Abstract
Long-chain PUFAs (LCPUFAs) occur in foods primarily in the natural lipid classes, triacylglycerols (TAGs) or phospholipids (PLs). We studied the relative efficacy of the neural omega-3 DHA provided in formula to growing piglets as a dose of 13C-DHA bound to either TAG or phosphatidylcholine (PC). Piglets were assigned to identical formula-based diets from early life and provided with TAG-13C-DHA or PC-13C-DHA orally at 16 days. Days later, piglet organs were analyzed for 13C-DHA and other FA metabolites. PC-13C-DHA was 1.9-fold more efficacious for brain gray matter DHA accretion than TAG-13C-DHA, and was similarly more efficacious in gray matter synaptosomes, retina, liver, and red blood cells (RBCs). Liver labeling was greatest, implying initial processing in that organ followed by export to other organs, and suggesting that transfer from gut to bloodstream to liver in part drove the difference in relative efficacy for tissue accretion. Apparent retroconversion to 22:5n-3 was more than double for PC-13C-DHA and was more prominent in neural tissue than in liver or RBCs. These data directly support greater efficacy for PC as a carrier for LCPUFAs compared with TAG, consistent with previous studies of arachidonic acid and DHA measured in other species.
Keywords: docosahexaenoic acid, triacylglycerol, phosphatidylcholine, nutrition
DHA is the most abundant omega-3 FA in the mammalian brain (1), accounting for 8–14% of FAs perinatally in primates (2) and humans (3, 4). In humans, DHA accumulates at an accelerating rate from mid-gestation reaching an inflection point during the first months after birth (5) and continuing well past the plateau of brain weight to reach a plateau around 18 years of age and remain stable to the end of life (6).
DHA can be synthesized de novo by term and preterm human infants from precursor omega-3 FAs, α-linolenic acid, or EPA (7–9), but the process is generally considered to be inefficient, also challenged by current dietary intakes of omega-6 linoleic acid at relatively high levels (10, 11). Direct measurement of percent incorporation of DHA into the primate brain from precursor α-linolenic acid indicates that it is 7-fold less efficient than preformed DHA. This finding is in contrast to that for arachidonic acid (ARA), present in the brain at similar/comparable levels but not strongly affected by dietary preformed ARA (12). Numerous human studies have shown efficacy of dietary preformed DHA to support brain and visual development (13), and follow-up studies suggest that preformed DHA intake in infancy provides long-term benefits on specific tests of higher cognitive function, although it is widely agreed that no effects can be seen using the Bayley scales, a standard test for normalcy (14).
While all preformed DHA is structurally identical, in human foods and food supplements DHA is normally esterified to one of three lipid classes as molecular carriers, triacylglycerols (TAGs), phospholipids (PLs), or ethyl esters. In primate milk, including human breast milk, DHA content is higher in PLs than in TAGs, but the bulk of DHA is still delivered via TAGs because of the overwhelming prevalence of TAGs over PLs (15). TAG-DHA is a common molecular form of DHA used as an ingredient in infant formula. Ongoing development of alternative sources of DHA has identified components potentially suitable for use in infant formulas that use PL-DHA. PL-DHA- and PL-ARA-supplemented infant formula has been studied since the 1990s (16, 17), and infant formula products were also widely available on the European market before this market expanded and changed for single-cell- and fish oil-based sources of TAG-bound ARA and DHA.
Once hydrolyzed from their esterified molecular carrier, DHA and ARA may be handled identically by normal metabolic processes. In vivo and in vitro studies show that pancreatic lipase hydrolyzes TAG in the sn-1 and sn-3 positions consistent with the survival of the predominantly saturated sn-2 FA of breast milk postabsorption (18, 19), while intestinal phospholipases in the rat hydrolyze in the sn-2 position leaving the predominantly saturated sn-1 position intact (20). Positional specificity of intestinal glycerolipid hydrolysis insures that the postabsorptive reassembled TAGs and PLs will have a nonrandomized distribution of FAs, though sn-1/3 FAs hydrolyzed by intestinal lipases and phospholipases are reesterified in a random fashion onto TAGs and PLs postabsorption.
Positional specificity may be metabolically relevant for subsequent metabolism, as FAs are incorporated into membranes, oxidized, or excreted on the skin. Previously we showed by 13C labeling that ARA-PL, specifically as ARA-phosphatidylcholine (PC), when added to artificial formula is about twice as efficacious as a substrate for supplying ARA to the brain as ARA-TAG in neonatal baboons (21). We report here a similar study in neonatal piglets using 13C-DHA, specifically investigating efficacy for supplying the gray matter of the cerebral cortex with DHA.
MATERIALS AND METHODS
Chemical
Uniformly labeled DHA (PERlabeled, [U-13C]DHA) was prepared in our labs using published methods (22) and esterified into lipid classes by Avanti Polar Lipids (Alabaster, AL). The TAG tracer (TAG-13C-DHA) had 13C-DHA in the sn-2 position with unlabeled 16:0 in the sn-1 and sn-3 positions. The PL tracer used was specifically PC with 13C-DHA in the sn-2 position and unlabeled 16:0 in the sn-1 position (PC-13C-DHA). Solvents for tissue lipid extraction were HPLC grade from Sigma-Aldrich (St. Louis, MO) or Burdick and Jackson (Muskegon, MI).
Animals and diet
The overall study and all procedures involving live piglets were approved by the Institutional Animal Care and Use Committee at Cornell University. Sows (Yorkshire and Landrace cross) were bred with Hampshire boars. After birth, piglets were left to nurse with the sow for at least 48 h. Twenty piglets, 2 to 4 days of age, were chosen for the study based on the following criteria: weight (about 2.0 kg), gender (10 males and 10 females), health (active, apparently normal, and not the smallest animal in the litter), and maternal sow. Piglets were given a unique number/color combination identifier and then were transported to the Large Animal Research and Teaching Unit at Cornell University.
At the study site, piglets were housed in individual stainless steel cages and were placed on a single, fully balanced, commercially available young farm animal milk replacer formula immediately upon arrival.
The FA profile of the milk replacer (piglet milk formula) is presented in Table 1. DHA was present in low levels in the form of TAG (0.02%, w/w) and PL (0.11%, w/w). TAG, PL, and related DHA measurements are presented in Table 2, and when appropriate tabulated for a 500 ml feeding used with the dose. As with human milk and human infant formulas, total energy from fat was about 47% and mostly in the form of TAG (94%). Despite the low absolute DHA concentration in TAG, the predominant mass of TAG over PL provided 73% of total DHA compared with 27% from PL, in line with human milk and other mammalian milks.
TABLE 1.
FA profile of piglet milk formula (replacer)
| TAG [% (w/w)] | PL [% (w/w)] | |
| Saturated FA | 42.32 | 55.84 |
| Branched/CLAa | 0.67 | 2.90 |
| Monoene FA | 41.14 | 28.71 |
| 18:2n-6 | 14.12 | 7.35 |
| 20:2n-6 | 0.57 | 0.34 |
| 20:3n-6 | 0.10 | 0.95 |
| 20:4n-6 | 0.25 | 2.63 |
| 22:4n-6 | 0.10 | 0.12 |
| 18:3n-3 | 0.53 | 0.69 |
| 20:3n-3 | 0.12 | 0.00 |
| 22:5n-3 | 0.04 | 0.38 |
| 22:6n-3 | 0.02 | 0.11 |
Indicative of ruminant fat.
TABLE 2.
Lipid and DHA distribution in piglet milk replacer
| TAG | PL | |
| Grams of FA per 500 ml | 23.9 | 1.6 |
| Approximate energy from FA (%) | 43 | 4 |
| FA (%) | 94 | 6 |
| Milligrams of DHA per 500 ml | 4.8 | 1.8 |
| DHA (%) | 73 | 27 |
Fresh formula was provided every 6 h for the first week and every 8 h thereafter in individual troughs as in our previous studies (23, 24). All piglets had free access to fresh water. Formula consumption for each group was recorded daily for the first week to insure the piglets thrived, and was monitored every third day thereafter. Piglet body weight was recorded every other day in the first week and every third day thereafter. Home cage enrichment included bedding, contact with other piglets, positive human contact, and rubber toys.
Dose and sampling
On day 16 of life, 16 robust piglets out of a total set of 20 piglets were assigned to two dosing groups, PC and TAG, balanced with respect to gender, weight, and age but otherwise distributed randomly. Group PC was orally dosed with PC-13C-DHA as described below. Group TAG was dosed with TAG-13C-DHA. The four smallest piglets were used as natural abundance isotopic controls and did not receive a dose but were otherwise treated identically to the other animals.
Doses were prepared by dissolving CHCl3 solutions of PC-13C-DHA or TAG-13C-DHA in about 1 ml of refined olive oil and driving off the CHCl3 by gentle heating under flowing dry N2 over 4 h. The resulting olive oils were carefully placed on the surface of a few milliliters of reconstituted piglet formula and sonicated until homogeneous. The resulting labeled formula was split gravimetrically into 1–2 ml aliquots and administered orally to the piglets via syringe. Labeled formula was withdrawn by syringe from the vials which were subsequently washed several times with unlabeled formula, which was then also administered to the piglets at the beginning of a normal feeding time. Piglets were therefore hungry but could be considered as fasted insofar as their normal meal interval was concerned. Care was taken to insure all of the labeled formula and the washes, consisting of a total of up to 10 ml, were swallowed by the piglets. Piglets were then provided with their normal meal of 500 ml milk replacer which they routinely consumed completely. Doses to piglets corresponded to about 20 mg 13C-DHA delivered in PC and 86 mg of 13C-DHA delivered in TAG, and the difference was only due to the amount of each labeled tracer that was available.
Six days after the receiving the labeled DHA dose and in total 20 days of formula and 22–24 days of life, all piglets were euthanized by exsanguination under anesthesia on a single day. Blood for FA analysis was collected in tubes with EDTA and spun to prepare red blood cells (RBCs). The surface few millimeters of the gray matter on the cerebral cortex superior surface was rapidly collected at necropsy. A piece approximately 5 × 10 × 3 mm was immediately used for preparation of synaptosomes, and the remaining gray matter was snap-frozen in liquid nitrogen for FA analysis. Retina, heart, liver, biceps, and femoris muscle were also harvested, and all tissues were snap-frozen and stored at −80°C until prepared for analysis.
Synaptosomes were prepared using Syn-PER synaptic protein extraction reagent (Thermo Scientific, Waltham, MA) according to manufacturer's instructions. Briefly, 1 ml of Syn-PER reagent was added to ∼100 mg brain tissue sample. Samples were homogenized and the homogenate centrifuged at 1,200 g for 10 min. The supernatant was collected and further centrifuged at 15,000 g for 20 min. Synaptosomes were recovered from the pellet and lipids were immediately extracted as described below.
Lipid extraction and analysis
Total lipids were extracted from samples of brain gray matter, the synaptosome pellet, liver, heart, and retina. Samples were simultaneously digested and FA methyl esters (FAMEs) prepared using a one step method as described in detail previously (25). For plasma and erythrocytes, the Bligh and Dyer method (26) was employed to extract total lipids and FAMEs were prepared using 14% BF3 in methanol. A known quantity of freshly prepared heptadecanoic acid in chloroform (99% pure, Sigma Chemical) was added as an internal standard to tissue samples just before extraction. FAMEs were dissolved in heptane and stored at −20°C until analysis.
FAMEs were analyzed using a Hewlett Packard 5890 series II GC-flame ionization detector with a BPX 70 column (60 m, 0.32 mm inner diameter, 0.25 μm film; Hewlett Packard, Palo Alto, CA) and H2 as carrier gas. Quantitative profiles were calculated using the internal standard and an equal weight FAME mixture to derive response factors for each FA. GC-flame ionization detector conditions and calibration details have been reported (21).
13C-DHA analysis
Tracer analysis for 13C-DHA was performed on the FAME mixtures using similar GC column conditions as for the quantitative analysis, as has been described in detail previously (27). Instrumentation for tracer analysis was an Agilent 6890 gas chromatograph coupled to a combustion furnace interface, and to a Thermo Scientific 253 isotope ratio mass spectrometer. FAMEs eluted from the gas chromatograph were combusted to CO2, dried, and admitted to the isotope ratio mass spectrometer. Data processing was as described previously (21). Isotope ratios in the conventional high precision notation, δ13C [defined previously (27)], were converted to fraction of 13C. For each FA, the mean isotope ratio of the control group was subtracted from the isotope ratio of the means for the enriched groups to yield an atom fraction enrichment, which was subsequently converted to %Dose, which reflects the appearance of tracer in the specific pool. The primary outcome was a relative comparison of the %Dose appearing in the brain gray matter for TAGs and PLs, respectively. Total %Dose found in liver and retina was calculated directly from their respective weights. The total labeled DHA in cerebral gray matter and RBCs was estimated based on brain weight, and using the relative amount of gray matter in the brain as 60%, estimated from human imaging data (28). For RBCs, the blood volume was estimated as 8.5% of body weight (29) and hematocrit was about 35%. For gray matter synaptosomes, we did not attempt to estimate the total amount and normalized the %Dose to the highest value found. In all cases, estimated masses apply to both experimental groups and cancel in the primary and secondary outcome calculations, and thus do not affect the final results. Detailed calculations have been previously presented (21).
Statistics
Primary outcome.
The primary outcome was the relative %Dose of 13C-DHA found in the gray matter of the cerebral cortex in the PC-13C-DHA- versus the TAG-13C-DHA-dosed groups. The %Dose in the two dosing groups was tested for equivalence by one-way ANOVA with P < 0.05 considered significant.
Secondary outcomes.
Total unlabeled FAs in the various pools were compared in a pairwise manner in the two dosing groups and were not significantly different, and were therefore pooled. Because these two groups were fed the same formulas and treated identically except for a few milligram doses, no differences were expected based on treatment.
Relative 13C-DHA %Doses for synaptosomes, retina, liver, and RBCs were compared for similarity to the primary outcome. Relative meal-wise amounts of total DHA delivered in TAG and PL were calculated from the determination of relative amounts of unlabeled DHA in formula in a 500 ml meal.
RESULTS
Piglets in both dosing groups grew at comparable rates and attained nonsignificantly different final weights of 9.4 ± 0.3 kg and 9.1 ± 0.4 kg for the PC- and TAG-dosed groups, respectively. The isotopic control groups were purposely chosen as the smallest animals but grew in parallel to the other groups, starting at about 6% lower body weight and finishing at about 12% lower weight. These animals were expected to yield accurate estimates of baseline isotope ratios, which were applied identically to both experimental groups.
The FA profile of neural tissue is presented in Table 3. Retina was richest in DHA (22:6) at 12%, followed by gray matter (8.3%), and white matter (5.2%). Docosapentaenoic acid (22:5n-6), the DHA analog among omega-6 FAs, mean value was 9.7% in gray matter, and thus slightly greater than DHA. Domestic piglets have long been considered to require small amounts of linoleic acid, and the n-3 incidental to practical diets is sufficient to support rapid growth (30). These levels are apparently insufficient to enable DHA levels similar to wild animals (1), though they are apparently developing normally.
TABLE 3.
FA composition of neonatal piglet tissues (pooled)
| FA | Gray Matter | White Matter | GM Synaptosomes | Retina | Liver | RBCs |
| SFA | ||||||
| 16:0 | 20.45 ± 0.67 | 19.04 ± 1.03 | 22.49 ± 0.68 | 20.42 ± 0.78 | 17.00 ± 0.65 | 23.07 ± 1.47 |
| 18:0 | 20.05 ± 0.40 | 18.48 ± 0.76 | 18.06 ± 0.27 | 21.85 ± 0.72 | 24.04 ± 0.84 | 15.97 ± 0.75 |
| ∑SFA | 41.95 ± 1.00 | 39.29 ± 1.53 | 42.83 ± 1.06 | 44.67 ± 1.05 | 42.22 ± 0.44 | 41.42 ± 2.10 |
| MUFA | ||||||
| 16:1 | 1.18 ± 0.15 | 2.85 ± 0.48 | 2.26 ± 0.22 | 1.64 ± 0.13 | 1.10 ± 0.13 | 1.02 ± 0.19 |
| 18:1 | 15.51 ± 0.75 | 25.77 ± 3.25 | 15.35 ± 0.55 | 16.74 ± 0.67 | 12.67 ± 0.68 | 34.36 ± 1.75 |
| 20:1 | 0.47 ± 0.08 | 1.50 ± 0.45 | 0.41 ± 0.09 | 0.30 ± 0.07 | 0.65 ± 0.19 | 0.34 ± 0.28 |
| ∑MUFA | 18.12 ± 0.83 | 30.66 ± 4.01 | 18.28 ± 0.68 | 19.00 ± 0.73 | 14.50 ± 0.78 | 36.28 ± 1.70 |
| n-6 PUFA | ||||||
| ‘18:2n-6 | 1.15 ± 0.07 | 1.23 ± 0.14 | 1.09 ± 0.11 | 2.29 ± 0.25 | 14.05 ± 0.79 | 15.40 ± 1.45 |
| 18:3n-6 | 0.09 ± 0.14 | 0.11 ± 0.03 | 0.11 ± 0.06 | 0.23 ± 0.10 | 0.29 ± 0.11 | 0.15 ± 0.07 |
| ‘20:4n-6 | 11.15 ± 0.37 | 9.57 ± 0.74 | 9.77 ± 0.34 | 9.37 ± 0.47 | 17.19 ± 0.96 | 3.23 ± 1.23 |
| ‘22:4n-6 | 6.64 ± 0.61 | 6.88 ± 1.00 | 6.97 ± 0.55 | 4.33 ± 0.28 | 2.16 ± 0.23 | 0.75 ± 0.29 |
| ‘22:5n-6 | 9.69 ± 0.73 | 3.68 ± 0.89 | 9.47 ± 0.59 | 5.67 ± 0.72 | 3.11 ± 0.57 | 0.45 ± 0.17 |
| ∑n-6 PUFA | 29.92 ± 0.97 | 22.78 ± 2.28 | 28.65 ± 1.02 | 22.91 ± 1.16 | 38.75 ± 0.60 | 21.00 ± 2.65 |
| n-3 PUFA | ||||||
| 18:3n-3 | 0.05 ± 0.03 | 0.08 ± 0.04 | 0.13 ± 0.08 | 0.10 ± 0.09 | 0.09 ± 0.07 | 0.12 ± 0.05 |
| ‘22:5n-3 | 0.54 ± 0.17 | 0.35 ± 0.07 | 0.50 ± 0.07 | 0.74 ± 0.06 | 1.51 ± 0.17 | 0.48 ± 0.24 |
| ‘22:6n-3 | 8.32 ± 0.68 | 5.21 ± 1.01 | 8.16 ± 0.65 | 12.02 ± 0.83 | 2.44 ± 0.23 | 0.47 ± 0.17 |
| ∑n-3 PUFA | 8.93 ± 0.77 | 5.82 ± 1.03 | 8.86 ± 0.65 | 12.87 ± 0.82 | 4.04 ± 0.32 | 1.06 ± 0.39 |
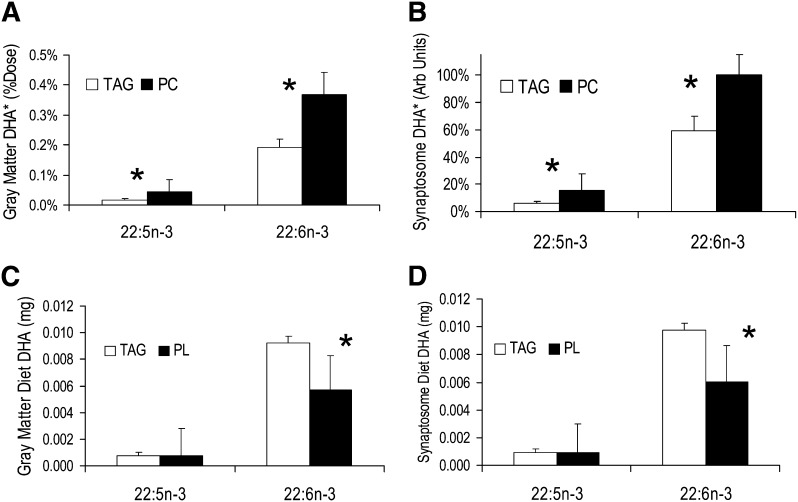
Results for the primary outcome in gray matter and gray matter synaptosomes are presented in Fig. 1. 13C label was detected only for DHA and 22:5n-3 (DPAn-3) among all FAs. The %Dose detected in cerebral cortex gray matter in the 13C-DHA-PC-dosed animals was about 0.4%, greater than that for the TAG dose. 13C-DHA from the TAG dose was about half this value, thus dietary PC was 1.9-fold more efficacious to supply DHA to the developing piglet brain compared with TAG. Results in gray matter synaptosomes, also shown in Fig. 1, were consistent with the relative values. The ratio of relative accretion of labeled DHA in the synaptosomes was 1.7-fold greater for PC than for TAG.
Fig. 1.
Results for primary outcome, cerebral cortex gray matter. A: DHA* (13C-22:6n-3) and DPAn-3* (13C-22:5n-3) expressed as %Dose found in gray matter. PC was 1.9-fold more efficacious for supplying DHA to the neonatal piglet cortex than TAG. The ratio of efficacy was 2.8 for the retroconversion product DPAn-3. B: 13C FAs found in gray matter synaptosomes normalized to the 13C-DHA-PC value. The ratio of efficacies for DHA and DPAn-3 in synaptosomes was 1.7 and 2.8, respectively. The mass of unlabeled (tracee) DHA and DPAn-3 from a 500 ml meal from PC and TAG for gray matter (C) and synaptosomes (D). TAG is favored because it predominates in formula; no difference was found for DPAn-3. Unlabeled material is calculated from the tracer percentages and concentrations of tracee DHA in formula. *P < 0.05 between adjacent white and black (TAG and PC) bars.
The retroconversion product, DPAn-3, was labeled below 0.05%Dose for the PC-dosed group and less than half of that for the TAG-dosed group. The relative efficacy of PC over TAG for the retroconversion of DHA to DPAn-3 was about 2.8. For gray matter synaptosomes, the superiority of PC over TAG was the same with a relative efficacy of 2.8. Gray matter and synaptosome 13C-DHA were approximately 10-fold greater than 13C-DPA.
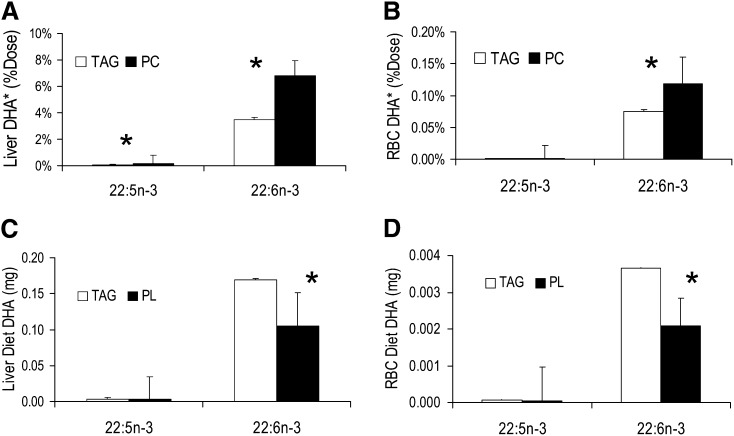
Figure 2 presents the results for liver and RBCs. Liver retained 6.8% of the PC 13C-dose compared with 3.5% of the TAG dose at 6 days postdose. The relative efficacy was 1.9, similar to gray matter and synaptosomes. The ratio of 13C-DHA to 13C-DPA was about 50, substantially greater than found for gray matter. RBC relative labeling paralleled liver levels, with PC-derived 13C-DHA 1.6-fold that of TAG-derived 13C-DHA, and a similar 50-fold dominance of 13C-DHA over 13C-DPA.
Fig. 2.
Liver and RBC tracer and tracee. A: 13C-DHA in liver from PC and TAG. Liver retained about 7% of the DHA from the PC dose at 6 days compared with about 3.8% for the TAG dose, corresponding to a relative efficacy of about 1.9; PC-derived DPAn-3 was significantly greater than TAG-derived DPAn-3, with a relative efficacy of 2.6. B: Similar relative efficacy was found for RBC DHA (1.6); RBC DPAn-3 was not significantly different. C, D: The mass (in milligrams) of DHA and DPAn-3 derived from unlabeled (tracee) TAG in the 500 ml meal predominated in whole liver and all RBCs due to the relative amounts of tracee in the respective pools. Liver weight was as measured at necropsy and RBC mass was estimated as described in the Materials and Methods. *P < 0.05 between adjacent white and black (TAG and PC) bars.
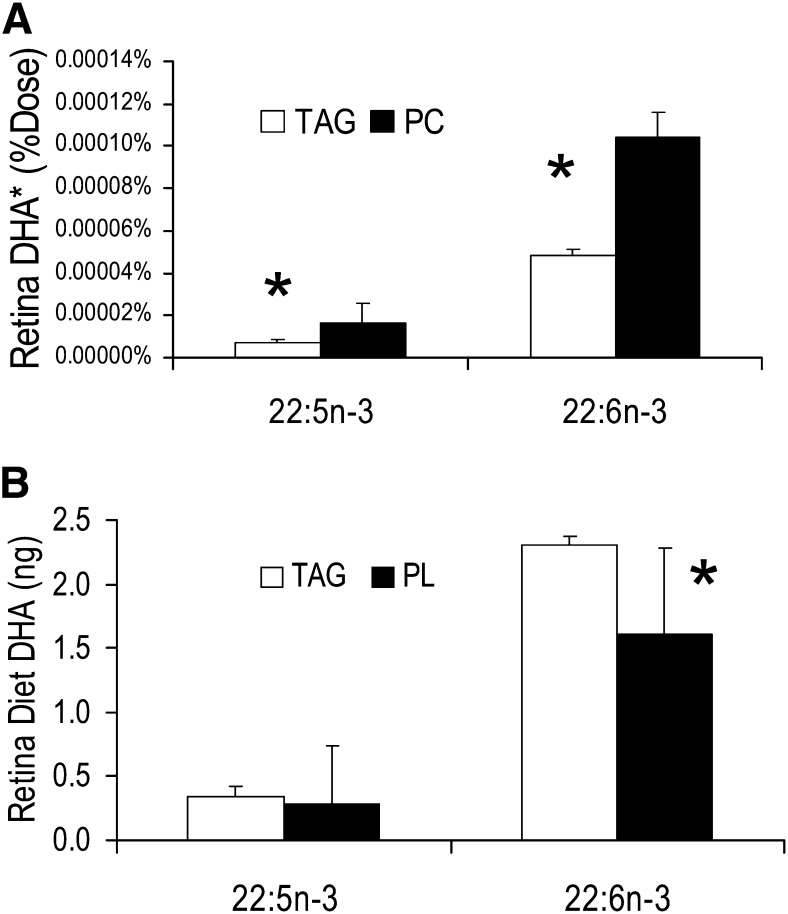
Figure 3 presents the results for retinal tissue. Relative efficacy of PC over TAG was 2.2-fold for DHA, and about 2.4-fold for DPAn-3, all similar and consistent with results in the other tissues examined. Consistent with the brain gray matter but in contrast to the liver, 13C-DHA was 6.3-fold greater in the retina than the retroconversion product 13C-DPAn-3, again suggesting that retroconversion took place outside the liver and possibly in the retina. As seen before, TAG predominated as the source of retinal DHA.
Fig. 3.
Retina. A: Relative efficacy of PC compared with TAG was about 2.2 for DHA and 2.4 for DPAn-3. B: More retinal DHA was derived from diet TAG than PC. *P < 0.05 between adjacent white and black (TAG and PC) bars.
DISCUSSION
Our study investigated the relative efficacy of dietary DHA carried by PC compared with DHA carried by TAG. The results show that dietary DHA carried as PC is more efficiently used for brain gray matter DHA accretion than when carried as TAG. The data in isolated white matter synaptosomes are consistent with those for whole gray matter, and match results obtained in other tissues. Moreover, the data is quantitatively consistent with our previous results using a similar approach to study ARA membrane incorporation in neonatal baboons (21). The similarity of these findings with respect to specific long-chain PUFAs (LCPUFAs) (ARA or DHA), various species and different laboratories, supports the conclusion that LCPUFAs carried as PLs are utilized differently and more efficiently deliver LCPUFAs as source of membrane components than when provided as TAG.
Despite the well-recognized importance of brain DHA accretion, transport forms of DHA into the brain are not fully understood. Deletion of CD36, an unesterified FA binding protein, in mice does not reduce brain DHA (31). Similar results were obtained for VLDL receptor-null mice (32). Consistent with the present data, albumin-bound sn-1-lyso-2-DHA-PC is more efficiently incorporated into brain PLs in the rat compared with unesterified DHA (33, 34). In our study, 1-lyso-2-13C-DHA-PC can be synthesized by a phospholipase A1 action on 13C-DHA-PC, recently proposed to be mediated by an endothelial lipase (35), analogous to results suggesting extensive phospholipase A1 activity mediated by hepatic lipase (36). This pathway would not be available to DHA-TAG, and could at least in part mediate some of our whole body results. A very recent report shows that mice expressing human apoE4 take up less unesterified DHA and have lower brain DHA compared with mice expressing apoE2 or apoE3, suggesting a mechanism involving free DHA as well (37). A DHA-specific lysophosphatidic acid acyl transferase that may mediate specific transfer of unesterified DHA into phospholipids was also recently reported (38).
The majority of dietary DHA was carried as TAGs because of its greater proportion of DHA compared with PLs in the formula (piglet milk replacer) (Figs. 1C, D;2C, D;3BFigs. 1C, D; 2C, D; 3B). Qualitatively, this is comparable with the composition of human/mammalian milks of other species. However, the amount of DHA in our piglet formula was very low, probably only being a minor component of bovine milk protein as base of the formula. Variation in human milk DHA is over at least an order of magnitude, from 0.1% to over 1% of FAs (39), in apparently well-nourished mothers and is strongly related to the mother's DHA intake (13). The lowest DHA content of human milk of which we are aware has been reported in resource-limited northern Sudanese mothers with carbohydrate rich diets (0.06%, w/w) (40). Because of the low dietary DHA, our study is more similar to previous studies conducted with LCPUFA-free control formulas reported until the early years of the present century compared with recent studies comparing formulas with various DHAs. However, the relative advantage of about 1.9-fold for PC-DHA compared with TAG-DHA applies closely to current formulas because it is relatively unaffected by overall formula DHA levels and reflects the handling of a single physiological dose of DHA; it should therefore apply also to greater formula DHA levels as have been investigated in primates and humans (12, 41).
Liver 13C-DHA was about 20-fold greater overall than the 13C-DHA in the gray matter, in contrast to the results reported previously for the baboon neonate (21). One explanation for this effect is likely to be a species difference in the relative mass of the organs, which in the baboon is near 10% of body weight, while in our piglets was only 0.57 ± 0.04% of body weight. In absolute terms, relative sizes of the different organs are relevant: the piglet gray matter represented about 29 g while the liver was about 321 g. Even with these considerations, the relative efficacy of 13C-DHA from PC and TAG was similar to that reported for 20:4n-6, ARA, in the baboon (21).
The retroconversion product DPAn-3, though also almost 3-fold greater in the liver for PC compared with TAG, was a smaller proportion of the liver 13C-DHA than found in gray matter. In gray matter, 13C-DHA was about 8-fold greater than 13C-DPA while in liver the 13C-DHA was 40-fold greater. If DPAn-3 had been synthesized in the liver and transported to the brain, similar ratios would be expected. These observations suggest that retroconversion takes place in organs other than the liver and may well be more active in the brain than in the liver.
The piglet has long been used as a model for infant nutrition because of its similar metabolism to humans, and in numerous studies it has been used to investigate the aspects of LCPUFA delivery from TAGs versus PLs [e.g., (42, 43)]. Piglet growth is several-fold more rapid than for human infants, thus also reflecting several fold greater intake and exposure to test substances than human infants (44). Others have shown that piglet lipoprotein characteristics depend on the source of DHA, whether TAG oil or egg PLs (45, 46), a mechanism that may have been operating in our piglets to influence the relative amounts captured by the liver. Notably, the major difference in 13C-DHA among the measured pools was in total labeling, with liver enrichment much greater than RBCs or neural tissue (%Dose in all figures). For instance, liver PC-13C-DHA was about 7%Dose while cerebral gray matter %Dose was about 0.4%. These results are consistent with the liver's central role as a processor/repackager of PUFAs including DHA, and that the form of DHA emerging from the lymph was of key importance. Within an organ, the relative efficacy is expected to accurately reflect human metabolism.
PLs as a carriers of LCPUFAs became of interest for infant nutrition in the 1990s with the introduction of sources that provided LCPUFAs in PL form, and the recognition that, although PLs are richer in LCPUFAs than TAGs, the vast bulk of LCPUFAs in breast milk is carried by TAGs because of their relative abundance (21). More recently, interest in the potential efficacy of PLs to deliver DHA has become of renewed interest also for use in adults with the widespread availability of krill oil. Krill oil carries greater than one-third of its high level of EPA and DHA as PLs (47), though notably, a recent report notes that about 22% of the EPA and DHA in one krill oil product is in the form of free FAs, which is at least several fold greater than normally found in foods or supplements (48). In the first day postdose, the timing and peak of blood levels are generally greater when ingested as free FAs, and similar to those of TAGs (49, 50) and/or as PLs compared with ethyl esters. However, these short-term kinetics may not correspond to long-term differences in DHA status as measured in human blood pools (48). Notably, however, blood FA status reflects FAs in circulation and does not address the uptake and incorporation of specific FAs into target tissues, which can be measured directly with isotopically labeled precursors in animal models. The greater efficacy of PC compared with TAG as a carrier for ARA found with 13C-precursors in previous studies (21, 51) led to speculation that the same would be the case for DHA. Others have considered the relative efficacy of labeled DHA as carried by TAG and PC in the rat (52). In a previous study with the radiolabeled (14C) version of the same lipids we considered here, PC-DHA was twice as efficacious for brain DHA accretion as TAG-DHA at 10 weeks but not earlier. The peak of the rat brain growth spurt is maximal between 1 and 2 weeks (53), and accordingly the rat is long past the developmental time when the brain is growing. It is possible, however, that rat brain DHA may continue past the time when the brain ceases to increase in weight, similar to humans (6). The brains of our piglets were certainly increasing in weight during the period of our study and indicate that the advantage of PC is found perinatally. The pig is generally considered a good model for human brain development because it is among the few experimental animals with a brain growth spurt similar to humans (53), and it is a large nonruminant omnivore.
Our data also show apparent retroconversion of DHA to 13C-22:5n-3, and a parallel advantage for PC. No label appeared in 20:5n-3, indicating chain shortening to 20:5n-3 was found in contrast to results in humans (54). Retroconversion in this context refers to the flow of 13C label from its original molecular form, 22:6n-3, to the more saturated 22:5n-3. Our measurements do not indicate the location of the labeling, whether all 22 carbons are labeled or possibly that 22:6n-3 might be β-oxidized to acetate and used for new synthesis of 22:5n-3 from 18:3n-3 or 20:5n-3. We did not find labeling in 20:5n-3, which would have been expected if 18:3n-3 was elongated with labeled acetate, and no evidence of label in other FAs, suggesting that the acetate pool was not a factor. In humans, 22:6n-3 β-oxidation is low compared with other FAs (55). These considerations all suggest that the label in 22:5n-3 did not proceed via acetate.
The pathway by which this transition occurs is not clear. The last few steps of the widely accepted coupled mitochondrial-peroxisomal pathway of 22:6n-3 synthesis proceed as (20:5→22:5→24:5→24:6)→22:6, where all but the last step occur on the endoplasmic reticulum (ER), and 24:6 is transported to the peroxisomes for one round of β-oxidation (56), the latter of which is a four step FAD- and NAD-dependent process. Retroconversion in the sense of reversal of this pathway would require entry of 22:6n-3 into peroxisomes for an energetically and entropically expensive reversal of β-oxidation to yield 24:6n-3, followed by transport to the ER for a reversal of 6-desaturation and elongation, another four step process. Conventional elongation of 22:6n-3 to 24:6n-3 in the ER is a known process and is therefore the simplest explanation for a first (set of) step(s), but it would then require reversal of desaturation and presumably β-oxidation of the resulting product, as 24:6→24:5→22:5. Direct conversion of 22:6n-3 to 22:5n-3 would require the equivalent of a reversal of Δ4-desaturation in one step and is the most parsimonious explanation. Further studies are required to establish the molecular details of this pathway, which have been hypothesized (57), and which operate in numerous species including vertebrate fish (58).
Gray matter DPA was greater than reported in other species, and DHA lower (Table 3). Gray matter DHA in normal neonatal baboons has recently been found to be around 12–14%, while DPA is between 1 and 2% (2). DPA is well-known to rise in neural tissue when omega-3 FAs are limited in the diet and with omega-6 being more abundant. It has been used as an index of relative omega-3 deficiency (59). In well-nourished normally growing piglets, the relatively high amount of DPA indicates active demand for highly unsaturated FAs and presumably a maximally upregulated pathway for their biosynthesis. The sum of DHA and DPA in gray matter was 18% (w/w), similar to that for normal primates of about 16% (w/w). White matter consisting of a large proportion of mostly saturated myelin has 5.2% DHA and 3.7% DPA, suggesting that the DHA supply more adequately satisfies the demand in white matter. Although retinal DHA concentration is high, the total retinal mass is small and the total DHA required is correspondingly small. We previously showed that small neural structures normalize DHA levels at lower dietary concentrations of DHA than the very large cerebral cortex in primates (2); these data are thus consistent.
In summary, stable isotope tracer doses of DHA bound in the sn-2 position of PC were 1.9-fold more efficacious for supply of cerebral cortex gray matter than DHA bound to the sn-2 position of TAG. These data are generally consistent with numerous previous measurements on other LCPUFAs and on DHA studied in other species. Together with previous work, the results indicate that PC is a highly efficacious source of both DHA and ARA, the two major LCPUFAs of human breast milk (60).
Acknowledgments
The authors thank Jiyao Zhang and James Zhang for technical assistance.
Footnotes
Abbreviations:
- ARA
- arachidonic acid
- DPA
- docosapentaenoic acid
- ER
- endoplasmic reticulum
- FAME
- FA methyl ester
- LCPUFA
- long-chain PUFA
- PC
- phosphatidylcholine
- RBC
- red blood cell
- TAG
- triacylglycerol
This study was financially supported by Nutricia Research and BNL Foods. N.B. and J.B. are employees of Nutricia Research; H.V.D. is an employee of BNL Foods, a producer of egg phospholipids. The sponsors were involved in the design of the study and discussion of the results but had no influence on experimental execution and result reporting. All other authors declare no conflicts of interest.
REFERENCES
- 1.Crawford M. A., Casperd N. M., Sinclair A. J. 1976. The long chain metabolites of linoleic avid linolenic acids in liver and brain in herbivores and carnivores. Comp. Biochem. Physiol. B. 54: 395–401 [DOI] [PubMed] [Google Scholar]
- 2.Diau G. Y., Hsieh A. T., Sarkadi-Nagy E. A., Wijendran V., Nathanielsz P. W., Brenna J. T. 2005. The influence of long chain polyunsaturate supplementation on docosahexaenoic acid and arachidonic acid in baboon neonate central nervous system. BMC Med. 3: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makrides M., Neumann M. A., Byard R. W., Simmer K., Gibson R. A. 1994. Fatty acid composition of brain, retina, and erythrocytes in breast- and formula-fed infants. Am. J. Clin. Nutr. 60: 189–194 [DOI] [PubMed] [Google Scholar]
- 4.Farquharson J., Cockburn F., Patrick W. A., Jamieson E. C., Logan R. W. 1992. Infant cerebral cortex phospholipid fatty-acid composition and diet. [see comments] Lancet. 340: 810–813 [DOI] [PubMed] [Google Scholar]
- 5.Martinez M. 1992. Tissue levels of polyunsaturated fatty acids during early human development. J. Pediatr. 120: S129–S138 [DOI] [PubMed] [Google Scholar]
- 6.Carver J. D., Benford V. J., Han B., Cantor A. B. 2001. The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects. Brain Res. Bull. 56: 79–85 [DOI] [PubMed] [Google Scholar]
- 7.Carnielli V. P., Wattimena D. J., Luijendijk I. H., Boerlage A., Degenhart H. J., Sauer P. J. 1996. The very low birth weight premature infant is capable of synthesizing arachidonic and docosahexaenoic acids from linoleic and linolenic acids. Pediatr. Res. 40: 169–174 [DOI] [PubMed] [Google Scholar]
- 8.Salem N., Jr, Wegher B., Mena P., Uauy R. 1996. Arachidonic and docosahexaenoic acids are biosynthesized from their 18-carbon precursors in human infants. Proc. Natl. Acad. Sci. USA. 93: 49–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demmelmair H., Sauerwald T., Koletzko B., Richter T. 1997. New insights into lipid and fatty acid metabolism via stable isotopes. Eur. J. Pediatr. 156(Suppl 1): S70–S74 [DOI] [PubMed] [Google Scholar]
- 10.Brenna J. T. 2002. Efficiency of conversion of alpha-linolenic acid to long chain n-3 fatty acids in man. Curr. Opin. Clin. Nutr. Metab. Care. 5: 127–132 [DOI] [PubMed] [Google Scholar]
- 11.Brenna J. T., Salem N., Jr, Sinclair A. J., Cunnane S. C.; International Society for the Study of Fatty Acids and Lipids, ISSFAL 2009. alpha-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot. Essent. Fatty Acids. 80: 85–91 [DOI] [PubMed] [Google Scholar]
- 12.Hsieh A. T., Brenna J. T. 2009. Dietary docosahexaenoic acid but not arachidonic acid influences central nervous system fatty acid status in baboon neonates. Prostaglandins Leukot. Essent. Fatty Acids. 81: 105–110 [DOI] [PubMed] [Google Scholar]
- 13.Brenna J. T., Lapillonne A. 2009. Background paper on fat and fatty acid requirements during pregnancy and lactation. Ann. Nutr. Metab. 55: 97–122 [DOI] [PubMed] [Google Scholar]
- 14.Colombo J., Carlson S. E., Cheatham C. L., Shaddy D. J., Kerling E. H., Thodosoff J. M., Gustafson K. M., Brez C. 2013. Long-term effects of LCPUFA supplementation on childhood cognitive outcomes. Am. J. Clin. Nutr. 98: 403–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen R. G. 1995. Handbook of Human Milk Composition. Academic Press, New York. [Google Scholar]
- 16.Willatts P., Forsyth J. S., DiModugno M. K., Varma S., Colvin M. 1998. Effect of long-chain polyunsaturated fatty acids in infant formula on problem solving at 10 months of age. Lancet. 352: 688–691 [DOI] [PubMed] [Google Scholar]
- 17.Willatts P., Forsyth S., Agostoni C., Casaer P., Riva E., Boehm G. 2013. Effects of long-chain PUFA supplementation in infant formula on cognitive function in later childhood. Am. J. Clin. Nutr. 98: 536S–542S [DOI] [PubMed] [Google Scholar]
- 18.Martin J. C., Bougnoux P., Antoine J. M., Lanson M., Couet C. 1993. Triacylglycerol structure of human colostrum and mature milk. Lipids. 28: 637–643 [DOI] [PubMed] [Google Scholar]
- 19.Innis S. M., Dyer R. A., Lien E. L. 1997. Formula containing randomized fats with palmitic acid (16:0) in the 2-position increases 16:0 in the 2-position of plasma and chylomicron triglycerides in formula-fed piglets to levels approaching those of piglets fed sow's milk. J. Nutr. 127: 1362–1370 [DOI] [PubMed] [Google Scholar]
- 20.Le Kim D., Betzing H. 1976. Intestinal absorption of polyunsaturated phosphatidylcholine in the rat. Hoppe Seylers Z. Physiol. Chem. 357: 1321–1331 [DOI] [PubMed] [Google Scholar]
- 21.Wijendran V., Huang M. C., Diau G. Y., Boehm G., Nathanielsz P. W., Brenna J. T. 2002. Efficacy of dietary arachidonic acid provided as triglyceride or phospholipid as substrates for brain arachidonic acid accretion in baboon neonates. Pediatr. Res. 51: 265–272 [DOI] [PubMed] [Google Scholar]
- 22.Le P. M., Fraser C., Gardner G., Liang W. W., Kralovec J. A., Cunnane S. C., Windust A. J. 2007. Biosynthetic production of universally (13)C-labelled polyunsaturated fatty acids as reference materials for natural health product research. Anal. Bioanal. Chem. 389: 241–249 [DOI] [PubMed] [Google Scholar]
- 23.Tyburczy C., Kothapalli K. S., Park W. J., Blank B. S., Bradford K. L., Zimmer J. P., Butt C. M., Salem N., Jr, Brenna J. T. 2011. Heart arachidonic acid is uniquely sensitive to dietary arachidonic acid and docosahexaenoic acid content in domestic piglets. Prostaglandins Leukot. Essent. Fatty Acids. 85: 335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tyburczy C., Kothapalli K. S., Park W. J., Blank B. S., Liu Y. C., Nauroth J. M., Zimmer J. P., Salem N., Jr, Brenna J. T. 2012. Growth, clinical chemistry and immune function in domestic piglets fed varying ratios of arachidonic acid and DHA. Br. J. Nutr. 107: 809–816 [DOI] [PubMed] [Google Scholar]
- 25.Zhou Y., Nijland M., Miller M., Ford S., Nathanielsz P. W., Brenna J. T. 2008. The influence of maternal early to mid-gestation nutrient restriction on long chain polyunsaturated fatty acids in fetal sheep. Lipids. 43: 525–531 [DOI] [PubMed] [Google Scholar]
- 26.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917 [DOI] [PubMed] [Google Scholar]
- 27.Goodman K. J., Brenna J. T. 1992. High sensitivity tracer detection using high-precision gas chromatography-combustion isotope ratio mass spectrometry and highly enriched [U-13C]-labeled precursors. Anal. Chem. 64: 1088–1095 [DOI] [PubMed] [Google Scholar]
- 28.Miller A. K., Alston R. L., Corsellis J. A. 1980. Variation with age in the volumes of grey and white matter in the cerebral hemispheres of man: measurements with an image analyser. Neuropathol. Appl. Neurobiol. 6: 119–132 [DOI] [PubMed] [Google Scholar]
- 29.Ramirez C. G., Miller E. R., Ullrey D. E., Hoefer J. A. 1963. Swine hematology from birth to maturity. III. Blood volume of the nursing pig. J. Anim. Sci. 22: 1068–1074 [Google Scholar]
- 30.National Research Council. 1998. Nutrient Requirements of Swine. 10th edition. National Academy Press, Washington, DC. [Google Scholar]
- 31.Song B. J., Elbert A., Rahman T., Orr S. K., Chen C. T., Febbraio M., Bazinet R. P. 2010. Genetic ablation of CD36 does not alter mouse brain polyunsaturated fatty acid concentrations. Lipids. 45: 291–299 [DOI] [PubMed] [Google Scholar]
- 32.Rahman T., Taha A. Y., Song B. J., Orr S. K., Liu Z., Chen C. T., Bazinet R. P. 2010. The very low density lipoprotein receptor is not necessary for maintaining brain polyunsaturated fatty acid concentrations. Prostaglandins Leukot. Essent. Fatty Acids. 82: 141–145 [DOI] [PubMed] [Google Scholar]
- 33.Thies F., Pillon C., Moliere P., Lagarde M., Lecerf J. 1994. Preferential incorporation of sn-2 lysoPC DHA over unesterified DHA in the young rat brain. Am. J. Physiol. 267: R1273–R1279 [DOI] [PubMed] [Google Scholar]
- 34.Lagarde M., Bernoud N., Brossard N., Lemaitre-Delaunay D., Thiès F., Croset M., Lecerf J. 2001. Lysophosphatidylcholine as a preferred carrier form of docosahexaenoic acid to the brain. J. Mol. Neurosci. 16: 201–204; discussion 215–221 [DOI] [PubMed] [Google Scholar]
- 35.Chen S., Subbaiah P. V. 2007. Phospholipid and fatty acid specificity of endothelial lipase: potential role of the enzyme in the delivery of docosahexaenoic acid (DHA) to tissues. Biochim. Biophys. Acta. 1771: 1319–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scagnelli G. P., Cooper P. S., VandenBroek J. M., Berman W. F., Schwartz C. C. 1991. Plasma 1-palmitoyl-2-linoleoyl phosphatidylcholine. Evidence for extensive phospholipase A1 hydrolysis and hepatic metabolism of the products. J. Biol. Chem. 266: 18002–18011 [PubMed] [Google Scholar]
- 37.Vandal M., Alata W., Tremblay C., Rioux-Perreault C., Salem N., Jr, Calon F., Plourde M. Reduction in DHA transport to the brain of mice expressing human APOE4 compared to APOE2. J. Neurochem. Epub ahead of print. December 18, 2013; 10.1111/jnc.12640. [DOI] [PubMed] [Google Scholar]
- 38.Eto M., Shindou H., Shimizu T. 2014. A novel lysophosphatidic acid acyltransferase enzyme (LPAAT4) with a possible role for incorporating docosahexaenoic acid into brain glycerophospholipids. Biochem. Biophys. Res. Commun. 443: 718–724 [DOI] [PubMed] [Google Scholar]
- 39.Brenna J. T., Varamini B., Jensen R. G., Diersen-Schade D. A., Boettcher J. A., Arterburn L. M. 2007. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am. J. Clin. Nutr. 85: 1457–1464 [DOI] [PubMed] [Google Scholar]
- 40.Nyuar K. B., Min Y., Ghebremeskel K., Khalil A. K., Elbashir M. I., Cawford M. A. 2010. Milk of northern Sudanese mothers whose traditional diet is high in carbohydrate contains low docosahexaenoic acid. Acta Paediatr. 99: 1824–1827 [DOI] [PubMed] [Google Scholar]
- 41.Birch E. E., Carlson S. E., Hoffman D. R., Fitzgerald-Gustafson K. M., Fu V. L., Drover J. R., Castaneda Y. S., Minns L., Wheaton D. K., Mundy D., et al. 2010. The DIAMOND (DHA Intake And Measurement Of Neural Development) Study: a double-masked, randomized controlled clinical trial of the maturation of infant visual acuity as a function of the dietary level of docosahexaenoic acid. Am. J. Clin. Nutr. 91: 848–859 [DOI] [PubMed] [Google Scholar]
- 42.Foote K. D., Hrboticky N., MacKinnon M. J., Innis S. M. 1990. Brain synaptosomal, liver, plasma, and red blood cell lipids in piglets fed exclusively on a vegetable-oil-containing formula with and without fish-oil supplements. Am. J. Clin. Nutr. 51: 1001–1006 [DOI] [PubMed] [Google Scholar]
- 43.Mathews S. A., Oliver W. T., Phillips O. T., Odle J., Diersen-Schade D. A., Harrell R. J. 2002. Comparison of triglycerides and phospholipids as supplemental sources of dietary long-chain polyunsaturated fatty acids in piglets. J. Nutr. 132: 3081–3089 [DOI] [PubMed] [Google Scholar]
- 44.Huang M. C., Chao A., Kirwan R., Tschanz C., Peralta J. M., Diersen-Schade D. A., Cha S., Brenna J. T. 2002. Negligible changes in piglet serum clinical indicators or organ weights due to dietary single-cell long-chain polyunsaturated oils. Food Chem. Toxicol. 40: 453–460 [DOI] [PubMed] [Google Scholar]
- 45.Amate L., Gil A., Ramirez M. 2001. Feeding infant piglets formula with long-chain polyunsaturated fatty acids as triacylglycerols or phospholipids influences the distribution of these fatty acids in plasma lipoprotein fractions. J. Nutr. 131: 1250–1255 [DOI] [PubMed] [Google Scholar]
- 46.Amate L., Gil A., Ramirez M. 2002. Dietary long-chain PUFA in the form of TAG or phospholipids influence lymph lipoprotein size and composition in piglets. Lipids. 37: 975–980 [DOI] [PubMed] [Google Scholar]
- 47.Winther B., Hoem N., Berge K., Reubsaet L. 2011. Elucidation of phosphatidylcholine composition in krill oil extracted from Euphausia superba. Lipids. 46: 25–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schuchardt J. P., Schneider I., Meyer H., Neubronner J., von Schacky C., Hahn A. 2011. Incorporation of EPA and DHA into plasma phospholipids in response to different omega-3 fatty acid formulations–a comparative bioavailability study of fish oil vs. krill oil. Lipids Health Dis. 10: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.el Boustani S., Colette C., Monnier L., Descomps B., Crastes de Paulet A., Mendy F. 1987. Enteral absorption in man of eicosapentaenoic acid in different chemical forms. Lipids. 22: 711–714 [DOI] [PubMed] [Google Scholar]
- 50.Dyerberg J., Madsen P., Moller J. M., Aardestrup I., Schmidt E. B. 2010. Bioavailability of marine n-3 fatty acid formulations. Prostaglandins Leukot. Essent. Fatty Acids. 83: 137–141 [DOI] [PubMed] [Google Scholar]
- 51.Wijendran V., Lawrence P., Diau G. Y., Boehm G., Nathanielsz P. W., Brenna J. T. 2002. Significant utilization of dietary arachidonic acid is for brain adrenic acid in baboon neonates. J. Lipid Res. 43: 762–767 [PubMed] [Google Scholar]
- 52.Graf B. A., Duchateau G. S., Patterson A. B., Mitchell E. S., van Bruggen P., Koek J. H., Melville S., Verkade H. J. 2010. Age dependent incorporation of 14C-DHA into rat brain and body tissues after dosing various 14C-DHA-esters. Prostaglandins Leukot. Essent. Fatty Acids. 83: 89–96 [DOI] [PubMed] [Google Scholar]
- 53.Dobbing J., Sands J. 1979. Comparative aspects of the brain growth spurt. Early Hum. Dev. 3: 79–83 [DOI] [PubMed] [Google Scholar]
- 54.Brossard N., Croset M., Pachiaudi C., Riou J. P., Tayot J. L., Lagarde M. 1996. Retroconversion and metabolism of [13C]22:6n-3 in humans and rats after intake of a single dose of [13C]22:6n-3-triacylglycerols. Am. J. Clin. Nutr. 64: 577–586 [DOI] [PubMed] [Google Scholar]
- 55.Plourde M., Chouinard-Watkins R., Vandal M., Zhang Y., Lawrence P., Brenna J. T., Cunnane S. C. 2011. Plasma incorporation, apparent retroconversion and beta-oxidation of 13C-docosahexaenoic acid in the elderly. Nutr. Metab. (Lond). 8: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sprecher H. 1999. An update on the pathways of polyunsaturated fatty acid metabolism. Curr. Opin. Clin. Nutr. Metab. Care. 2: 135–138 [DOI] [PubMed] [Google Scholar]
- 57.Infante J. P., Tschanz C. L., Shaw N., Michaud A. L., Lawrence P., Brenna J. T. 2002. Straight-chain acyl-CoA oxidase knockout mouse accumulates extremely long chain fatty acids from alpha-linolenic acid: evidence for runaway carousel-type enzyme kinetics in peroxisomal beta-oxidation diseases. Mol. Genet. Metab. 75: 108–119 [DOI] [PubMed] [Google Scholar]
- 58.Li Y., Monroig O., Zhang L., Wang S., Zheng X., Dick J. R., You C., Tocher D. R. 2010. Vertebrate fatty acyl desaturase with Δ4 activity. Proc. Natl. Acad. Sci. USA. 107: 16840–16845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neuringer M., Connor W. E., Lin D. S., Barstad L., Luck S. 1986. Biochemical and functional effects of prenatal and postnatal omega 3 fatty acid deficiency on retina and brain in rhesus monkeys. Proc. Natl. Acad. Sci. USA. 83: 4021–4025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yuhas R., Pramuk K., Lien E. L. 2006. Human milk fatty acid composition from nine countries varies most in DHA. Lipids. 41: 851–858 [DOI] [PubMed] [Google Scholar]