Abstract
Total parenteral nutrition (TPN) is associated with the development of parenteral nutrition-associated liver disease (PNALD) in infants. Fish oil-based lipid emulsions can reverse PNALD, yet it is unknown if they can prevent PNALD. We studied preterm pigs administered TPN for 14 days with either 100% soybean oil (IL), 100% fish oil (OV), or a mixture of soybean oil, medium chain triglycerides (MCTs), olive oil, and fish oil (SL); a group was fed formula enterally (ENT). In TPN-fed pigs, serum direct bilirubin, gamma glutamyl transferase (GGT), and plasma bile acids increased after the 14 day treatment but were highest in IL pigs. All TPN pigs had suppressed hepatic expression of farnesoid X receptor (FXR), cholesterol 7-hydroxylase (CYP7A1), and plasma 7α-hydroxy-4-cholesten-3-one (C4) concentrations, yet hepatic CYP7A1 protein abundance was increased only in the IL versus ENT group. Organic solute transporter alpha (OSTα) gene expression was the highest in the IL group and paralleled plasma bile acid levels. In cultured hepatocytes, bile acid-induced bile salt export pump (BSEP) expression was inhibited by phytosterol treatment. We show that TPN-fed pigs given soybean oil developed cholestasis and steatosis that was prevented with both OV and SL emulsions. Due to the presence of phytosterols in the SL emulsion, the differences in cholestasis and liver injury among lipid emulsion groups in vivo were weakly correlated with plasma and hepatic phytosterol content.
Keywords: soybean oil, fish oil, cholestasis, FGF19, parenteral nutrition-associated liver disease
Cholestatic liver disease is one of the most common metabolic problems associated with total parenteral nutrition (TPN) in preterm infants, and it is strongly related to the duration of TPN (1). The incidence of parenteral nutrition-associated liver disease (PNALD) in infants who receive parenteral nutrition for at least 2 months can be as high as 50% (2). Risk factors associated with PNALD are multifactorial and include immature hepatic function, lack of enteral feeding, sepsis or infection, toxin exposure, or nutrient deficiencies. Currently, there is much interest in the role of lipid emulsions in the development of PNALD.
Soybean oil-based emulsions, rich in PUFAs, are the most common form of lipid emulsion administered to preterm infants in the world (3). However, an excess intake of n-6 PUFA in parenteral nutrition is associated with an unbalanced fatty acid pattern and increased harmful lipid peroxidation (3). Other studies have shown that phytosterols present in soybean oil are associated with phytosterolemia and the severity of cholestasis (4). The cholestatic effect of phytosterols is thought to be mediated by their antagonism of the farnesoid X receptor (FXR), the dominant bile acid sensor involved in bile acid homeostasis, as shown in cultured hepatocytes (5). The cholestatic effect of lipid emulsions has also been shown to be closely associated with the lipid dose itself (6, 7).
New lipid emulsions have emerged that contain either only fish oil or a mixture of soybean oil, medium chain triglycerides (MCTs), olive oil, and fish oil. Several small trials with these emulsions have shown good tolerance; in infants and children with PNALD, fish oil emulsions in low doses reduced serum triglyceride and bilirubin concentrations and improved liver function, and in some of these children, fish oil administration even reversed PNALD (8–10). However, it is unknown if fish oil-containing lipid emulsions can prevent the development of PNALD.
We hypothesized that parenteral lipid emulsions containing pure fish oil or a mixture of soybean oil, MCTs, olive oil, and fish oil can prevent hepatic cholestasis compared with a purely soybean oil-based lipid emulsion when given to TPN-fed preterm pigs. We and others have established the piglet as an excellent animal model of the term and preterm infant to study the effects of parenteral and enteral nutrition (11–14). Our first aim was to test whether fish oil-containing emulsions compared with soybean oil emulsions can prevent the development of PNALD in preterm, TPN-fed pigs. Our second aim was to measure the presence of systemic and hepatic phytosterolemia in preterm TPN-fed pigs and establish the mechanistic effects of phytosterols on hepatocyte FXR function.
MATERIALS AND METHODS
Animals and surgery
The study protocol was approved by the Animal Care and Use Committee of Baylor College of Medicine and was conducted in accordance with the Guide for the Care and Use of Laboratory Animals (DRR/NIH, Bethesda, MD). Pregnant crossbred sows were obtained from the Texas Department of Criminal Justice (Huntsville, TX). Sows were housed in the Children's Nutrition Research Center and were given food and water ad libitum. At gestation day 108, piglets were delivered 7 days preterm by cesarean section and immediately placed in cages housed at 31°C to 32°C, as described previously (12). Based on body weight, pigs delivered from each sow were randomly assigned to one of the three TPN treatment groups or to enteral nutrition (ENT). After delivery, pigs were surgically implanted with catheters into the jugular vein and umbilical artery. Pigs in the enteral group also were implanted with an orogastric feeding tube, whereas TPN groups received a sham puncture. Maternal plasma (16 ml/kg intravenously during the first 24 h) was administered for passive immunological protection. During the 14 day study, pigs received antibiotics (enrofloxacin 5 mg/kg) intravenously on alternating days.
Nutritional support and study design
TPN consisted of an elemental solution containing a complete nutrient mixture of amino acids, glucose, electrolytes, vitamins, and trace minerals, and a parenteral lipid emulsion, which was infused separately. Pigs in the TPN groups randomly received one of the following lipid emulsions: 100% soybean oil (IL) (Intralipid), 100% fish oil (OV) (Omegaven), or a mixture of 30% soybean oil, 30% MCTs, 25% olive oil, and 15% fish oil (SL) (SMOFlipid); all three lipid emulsions were provided by Fresenius Kabi (Bad Homburg, Germany). ENT pigs were fed a milk-based formula (Litter Life; Merrick, Middletown, WI) at 240 ml/kg in eight feeds per day. Postsurgery, TPN was started at 5 ml/(kg·h) and gradually increased to 10 ml/(kg·h). ENT pigs also received TPN with IL following surgery but started to be fed enterally the day thereafter and were weaned from TPN by day 2. On day 7, all TPN and enterally fed pigs received full amounts of nutrition per kilogram body weight: fluid, 240 ml; energy, 195 kcal; carbohydrate, 25 g; protein, 14 g; and lipid, 5 g, as described (12).
Pigs were weighed every other day, and arterial blood samples were drawn during surgery (day 0) and on days 7 and 14. Immediately after the last blood sample was taken on day 14, the animals were anesthetized with isoflurane and euthanized with injection of Beuthanasia (pentobarbital sodium, phenytoin sodium). Organs were isolated and weighed. Liver tissue samples were frozen in liquid nitrogen and stored at −70°C until analysis. Liver samples also were fixed in 10% formalin for histopathology.
Sample preparation and analysis
Blood samples were collected in tubes containing Na2EDTA and centrifuged at 3,000 g at 4°C for 10 min. Plasma and red cells were stored at −70°C until analysis. Blood samples for serum chemistry were left at room temperature for 1 h and centrifuged at 3,000 g for 10 min; serum was stored at −70°C until analysis. Serum was assayed for aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma glutamyl transferase (GGT), and bilirubin (total, direct, and indirect) using commercially available kits (Thermo Scientific, Waltham, MA). Liver tissue triglyceride (Thermo Fisher) and glycogen (Sigma-Aldrich, St. Louis, MO) were determined using commercial kits as described previously (12). Portal plasma was assayed for fibroblast growth factor (FGF)19 using a porcine-specific FGF19 ELISA assay (Cusabio Biotech Co. Ltd). This kit is designed using a full-length porcine FGF19 protein expressed in eukaryotic cells and employs rabbit polyclonal antibody detection.
Phytosterol concentrations were measured in plasma and red blood cells, liver, distal ileum, adipose tissue, and dietary lipid and formula samples as their trimethylsilyl derivatives by GC-MS, after hydrolysis, extraction, and derivatization, as previously described (15). Analysis was performed on a Shimadzu GC/MS-QP2010 system with a Varian VF-1701 MS WCOT column, and ions were recorded in total ion current and selected ion monitoring modes. Individual phytosterols were quantified by normalization with the internal standard and calibration with weighed standards. Phytosterol concentrations lower than 1 µmol/l were set as 0.5 µmol/l. Total plasma bile acid was determined using a total bile acid kit (BQ Kits Inc., San Diego, CA). Individual bile acid concentrations in plasma (day 14), bile, and frozen liver samples (day 14) were quantified by capillary gas chromatography and further characterized by LC-MS/MS as described previously (16–18). Plasma 7α-hydroxy-4-cholesten-3-one (C4) concentrations were determined as described previously (19). Plasma bile acid pool was calculated as the product of plasma total bile acid concentration (μM) and blood volume assuming 80 ml/kg body weight. Liver bile acid pool was calculated as the product of total liver tissue total bile acid concentration (μmol/g) and liver weight (g liver/kg body weight). The phytosterol load was calculated based on the phytosterol concentration measured in each of the treatment solutions (i.e., formula and individual lipid emulsions) and the daily feeding or infusion rate per kilogram body weight.
Histology
Liver tissues were fixed in 10% formalin and embedded in paraffin and reviewed by experienced pathologists (M.L.F. and D.S.) who were blinded to the treatment groups. Histological scores for tissue injury were developed and quantified for each pig, and a composite total score was calculated. Fresh liver was fixed in OCT Compound (Sakura Finetek) and frozen in liquid nitrogen, and then frozen sections were stained with Oil Red O.
Real-time PCR
Quantitative real-time PCR was performed on frozen liver samples. Total RNA was isolated from 100 to 150 mg of frozen liver or ileum tissue with RNeasy Midi kits (Qiagen) and DNase treated using an Ambion (AM1906) kit. DNase-treated RNA was used for the reverse transcription reactions using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystem), and cDNA products were stored at 4°C prior to real-time quantitative PCR (qPCR). Real-time qPCR was performed using commercially available kits made by Invitrogen (Superscript III Platinum Two-Step qPCR Kit with SYBR Green) on real-time PCR machines made by Applied Biosystem 7900HT and Bio Rad CFX96. Primers were designed using software from NCBI Primer BLAST (Table 1). The primers for porcine FGF19 were designed using the predicted porcine sequence available on Ensemble Genome Browser (Gene ID: ENSSSCG00000012871). Amplification efficiency was controlled by the use of an internal control (GAPDH or β-actin). Relative quantification of target mRNA expression was calculated and normalized to GAPDH or β-actin expression. All reactions were performed under the following thermal cycling conditions: 10 min at 95°C, followed by 40 cycles of 95°C for 15 s and 60°C for 60 s. The 2−ΔΔCT method was used to compare gene expression levels between samples, which were analyzed to determine the fold induction of mRNA expression.
TABLE 1.
Forward and reverse primers for porcine gene quantification by quantitative RT-PCR
| Gene | Primer | Accession Number |
| ABCG5 | 5′TCCAATGTGTGCCTTGAGTC | EF472591 |
| 5′CCAGGATGACAAGAGTTGGG | ||
| ABCG8 | 5′GTCTGGCACCACCATCTACT | XM_003354847 |
| 5′TCCACCCCATAGAAGTCAGC | ||
| β-Actin | 5′GGACCTGACCGACTACCTCA | DQ452569 |
| 5′GCGACGTAGCAGAGCTTCTC | ||
| BSEP | 5′ TTTCATTCAGCGCCTGACCA | XM_003133457 |
| 5′ ACTCCAATGAGAGGGCTGAC | ||
| CYP27A1 | 5′ACTGAAGACCGCGATGAAAC | NM_001243304 |
| 5′CAAAGGCGAATCAGGAAGGG | ||
| CYP3A29 | 5′GTGGAGTGTTACATACGGGC | NM_214423 |
| 5′AGGTGATACTAGGTGGGGGT | ||
| CYP7A1 | 5′GAAAGAGAGACCACATCTCGG | NM_001005352 |
| CYP8B1 | 5′GAATGGTGTTGGCTTGCGAT | NM_214426.1 |
| 5′CCGGAAGAATATGTTGGAAT | ||
| 5′AAGTCTAGTTTTCTCTTCGC | ||
| FGF19 | 5′AAGATGCAAGGGCAGACTCA | ENSSSCG00000012871 |
| 5′AGATGGTGTTTCTTGGACCAGT | ||
| FXR | 5′TTTGTGTCGTTTGCGGAGAG | XM_003481738 |
| 5′GTTGCCCCCATTTTTACACTTG | ||
| GAPDH | 5′CAGGTTGTGTCCTCTGACTTT | NM_001206359 |
| MRP3 | 5′ACCCTGTTGCTGTAGCCAAA | XM_003126534 |
| 5′TGGACAAAGGGACAATAGCTGAGT | XM_003131575.3 | |
| 5′TGGCCATCCCGTAGAAGATG | ||
| NTCP | 5′ ACTTTCGGAAACCTAAGGGACT | XM_001927695 |
| 5′ AAGAGCTTGCCCAGTGCAAAG | ||
| OSTα | 5′ TGTACAAGAACACTCGCTGC | NM_001244266 |
| 5′ GAACACACACACTATCGTGGG | XM_003132614 | |
| SHP | 5′ GCCTACCTGAAAGGGACCAT | DQ002896 |
| 5′ CAACGGGTGTCAAGCCTTTA |
BSEP, bile salt export pump; CYP8B1, sterol 12-alpha-hydroxylase; CYP7A1, cholesterol 7-hydroxylase; CYP3A29, cytochrome P450 3A29; CYP27A1, sterol 27-hydroxylase; MRP3, multidrug resistant protein 3; NTCP, Na+/ taurocholate cotransporter; OSTα, organic solute transporter alpha; SHP, small heterodimer partner.
Immunoblot analysis
Western immunoblotting of tissue samples was described previously (12). Briefly, frozen liver was homogenized in buffer containing 50 mM HEPES (pH 7.4), 1 mM EDTA, 1 mM dithiothreitol, 5 mg/l phenylmethylsulfonyl-fluoride, 5 mg/l aprotinin, 5 mg/l chymostatin, and 5 mg/l pepstatin. The homogenate was sonicated and centrifuged at 12,000 g for 15 min at 4°C. The extracts (120 μg protein/lane) were separated via 10% SDS-PAGE, transferred to nitrocellulose membranes, and after blocking with 5% nonfat milk in Tris-buffered saline (TBS, 20 mM Tris, 150 mM NaOH, pH 7.4) incubated with a primary antibody diluted in 5% nonfat milk in TBS + 0.1% Tween-20. The membranes were probed with CYP7A1 (1:250, goat polyclonal antibody; Santa Cruz Biotech Inc.), which produced a single band at approximately molecular mass 58 kDa. The loading control was α-tubulin (1:10,000 dilution, mouse monoclonal antibody; Sigma). Membranes were incubated with a secondary antibody (goat anti-rabbit IgG-HRP or goat anti-mouse IgG-HRP, 1:5,000; Santa Cruz Biotech Inc.). Signal was developed by ECL-plus (Life Technologies) and then detected by Bio-Rad Chemi Doc (Bio-Rad Laboratories, Hercules, CA), and the image was quantified by ImageQuant 5.0 software (Molecular Dynamics). We expressed the abundances of specific target proteins relative to that of tubulin measured after stripping and reprobing membranes. All Western blots were run with six samples from each treatment group and used for statistical analysis. The ratios of each protein relative to tubulin are shown relative to the enteral control group.
Primary pig hepatocyte culture
Newborn pigs were anesthetized with isoflurane, the abdominal cavity was opened surgically, and the portal vein and inferior vena cava were isolated. The portal vein was cannulated, and the liver perfused with calcium-free EGTA-containing Krebs-Henseleit (KH) buffer. After 10 min, the buffer was replaced with collagenase-containing KH buffer, and the perfusion continued for an additional 15 min to promote collagen digestion. The liver was then excised and transferred in calcium-containing KH buffer, and hepatocytes were filtered through a 100 μm mesh. Cell suspensions were washed twice with KH buffer by centrifugation (15 g, 2 min) to remove dead cells, and cell viability was determined using trypan blue dye exclusion. Cell concentration was determined, and 5 × 105 cells per well were plated in 6-well plates for all subsequent experiments.
Cells were first grown in a medium containing Williams E buffer, penicillin, streptomycin, gentamycin, glutamine, glucagon, insulin-transferrin-selenium, and 10% FBS for 12 h and then in a medium containing Williams E buffer, penicillin, streptomycin, gentamycin, glutamine, and 0.5% albumin for another 12 h. Stock solutions of chenodeoxycholic acid (CDCA), obeticholic acid (OBCA), and phytosterols were prepared in DMSO. Then, 24 h after initial plating, cells were treated for 24 h with DMSO (control); CDCA (5, 10, 50, or 100 μM); OBCA (0.5, 1, 5, or 10 μM); OBCA (10 μM) plus either β-sitosterol, campesterol, or stigmasterol (10 or 25 μM); OBCA (10 μM) plus IL 20% (500 μl); OBCA (10 μM) plus OV 10% (1,000 μl); and IL 20% (500 μl) or OV 10% (1,000 μl) alone. After 24 h incubation, cells were treated with 1 ml Trizol (for RNA extraction), and cell extracts stored at −70°C.
Porcine FXR luciferase reporter assay
Porcine FXR was cloned into pcDNA3.1/V5-His TOPO vector (Invitrogen Corp., Burlington, ON, Canada) as previously described (20). HepG2 cells were cultured in Eagle MEM (Earle's balanced salt solution, nonessential amino acids, 1 mM sodium pyruvate, 2 mM L-glutamine, 1,500 mg sodium bicarbonate/l) (ATCC, Manassas, VA) supplemented with 10% FBS and 1% v/v penicillin/streptomycin (Invitrogen Corp.). Cells were plated in 24-well plates at a seeding density of 1.0 × 106 cells per well. Twenty-four hours after plating, cells were transfected with an FXR expression plasmid (250 ng/well), a pRL-tk control plasmid (5 ng/well), and an IR-1-tk-luciferase reporter plasmid for FXR (250 ng/well) (19), using Lipofectamine 2000 (Invitrogen Corp.) following the manufacturer's instructions. Twenty-four hours after transfection, cells were treated with CDCA (100 µM; Sigma-Aldrich) along with various individual phytosterols (12.5 µM) or lipid emulsions [IL (500 µL) or OV (1,000 µL)]. CDCA and phytosterol stocks were made in DMSO and then diluted to final concentrations in culture media. A DMSO control treatment was also used, with DMSO at 0.05% (v/v) in culture media. Twenty-four hours after ligand treatment, the media were removed, cells were washed in 1× PBS, and cells were lysed following the Dual-Luciferase Assay protocol (Promega, Madison, WI). The Dual-Luciferase Assay was carried out following the manufacturer's instructions using a Sirius single tube luminometer with dual injectors (Berthold Detection Systems, Oak Ridge, TN).
Statistics
Statistical analyses were performed using SPSS 16 software (Armonk, NY). Differences among the four groups were first analyzed using ANOVA, and differences between individual group means using Tukey's test as described in the figure legends. Histological scores were tested using a Kruskal-Wallis test for nonparametric variables. P values < 0.05 were considered significant. Results are presented as mean ± SEM.
RESULTS
Body weight gain from birth to day 14 was statistically lower (P < 0.05) in IL versus all other groups [mean ± SEM in g/(kg·day): ENT, 58.7 ± 2.2; IL, 49.5 ± 1.5; OV, 53.3 ± 1.7; SL, 54.3 ± 2.2]. Liver weight was significantly higher in TPN pigs than in ENT pigs, and among the TPN pigs, significantly (P < 0.05) higher in IL and OV pigs than in SL pigs (mean liver weight ± SEM in g/kg body weight: ENT, 32.4 ± 1.3; IL, 47.8 ± 1.8; OV, 47.6 ± 1.5; SL, 41.6 ± 1.3).
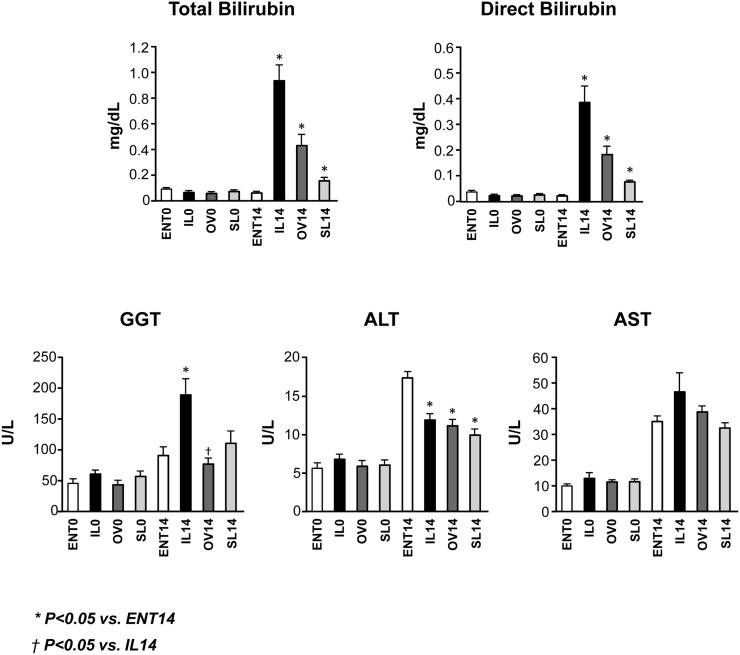
Serum chemistry concentrations are presented in Fig. 1. After 14 days of treatment, both direct and total bilirubin concentrations were elevated in the TPN groups compared with day 0. However, the concentrations were the highest in the IL group. In addition, the IL group also had the highest GGT value as compared with the other three groups, whereas the GGT levels in the ENT and OV groups were similar. In contrast, we unexpectedly found that serum concentrations of ALT were slightly lower in the TPN versus ENT groups and that AST levels were similar among the four groups.
Fig. 1.
Serum markers of hepatic cholestasis and injury including total bilirubin, direct bilirubin, GGT, ALT, and AST in preterm pigs at birth (day 0) and 14 days of treatment with enteral formula (ENT) or TPN with IL, OV, or SL. Values are means ± SEM; n = 7–12 pig group. Differences between groups were analyzed using ANOVA and Tukey's means comparison test. * P < 0.05 versus ENT14; † P < 0.05 versus IL14.
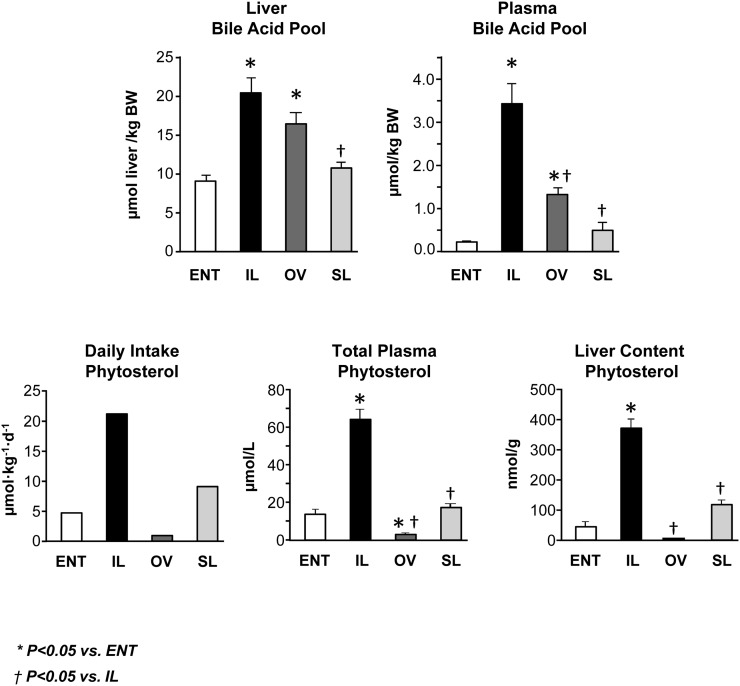
We next measured the bile acid pools in both the liver and plasma after 14 days of treatment (Fig. 2 and Table 2). Both the IL and OV groups had higher (P < 0.05) liver bile acid pool relative to ENT, yet the SL and ENT groups had comparable liver bile acid concentrations. The plasma bile acid pool in the IL group was 7-fold and 2.5-fold higher than the ENT and OV groups, respectively. The two most abundant plasma primary bile acids found were CDCA and UDCA with much lower concentrations of CA. In general, the glyco- versus tauro-conjugated form of all three major bile acids was higher than the unconjugated forms, and the glyco-conjugated forms of CDCA, UDCA, and CA were markedly increased (P < 0.05) in the IL versus ENT and SL groups.
Fig. 2.
Liver and plasma bile acid pool sizes, daily phytosterol load, and phytosterol concentrations in plasma and liver tissue in preterm pigs at 14 days of treatment with ENT or TPN with IL, OV, or SL (see calculations of daily load and pool sizes in methods). Values are means ± SEM; n = 7–12 pig group. Differences between groups were analyzed using ANOVA and Tukey's means comparison test. * P < 0.05 versus ENT; † P < 0.05 versus IL.
TABLE 2.
Plasma concentrations of individual bile acids determined in preterm piglets after 14 days with either ENT or TPN treatments
| Bile Acid | ENT | IL | OV | SL |
| CDCA | 92.1 ± 25a | 185 ± 58.8b | 396 ± 85.7b | 122 ± 46a |
| t-CDCA | 298 ± 50a | 3,198 ± 1,581b | 488 ± 215a | 109 ± 20.4a |
| g-CDCA | 523 ± 83.8a | 21,792 ± 12,155b | 10,087 ± 4,804b | 1,372 ± 491c |
| Total CDCA | 913 ± 136.5a | 25,176 ± 13,721b | 10,973 ± 5,012b | 1,604 ± 556c |
| UDCA | 48.2 ± 12.4a | 322.2 ± 153.5b | 772.8 ± 175.3b | 145.3 ± 54.8a |
| t-UDCA | 169 ± 25.3a | 526 ± 246a | 90.4 ± 30.7a | 24.6 ± 6.9b |
| g-UDCA | 386 ± 63.8a | 6,296 ± 2,038b | 3,191 ± 828b | 787 ± 214.3a |
| Total UDCA | 604 ± 88.0a | 7,144 ± 2,334b | 4,054 ± 911.9b | 956 ± 272c |
| CA | 27.7 ± 3.7a | 53.0 ± 11.2ab | 83.4 ± 10.3b | 78.6 ± 23.5b |
| t-CA | 30.5 ± 5.5a | 208.5 ± 3.5b | 35.2 ± 8.9a | 19.1 ± 4.5c |
| g-CA | 17.1 ± 4.5a | 601 ± 240b | 158 ± 66.8b | 40.4 ± 14.9a |
| Total CA | 75.3 ± 9.8a | 862 ± 297b | 276 ± 81.5b | 138 ± 36.7a,c |
| THCA | 11.00 ± 3.5a | 13.00 ± 2.9a | nd | nd |
| OH-THCA | 10.00 ± 4.7a | 109 ± 22.1b | 57.5 ± 21.8b | 11.00 ± 3.4a |
| t-OH-THCA | nd | 13.00 | nd | nd |
| DHCA | 17.8 ± 2.5a | 127 ± 18.2b | 306 ± 121b | 47.6 ± 11.4c |
Concentrations in nmol/l. Values are means ± SEM. Means with different superscripts are significantly different (P < 0.05). Conjugated bile acid forms: g, glycine; t, tauro. CA, cholic acid; DHCA, 3a,7a-dihydroxy-5β-cholestanoic acid; THCA, 3a,7a,12a-trihydroxy-5β-cholestanoic acid; UDCA, ursodeoxycholic acid.
In order to examine the link between phytosterols and bile acid homeostasis, we calculated the daily phytosterol intake as well as the liver and plasma phytosterol concentrations (Fig. 2 and Table 3). The phytosterol load was more than 4-fold higher in the IL than in the ENT group. It is important to note that OV was devoid of phytosterols and SL contained one-third of the total phytosterol found in IL. Accordingly, phytosterol concentrations in plasma and liver (Fig. 2) and in other tissues (data not shown) were statistically highest in the IL group compared with the other groups, and phytosterol concentrations in liver were higher than concentrations in other tissues.
TABLE 3.
Daily intake and plasma and liver concentrations for cholesterol and individual phytosterols (stigmasterol, campesterol, and β-sitosterol) in preterm piglets after 14 days with ENT or TPN treatments
| ENT | IL | OV | SL | |
| Lipid intake, g/(kg·day) | 5 | 5 | 5 | 5 |
| Sterol intake, μmol/(kg·day)a | ||||
| Stigmasterol | 0.19 | 3.38 | 0.70 | 1.25 |
| Campesterol | 1.54 | 3.78 | 0.05 | 1.25 |
| β-Sitosterol | 2.88 | 14.50 | 0.05 | 6.45 |
| Total phytosterol | 4.61 | 21.65 | 0.80 | 8.95 |
| Cholesterol | 81.4 | 15.6 | 39.5 | 20.5 |
| Plasma sterol, μM | ||||
| Stigmasterol | 3.17 ± 1.4a | 6.75 ± 1.5b | 1.00c | 0.00c |
| Campesterol | 9.00 ± 1.0a | 13.88 ± 1.9b | 2.00c | 3.75 ± 0.3c |
| β-Sitosterol | 8.67 ± 1.1a | 47.17 ± 3.5b | 0.00c | 17.17 ± 1.7d |
| Total phytosterol | 14.38 ± 1.7a | 58.67 ± 5.5b | 0.69 ± 0.2c | 16.86 ± 2.9a |
| Cholesterol | 2,501 ± 289a | 1,220 ± 183b | 1,322 ± 218b | 1,094 ± 158b |
| Liver sterol, nmol/g | ||||
| Stigmasterol | 2.9 ± 0.7a | 53.5 ± 5.7b | 2.2a | 11.0 ± 1.0c |
| Campesterol | 20.2 ± 1.4a | 69.8 ± 6.0b | <1c | 19.7 ± 1.4a |
| β-Sitosterol | 17.4 ± 1.5a | 226.5 ± 21.5b | <1c | 85.6 ± 4.0d |
| Total phytosterol | 38.8 ± 2.4a | 349.8 ± 31.7b | 2.2c | 114.1 ± 6.0d |
| Cholesterol | 3,245 ± 168 | 3,731 ± 364 | 3,462 ± 102 | 3,202 ± 259 |
Means with different superscripts are significantly different (P < 0.05).
Sterol intakes were calculated based on analysis of cholesterol and phytosterols in each lipid emulsion and the enteral formula and the daily infusion rate of lipid emulsions and formula in the respective treatment groups. nd, not detected.
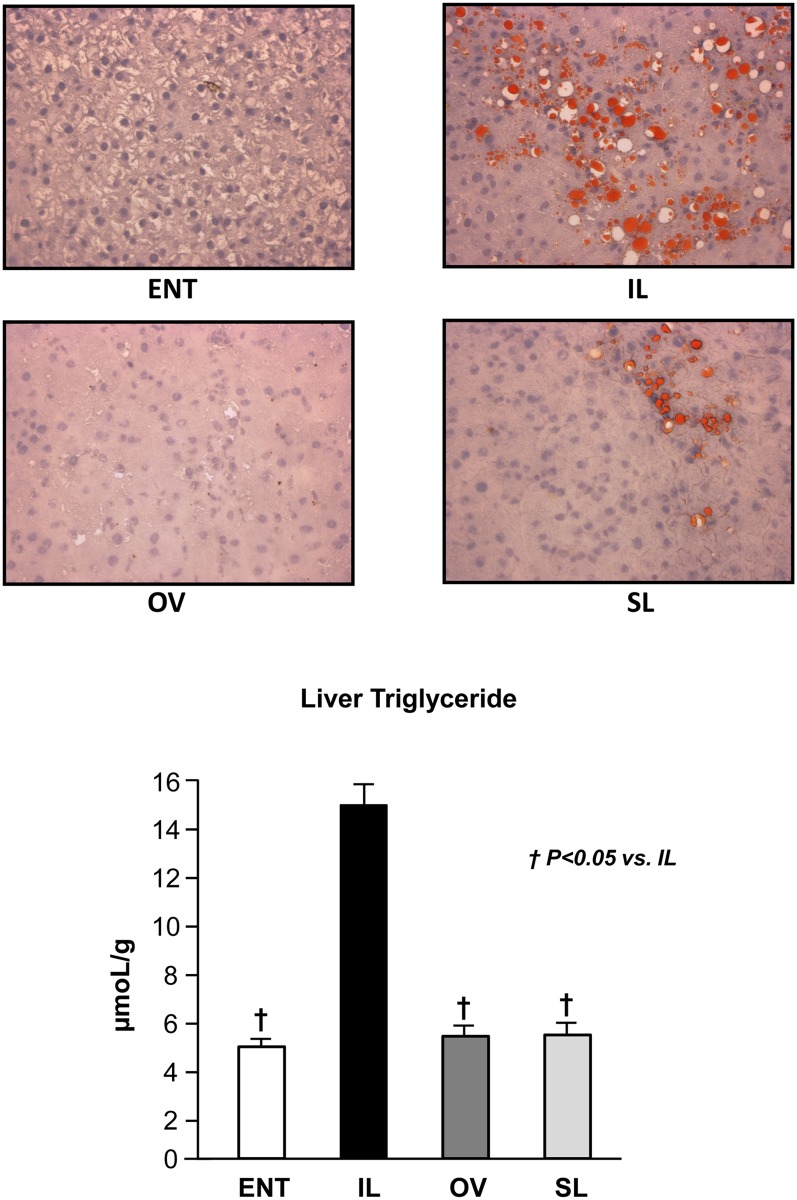
Liver triglyceride analysis supported the histological findings of Oil Red O staining (Fig. 3). The IL group had a significantly (P < 0.05) higher liver triglyceride content compared with ENT, OV, and SL (Fig. 3). Furthermore, the liver triglyceride contents were similar among ENT, OV, and SL. The liver glycogen content was significantly (P < 0.05) lower in OV compared with all other groups but was highest in SL (mean ± SEM, mg/g tissue: ENT, 49.3 ± 6.9; IL, 43.2 ± 8.3; OV, 27.2 ± 5.1; SL, 59.7 ± 7.2). Liver histopathology after 14 day treatments was scored according to the features described in Table 4. The histological sections showed evidence of microsteatosis, portal triad infiltration, cholangitis, pericholangitis, and lobular inflammation that were highest in the IL versus other TPN groups; whereas hepatocyte swelling was prominent in SL pigs (Table 5). Representative images are shown (Fig. 4).
Fig. 3.
Oil Red O staining of liver sections from the four groups after 14 day treatment (top panels). Representative images labeled as ENT, IL, OV, and SL show lipid droplets with red staining in IL and SL groups. Liver triglyceride content in pigs after 14 day treatment labeled as ENT, IL, OV, and SL (bottom panel). Values are means ± SEM; n = 7–12 pig group. Differences between groups were analyzed using ANOVA and Tukey's means comparison test. * P < 0.05 versus ENT; † P < 0.05 versus IL.
TABLE 4.
Liver histopathology score definitions
| Feature | Score Definition |
| Hepatocellular glycogenation | Absent = 0 |
| Present = 1 | |
| Steatosis | Absent/rare (0–5%) = 0 |
| 5–33% of hepatocytes = 1 | |
| 33–66% of hepatocytes = 2 | |
| >66% of hepatocytes = 3 | |
| Hepatocellular swelling | Absent/rare = 0 |
| Few = 1 | |
| Many/prominent = 2 | |
| Extramedullary hematopoiesis | Absent/rare = 0 |
| Present = 1 | |
| Extensive = 2 | |
| Portal tract edema | Absent = 0 |
| Present = 1 | |
| Portal cellular infiltrate | Absent/minimal = 0 |
| Mild = 1 | |
| Moderate/marked = 2 | |
| Ductulitis (mononuclear) | Absent = 0 |
| Mild, present in occasional ducts = 1 | |
| Multiple foci = 2 | |
| Lobular inflammation | Grade 0, none = 0 |
| Grade 1, <2 foci per 200× field = 1 | |
| Grade 2, 2–4 foci per 200× field = 2 | |
| Grade 3, >4 foci per 200× field = 3 |
TABLE 5.
Liver histopathology scores in preterm piglets after 14 d with ENT or TPN treatments
| ENT | IL | OV | SL | |
| Hepatocellular glycogenation | 0.200 ± 0.133 | 0.167 ± 0.112 | 0.100 ± 0.100 | 0.571 ± 0.202 |
| Steatosisa | 0.00 ± 0 | 1.833 ± 0.241 | 0.200 ± 0.133 | 0.286 ± 0.184 |
| Hepatocellular swellinga | 0.800 ± 0.249 | 0.333 ± 0.225 | 0.300 ± 0.153 | 1.286 ± 0.360 |
| Extramedullary hematopoiesis | 1.200 ± 0.133 | 0.917 ± 0.149 | 1.400 ± 0.221 | 1.143 ± 0.143 |
| Portal tract edema | 0.300 ± 0.153 | 0.583 ± 0.149 | 0.400 ± 0.163 | 0.857 ± 0.143 |
| Portal cellular infiltratea | 0.00 ± 0.0 | 0.667 ± 0.225 | 0.300 ± 0.153 | 0.143 ± 0.143 |
| Ductulitisa | 0.200 ± 0.133 | 1.250 ± 0.179 | 0.200 ± 0.133 | 0.286 ± 0.184 |
| Lobular inflammationa | 0.600 ± 0.221 | 2.250 ± 0.218 | 1.600 ± 0.267 | 1.143 ± 0.340 |
Values are means ± SEM.
Significant difference (P < 0.05) among groups based on Kruskal-Wallis test.
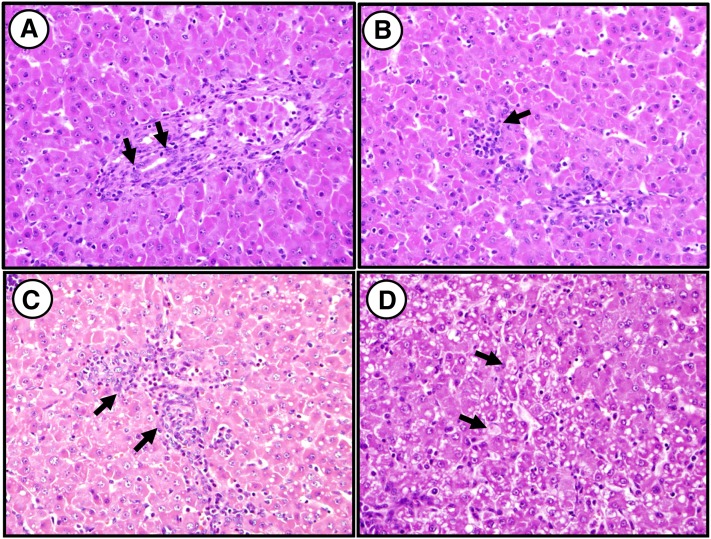
Fig. 4.
Representative histological images of liver tissue from the four groups after 14 day treatment. A: Hematoxylin and eosin staining from IL pig liver shows ductulitis. B: Staining from IL pig shows evidence of lobular inflammation. C: Staining from IL pig shows evidence of portal inflammation. D: Staining from SL pig shows hepatocyte swelling (hematoxylin and eosin stain, ×400). Arrows indicate areas in the image that contain evidence of (A) ductulitis, (B) lobular inflammation, (C) portal inflammation, and (D) hepatocyte swelling.
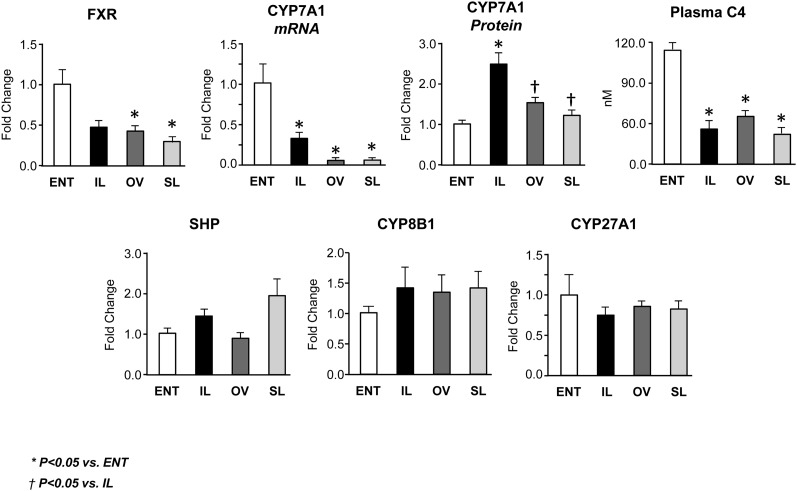
In order to elucidate the mechanism of cholestasis, we measured the expression of FXR and its downstream target genes (Figs. 5, 6). First, we discovered that FXR expression was suppressed in all three TPN groups and significantly (P < 0.05) lower in the OV and SL groups versus ENT. We next measured the FXR gene target, CYP7A1, involved in bile acid synthesis (20). CYP7A1 gene expression was suppressed in all three TPN groups, suggesting decreased bile acid synthesis in TPN-fed pigs. This finding was supported by measurement of the intermediate in the conversion of cholesterol to bile acid, C4, which was also significantly (P < 0.05) lower in all three TPN groups compared with ENT. Surprisingly, based on immunoblot analysis, we found an increase (P < 0.05) in the CYP7A1 protein abundance in the IL group compared with all other groups. These opposing findings raise the possibility of a posttranscriptional or posttranslational process that may be contributing to increased CYP7A1 protein production, relative to CYP7A1 mRNA expression. Bile acid synthesis from cholesterol occurs via either the classic or alternative pathway, driven by CYP7A1 or CYP27A1, respectively (20). The CYP27A1 and CYP8B1 expression was unchanged in the four groups, implying that the alternative bile acid synthesis pathway was not affected by parenteral nutrition.
Fig. 5.
Expression of hepatic FXR and target genes involved in bile acid synthesis in pigs after 14 day treatment labeled as ENT, IL, OV, and SL. Quantitative RT-PCR analysis showing relative transcript levels of FXR, CYP7A1, SHP, CYP8B1, and CYP27A1 mRNA. Also shown in the top panel is relative expression of CYP7A1 protein results from Western blotting and plasma concentrations of C4, an intermediate marker of bile acid synthesis. Values are means ± SEM; n = 7–12 pig group. Differences between groups were analyzed using ANOVA and Tukey's means comparison test. * P < 0.05 versus ENT; † P < 0.05 versus IL.
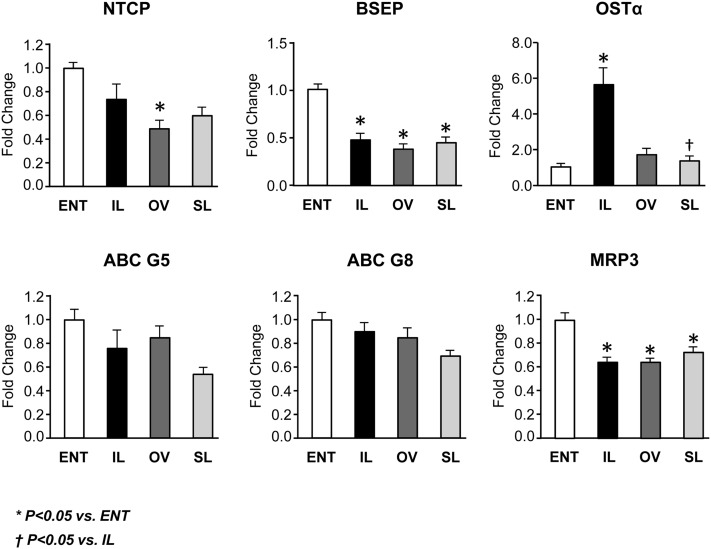
Fig. 6.
Hepatic expression of genes involved in transport of bile acids and phytosterols in pigs after 14 day treatment labeled as ENT, IL, OV, and SL. Quantitative RT-PCR analysis showing relative transcript levels of NTCP, BSEP, OSTα, ABCG5, ABCG8, and MRP3. Values are means ± SEM; n = 7–12 pig group. Differences between groups were analyzed using ANOVA and Tukey's means comparison test. * P < 0.05 versus ENT; † P < 0.05 versus IL.
We next examined the expression of FXR targets involved in bile acid transport (Fig. 6). The NTCP imports ∼80% of bile acids into hepatocytes (20). TPN-treated pigs tended to have decreased NTCP mRNA expression, with the OV group having the lowest (P < 0.05) expression. We found the mRNA expression of BSEP, which is the major bile acid exporter involved in canalicular excretion (21), was lower (P < 0.05) in all three TPN groups, suggesting decreased bile acid export. Given the increased plasma bile acid concentrations, we measured OSTα. This is a basolateral heterodimeric bile acid transporter (along with OSTβ) that is regulated by FXR and is upregulated to promote bile acid export into the systemic circulation (21). We discovered an ∼5-fold increase in the expression of OSTα in the IL group, yet the OV and SL groups were similar to the ENT group. In contrast, the expression of another basolateral bile efflux transporter, MRP3, was downregulated (P < 0.05) in all TPN groups. This suggests that hepatocytes in IL pigs accumulate bile acids, which in turn drive a compensatory bile acid efflux into the systemic circulation. Phytosterols are exported into bile via the basolateral heterodimers ABCG5 and ABCG8 (22), and the expression trended downward in the TPN groups but was not statistically different from the ENT group.
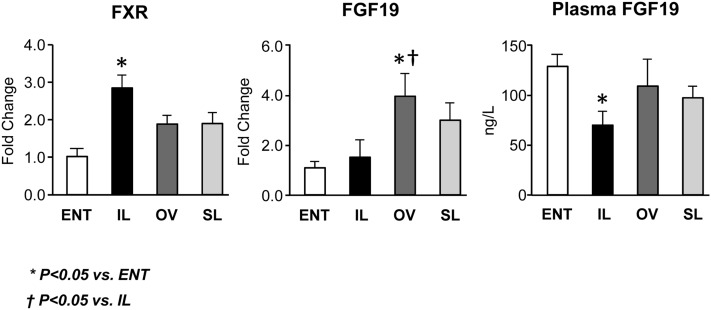
We measured the expression in ileum tissue of FXR and its key target gene FGF19, which functions as an enterohepatic negative feedback on hepatic CYP7A1 (Fig. 7). In general, the ileal mRNA expression of FXR tended to be higher in all TPN groups, yet FXR was statistically higher (P < 0.05) only in IL versus ENT pigs. Interestingly, the ileal mRNA expression of FGF19 was similar in ENT and IL, but higher in OV (P < 0.05) and SL pigs versus ENT. As expected from our previous studies (23), we found that portal plasma FGF19 concentration in ENT pigs was higher (P < 0.05) than in IL, but not OV or SL pigs.
Fig. 7.
Intestinal (ileum) expression of genes involved in FXR-FGF19 signaling in pigs after 14 day treatment labeled as ENT, IL, OV, and SL. Quantitative RT-PCR analysis showing relative transcript levels of ileal tissue FXR and FGF19 mRNA and portal venous plasma concentrations of FGF19. Plasma FGF19 concentrations were measured by porcine-specific ELISA as described in “Materials and Methods.” Values are means ± SEM; n = 7–12 pig group. Differences between groups were analyzed using ANOVA and Tukey's means comparison test. * P < 0.05 versus ENT; † P < 0.05 versus IL.
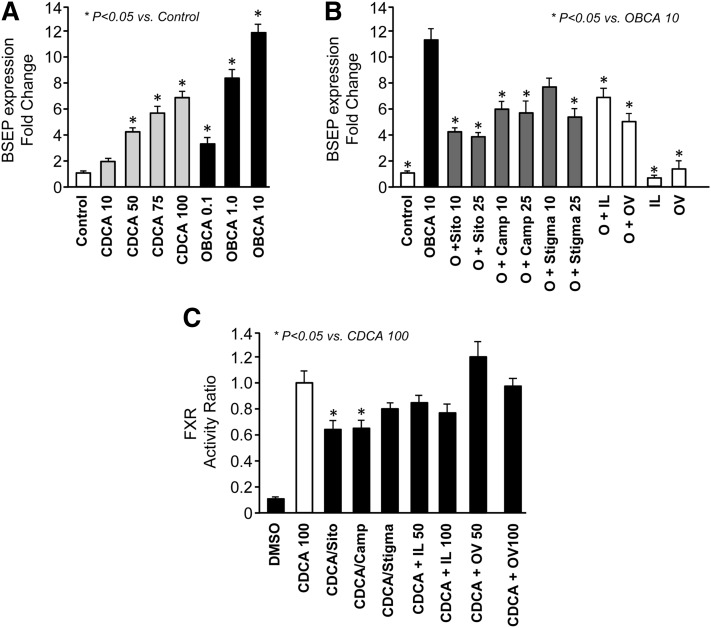
Given the structural similarity between bile acids, cholesterols, and phytosterols, as well as earlier studies that showed phytosterols downregulating FXR in mouse hepatocytes, we measured their effects in pig hepatocytes. We examined BSEP expression as a direct FXR target that plays an integral role in bile acid homeostasis (5). We first demonstrated that BSEP expression increased dose dependently in response to the FXR agonists CDCA (natural FXR ligand) and OBCA (selective FXR agonist) (Fig. 8). We next measured BSEP expression after 24 h treatment with OBCA plus each of the three principal phytosterols (β-sitosterol, campesterol, and stigmasterol), OBCA plus IL, and OBCA plus OV, as well as IL alone and OV alone. We used OBCA because it is a more selective FXR agonist than CDCA (24). OBCA-activated BSEP expression was significantly decreased by all three phytosterols at 10 and 25 µM, as well as IL and OV. Next, we used an FXR luciferase assay to confirm that FXR activity was indeed changed after exposure to phytosterols. The results showed that CDCA induced a robust increase in FXR activity; this CDCA induction was decreased with addition of either β-sitosterol or campesterol, but not stigmasterol. CDCA-activated FXR activity also tended to decrease in a dose-dependent manner with IL, but this was not statistically significant.
Fig. 8.
Fold-changes in BSEP mRNA expression in primary pig hepatocytes in response to 24 h treatment with DMSO (control), CDCA (10–100 µM), or OBCA (0.1–10 µM) (A) at the concentrations indicated. Relative transcript levels of BSEP mRNA expression in treated hepatocytes were measured by qualitative RT-PCR analysis. All treated groups were compared statistically to control DMSO-treated cells using ANOVA. * P < 0.05 versus ENT; † P < 0.05 versus IL. Fold-changes in BSEP mRNA expression in primary pig hepatocytes in response to 24 h treatment with OBCA (10 µM, O) with either individual phytosterols (sitosterol, sito; campesterol, camp; stigmasterol, stigma) or lipid emulsions (IL, OV) for 24 h (B) as described in detail in “Materials and Methods.” Individual phytosterols were solubilized in stock solutions with DMSO and diluted to final concentrations in media. All treated groups were compared statistically to OBCA 10 alone using ANOVA. * P < 0.05 versus ENT; † P < 0.05 versus IL. (C) Results from the luciferase assay in which HepG2 cells were transiently transfected with porcine FXR constructs and luciferase activity was measured after treatment for 24 h with CDCA (100 µM); CDCA (100 µM) plus individual β-sitosterol, campesterol, or stigmasterol (12.5 µM); or CDCA (100 µM) plus IL (50 and 100 µL) or OV (50 and 100 µL) lipid emulsions as described in detail in “Materials and Methods.” All treated groups were compared statistically to CDCA alone using ANOVA. * P < 0.05 versus ENT; † P < 0.05 versus IL.
DISCUSSION
Prolonged TPN exposure increases the risk of PNALD, especially in preterm infants. However, studies have shown that switching to fish oil emulsions can reverse PNALD in infants previously given soybean oil lipid emulsions (8, 9). Possible mechanisms for the protective effect of fish oil emulsions include reduction of lipid dose, lower phytosterol content, higher n-3 PUFA content (i.e., fatty acid composition of emulsion), higher α-tocopherol content, and direct regulation of bile flow through eicosanoid-mediated mechanisms (3, 25). Much debate has emerged in recent reports questioning whether the lower lipid load [1 g/(kg·day)] in fish oil emulsions is itself the primary protective factor against PNALD, rather than the differences in the ingredient composition of the emulsion. Indeed, minimization of the lipid load itself has been shown to reverse cholestasis (6, 7). We sought to test these questions using our preterm pig model by comparing the end points of cholestatic liver disease in response to chronic treatment with different commercial soybean oil-containing and fish oil-containing lipid emulsions administered at equal lipid loads. We also hypothesized that phytosterols in soybean oil emulsions, but absent in pure fish oil emulsions, are a contributing factor in the development of PNALD.
Two weeks of continuous TPN in our preterm pigs resulted in remarkable differences in measures of hepatic cholestasis and injury among the three TPN groups. Serum markers of cholestasis were increased in all TPN groups, but not ENT pigs between days 0 and 14. Serum direct and total bilirubin were 8- to 10-fold in higher in IL versus ENT pigs at day 14. Serum GGT was highest in the IL group and statistically different from the OV and ENT groups. Surprisingly, we observed that ALT and AST levels were both increased in all treatment groups after 14 days, yet ALT levels were lower in the TPN-fed piglets relative to the ENT group. These changes in liver enzymes suggest a pattern of biliary tract rather than hepatocellular injury. Likewise, we found that the estimated plasma bile acid pool size was highest in the IL pigs and significantly lower in OV and SL pigs. The two predominant plasma bile acids found were CDCA and UDCA, and glyco-conjugated CDCA was markedly increased in IL versus all other groups. These findings suggest that IL-induced intrahepatic accumulation of conjugated bile acid is associated with biliary injury, which may precede hepatocellular injury. Direct examination of the liver showed hepatomegaly in all TPN groups compared with enteral formula feeding, consistent with our previous reports (4, 26). Livers from all TPN pigs showed evidence of histopathological injury, including biliary duct dilation, portal tract cellular infiltration, and microvascular steatosis. However, the histological scores suggested that the features biliary injury and inflammation were highest among the IL versus other lipid emulsion groups. Mouse models of cholestasis have shown blunted histological hepatotoxicity with fish oil (26, 27). Collectively, these results suggest that fish oil-containing emulsions prevent the development of PNALD in TPN-fed neonatal pigs.
Our second aim was to measure the presence of systemic and hepatic phytosterolemia in preterm TPN-fed pigs and establish whether this would be associated with markers of cholestasis and liver injury among the three lipid emulsion groups. Soybean oil-derived IL is abundant in phytosterols, whereas fish oil-derived OV is devoid of phytosterols. SL is a blend of lipids including soybean oil, MCTs, olive oil, and fish oil and thus contains phytosterols. The attention on phytosterols stems from their structural similarity with bile acids and earlier reports of phytosterolemia associated with PNALD in TPN-fed pediatric patients (4, 26, 28). After 2 weeks, the differences in phytosterol intake among the groups were directly reflected in both the plasma and liver phytosterol concentrations, with the highest and lowest phytosterol levels in IL and OV pigs, respectively. Comparison of phytosterol concentrations among various tissues indicated that they were concentrated in liver tissue. Importantly, we found that the relative patterns in plasma and liver phytosterol concentrations were weakly correlated with markers of cholestasis and liver injury among lipid emulsion groups, mainly due to the presence of substantial phytosterols in the SL emulsion. Thus, our results do not support the prevailing hypothesis that phytosterolemia contributes to hepatic cholestasis and injury.
We examined the molecular evidence linking phytosterolemia with disruption of hepatic expression of FXR and its target genes. Our results show that all three TPN groups had markedly decreased FXR mRNA expression compared with ENT pigs. Surprisingly, we found that CYP7A1 expression was lower in all three TPN groups versus the ENT group, with the greatest decrease in both the OV and SL groups. Consistent with CYP7A1 expression, we also observed that the concentration of plasma C4, an intermediate metabolite in bile acid synthesis, was also lower in all three TPN groups versus the ENT group. In contrast, the CYP7A1 protein abundance based on Western blots showed that the IL group was more than 1.5-fold higher relative to the ENT group and higher than the OV and SL groups. This difference between mRNA expression and protein abundance of CYP7A1 may be explained by a possible posttranslational mechanism that is allowing either prolonged protein production or delayed protein breakdown. The mRNA expression of other bile acid synthetic enzymes (CYP27A1 and CYP8B1) was not markedly different among groups. We also found that expression of BSEP, MRP3, and NTCP was decreased in all three TPN groups. Importantly, we found a marked upregulation of OSTα expression in IL versus OV and SL pigs. This evidence suggests that TPN suppressed both bile acid production (CYP7A1) and hepatocyte export (BSEP and MRP3) leading to intrahepatic bile acid accumulation especially in IL pigs, which triggered a compensatory upregulation of OSTα expression and increased shunting of bile acid into the bloodstream. The greater accumulation of hepatic bile acids in IL versus OV and SL groups despite general TPN-induced suppression of bile acid synthesis and export suggests that OV and SL may increase hepatocyte pathways of bile acid disposal such as oxidation, sulfation, or glucuronidation. The changes in hepatic FXR and its target gene expression patterns among the lipid emulsion groups were also not closely correlated with the liver phytosterol content, which does not support a molecular mechanism of FXR antagonism.
Another potential factor regulating bile acid homeostasis is the gut enterokine FGF19, which functions to act as a negative feedback signal to suppress hepatic bile acid synthesis. We previously showed that TPN results in reduced circulating FGF19 concentrations in TPN-fed compared with enterally fed piglets (23). In the current study with preterm piglets, we also found a lower portal plasma FGF19 concentration in IL versus ENT pigs, but not in the OV or SL groups. The changes in plasma FGF19 tended to parallel the relative difference in ileum FGF19 mRNA expression for the three TPN groups, but not the ENT group, which had the lowest expression. More importantly, the changes in ileum and plasma FGF19 were inversely related to hepatic CYP7A1 mRNA and plasma C4 levels as would be expected based on previous literature evidence mainly in mice showing gut FGF15 suppression of bile acid synthesis. However, these parallel patterns of circulating protein and ileum tissue FGF19 are in contrast to a recent report in rats and rabbits showing that the plasma FGF15/19 concentrations do not reflect changes in ileal FGF15/19 or hepatic CYP7A1 (29). These results suggest that further study is warranted to resolve whether changes in circulating FGF19 in response to TPN versus enteral feeding or gut FXR activation lead to physiological changes in direct measurements of bile acid synthesis.
We also tested the ability of phytosterols to affect FXR function in pig hepatocytes. We found robust induction of BSEP in response to both CDCA and the selective FXR agonist OBCA. Consistent with our hypothesis, we found that OBCA-induced BSEP expression was decreased with the addition of each of the three principal phytosterols, with β-sitosterol and stigmasterol demonstrating dose-dependent effects. To further demonstrate the direct negative effect of phytosterols, we studied their effect in a porcine FXR-linked luciferase assay. Phytosterol-treated cells had decreased CDCA-stimulated FXR activity, whereby campesterol and sitosterol showed the largest suppression. Thus, while this result supports the previous reports of stigmasterol antagonism of FXR in hepatocytes (5) and induction of liver injury in TPN-fed mice (30), it is not consistent with our in vivo findings in the current study of cholestasis and FXR target gene expression among the three lipid groups in TPN-fed piglets.
In conclusion, our results provide novel evidence that pure fish oil and a multicomponent lipid emulsion containing 15% fish oil protect against PNALD in preterm TPN-fed pigs. The data provide strong support for the idea that the composition of the lipid emulsion is a significant determinant of PNALD even when the lipid load is held constant. Our work does not exclude the possibility that lipid load also contributes to PNALD. Our results in vivo, but not in vitro, do not support the idea that phytosterols are strongly associated with PNALD and antagonism of hepatic FXR function. This interpretation contrasts with the prevailing idea that phytosterols cause liver injury and is mainly explained by the fact that SMOFlipid contains substantial amounts of phytosterol, yet is protective. These findings suggest that the protective effects of these new generation lipid emulsions are explained by other lipid emulsion components that are known to be present in widely different concentrations among these three lipid emulsions, such as n-3 PUFA and vitamin E. Both n-3 PUFA and vitamin E have been shown to be protective in conditions of liver disease and inflammation (31–33). Our findings are in line with studies in infants and children with PNALD in whom the switch from soybean oil-based emulsions to the use of fish oil-based emulsions as rescue therapy also resulted in decreased concentrations of serum markers of liver injury (6, 8, 10, 27). The results provide a compelling rationale to further examine the clinical and metabolic effects of new generation lipid emulsions to establish their safety and optimal nutritional availability in pediatric populations, especially neonatal infants.
Acknowledgments
The authors wish to acknowledge Liwei Cui and the staff of the Texas Medical Center Digestive Disease Center Cores for technical assistance, especially Sundararajah Thevananther, Angela Major, and Pamela Parsons. The authors thank Luciano Adorini, Intercept Pharmaceuticals for the gift of obeticholic acid.
Footnotes
Abbreviations:
- ALT
- alanine aminotransferase
- AST
- aspartate aminotransferase
- BSEP
- bile salt export pump
- CA
- cholic acid
- CDCA
- chenodeoxycholic acid
- C4
- 7α-hydroxy-4-cholesten-3-one
- CYP8B1
- sterol 12-alpha-hydroxylase
- CYP7A1
- cholesterol 7-hydroxylase
- CYP3A29
- cytochrome P450 3A29
- CYP27A1
- sterol 27-hydroxylase
- FGF
- fibroblast growth factor
- FXR
- farnesoid X receptor
- GGT
- gamma glutamyl transferase
- MCT
- medium chain triglyceride
- MRP3
- multidrug resistant protein 3
- NTCP
- Na+/ taurocholate cotransporter
- OBCA
- obeticholic acid
- OSTα/β
- organic solute transporters alpha and beta
- PNALD
- parenteral nutrition-associated liver disease
- TPN
- total parenteral nutrition
- UDCA
- ursodeoxycholic acid
This work was supported in part by federal funds from the USDA, Agricultural Research Service under Cooperative Agreement Number 58-6250-6-001, National Institutes of Health Grant DK-094616 (D.G.B.), the American Society for Parenteral and Enteral Nutrition, and the Texas Medical Center Digestive Diseases Center (National Institutes of Health Grant P30 DK-56338). H. Vlaardingerbroek was supported by a grant obtained from the Ter Meulen Fund of the Royal Netherlands Academy of Arts and Sciences, a grant from the Young Investigator Exchange Program of the International Pediatric Research Foundation, and a Research fellowship grant of the Sophia Kinderziekenhuis Fonds, Rotterdam, The Netherlands. K. Ng was supported by a grant from the American Liver Foundation. Financial disclosure: D. G. Burrin received lipid emulsions donated from Fresenius Kabi for the study.
REFERENCES
- 1.Christensen R. D., Henry E., Wiedmeier S. E., Burnett J., Lambert D. K. 2007. Identifying patients, on the first day of life, at high-risk of developing parenteral nutrition-associated liver disease. J. Perinatol. 27: 284–290 [DOI] [PubMed] [Google Scholar]
- 2.Carter B. A., Shulman R. J. 2007. Mechanisms of disease: update on the molecular etiology and fundamentals of parenteral nutrition associated cholestasis. Nat. Clin. Pract. Gastroenterol. Hepatol. 4: 277–287 [DOI] [PubMed] [Google Scholar]
- 3.Waitzberg D. L., Torrinhas R. S., Jacintho T. M. 2006. New parenteral lipid emulsions for clinical use. JPEN J. Parenter. Enteral Nutr. 30: 351–367 [DOI] [PubMed] [Google Scholar]
- 4.Clayton P. T., Bowron A., Mills K. A., Massoud A., Casteels M., Milla P. J. 1993. Phytosterolemia in children with parenteral nutrition-associated cholestatic liver disease. Gastroenterology. 105: 1806–1813 [DOI] [PubMed] [Google Scholar]
- 5.Carter B. A., Taylor O. A., Prendergast D. R., Zimmerman T. L., Von Furstenberg R., Moore D. D., Karpen S. J. 2007. Stigmasterol, a soy lipid-derived phytosterol, is an antagonist of the bile acid nuclear receptor FXR. Pediatr. Res. 62: 301–306 [DOI] [PubMed] [Google Scholar]
- 6.Colomb V., Jobert-Giraud A., Lacaille F., Goulet O., Fournet J. C., Ricour C. 2000. Role of lipid emulsions in cholestasis associated with long-term parenteral nutrition in children. JPEN J. Parenter. Enteral Nutr. 24: 345–350 [DOI] [PubMed] [Google Scholar]
- 7.Cober M. P., Killu G., Brattain A., Welch K. B., Kunisaki S. M., Teitelbaum D. H. 2012. Intravenous fat emulsions reduction for patients with parenteral nutrition-associated liver disease. J. Pediatr. 160: 421–427 [DOI] [PubMed] [Google Scholar]
- 8.de Meijer V. E., Gura K. M., Le H. D., Meisel J. A., Puder M. 2009. Fish oil-based lipid emulsions prevent and reverse parenteral nutrition-associated liver disease: the Boston experience. JPEN J. Parenter. Enteral Nutr. 33: 541–547 [DOI] [PubMed] [Google Scholar]
- 9.Gura K. M., Lee S., Valim C., Zhou J., Kim S., Modi B. P., Arsenault D. A., Strijbosch R. A., Lopes S., Dugan C., et al. 2008. Safety and efficacy of a fish-oil-based fat emulsion in the treatment of parenteral nutrition-associated liver disease. Pediatrics. 121: e678–e686 [DOI] [PubMed] [Google Scholar]
- 10.Diamond I. R., Sterescu A., Pencharz P. B., Kim J. H., Wales P. W. 2009. Changing the paradigm: omegaven for the treatment of liver failure in pediatric short bowel syndrome. J. Pediatr. Gastroenterol. Nutr. 48: 209–215 [DOI] [PubMed] [Google Scholar]
- 11.Sangild P. T., Petersen Y. M., Schmidt M., Elnif J., Petersen T. K., Buddington R. K., Greisen G., Michaelsen K. F., Burrin D. G. 2002. Preterm birth affects the intestinal response to parenteral and enteral nutrition in newborn pigs. J. Nutr. 132: 3786–3794 [DOI] [PubMed] [Google Scholar]
- 12.Stoll B., Horst D. A., Cui L., Chang X., Ellis K. J., Hadsell D. L., Suryawan A., Kurundkar A., Maheshwari A., Davis T. A., et al. 2010. Chronic parenteral nutrition induces hepatic inflammation, steatosis, and insulin resistance in neonatal pigs. J. Nutr. 140: 2193–2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoll B., Puiman P. J., Cui L., Chang X., Benight N. M., Bauchart-Thevret C., Hartmann B., Holst J. J., Burrin D. G. 2012. Continuous parenteral and enteral nutrition induces metabolic dysfunction in neonatal pigs. JPEN J. Parenter. Enteral Nutr. 36: 538–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puiman P., Stoll B. 2008. Animal models to study neonatal nutrition in humans. Curr. Opin. Clin. Nutr. Metab. Care. 11: 601–606 [DOI] [PubMed] [Google Scholar]
- 15.Koopman B. J., Wolthers B. G., van der Molen J. C., Nagel G. T., Waterreus R. J., Oosterhuis H. J. 1984. Capillary gas chromatographic determinations of urinary bile acids and bile alcohols in CTX patients proving the ineffectivity of ursodeoxycholic acid treatment. Clin. Chim. Acta. 142: 103–111 [DOI] [PubMed] [Google Scholar]
- 16.Bootsma A. H., Overmars H., van Rooij A., van Lint A. E., Wanders R. J., van Gennip A. H., Vreken P. 1999. Rapid analysis of conjugated bile acids in plasma using electrospray tandem mass spectrometry: application for selective screening of peroxisomal disorders. J. Inherit. Metab. Dis. 22: 307–310 [DOI] [PubMed] [Google Scholar]
- 17.Ferdinandusse S., Denis S., Overmars H., Van E. L., Van Veldhoven P. P., Duran M., Wanders R. J., Baes M. 2005. Developmental changes of bile acid composition and conjugation in L- and D-bifunctional protein single and double knockout mice. J. Biol. Chem. 280: 18658–18666 [DOI] [PubMed] [Google Scholar]
- 18.Denk G. U., Maitz S., Wimmer R., Rust C., Invernizzi P., Ferdinandusse S., Kulik W., Fuchsbichler A., Fickert P., Trauner M., et al. 2010. Conjugation is essential for the anticholestatic effect of NorUrsodeoxycholic acid in taurolithocholic acid-induced cholestasis in rat liver. Hepatology. 52: 1758–1768 [DOI] [PubMed] [Google Scholar]
- 19.Steiner C., von Eckardstein A., Rentsch K. M. 2010. Quantification of the 15 major human bile acids and their precursor 7alpha-hydroxy-4-cholesten-3-one in serum by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 878: 2870–2880 [DOI] [PubMed] [Google Scholar]
- 20.Gray M. A., Pollock C. B., Schook L. B., Squires E. J. 2010. Characterization of porcine pregnane X receptor, farnesoid X receptor and their splice variants. Exp. Biol. Med. (Maywood). 235: 718–736 [DOI] [PubMed] [Google Scholar]
- 21.Lefebvre P., Cariou B., Lien F., Kuipers F., Staels B. 2009. Role of bile acids and bile acid receptors in metabolic regulation. Physiol. Rev. 89: 147–191 [DOI] [PubMed] [Google Scholar]
- 22.Kidambi S., Patel S. B. 2008. Cholesterol and non-cholesterol sterol transporters: ABCG5, ABCG8 and NPC1L1: a review. Xenobiotica. 38: 1119–1139 [DOI] [PubMed] [Google Scholar]
- 23.Jain A. K., Stoll B., Burrin D. G., Holst J. J., Moore D. D. 2012. Enteral bile acid treatment improves parenteral nutrition-related liver disease and intestinal mucosal atrophy in neonatal pigs. Am. J. Physiol. Gastrointest. Liver Physiol. 302: G218–G224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pellicciari R., Costantino G., Camaioni E., Sadeghpour B. M., Entrena A., Willson T. M., Fiorucci S., Clerici C., Gioiello A. 2004. Bile acid derivatives as ligands of the farnesoid X receptor. Synthesis, evaluation, and structure-activity relationship of a series of body and side chain modified analogues of chenodeoxycholic acid. J. Med. Chem. 47: 4559–4569 [DOI] [PubMed] [Google Scholar]
- 25.Diamond I. R., Pencharz P. B., Wales P. W. 2009. Omega-3 lipids for intestinal failure associated liver disease. Semin. Pediatr. Surg. 18: 239–245 [DOI] [PubMed] [Google Scholar]
- 26.Kurvinen A., Nissinen M. J., Gylling H., Miettinen T. A., Lampela H., Koivusalo A. I., Rintala R. J., Pakarinen M. P. 2011. Effects of long-term parenteral nutrition on serum lipids, plant sterols, cholesterol metabolism, and liver histology in pediatric intestinal failure. J. Pediatr. Gastroenterol. Nutr. 53: 440–446 [DOI] [PubMed] [Google Scholar]
- 27.Puder M., Valim C., Meisel J. A., Le H. D., de Meijer V. E., Robinson E. M., Zhou J., Duggan C., Gura K. M. 2009. Parenteral fish oil improves outcomes in patients with parenteral nutrition-associated liver injury. Ann. Surg. 250: 395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurvinen A., Nissinen M. J., Andersson S., Korhonen P., Ruuska T., Taimisto M., Kalliomäki M., Lehtonen L., Sankilampi U., Arikoski P., et al. 2012. Parenteral plant sterols and intestinal failure-associated liver disease in neonates. J. Pediatr. Gastroenterol. Nutr. 54: 803–811 [DOI] [PubMed] [Google Scholar]
- 29.Shang Q., Guo G. L., Honda A., Saumoy M., Salen G., Xu G. 2013. FGF15/19 protein levels in the portal blood do not reflect changes in the ileal FGF15/19 or hepatic CYP7A1 mRNA levels. J. Lipid Res. 54: 2606–2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El Kasmi K. C., Anderson A. L., Devereaux M. W., Vue P. M., Zhang W., Setchell K. D. R., Karpen S. J., Sokol R. J. 2013. Phytosterols promote liver injury and Kupffer cell activation in parenteral nutrition-associated liver disease. Sci. Transl. Med. 5: 206ra137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J. Y., Plakidas A., Lee W. H., Heikkinen A., Chanmugam P., Bray G., Hwang D. H. 2003. Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J. Lipid Res. 44: 479–486 [DOI] [PubMed] [Google Scholar]
- 32.Sanyal A. J., Chalasani N., Kowdley K. V., McCullough A., Diehl A. M., Bass N. M., Neuschwander-Tetri B. A., Lavine J. E., Tonascia J., Unalp A., et al. 2010. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 362: 1675–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alwayn I. P., Gura K., Nosé V., Zausche B., Javid P., Garza J., Verbesey J., Voss S., Ollero M., Andersson A., et al. 2005. Omega-3 fatty acid supplementation prevents hepatic steatosis in a murine model of nonalcoholic fatty liver disease. Pediatr. Res. 57: 445–452 [DOI] [PubMed] [Google Scholar]