Abstract
There is an increasing need to develop new neuropsychometric tools sensitive enough to detect subtle declines in cognitive performance during normal aging, as well as to distinguish between normal aging and the earliest stages of dementia. In this study, we report our findings regarding a new confrontational naming test, the Memory for Names test. We conducted evaluations utilizing a cohort of 234 elderly participants who comprised a spectrum of cognitive function ranging from normal for age (Uniform Data Set Overall Appraisal = 2, Clinical Dementia Rating = 0) to demented (Clinical Dementia Rating = 1–2, Mini Mental Status Examination Total Score <25). The Memory for Names test was found to measure the same cognitive construct as the Boston Naming Test. In conclusion, the Memory for Names test is a reliable and valid measure of age-related cognitive function that can discriminate between normal aging and mild cognitive impairment, and between mild cognitive impairment and dementia.
Keywords: Alzheimer’s disease, anomia, Boston naming, facial recognition, mild cognitive impairment
INTRODUCTION
Distinguishing normal age-related decline in cognitive function from the cognitive disturbances observed in Alzheimer’s disease (AD) and other age-related dementias is most difficult in the initial and early stages of disease onset. A number of research studies have identified that mild cognitive impairment (MCI) may represent one of the earliest stages of AD [1], although the ability to reliably detect MCI in an elderly population remains a significant clinical challenge [2]. Current research is focused on developing tools which identify the earliest stages of AD in order to understand the basis for dementia, as well as to allow for beginning pharmaceutical interventions in the earliest stages of AD. Taken together, these findings implicate the growing need for an array of new psychometric tests which can distinguish normal aging from MCI and the early stages of dementia in AD and other age-related dementias.
The Boston Naming Test (BNT) is a test of confrontational naming, consisting of 60 simple line-drawings. The initial authors of the BNT reported a decline in naming ability associated with age, particularly after the age of 70 [3]. This was supported by subsequent studies involving an increasingly aged cohort [4]. Many studies have demonstrated the utility of the BNT in distinguishing between late stage AD and age-related decline [5-7]. More recent studies of the BNT in the context of normal aging, MCI, and AD populations have repeatedly shown MCI patients performing normally or similar to age-matched controls on the BNT [8, 9]. These data suggest that while the BNT has utility in normal aging and distinguishing normal aging from AD, it has less utility in identifying MCI.
This report outlines the potential utility of the Memory for Names (Mem4Names) test, a test of confrontational naming, for the study of normal aging and dementia. Additionally, our results provide evidence to support our hypothesis that Mem4Names can discriminate between MCI and normal aging, and between MCI and dementia.
METHODS
Participants
A total of 234 participants were involved in this study. All were participants in the ongoing longitudinal cognitive assessment studies as part of the Institute for Dementia Research and Prevention (IDRP) at the Pennington Biomedical Research Center.
Normal controls
The normal control group was comprised of 169 participants in the Louisiana Aging Brain Study (LABrainS). Volunteers for the LABrainS study are recruited for the study through regular outreach efforts of the IDRP throughout Louisiana, with LABrainS participants consisting of non-demented elderly individuals willing to undergo annual cognitive assessments. The cognitive assessments for these participants are conducted utilizing the Uniform Data Set (UDS) Neuropsychological battery established by the National Alzheimer’s Coordinating Center (NACC). All participants in this control group were classified as UDS 2, indicating a neurocognitive status considered normal for age.
Mild cognitive impairment
The MCI group consisted of 41 participants in LABrainS. These participants receive annual cognitive evaluations utilizing the UDS Neuropsychological battery, with all participants in the MCI group classified as UDS 3, indicating test performance on one or two components of the UDS battery abnormal for age. These participants represented individuals with both amnestic and non-amnestic forms of MCI. Additionally, each of these participants was designated as having a Clinical Dementia Rating (CDR) of 0 and a Mini-Mental Status Exam (MMSE) >25, consistent with the absence of dementia.
Dementia
The dementia group consisted of 24 participants recruited from the IDRP’s longitudinal study of dementia. The demented individuals had MMSE scores <25 and CDR scores of 1–2, consistent with the presence of dementia.
Uniform Data Set
The neuropsychological test battery from the Uniform Data Set (UDS) of the Alzheimer’s Disease Centers’ (ADC) program of the National Institute on Aging consists of brief measures of attention, processing speed, executive function, episodic memory, and language [10]. The battery consists of the MMSE, Digit Span Forward and Backward from the Wechsler Memory Scale-Revised, Digit Symbol Subtest from the Wechsler Adult Intelligence Scale-Revised, Trails Making Test Parts A and B, Logical Memory Story A Immediate Recall from the Wechsler Memory Scale-Revised, Logical Memory Story A Delayed Recall from the Wechsler Memory Scale-Revised, Verbal Fluency (Animals and Vegetables), and the Boston Naming Test (30 odd items).
Memory for Names test
Study participants received the full 75 item version of Mem4Names. The faces chosen included a mixture of 75 famous political/historical figures and various entertainers from film, television, and music. Emphasis was placed on selecting images of people widely and easily recognizable by individuals over the age of 60. Image selection process closely mirrors item selection for similar studies of this type [11, 12]. Pilot studies were initially conducted on 25 study participants (as part of a separate study) to ensure the recognizability of the faces selected. Potentially troublesome items were eliminated prior to the development of the final list of faces. All images were obtained via public internet search and placed on a standardized background.
The Mem4Names test was administered to each participant utilizing the following standardized instructions:
“This is a test of memory for names. I will show you a series of pictures of famous individuals. They may be famous actors, presidents or other historical figures. Please tell me both the first and last name of each person.” “If the person’s face is familiar to you but you cannot recall the name, please tell me what you know about them. Say … He’s a former president, TV actor, etc.”
Participants who spontaneously named the individual received a score of one for “Correct” and the next item was administered. Names were considered correct if first and last name were accurately provided or if correct title was incorporated in the participant’s response (e.g., President Truman).
Participants who struggled to formulate a response for a period of longer than 15 seconds received a score of zero for correct response and were then evaluated to determine if they knew the context of the individual pictured. This was determined either by the participant’s open elaborations (e.g., “I know he’s the president who dropped the bomb” or “He was a football player accused of killing his wife”, etc.) or by the examiner query of:
“What is he/she famous for?”
If the participant expressed proper context a score of one was recorded for “Context”. Participants unable to provide context or providing erroneous context received a score of zero. After determining the participants’ identification of context they were cued with the first name of the individual and scored on their ability to provide the last name.
Statistical analyses
As detailed above, the Mem4Names test consists of three subscales (Correct, Context, and Cued). The Correct score reflects patients’ ability to name a face without facilitation and this subscale was used to evaluate items. We also report the reliability and validity results for this subscale, though test-retest correlation coefficients and means are also reported for the Context and Cued subscales.
Item evaluation
Item evaluation was performed using item total correlations on the initial set of 75 items. This analysis relied on the entire sample of participants, including Normal Controls, MCI, and participants with Dementia. Items with correlation coefficients less than 0.30 [13] were eliminated. Item total correlations were conducted until all test items demonstrated correlation coefficients greater than or equal to 0.30.
Reliability
Internal consistency reliability was assessed using Cronbach’s alpha with the entire sample of participants. Test-retest reliability was examined using a random sub-sample of 50 Normal Controls and MCI participants who completed the Mem4Names on two occasions separated by approximately two weeks (mean ± SD days between tests = 15 ± 2.8).
Validity
Construct validity for Mem4Names was examined using a multi-tiered approach. First, all participants’ data were used in an exploratory principle components analysis on Mem4Names Correct items to confirm that a single construct was being measured. Second, convergent validity correlation coefficients were calculated between Mem4Names Correct Score performance and the following UDS tests (this analyses was conducted only with participants who had UDS data; i.e., Normal Controls and MCI): a) the Boston Naming Test (BNT), which assesses verbal memory, fluency, and naming, and b) Controlled Oral Word Association Test (COWAT; vegetables and animals), which measures verbal memory, fluency, and naming. We hypothesized that Mem4Names would correlate significantly with the BNT and COWAT to demonstrate convergent validity. Third, an exploratory principle components analysis was conducted to determine if Mem4Names Correct score performance loaded on the same factor as the BNT and COWAT. Fourth, a multivariate analysis of variance (MANOVA) was used to determine if performance on Mem4Names differed among the Normal Control, MCI, and Dementia groups. Gender, age, and education were entered as covariates.
Creation of forms A and B for serial testing
Following completion of validity analyses, Mem4Names items were randomly allocated into two forms (A and B) to facilitate serial testing and reduce practice effects. All reliability and validity analyses, except principle components analysis, were repeated for each of the two short forms.
Identification of Mem4Names cut-scores and evaluation of sensitivity and specificity
Receiver operating characteristic (ROC) analyses were used to determine the optimal cut-scores for classifying participants as normal controls, MCI, or demented. A ROC curve is a plot of sensitivity (y-axis) versus 1-specificity (x-axis) of a diagnostic test with a binary result such as either test positive or test negative. Sensitivity is the proportion or percent of true positive participants who are classified as positive by the diagnostic test, whereas specificity is the proportion or percent of true negative participants who are classified as negative by the diagnostic test. Thus, 1-specificity is the proportion or percent of true negative participants who are classified as positive by the diagnostic test and in this context is the relative number of false positive test results. By plotting the sensitivity against 1-specificity for incremental choices of cut-scores, we obtain a visual aid (ROC curve) for determining the optimal diagnostic cut-scores. An ideal diagnostic test has both a high sensitivity and a high specificity. High specificity is equivalent to 1-specificity being low: hence, ideally, the ROC curve approaches the upper left corner of the plot. The optimal cut-scores for distinguishing normal controls from MCI, and MCI from demented participants were then selected balancing sensitivity and specificity. Area Under the Curve (AUC) analysis was then conducted to quantify and summarize Mem4Names’ diagnostic accuracy. AUC values range from 0.50 to 1.0, with 0.50 indicating a very poor test and 1.0 indicating a test that perfectly separates two groups. Because three groups of participants were included in this study, separate ROC analyses were conducted to identify optimal Mem4Names cut-points to distinguish: 1) normal controls from MCI, and 2) MCI from dementia. In the first analysis, sensitivity was defined as the percentage of MCI participants correctly classified and specificity was defined as the percentage of normal controls correctly classified. In the second analysis, sensitivity was defined as the percentage of dementia participants correctly classified and specificity was defined as the percentage of MCI participants correctly classified.
RESULTS
Characteristics of the study sample
The sample included 234 participants (58.5% female; mean age ± SD, 70.3 ± 8.6 years). The characteristics of the entire study sample and by group (Control/Normals, MCI, and Demented) are provided in Table 1. Characteristics among the subsamples used to examine test-retest reliability and the validity of the instruments were very similar (data not shown).
Table 1.
Characteristics of the entire study sample and by group
| Total sample (n = 234) |
Normal controls (n = 169) |
MCI (n = 41) |
Demented (n = 24) |
|
|---|---|---|---|---|
| Gender (n, %) | ||||
| Male | 97 (41.5) | 55 (32.5) | 27 (65.9) | 15 (62.5) |
| Female | 137 (58.5) | 114 (67.5) | 14 (34.1) | 9 (37.5) |
| Race (n, %) | ||||
| Caucasian | 231 (98.7) | 167 (98.8) | 40 (97.6) | 24 (100.0) |
| African American | 3 (1.3) | 2 (1.2) | 1 (2.4) | 0 (0.0) |
| Age (y) | 70.3 ± 8.6 | 69.7 ± 8.3 | 71.3 ± 8.5 | 72.8 ± 10.2 |
| Education (y) | 15.8 ± 2.5 | 16.0 ± 2.5 | 15.6 ± 2.5 | 15.1 ± 2.6 |
| MMSE | 28.5 ± 2.6 | 29.3 ± 1.0 | 28.4 ± 1.4 | 22.5 ± 4.2 |
Note: Gender and race are reported as number (percent). All other values represent mean ± SD.
Item evaluation
Two items were eliminated for being closely related to each other, which created the possibility of a priming effect (e.g., both Bill and Hillary Clinton were included in the original 75 items, and Bill Clinton was eliminated). One item was eliminated due to an item-total correlation coefficient <0.30. The resulting Mem4Names test consisted of 72 items.
Reliability
Test-retest reliability coefficients between visits were considered large [14] for Correct (r = 0.89, p < 0.001), Context (r = 0.87, p < 0.001), and Cued (r = 0.79, p < 0.001) sub-scale scores. Cronbach’s alpha was high (0.96), demonstrating good internal consistency reliability and suggesting that the 72 items assess the same construct.
Validity
The exploratory principal components analysis on Mem4Names Correct items supported a single factor solution. Although more than one factor had an eigenvalue >1.0, the difference between the first factor (eigenvalue = 19.5) and the second factor (eigenvalue = 3.9) indicates that a single factor solution is appropriate. This conclusion was supported by inspection of the scree plot that showed a very steep slope between factors 1 and 2 and a flattening of the plot thereafter. The single factor accounted for 27% of the variance and the Cronbach’s alpha (0.96) reported earlier is high.
Convergent validity was supported by significant correlation coefficients between the Correct subscale of Mem4Names and the: 1) BNT (r = 0.65, p < 0.001) and 2) the animals (r = 0.47, p < 0.001) and vegetables (r = 0.37, p < 0.001) subscales of the COWAT. Additionally, the exploratory principal components analysis demonstrated that the Correct score from Mem4Names loaded on the same factor as the BNT and COWAT. Only one factor had an eigenvalue >1.0 [15] and inspection of the scree plot showed a large slope between factors 1 and 2 with the plot flattening thereafter. This single factor accounted for 62% of the variance. The factor loadings for the Mem4Names Correct score, animal naming, vegetable naming, and the BNT were 0.80, 0.80, 0.72, and 0.82, respectively.
MANOVA indicated that performance on the Correct, Context, and Cued subscales of Mem4Names differed significantly (p-values <0.01) by group (Normal Controls, MCI, and participants with Dementia). As expected, post-hoc tests indicated that increased levels of impairment were associated with lower scores on the Correct subscale and higher scores on the Context and Cued subscales (Table 2).
Table 2.
Mean (±SD) Mem4Names scores by participant subsample
| Total sample (n = 234) |
Normal controls (n = 169) |
MCI (n = 41) |
Dementia (n = 24) |
|
|---|---|---|---|---|
| Mem4Names test (72 items) | ||||
| Correct | 47.4 ± 15.9 | 53.1 ± 10.8 | 40.3 ± 14.8 | 19.3 ± 13.3 |
| Context | 13.7 ± 8.2 | 11.8 ± 7.5 | 18.3 ± 8.5 | 18.7 ± 7.9** |
| Cued | 15.4 ± 8.3 | 13.0 ± 6.9 | 19.0 ± 7.9 | 26.2 ± 6.9 |
| Form A (36 items) | ||||
| Correct | 22.7 ± 8.2 | 25.6 ± 5.8 | 19.0 ± 7.6 | 8.5 ± 6.1 |
| Context | 7.4 ± 4.3 | 6.5 ± 4.1 | 9.7 ± 4.3 | 9.2 ± 3.7* |
| Cued | 8.9 ± 4.7 | 7.7 ± 4.1 | 11.1 ± 4.5 | 13.8 ± 4.4 |
| Form B (36 items) | ||||
| Correct | 24.6 ± 8.0 | 27.4 ± 5.5 | 21.1 ± 7.5 | 10.8 ± 7.7 |
| Context | 6.4 ± 4.5 | 5.3 ± 3.9 | 8.7 ± 4.8 | 9.5 ± 4.6** |
| Cued | 6.5 ± 4.3 | 5.3 ± 3.5 | 7.9 ± 4.2 | 12.4 ± 3.7 |
Note: A MANOVA was used to determine if Correct, Context, and Cued scores differed by group (no comparisons were made to the mean from the total sample; comparisons were only performed among the Normal controls, Cognitively impaired, and Demented groups). Means without asterisks differed significantly (p <0.05) from each other, with the following exceptions:
did not differ significantly from normal controls or cognitively impaired
did not differ significantly from cognitively impaired.
Creation of Forms A and B for serial testing
The 72 item Mem4Names test was split into two 36 item tests (Forms A and B). With the exception of 3 items on Form A, item total correlations on Forms A and B were ≥0.30. Internal consistency reliability was supported for both forms, with Cronbach’s alpha equal to 0.92 for Form A and Form B. Test-retest reliability was also demonstrated for Correct (Form A, r = 0.84, p < 0.001; Form B, r = 0.86, p < 0.001), Context (Form A, r = 0.75, p < 0.001; Form B, r = 0.81, p < 0.001), and Cued (Form A, r = 0.71, p < 0.001; Form B, r = 0.73, p < 0.001) sub-scales.
Correct subscale performance on Forms A and B also demonstrated convergent validity with the BNT (Form A, r = 0.63, p < 0.001; Form B, r = 0.62, p < 0.001) and the animals (Form A, r = 0.44, p < 0.001; Form B, r = 0.47, p < 0.001) and vegetables (Form A, r = 0.35, p < 0.001; Form B, r = 0.36, p < 0.001) sub-scales of the COWAT. Finally, MANOVA indicated that Mem4Names performance differences among groups (Normal Controls, MCI, and participants with Dementia) were very similar to those obtained with the 72-item test (Table 2).
Receiver operating characteristic (ROC) analyses
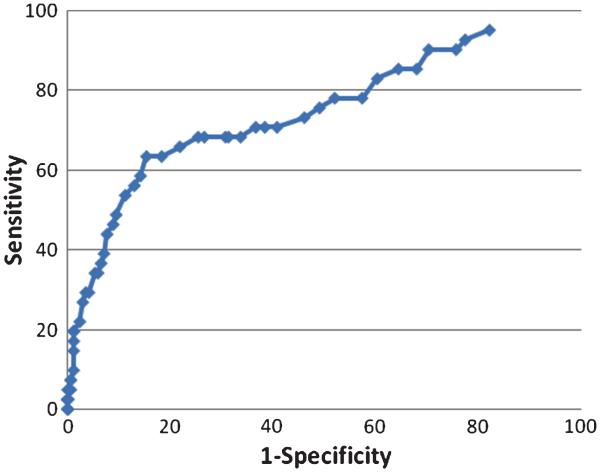
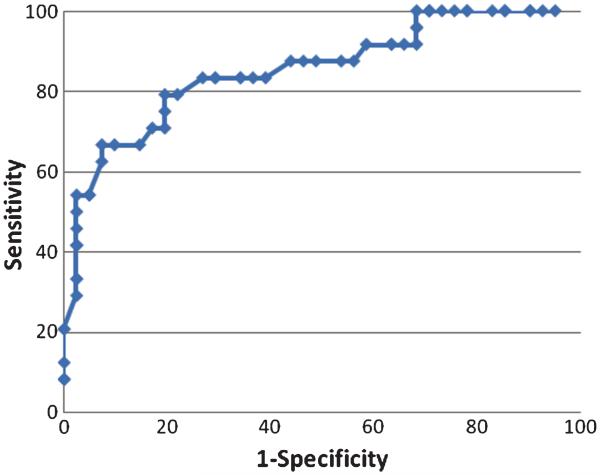
The ROC analysis of Mem4Names cut-scores for classifying normal controls from MCI participants indicated that a cut-score of 49 had sensitivity of 68.3% and specificity of 68.6%, with an AUC value of 0.75 (95th CI: 0.66–0.84) (Fig. 1). The ROC analysis of Mem4Names cut-scores for classifying MCI from demented participants indicated that a cut-score of 28 had sensitivity of 79.2% and specificity of 80.5%, with an AUC value of 0.85 (95th CI: 0.75–0.95) (Fig. 2).
Fig. 1.
Receiver operating characteristic (ROC) plot of Mem4Names cut-scores for classifying normal controls from MCI participants. The Mem4Names cut-score of 49 had sensitivity of 68.3% and specificity of 68.6%, with an AUC value of 0.75 (95th CI: 0.66–0.84) for discrimination between controls and MCI participants.
Fig. 2.
Receiver operating characteristic (ROC) plot of Mem4Names cut-scores for classifying MCI from demented participants. The Mem4Names cut-score of 28 had sensitivity of 79.2% and specificity of 80.5%, with an AUC value of 0.85 (95th CI: 0.75–0.95) for discrimination between MCI and demented participants.
DISCUSSION
The reliability and validity of Mem4Names, a test of confrontational naming and facial recognition, was supported by our study. The focus of this study was not to provide normative data, but to demonstrate the application, sensitivity, and specificity of the Mem4Names test. Mem4Names is one of the first tests of facial recognition and naming that includes stimuli familiar to people in North America. Previous test development efforts relied on stimuli familiar to people in the United Kingdom or Europe [11, 12]. Importantly, Mem4Names is the first test to include alternate forms, which facilitates serial assessment while controlling practice effects. Hence, Mem4Names can be used to quantify change in cognitive function over time.
Mem4Names performance was sensitive to differences in cognitive function among groups of people who were healthy, cognitively impaired, as well as those diagnosed with dementia. These findings are consistent with previous studies that found facial recognition and naming decrements among patients diagnosed with early AD compared to healthy controls [11]. Similarly, patients diagnosed with semantic dementia performed worse on a measure of facial recognition and naming compared to patients diagnosed with AD, with both groups performing worse than a healthy control group [12]. Cognitive decline is often associated with difficulty recognizing faces and names. Compared to other types of visual stimuli, facial recognition and face-name association impose a unique complexity that provides for greater utility for early detection in the initial stages of AD [16]. Mem4Names is a useful tool to quickly quantify this decrement. In particular, Mem4Names is a useful test for quantifying normal versus pathological degrees of facial recognition (in regards to recognition of famous individuals). It is possible that facial recognition and naming is sensitive to the early detection of cognitive decline compared to tests of verbal fluency, which assess a construct that is frequently used and possibly over-practiced. Additional research is warranted to determine if Mem4Names performance is more sensitive to the early detection of cognitive decline.
The ROC analyses identified Mem4Names cut-scores that differentiated normal controls from MCI participants, and MCI from demented participants. When classifying normal controls from MCI participants with a cut-score of 49, the sensitivity, specificity, and AUC values were fair and support the use of this cut-score. The sensitivity, specificity, and AUC values were higher when classifying MCI from demented participants with a cut-score of 28, which clearly supports this cut-score. The ROC analysis results support the clinical and diagnostic utility of Mem4Names. Importantly, Mem4Names can classify normal controls from MCI, and MCI from dementia, which is noteworthy for a single, brief instrument. Indeed, correctly classifying these groups even with a battery of instruments can be difficult.
In a recent study utilizing a small sample of participants with probable AD, the authors trained 4 older adults to recall name-face-occupation associations utilizing the spaced retrieval technique. Interestingly, the authors found that the AD participants remembered the target person’s occupation more often than their name [17]. While the basis for this observation is not known, these data raise the potential for utilizing multiple variations and permutations of the name-face recognition test model to probe our understanding of how cognition is altered during the progression of dementia.
It is interesting to note that previous studies utilizing name and face recognition in the context of functional MRI found that distinct areas of activation were observed for face recognition versus name recognition [18]. Activation for faces was primarily right lateralized while recognition of names was observed to be left lateralized [18]. Importantly, regardless of whether discussing face or name recognition, there was observed to be an extensive amount of bilateral activity within multiple brain structures including hippocampal gyri, posterior cingulated, and middle temporal gyri. All of these anatomical areas are known to undergo significant increases in neuropathology during normal aging as well as age-related dementias such as AD [19-21]. Such studies, in conjunction with our present findings support continued research to explore the utility of Mem4Names in studying cognitive function in context of aging and age-related dementias. In particular, future studies of the Mem4Names should seek to administer the test to larger segments of the LABrainS cohort for the purpose of developing more extensive norms including among minority populations, focus on the impact of demographics on test performance, the utility of the instrument in the neuropsychological assessment of other neurological conditions (brain injury, stroke, etc.), and its possible use as a measure of memory malingering.
ACKNOWLEDGMENTS
The authors would like to acknowledge the following for their part in the completion of this project: The Hibernia National Bank/Edward G. Schlieder Endowed Chair at Pennington Biomedical Research Center, and the Institute for Dementia Research and Prevention study participants. Corby Martin is supported by NIH grant K23 DK068052.
Footnotes
Authors’ disclosures available online (http://www.jalz.com/disclosures/view.php?id=659).
REFERENCES
- 1.Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 2.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, De Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, Van Duijn C, Visser P, Petersen R. Mild cognitive impairment – beyond controversies, towards a consensus: report of the International Working Group on mild cognitive impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan E, Goodglass H, Weintraub S. TheBostonNaming Test. Lee and Febiger; Philadelphia: 1983. [Google Scholar]
- 4.Albert MS, Heller HS, Milberg W. Changes in naming ability with age. Psychol Aging. 1988;3:173–178. doi: 10.1037//0882-7974.3.2.173. [DOI] [PubMed] [Google Scholar]
- 5.Huff FJ, Corkin S, Growdon JH. Semantic impairment and anomia in Alzheimer’s disease. Brain Lang. 1986;28:235–249. doi: 10.1016/0093-934x(86)90103-3. [DOI] [PubMed] [Google Scholar]
- 6.Margolin D, Pate DS, Friedrich FJ, Elia E. Dysnomiain dementia and in stroke patients: different underlying cognitive deficits. J Clin Exp Neuropsychol. 1990;12:597–612. doi: 10.1080/01688639008401004. [DOI] [PubMed] [Google Scholar]
- 7.Storandt M, Hill RD. Very mild senile dementia of the Alzheimer’s type II. Psychometric test performance. Arch Neurol. 1989;46:383–386. doi: 10.1001/archneur.1989.00520400037017. [DOI] [PubMed] [Google Scholar]
- 8.Balthazar ML, Martinelli JE, Cendes F, Damasceno BP. Lexical semantic memory in amnestic mild cognitive impairment and mild Alzheimer’s disease. Arq Neuropsiquiatr. 2007;65:619–622. doi: 10.1590/s0004-282x2007000400014. [DOI] [PubMed] [Google Scholar]
- 9.Balthazar ML, Cendes F, Damasceno BP. Semantic error patterns on the Boston Naming Test in normal aging, amnestic mild cognitive impairment, and mild Alzheimer’s disease: is there semantic disruption? Neuropsychology. 2008;22:703–709. doi: 10.1037/a0012919. [DOI] [PubMed] [Google Scholar]
- 10.Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Cummings J, DeCarli C, Foster NL, Galasko D, Peskind E, Dietrich W, Beekly DL, Kukull WA, Morris J. The Alzheimer’s disease centers’ Uniform Data Set (UDS), The neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greene JD, Hodges JR. Identification of famous faces and famous names in early Alzheimer’s disease. Relationship to anterograde episodic and general semantic memory. Brain. 1996;119:111–128. doi: 10.1093/brain/119.1.111. [DOI] [PubMed] [Google Scholar]
- 12.Snowden JS, Thompson JC, Neary D. Knowledge of famous faces and names in semantic dementia. Brain. 2004;127:860–872. doi: 10.1093/brain/awh099. [DOI] [PubMed] [Google Scholar]
- 13.Cronk BC. How to Use SPSS: A Step-by-Step Guide Analysis and Interpretation. 5th Pyrczak Publishing; Glendale, CA: 2008. [Google Scholar]
- 14.Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 15.Tabachnick BG, Fidell LS. Using Multivariate Statistics. 5th Allyn & Bacon; Boston: 2007. [Google Scholar]
- 16.Werheid K, Clare L. Are faces special in alzheimer’s disease? Cognitive conceptualisation, neural correlates, and diagnostic relevance of impaired Memory for faces and names. Cortex. 2006;43:898–906. doi: 10.1016/s0010-9452(08)70689-0. [DOI] [PubMed] [Google Scholar]
- 17.Cherry KE, Walvoord AA, Hawley KS. Spaced retrieval enhances memory for a name-face-occupation association in older adults with probable Alzheimer’s disease. J Genet Psychol. 2010;171:168–181. doi: 10.1080/00221320903548118. [DOI] [PubMed] [Google Scholar]
- 18.Nielson KA, Seidenberg M, Woodard JL, Durgerian S, Zhang Q, Gross WL, Gander A, Guidotti LM, Antuono P, Rao SM. Common neural systems associated with the recognition of famous faces and names: an event-related fMRI study. Brain Cogn. 2010;72:491–498. doi: 10.1016/j.bandc.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perl DP. Neuropathology of Alzheimer’s disease. Mt Sanai J Med. 2010;77:32–42. doi: 10.1002/msj.20157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, Markesbery WR. “Preclinical” AD revisited: neuropathology of cognitively normal older adults. Neurology. 2000;55:370–376. doi: 10.1212/wnl.55.3.370. [DOI] [PubMed] [Google Scholar]
- 21.Keller JN. Age-related neuropathology, cognitive decline, and Alzheimer’s disease. Ageing Res Rev. 2006;5:1–13. doi: 10.1016/j.arr.2005.06.002. [DOI] [PubMed] [Google Scholar]