion. This effect differed from the findings of Thijssen's group (124) in that the antioxidant activity was not inhibited by chloro-K1 or by warfarin, showing that the protective effect of vitamin K compounds was independent of γ-carboxylation and recycling of vitamin K by VKOR, the only enzyme known to possess this function at the time. However, the possibility that the effect was mediated by KH2 generated by other pathways was not ruled out (125). Another pathway, which leads to oxidative damage, is the release of arachidonic acid, which induces cell death through the activation of 12-lipoxygenase (12-LOX). Since their earlier study, Li, Wang, and Rosenberg (126) have shown that both K1 and MK-4 in the nanomolar range inhibit 12-LOX activation and prevent ROS accumulation and oxidative death in developing oligodendrocytes. Vitamin K compounds did not interact directly with 12-LOX, but probably act upstream of its activation by an as-yet unidentified mechanism (126).
Taking into account this earlier work, and the properties of VKORL1 from their own work, Westhofen et al. (110) hypothesized that the apparent independence of antioxidant protection of vitamin K from the VKOR might be explained if this paralog enzyme was carrying out the synthesis of KH2. They therefore investigated the ability of VKOR and VKORL1 to protect against oxidative stress in cultured HEK293 cells overexpressing these enzymes. Cells subjected to hydrogen peroxide-induced oxidative stress showed dramatic increases in K>O reductase activity and in the short-term showed upregulation of VKORL1 expression and downregulation of VKOR expression. In addition, the viability of cells growing without imposed oxidative stress was increased by both K1 and MK-4, but not by ubquinone-10, and was specifically dependent on VKORL1 expression. Finally, the level of expression of VKORL1 positively correlated with the reduction in intracellular ROS induced by chemical agents, and inversely correlated with oxidative protein damage (110). Based on this data, Westhofen et al. (110) proposed that the primary role of VKORL1 is to reduce vitamin K and thus to provide a VKD mechanism to provide universal tissue protection against oxidative injury.
BIOSYNTHESIS AND CELL BIOLOGY OF MK-4
Discovery of UBIAD1 as a novel MK-4 biosynthetic enzyme
The potential transformation of K1 to MK-4 has been studied since the 1950s and was reviewed extensively in our previous review (11). At this time, Okano et al. (127) had used NMR and LC-MS/MS technology to show that oral administration of deuterium-labeled menadione or K1 led to accumulation of MK-4 in the brains of experimental mice. When both ring and side chain deuterated K1 was used as the substrate, the side chain of the MK-4 formed was unlabeled suggesting that the side chain had been completely removed and replaced, presumably with geranylgeranyl pyrophosphate as the source of the side chain (127). Since then, the same group has gone on to attribute this conversion to the enzyme UBIAD1 (15). The organ of choice in this study was bone, as opposed to brain, reflecting the weight of literature on vitamin K in bone at the time, although brain is also rich in MK-4 (50–52). The authors speculated that the enzyme responsible for the conversion might be a mammalian homolog of one of the bacterial enzymes that are involved in the biosynthesis of MK-4, and considered two candidate mammalian genes, UBIAD1 and COQ2. COQ2 is a human homolog of a yeast prenyl transferase and UBIAD1 is the human homolog of Escherichia coli MenA; the latter encodes the enzyme that converts 1,4-dihydroxy-2-naphthoic acid to dimethylmenaquinone, though the human gene had no known function. The ability of these genes to encode for the enzyme that synthesizes MK-4 was tested using stable isotope substrates of menadione or MK-4 and siRNA inhibition in cultured MG-63 cells. UBIAD1 siRNA strongly inhibited this conversion while COQ2 siRNA did not. When cells were transfected with a UBIAD1 expression vector, the production of MK-4 was enhanced. Furthermore, the insect cell line Sf9 had no intrinsic conversion activity, but could produce MK-4 when transfected with the expression vector. UBIAD1 is located in the ER and is not inhibited by warfarin (15).
Menadione as an intermediate and circulating precursor of tissue MK-4 biosynthesis
As discussed earlier (see section above on storage and catabolism), MK-4 has an unusual tissue distribution with high concentrations in some tissues (e.g., brain, kidney, and pancreas) and low concentrations in others (e.g., plasma and liver) (50–52). This uneven distribution raises the question of whether MK-4 is synthesized from K1 locally or is transported to the target tissue from another site such as the liver or intestine, either as MK-4 or as a precursor such as menadione (50–52). The recent discovery of UBIAD1 clearly showed that this enzyme has a strong activity for prenylation of menadione but much weaker activity for phytyl side-chain cleavage (15). This raises questions of whether UBIAD1 has the capacity to generate sufficient menadione from K1 to account for the high MK-4 concentrations in some tissues and whether another enzyme(s) besides UBIAD1 catalyzes the removal of the side chain, possibly in tissues distant from the site of prenylation. The likelihood of different cleavage and prenylation sites is supported by evidence that, although a number of cell lines readily convert menadione to MK-4 (120, 121, 127, 128), the same cell lines showed either no (120, 121, 128) or some (127, 128) capacity to utilize K1 as a substrate. As pointed out by Thijssen et al. (120), a caveat to some previous observations of MK-4 synthesis from K1 in cell culture or in vivo is that commercial preparations of K1 contain traces of menadione. This failure to demonstrate conversion of K1 to MK-4 in cell culture included pancreatic cell lines (120), which, based on the very high capacity of the pancreas to accumulate MK-4 (50–52) and to utilize menadione as substrate might have been expected to possess high activity for side-chain cleavage (120). Until the recent identification of a candidate mammalian enzyme that could remove the phytyl side chain, one hypothesis was that side-chain cleavage of K1 was carried out by the intestinal microflora, but this was discounted by studies showing that germ-free rats carry out MK-4 synthesis from K1 as efficiently as control animals (128, 129).
The first in vivo evidence that the alkyl side chains of vitamin K could be completely cleaved in humans was a study by Thijssen et al. (120), who showed that oral administration of K1, MK-4, or MK-7 rapidly stimulated the urinary excretion of menadione. Importantly, menadione excretion was not increased above basal concentrations when K1 was given as a subcutaneous injection indicating that intestinal absorption is required for menadione formation (120). The same investigators hypothesized that side-chain cleavage most likely occurred within enterocytes and that the released menadione was transported to target organs for MK-4 synthesis (120). The necessity for intestinal metabolism was subsequently confirmed in stable-isotope studies that showed that brain synthesis of MK-4 in mice occurred only after oral or enteral administration of K1, and not after direct injection into the blood stream or into the brain ventricles (127).
Direct evidence that menadione is an intermediate of MK-4 synthesis was obtained by the detection of labeled menadione in both urine and serum within 1 day of feeding rats with deuterium-labeled collard greens (121). In addition the finding that the de novo labeled MK-4 detected in various tissues carried the deuterium label in the naphthoquinone ring, but not the side chain, confirmed the need for phytyl side-chain cleavage prior to MK-4 synthesis (121). Interestingly, measurements of both labeled and total MK-4 in individual tissues showed that the overall concentration of MK-4 in some tissues (including testes, brain, and visceral fat) remained constant, although the proportion of labeled MK-4 in the same tissues had increased after feeding labeled K1. This suggested that these tissues have some means of regulating their MK-4 concentrations. Other tissues had fluctuating levels of MK-4, which seem to respond to the dietary availability of K1 (121).
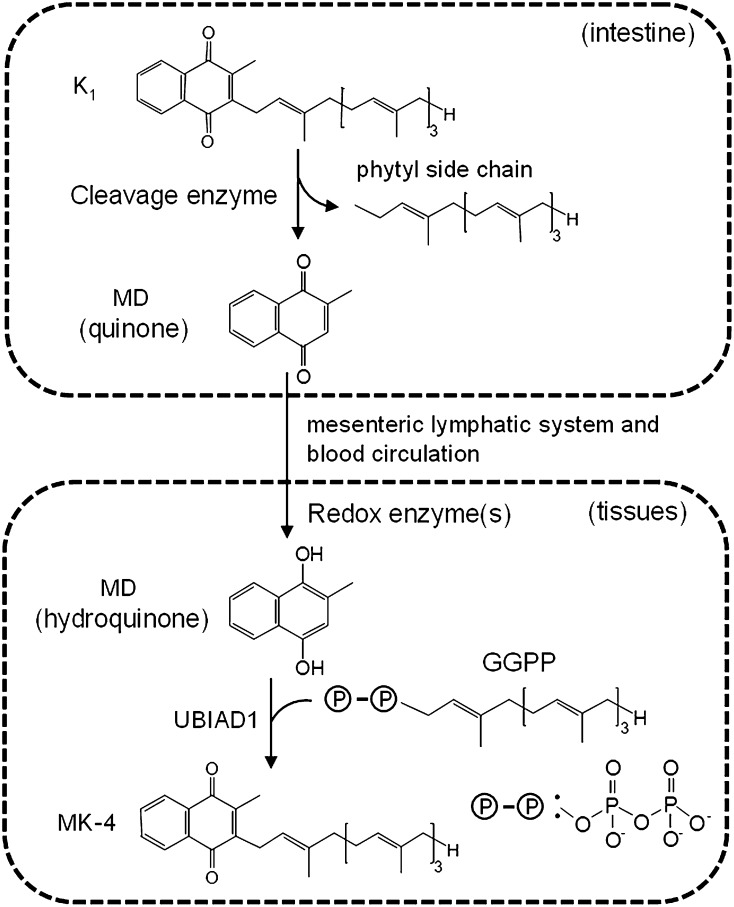
Very recently, a new study from Okano's group has provided definitive evidence that a small proportion of dietary phylloquinone is converted to menadione in the intestine and transported to tissues where it is prenylated to form MK-4 (122). Experiments were done with rats in which vessels transporting blood and lymph were cannulated allowing their contents to be sampled and analyzed at various time points after the oral administration of deuterium-labeled K1. The sensitive detection of menadione in tissues was achieved by either high-resolution MS or by a novel bioassay using recombinant UBIAD1 protein. Labeled menadione appeared in the thoracic lymph duct by 1 h after administration of K1, and peaked at about 5 h, which was about 2 h later than the peak for labeled K1. Quantitatively, approximately 2 and 0.1% of the absorbed labeled K1 was converted to menadione and MK-4 in the intestine, respectively. Analysis of representative organs showed high concentrations of deuterium-labeled MK-4 in the cerebrum, and low concentrations in the liver in which the label was predominately found as K1. This is in agreement with the distribution of unlabeled compounds in previous studies (50–52). The authors also showed that rat intestinal IEC-6 cells were capable of removing the side chain of K1 to generate menadione, which could be converted to MK-4. This has been observed only rarely in other studies with cultured cells, and is possibly due to the higher sensitivity of the methods used here to detect the conversion of K1 to MK-4. It was also demonstrated that menadione has to be in the hydroquinone form in order to be prenylated by UBIAD1 and that an unidentified redox enzyme in the tissues carries out this reduction. On the basis of their latest data and previous studies, Okano and coworkers (122) proposed a working hypothesis for MK-4 biosynthesis from K1 which proposes that menadione is released from K1 in the intestine, the free menadione delivered via the mesenteric lymphatics into the blood and then transported to peripheral tissues where menadione is reduced to menadiol and prenylated to MK-4 by UBIAD1 (Fig. 3).
Fig. 3.
Scheme showing the putative catabolism of K1 to menadione (MD) in the intestine, its uptake into the blood via the mesenteric lymphatic system and delivery to target tissues for local synthesis of MK-4 by UBIAD1. Taken from (122) with permission of authors.
Cell biological effects of UBIAD1 and its product MK-4
An insight into the role of MKs in eukaryotic cells came from the use of a novel genetic approach to show that the gene heixuedian (heix), a Drosophila homolog of mammalian UBIAD1, acts as a modifier of pink1 which encodes a mitochondrial kinase and which in humans is often mutated in individuals with Parkinson's disease (130). Heix mutants that had defects in their mitochondrial electron transport could be rescued by the addition of MK-4 to the medium on which Drosophila larvae were grown, suggesting that it can act as a mitochondrial electron carrier facilitating ATP production in purified preparations of mitochondria. It thus appears that MK-4 may have a role in the mitochondrial electron transport chain of Drosophila as in prokaryotes, but it has not been established whether it may have a similar role in humans.
In a further development, UBIAD1 has been implicated in the maintenance of endothelial cells in the vasculature of zebrafish (131). In a study of the reddish (reh) mutant zebrafish, Hegarty, Yang, and Chi (131) found that embryos appear to develop a normal vascular system initially but then experience a degeneration of the blood vessels and hemorrhages in areas of the head by 48 h followed by cardiac edema and death. Genetic analysis of the reh mutant locus pointed to the ubiad1 gene causing a Leu65Gln substitution in a transmembrane region of the protein. Loss of UBIAD1 function in zebrafish embryos induced by the injection of antisense oligos resulted in a similar vascular phenotype to the reh mutants. When reh embryos were injected with wild-type ubiad1 mRNA, a significant percentage of them were rescued from the vascular phenotype, while injection of rehs587 ubiad1 mRNA, which is the transcript of the reh mutant ubiad1 gene, failed to rescue embryos.
The role of MK-4, the product of the UBIAD1 enzyme, in vascular maintenance was first investigated in wild-type embryos by blocking the reduction of oxidized forms of vitamin K with warfarin (131). This caused a similar cranial hemorrhage and vascular phenotype to the reh mutants and the ubiad1 knockdown embryos except that they did not develop cardiac edema. This phenotype could be rescued by treatment with either MK-4 (94%) or by K1 (75%). The greater efficiency of MK-4 compared with K1 was also seen when the compounds were used to treat the reh mutants. Embryos were injected at 36 h postfertilization and 12 h later 58% of MK-4-treated embryos had been rescued compared with only 6% of those treated with K1. This may suggest that K1 needs to be converted into MK-4 in order to enable the rescue, hence the relatively low number of reh embryos that appeared normal after K1 injection. It is interesting that the vascular and cardiac phenotypes differed in their responses to either targeted expression of ubiad1 or injection with vitamin K. Neither K1 nor MK-4 was able to rescue the cardiac phenotype, which may imply that UBIAD1 could also have non-VKD means of regulating cardiac development and function.
Missense mutations in the UBIAD1 gene have been linked to the autosomal dominant disease, Schnyder corneal dystrophy, that causes slow deposition of cholesterol in the cornea and loss of transparency of the tissue. Several of these mutations lead to modest reductions in MK-4 synthesis by microsomal fractions of cell lysates from Schnyder corneal dystrophy subjects compared with normal controls, though the physiological significance of this is unknown (132). There is also some evidence based on yeast two-hybrid screening and immunoprecipitation that UBIAD1 interacts directly with the cholesterol metabolic and storage enzymes HMGCR and SOAT1 (132). Although there is no supporting evidence of direct protein-protein interaction from other laboratories, this is in agreement with the effects of MK-4 on the steroid and xenobiotic receptor (SXR) target genes (see below).
UBIAD1, MKs, and cancer
The gene UBIAD1 had also been previously identified independently as a tumor-suppressor gene referred to as transitional epithelial response gene 1 (TERE1), whose expression is reduced or absent in many bladder, prostate, or renal tumors (133). This was many years before the prenyltransferase function of UBIAD1 was known. TERE1 expression was found to be greatly reduced in many prostate tumors, often absent as protein in metastatic prostate tumors, and when its expression was induced in prostate carcinoma cell lines, their rate of proliferation was greatly reduced (134). In a recent study, TERE1 transcript and protein expression was inversely related to cholesterol synthesis in a cell culture model of castration-resistant prostate cancer, using the cell line LnCap-C81 (135). The mechanism was predominantly through genes regulated by the SXR nuclear hormone receptor which is downregulated in this cell line compared with the parental line from which it was derived (135). Groups of genes involved in the hydroxylation and cellular efflux of cholesterol and in steroid catabolism were all induced by the expression of TERE1 protein or the addition of MK-4 to the cells implying that UBIAD1/TERE1 links cholesterol metabolism and the castration-resistant prostate cancer phenotype through its product MK-4 (135).
In our earlier review (11), we covered the effects of MK on certain types of cancer cells. Some of these anti-cancer properties of MK have been attributed to the induction of apoptosis in cancer cells (136), but no mechanism has been established. One recent study has claimed that MK-2, a compound not widely known in living organisms, but which has been found to induce apoptosis in certain leukocyte cell lines, can actually interact with the protein Bcl-2 antagonist killer 1 (Bak) (137). Karasawa et al. (137) claim that MK-2 epoxide forms a covalent bond with the thiol group of Cys-166 of Bak and also that both MK-2 and MK-2 epoxide form a noncovalent interaction with a different part of Bak. However no mechanism for the covalent bond formation was found, the reaction could not be recapitulated in vitro, and the finding remains to be substantiated.
MKs and bone metabolism
Much of the diverse literature on vitamin K and bone metabolism has been carried out using MK-4, its geranylgeranyl side chain, or related molecules, and the results reported have not always been entirely in agreement. This is probably due to the different experimental systems used and individual approaches taken by different labs, but the recurrent theme has been that MK-4 and its derivatives generally suppress osteoclastogenesis and favor bone formation by osteoblasts [see Shearer and Newman (11)]. MK-4 has been used clinically to treat postmenopausal osteoporosis in Japan for around 30 years, which probably explains why it has been the subject of so much scientific attention in Japan. However in recent years other MKs have been studied too, in particular MK-7, and it remains unclear whether the molecular action of these compounds is due to the side chain, the naphthoquinone ring, or both.
In our previous review of this topic (11), we mentioned the significance of an apparent inhibition of the transcription factor NF-κB by MK-4 in hepatocellular carcinoma cell lines (138), and also of how MK-4 or its geranylgeranyl side-chain derivatives seemed to modulate bone resorption and formation through the activities of osteoclasts and osteoblasts in bone. Since then, it has been reported that a different MK, MK-7, suppresses the activation of NF-κB in preosteoclasts and osteoblasts and thereby both opposes bone resorption through the formation of osteoclasts and stimulates the creation of new bone matrix (139). In cell cultures of preosteoblastic MC3T3 cells, MK-7 dose-dependently promoted differentiation and mineralization, while in monocytes stimulated to differentiate into osteoclasts with RANKL, MK-7 suppressed osteoclast formation. In MC3T3 cells, MK-7 could overcome the opposing effect of another soluble factor, TNFα, which is known to inhibit osteoblastic differentiation. MK-7 was shown to suppress the activation of NF-κB in both cell types through the elevation of the mRNA and protein of its inhibitor IκBα, but apparently without altering its phosphorylation state (139).
SUMMARY AND FUTURE PERSPECTIVES
In reviewing the literature on vitamin K metabolism over the last 5 years, we consider that the most significant advances have been made in delineating the importance and mechanisms of either cellular vitamin K recycling or the de novo mammalian synthesis of MK-4. In both areas, new knowledge has been driven by the introduction of new models and techniques.
Studies of Vkor−/− mice that do not survive after birth have shown, for the first time, that the VKOR enzyme plays a life-preserving role in salvaging vitamin K molecules that have been metabolized to K>O during the synthesis of VKD coagulation proteins in the liver (60). In the future, this Vkor-knockout model offers a way of quantifying nutritional requirements for hepatic and nonhepatic VKD functions in the presence and absence of functional vitamin K recycling. This has direct clinical relevance, for example, to vitamin K treatment and maintenance in patients in whom recycling is completely blocked by VKAs, especially in patients poisoned by long-acting superwarfarin rodenticides. In the light of the importance of vitamin K recycling to newborn mice, studies are also indicated to understand the extent to which impaired recycling contributes to the known susceptibility of human newborns to vitamin K deficiency. This increased risk of deficiency in the first 6 months of life has led to universal acceptance of the need for vitamin K prophylactic regimens to prevent a few susceptible infants from developing a potentially life-threatening bleed with the brain being a common bleeding site. The possible contribution of already identified VKOR genetic variants to newborn risk is unknown, but is relevant in view of evidence that different genotypes of VKOR modulate warfarin dose requirements, presumably by affecting vitamin K availability (140). Here again, nutritional studies in Vkor−/− mice might help to determine optimal vitamin K prophylactic regimens for preventing neonatal vitamin K-deficiency bleeding.
There are also gaps in our knowledge of the identity of the enzyme(s) and natural cofactors that participate in vitamin K recycling which include the enzyme(s) that reduces vitamin K to the hydroquinone and the physiological reductant(s) that provides reducing equivalents for VKOR function. In this area, the development of cell-based reporter assays in which normal or chimeric VKD proteins are stably expressed in cell lines have provided a new tool to identify and characterize candidate enzymes of the vitamin K-epoxide cycle and to study warfarin resistance (85, 86, 98). In marked contrast to DTT-driven in vitro assays, these cell-based reporter assays showed improved correlations of VKOR activity with the clinical phenotype and warfarin dose requirements (86, 98). One future refinement to these new cell-based assays would be to deliver the vitamin K to the cells in the physiological lipoprotein milieu in which they are found in the blood. This would enable the study of the relative activities and metabolism of different forms of vitamin K by the enzymes of the vitamin K cycle that would more closely mirror the in vivo delivery of vitamin K to cells. Lipoprotein delivery of vitamin K has already been used in studies of the mechanism of receptor uptake of vitamin K by cultured bone cells (45–47).
An intriguing hypothesis to recently emerge from Oldenburg's group is that the primary role of the paralogous VKORL1 is to reduce vitamin K to the hydroquinone with the latter functioning as an intracellular antioxidant (110). If true, this hypothesis that specifically relates to antioxidant protection of cell membranes would have far-reaching consequences to current concepts of antioxidant mechanisms. An alternative hypothesis is that VKORL1 is important for the reduction of K>O to K in extrahepatic tissues and can serve to rescue the recycling function in some extrahepatic tissues during treatment with VKAs (111). Clearly further studies are needed to clarify the physiological role of VKORL1, not least because currently there are disparities regarding the ability of VKORL1 to reduce K>O and its sensitivity to warfarin (86, 110, 111).
The recent confirmation that the in vivo conversion of dietary K1 to MK-4 involves the complete removal of the phytyl side chain and that the naphthoquinone nucleus serves as the intermediate for cellular MK-4 synthesis finally confirms a prediction made by Martius over 50 years previously [for review of early work see Shearer and Newman (11)]. Many questions remain, not least that while the discovery of UBIAD1 explains the prenyltransferase activity in humans, it is likely that another enzyme(s) contributes to the proposed side-chain cleavage by intestinal cells (122). A significant finding from Okano's group is that the menadione released from K1 in the intestine (which represents only a very small fraction of absorbed K1) is transported into the blood via the lymphatic system (122). This suggests that the released menadione, like K1 and MK, is incorporated into lipoproteins with this route of menadione generation obviously being dependent on the initial efficiency of the bile salt-mediated absorption of K1 itself. In contrast, the absorption efficiency of an exogenous dose of free [14C]menadione in rats was unimpaired by the absence of bile and was efficiently transported to the liver via the portal vein (141). However, in dogs about 10% of absorbed menadione was recovered in thoracic duct lymph, showing that some lymphatic transport is possible (142). In the scheme proposed by Okano's group (Fig. 3), menadione is shown as being transported in blood in the quinone form, which is then reduced to the quinol in the target tissues before MK-4 synthesis. However, as discussed in this review, the 3-position of menadione is highly reactive and combines indiscriminately with free and bound thiol groups in blood contributing to its toxicity. In other words, targeted transport of menadione in plasma would seem to present a problem unless highly regulated. Although vitamin K is present in the diet as the quinone form, we do not know the redox status of vitamin K during its transportation in the plasma. It is known that a closely related member of the isoprenoid quinone family, ubiquinone-10 (Q-10), is present in plasma almost entirely (∼95%) in its reduced form, ubiquinol, and that this proportion is not significantly altered by oral administration of Q-10 supplements, regardless of the dose given or whether ingested as ubiquinone or ubiquinol (143). After administration of Q-9 and Q-10 to rats, both were recovered in mesenteric lymph as the corresponding ubiquinols (143). There is also evidence that enterocytes in cell culture can carry out the reduction of Q-10 (143). By analogy, menadione might also be reduced by the enterocytes and transported in plasma as the quinol, which is the reduction state required for prenylation by UBIAD1 (122). The enzyme NQO1 would be a prime candidate enzyme for carrying out this reduction of menadione.
To date there are no clues of the mechanisms whereby certain tissues such as brain and pancreas are able to preferentially accumulate MK-4 and the reasons for this local biosynthesis are unclear. As discussed in this review and previously (11), there is evidence that MK-4 has roles in cellular functions that are independent of its cofactor role. It is interesting that the concentrations of MK-4 in the brains of mice, rats, and humans are several-fold higher than those of K1 (50, 122). Perhaps a mechanism that utilizes a side-chain-depleted precursor of dietary vitamin K as a transport form facilitates the passage of this smaller molecule across the blood-brain barrier to enable MK-4 biosynthesis (50, 122)?
Footnotes
Abbreviations:
- Bak
- Bcl-2 antagonist killer 1
- CR
- chylomicron remnant
- ER
- endoplasmic reticulum
- GGCX
- γ-carboxyglutamyl carboxylase
- Gla
- γ-carboxyglutamate
- K
- vitamin K
- K>O
- vitamin K 2,3-epoxide
- K1
- phylloquinone
- 12-LOX
- 12-lipoxygenase
- LRP1
- LDL receptor-related protein 1
- MK
- menaquinone
- NQO1
- NAD(P)H-dependent quinone oxidoreductase 1
- ROS
- oxygen reactive species
- TERE1
- transitional epithelial response gene 1
- TRL
- triacylglycerol-rich lipoprotein
- SXR
- steroid and xenobiotic receptor
- UBIAD1
- UbiA prenyltransferase-containing domain 1
- VKA
- vitamin K antagonist
- VKD
- vitamin K-dependent
- VKOR
- vitamin K epoxide reductase
- VKORL1
- vitamin K epoxide reductase-like 1
REFERENCES
- 1.Stenflo J., Fernlund P., Egan W., Roepstorff P. 1974. Vitamin K dependent modifications of glutamic acid residues in prothrombin. Proc. Natl. Acad. Sci. USA. 71: 2730–2733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelsestuen G. L., Zytkovicz T. H., Howard J. B. 1974. The mode of action of vitamin K. Identification of gamma-carboxyglutamic acid as a component of prothrombin. J. Biol. Chem. 249: 6347–6350 [PubMed] [Google Scholar]
- 3.Booth S. L. 2009. Roles for vitamin K beyond coagulation. Annu. Rev. Nutr. 29: 89–110 [DOI] [PubMed] [Google Scholar]
- 4.Gundberg C. M., Lian J. B., Booth S. L. 2012. Vitamin K-dependent carboxylation of osteocalcin: friend or foe? Adv. Nutr. 3: 149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancela M. L., Conceição N., Laizé V. 2012. Gla-rich protein, a new player in tissue calcification. Adv. Nutr. 3: 174–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schurgers L. J., Uitto J., Reutelingsperger C. P. 2013. Vitamin K-dependent carboxylation of matrix Gla-protein: a crucial switch to control ectopic mineralization. Trends Mol. Med. 19: 217–226 [DOI] [PubMed] [Google Scholar]
- 7.Booth S. L., Centi A., Smith S. R., Gundberg C. 2013. The role of osteocalcin in human glucose metabolism: marker or mediator? Nat. Rev. Endocrinol. 9: 43–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferland G. 2012. Vitamin K and the nervous system: an overview of its actions. Adv. Nutr. 3: 204–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellido-Martín L., de Frutos P. G. 2008. Vitamin K-dependent actions of gas-6. Vitam. Horm. 78: 185–209 [DOI] [PubMed] [Google Scholar]
- 10.Laurance S., Lemarié C. A., Blostein M. D. 2012. Growth arrest-specific gene 6 (gas 6) and vascular hemostasis. Adv. Nutr. 3: 196–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shearer M. J., Newman P. 2008. Metabolism and cell biology of vitamin K. Thromb. Haemost. 100: 530–547 [PubMed] [Google Scholar]
- 12.Shearer M. J., Fu X., Booth S. L. 2012. Vitamin K nutrition, metabolism, and requirements: current concepts and future research. Adv. Nutr. 3: 182–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rost S., Fregin A., Ivaskevicius V., Conzelmann E., Hörtnagel K., Pelz H. J., Lappegard K., Seifried E., Scharrer I., Tuddenham E. G., et al. 2004. Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2. Nature. 427: 537–541 [DOI] [PubMed] [Google Scholar]
- 14.Li T., Chang C. Y., Jin D. Y., Lin P. J., Khvorova A., Stafford D. W. 2004. Identification of the gene for vitamin K epoxide reductase. Nature. 427: 541–544 [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa K., Hirota Y., Sawada N., Yuge N., Watanabe M., Uchino Y., Okuda N., Shimomura Y., Suhara Y., Okano T. 2010. Identification of UBIAD1 as a novel human menaquinone-4 biosynthetic enzyme. Nature. 468: 117–121 [DOI] [PubMed] [Google Scholar]
- 16.Langemann A., Isler O. 1965. Chemistry of isoprenoid quinones. In Biochemistry of Quinones. R. A. Morton, editor. Academic Press, London. 89–147 [Google Scholar]
- 17.Bolton-Smith C., Price R. J. G., Fenton S. T., Harrington D. J., Shearer M. J. 2000. Compilation of a provisional UK database for the phylloquinone (vitamin K1) content of foods. Br. J. Nutr. 83: 389–399 [PubMed] [Google Scholar]
- 18.Thane C. W., Paul A. A., Bates C. J., Bolton-Smith C., Prentice A., Shearer M. J. 2002. Intake and sources of phylloquinone (vitamin K1): variation with socio-demographic and lifestyle factors in a national sample of British elderly people. Br. J. Nutr. 87: 605–613 [DOI] [PubMed] [Google Scholar]
- 19.Holmes M. V., Hunt B. J., Shearer M. J. 2012. The role of dietary vitamin K in the management of oral vitamin K antagonists. Blood Rev. 26: 1–14 [DOI] [PubMed] [Google Scholar]
- 20.Nowicka B., Kruk J. 2010. Occurrence, biosynthesis and function of isoprenoid quinones. Biochim. Biophys. Acta. 1797: 1587–1605 [DOI] [PubMed] [Google Scholar]
- 21.Collins M. D., Jones D. 1981. Distribution of isoprenoid quinone structural types in bacteria and their taxonomic implications. Microbiol. Rev. 45: 316–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandez F., Collins M. D. 1987. Vitamin K composition of anaerobic gut bacteria. FEMS Microbiol. Lett. 41: 175–180 [Google Scholar]
- 23.Conly J. M., Stein K. 1992. Quantitative and qualitative measurements of K vitamins in human intestinal contents. Am. J. Gastroenterol. 87: 311–316 [PubMed] [Google Scholar]
- 24.Schurgers L. J., Geleijnse J. M., Grobbee D. E., Pols H. A. P., Hofman A., Witteman J. C. M., Vermeer C. 1999. Nutritional intake of vitamins K1 (phylloquinone) and K2 (menaquinone) in the Netherlands. J. Nutr. Environ. Med. 9: 115–122 [Google Scholar]
- 25.Nimptsch K., Rohrmann S., Linseisen J. 2008. Dietary intake of vitamin K and risk of prostate cancer in the Heidelberg cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Heidelberg). Am. J. Clin. Nutr. 87: 985–992 [DOI] [PubMed] [Google Scholar]
- 26.Schurgers L. J., Vermeer C. 2000. Determination of phylloquinone in food. Effect of food matrix on circulating vitamin K concentrations. Haemostasis. 30: 298–307 [DOI] [PubMed] [Google Scholar]
- 27.Hojo K., Watanabe R., Mori T., Taketomo N. 2007. Quantitative measurement of tetrahydromenaquinone-9 in cheese fermented by propionibacteria. J. Dairy Sci. 90: 4078–4083 [DOI] [PubMed] [Google Scholar]
- 28.Burt V. T., Bee E., Pennock J. F. 1977. The formation of menaquinone-4 (vitamin K) and its oxide in some marine invertebrates. Biochem. J. 162: 297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walther B., Karl J. P., Booth S. L., Boyaval P. 2013. Menaquinones, bacteria, and the food supply: the relevance of dairy and fermented food products to vitamin K requirements. Adv. Nutr. 4: 463–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones K. S., Bluck L. J., Wang L. Y., Stephen A. M., Prynne C. J., Coward W. A. 2009. The effect of different meals on the absorption of different meals on the absorption of stable isotope-labelled phylloquinone. Br. J. Nutr. 102: 1195–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suttie J. W. 2009. Vitamin K in Health and Disease. CRC Press, Boca Raton, FL. [Google Scholar]
- 32.Schurgers L. J., Shearer M. J., Hamulyák K., Stöcklin E., Vermeer C. 2004. Effect of vitamin K intake on the stability of oral anticoagulant treatment: dose-response relationships in healthy subjects. Blood. 104: 2682–2689 [DOI] [PubMed] [Google Scholar]
- 33.Schurgers L. J., Teunissen K. J., Hamulyák K., Knapen M. H., Vik H., Vermeer C. 2007. Vitamin K-containing dietary supplements: comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood. 109: 3279–3283 [DOI] [PubMed] [Google Scholar]
- 34.Theuwissen E., Teunissen K. J., Spronk H. M., Hamulyák K., Ten Cate H., Shearer M. J., Vermeer C., Schurgers L. J. 2013. Effect of low-dose supplements of menaquinone-7 (vitamin K2) on the stability of oral anticoagulant treatment: dose−response relationship in healthy volunteers. J. Thromb. Haemost. 11: 1085–1092 [DOI] [PubMed] [Google Scholar]
- 35.O'Byrne S. M., Blaner W. S. 2013. Retinol and retinyl esters: biochemistry and physiology. J. Lipid Res. 54: 1731–1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Traber M. G. 2013. Mechanisms for the prevention of vitamin E excess. J. Lipid Res. 54: 2295–2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Card D. J., Shearer M. J., Schurgers L. J., Harrington D. J. 2009. The external quality assurance of phylloquinone (vitamin K1) analysis in human serum. Biomed. Chromatogr. 23: 1276–1282 [DOI] [PubMed] [Google Scholar]
- 38.Tsugawa N., Shiraki M., Suhara Y., Kamao M., Tanaka K., Okano T. 2006. Vitamin K status of healthy Japanese women: age-related vitamin K requirement for gamma-carboxylation of osteocalcin. Am. J. Clin. Nutr. 83: 380–386 [DOI] [PubMed] [Google Scholar]
- 39.Kohlmeier M., Salomon A., Saupe J., Shearer M. J. 1996. Transport of vitamin K to bone in humans. J. Nutr. 126: 1192S–1196S [DOI] [PubMed] [Google Scholar]
- 40.Lamon-Fava S., Sadowski J. A., Davidson K. W., O'Brien M. E., McNamara J. R., Schaefer E. J. 1998. Plasma lipoproteins as carriers of phylloquinone (vitamin K1) in humans. Am. J. Clin. Nutr. 67: 1226–1231 [DOI] [PubMed] [Google Scholar]
- 41.Schurgers L. J., Vermeer C. 2002. Differential lipoprotein transport pathways of K-vitamins in healthy adults. Biochim. Biophys. Acta. 1570: 27–32 [DOI] [PubMed] [Google Scholar]
- 42.Erkkilä A. T., Lichtenstein A. H., Dolnikowski G. G., Grusak M. A., Jalbert S. M., Aquino K. A., Peterson J. W., Booth S. L. 2004. Plasma transport of vitamin K in men using deuterium-labeled collard greens. Metabolism. 53: 215–221 [DOI] [PubMed] [Google Scholar]
- 43.Shearer M. J., McBurney A., Barkhan P. 1974. Studies on the absorption and metabolism of phylloquinone (vitamin K1) in man. Vitam. Horm. 32: 513–542 [DOI] [PubMed] [Google Scholar]
- 44.Jones K. S., Bluck L. J., Wang L. Y., Coward W. A. 2008. A stable isotope method for the simultaneous measurement of vitamin K1 (phylloquinone) kinetics and absorption. Eur. J. Clin. Nutr. 62: 1273–1281 [DOI] [PubMed] [Google Scholar]
- 45.Newman P., Bonello F., Wierzbicki A. S., Lumb P., Savidge G. F., Shearer M. J. 2002. The uptake of lipoprotein-borne phylloquinone (vitamin K1) by osteoblasts and osteoblast-like cells: role of heparan sulfate proteoglycans and apolipoprotein E. J. Bone Miner. Res. 17: 426–433 [DOI] [PubMed] [Google Scholar]
- 46.Niemeier A., Kassem M., Toedter K., Wendt D., Ruether W., Beisiegel U., Heeren J. 2005. Expression of LRP1 by human osteoblasts: a mechanism for the delivery of lipoproteins and vitamin K1 to bone. J. Bone Miner. Res. 20: 283–293 [DOI] [PubMed] [Google Scholar]
- 47.Niemeier A., Niedzielska D., Secer R., Schilling A., Merkel M., Enrich C., Rensen P. C., Heeren J. 2008. Uptake of postprandial lipoproteins into bone in vivo: impact on osteoblast function. Bone. 43: 230–237 [DOI] [PubMed] [Google Scholar]
- 48.Cooper A. D. 1997. Hepatic uptake of chylomicron remnants. J. Lipid Res. 38: 2173–2192 [PubMed] [Google Scholar]
- 49.Shearer M. J., McCarthy P. T., Crampton O. E., Mattock M. B. 1988. The assessment of human vitamin K status from tissue measurements. In Current Advances in Vitamin K Research. J. W. Suttie, editor. Elsevier, New York. 437–452 [Google Scholar]
- 50.Thijssen H. H., Drittij-Reijnders M. J. 1996. Vitamin K status in human tissues: tissue-specific accumulation of phylloquinone and menaquinone-4. Br. J. Nutr. 75: 121–127 [DOI] [PubMed] [Google Scholar]
- 51.Thijssen H. H., Drittij-Reijnders M. J. 1994. Vitamin K distribution in rat tissues: dietary phylloquinone is a source of tissue menaquinone-4. Br. J. Nutr. 72: 415–425 [DOI] [PubMed] [Google Scholar]
- 52.Thijssen H. H., Drittij-Reijnders M. J., Fisher M. A. 1996. Phylloquinone and menaquinone-4 distribution in rats: synthesis rather than uptake determines menaquinone-4 organ concentrations. J. Nutr. 126: 537–543 [DOI] [PubMed] [Google Scholar]
- 53.Usui Y., Tanimura H., Nishimura N., Kobayashi N., Okanoue T., Ozawa K. 1990. Vitamin K concentrations in the plasma and liver of surgical patients. Am. J. Clin. Nutr. 51: 846–852 [DOI] [PubMed] [Google Scholar]
- 54.Shearer M. J., Bach A., Kohlmeier M. 1996. Chemistry, nutritional sources, tissue distribution and metabolism of vitamin K with special reference to bone health. J. Nutr. 126: 1181S–1186S [DOI] [PubMed] [Google Scholar]
- 55.McBurney A., Shearer M. J., Barkhan P. 1980. Preparative isolation and characterization of the urinary aglycones of vitamin K (phylloquinone) in man. Biochem. Med. 24: 250–267 [DOI] [PubMed] [Google Scholar]
- 56.Harrington D. J., Soper R., Edwards C., Savidge G. F., Hodges S. J., Shearer M. J. 2005. Determination of the urinary aglycone metabolites of vitamin K by HPLC with redox-mode electrochemical detection. J. Lipid Res. 46: 1053–1060 [DOI] [PubMed] [Google Scholar]
- 57.Thierry M. J., Suttie J. W. 1971. Effect of warfarin and the chloro analog of vitamin K1 on phylloquinone metabolism. Arch. Biochem. Biophys. 147: 430–435 [DOI] [PubMed] [Google Scholar]
- 58.McDonald M. G., Rieder M. J., Nakano M., Hsia C. K., Rettie A. E. 2009. CYP4F2 is a vitamin K1 oxidase: an explanation for altered warfarin dose in carriers of the V433M variant. Mol. Pharmacol. 75: 1337–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caldwell M. D., Awad T., Johnson J. A., Gage B. F., Falkowski M., Gardina P., Hubbard J., Turpaz Y., Langaee T. Y., Eby C., et al. 2008. CYP4F2 genetic variant alters required warfarin dose. Blood. 111: 4106–4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spohn G., Kleinridders A., Wunderlich F. T., Watzka M., Zaucke F., Blumbach K., Geisen C., Seifried E., Müller C., Paulsson M., et al. 2009. VKORC1 deficiency in mice causes early postnatal lethality due to severe bleeding. Thromb. Haemost. 101: 1044–1050 [PubMed] [Google Scholar]
- 61.Matschiner J. T., Bell R. G., Amelotti J. M., Knauer T. E. 1970. Isolation and characterization of a new metabolite of phylloquinone in the rat. Biochim. Biophys. Acta. 201: 309–315 [DOI] [PubMed] [Google Scholar]
- 62.Bell R. G., Matschiner J. T. 1972. Warfarin and the inhibition of vitamin K activity by an oxide metabolite. Nature. 237: 32–33 [DOI] [PubMed] [Google Scholar]
- 63.Fregin A., Rost S., Wolz W., Krebsova A., Muller C. R., Oldenburg J. 2002. Homozygosity mapping of a second gene locus for hereditary combined deficiency of vitamin K-dependent clotting factors to the centromeric region of chromosome 16. Blood. 100: 3229–3232 [DOI] [PubMed] [Google Scholar]
- 64.Kohn M. H., Pelz H. J. 2000. A gene-anchored map position of the rat warfarin-resistance locus, Rw, and its orthologs in mice and humans. Blood. 96: 1996–1998 [PubMed] [Google Scholar]
- 65.Chu P. H., Huang T. Y., Williams J., Stafford D. W. 2006. Purified vitamin K epoxide reductase alone is sufficient for conversion of vitamin K epoxide to vitamin K and vitamin K to vitamin KH2. Proc. Natl. Acad. Sci. USA. 103: 19308–19313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oldenburg J., Marinova M., Müller-Reible C., Watzka M. 2008. The vitamin K cycle. Vitam. Horm. 78: 35–62 [DOI] [PubMed] [Google Scholar]
- 67.Tie J-K., Stafford D. W. 2008. Structure and function of vitamin K epoxide reductase. Vitam. Horm. 78: 103–130 [DOI] [PubMed] [Google Scholar]
- 68.Van Horn W. D. 2013. Structural and functional insights into human vitamin K epoxide reductase and vitamin K epoxide reducatase-like1. Crit. Rev. Biochem. Mol. Biol. 48: 357–372 [DOI] [PubMed] [Google Scholar]
- 69.Link K. P. 1959. The discovery of dicumarol and its sequels. Circulation. 19: 97–107 [DOI] [PubMed] [Google Scholar]
- 70.Shearer M. J., Bechtold H., Andrassy K., Koderisch J., McCarthy P. T., Trenk D., Jähnchen E., Ritz E. 1988. Mechanism of cephalosporin-induced hypoprothrombinemia: relation to cephalosporin side chain, vitamin K metabolism, and vitamin K status. J. Clin. Pharmacol. 28: 88–95 [DOI] [PubMed] [Google Scholar]
- 71.Gebauer M. 2007. Synthesis and structure activity-relationships of novel warfarin derivatives. Bioorg. Med. Chem. 15: 2414–2420 [DOI] [PubMed] [Google Scholar]
- 72.Price P. A., Kaneda Y. 1987. Vitamin K counteracts the effect of warfarin in liver but not in bone. Thromb. Res. 46: 121–131 [DOI] [PubMed] [Google Scholar]
- 73.Wallin R., Martin L. F. 1987. Warfarin poisoning and vitamin K antagonism in rat and human liver. Design of a system in vitro that mimics the situation in vivo. Biochem. J. 241: 389–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wallin R., Patrick S. D., Martin L. F. 1989. Vitamin K1 reduction in human liver. Location of the coumarin-drug-insensitive enzyme. Biochem. J. 260: 879–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wallin R., Gebhardt O., Prydz H. 1978. NAD(P)H dehydrogenase and its role in the vitamin K (2-methyl-3-phytyl-1,4-naphthaquinone)-dependent carboxylation reaction. Biochem. J. 169: 95–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wallin R., Hutson S. 1982. Vitamin K-dependent carboxylation. Evidence that at least two microsomal dehydrogenases reduce vitamin K1 to support carboxylation. J. Biol. Chem. 257: 1583–1586 [PubMed] [Google Scholar]
- 77.Wallin R., Martin L. F. 1985. Vitamin K-dependent carboxylation and vitamin K metabolism in the liver. Effects of warfarin. J. Clin. Invest. 76: 1879–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wallin R. 1986. Vitamin K antagonism of coumarin anticoagulation. A dehydrogenase pathway in rat liver is responsible for the antagonistic effect. Biochem. J. 236: 685–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Finkelstein Y., Chitayat D., Schechter T., Keating S., Toi A., Koren G. 2005. Motherrisk rounds. Warfarin embryopathy following low-dose maternal exposure. J. Obstet. Gynaecol. Can. 27: 702–706 [DOI] [PubMed] [Google Scholar]
- 80.Pauli R. M., Lian J. B., Mosher D. F., Suttie J. W. 1987. Association of congenital deficiency of multiple vitamin K-dependent coagulation factors and the phenotype of the warfarin embryopathy: clues to the mechanism of teratogenicity of coumarin derivatives. Am. J. Hum. Genet. 41: 566–583 [PMC free article] [PubMed] [Google Scholar]
- 81.Menger H., Lin A. E., Toriello H. V., Bernert G., Spranger J. W. 1997. Vitamin K deficiency embryopathy: a phenocopy of the warfarin embryopathy due to a disorder of embryonic vitamin K metabolism. Am. J. Med. Genet. 72: 129–134 [PubMed] [Google Scholar]
- 82.Munroe P. B., Olgunturk R. O., Fryns J. P., van Maldergem L., Ziereisen F., Yuksel B., Gardiner R. M., Chung E. 1999. Mutations in the gene encoding the human matrix Gla protein cause Keutal syndrome. Nat. Genet. 21: 142–144 [DOI] [PubMed] [Google Scholar]
- 83.Tie J. K., Nicchitta C., von Heijne G., Stafford D. W. 2005. Membrane topology mapping of vitamin K epoxide reductase by in vitro translation/cotranslocation. J. Biol. Chem. 280: 16410–16416 [DOI] [PubMed] [Google Scholar]
- 84.Jin D. Y., Tie J. K., Stafford D. W. 2007. The conversion of vitamin K epoxide to vitamin K quinone and vitamin K quinone to vitamin K hydroquinone uses the same active site cysteines. Biochemistry. 46: 7279–7283 [DOI] [PubMed] [Google Scholar]
- 85.Tie J. K., Jin D. Y., Straight D. L., Stafford D. W. 2011. Functional study of the vitamin K cycle in mammalian cells. Blood. 117: 2967–2974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tie J. K., Jin D. Y., Tie K., Stafford D. W. 2013. Evaluation of warfarin resistance using transcription activator-like effector nucleases-mediated vitamin K epoxide reductase knockout HEK293 cells. J. Thromb. Haemost. 11: 1556–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.O'Reilly R. A., Aggeler P. M., Hoag M. S., Leong L. S., Kropatkin M. L. 1964. Hereditary transmission of exceptional resistance to coumarin anticoagulant drugs. The first reported kindred. N. Engl. J. Med. 271: 809–815 [DOI] [PubMed] [Google Scholar]
- 88.Boyle C. M. 1960. Case of apparent resistance of Rattus norvegicus Berkenhout to anticoagulant poisons. Nature. 188: 517 [Google Scholar]
- 89.Lund M. 1964. Resistance to warfarin in the common rat. Nature. 203: 778. [DOI] [PubMed] [Google Scholar]
- 90.Rowe F. P., Plant C. J., Bradfield A. 1981. Trials of the anticoagulant rodenticides bromadiolone and difenacoum against the house mouse (Mus musculus L.). J. Hyg. (Lond.). 87: 171–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Greaves J. H., Shepherd D. S., Quy R. 1982. Field trials of second-generation anticoagulants against difenacoum-resistant Norway rat populations. J. Hyg. (Lond.). 89: 295–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hodroge A., Matagrin B., Moreau C., Foure I., Hammed A., Benoit E., Lattard V. 2012. VKORC1 mutations detected in patients resistant to vitamin K antagonists are not all associated with a resistant VKOR activity. J. Thromb. Haemost. 10: 2535–2543 [DOI] [PubMed] [Google Scholar]
- 93.Pelz H. J., Rost S., Hünerberg M., Fregin A., Heiberg A. C., Baert K., MacNicoll A. D., Prescott C. V., Walker A. S., Oldenburg J., et al. 2005. The genetic basis of resistance to anticoagulants in rodents. Genetics. 170: 1839–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rost S., Pelz H. J., Menzel S., MacNicoll A. D., Leon V., Song K. J., Jakel T., Oldenburg J., Muller C. R. 2009. Novel mutations in the VKORC1 gene of wild rats and mice–a response to 50 years of selection pressure by warfarin? BMC Genet. 10: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hodroge A., Longin-Sauvageon C., Fourel I., Benoit E., Lattard V. 2011. Biochemical characterization of spontaneous mutants of rat VKORC1 involved in the resistance to antivitamin K anticoagulants. Arch. Biochem. Biophys. 515: 14–20 [DOI] [PubMed] [Google Scholar]
- 96.Ma Q., Cui K., Xiao F., Lu A. Y., Yang C. S. 1992. Identification of a glycine-rich sequence as an NAD(P)H-binding site and tyrosine 128 as a dicumarol-binding site in rat liver NAD(P)H:quinone oxidoreductase by site-directed mutagenesis. J. Biol. Chem. 267: 22298–22304 [PubMed] [Google Scholar]
- 97.Oldenburg J., Bevans C. G., Muller C. R., Watzka M. 2006. Vitamin K epoxide reductase complex subunit 1 (VKORC1): the key protein of the vitamin K cycle. Antioxid. Redox Signal. 8: 347–353 [DOI] [PubMed] [Google Scholar]
- 98.Fregin A., Czogalla K. J., Gansler J., Rost S., Taverna M., Watzka M., Bevans C. G., Muller C. R., Oldenburg J. 2013. A new cell culture-based assay quantifies vitamin K 2,3-epoxide reductase complex subunit 1 function and reveals warfarin resistance phenotypes not shown by the dithiothreitol-driven VKOR assay. J. Thromb. Haemost. 11: 872–880 [DOI] [PubMed] [Google Scholar]
- 99.Harrington D. J., Siddiq S., Allford S. L., Shearer M. J., Mumford A. D. 2011. More on: endoplasmic reticulum loop VKORC1 substitutions cause warfarin resistance but do not diminish gamma-carboxylation of the vitamin K-dependent coagulation factors. J. Thromb. Haemost. 9: 1093–1095 [DOI] [PubMed] [Google Scholar]
- 100.Ernster L. 1967. DT-diaphorase. Methods Enzymol. 10: 309–317 [DOI] [PubMed] [Google Scholar]
- 101.Lind C., Cadenas E., Hochstein P., Ernster L. 1990. DT-diaphorase: purification, properties, and function. Methods Enzymol. 186: 287–301 [DOI] [PubMed] [Google Scholar]
- 102.Fasco M. J., Principe L. M. 1982. Vitamin K1 hydroquinone formation catalyzed by DT-diaphorase. Biochem. Biophys. Res. Commun. 104: 187–192 [DOI] [PubMed] [Google Scholar]
- 103.Gong X., Gutala R., Jaiswal A. K. 2008. Quinone oxidoreductases and vitamin K metabolism. Vitam. Horm. 78: 85–101 [DOI] [PubMed] [Google Scholar]
- 104.Hollander P. M., Ernster L. 1975. Studies on the reaction mechanism of DT Diaphorase. Action of dead-end inhibitors and effects of phospholipids. Arch. Biochem. Biophys. 169: 560–567 [DOI] [PubMed] [Google Scholar]
- 105.Preusch P. C., Smalley D. M. 1990. Vitamin K1 2,3-epoxide and quinone reduction: mechanism and inhibition. Free Radic. Res. Commun. 8: 401–415 [DOI] [PubMed] [Google Scholar]
- 106.Park B. K., Leck J. B. 1982. A comparison of vitamin K antagonism by warfarin, difenacoum and brodifacoum in the rabbit. Biochem. Pharmacol. 31: 3635–3639 [DOI] [PubMed] [Google Scholar]
- 107.Martius C., Ganser R., Viviani A. 1975. The enzymatic reduction of K-vitamins incorporated in the membrane of liposomes. FEBS Lett. 59: 13–14 [DOI] [PubMed] [Google Scholar]
- 108.Ingram B. O., Turbyfill J. L., Bledsoe P. J., Jaiswal A. K., Stafford D. W. 2013. Assessment of the contribution of NAD(P)H-dependent quinone oxidoreductase 1 (NQO1) to the reduction of vitamin K in wild-type and NQO1-deficient mice. Biochem. J. 456: 47–54 [DOI] [PubMed] [Google Scholar]
- 109.Robertson H. M. 2004. Genes encoding vitamin-K epoxide reductase are present in Drosophila and trypanosomatid protists. Genetics. 168: 1077–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Westhofen P., Watzka M., Marinova M., Hass M., Kirfel G., Muller J., Bevans C. G., Muller C. R., Oldenburg J. 2011. Human vitamin K 2,3-epoxide reductase complex subunit 1-like 1 (VKORC1L1) mediates vitamin K-dependent intracellular antioxidant function. J. Biol. Chem. 286: 15085–15094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hammed A., Matagrin B., Spohn G., Prouillac C., Benoit E., Lattard V. 2013. VKORC1L1, an enzyme rescuing the vitamin K 2,3-epoxide reductase activity in some extrahepatic tissues during anticoagulation therapy. J. Biol. Chem. 288: 28733–28742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.WHO IARC-Monographs on the evaluation of carcinogenic risks to humans. Vol. 76: Some antiviral and antineoplastic drugs, and other pharmaceutical agents. Lyon, France: IARC Press. 2000. 417–486. [PMC free article] [PubMed] [Google Scholar]
- 113.Vest M. 1966. Vitamin K in medical practice: pediatrics. Vitam. Horm. 24: 649–663 [DOI] [PubMed] [Google Scholar]
- 114.Thor H., Smith M. T., Hartzell P., Bellomo G., Jewell S. A., Orrenius S. 1982. The metabolism of menadione (2-methyl-1,4-naphthoquinone) by isolated hepatocytes. A study of the implications of oxidative stress in intact cells. J. Biol. Chem. 257: 12419–12425 [PubMed] [Google Scholar]
- 115.Brown P. C., Dulik D. M., Jones T. W. 1991. The toxicity of menadione (2-methyl-1,4-naphthoquinone) and two thioether conjugates studied with isolated renal epithelial cells. Arch. Biochem. Biophys. 285: 187–196 [DOI] [PubMed] [Google Scholar]
- 116.Di Monte D., Ross D., Bellomo G., Eklöw L., Orrenius S. 1984. Alterations in intracellular thiol homeostasis during the metabolism of menadione by isolated rat hepatocytes. Arch. Biochem. Biophys. 235: 334–342 [DOI] [PubMed] [Google Scholar]
- 117.Canady W. J., Roe J. H. 1956. Studies on the reaction of menadione with blood and denatured proteins. J. Biol. Chem. 220: 571–582 [PubMed] [Google Scholar]
- 118.Lee J. Y., Bae O. N., Chung S. M., Lee M. Y., Chung J. H. 2001. Menadione induces endothelial dysfunction mediated by oxidative stress and arylation. Chem. Biol. Interact. 137: 169–183 [DOI] [PubMed] [Google Scholar]
- 119.Radjendirane V., Joseph P., Lee Y. H., Kimura S., Klein-Szanto A. J., Gonzalez F. J., Jaiswal A. K. 1998. Disruption of the DT diaphorase (NQO1) gene in mice leads to increased menadione toxicity. J. Biol. Chem. 273: 7382–7389 [DOI] [PubMed] [Google Scholar]
- 120.Thijssen H. H., Vervoort L. M., Schurgers L. J., Shearer M. J. 2006. Menadione is a metabolite of oral vitamin K. Br. J. Nutr. 95: 260–266 [DOI] [PubMed] [Google Scholar]
- 121.Al Rajabi A., Booth S. L., Peterson J. W., Choi S. W., Suttie J. W., Shea M. K., Miao B., Grusak M. A., Fu X. 2012. Deuterium-labeled phylloquinone has tissue-specific conversion to menaqunone-4 among Fischer 344 male rats. J. Nutr. 142: 841–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hirota Y., Tsugawa N., Nakagawa K., Suhara Y., Tanaka K., Uchino Y., Takeuchi A., Sawada N., Kamao M., Wada A., et al. 2013. Menadione (vitamin K3) is a catabolic product of oral phylloquinone (vitamin K1) in the intestine and a circulating precursor of tissue menaquinone-4 (vitamin K2) in rats. J. Biol. Chem. 288: 33071–33080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mukai K., Morimoto H., Kikuchi S., Nagaoka S. 1993. Kinetic study of free-radical-scavenging action of biological hydroquinones (reduced forms of ubiquinone, vitamin K and tocopherol quinone) in solution. Biochim. Biophys. Acta. 1157: 313–317 [DOI] [PubMed] [Google Scholar]
- 124.Vervoort L. M. T., Ronden J. E., Thijssen H. H. W. 1997. The potent antioxidant activity of the vitamin K cycle in microsomal lipid peroxidation. Biochem. Pharmacol. 54: 871–876 [DOI] [PubMed] [Google Scholar]
- 125.Li J., Lin J. C., Wang H., Peterson J. W., Furie B. C., Furie B., Booth S. L., Volpe J. J., Rosenberg P. A. 2003. Novel role of vitamin K in preventing oxidative injury to developing oligodendrocytes and neurons. J. Neurosci. 23: 5816–5826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li J., Wang H., Rosenberg P. A. 2009. Vitamin K prevents oxidative cell death by inhibiting activation of 12-lipoxygenase in developing oligodendrocytes. J. Neurosci. Res. 87: 1997–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Okano T., Shimomura Y., Yamane M., Suhara Y., Kamao M., Sugiura M., Nakagawa K. 2008. Conversion of phylloquinone (vitamin K1) into menaquinone-4 (vitamin K2) in mice: two possible routes for menaquinone-4 accumulation in cerebra of mice. J. Biol. Chem. 283: 11270–11279 [DOI] [PubMed] [Google Scholar]
- 128.Davidson R. T., Foley A. L., Engelke J. A., Suttie J. W. 1998. Conversion of dietary phylloquinone to tissue menaquinone-4 in rats is not dependent on gut bacteria. J. Nutr. 128: 220–223 [DOI] [PubMed] [Google Scholar]
- 129.Ronden J. E., Drittij-Reijnders M. J., Vermeer C., Thijssen H. H. 1998. Intestinal flora is not an intermediate in the phylloquinone-menaquinone-4 conversion in the rat. Biochim. Biophys. Acta. 1379: 69–75 [DOI] [PubMed] [Google Scholar]
- 130.Vos M., Esposito G., Edirisinghe J. N., Vilain S., Haddad D. M., Slabbaert J. R., Van Meensel S., Schaap O., De Strooper B., Meganathan R., et al. 2012. Vitamin K2 is a mitochondrial electron carrier that rescues pink1 deficiency. Science. 336: 1306–1310 [DOI] [PubMed] [Google Scholar]
- 131.Hegarty J. M., Yang H., Chi N. C. 2013. UBIAD1-mediated vitamin K2 synthesis is required for vascular endothelial cell survival and development. Development. 140: 1713–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nickerson M. L., Bosley A. D., Weiss J. S., Kostiha B. N., Hirota Y., Brandt W., Esposito D., Kinoshita S., Wessjohan L., Morham S. G., et al. 2013. The UBIAD1 prenyltransferase links menaquinone-4 synthesis to cholesterol metabolic enzymes. Hum. Mutat. 34: 317–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McGarvey T. W., Nguyen T., Tomaszewski J. E., Monson F. C., Malkowicz S. B. 2001. Isolation and characterization of the TERE1 gene, a gene down-regulated in transitional cell carcinoma of the bladder. Oncogene. 20: 1042–1051 [DOI] [PubMed] [Google Scholar]
- 134.McGarvey T. W., Nguyen T., Puthiyaveettil R., Tomaszewski J. E., Malkowicz S. B. 2003. TERE1, a novel gene affecting growth regulation in prostate carcinoma. Prostate. 54: 144–155 [DOI] [PubMed] [Google Scholar]
- 135.Fredericks W. J., Sepulveda J., Lal P., Tomaszewski J. E., Lin M-F., McGarvey T., Rauscher F. J., III, Malkowicz S. B. 2013. The tumor suppressor TERE1 (UBIAD1) prenyltransferase regulates the elevated cholesterol phenotype in castration resistant prostate cancer by controlling a program of ligand dependent SXR target genes. Oncotarget. 4: 1075–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yaguchi M., Miyazawa K., Katagiri T., Nishimaki J., Kizaki M., Tohyama K., Toyama K. 1997. Vitamin K2 and its derivatives induce apoptosis in leukemia cells and enhance the effect of all-trans retinoic acid. Leukemia. 11: 779–787 [DOI] [PubMed] [Google Scholar]
- 137.Karasawa S., Azuma M., Kasama T., Sakamoto S., Kabe Y., Imai T., Yamaguchi Y., Miyazawa K., Handa H. 2013. Vitamin K2 covalently binds to Bak and induces Bak-mediated apoptosis. Mol. Pharmacol. 83: 613–620 [DOI] [PubMed] [Google Scholar]
- 138.Ozaki I., Zhang H., Mizuta T., Ide Y., Eguchi Y., Yasutake T., Sakamaki T., Pestell R. G., Yamamoto K. 2007. Menatetrenone, a vitamin K2 analogue, inhibits hepatocellular carcinoma cell growth by suppressing cyclin D1 expression through inhibition of nuclear factor kappaB activation. Clin. Cancer Res. 13: 2236–2245 [DOI] [PubMed] [Google Scholar]
- 139.Yamaguchi M., Weitzmann M. N. 2011. Vitamin K2 stimulates osteoblastogenesis and suppresses osteoclastogenesis by suppressing NF-κB activation. Int. J. Mol. Med. 27: 3–14 [DOI] [PubMed] [Google Scholar]
- 140.Wadelius M., Chen L. Y., Lindh J. D., Eriksson N., Ghori M. J., Bumpstead S., Holm L., McGinnis R., Rane A., Deloukas P. 2009. The largest prospective warfarin-treated cohort supports genetic forecasting. Blood. 113: 784–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jaques L. B., Millar G. J., Spinks J. W. T. 1954. The metabolism of the K-vitamins. Schweiz. Med. Wochenschr. 84: 792–796 [PubMed] [Google Scholar]
- 142.Mezick J. A., Tompkins R. K., Cornwell D. G. 1968. Absorption and intestinal lymphatic transport of 14C-menadione. Life Sci. 7: 153–158 [DOI] [PubMed] [Google Scholar]
- 143.Bhagavan H. N., Chopra R. K. 2007. Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations. Mitochondrion. 7(Suppl): S78–S88 [DOI] [PubMed] [Google Scholar]