Abstract
Objectives
The purpose of this study was to estimate the degree of obesity misclassification between body mass index (BMI) and body fat percentage in adults with functional mobility impairment, and to determine cardiometabolic risk profiles.
Design and Methods
Data from the combined 2003–2006 National Health and Nutrition Examination Survey (NHANES) were incorporated. The representative sample included 852 individuals, aged 20–85 years, reporting at least one major physical limitation related to mobility or lower body function, and 4,724 individuals reporting no impairments. Body mass index, percent body fat (%BF) as determined by dual energy X-ray absorptiometry (DXA), objectively measured sedentary behavior and activity, and markers of cardiometabolic risk were compared between adults with and without functional mobility impairments. Among functional mobility impaired individuals, sensitivity, specificity, and receiver operating characteristic curves were used to evaluate the performance of BMI as a continuous variable, as well as various BMI thresholds to detect obesity defined by sex-specific %BF cutoffs.
Results
Adults with functional mobility impairments were older, had larger waist circumferences (WC), had greater prevalence of obesity according to BMI and %BF, were more sedentary, had less physical activity, and had higher overall cardiometabolic risk. The standard BMI cutoff for obesity had excellent specificity in both men (100%) and women (98.4%) with functional mobility impairment, but sensitivity was poor (<55%). Whereas approximately 36% and 43% of impaired men and women fell into the obese BMI category, over 80% of men and women were obese according to %BF. Individuals with high %BF who were misclassified as not obese, according to BMI, had a significantly higher prevalence of the metabolic syndrome (17.6%) compared to subjects with normal BMI and low %BF (2.1%).
Conclusions
Obesity misclassification and cardiometabolic risk is prevalent among individuals with functional mobility impairments, and thus diagnostic screening for obesity should be modified to account for %BF and/or waist circumference. Behavioral interventions to decrease sedentary behavior, increase activity, and reduce abdominal obesity are warranted.
Introduction
Obesity is an established predictor of insulin resistance, glucose intolerance, hyperglycemia, hypercholesterolemia, and hypertension. Left untreated, this combination of pathophysiologic factors precipitates significantly increased risk for early all-cause mortality (Despres and Lemieux, 2006; Kuk et al., 2006; Neter et al., 2011). Though clearly disturbing, these statistics fail to account for individuals with chronic or congenital physical disabilities-a sub-population in which overweight and obesity prevalence is significantly higher (Bandini et al., 2005; K. I. Lim et al., 2010; S. Lim et al., 2010; Neter et al., 2011; Rimmer and Wang, 2005; Weil et al., 2002; Weinheimer et al., 2010). Considering that individuals with disabilities are often excluded from public health surveillance, and moreover that the criterion for obesity using body mass index (BMI) may be a poor indicator of risk, these data inevitably underestimate the actual negative health implications specific to these populations.
Nearly all research related to the influence of obesity as a contributing factor for, or consequence of functional impairment, has been conducted among the general elderly population (Batsis et al., 2013; Delmonico et al., 2009; Goodpaster et al., 2001; Goodpaster et al., 2000; Visser et al., 2005; Woo et al., 2007). From a clinical perspective, there is an increasing interest in understanding disparate declines in functional capacity and changes in body composition, as a way to explain the health risks of disease, lifestyle and/or aging. As is frequently reported in the geriatrics and gerontology literature (Goodpaster et al., 2001), if muscle strength deteriorates at a greater rate or to a larger extent than skeletal muscle mass, muscle quality is significantly diminished, placing individuals at heightened risk of gross motor functional decline, falls, and early mortality (Pijnappels et al., 2008; Ruiz et al., 2008). However, it is also well established that among older adults, weakness is paralleled with increases in (Schrager et al., 2007); the confluence of which represents a predictor for subsequent declines in muscle quality (Delmonico et al., 2009; Goodpaster et al., 2001) and cardiometabolic disease (S. Lim et al., 2010).
It is conceivable that adults with chronic motor disabilities are at a substantially increased risk for cardiometabolic abnormalities (Peterson et al., 2013). Although BMI is a valid metric for stratifying the population into different risk categories, it does not discriminate adipose tissue and muscle, and lacks sensitivity to identify non-obese individuals with excess body fat (Okorodudu et al.). Previous studies have focused on validating measurement strategies to predict body fat distribution among patients with mobility impairments; however, many individuals with chronic disabilities have altered growth and distribution of adiposity, as well as premature musculoskeletal deterioration. As a result, such individuals may likely have a “normal” BMI, and yet still have excessive body fat or high risk for cardiometabolic abnormalities (Romero-Corral et al., 2010).
Therefore, the purpose of this study was to estimate the degree of obesity classification discrepancy among a nationally representative sample of adults with chronic motor impairments, and to assess the sensitivity and specificity of various BMI thresholds to detect obesity by percent body fat. Although obesity prevalence disparities have been linked to disabilities in older adults (Al Snih et al., 2010), there is no research to date that has exclusively examined these issues across a larger spectrum of ages. As a secondary objective, we sought to compare the cardiometabolic risk profiles among persons with and without functional mobility impairments.
Methods
Study Design and Sample
The National Health and Nutrition Examination Survey (NHANES) 2003/2004 and 2005/2006 surveys and data were specifically chosen based on the availability of relevant information pertaining to physical disabilities, anthropometrics, body composition, objective physical activity counts, and markers of cardiometabolic health. Of the 8,034 participants who were 20 years and older, and from whom valid body composition data from dual-energy x-ray absorptiometry (DXA) existed, a total of 852 individuals reported at least one major functional limitation related to mobility. A sample of 2,321 men and 2,403 women without functional limitations were used as controls for comparing cardiometabolic and activity characteristics.
Demographic and Anthropometrics Factors
Age, sex, and race/ethnicity were all assessed by self-report during the in-home interview. Age was used as both a continuous factor, as well as a categorical factor: (1) ≥ 20 years and <40 years, (2) ≥ 40 years and < 60 years, (3) ≥ 60 years. Race/ethnicity was categorized as: (1) non-Hispanic white, (2) non-Hispanic black, (3) Mexican American or other Hispanic, and (5) other. Annual household income was categorized as: (1) ≤ $24,999, (2) $25,000–$54,999, and (3) ≥ $55,000. Education was categorized as: (1) less than high school graduate, (2) high school graduate/general educational development (GED) or equivalent, and/or some college or Associate’s degree (e.g., A.A. A.S.), and (3) college graduate or above.
Weight was measured using a digital Toledo scale (Mettler-Toledo International, Inc., Columbus, OH), and participants wore only underwear, gown and foam slippers. Height was measured using a fixed stadiometer. BMI was calculated as weight in kilograms divided by height in meters squared (kg/m2), and standard categories were applied to determine if each participant was normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), or obese (≥ 30 kg/m2). Waist circumference was measured to the nearest 0.1 cm at the level of the iliac crest. Standard cutpoints for abdominal obesity in men (>102 cm) and women (>88cm) were used, as outlined by the Adult Treatment Panel (ATP) III report.
Body Composition
The NHANES dual energy X-ray absorptiometry scans were administered using a Hologic QDR-4500A fan-beam densitometer with Hologic software (Hologic Corp., Bedford, MA). The ratio of tissue penetration of the x-ray scans was used to distinguish bone from soft tissue and the percent body fat (%BF) in the soft tissue once bone was excluded. Obesity was defined on the basis of sex-specific cutoffs for %BF, at a level (≥25% for men, and ≥35% for women) associated with significant increases with cardiometabolic risk, and most frequently used in the literature (Gomez-Ambrosi et al., 2012; Okorodudu et al., 2010; Romero-Corral et al., 2007; Romero-Corral et al., 2008). Normal weight obesity (NWO) was defined as BMI ≤ 25 kg/m2, and %BF ≥ 25% for men or 35% for women, respectively.
Cardiometabolic Parameters
Resting systolic and diastolic blood pressures were measured three to four times with a mercury sphygmomanometer by trained staff. Non-fasting serum measures of HDL-cholesterol and high sensitivity C-reactive protein (hsCRP) concentrations were measured. Additionally, fasting measures were obtained for triglycerides, plasma glucose, and insulin. The homeostasis model assessment (HOMA) was calculated according to the formula: [I0 (μU/ml) × G0 (mmol/l)]/22.5 (Matthews et al., 1985). Insulin resistance was set at a HOMA score of ≥5.9, as recently determined and validated against hyperinsulinemic-euglycemic clamp by Tam and colleagues (Tam et al., 2012).
Metabolic Syndrome (MetS) Classification
Subjects were also classified with/without MetS, based on the recent harmonized definition (Alberti et al., 2009). MetS reflected the presence of any three or more abnormal findings from the following: (1) abdominal obesity (≥102 cm for men; ≥88cm for women), (2) elevated triglycerides (≥150 mg/dL [1.7 mmol/L]), (3) reduced HDL-cholesterol (<40 mg/dL [1.0 mmol/L] in men; <50 mg/dL [1.3 mmol/L] in women); (4) hypertension (≥ 130 mm Hg systolic and/or ≥85 mm Hg diastolic); and (5) elevated fasting glucose (≥ 100 mg/dL).
The NHANES Physical Functioning Questionnaire (PFQ)
The PFQ was designed as a way of assessing level of disability, as reported as difficulty performing specific, everyday tasks. The questions chosen as most relevant for this research involved the ranking of difficulty in performing specific tasks of everyday living, and those that are most relevant to mobility and lower extremity functional capacity. Answers to the following questions were used as a general screen for more specific PFQ questions related to different domains of physical disabilities:
PFQ049: Does a physical, mental or emotional problem now keep you from working at a job or business?
PFQ054: Because of a health problem, do you have difficulty walking without using any special equipment?
PFQ057: Are you limited in any way because of difficulty remembering or because you experience periods of confusion?
PFQ059: Are you limited in any way in any activity because of a physical, mental or emotional problem?
If the participant answered “yes” to any of the previous questions, the following PFQ items were also incorporated to reflect specific factors pertaining to self-reported mobility, strength and dynamic balance. The six physical limitation questions chosen for use in these analyses include difficulty ratings with the following questions: “By yourself and without using any special equipment, how much difficulty do you have: (PFQ061B) walking for a quarter of a mile (that is about two or three blocks)?; (PFQ061C) walking up ten steps without resting?; (PFQ061D) stooping, crouching or kneeling?; (PFQ061E) lifting or carrying something as heavy as ten pounds (like a sack of potatoes or rice)?; (PFQ061H) walking from one room to another on the same level?; (PFQ061I) Standing up from an armless straight chair
Participants were instructed to not report temporary conditions such as broken limbs or pregnancy. Response options for each task include: “no difficulty,” “some difficulty,” “much difficulty,” “unable to do,” “do not do this activity,” “refused” and “don’t know.” Subjects were classified on the basis of functional disability, defined as any difficulty in a physical task, consistent with prior studies (Alexander et al., 2000; Hoeymans et al., 1996). Moreover, in the event that subjects answered “yes” to question PFQ054 about difficulty walking without the use of special equipment, they were not asked to rate difficulty performing two of the functioning tasks: walking ¼ mile, and walking up ten steps without resting.
Objective Activity assessment
Habitual physical activity and sedentary behavior were assessed in NHANES with an accelerometer (Actigraph 7164; Actigraph, LLC, Fort Walton Beach, FL), which provided an objective estimate of the intensity of bodily movement. Lack of, or minimal movement (i.e. <100 counts per minute (cpm)) recorded by the accelerometer was used to derive the non-sleeping time spent in sedentary behavior, as previously documented (Maher et al., 2013). The accelerometer was worn on the right hip during waking hours by participants for 7 days. In order to represent an individual’s typical behavior in the assessment of activity, at least 4 days of monitoring with at least 10 hours per day were necessary for inclusion. Accelerometer counts were used to classify all worn time as either sedentary (<100 cpm), light activity (100 – 759 cpm), lifestyle moderate activity (760–2019 cpm), moderate physical activity (2020–5998 cpm), and/or vigorous physical activity (exercise, ≥5999 cpm). Total volumes of sedentary behavior and each activity category were summed from all time spent. However, since subjects wore the monitors for differing amounts of time, proportion of wear-time values were calculated for each subject to account for total number of minutes spent in SB and each activity category, relative to total time spent wearing the accelerometer.
Statistical Analysis
All statistical analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC). To obtain population-representative findings, analyses were conducted using sample weights for the combined 2003/2004 and 2005/2006 NHANES cycles which account for the complex survey design (including oversampling), survey nonresponse, and post-stratification. Descriptive characteristics were stratified by sex and function/mobility impairment status and are provided as means, standard errors, and percentages. Differences in these characteristics across function/mobility impairment status were tested using linear regression (proc surveyreg) and logistic regression (proc surveylogistic) for continuous (e.g., age, BMI, WC, glucose, etc.) and categorical variables (e.g., prevalence of NWO, abdominal obesity, insulin resistance, etc.) respectively, after creating appropriate categories and dummy coding for each. Moreover, adjusted regression analyses were performed to account for age and race/ethnicity. To assess the odds of the MetS in the adults with functional mobility impairments, separate weighted, adjusted logistic regression models (i.e., adjusted for sociodemographic factors such as BMI, age, education, race/ethnicity, and annual income) were performed. The effects of sedentary behavior and physical activity on MetS risk were assessed separately with adjusted models.
Analyses of receiver operating characteristic (ROC) curves were used to evaluate the overall performance of BMI as a continuous variable, as well as various specific BMI thresholds to detect obesity defined by %BF among subjects with functional mobility impairments. In conjunction with the ROC curves, individual two-by-two tables were constructed to assess the sensitivity, specificity, and positive/negative predictive values for each potential threshold. This was conducted to identify the best gender-specific BMI thresholds for detecting %BF-defined obesity.
RESULTS
Cardiometabolic Risk between Adults with/without Functional Mobility Impairments
Descriptive data are presented as weighted means, standard errors, and percentages across sexes and functional mobility impairment in Table 1. Among adults without impairments, obesity according to BMI was prevalent in both men (29.4%) and women (35.1%), while the prevalence of obesity in the mobility impaired adults was significantly greater for men (36.2%) and women (43.1%) (p < 0.001). Moreover, adults with mobility impairments were older, had a greater prevalence of abdominal obesity, were more sedentary, had less physical activity, and had higher cardiometabolic risk profiles for nearly every outcome. After adjusting for age and race/ethnicity (two known correlates of cardiometabolic outcomes), %BF and WC were still significantly higher for both men and women with functional mobility impairments, but only prevalence of obesity per %BF and WC (i.e., abdominal obesity) were greater in men with functional mobility impairments. Insulin resistance (HOMA>5.9), the MetS, and hsCRP were also significantly different between functionally unimpaired and impaired adults after adjusting for age and race/ethnicity. Although fasting glucose remained significantly different by impairment status after adjusting for age and race/ethnicity in both men and women, systolic and diastolic blood pressure remained significantly greater only among men with impairments. There were no differences in lean body mass among men or women with functional mobility impairment, as compared to unimpaired men and women (p>0.05).
Table 1.
Demographic and cardiometabolic characteristics of the study population by sex and functional impairment status.
| Men
|
Women
|
|||
|---|---|---|---|---|
| Functionally Unimpaired | Mobility Impaired | Functionally Unimpaired | Mobility Impaired | |
| N | 2321 | 402 | 2403 | 450 |
| Age, years | 43.95 (0.49) | 53.94 (0.68)* | 44.99 (0.44)a | 53.09 (1.08)* |
| Body Mass Index (BMI), kg/m2 | 28.12 (2.32) | 29.79 (0.44)* † | 28.02 (0.18) | 29.33 (0.46)* † |
| BMI 18.5 – < 25 kg/m2, % | 29.55 (1.12)b | 23.58 (3.18)ab | 34.63 (1.29) | 29.19 (0.23)a |
| BMI 25 - 24.9 kg/m2, % | 41.06 (1.16)b | 40.25 (5.21)b | 30.30 (0.81) | 27.75 (0.29) |
| BMI ≥ 30 kg/m2, % | 29.39 (1.41) | 36.17 (3.95)a | 35.07 (0.91) | 43.06 (0.37)ab |
| Body Fat, % | 27.70 (0.16) | 30.52 (0.41)* † | 39.37 (0.21)a | 41.41 0.43)*†b |
| Obesity (%BF-sex specific), % | 69.38 (1.20) | 80.72 (0.42)* † | 74.20 (1.02)a | 82.60 (0.64)* |
| NWO, % | 8.80 (0.57) | 7.10 (0.12) | 16.17 (0.72)a | 16.50 (0.27)b |
| Waist Circumference (WC), cm | 99.91 (0.46) | 107.04 (1.06)* † | 93.24 (0.43) | 97.28 (1.00)* † |
| Abdominal Obesity (sex-specific WC), % | 39.78 (1.43) | 56.81 (0.37)* † | 58.60 (1.13)a | 67.90 (0.53)*b |
| Glucose, mg/dL | 102.54 (0.77)a | 112.63 (2.97)* † | 98.09 (0.71) | 110.31 (3.77)* † |
| Insulin, μU/mL | 11.60 (0.32)a | 12.14 (1.08) | 9.86 (0.33) | 14.29 (1.56)* † |
| HOMA | 3.08 (0.09)a | 3.50 (0.32) | 2.52 (0.10) | 4.30 (0.52)* † |
| HOMA-IR (≥ 5.9), % | 4.98 (0.36) | 9.00 (1.85)a† | 4.33 (0.36) | 6.50 (1.58)a† |
| Triglycerides, mg/dL | 141.15 (2.49)a | 160.55 (9.86)* | 121.02 (2.00) | 144.58 (6.97)*† |
| Total Cholesterol, mg/dL | 200.34 (1.21) | 204.16 (2.79) | 201.88 (1.11) | 205.47 (4.22) |
| HDL-Cholesterol, mg/dL | 48.70 (0.33) | 47.66 (1.20) | 60.37 (0.45) | 56.50 (1.26)* |
| hsCRP, mg/dL | 0.32 (0.01) | 0.55 (0.06)* † | 0.48 (0.02)a | 0.66 (0.08)* † |
| Systolic Blood Pressure, mm Hg | 124.13 (0.41)a | 130.73 (1.68)*†b | 120.82 (0.42) | 125.50 (1.48)* |
| Diastolic Blood Pressure, mm Hg | 73.18 (0.30)a | 76.00 (0.61)*†b | 70.71 (0.28) | 73.11 (0.81)* |
| Metabolic Syndrome, % | 16.04 (0.57) | 24.69 (0.21)* † | 16.17 (0.73) | 23.16 (0.27)* † |
| Objectively Measured Activity Categories | ||||
| Total Monitor Wear Time, min | 871.02 (2.47) | 845.40 (9.18) | 849.03 (2.29) | 836.84 (9.75) |
| Sedentary (<100 cpm), min (% wear time) | 479.06 (55.0) | 513.50 (60.7*†) | 477.16 (56.2) | 494.57 (59.1*†) |
| Light (100 – 759 cpm), min (% wear time) | 251.72 (28.9)a | 235.02 (27.8*)b | 264.05 (31.1) | 268.63 (32.1) |
| Lifestyle Moderate (760 – 2019 cpm), min (% wear time) | 97.55 (11.2) | 79.47 (9.4*†) | 83.21 (9.8)a | 58.58 (7.3*†)b |
| Moderate PA (2020 – 5998 cpm), min (% wear time) | 30.49 (3.5) | 16.91 (2.0*†) | 23.77 (2.8)a | 10.88 (1.3*†)b |
| Vigorous PA (≥ 5999 cpm), min (% wear time) | 1.22 (1.4) | 0.51 (0.06*) | 0.68 (0.08) | 0.34 (0.04) |
Abreviations: N WO-normal weight obesity (BMI < 25 kg/m2 and body fat ≥ 25% (males) or 35% (females)); BMI-body mass index; %BF-percentage of fat; WC -waist circumference; HOMA-Homeostasis Model of Assessment; HOMA-IR-Homeostasis Model of Assessment, Insulin Resistant; HDL-high density lipoprotein; hsCRP-high sensitivity C-reactive protein; PA-physical activity.
Significant difference between functionally unimpaired and mobility impaired adults, within sex. Denoted as group with higher risk.
Significant difference between functionally unimpaired and mobility impaired adults, within sex, after adjusting for age and race/ethnicity. Denoted as group with higher risk.
Significant difference between sexes for functionally unimpaired adults. Denoted as group with higher risk.
Significant difference between sexes for adults with mobility impairment.
Obesity Misclassification
For adults both with and without functional mobility impairment, obesity according to %BF was more prevalent (69–82%) than per BMI, and thus there was significant overall obesity misclassification. Contrary to the primary hypothesis, NWO prevalence was not significantly different between those with mobility functional impairment as compared to those who were functionally unimpaired (men: 7.1% [mobility impaired] versus 8.8% [functionally unimpaired]; women: 16.5% [mobility impaired] versus 16.2% [functionally unimpaired]). However, when examined within the “normal” BMI category (i.e. 18.5 – 24.9 kg/m2), 32.1% of men and 47.5% of the women with impairments were obese according to %BF.
ROC and Sensitivity Analyses for Functional Mobility Impaired Adults
For adults with functional mobility impairments, ROC curves and sensitivity analyses demonstrated that the standard BMI cutoff for obesity (BMI ≥ 30 kg/m2) had excellent specificity in both men (100%) and women (98.4%), but very poor sensitivity (i.e. thus failed to classify many individuals with true obesity as such) in both men (47.0%) and women (52.5%). Figure 1 and Table 1 reveal visual ROCs with AUC values, and sensitivity/specificity data for BMI thresholds ranging from 24–31 kg/m2, for men and women with impairments respectively. The optimal cutoff for BMI was 25 kg/m2 for both sexes.
Figure 1.
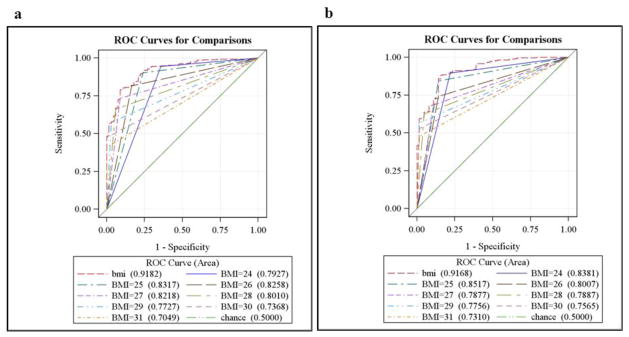
Receiver operating characteristic and area under the curve (AUC) values for men (2a) and women (2b) with functional mobility impairments, depicting the performance of BMI as a continuous variable, as well as individual thresholds to detect obesity according to %BF.
Objectively Measured Sedentary Behavior and Physical Activity
Men and women with functional mobility impairments spent on average 50 and 25 more minutes in sedentary behavior (p<0.001), respectively, than men and women that were functionally unimpaired. The greatest discrepancies in activity were in lifestyle moderate activity and moderate-intensity physical activity combined, where functionally unimpaired men and women spent an average of 30 and 36 more minutes of activity (p<0.05), respectively, than mobility impaired adults. Light and vigorous activity were less in functional mobility impaired men (p<0.05), but no differences in these categories were found among women.
Predictors of the MetS
The prevalence of the MetS was 24.7% in men and 23.2% in women with functional mobility impairments. After adjustment for all model predictors and sex, only education (lowest versus highest: OR 3.91, 95% CI 1.45–10.55), obesity per %BF (OR 13.77, 95% CI 1.82–95.03), and sedentary behavior (for every 60-minute increase: OR 1.25, 95% CI 1.00–1.50) were independently associated with higher odds of MetS. Conversely, only lifestyle moderate activity (for every 60-minute increase: OR 1.93, 95% CI 1.18–3.16) was independently associated with lower odds of MetS. Moreover, individuals with high %BF who were misclassified as not obese according to BMI, had a significantly higher prevalence of the MetS (17.6%) compared to correctly classified subjects with normal BMI and low %BF (2.1%).
Discussion
This is one of the first studies to examine the extent of obesity misclassification and predictors of cardiometabolic risk among a U.S. representative sample of adults with functional mobility impairments. Based on these findings there is significant discrepancy in the classification of obesity according to BMI versus %BF, and thus since BMI is readily used in clinical settings, many individuals with functional mobility impairments may go undetected for risk of chronic diseases. Whereas approximately 36% and 43% of men and women fell into the obese BMI category (a higher prevalence than in functionally unimpaired participants), over 80% of men and women were obese according to %BF. As a result, approximately one-third of men and nearly 50% of the women who were normal weight according to BMI (18.5–24.9 kg/m2) were actually obese according to %BF.
Although current BMI cutoffs had excellent specificity (i.e. accurately classified individuals who were not obese, as not obese), sensitivity was very poor. Therefore, many individuals with functional mobility impairments who are normal or overweight according to BMI may actually be at increased risk for cardiometabolic disease. A lower BMI cutoff for obesity in both men and women would effectively capture a greater proportion of at-risk individuals. While there are certainly potential psychosocial implications of labeling healthy individuals as overweight or obese, the risk-to-benefit ratio of altering the BMI to decrease false negative rates (i.e., a negative result obtained from individuals in whom obesity is actually present) is comparatively inconsequential.
However, and in contrast to our original hypothesis, the overall prevalence of NWO among adults with impairments was not greater than that of unimpaired individuals. Rather, BMI was actually significantly higher in both men and women with functional mobility impairments, with approximately 6% lower prevalence of “normal” BMI. One obvious limitation of this study that must be considered pertains to the method by which individuals were classified as functional mobility impaired. By using a self-reported physical function questionnaire, individuals did not report any specific diagnostic disability category, but rather the presence/absence of function or mobility restrictions. This and similar surveys have been readily used in the literature (Alley and Chang, 2007; Davison et al., 2002), and are clinically relevant for identifying modifiable predictors of functional deterioration, especially among older adults. Thus, since we did not limit inclusion to older adults, it is quite plausible that many individuals were labeled as mobility impaired for reasons directly attributable to high body mass (i.e., as opposed to a chronic or congenital motor-related disability such as cerebral palsy or muscular dystrophy). Indeed, many tasks such as standing up from an armless chair or walking without special equipment are difficult for obese individuals, due to the inherent “handicap” of lifting/maneuvering greater dead-weight adipose tissue.
It is also possible that age may have confounded the results of greater cardiometabolic risk in the impaired adults, as advancing age is a robust predictor of the metabolic syndrome (Ford et al., 2010), insulin resistance (Rowe et al., 1983), weakness (Cruz-Jentoft et al., 2010; Pijnappels et al., 2008; Ruiz et al., 2008), and mobility disability (Seeman et al., 2010). When adjusted for age, however, there were still various primary differences between adults with and without impairments. Specifically, %BF and WC were still significantly higher among both men and women with functional mobility impairments, but prevalence of obesity per %BF and abdominal obesity (i.e., according to the sex-specific WC measures) were only greater in impaired men. Insulin resistance, the MetS, and hsCRP were also significantly greater among functional mobility impaired men and women after adjusting for age. Likewise, although fasting glucose remained significantly different by impairment status after adjusting for age in both men and women, systolic and diastolic blood pressure remained significantly greater only in men with functional mobility impairments. Thus, age is a strong mediating factor between functional mobility impairment and cardiometabolic risk in women, and future research should attempt to identify the sex-specific trajectories of cardiometabolic decline in persons at risk for both obesity and disability.
There is increasingly strong evidence to suggest that physical inactivity may underlie the cardiometabolic risk attributed to obesity and age-related functional decline (Amati et al., 2009). It is thus possible that chronic sedentary behavior and diminished activity levels may be the primary drivers of MetS in functional mobility impairment, rather than the disproportionate prevalence of obesity per se. Our results indicate that mobility impaired individuals do spend a significantly greater volume of time in sedentary behavior, and less overall time in nearly every activity category. The largest differences for both men and women were seen in lifestyle moderate activity and moderate-intensity physical activity. Thus, replacing some sedentary behavior with moderate activity may have profound health implications for adults at risk for obesity and functional mobility impairments.
Conclusions
Obesity misclassification is prevalent among adults with functional mobility impairments. Men and women with functional and mobility impairments displayed increased age-adjusted prevalence of the MetS, insulin resistance, and obesity by BMI for both sexes, and by %BF and WC for men. Impaired individuals also demonstrated significantly higher volumes of sedentary behavior and decreased volumes of lifestyle moderate activity and moderate intensity physical activity. These results suggest an increased risk profile for mobility impaired individuals, which may be modified through reduction of sedentary behavior and increased moderate activity. There is a dire need for more longitudinal work to investigate the viability of behavioral interventions to reduce adiposity and to increase activity levels in these populations.
Table 2.
Sensitivity and specificity for individual BMI thresholds to detect obesity by %BF, among men and women with functional mobility impairments.
| BMI Threshold | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | Concordance C (AUC) | 95% Wald CL (AUC) | Age-Adjusted AUC |
|---|---|---|---|---|---|---|---|
| Men | |||||||
| 24 (kg/m2) | 93.66 | 64.18 | 0.91 | 0.72 | 0.793 (0.03) | 0.73 – 0.85 | 0.86 |
| 25 (kg/m2)* | 89.56 | 76.12 | 0.94 | 0.65 | 0.832 (0.03) | 0.77 – 0.88 | 0.86 |
| 26 (kg/m2) | 80.97 | 83.58 | 0.95 | 0.52 | 0.826 (0.02) | 0.77 – 0.87 | 0.87 |
| 27 (kg/m2) | 72.76 | 91.04 | 0.97 | 0.46 | 0.822 (0.02) | 0.78 – 0.86 | 0.88 |
| 28 (kg/m2) | 65.67 | 94.03 | 0.98 | 0.41 | 0.801 (0.02) | 0.76 – 0.84 | 0.86 |
| 29 (kg/m2) | 57.09 | 97.01 | 0.99 | 0.36 | 0.773 (0.02) | 0.73 – 0.81 | 0.85 |
| 30 (kg/m2) | 47.01 | 100.00 | 1.00 | 0.32 | 0.737 (0.01) | 0.71 – 0.77 | 0.83 |
| 31 (kg/m2) | 40.67 | 100.00 | 1.00 | 0.30 | 0.705 (0.01) | 0.67 – 0.73 | 0.81 |
| Women | |||||||
| 24 (kg/m2) | 88.92 | 78.13 | 0.95 | 0.59 | 0.838 (0.03) | 0.78 – 0.89 | 0.85 |
| 25 (kg/m2)* | 83.86 | 85.94 | 0.97 | 0.52 | 0.852 (0.02) | 0.80 – 0.89 | 0.85 |
| 26 (kg/m2) | 73.73 | 85.94 | 0.96 | 0.40 | 0.801 (0.03) | 0.75 – 0.85 | 0.82 |
| 27 (kg/m2) | 68.04 | 89.06 | 0.97 | 0.36 | 0.788 (0.02) | 0.74 – 0.83 | 0.82 |
| 28 (kg/m2) | 62.03 | 95.31 | 0.98 | 0.33 | 0.788 (0.02) | 0.75 – 0.82 | 0.82 |
| 29 (kg/m2) | 56.33 | 98.44 | 0.99 | 0.31 | 0.776 (0.01) | 0.74 – 0.81 | 0.82 |
| 30 (kg/m2) | 52.53 | 98.44 | 0.99 | 0.30 | 0.756 (0.01) | 0.72 – 0.79 | 0.81 |
| 31 (kg/m2) | 47.47 | 98.44 | 0.99 | 0.28 | 0.731 (0.02) | 0.70 – 0.76 | 0.79 |
Denotes the best overall threshold for BMI to detect obesity by percent body fat.
Highlights.
Sedentary behavior is exaggerated among individuals who report mobility impairment.
Adults with mobility impairments have greater adiposity and prevalence of obesity.
Adults with impairments have higher risk for age-adjusted cardiometabolic disease.
The BMI cutoff for obesity has poor sensitivity among functionally impaired adults.
Acknowledgments
The authors would like to acknowledge the University of Texas Medical Branch Claude D. Pepper Center (P30-AG024832), and the Michigan Institute for Clinical & Health Research (2UL1TR000433-06). The authors would also like to acknowledge the assistance of James McClain, PhD, MPH, (Division of Cancer Control and Population Sciences-National Cancer Institute, National Institutes of Health) in preparing the physical activity monitoring data. Dr. McClain received no special remuneration for this assistance.
Funding/Support: This study was funded by the National Institutes of Health: R24 HD065702-03 (M. Peterson & S. Al Snih).
Footnotes
Conflict of interests:
The authors have no conflicts of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al Snih S, Graham JE, Kuo YF, Goodwin JS, Markides KS, Ottenbacher KJ. Obesity and disability: relation among older adults living in Latin America and the Caribbean. American journal of epidemiology. 2010;171:1282–8. doi: 10.1093/aje/kwq087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- Alexander NB, Guire KE, Thelen DG, Ashton-Miller JA, Schultz AB, Grunawalt JC, Giordani B. Self-reported walking ability predicts functional mobility performance in frail older adults. Journal of the American Geriatrics Society. 2000;48:1408–13. doi: 10.1111/j.1532-5415.2000.tb02630.x. [DOI] [PubMed] [Google Scholar]
- Alley DE, Chang VW. The changing relationship of obesity and disability, 1988–2004. Jama. 2007;298:2020–7. doi: 10.1001/jama.298.17.2020. [DOI] [PubMed] [Google Scholar]
- Amati F, Dube JJ, Coen PM, Stefanovic-Racic M, Toledo FG, Goodpaster BH. Physical inactivity and obesity underlie the insulin resistance of aging. Diabetes Care. 2009;32:1547–9. doi: 10.2337/dc09-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandini LG, Curtin C, Hamad C, Tybor DJ, Must A. Prevalence of overweight in children with developmental disorders in the continuous national health and nutrition examination survey (NHANES) 1999–2002. J Pediatr. 2005;146:738–43. doi: 10.1016/j.jpeds.2005.01.049. [DOI] [PubMed] [Google Scholar]
- Batsis JA, Barre LK, Mackenzie TA, Pratt SI, Lopez-Jimenez F, Bartels SJ. Variation in the Prevalence of Sarcopenia and Sarcopenic Obesity in Older Adults Associated with Different Research Definitions: Dual-Energy X-Ray Absorptiometry Data from the National Health and Nutrition Examination Survey 1999–2004. J Am Geriatr Soc. 2013 doi: 10.1111/jgs.12260. [DOI] [PubMed] [Google Scholar]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010 doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison KK, Ford ES, Cogswell ME, Dietz WH. Percentage of body fat and body mass index are associated with mobility limitations in people aged 70 and older from NHANES III. J Am Geriatr Soc. 2002;50:1802–9. doi: 10.1046/j.1532-5415.2002.50508.x. [DOI] [PubMed] [Google Scholar]
- Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, Boudreau R, Manini TM, Nevitt M, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–85. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–7. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- Ford E, Li C, Zhao G. Prevalence and correlates of metabolic syndrome based on a harmonious definition among adults in the US. J Diabetes. 2010;2:180–93. doi: 10.1111/j.753-0407.2010.00078.x. [DOI] [PubMed] [Google Scholar]
- Gomez-Ambrosi J, Silva C, Galofre JC, Escalada J, Santos S, Millan D, Vila N, Ibanez P, Gil MJ, et al. Body mass index classification misses subjects with increased cardiometabolic risk factors related to elevated adiposity. International journal of obesity (2005) 2012;36:286–94. doi: 10.1038/ijo.2011.100. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, Stamm E, Newman AB. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001;90:2157–65. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71:885–92. doi: 10.1093/ajcn/71.4.885. [DOI] [PubMed] [Google Scholar]
- Hoeymans N, Feskens EJ, van den Bos GA, Kromhout D. Measuring functional status: cross-sectional and longitudinal associations between performance and self-report (Zutphen Elderly Study 1990–1993) J Clin Epidemiol. 1996;49:1103–10. doi: 10.1016/0895-4356(96)00210-7. [DOI] [PubMed] [Google Scholar]
- Kuk JL, Katzmarzyk PT, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat is an independent predictor of all-cause mortality in men. Obesity (Silver Spring) 2006;14:336–41. doi: 10.1038/oby.2006.43. [DOI] [PubMed] [Google Scholar]
- Lim KI, Yang SJ, Kim TN, Yoo HJ, Kang HJ, Song W, Baik SH, Choi DS, Choi KM. The association between the ratio of visceral fat to thigh muscle area and metabolic syndrome: the Korean Sarcopenic Obesity Study (KSOS) Clin Endocrinol (Oxf) 2010;73:588–94. doi: 10.1111/j.1365-2265.2010.03841.x. [DOI] [PubMed] [Google Scholar]
- Lim S, Kim JH, Yoon JW, Kang SM, Choi SH, Park YJ, Kim KW, Lim JY, Park KS, et al. Sarcopenic obesity: prevalence and association with metabolic syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA) Diabetes Care. 2010;33:1652–4. doi: 10.2337/dc10-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher CA, Mire E, Harrington DM, Staiano AE, Katzmarzyk PT. The independent and combined associations of physical activity and sedentary behavior with obesity in adults: NHANES 2003–06. Obesity (Silver Spring) 2013 doi: 10.1002/oby.20430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Neter JE, Schokker DF, de Jong E, Renders CM, Seidell JC, Visscher TLS. The Prevalence of Overweight and Obesity and Its Determinants in Children with and without Disabilities. J Pediatr-Us. 2011;158:735–39. doi: 10.1016/j.jpeds.2010.10.039. [DOI] [PubMed] [Google Scholar]
- Okorodudu DO, Jumean MF, Montori VM, Romero-Corral A, Somers VK, Erwin PJ, Lopez-Jimenez F. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obesity. 2010;34:791–99. doi: 10.1038/ijo.2010.5. [DOI] [PubMed] [Google Scholar]
- Peterson MD, Gordon PM, Hurvitz EA. Chronic disease risk among adults with cerebral palsy: the role of premature sarcopoenia, obesity and sedentary behaviour. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2013;14:171–82. doi: 10.1111/j.1467-789X.2012.01052.x. [DOI] [PubMed] [Google Scholar]
- Pijnappels M, Reeves ND, Maganaris CN, van Dieen JH. Tripping without falling; lower limb strength, a limitation for balance recovery and a target for training in the elderly. J Electromyogr Kinesiol. 2008;18:188–96. doi: 10.1016/j.jelekin.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Rimmer JH, Wang E. Obesity prevalence among a group of Chicago residents with disabilities. Arch Phys Med Rehab. 2005;86:1461–64. doi: 10.1016/j.apmr.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Romero-Corral A, Somers VK, Sierra-Johnson J, Jensen MD, Thomas RJ, Squires RW, Allison TG, Korinek J, Lopez-Jimenez F. Diagnostic performance of body mass index to detect obesity in patients with coronary artery disease. European heart journal. 2007;28:2087–93. doi: 10.1093/eurheartj/ehm243. [DOI] [PubMed] [Google Scholar]
- Romero-Corral A, Somers VK, Sierra-Johnson J, Korenfeld Y, Boarin S, Korinek J, Jensen MD, Parati G, Lopez-Jimenez F. Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J. 2010;31:737–46. doi: 10.1093/eurheartj/ehp487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazo-Clavell ML, Korinek J, Allison TG, Batsis JA, Sert-Kuniyoshi FH, et al. Accuracy of body mass index in diagnosing obesity in the adult general population. International journal of obesity (2005) 2008;32:959–66. doi: 10.1038/ijo.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JW, Minaker KL, Pallotta JA, Flier JS. Characterization of the insulin resistance of aging. J Clin Invest. 1983;71:1581–7. doi: 10.1172/JCI110914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz JR, Sui X, Lobelo F, Morrow JR, Jr, Jackson AW, Sjostrom M, Blair SN. Association between muscular strength and mortality in men: prospective cohort study. Bmj. 2008;337:a439. doi: 10.1136/bmj.a439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrager MA, Metter EJ, Simonsick E, Ble A, Bandinelli S, Lauretani F, Ferrucci L. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol. 2007;102:919–25. doi: 10.1152/japplphysiol.00627.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman TE, Merkin SS, Crimmins EM, Karlamangla AS. Disability trends among older Americans: National Health And Nutrition Examination Surveys, 1988–1994 and 1999–2004. Am J Public Health. 2010;100:100–7. doi: 10.2105/AJPH.2008.157388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam CS, Xie W, Johnson WD, Cefalu WT, Redman LM, Ravussin E. Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabetes Care. 2012;35:1605–10. doi: 10.2337/dc11-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–33. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- Weil E, Wachterman M, McCarthy EP, Davis RB, O’Day B, Iezzoni LI, Wee CC. Obesity among adults with disabling conditions. Jama-J Am Med Assoc. 2002;288:1265–68. doi: 10.1001/jama.288.10.1265. [DOI] [PubMed] [Google Scholar]
- Weinheimer EM, Sands LP, Campbell WW. A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: implications for sarcopenic obesity. Nutr Rev. 2010;68:375–88. doi: 10.1111/j.1753-4887.2010.00298.x. [DOI] [PubMed] [Google Scholar]
- Woo J, Leung J, Kwok T. BMI, body composition, and physical functioning in older adults. Obesity (Silver Spring) 2007;15:1886–94. doi: 10.1038/oby.2007.223. [DOI] [PubMed] [Google Scholar]


