Abstract
Long-chain aldehydes are commonly produced in various processes, such as peroxisomal α-oxidation of long-chain 3-methyl-branched and 2-hydroxy fatty acids and microsomal breakdown of phosphorylated sphingoid bases. The enzymes involved in the aldehyde-generating steps of these processes are 2-hydroxyacyl-CoA lyase (HACL1) and sphingosine-1-phosphate lyase (SGPL1), respectively. In the present work, nonradioactive assays for these enzymes were developed employing the Hantzsch reaction. Tridecanal (C13-al) and heptadecanal (C17-al) were selected as model compounds and cyclohexane-1,3-dione as 1,3-diketone, and the fluorescent derivatives were analyzed by reversed phase (RP)-HPLC. Assay mixture composition, as well as pH and heating, were optimized for C13-al and C17-al. Under optimized conditions, these aldehydes could be quantified in picomolar range and different long-chain aldehyde derivatives were well resolved with a linear gradient elution by RP-HPLC. Aldehydes generated by recombinant enzymes could easily be detected via this method. Moreover, the assay allowed to document activity or deficiency in tissue homogenates and fibroblast lysates without an extraction step. In conclusion, a simple, quick, and cheap assay for the study of HACL1 and SGPL1 activities was developed, without relying on expensive mass spectrometric detectors or radioactive substrates.
Keywords: α-oxidation, Hantzsch reaction, peroxisomes, phytanic acid, sphingosine-1-phosphate
Aldehydes are highly susceptible to both enzymatic and chemical degradation. On the one hand, the high reactivity of aldehydes makes their detection in tissue homogenates rather tricky; on the other hand, it can be exploited to develop assays for their detection.
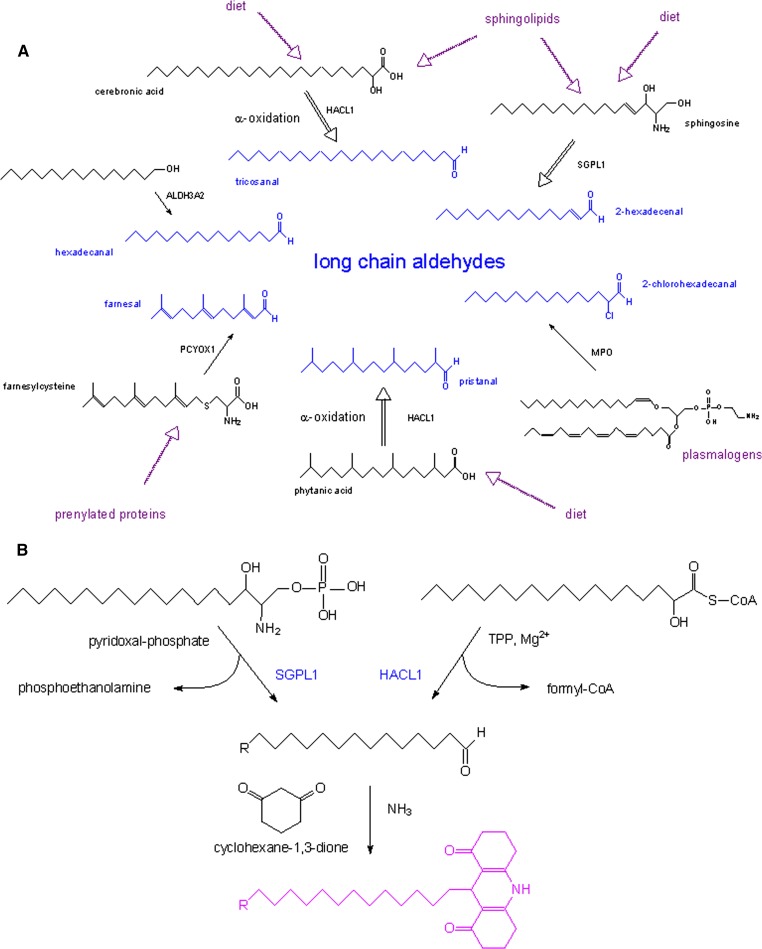
Long-chain aliphatic aldehydes are produced during various metabolic processes, such as microsomal oxidation of long-chain alcohols, lysosomal degradation of prenylated proteins, peroxisomal α-oxidation of 3-methyl-branched fatty acids and 2-hydroxy long-chain fatty acids, microsomal breakdown of phosphorylated sphingoid bases, attack of plasmalogens by myeloperoxidase (MPO)-derived hypochlorous acid, and microsomal degradation of (lyso)plasmalogens (see Fig. 1A). The involved enzymes are fatty aldehyde dehydrogenase (ALDH3A2) (1, 2), prenylcysteine oxidase 1 (PCYOX1) (3, 4), 2-hydroxyacyl-CoA lyase (HACL1) (5, 6), sphingosine-1-phosphate lyase (SGPL1) (7–9), MPO (10), and lysoplasmalogenase (11, 12). In particular, two of these enzymes, HACL1 and SGPL1, have intensively been studied in our laboratory. Their activity measurements are complicated by their low specific activity [∼115 (5) and ∼15 mU/g rat liver (13), respectively], low Km (10–15 μM), and the considerable interference of enzymes acting on their substrates (acyl-CoA hydrolases for HACL1; phosphatases for SGPL1) or on their cofactors (phosphatases acting on TPP, cofactor for HACL1, and on pyridoxal-phosphate, cofactor for SGPL1). In addition, the generated aldehyde is chemically not stable and subject to further metabolism, and compounds normally used to trap the aldehyde or block its metabolism interfere with the cofactor (pyridoxal-phosphate). Moreover, the presence of plasmalogens in mammalian tissues, which give rise to aldehydes under acidic conditions (14), can cause a high background when using nonradioactive substrates, especially in nervous tissue.
Fig. 1.
Long-chain aliphatic aldehydes are produced during various metabolic processes. A: Schematic representation of aldehyde production in mammals during various enzymatic reactions, such as microsomal oxidation of long-chain alcohols, lysosomal degradation of prenylated proteins, peroxisomal α-oxidation of 3-methyl-branched fatty acids and 2-hydroxy long-chain fatty acids, attack of plasmalogens by MPO-derived hypochlorous acid, and microsomal breakdown of phosphorylated sphingoid bases. Double black arrows point to multiple steps. B: Scheme for the Hantzsch reaction.
To measure HACL1 and SGPL1 activities in tissue or cell lysates, various protocols were developed by our group. Initially, home-made radioactive substrates were used, such as 2-hydroxy-3-methyl-[1-14C]hexadecanoyl-CoA (5) or 2-hydroxy-[1-14C]octadecanoyl-CoA (6), converted by HACL1 into [1-14C]formyl-CoA and 2-methylpentadecanal or heptadecanal, and [4,5-3H]D,e-sphinganine-1-phosphate (9), converted by SGPL1 into [2,3-3H]hexadecanal and phosphoethanolamine. Recently, given the issues concerning the use, synthesis, and waste disposal of radioactive substrates, nonlabeled 2-hydroxyoctadecanoyl-CoA was used as substrate for HACL1 (6), followed by derivatization of the generated aldehyde to the corresponding 2,4-dinitrophenylhydrazone, extraction, and reversed-phase (RP)-HPLC analysis. In addition, Jansen et al. (15) used 2-hydroxyphytanoyl-CoA as a substrate for HACL1 and analyzed the produced pristanal as an ethoxime derivative by GC-MS. Related to SGPL1, other groups introduced alternative substrates which give rise to a fluorescent aldehyde, either directly such as ω-NBD-sphingosine-1-phosphate (16) and ω-BODIPY-C13-sphingosine-1-phosphate (17), or via β-elimination such as ω-(2-oxo-2H-chromen-7-yloxy)-C5-sphingosine-1-phosphate (18). Alternatively, SGPL1-produced aldehydes were detected by mass spectrometry after suitable derivatization with semicarbazide followed by LC-ESI (sphingosine-1-phosphate as substrate) (19), pentafluorobenzyloxime followed by GC-EI (C17-sphinganine-1-phosphate as substrate) (20), or 2-diphenylacetyl-1,3-indandione-1-hydrazone followed by LC-MS/MS (sphingosine-1-phosphate as substrate) (21).
In the present work, we investigated the Hantzsch reaction for carbonyl compounds in order to allow the use of nonlabeled substrates and an inexpensive derivatization reagent (see Fig. 1B). Tridecanal (C13-al) and heptadecanal (C17-al) were employed as model compounds and cyclohexane-1,3-dione (CHD) as 1,3-diketone, followed by RP-HPLC of the fluorescent decahydroacridine derivatives. The first application of CHD to measure aldehydes, in casu formaldehyde, was described by Sawicki and Carnes in 1968 (22).
EXPERIMENTAL PROCEDURES
Animals and cells
Animal studies were approved by the University Ethics Committee, and all animal experiments were performed in accordance with the institutional and national guidelines for care and use of laboratory animals. Swiss Webster mice were kept under conventional conditions in the animal housing facility of the KU Leuven, with a 12 h light and dark cycle, and fed a regular chow diet. At the time of the experiment, mice were anesthetized with an overdose of Nembutal (approximately 150 μg/g body weight), and tissues were dissected. Human fibroblasts (23) and murine fibroblasts, derived from C57BL6 or Sgpl1−/− mice (24, 25), were cultured as described in the cited references.
Recombinant enzymes
Human HACL1 was expressed in Saccharomyces cerevisiae CB80 strain (MATα, ura3-52, leu2-1, trp1-63, his3-200) transformed with pPF17, coding for an N-His6-tagged fusion (26). Human SGPL1 was expressed in Escherichia coli Top10F’ cells transformed with plasmid pVB001, coding for an N-His6-tagged fusion (7).
Synthesis of lipids
Saturated long-chain aldehydes were commercially obtained or synthesized in a similar way as described previously for C17-al (5); 2-hexadecenal was obtained by periodate treatment of D,erythro-sphingosine (27). Purity of aldehydes was verified by GC-MS and/or derivatization with 2,4-dinitrophenylhydrazine (6). 2-Hydroxyoctadecanoyl-CoA was prepared as described before (6). D,erythro-sphingosine, D,erythro-sphinganine-1-phosphate, and 1-O-1’-(Z)-octadecenyl-2-oleoyl-sn-glycerol-3-phosphocholine were obtained from Avanti Polar Lipids. Decahydroacridine derivatives of C13-al and C17-al were prepared by mixing the aldehydes (100 nmol dissolved in 50 μl methanol) with 50 μl derivatization mixture and 50 μl water (see below). The reaction mixtures were loaded on an activated Bond Elut C18 cartridge (100 mg; Varian), and the derivatives were eluted with methanol. After drying, residues were reconstituted in 80% (v/v) methanol containing 10 mM ammonium acetate (∼80 μM), and an aliquot was injected at 10 μl/min on line in a 1100 Series LC/MSD Trap-VL (Agilent) equipped with ESI (nebulizer 15 psi, dry gas 5 l/min, dry temperature 325°C) and scanned for m/z 100–650 for 2 min.
Enzyme measurements
The substrate 2-hydroxyoctadecanoyl-CoA or sphinganine-1-phosphate was incubated at 37°C with purified recombinant enzyme, tissue homogenate, or cell lysate in baked Pyrex 5 ml glass tubes. Reaction mixtures for activity measurement consisted of 50 mM Tris-HCl (pH 7.5), 0.8 mM MgCl2, 20 μM TPP, 6.6 μM BSA, and 40 μM 2-hydroxyoctadecanoyl-CoA for HACL1 (6); and 0.1 M K-phosphate buffer (pH 7.5), 25 mM NaF, 0.1 mM Na-orthovanadate, 0.25 mM pyridoxal-phosphate, 1 mM DTT, 1 mM EDTA, 0.1% (v/v) Triton X-100, and 40 μM sphinganine-1-phosphate for SGPL1 (8). Reactions for tissue homogenates and cell lysates were halted after 5 min (HACL1) or 60 min (SGPL1) by adding 1 vol of methanol (see below).
Acidic pretreatment of tissue homogenates
Mouse livers and brains were homogenized (1 part/20 vol) in isotonic buffer [0.25 M sucrose, 5 mM MOPS (pH 7.2), 0.1% (v/v) ethanol], and 40 μl homogenates were exposed to either 0.3 M HCl or NaCl for 15 min at 65°C. Subsequently, the acid-treated samples were neutralized with NaOH. The generated aldehydes were then analyzed by RP-HPLC.
RP-HPLC analysis of aldehydes
According to our optimized protocol, assay mixtures [100–200 μl spiked with internal standard (IS, 0.5–2.0 nmol)] were mixed with 1 vol of ice-cold HPLC-grade methanol, followed by 1 vol derivatization medium [3.6 M ammonium acetate, 100 mM CHD, 42% (v/v) acetic acid], capping and heating for 60 min at 65°C. Upon cooling, 2 vol of methanol, relative to the initial sample volume, were added to reach a methanol concentration of 60%. The latter step was omitted when optimizing the derivatization of aldehyde standards. After a 1000 g spin, the supernatant was transferred to inserts of HPLC-vials (Alltech) and 1/50 up to 1/5 of the total volume was injected on a Symmetry® C18 column (4.6 × 150 mm; 5 μm; 100 Å Waters) equilibrated with 80% (v/v) methanol. Bound derivatives were eluted with a gradient (80–100% methanol) over 12 min and detected by fluorimetry (Waters 2475 Multi-Wavelength Fluorescence Detector; Ex 390 nm; Em 460 nm; emission mode; EUFS 3000 or lower). Wavelength settings were based on Ex/Em spectra obtained in Aminco SPF500 fluorimeter for the decahydroacridine derivatives of formaldehyde and tetradecanal (C14-al) (data not shown).
RESULTS AND DISCUSSION
Optimization of assay conditions
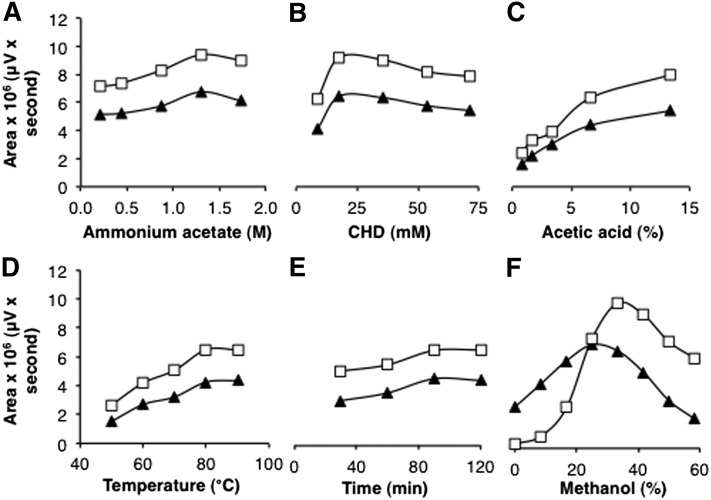
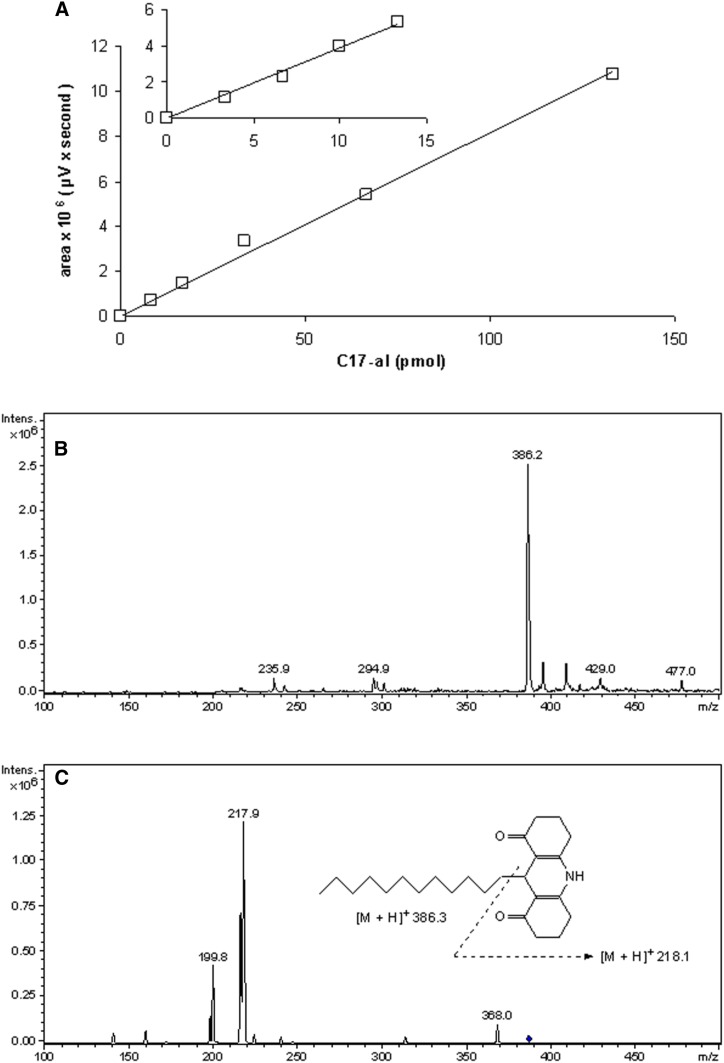
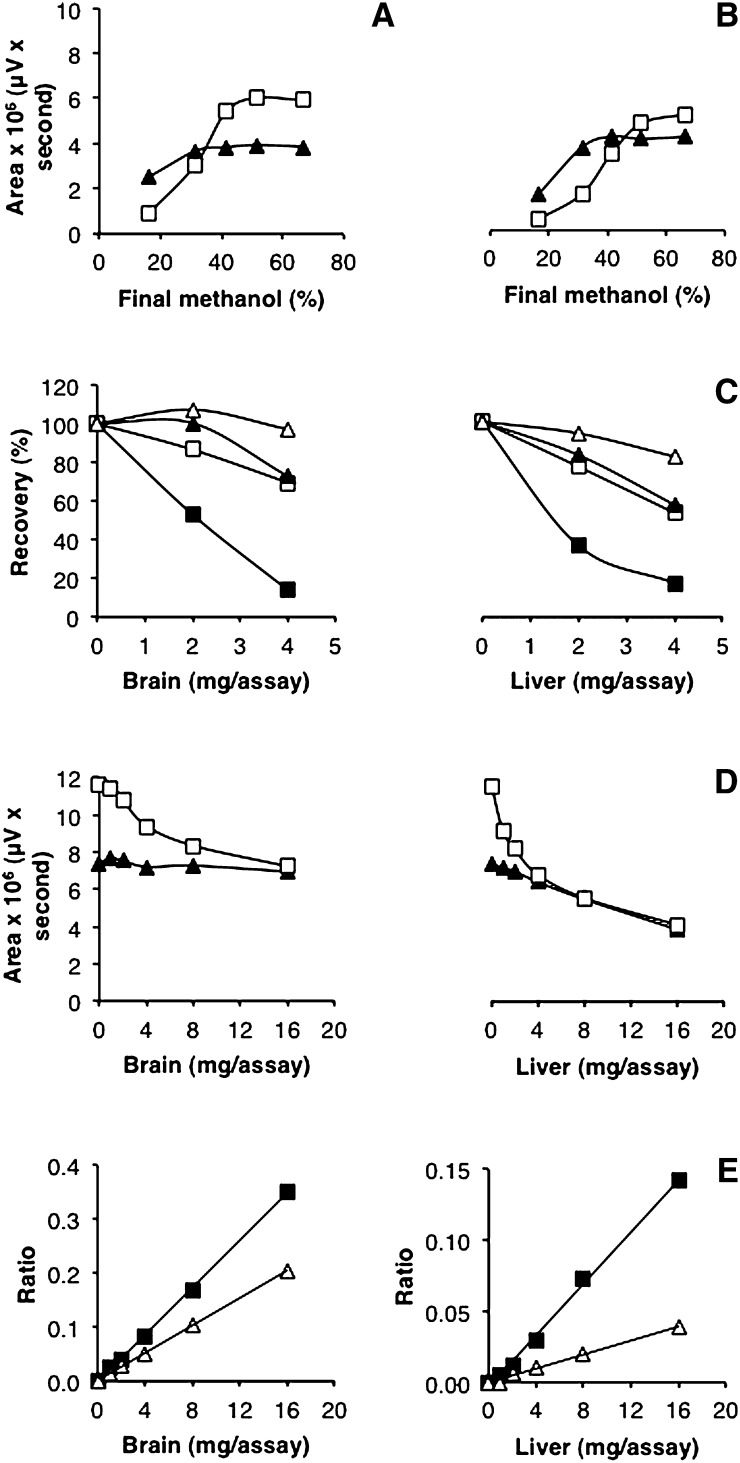
To date, the longest aldehyde that has been analyzed via the Hantzsch reaction using CHD as 1,3-diketone was decanal (C10-al) (28, 29). Based on this report, different concentrations of components, as well as pH and heating parameters, were tested to optimize the detection of C13-al and C17-al (Fig. 2A–E). In addition, the presence of methanol as a cosolvent, not investigated in previous reports, was demonstrated to be essential for the quantitative conversion of C17-al into its fluorescent derivative (Fig. 2F). Other cosolvents, such as ethanol, dimethylsulfoxide, tetrahydrofuran, acetonitrile, and dimethylformamide, were also stimulatory but not to the same extent (ethanol, dimethylsulfoxide), or they displayed a sharp concentration optimum (data not shown). For shorter aldehydes, e.g., C13-al, the requirement for methanol was less stringent (Fig. 2F). From the data obtained, we evaluated the best assay conditions, which allowed for the quantification of both C13-al and C17-al. The selected parameters, based on three subsequent rounds of optimizations on standards, were 1.2 M ammonium acetate, 33 mM CHD, 14% (v/v) acetic acid, 33% (v/v) methanol, and heating at 65°C for 60 min. Other factors, which could cause interference when analyzing biological samples, were considered, such as plasmalogen breakdown at low pH or high temperature (see below) or peroxidation of polyenoic acids by extreme heat; hence, 65°C instead of 90°C was chosen (see also Ref. 28). The selected conditions allowed for the quantification of C17-al in picomolar range (low sensitivity, 10–150 pmol; medium sensitivity, 1–10 pmol) (Fig. 3A). LC-ESI-MS analysis of the C13-al and C17-al derivatives revealed a compound with m/z 386.2 for C13-al (Fig. 3B) and m/z 442.2 for C17-al (data not shown), which upon fragmentation, yielded a fragment of m/z 217.9, corresponding to the decahydroacridine moiety and confirming the postulated structures (Fig. 3C).
Fig. 2.
Optimization of aldehyde derivatization. Concentration of the different assay components, pH, and heating parameters were varied to optimize the derivatization of C13-al (triangles) and C17-al (open squares) (1.5 and 2 nmol, respectively). Fluorescent derivatives were directly separated by RP-HPLC, without dilution with methanol, and the integrated area of the signal was plotted against the variable parameter, while other parameters (1.3 M ammonium acetate, 35.7 mM CHD, 13.3% (v/v) acetic acid, 80°C temperature, 90 min heating time, 40% (v/v) methanol, based on initial tests) were kept constant, unless otherwise mentioned. A: Effect of ammonium acetate. B: Effect of CHD. C: Effect of acetic acid. D: Effect of temperature (1.6 M ammonium acetate, 14% (v/v) acetic acid, 60 min heating time). E: Effect of heating time (1.6 M ammonium acetate, 14% (v/v) acetic acid). F: Effect of methanol (1.2 M ammonium acetate, 14% (v/v) acetic acid, 65°C temperature, 60 min heating time).
Fig. 3.
RP-HPLC and MS analyses of fluorescent derivatives. A: RP-HPLC analysis of the C17-al derivative. Integrated fluorescence peak area, obtained with EUFS setting of the detector at 3,000, was plotted versus amount of injected C17-al present in 10 μl of reaction mixture (600 μl in total). Insert shows analysis of lower amounts of aldehyde at a higher sensitivity (EUFS 1000). B: Mass spectrum scan of the C13-al derivative [positive mode, smoothed (0.2; 1; GA)]. Semi-purified derivatives were subjected to LC-ESI-MS, revealing a major ion of m/z 386.2 for the C13-al derivative (calculated mass 385.3) and m/z 442.2 for the C17-al derivative (calculated mass 441.36) (not shown). Smaller peaks represent contaminants present in both spectra. C: Fragmentation spectrum of the compound with m/z 386.2 (position indicated by diamond) with a characteristic peak at m/z 217.9, corresponding to the aromatic ring structure.
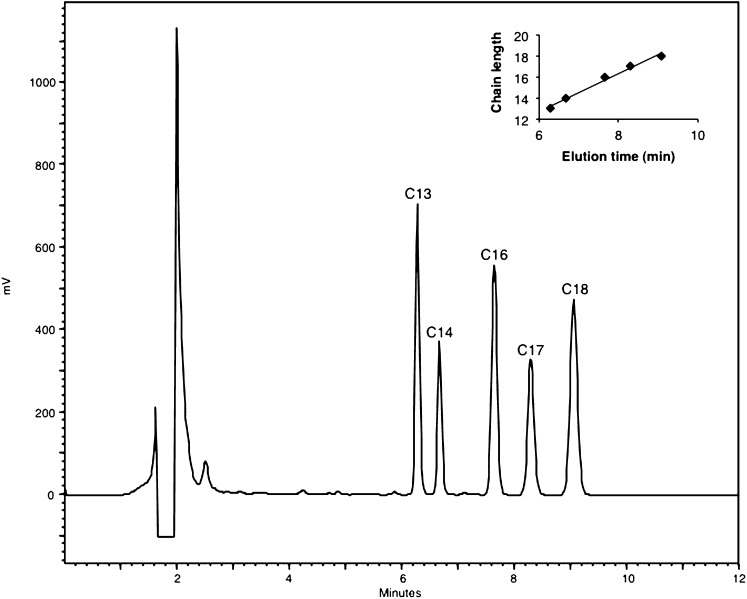
Subsequently, we checked to which extent aldehydes with rather similar chain length could be discriminated: a good resolution of different long-chain aldehydes was obtained, and the elution time related to the number of carbon atoms (Fig. 4).
Fig. 4.
RP-separation of long-chain aldehydes according to chain length. Aldehydes (1-3 nmol, based on derivatization with 2,4-dinitrophenylhydrazine) with different chain lengths (13–18 carbon atoms) were mixed, converted to CHD derivatives, separated by RP-HPLC, and detected by fluorimetry (Ex 390 nm, Em 460 nm). The insert shows the relationship between elution time and aldehyde chain length. The drop in fluorescence near 1.8 min is due to a transient closure of the emission filter to protect the detector against too high intensity.
The presence of proteins can affect the recovery of aliphatic aldehydes (e.g., Schiff base with amino groups, hydrophobic interactions, trapping in pelleted proteins or in interphase during extraction), and negative effects of proteins have been observed also in CHD-based assays containing no or less methanol [59% recovery for C6-al in presence of 16.7 mg rat liver/ml assay mixture (28); 65–72% recovery for C7-al in presence of 50 μl human plasma/ml assay mixture (30); chain length-dependent decrease of recovery, down to 8% for C10-al, in presence of 0.33 ml human plasma/ml assay mixture (29)]. Nonetheless, recovery of medium-chain aldehydes (C13-al) was excellent under our conditions [33% (v/v) methanol during derivatization] in presence of increasing amounts of tissue homogenate. However, recovery of C17-al decreased (15% at 2 mg tissue/assay). Attempts to prevent this by adding detergents or increasing the amount of cosolvent were not successful (data not shown). During these trials, extra methanol was added to the derivatized samples: this increased the amount of C17-al signal considerably, without (Fig. 5A) or with proteins (Fig. 5B). Hence, the formed C17-al derivative is partially lost after cooling and spinning, perhaps due to solubility problems, and this can be counteracted by adding extra methanol after derivatization, or in the opposite sense, worsened by adding water. With this modification, recoveries of IS with brain or liver homogenate were improved (Fig. 5C, D). More importantly, the ratio of endogenous aldehydes over C17-al (Fig. 5E) or C13-al (data not shown) was linear with the amount of tissue analyzed, up to 16 mg tissue/assay, which allows for reliable quantifications. When applying the method for the analysis of enzyme-derived aldehydes in biological samples, it is suggested to use less than 4 mg tissue or less than 0.8 mg protein per 200 μl assay (see Fig. 5D).
Fig. 5.
Recovery of long-chain aldehydes in presence of proteins. A: Effect of methanol on standards. C13-al (triangles) and C17-al (open squares) (1.5 and 2 nmol, respectively) were derivatized following the optimized protocol in the presence of 33% (v/v) methanol. Upon derivatization, equal volumes of water, water/methanol, or pure methanol were added to the assay to obtain the indicated methanol concentration. After centrifugation, the supernatant was subjected to RP-HPLC, and the fluorescent derivatives were detected by fluorimetry (Ex 390 nm, Em 460 nm). Values represent means of two determinations. B: Effect of methanol on standards in presence of proteins. Brain homogenates (2 mg tissue) were spiked with C13-al (triangles) and C17-al (open squares) before derivatization. Upon derivatization, mixtures were processed as described for (A). Integrated peak areas of C13-al and C17-al derivatives are plotted versus final methanol concentration. C. Recovery of IS in presence of proteins. Increasing amounts of brain and liver homogenates were spiked with C13-al and C17-al before derivatization. Water/methanol or methanol was added to the derivatized sample in order to have a final concentration of either 30% or 60% (v/v). Derivatives were separated by RP-HPLC. Recoveries of C13-al and C17-al derivatives were plotted versus amount of tissue per assay. Open triangles: C13-al, 60% methanol; filled triangles: C13-al, 30% methanol; open squares: C17-al, 60% methanol; filled squares: C17-al, 30% methanol. D. Recovery of IS in presence of proteins. Increasing amounts of brain and liver homogenates were spiked with C13-al (triangles) and C17-al (open squares) before derivatization. Methanol was added to the derivatized sample to a final concentration of 60% (v/v). Derivatives were separated by RP-HPLC. Integrated peak areas of C13-al and C17-al derivatives were plotted versus amount of tissue per assay. E. Analysis of endogenous aldehydes. Increasing amounts of brain and liver homogenates were spiked with C17-al before derivatization and separation of the fluorescent derivatives by RP-HPLC. The ratio of endogenous C16-al (squares) and C18-al (open triangles) over C17-al standard was plotted versus amount of tissue per assay.
Application to biological samples
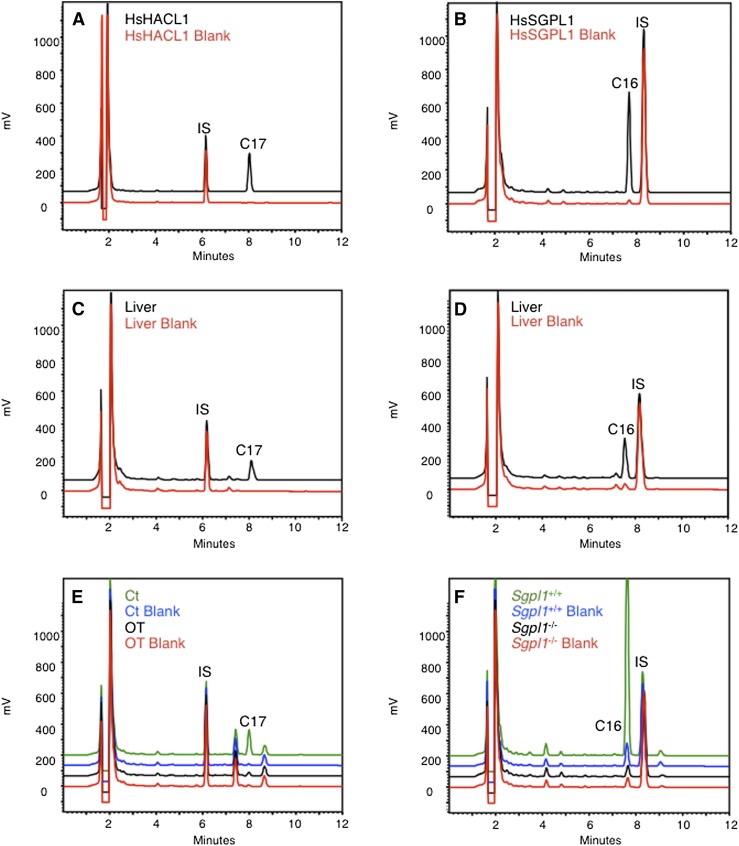
First, we verified whether our optimized method was suitable for the measurement of enzyme-produced aldehydes. As expected, incubation of recombinant human HACL1 with 2-hydroxyoctadecanoyl-CoA resulted in the formation of C17-al (Fig. 6A); similarly, the cleavage of sphinganine-1-phosphate by recombinant human SGPL1 could be demonstrated (Fig. 6B).
Fig. 6.
Analysis of enzymes producing fatty aldehydes. A: Human recombinant HACL1 (5 μg purified protein) was incubated at 37°C for 5 min with 2-hydroxyoctadecanoyl-CoA as substrate (40 μM). A blank reaction was also included, where no substrate was added. The reaction mixtures were fortified with IS (C13-al; 1.5 nmol) before derivatization. B: Human recombinant SGPL1 (0.8 mg protein of soluble E. coli extract) was incubated at 37°C for 60 min with sphinganine-1-phosphate as substrate (40 μM). A blank reaction was also included, where no substrate was added. The reaction mixtures were fortified with IS (C17-al; 2 nmol) before derivatization. C: Liver homogenates of Swiss mice (1 mg tissue, corresponding to ∼0.2 mg protein) were incubated at 37°C for 5 min with 2-hydroxyoctadecanoyl-CoA (40 μM), mixtures were spiked with IS (C13-al; 0.74 nmol), and formed aldehydes were analyzed as described in (A). D: Liver homogenates of Swiss mice (2 mg tissue, corresponding to ∼0.4 mg protein) were incubated at 37°C for 60 min with sphinganine-1-phosphate (40 μM), and formed aldehydes were analyzed as described in (B). E: Human fibroblasts were grown either in standard medium (Ct) or in the presence of 1 mM oxythiamine (OT) for two days before HACL1 activity measurement. Fibroblast lysates (0.2–0.5 mg protein) were then incubated at 37°C for 5 min with 2-hydroxyoctadecanoyl-CoA (40 μM). Blank reactions were also included, where no substrate was added. The reaction mixtures were spiked with IS (C13-al; 0.74 nmol) before derivatization. Note the presence in fibroblasts of endogenous C16-al and C18-al, eluting at 7.4 and 8.7 min, respectively. F: Lysates of mouse fibroblasts (∼0.2 mg protein), obtained from wild-type or Sgpl1−/− C57BL6 embryos, were incubated at 37°C for 60 min with sphinganine-1-phosphate (40 μM). Blank reactions were also included, where no substrate was added. The reaction mixtures were spiked with IS (C17-al; 2 nmol) before derivatization, followed by analysis of the generated C16-al. Assays without substrate serve to correct for nonSGPL1-derived C16-al.
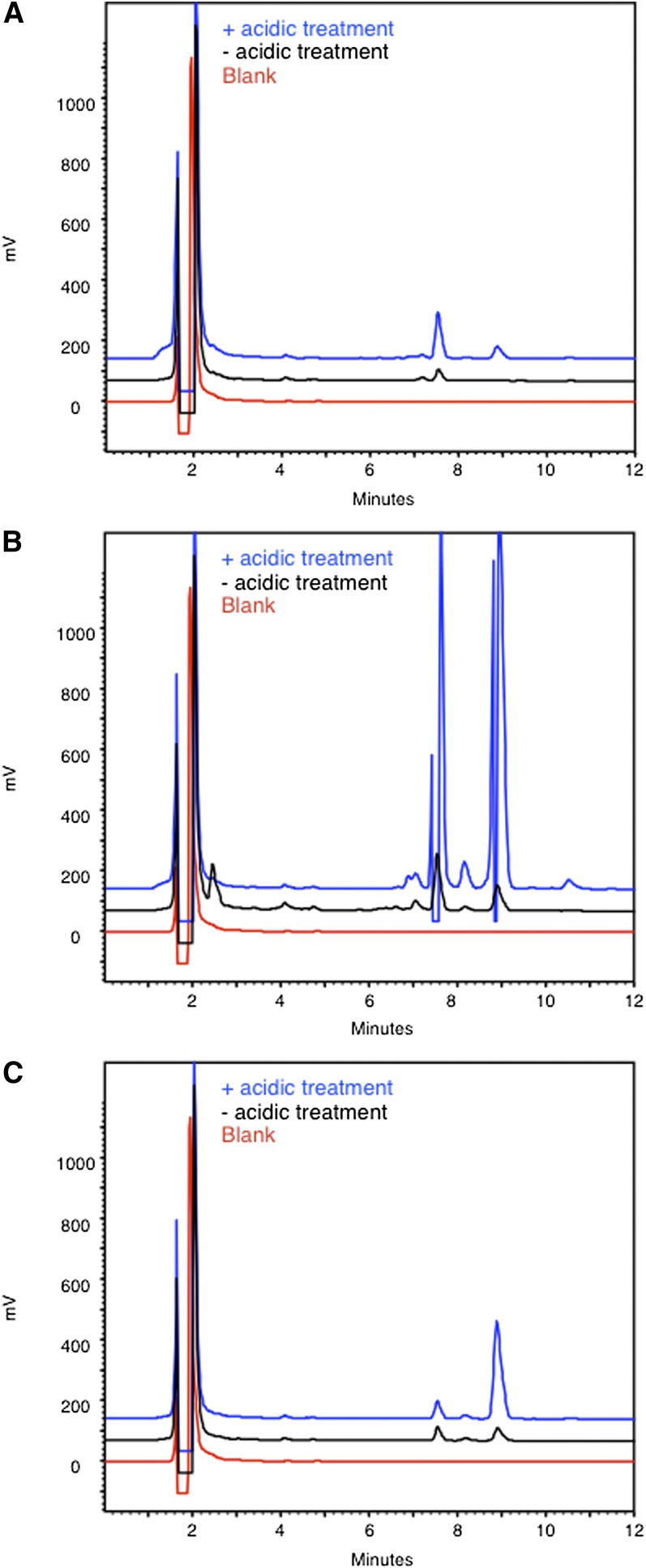
Then, given our intention to measure HACL1 activity in cells or tissues of patients or mice with peroxisomal disorders (31), the possible interference of plasmalogens, which are present in variable amounts in different tissues and whose levels depend on functional peroxisomes, was verified. Indeed, the vinyl-ether bond of plasmalogens, present at 1–10 μmol/g in some tissues (brain, sebaceous glands), is sensitive to acidic conditions (14), resulting in formation of aldehydes and, consequently, in a high background when analyzing enzymatically formed aldehydes. Comparison of the fatty aldehyde profiles of liver or brain samples with or without pretreatment with acid showed that plasmalogen hydrolysis was minimal when derivatization was performed under optimized conditions (Fig. 7A, B), but it was increased at 90°C (data not shown). The acidic pretreatment caused the expected generation of aldehydes, especially hexadecanal (C16-al) and octadecanal (C18-al) in brain (Fig. 7B). Through derivatization of synthetic plasmalogen (1-O-1’-(Z)-octadecenyl-2-oleoyl-sn-glycero-3-phosphocholine), the amount of hydrolysis was estimated at 6.2% (Fig. 7C). Hence, the peaks seen in the nontreated conditions likely represent both free fatty aldehydes (32) and plasmalogen-derived aldehydes. This might, depending on tissue/cell type investigated and on the aldehyde produced, call for correction by appropriate blank incubations (see Fig. 6D, F). Compared with the classical derivatization with DNPH, the CHD method is less aggressive. In fact, derivatization with CHD is performed at pH ∼5.5, while derivatization with DNPH, being only soluble at low pH, is generally carried out under acidic conditions (e.g., 1–2 N HCl or H2SO4). Hence, peroxidation of polyenoic acids, resulting in formation of secondary products, such as 2,4-hexadienal, is less with CHD, as was also pointed out by Yoshino et al. (28). Moreover, as shown in this work, plasmalogen hydrolysis is limited. Furthermore, DNPH-based measurements rely on a photometric detector, sensitivity being determined by its ε value (23,000–28,700; solvent dependent), whereas CHD relies on a fluorimetric detector (lowest limit at highest sensitivity, approximately 5 fmol). In addition, CHD is preferred over DNPH, as the latter is classified as a dangerous chemical (risk class E4), whereas CHD is considered a safe chemical (at least in the European Union). Moreover, CHD is much cheaper than dansylhydrazine or other (even more expensive) fluorescent hydrazides (33–37) that have been used to analyze (short-chain) fatty aldehydes via HPLC. Like DNPH, these compounds react optimally under acidic conditions.
Fig. 7.
Plasmalogen cleavage. Liver homogenates (∼2 mg tissue) (A), brain homogenates (∼2 mg tissue) (B), and 1-O-1’-(Z)-octadecenyl-2-oleoyl-sn-glycero-3-phosphocholine (2 nmol) (C) were pretreated with either HCl or NaCl for 15 min at 65°C before aldehyde derivatization and separation of the fluorescent derivatives by RP-HPLC. Also a blank, containing no tissue or lipid, was analyzed. C16-al and C18-al eluted at 7.4 and 8.8 min, respectively. The drop of the blue line at 7.4 min and 8.8 min in (B) is due to a transient closure of the emission filter to protect the detector against too high intensity. The peak at 7.4 min in (C) is likely due to the presence of a contaminant in the stock solution of 1-O-1’-(Z)-octadecenyl-2-oleoyl-sn-glycero-3-phosphocholine.
Subsequently, we measured HACL1 activity in mouse liver homogenates (Fig. 6C). A gender dependency, so far not reported, was found (106 ± 12 mU/g liver in females, n = 4, and 71 ± 19 mU/g liver in males, n = 6). The values obtained were comparable to those previously measured in rodent liver homogenates using long-chain 2-hydroxyacyl-CoA as substrate (5, 6, 23). Linearity of HACL1 activity in mouse liver homogenates is limited to 5 min (at 1 mg tissue/assay), while activity of the recombinant enzyme was linear at least up to 30 min. This difference can be explained by the presence of a high acyl-CoA hydrolase activity in mouse liver homogenates (approximately 1.8 U/g mouse liver under the HACL1 assay conditions), responsible for the degradation of the substrate during incubation. SGPL1 activity could easily be measured in mouse liver homogenates (4.2 ± 1.9 mU/g liver in females, n = 3; 3.4 ± 0.4 mU/g liver in males, n = 3) (Fig. 6D). In agreement with previous reports (13), SGPL1 activity in liver homogenates (<4 mg tissue/assay) was linear up to 1 h of incubation.
Of clinical importance, lyase activities could also be measured in human fibroblast lysates [187 ± 29 pmol/min/mg protein for HACL1 (n = 4), and 19 pmol/min/mg protein for SGPL1 (n = 2)]. Hence, the assay can be employed to study enzyme deficiencies in patients. To simulate HACL1 deficiency, so far not yet reported, HACL1 activity was measured in human fibroblasts exposed to oxythiamine (OT), which blocks the coenzyme functions of thiamine. In fact, OT-diphosphate competes with TPP for binding to apo-enzymes (23). As reported before, OT exposure resulted in inhibition of α-oxidation (23) and reduced HACL1 activity (Fig. 6E). Likewise, SGPL1 deficiency in man has not been diagnosed, but a clear drop in aldehyde production was seen in Sgpl1−/− mouse fibroblasts (Fig. 6F).
Note that the use of sphingosine-1-phosphate as SGPL1 substrate is not recommended for this assay. The recovery of the produced 2-hexadecenal from homogenates is not complete (unpublished data), as was also noticed by Berdyshev et al. (19). Furthermore, the Hantzsch reaction with synthetic 2-hexadecenal does not proceed well, giving rise to spurious peaks, whereas derivatization with 2,4-dinitrophenylhydrazine works smoothly (unpublished data). Both observations are likely due to the reactivity of the α,β-unsaturated carbonyl group toward sulphydryl and amino compounds. Adduct formation of α,β-unsaturated aldehydes with proteins and DNA has long been known (38), and more recently, it has also been demonstrated for the lyase metabolite of sphingosine-1-phosphate (39, 40).
In conclusion, a simple and cheap assay for aliphatic aldehydes is described, without an extraction step or need for a mass spectrometric detector, and it is generally applicable to enzymes producing aldehydes, as demonstrated for HACL1 and SGPL1. For quantitative measurements, an appropriate IS has to be selected, depending on the chain length of the produced aldehyde and the endogenous fatty aldehydes present in tissue/cells investigated. Likewise, the assay can be adapted to other analytical techniques in which aldehydes are quantified, such as plasmalogen content/composition analyses.
Acknowledgments
The authors appreciate the help of S. Asselberghs (KU Leuven, LIPIT) with the MS analysis and the interest of Dr. M. Baes (Laboratory for Cell Metabolism, Dept. Pharmaceutical and Pharmacological Sciences, KU Leuven) in our work.
Footnotes
Abbreviations:
- ALDH3A2
- fatty aldehyde dehydrogenase
- CHD
- cyclohexane-1,3-dione
- Cn-al
- saturated straight chain aldehyde containing n carbon atoms
- Cn-sphingosine
- sphingosine analogue containing n carbon atoms
- HACL1
- 2-hydroxyacyl-CoA lyase
- IS
- internal standard
- MPO
- myeloperoxidase
- OT
- oxythiamine
- PCYOX1
- prenylcysteine oxidase 1
- RP-HPLC
- reversed-phase HPLC
- SGPL1
- sphingosine-1-phosphate lyase
- TPP
- thiamine pyrophosphate
This work was supported by grants from the Flemish “Fonds voor Wetenschappelijk onderzoek” (G.0581.09N; G.0721.10N) and the Katholieke Universiteit Leuven (OT/08/040). S.M. was supported in part by ELA no. 2010-00215 and OT/08/040, both granted to Dr. M. Baes.
REFERENCES
- 1.Rizzo W. B. 2007. Sjogren-Larsson syndrome: molecular genetics and biochemical pathogenesis of fatty aldehyde dehydrogenase deficiency. Mol. Genet. Metab. 90: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rizzo W. B., Craft D. A., Somer T., Carney G., Trafrova J., Simon M. 2008. Abnormal fatty alcohol metabolism in cultured keratinocytes from patients with Sjogren-Larsson syndrome. J. Lipid Res. 49: 410–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tschantz W. R., Zhang L., Casey P. J. 1999. Cloning, expression, and cellular localization of a human prenylcysteine lyase. J. Biol. Chem. 274: 35802–35808 [DOI] [PubMed] [Google Scholar]
- 4.Lu J. Y., Hofmann S. L. 2006. Thematic review series: lipid posttranslational modifications. Lysosomal metabolism of lipid-modified proteins. J. Lipid Res. 47: 1352–1357 [DOI] [PubMed] [Google Scholar]
- 5.Foulon V., Antonenkov V. D., Croes K., Waelkens E., Mannaerts G. P., Van Veldhoven P. P., Casteels M. 1999. Purification, molecular cloning, and expression of 2-hydroxyphytanoyl-CoA lyase, a peroxisomal thiamine pyrophosphate-dependent enzyme that catalyzes the carbon-carbon bond cleavage during alpha-oxidation of 3-methyl-branched fatty acids. Proc. Natl. Acad. Sci. USA. 96: 10039–10044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foulon V., Sniekers M., Huysmans E., Asselberghs S., Mahieu V., Mannaerts G. P., Van Veldhoven P. P., Casteels M. 2005. Breakdown of 2-hydroxylated straight chain fatty acids via peroxisomal 2-hydroxyphytanoyl-CoA lyase: a revised pathway for the alpha-oxidation of straight chain fatty acids. J. Biol. Chem. 280: 9802–9812 [DOI] [PubMed] [Google Scholar]
- 7.Van Veldhoven P. P., Gijsbers S., Mannaerts G. P., Vermeesch J. R., Brys V. 2000. Human sphingosine-1-phosphate lyase: cDNA cloning, functional expression studies and mapping to chromosome 10q22(1). Biochim. Biophys. Acta. 1487: 128–134 [DOI] [PubMed] [Google Scholar]
- 8.Van Veldhoven P. P. 2000. Sphingosine-1-phosphate lyase. Methods Enzymol. 311: 244–254 [DOI] [PubMed] [Google Scholar]
- 9.Van Veldhoven P. P., Mannaerts G. P. 1994. Sphinganine 1-phosphate metabolism in cultured skin fibroblasts: evidence for the existence of a sphingosine phosphatase. Biochem. J. 299: 597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thukkani A. K., Hsu F. F., Crowley J. R., Wysolmerski R. B., Albert C. J., Ford D. A. 2002. Reactive chlorinating species produced during neutrophil activation target tissue plasmalogens: production of the chemoattractant, 2-chlorohexadecanal. J. Biol. Chem. 277: 3842–3849 [DOI] [PubMed] [Google Scholar]
- 11.Wu L. C., Pfeiffer D. R., Calhoon E. A., Madiai F., Marcucci G., Liu S., Jurkowitz M. S. 2011. Purification, identification, and cloning of lysoplasmalogenase, the enzyme that catalyzes hydrolysis of the vinyl ether bond of lysoplasmalogen. J. Biol. Chem. 286: 24916–24930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunawan J., Debuch H. 1982. Lysoplasmalogenase–a microsomal enzyme from rat brain. J. Neurochem. 39: 693–699 [DOI] [PubMed] [Google Scholar]
- 13.Van Veldhoven P. P., Mannaerts G. P. 1991. Subcellular localization and membrane topology of sphingosine-1-phosphate lyase in rat liver. J. Biol. Chem. 266: 12502–12507 [PubMed] [Google Scholar]
- 14.Piantadosi C., Snyder F. 1970. Plasmalogens and related derivatives: their chemistry and metabolism. J. Pharm. Sci. 59: 283–297 [DOI] [PubMed] [Google Scholar]
- 15.Jansen G. A., Verhoeven N. M., Denis S., Romeijn G., Jakobs C., ten Brink H. J., Wanders R. J. 1999. Phytanic acid alpha-oxidation: identification of 2-hydroxyphytanoyl-CoA lyase in rat liver and its localisation in peroxisomes. Biochim. Biophys. Acta. 1440: 176–182 [DOI] [PubMed] [Google Scholar]
- 16.Bandhuvula P., Fyrst H., Saba J. D. 2007. A rapid fluorescence assay for sphingosine-1-phosphate lyase enzyme activity. J. Lipid Res. 48: 2769–2778 [DOI] [PubMed] [Google Scholar]
- 17.Bandhuvula P., Li Z., Bittman R., Saba J. D. 2009. Sphingosine 1-phosphate lyase enzyme assay using a BODIPY-labeled substrate. Biochem. Biophys. Res. Commun. 380: 366–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bedia C., Camacho L., Casas J., Abad J. L., Delgado A., Van Veldhoven P. P., Fabrias G. 2009. Synthesis of a fluorogenic analogue of sphingosine-1-phosphate and its use to determine sphingosine-1-phosphate lyase activity. ChemBioChem. 10: 820–822 [DOI] [PubMed] [Google Scholar]
- 19.Berdyshev E. V., Goya J., Gorshkova I., Prestwich G. D., Byun H. S., Bittman R., Natarajan V. 2011. Characterization of sphingosine-1-phosphate lyase activity by electrospray ionization-liquid chromatography/tandem mass spectrometry quantitation of (2E)-hexadecenal. Anal. Biochem. 408: 12–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reina E., Camacho L., Casas J., Van Veldhoven P. P., Fabrias G. 2012. Determination of sphingosine-1-phosphate lyase activity by gas chromatography coupled to electron impact mass spectrometry. Chem. Phys. Lipids. 165: 225–231 [DOI] [PubMed] [Google Scholar]
- 21.Luth A., Neuber C., Kleuser B. 2012. Novel methods for the quantification of (2E)-hexadecenal by liquid chromatography with detection by either ESI QTOF tandem mass spectrometry or fluorescence measurement. Anal. Chim. Acta. 722: 70–79 [DOI] [PubMed] [Google Scholar]
- 22.Sawicki E., Carnes R. A. 1968. Spectrophotofluorimetric determination of aldehydes with dimedone and other reagents. Mikrochim. Acta. 1968: 148–159 [DOI] [PubMed] [Google Scholar]
- 23.Sniekers M., Foulon V., Mannaerts G. P., Van Maldergem L., Mandel H., Gelb B. D., Casteels M., Van Veldhoven P. P. 2006. Thiamine pyrophosphate: an essential cofactor for the alpha-oxidation in mammals–implications for thiamine deficiencies? Cell. Mol. Life Sci. 63: 1553–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colie S., Van Veldhoven P. P., Kedjouar B., Bedia C., Albinet V., Sorli S. C., Garcia V., Djavaheri-Mergny M., Bauvy C., Codogno P., et al. 2009. Disruption of sphingosine 1-phosphate lyase confers resistance to chemotherapy and promotes oncogenesis through Bcl-2/Bcl-xL upregulation. Cancer Res. 69: 9346–9353 [DOI] [PubMed] [Google Scholar]
- 25.Weber C., Krueger A., Munk A., Bode C., Van Veldhoven P. P., Graler M. H. 2009. Discontinued postnatal thymocyte development in sphingosine 1-phosphate-lyase-deficient mice. J. Immunol. 183: 4292–4301 [DOI] [PubMed] [Google Scholar]
- 26.Fraccascia P., Casteels M., De Schryver E., Van Veldhoven P. P. 2011. Role of thiamine pyrophosphate in oligomerisation, functioning and import of peroxisomal 2-hydroxyacyl-CoA lyase. Biochim. Biophys. Acta. 1814: 1226–1233 [DOI] [PubMed] [Google Scholar]
- 27.Baumann W. J., Schmid H. H., Mangold H. K. 1969. Oxidative cleavage of lipids with sodium metaperiodate in pyridine. J. Lipid Res. 10: 132–133 [PubMed] [Google Scholar]
- 28.Yoshino K., Matsuura T., Sano M., Saito S., Tomita I. 1986. Fluorometric liquid chromatographic determination of aliphatic aldehydes arising from lipid peroxides. Chem. Pharm. Bull. (Tokyo). 34: 1694–1700 [DOI] [PubMed] [Google Scholar]
- 29.Holley A. E., Walker M. K., Cheeseman K. H., Slater T. F. 1993. Measurement of n-alkanals and hydroxyalkenals in biological samples. Free Radic. Biol. Med. 15: 281–289 [DOI] [PubMed] [Google Scholar]
- 30.Matsuoka M., Imado N., Maki T., Banno K., Sato T. 1996. Determination of free aliphatic aldehydes in plasma by high-performance liquid chromatography of the 1,3-cyclohexanedione derivatives. Chromatographia. 43: 501–506 [Google Scholar]
- 31.Baes M., Van Veldhoven P. P. 2012. Mouse models for peroxisome biogenesis defects and beta-oxidation enzyme deficiencies. Biochim. Biophys. Acta. 1822: 1489–1500 [DOI] [PubMed] [Google Scholar]
- 32.Ingrand S. S., Wahl A., Favreliere S., Barbot F., Tallineau C. 2000. Quantification of long-chain aldehydes by gas chromatography coupled to mass spectrometry as a tool for simultaneous measurement of plasmalogens and their aldehydic breakdown products. Anal. Biochem. 280: 65–72 [DOI] [PubMed] [Google Scholar]
- 33.Anderson J. M. 1986. Fluorescent hydrazides for the high-performance liquid chromatographic determination of biological carbonyls. Anal. Biochem. 152: 146–153 [DOI] [PubMed] [Google Scholar]
- 34.Uzu S., Kanda S., Imai K., Nakashima K., Akiyama S. 1990. Fluorogenic reagents: 4-aminosulphonyl-7-hydrazino-2,1,3-benzoxadiazole, 4-(N,N-dimethylaminosulphonyl)-7-hydrazino-2,1,3-benzoxadiazole and 4-hydrazino-7-nitro-2,1,3-benzoxadiazole hydrazine for aldehydes and ketones. Analyst (Lond.). 115: 1477–1482 [Google Scholar]
- 35.Iwata T., Hirose T., Nakamura M., Yamaguchi M. 1993. 6,7-Dimethoxy-1-methyl-2-oxo-1,2-dihydroquinoxalin-3-ylpropionohydrazide as a fluorescence derivatization reagent for aldehydes in high-performance liquid-chromatography. Analyst (Lond.). 118: 517–519 [Google Scholar]
- 36.Nakashima K., Hidaka Y., Yoshida T., Kuroda N., Akiyama S. 1994. High-performance liquid chromatographic determination of short-chain aliphatic aldehydes using 4-(N,N-dimethylaminosulphonyl)-7-hydrazino-2,1, 3-benzoxadiazole as a fluorescence reagent. J. Chromatogr. B Biomed. Appl. 661: 205–210 [DOI] [PubMed] [Google Scholar]
- 37.Xiong X. J., Wang H., Rao W. B., Guo X. F., Zhang H. S. 2010. 1,3,5,7-Tetramethyl-8-aminozide-difluoroboradiaza-s-indacene as a new fluorescent labeling reagent for the determination of aliphatic aldehydes in serum with high performance liquid chromatography. J. Chromatogr. A. 1217: 49–56 [DOI] [PubMed] [Google Scholar]
- 38.Witz G. 1989. Biological interactions of alpha,beta-unsaturated aldehydes. Free Radic. Biol. Med. 7: 333–349 [DOI] [PubMed] [Google Scholar]
- 39.Brahmbhatt V. V., Hsu F. F., Kao J. L., Frank E. C., Ford D. A. 2007. Novel carbonyl and nitrile products from reactive chlorinating species attack of lysosphingolipid. Chem. Phys. Lipids. 145: 72–84 [DOI] [PubMed] [Google Scholar]
- 40.Upadhyaya P., Kumar A., Byun H. S., Bittman R., Saba J. D., Hecht S. S. 2012. The sphingolipid degradation product trans-2-hexadecenal forms adducts with DNA. Biochem. Biophys. Res. Commun. 424: 18–21 [DOI] [PMC free article] [PubMed] [Google Scholar]