Abstract
Pancreatic β-cell dysfunction plays an important role in the pathogenesis of both type 1 and type 2 diabetes. Insulin, which is produced in β-cells, is a critical regulator of metabolism. Insulin is synthesized as preproinsulin and processed to proinsulin. Proinsulin is then converted to insulin and C-peptide and stored in secretary granules awaiting release on demand. Insulin synthesis is regulated at both the transcriptional and translational level. The cis-acting sequences within the 5′ flanking region and trans-activators including paired box gene 6 (PAX6), pancreatic and duodenal homeobox-1(PDX-1), MafA, and B-2/Neurogenic differentiation 1 (NeuroD1) regulate insulin transcription, while the stability of preproinsulin mRNA and its untranslated regions control protein translation. Insulin secretion involves a sequence of events in β-cells that lead to fusion of secretory granules with the plasma membrane. Insulin is secreted primarily in response to glucose, while other nutrients such as free fatty acids and amino acids can augment glucose-induced insulin secretion. In addition, various hormones, such as melatonin, estrogen, leptin, growth hormone, and glucagon like peptide-1 also regulate insulin secretion. Thus, the β-cell is a metabolic hub in the body, connecting nutrient metabolism and the endocrine system. Although an increase in intracellular [Ca2+] is the primary insulin secretary signal, cAMP signaling-dependent mechanisms are also critical in the regulation of insulin secretion. This article reviews current knowledge on how β-cells synthesize and secrete insulin. In addition, this review presents evidence that genetic and environmental factors can lead to hyperglycemia, dyslipidemia, inflammation, and autoimmunity, resulting in β-cell dysfunction, thereby triggering the pathogenesis of diabetes.
Keywords: β-cell, diabetes, glucose, hormones, insulin secretion, insulin synthesis
INSULIN
Insulin structure
The crystal structure of insulin is well documented as well as the structural features that confer receptor binding affinity and activity. This has been extensively reviewed and readers are encouraged to visit [1] and [2] for excellent discussions on insulin structure and structure-activity relationships. As discussed in this review, insulin receptor downstream signaling intersects with the signaling pathways of other growth factors, including IGF1 and IGF2 [3]. This demonstrates the importance of identifying receptor ligand agonists as potential insulin-mimetic therapeutic agents in diabetes. This section of the review will focus on the native structure of insulin. For an excellent review on the insulin receptor structure and binding domains, readers are encouraged to visit several references [2, 3].
The 3-D structure of monomeric insulin was first discovered by x-ray crystallography and reported in 1926 [4]. More than 40 years later, the structure of the zinc-containing hexameric insulin was solved [5–8]. 2D NMR studies have also contributed to knowledge on the monomeric, dimeric and hexameric conformations of insulin, all revealing information on the native structure of insulin and the amino acids that confer binding specificity to the insulin receptor [1]. Insulin concentration and surrounding pH influence the conformational state of insulin. The monomers tend to form dimers as the concentration of insulin rises, and in the presence of zinc and favorable pH (10 mM Zn++, pH ~6.0) the monomers assemble into higher order conformations called hexamers [9]. As discussed below, interactions among hydrophobic amino acids in insulin dimer structures favor aggregation as concentrations rise. Once the hexamers are secreted from the β-cell and diffuse into the blood down their concentration gradient, a combination of electrostatic repulsion and decreased concentration of insulin favors the dissociation of insulin into its monomeric form [1]. Hence, the monomer is the active form of insulin, while the hexamer is the storage form of insulin.
The monomeric insulin consists of the 21 amino acid residue “A” chain and 30 amino acid residue “B” chain bound by disulfide linkages. The monomer consists of three disulfide linkages, including two between the A and B chains (A7-B7, A20-B19) and one within the A chain (A7-A11) [4]. The secondary structure of the A chain contains two antiparallel α-helices, formed between the residues A2-A8 and A13 to A19 [1]. These two helices are connected by residues A9 to A12. This conformation brings the two ends of the A chain into close proximity, allowing them to exist side by side.
The secondary structure of the B chain contains both alpha helices and β-sheets [1]. The B chain may exist in two different conformations when crystallized [8]. In the T-state, there is a central alpha helix from B9 to B19 (1→5 helix hydrogen bonding pattern). There is a 1→4 β turn from B20-B23, with the Gly20 and Gly23 residues allowing the chain to fold into a “V” shape. Residues B24 to B30 form an extended β strand structure; the β-turn allows the chain to be in close enough proximity to form a β-sheet with PheB24 and TyrB26 in contact with leucines B11 and B15 of the B-chain alpha helix. In the R state, there is a continuous alpha helix from B1-B19. The disulfide bonds between Cys residues A7-B7 and A20-B19 contribute to the stability of the native insulin structure. The secondary structure of both the A and B chains is surprisingly complex for such small peptides, and these intricate side-chain interactions determine insulin receptor affinity.
The overall tertiary structure of the insulin monomer (A and B chain joined by disulfide bonds) is highly organized and stabilized by specific amino acid side chain interactions. These interactions influence ligand-receptor binding kinetics [1]. The residues A6-A11 and Leu A11, B1 and B15, Ile A2, Phe B24, Val A3, Ile A13, Val B18 and Val B12 comprise the hydrophobic core of the monomeric protein structure. The latter AA residues on the B chain also serve to stabilize the β-turn of the B chain (B20-B23) allowing the β sheet (B23-B30) to fold against the helix and hydrophobic residues. These nonpolar amino acid residues become buried when the monomers form dimers.
At micromolar concentrations insulin monomers form dimers [2]. The insulin dimer is maintained by the antiparallel β sheets at the carboxy terminus of the B chains on each monomer. These β sheets are exposed to the surface of the dimer structure. The interface of the dimer contains the nonpolar residues discussed above that contribute to the hydrophobic core. Solvent exclusion as a result of interactions among hydrophobic AA residues leads to aggregation, favoring the formation of a higher order structure.
Insulin stored in β-cells is packed into densely clustered “granules” consisting of insoluble crystalline hexameric insulin. The concentration of insulin in these granules is roughly 40 mM [2]. The hexameric form of insulin consists of 6 molecules of insulin peptide arranged as 3 dimers. Although each monomer in the dimer consists of the same peptide sequence, there are some differences in side chain spatial conformation such that there is not perfect 2-fold symmetry [1]. One example is Phe (B-chain, residue 25), which is oriented towards the hydrophobic core of the peptide on one side of the dimer, and folded away from the peptide on the other side of the dimer. The insulin hexamer coordinates two zinc atoms with the imidazole groups of three histidine residues (B chain, residue 10) and three water molecules. In the 2 Zn crystalline structure, all six monomers are in the T conformation discussed above [2]. In the presence of high chloride concentrations in the β-cell, a 4 Zn hexameric structure forms, whereby three of the monomers exist in the R conformation and three in the T form [2]. The R form predominates in phenol-containing crystals [10]. From a pharmacological standpoint, these are important observations, as phenol serves as an antimicrobial and chloride as an isotonic agent in insulin formulations [2]. Interactions between the dimers in the hexamer are weaker than interactions within the dimer, with greater vander Waals separations among the dimers in the hexamer as compared with separations among the monomers in the dimer [1]. Hence, the hexamer configuration is less stable and subject to dissociation when concentrations of insulin fluctuate (e.g., secretion into bloodstream).
Insulin structure-function relationships
Solving the 3D structure of insulin allowed for prediction of regions important in insulin activity. For such a small protein, the secondary and tertiary structures are complex and impressive. As reviewed by Pittman et al [1], the residues that are evolutionarily conserved and important for conformational flexibility are likely those important for determining insulin receptor binding affinity.
There are several regions of the insulin monomer that are important for facilitating receptor binding. The importance of these amino acid residues was discovered by screening insulin analogs with various substitutions and deletions for their ability to activate the insulin receptor. Recombinant DNA technology, allowing for production of active insulin, allowed for development of high-throughput screening by site-directed mutatgenesis [2]. These studies revealed several evolutionarily conserved amino acids near the surface of the insulin monomer [5]. Among these, several are located at the amino terminus of the A chain (GlyA1, IleA2, ValA3, GluA4), the carboxy terminus of the A chain (TyrA19, CysA20, AsnA21) and the carboxy terminus of the B chain (glyB23, PheB24, PheB25, TyrB26) [11]. Several of these residues (A21, B23–25) through negative cooperativity, define the “cooperative” site on the insulin structure [2]. The IleA2 and ValA3 are not exposed to the surface of the native insulin structure, but upon displacement of the B chain carboxy terminus during receptor binding become exposed to the surface [12, 13]. Several mutations were identified in these regions that reduce the affinity to the insulin receptor, including LeuB25 for PheB25 (insulin Chicago), SerB24 for PheB24 (insulin Los Angeles) and LeuA3 for ValA3 (insulin Wakayama). Patients harboring these mutations display glucose intolerance and hyperinsulinemia [14–19].
The A chain contains several regions that are important for receptor binding. The amino terminus, when N-acetylated, shows reduced receptor binding by 30 %, indicating that a free positively charged amino terminus is critical for receptor binding [20]. Deleting Gly1 reduces activity by 15 %, indicating that the salt bridge formed between Gly1 and the carboxy terminus of the B chain is important for correct positioning of the peptide [6]. Additionally, the carboxy terminus of the A chain, particularly TyrA19, CysA20 and AsnA21 are considered to be important for insulin receptor affinity.
The B chain is the most studied in the context of structure-activity relationships, in particular the carboxy terminal domain. The first four residues of the B chain (amino terminus) can be deleted with only modest reductions in receptor binding activity (roughly 70 % activity retained) [21, 22], however; deletion of HisB5 leads to a major reduction in activity (15 % activity retained). Furthermore, LeuB6 is critical for binding activity and deletion results in a mutant with less than 1 % binding affinity [23]. CysB7 is also important, with obvious importance in maintaining the disulfide linkage between the A and B chains. HisB10 is key for maximal insulin activity and when substituted for AspB10, proinsulin is not processed to insulin, leading to increases in circulating proinsulin [24, 25]. Interestingly though, synthetic insulin containing the AspB10 substitution shows a 500 % increase in binding affinity as compared with the “wild type” synthetic insulin [26]. The B chain carboxy terminus contains evolutionarily conserved residues important for receptor binding, including GlyB23, PheB24, PheB25 and TyrB26. Specifically, PheB24 forms hydrogen bonds that are critical for dimer formation and PheB25 shows different conformations on the two molecules that comprise the dimer, indicating that it is important for conformation of the native insulin structure [6].
In summary, while numerous amino acid residues are important for insulin binding to the insulin receptor, namely N-terminal A chain residues and C-terminal B chain residues, it is likely that conformational changes in the insulin secondary and tertiary structure dictate the stability of the protein and affinity for the insulin receptor. The exact mechanism whereby insulin binds to its receptor has yet to be elucidated. Hence, a more detailed structure of the insulin-receptor complex combined with functional activity assays should lead to a greater understanding of the factors that determine receptor-ligand binding affinity and specificity.
PANCREATIC β-CELL PHYSIOLOGY
Insulin biosynthesis
The secreted insulin consists of 51 amino acids with a molecular weight of 5.8 kDa. However, the insulin gene encodes a 110-amino acid precursor known as preproinsulin. As with other secreted proteins, preproinsulin contains a hydrophobic N-terminal signal peptide, which interacts with cytosolic ribonucleoprotein signal recognition particles (SRP) [27]. SRP facilitates preproinsulin translocation across the rough endoplasmic reticulum (rER) membrane into the lumen. This process occurs via the peptide-conducting channel [28, 29], where the signal peptide from preproinsulin is cleaved by a signal peptidase to yield proinsulin [30]. Proinsulin then undergoes folding and formation of three disulfide bonds [31], a process requiring a diverse range of endoplasmic reticulum (ER) chaperone proteins such as the protein-thiol reductase [32]. Subsequent to maturation of the three dimensional conformation, the folded proinsulin is transported from the ER to the Golgi apparatus where proinsulin enters immature secretary vesicles and is cleaved to yield insulin and C-peptide. Insulin and C-peptide are then stored in these secretory granules together with islet amyloid polypeptide (IAPP or amylin) and other less abundant β-cell secretary products [33, 34].
Although insulin biosynthesis is controlled by multiple factors, glucose metabolism is the most important physiological event that stimulates insulin gene transcription and mRNA translation [35]. In 3-day fasted rats, glucose injection increased relative proinsulin mRNA levels by three- to four-fold within 24 h and this effect was blocked by pharmacological inhibition of transcription with actinomycin D [36]. These results suggest that glucose plays a central role in regulation of insulin biosynthesis which is controlled at least partially via alterations in proinsulin mRNA expression. In addition, glucose is an important factor for maintaining insulin mRNA stability. Results from in vitro studies demonstrated that insulin mRNA stability was reduced under lower glucose concentrations and increased under higher glucose concentrations [37, 38]. Interestingly, elevation of intracellular cAMP levels can prevent this reduction [39].
Most animals have only a single copy of the insulin gene, but rodents have two non-allelic insulin genes (insulin I and II). They differ in their number of introns and chromosomal locations [40]. In all insulin genes the 5′-flanking region determines its tissue- and cell-type-specific expression [41]. The transcriptional factor binding sites that determine insulin’s exclusive expression in β-cells are located between −520 and +1 base pairs (bp) relative to the transcription start site (TSS) in both rat and human insulin genes [35, 41, 42]. Among mammalian insulin genes, there is a conserved sequence located from −350 bp to the TSS, which controls cell-type-specific expression of insulin. Most transcriptional regulation occurs through interactions within these conserved sequences. Studies have shown that the sequence between −340 and +91 is the major insulin gene transcription enhancer region, which determines cell-specific and glucose-regulated insulin gene expression [43–47].
Regulation of insulin transcription
Insulin biosynthesis is regulated both at transcriptional and translational levels. In a mouse β-cell, there are roughly 13,000 insulin granules. They occupy more than 10% of the total cell volume [48]. Each granule contains approximately 200,000 insulin molecules [49]. However, insulin content in β-cells is highly dynamic. Insulin accumulates in the presence of nutrients and decreases in response to nutrient deprivation. The ability of β-cells to quickly respond to cellular signals is generally due to transcriptional regulation. A number of discrete sequence elements within the promoter region of insulin gene, named A, C, E, Z, and CRE elements determine localization of insulin in β-cells and also serve as binding sites for several β-cell transcription factors to regulate insulin gene expression [50]. The transcription factor binding sites that are located within a region spanning ~-400 base pairs (bp) relative to the TSS are determinants of β-cell-specific expression of insulin [50].
A number of cis- and trans- transcriptional factors are associated with the activation of the insulin enhancer region. In all characterized insulin enhancer sequences the A, C, and E elements are contained in core binding motifs [51].
A elements
The A elements are multiple A/T rich elements located in the conserved control region of the insulin gene [52]. There is a TAAT core in each of these A elements that serves as the central DNA binding recognition motif for homeodomain proteins [53, 54], including duodenal homeobox-1(PDX-1) [55–57], Cdx2/3 [58], and Isl-1 [59]. PDX-1 is the predominant binding factor detected with insulin A element probes in pancreatic β-cell extracts [55, 60, 61]. . This factor was first characterized as an insulin [55, 60–62] and somatostatin [43,44] transcriptional factor. The expression of PDX-1 in adult pancreas is generally restricted to islet β-cells (~91%). Only a small subset of δ-cells (~15%) express PDX-1 and levels in exocrine acinar cells are extremely low [57, 62–64]. The Cdx2/3, while expressed in β-cells and α-cells, appears to play a less important role in islet function, because Cdx2/3 mutant mice have defects in only intestinal function [65]. The Isl-1 is present in all types of islet cells [66] and can activate somatostatin [67], glucagon [68], and IAPP [69] gene expression. It also plays an essential role in islet formation during embryo development [70].
C element
There are two C elements in the insulin gene promoter. The C1 element is located between 118 and 107 bp upstream of the rat insulin TSS [71]. Rat insulin promoter element 3b (RIPE3b)1 and RIPE3b2 [71, 72] are two factors that form protein-DNA complexes within the C1 element. RIPE3b2 consists of the p58, p62, and p110 subunits [73]. RIPE3b2 does not contribute to β-cell-specific expression of the insulin gene [71, 73], and RIPE3b2-binding activity is present in a variety of other tissues [71]. The DNA-binding component of the RIPE3b1 was recently identified as MafA [74–76], which is expressed exclusively in β-cells [77]. MafA also mediates glucose-regulated and fatty acid-inhibited insulin expression. Prolonged exposure of islets to fatty acid and high glucose inhibits insulin gene transcription by impairing nuclear cellular expression of MafA. [78–80]. MafA deficient animals showed no defects in β-cell development, although impaired insulin expression in adult islets was observed [81].
The C2 element, which is located at −317/−311 bp in the rat I insulin gene was termed the pancreatic islet cell enhancer sequence (PISCES). It was found to contribute to insulin, glucagon, and somatostatin transcription in α-, β-, and ε-cells, respectively [82]. Furthermore, PISCES is a binding site for PAX6 both in insulin and glucagon genes [83]. Like other PAX transcriptional factors, PAX6 contains a paired box bipartite DNA binding domain. PAX6 is required for normal transcription of insulin genes and islet development [83]. Besides PAX6, PAX4 is also a paired/homeodomain protein expressed in the pancreas. Although both PAX4 and PAX6 can bind to PISCES [84], PAX4 is only detected transiently in β-cells during early development and absent in adult β-cells [85]. PAX4 is reported to suppress PAX6-induced trans-activation; however it is unclear if PAX4 regulates insulin expression in vivo because of its narrow window of expression.
E element
The E elements (5′-GCCATCTG-3′) are two separated mini-enhancer units within the insulin enhancer [43, 71, 86]. Rodents have two E elements (−241 to −233 bp and −112 to −104 bp) in the insulin I gene; while other mammals have only one (approximately −100 to −91 bp) [51]. The core insulin E element (5′-CANNTG-3′) is also found in the heavy-chain immunoglobulin and muscle creatine kinase control elements [87–89]. The factors that bind the E element contain a helix-loop-helix domain (HLH) that is important in facilitating protein-protein interactions, and a contiguous amino terminal basic region (b) that is necessary for DNA-protein binding. This motif is shared by a number of transcriptional factors required in cell type determination including the muscle determination proteins MyoD [90], Myf-5 [91], myogenin [92], and the proteins of the drosphila achaete-scute complex, which are important in neural development [93]. The E element activators include BETA2/NeuroD1, E2/5, E12 and E47. BETA2/NeuroD1 is enriched in islets [94, 95], while E2/5, E47 [71, 96, 97], and E12 [98] are widely distributed. BETA2/NeuroD1 is important in regulating insulin gene expression and β-cell survival, and the endocrine pancreas-specific deficiency of BETA2/NeuroD in mice causes massive β-cell apoptosis and subsequent diabetes and early death [99, 100].
Z element
The Z element is located upstream of the A element (−292 to −243 bp) and is unique to human insulin. A glucose-sensitive DNA-binding complex termed ZaI binds to the region of −287 to −271 bp within the Z element in primary islet cells [101]. Z element also functions as a transcriptional repressor in transformed β-cell lines and primary fibroblast cells [101, 102]. Recent studies show that A element activation depends on the present of the Z element [103]. PDX-1 and MafA regulate insulin gene transcription through activation of the Z element [104].
Cyclic AMP response element (CRE)
The human insulin gene promoter contains four CRE sites: CRE1 at −210 bp, CRE2 at −183 bp, CRE3 at +18 bp, and CRE4 at +61 bp [105]; within the core of each CRE there is a sequence similar to the CRE consensus sequence [106]. A variety of transcription factors can regulate insulin gene transcription by binding to the consensus CRE sequence of 5′-TGACGTCA-3′ [106]. These transcriptional factors are members of the CRE binding protein (CREB)/ATF family [107]. The CREB/ATF family of transcription factors are basic region leucine zipper (bZIP) proteins that share a common cluster of basic amino acids at the N-terminus of the bZIP domain, which binds to the CRE site to initiate insulin transcription [108].
Modes of gene regulation can be species-specific, and thus interpretation of data from animal models and extrapolation to humans must be exercised with caution. For example, hepatocyte nuclear factor (HNF)-1 [109] and Isl-1 [110] can bind to the A elements to stimulate rat insulin-I gene transcription. In addition, Cdx-3 [58] and HMGI(Y) [111] bind specifically to the A3/A4 element, which is unique to rat insulin-I. Besides regulation at the gene promoter regions as described above, control of ER load, granule counting, and cell cooperation provide essential feedback loops for controlling insulin transcription[112], which will be further elaborated upon in this review.
Regulation of insulin translation
In response to nutrients, β-cells enhance their overall speed of protein translation, which is at least partly controlled by dephosphorylation of eukaryotic initiation factor 2a (eIF2a) via protein phosphatase 1 (PP1) [113]. For example, exposure of β-cells to high glucose for 2 hours significantly decreases the ratio of phosphorylated eIF2a to eIF2a [114]. However, there are additional mechanisms to regulate glucose-induced insulin translation, since the overall protein translation induced by glucose compared to the fasting state in β-cells was only 3-fold compared with an up to 8-fold induction in proinsulin translation [115].
The pancreatic ER kinase (PERK) plays an important role in regulating translational events. It phosphorylates eIF2a [116], thereby regulating insulin translation [117]. PERK phosphorylation of eIF2a can be partially compensated for by other kinases [118, 119]. PERK mutation results in Wolcott-Rallison syndrome associated with permanent neonatal diabetes in humans [120]. PERK-deficient mice not only develop severe defects in insulin synthesis, but also in β-cell proliferation and differentiation, leading to permanent neonatal diabetes as seen in humans. Using pancreas and β-cell specific knockout mouse models, it was determined that while PERK is required during the fetal and neonatal stages for proper development of β-cell mass, it is not required in adults for maintaining β-cell mass [121]. The Wolfram Syndro gene WSF1, taking its name from the Wolfram syndrome, is unregulated by ER stress via Inositol-Requiring Protein 1(IRE1)-a and PERK [122, 123]. At the beginning of glucose exposure, IRE1 stimulates insulin synthesis via WFS1, while after prolonged exposure, it may reduce insulin production via X-box-binding protein 1 (XBP1) [124]. The β-cells have evolved a mechanism to detect the amount of insulin stored and secreted and adjust insulin synthesis accordingly. A granule transmembrane protein called islet cell autoantigen 512 (ICA512), is a crucial part of this feedback control. Insulin granules travel a long distance on tubulin tracks before arriving at the peripheral actin network [125]. Before becoming linked to the cytoskeleton, insulin granules are anchored to actin cortex via ICA512 and β2-synthrophin [126]. Upon activation, the granule membrane fuses transiently to the cell membrane to release insulin. Elevated Ca2+ levels in the meantime activate the protease μ-calpain to cleave away a cytosolic fragment from ICA512. The free ICA512 cytosolic fragment then moves to the nucleus and binds to the tyrosine-phosphorylated transcriptional factor STAT5 to prevent STAT 5 from dephosphorylation, which in turn upregulates insulin transcription [127]. Nuclear free ICA512 cytosolic fragments also bind to sumoylating enzyme PIASγ. The sumoylation of ICA512 by PIAS γ reverses the binding of ICA512 to STAT5 [127]. Hence, the release of insulin from secretory granules is communicated to the nucleus, which serves as a positive feedback mechanism to initiate insulin translation for maintaining an adequate amount of stored insulin.
In addition to transcriptional regulation, β-cells are also able to adjust insulin production in response to immediate environmental triggers by regulating the speed of insulin translation. For example, exposure of rat islets to 25 mM glucose for 1 hour led to an induction in intracellular proinsulin levels by up to ten-fold from baseline (2.8 mM glucose), whereas proinsulin mRNA quantities remained the same [128]. Results from an earlier study demonstrated that this acute glucose-stimulated insulin synthesis is independent of mRNA synthesis within the first 45 min because blockage of transcription only slowed insulin accumulation after that time frame [129]. In addition, insulin mRNA stability, which depends on nutrient status, is an important factor that influences insulin protein synthesis [36]. Results of in vitro studies showed that insulin mRNA stability decreases under lower glucose concentrations and increases under high glucose conditions [37, 38]. In the absence of glucose, insulin mRNA levels in β-cells decrease sharply, which is reversed by elevation of intracellular cAMP levels [39]. The same phenomena were observed in animal studies. Rats fasted for 3 days have only 15–20% of the levels of pancreatic insulin mRNA that were measured in the control animals [36]. Thus, post-transcriptional regulation controls the modulation of immediate insulin synthesis, while regulation at the transcriptional level contributes to the modulation of delayed insulin synthesis.
Polypyrimidine tract binding proteins (PTBPs) are proteins that regulate mRNA translation. They are involved in exon repression while mRNA is undergoing splicing in nuclei and stabilization and ribosome recruitment in the cytosol [130–132], They upregulate translation both by extending mRNA viability and by stimulating the initiation of translation. Cytosolic PTBP1 binds to a CU-rich sequence in the 3′ UTR of proinsulin, which stabilizes proinsulin mRNA [130–132]. PTBP1 also upregulates translation of several insulin granule proteins. PTBP1 binding to ICA512 mRNA decreases 3′ UTR decay. Deletion of the PTB binding site substantially reduced prohormone convertase 2 (PC2) translation. Insulin and insulin granule protein mRNA share a similar affinity to the RNA binding protein PTBP1, which enables their gene-specific activation by glucose. It was recently found that there is a conserved region (40–48nt) from the 5′ UTR of proinsulin mRNA that plays an essential role in glucose regulation of proinsulin translation, because removal of this region blocked glucose-stimulated proinsulin translation [115].
Regulation of insulin secretion
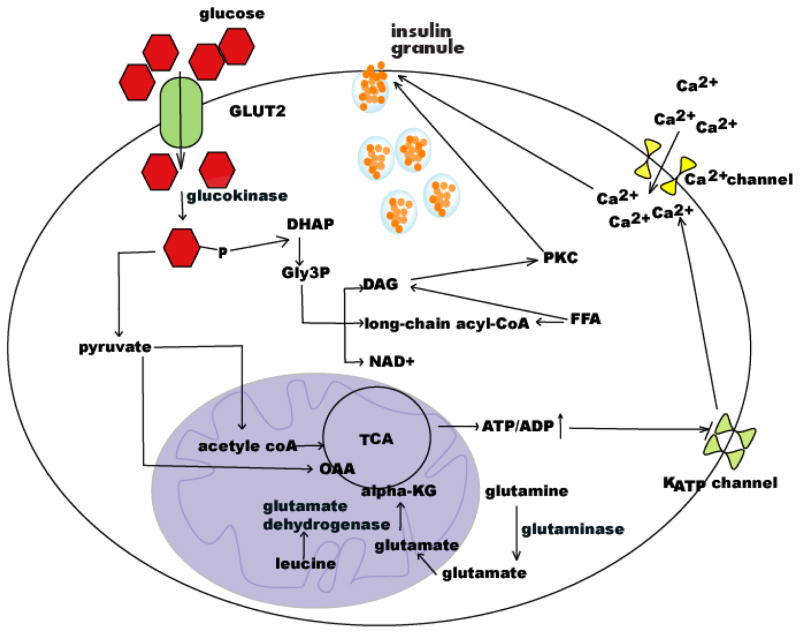
Insulin is an important hormone required for normal metabolism. In healthy subjects, insulin release is exquisitely exact to meet the metabolic demand. Specifically, β-cells sense changes in plasma glucose concentration and response by releasing corresponding amounts of insulin [133]. To sense the nutritional state, β-cells are clustered in islets that strategically connect to the vasculature. Islets form a dense network with small blood vessels and receive 10 times the amount of blood than cells in the surrounding exocrine regions. Capillaries surrounding islets show a remarkable number of small pores called fenestrae that allow for a greater nutrient exchange between the circulation and surrounding tissues. This structure enhances permeability, allowing unrestricted nutrient access so that β-cells can sense the nutritional state quickly. Fenestrations also permit rapid insulin diffusion into the blood [112]. In addition to glucose, some amino acids and fatty acids also regulate insulin secretion. A schematic illustration of nutrient regulated insulin secretion is shown in Fig. (1).
Fig. 1.
Schematic illustration of nutrient-regulated insulin secretion.
Glucose and insulin secretion
The β-cells respond to many nutrients in the blood circulation, including glucose, other monosaccharides, amino acids, and fatty acids. Glucose is evolutionarily the primary stimuli for insulin release in some animal species, because it is a principal food component and can accumulate immediately after food ingestion. Indeed, in rodents and humans, the amplitude of insulin secretion induced by glucose is much larger compared with that stimulated by protein or fat. Oral ingestion of 75 g of glucose will cause plasma insulin to rise from a basal level (20–30 pmol/L) to 250–300 pmol/L in 30 min, while intake of a similar amount of fat or a fat plus protein diet will only increase plasma insulin levels to 50 and 60 pmol/L, respectively, in human subjects [134]. While glucose is the obligate fuel source for neurons [112], other cells, including β-cells can use alternative fuel sources during brief periods of starvation, an adaptation that could predispose them to glucolipotoxicity, as discussed later in this review.
β-cells do not appear to contain membrane-bound glucose receptors but are equipped with several sensing devices that measure circulating glucose. Glucose transporter 2 (GLUT2), constitutively expressed in β-cells, is the first encountered glucose sensor in β-cells. Glucose equilibrates in β-cells via GLUT2-mediated facilitated diffusion. GLUT2 is the only glucose transporter expressed in β-cells. It is also expressed in the liver, and to a lesser extent, in renal and intestinal absorptive cells. Unlike GLUT4, which is primarily expressed in muscle and fat cells, mobilization of GLUT2 to the plasma membrane is insulin-independent and the transporter protein shows a low substrate affinity, ensuring high glucose influx. After entering β-cells, glucose is phosphorylated by the rate-limiting enzyme glucokinase, a subtype of hexokinase. Glucokinase is expressed in only four types of mammalian cells: hepatic cells, β-cells, enterocytes, and glucose-sensitive neurons [112]. Two important properties enable glucokinase to function as a glucose sensor in β-cells, distinguishing it from other hexokinases. The first property is its relatively lower affinity for glucose than other hexokinases. Its Km is only 6 mmol/L, falling in the middle of the normal blood glucose range (4–10 mmol/l), while other hexokinases function at maximal velocity at this glucose concentration. The second property is that it is not inhibited by its product, often a regulatory feature in metabolism. This feature enables its continued activity in spite of a high glycolysis load. Glucokinase is thus the rate-limiting step in β-cell glucose metabolism and it is considered to be an important glucose sensor [112].
The endpoint of glycolysis is the metabolic substrate pyruvate that is then oxidized through the tricarboxylic acid cycle by mitochondria in β-cells to produce ATP. In other types of cells, pyruvate can be converted to lactate by pyruvate dehydrogenase. However, because β-cells lack this enzyme, pyruvate is mainly metabolized to produce metabolic coupling factors through 2 routes: 1) After being metabolized to acetyl-coA it enters glucose oxidation and 2) anaplerosis. Pyruvate oxidation through the tricarboxylic acid cycle (TCA) by mitochondria is the major signaling pathway coupled to “ATP-sensitive potassium (KATP) channel-dependent insulin release”, which increases the intracellular ATP/ADP ratio, sequentially leading to closure of KATP channels, depolarization of the plasma membrane, opening of voltage-dependent Ca2+ channels, influx of Ca2+, and eventual activation of exocytosis of insulin-containing granules. Anaplerosis serves to replenish the carbon pool in the TCA cycle. After the cycle is filled with intermediates, these carbons can exit via cataplerosis. Some products derived in these processes can act as insulin secretion signals, which include NADPH, malonyl-CoA, and glutamate. These molecules reportedly amplify KATPchannel -dependent insulin secretion [134, 135].
A third glucose signal results from the formation of glycerol-3-phosphate (Gly3P). After glucose is phosphorylated into glucose-6-phosphate (G6P) by glucokinase, G6P can enter glycolysis to generate pyruvate. It can also be metabolized into dihydroxyacetone phosphate (DHAP) part of the way through the pathway to provide Gly3P. Gly3P is important for generating lipid metabolic coupling factors such as long-chain acyl-CoA and diacylglycerol (DAG) that can augment insulin secretion. Gly3p/DAG is an alternative pathway that is independent of mitochondrial metabolism of glucose to produce metabolic coupling factors to stimulate insulin release. Gly3P can also replenish NAD+ to promote β-cell glycolysis via the mitochondrial Gly3P NADH shuttle process, which then activates mitochondrial energy metabolism and triggers insulin secretion [ 136, 137].
Amino acids and insulin secretion
Individual amino acids at physiological concentrations are poor insulin secretagogues. However, certain combinations of amino acids at physiological concentrations or higher can augment GSIS [138]. For example, glutamine alone does not stimulate insulin secretion or enhance GSIS, but a combination of glutamine with leucine can enhance GSIS from β-cells [139]. Leucine can activate glutamate dehydrogenase, which converts glutamate to α-ketoglutarate. Glutamine, after converted into glutamate by glutaminase in the cytosol, can enter the TCA cycle via α-ketoglutarate, which results in ATP production, thereby enhancing insulin secretion [138]. Without leucine, glutamine is metabolized to γ-aminobutyric acid (GABA) and aspartate. Moreover, some amino acids can indirectly influence β-cell insulin secretion. During the fasting period, proteins in skeletal muscle are catabolized and amino acids are subsequently metabolized for generating energy. Free amino acids, including alanine and glutamine, are released into the blood and serve as potent glucagon secretagogues. This results in elevatation of blood glucose levels, which then triggers insulin secretion. Dietary amino acids can also induce insulin secretion via incretin-dependent mechanisms. Gastric inhibitory polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) are the two major incretin hormones secreted from the gastrointestinal tract. Ingestion of nutrients in the gut, including glucose and amino acids, stimulates secretion of these hormones from intestinal K-cells and L-cells. These hormones then directly act on β-cells by binding to their specific cell-surface receptors, augmenting GSIS [140–142].
Fatty acids and insulin secretion
Free fatty acids (FFAs) also influence β-cell secretion of insulin. They potentiate insulin secretion to compensate for increased insulin need as a consequence of insulin resistance in type 2 diabetes [143–145]. FFA can also enhance GSIS. Islets deprived of fatty acids lose GSIS, which can be reversed by replacement with exogenous fatty acids [146–148]. Recently, it was discovered that β-cells have a free fatty acid receptor, free fatty acid receptor (FFAR)-1, through which FFA can influence β-cell function [149, 150]. The intracellular metabolism of FFA is the source for synthesis of lipid signal molecules such as long-chain acyl-CoA [145] and DAG [145, 151]. Long-chain acyl-CoA could acylate essential proteins in insulin granule fusion, such as synaptosomal-associated protein-25 (SNAP-25) and synaptogamin [152, 153]. DAG activates protein kinase C, which is implicated in insulin secretion [154]. It also binds to synaptic vesicle priming protein Munc-13 to promote insulin secretion [155].
Cellular signaling transduction pathways in regulation of insulin secretion
Insulin secretion is a process that involves the fusion of insulin granules with the plasma membrane and exocytosis of granule content. Insulin secretion shows a characteristic biphasic pattern that consists of a transient first phase followed by a sustained second phase. In humans, when plasma glucose is ~7 mM, first phase insulin secretion peaks at 1.4 nmol/min. The first phase lasts for ~10 min and is then followed by the second phase with the secreting rate at ~0.4 nmol/min [156]. However, the biphasic pattern is less prominent in mice than in rats and humans. This might be explained by the relatively higher basal plasma blood insulin levels in mice (8–9 mmol/L in mice vs. 4–5 mmol/L in rats and humans) [157, 158]. Thus, even though insulin secretion is induced by 10 mM glucose in mouse islets, the clear peak of a first phase of insulin release is missing. It is likely that biphasic insulin secretion and insulin exocytosis have the same cellular background. Although no clear boundary exists, insulin granules can be categorized into distinct functional pools [159, 160]. A small fraction of the granules (1%) are immediately available for release, named the readily releasable pool (RRP), which contribute to the rapid insulin release triggered by glucose [161]. The remaining granules (99%) belong to the reserve pool. When the RRP depletes, it is refilled from the reserve pool. Granules in the reserve pool have to undergo the preparatory reactions before becoming a RRP granule. The priming process, involving both granule modification and translocation toward the plasma membrane, is the rate limiting step for insulin exocytosis. The following observations suggest that there is a relationship between biphasic insulin secretion and pools of granules: 1). Both the first phase of insulin secretion and the exocytosis from RRP can occur even in the absence of nutrients, while both the second phase of insulin secretion and RRP replacement are strictly metabolic-product-dependent; 2), The total number of granules in RRP is positively related to the amount of insulin released in the first phase of secretion [162]; and 3). Ablation of Munc13-1 selectively suppresses second phase insulin secretion and insulin granule exocytosis, but does not affect the first phase and insulin exocytosis from the RRP [163, 164]. However, there is discrepancy in kinetics. The replacement of RRP occurs in less than 1 sec, while the first phase of insulin secretion can last for about 10 min.
Several proteins participate in insulin exocytosis. The soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) plays an essential role in insulin granule membrane fusion. Four SNARE motifs form the extremely stable helical β-cell exocytotic core complex. The central part of this complex contains four highly conserved amino acids contributed by the four SNARE motifs: three glutamine (Q), and one arginine (R) residue [165]. In β-cells, the fusion of the insulin granules with the plasma membrane involves the assembly of a complex consisting of VAMP-2 (R-SNARE) on the granule membrane, syntaxin-1a (Qc-SNARE) on the plasma membrane, and the membrane-associated protein SNAP-25 (Qa-Qb SNARE). The assembly of this complex can be regulated by other accessory factors to achieve elegant regulation of insulin granule fusion. Tomosyn-1 is such a regulatory factor that can replace VAMP2 in the process of assembly [166]. It is required for granule fusion and/or priming of the granules, but its absence does not influence insulin granule transport and docking [167, 168].
The priming and fusion of insulin granules, which results in insulin exocytosis, is triggered by elevation of intracellular [Ca2+]. Insulin exocytosis can proceed at the rate of 500 granules per second when intracellular [Ca2+] is increased to 17 mmol/L, but it only proceeds at the rate of 3–4 granules per second when [Ca2+] is at 0.17 mmol/L. Exocytosis occurring at low [Ca2+] is due to a small portion of granules capable of releasing insulin, which are referred to as the high Ca2+-sensitive pool (HCSP). The exocytosis occurring at different rates is controlled by two Ca2+ sensing mechanisms: the low affinity Ca2+ sensor and the high affinity Ca2+ sensor. Exocytosis occurring at high [Ca2+] are controlled by the low affinity Ca2+ sensor. Synaptotagmin IX was reported to be a high-affinity Ca2+ sensor in β-cells [169] and it remains to be determined if synaptotagmin IX also functions as a low-affinity Ca2+ sensor. Another putative Ca2+ sensor is piccolo, which facilitates rapid Ca2+- induced exocytosis by interacting with essential proteins, including cAMP-regulated guanine nucleotide exchange factor/exchange proteins activated directly by cyclic AMP (cAMPGEFII/Epac2), sulfonylurea receptor1 (SUR1), and the L-type Ca2+ channels [158, 170, 171]. Thus, piccolo might act as a low-affinity Ca2+ sensor.
Intracellular [Ca2+] is determined by the open and closure of plasma membrane Ca2+ channels. Three subfamilies of voltage-gated Ca2+ channels exist: (1). L-type high voltage-activated (HVA) Ca2+ channels. They are sensitive to dihydropyridines (DHP) and include (1) CaV1.1, 1.2, 1.3, and 1.4 channels [172–174]; (2) the non-L-type HVA channels CaV2.1 (P/Q-type), 2.2 (N-type) and 2.3 (R-type) [172, 173, 175]; and (3) the low voltage-activated (LVA) T-type Ca2+ channel (CaV3.1, 3.2, and 3.3). LVA differs from HVA Ca2+ channels electrophysiologically. The LVA open transiently upon modest depolarization [176, 177]. They are pacemakers in most cell types [178]. A mixture of voltage-gated Ca2+ channels were reported to be present in β-cells [179–181]. The existence of L-type Ca2+ channels was first confirmed about 25 years ago by radioisotopic and electrophysiological measurements [182]. Later the expression of CaV1.2 of the L-type Ca2+ channels, and P/Q, N and R-type Ca2+ channels were confirmed by single-cell PCR [181]. The first phase of insulin secretion couples to the activation of L-type CaV1.2 channels, whereas the second phase secretion depends on R-type (CaV2.3 channels), which mediates a moderate global increase in intracellular [Ca2+]. The R-type Ca2+ channel- mediated Ca2+ influx is insufficient to cause insulin exocytosis, but accelerates granule mobilization, and increases the size of RRP [183].
Glucose induces insulin secretion by both triggering (i.e. involving closure of the KATP channels) and amplifying (i.e. post KATP channel closure) effects. Although the increase in intracellular [Ca2+] is the primary signal that triggers insulin exocytosis by glucose, there are other cell signals activated by glucose that also play roles in this process, such as cAMP, cGMP, inositol 1,4,5-trisphosphate (IP3), and DAG [183]. Among those signaling molecules, cAMP may be the most important one for potentiating insulin secretion [154, 184–187]. Around 50 years ago, it was found that an oral glucose load elicits greater insulin secretion than an intravenous glucose load even though similar circulating glucose levels were achieved by these two methods [187]. The potentiated insulin secretion by orally ingested glucose was due to the action of GIP and GLP-1, incretin hormones secreted by enteroendocrine K cells and L cells, respectively, upon glucose ingestion [188, 189]. Incretin hormones augment GSIS by stimulating the cAMP signaling pathway. Cyclic AMP’s action was generally thought to be mediated exclusively by the activation of protein kinase A (PKA), which phosphorylates proteins involved in insulin exocytosis [190]. However, the insulinotropic effect of cAMP can only be blocked partially by inhibition of PKA activity, suggesting that an alternative mechanism exists that mediates, in part, the cAMP effect on insulin exocytosis. Recently it was discovered that cAMP stimulates exocytosis of insulin granules from RRP, an effect that was unaffected by PKA inhibition, suggesting a PKA-independent mechanism [191]. A cAMP-binding protein called CAMPS (cAMP sensor) was identified by yeast two-hybrid screening of the insulinoma cell line MIN6, during the search for intracellular molecules that interact with the sulfonylurea receptor SUR1 [192]. CAMPS was later identified using a BLAST-search approach as a mouse homolog of rat cAMP-GEFII/Epac2, which is an isoform of cAMP-GEFI/Epac1 [193, 194]. Studies using a yeast two-hybrid system later confirmed the interaction between cAMP-GEFII/Epac2 and SUR1 [192, 195], revealing a novel cAMP-GEFII/Epac-dependent pathway activated by cAMP.
The cAMP-binding protein, cAMP-GEF/Epac participates in potentiating insulin secretion in a PKA-independent manner. cAMPGEFII/Epac2 is abundant in the brain and neuroendocrine and endocrine tissues including pituitary, adrenal, and pancreatic islets, while cAMP-GEFI/Epac1 is expressed at high levels in adult tissues including thyroid, kidney, ovary, skeletal muscle, and heart, and at low levels in the brain [192–194]. In addition to SUR1, which is the regulatory subunit of the KATP channel, cAMPGEFII/Epac2 also binds to Rab3-interacting molecule 2 (Rim2) [192] and Piccolo [196]. Rim2 is the target of the small G-protein Rab3, which is involved in exocytosis [197]. Piccolo defines and organizes the site of neurotransmitter release in neurons [198]. Piccolo also forms both homodimers and heterodimers with Rim2 in a Ca2+-dependent manner [196]. cAMP-GEF/Epac, which has a higher dissociation constant for cAMP (1.2–4μmol/L, PKA: 5–25 μmol/L), is a cAMP sensor when PKA activity is fully saturated [199–201]. The cAMP-GEF/Epac might be localized in cAMP compartments distinct from PKA, since a much higher concentration of cAMP is required for activating the cAMP-GEF/Epac-mediated signaling than that for stimulating PKA activity. In the absence of cAMP, Ras exchange motif (REM) binds to GEF/Epac to stabilize GEF/Epac and inhibit its activity. Cyclic AMP activates GEF/Epac by binding to its regulatory region. Activated GEF/Epac then activates Ras-like small GTP-binding proteins, Rap1 and Rap2 [193, 194, 199, 202]. Cyclic AMP-GEFII/Epac2 may be involved in both the first and second phase of insulin release, since treatment with antisense oligodeoxynucleotides (ODNs) against GEFII/Epac2 in pancreatic islets reduced both first and second phases of cAMP- potentiated insulin secretion [203]. Interaction between SUR1 and cAMP-GEFII/Epac2 is an essential step in this PKA-independent cAMP potentiated insulin secretion. This PKA-independent effect on cAMP-regulated insulin secretion is impaired in SUR1 knockout islets [204, 205] and early PKA-independent exocytosis is absent in SUR1 knockout β-cells [170]. Being recruited to the plasma membrane by SUR1, cAMP-GEFII/Epac2 mediates the cAMP-dependent activation of Rap GTPase activity. Rap then stimulates phospholipase C (PLC)-ε, which catalyzes hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2). The hydrolysis of PIP2 causes inhibition of KATP channels [206]. Therefore, the interaction between cAMP-GEFII/Epac2 and SUR1 is independent of intracellular ATP concentration [207]. After activated by cAMP, cAMP-GEFII/Epac2 dissociates from the SUR1-cAMP-GEFII/Epac2 complex, which then releases its inhibition on KATP channels. There is another hypothesis that after activated by cAMP, cAMP-GEFII/Epac2 will dissociate from granule SUR (gSUR, a putative SUR on insulin granules) to open a chloride channel (ClC-3), which is coupled with gSUR. Opening of the ClC-3 channel allows Cl− influx to promote granular acidification, which allows insulin granule priming and refilling of the RRP [208]. Accumulating evidence also suggests that activation of cAMP-GEFII/Epac2 mobilizes Ca2+ from intracellular Ca2+ storage, thereby increasing insulin secretion [209–212]. Three possible mechanisms might mediate this Ca2+ mobilization: 1). cAMP-GEFII/Epac2 interacts with IP3 receptors and ryanodine receptors to increase intracellular Ca2+ channel sensitivity to Ca2+ or Ca2+ mobilizing signals; 2). cAMP-GEFII/Epac2 might act through Rap and extracellular signal regulated kinase to sensitize intracellular Ca2+ release; and 3). cAMPGEFII/Epac2 acts through Rap to stimulate PLC-ε, thereby hydrolyzing PIP2 to release IP3,which signals release of Ca2+ from the ER[ 213].
Hormone regulation of insulin secretion
Estrogen
β-cells are not considered classic estrogen targets; however, estrogen receptors are present in islets [214] and the effects of 17β-estradiol on β-cells are well documented [215]. The main physiological consequence of 17β-estradiol action on β-cells is the enhancement of insulin secretion [216]. In humans, 17β-estradiol can increase insulin secretion in postmenopausal women [217, 218]. This insulinotropic effect is mediated by potentiating glucose-stimulated insulin secretion (GSIS) [219]. The effects of estradiol are initiated by its binding to estrogen receptors. Two types of estrogen receptor (ER) are present in β-cells: 1). the nuclear ERs (ERα and ERβ) and 2). the membrane ER (ERγ) [220]. It is reported that at physiological concentrations, 17β-estradiol can significantly decrease KATP channel activity in a reversible manner [216], which causes membrane depolarization and subsequent opening of voltage-gated Ca2+ channels, thereby potentiating glucose-induced intracellular [Ca2+] oscillations. The modulation of KATP channel activity by estradiol may be mediated by activation of the cGMP-dependent protein kinase (PKG) pathways [221]. The activated PKG can directly phosphorylate transcriptional factor CREB. After phosphorylation, CREB can bind to CRE, which in turn modulates transcription of genes containing cAMP/Ca2+ response elements to potentiate glucose-induced intracellular [Ca2+] oscillations that influence insulin secretion [222–225].
Melatonin
Melatonin is a hormone secreted by the pineal gland, which helps adjust the timing or reinforces oscillations of the biological clock [226]. The direct effect of melatonin on β-cells was confirmed by the discovery of melatonin receptors on both clonal β-cells [227, 228] and human islets [229]. Effects on insulin section are controversial. There are studies showing that melatonin exerts an inhibitory [230, 231], neutral [232], or stimulatory effect on insulin secretion [233]. However the inhibitory effect is consistent in replicated experiments with clonal β-cells [227, 229, 233, 234]. Melatonin attenuated glucose- and KCl-stimulated insulin secretion in rat islets [235]. The inhibitory effect of melatonin on insulin release was later confirmed in rat islets [236]. Consistently, chronic melatonin administration ameliorates hyperinsulinemia in vivo [237].
It was reported that melatonin receptor is coupled to Gi, which inhibits G protein [238]. G protein activation will further activate adenylate cyclase to catalyze cAMP production. Indeed, melatonin blocks the enhanced insulin secretion by cAMP agonists forskolin or GLP-1 [227, 228]. In contrast, cAMP levels in human islets are not influenced by melatonin, whereas the formation of cAMP in MIN-6 cells is impaired in the presence of melatonin [229]. Melatonin also decreased cGMP levels to inhibit insulin secretion. This effect is mediated by activation of melatonin receptor (MTNR) 1B [239]. However, when Gi coupling is blocked by pertussis toxin, MTNR1A also mediated a stimulatory effect on insulin secretion by coupling to Gq/11. The activation of Gq/11 provokes the release of IP3 by activating PLC-ε to potentiate insulin secretion [233, 240, 241].
GLP-1
GLP-1, an incretin hormone, is secreted from small intestinal L-cells together with GIP in response to nutrient load [242, 243]. Incretin is responsible for augmentation of insulin secretion to meet the increased demand for insulin after a meal. Experiments have shown nutrient load from the oral route stimulates more insulin secretion than intravenous nutrient load [244]. The analogs of both GLP-1 and GIP have been explored as a potential therapy for T2D for many years, and the long-lasting GLP-1 analog exenatide was introduced to clinics in 2005 as a prescription drug for T2D treatment [245]. Upon activation of the GLP-1 receptor (GLP-1R), adenylyl cyclase is activated, leading to the generation of cAMP [246]. The elevated cAMP then potentiates GSIS. This insulinotropic effect is dependent on glucose. When the extracellular glucose concentration is in the normal fasting range (lower than 4 mmol/L), GLP-1 is inactive in stimulating insulin secretion [245]. Such glucose-dependent action of GLP-1 is very important in preventing hypoglycemia.
Leptin
Leptin is secreted by adipocytes and is known to influence insulin action in fat and liver cells [247, 248]. It is generally accepted that leptin exerts an inhibitory effect on insulin secretion. Leptin deficiencies are associated with hyperinsulinemia in both mice and humans [247, 249]. A large body of literature shows that leptin plays an inhibitory role in insulin secretion in clonal β-cells [250–252], cultured rodent islets [251–259], human islets [251, 260, 261], perfused rodent pancreas [250, 262], as well as in mice [251]. It is hypothesized that leptin’s inhibitory effect is through antagonizing the action of elevated intracellular cAMP [263], since it was reported that leptin inhibits insulin secretion induced by 3-isobutyl-1-methylxanthine (IBMX), which elevates cAMP content by inhibiting phosphodiesterases (PDEs) [258], the enzymes catalyzing the hydrolysis of cAMP. Leptin also potently inhibits glucoincretin- or GLP-1-induced insulin secretion, which augments GSIS through activation of the cAMP signaling pathways [252, 262]. Leptin was shown to inhibit insulin secretion by activating PDE 3B, a subtype of PDE [252].
Growth hormone
Growth hormone (GH) has targets in variety of cells but one of its best-known actions is to stimulate production of insulin-like growth factor-I (IGF-I) and its binding proteins [264]. Recombinant human IGF-I was shown to decrease serum levels of insulin and C-peptide in normal human subjects [265]. Ex-vivo studies using isolated rat islets confirmed that IGF-1 directly suppresses insulin secretion [266]. This inhibitory effect is possibly mediated through activation of PDE3B [267], which is responsible for breaking down cAMP in β-cells, as stated above.
Pancreatic β-cell apoptosis and regeneration
Both T1D and T2D are characterized by progressive β-cell destruction, of which apoptosis is the main form. Although β-cell loss is caused by excessive nutrients in T2D and an autoimmune reaction in T1D, there is a convergence in cellular signaling pathways between the two types of diabetes [268], which is schematically illustrated in Fig (2).
Fig. 2.
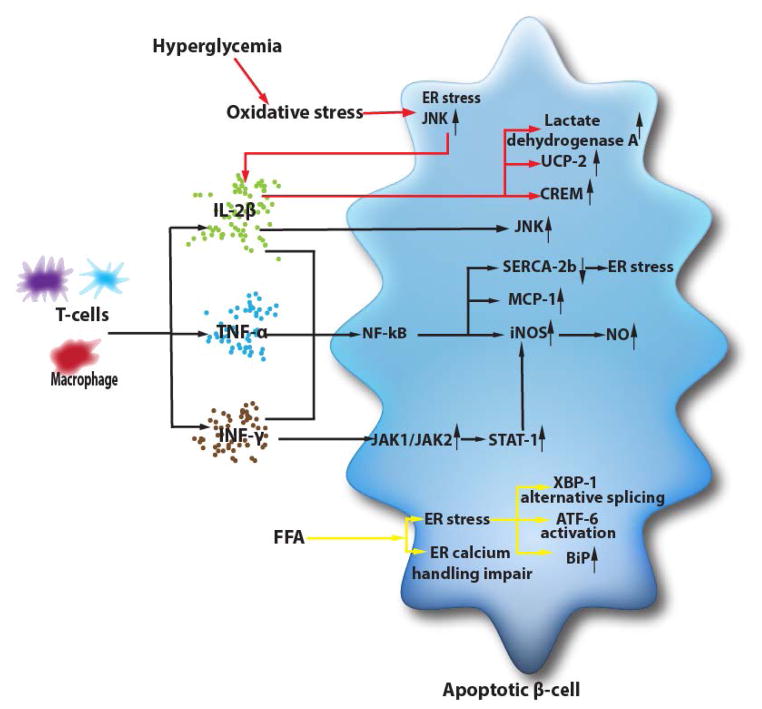
Schematic illustration of pancreatic beta-cell apoptosis and dysfunction.
T1D is a T-cell-mediated autoimmune disease resulting from selective destruction of pancreatic β-cells. The incidence of T1D is estimated to increase from 4.4 million in 2000 to about 5.4 million in 2010 [269]. However the pathogenic mechanisms and T-cell mediated autoimmune process that destroy pancreatic β-cells in T1D are complex and are not fully defined, the subject of many excellent reviews [269–272]. It is clear from past studies that infiltration of inflammatory cells, such as T helper type 1 (Th1) cells and macrophages into the islets in response to islet associated antigens and subsequent insulitis are hallmarks of the pathogenesis of T1D. Activated T cells and macrophages release several proinflammatory cytokines, such as interleukin-1β (IL-1β), interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α), which are believed to be important mediators leading to β-cell destruction in T1D [273–278]. These cytokines act on β-cells through their specific receptors to induce several signal transduction pathways that lead to alternations in gene and protein expressions [269]. Research evidence suggests that activation of NF-kB may be a common and crucial step for various cytokine-stimulated β-cell dysfunction. Activation of NF-kB will lead to induction of its downstream gene inducible nitric oxide (NO) synthase (iNOS) and subsequent NO production [279]. Cumulative evidence shows that IL-1β, IFN-γ and TNF-α induce the overexpression of iNOS in β-cells, leading to the overproduction of NO that causes the cytotoxicity to β-cells [278], suggesting an important role for NO in the pathogenesis of diabetes. Indeed, transgenic mice overexpressing iNOS in β-cells developed insulin-dependent diabetes [280]. Conversely, inhibition or knockout of iNOS in the islets protects β-cells in vitro and in vivo from the cytotoxic effects of cytokines [281–283]. While additional mechanisms may also be involved in the pathogenesis of T1D, these data clearly indicate that iNOS derived NO is at least partially responsible for cytokine-mediated destruction of β-cells, which is central to the development of T1D [284–287]. β-cell proliferation is increased during the pathogenesis of diabetes in humans and non-obese diabetic (NOD) mice, an animal model for human T1D [288, 289], which does not adequately compensate for autoimmune-mediated destruction of β-cells [290, 291]. As such, identifying agents that can simultaneously induce β-cell proliferation and survival can provide novel treatment for T1D.
β-cell apoptosis in the course of insulitis in T1D is caused by direct contact with islet infiltrated T cells and macrophages and exposure to soluble mediators secreted by those infiltrated immune cells such as oxygen free radicals, NO, and cytokines including IL-1β, IFN- γ and TNF-α [292]. IL-1 β and IFN- γ are considered to be two major soluble factors which mediate β-cell damage. In vitro cell culture showed that exposure of β-cells to IL-1β or IL-1β+IFN- γ causes β-cell destruction similar to that which was observed in pre-diabetic patients [293]. In response to the stimulation of IL-1β and INF- γ around 700 genes are up- or down-regulated in β-cells[ 294].
In β-cells, IL-1β can activate transcription factor nuclear factor (NF)-kB [292]. Although basal NF-kB activity is required for maintaining β-cell normal insulin secretary function [295], excessive activated NF-kB will result in up-regulated expression of inducible nitric oxide synthesis (iNOS) [296]. iNOS can produce massive amounts of NO which will result in decreased expression levels of transcription factors responsible for β-cell differentiation and function (e.g., PDX-1 and Isl-1) [297, 298]. This cytokine mediated NF-kB also up-regulates chemokines such as monocyte chemoattractant protein-1 (MCP-1) [299, 300] and down-regulates the Ca2+ pump sarcoendoplasmic reticulum Ca2+ ATPase type 2b (SERCA-2b) [298, 301] Decreased SERCA-2b expression leads to ER calcium depletion and severe ER stress which results in β-cell apoptosis [298, 302–304].
Exposure to IL-1β also activates the c-Jun NH2-terminal kinase (JNK), a member of the mitogen-activated protein kinases (MAPKs) [305, 306]. JNK plays an important role in the intracellular events during β-cell loss [307]. Cell-permeable peptide inhibitors of JNK prevent cytokine-induced β-cell apoptosis [308]. IFN-γ is reported to synergize with IL-1β to trigger β-cell apoptosis [292]. IFN-γ binds to cell surface receptors and activates JAK1 and JAK2, which phosphorylate the transcription factor STAT-1. Upon activation STAT-1 forms a dimer and translocates into the nucleus to activate γ-activated site of diverse genes including up-regulating iNOS expression [292, 296].
Cytokines and hyperglycemia share some common mechanisms to alter β-cell gene expression. C-Myc, A20, and heme-oxygenase are induced under both conditions. Hyperglycemia was reported to increase the production of IL-1 β from β-cell [309]. However, the patterns of hyperglycemia-induced genes in β-cells are not exactly the same as that induced by cytokines [294, 298, 310] suggesting that there is a divergence in mechanisms of β-cell apoptosis between these two conditions. NF-kB dependent genes which are strictly activated by IL-1β remained unchanged after high glucose exposure, while lactate dehydrogenase A, the mitochondrial uncoupling protein (UCP)-2, and the transcription factor cAMP-responsive element modulator (CREM) can be induced in hyperglycemia [310, 311]. β-cell glucotoxicity at least in part results from an increase in β-cell oxidative stress, and subsequent JNK activation is NF-kB independent [312, 313]. The main source of oxidative stress probably comes from the mitochondrial electron transport chain [314, 315]. In addition, ER stress and sustained elevation of cytosolic calcium concentrations could also be possible explanations of β-cell viability loss [316].
Besides glucotoxicity, high plasma concentration of free fatty acid (FFA) is another risk factor for β-cell destruction. The effect of dyslipidemia depends on the lipid profile. Saturated fatty acids such as palmitate are highly toxic at long-term exposure, whereas monounsaturated fatty acids such as oleate protect β-cells from palmitate and high glucose-induced β-cell apoptosis [317, 318]. FFA’s toxicity on β-cells is suggested to be iNOS/NO independent, because of the absence of iNOS expression or NO production in FFA-mediated β-cell apoptosis [319, 320]. It is also reported that FFA’s toxic effect is oxidative stress independent [320]. Moreover, oleate or palmitate did not activate NF-kB in β-cells [319]. FFA-induced β-cell toxicity might occur at the ER level where FFAs are esterified. Both oleate and palmitate can induce ER stress markers including alternative splicing of XBP-1, activation of ATF-6, and induction of the ER chaperone BiP [319]. Besides, FFA might also impair ER calcium handling [321]. Therefore, in the condition of increased insulin demand such as high glucose, ER stress induced by FFA might be amplified.
In mammals, β-cells have a slow turnover rate. In healthy adult individuals, there is an extremely low ratio of replicating β-cells to apoptotic ones [322]. However, β-cell proliferation can be increased in obese or/and insulin resistant individuals [323–325], during the progression of autoimmunity in type 1 diabetes [326], during pregnancy [327], and in response to mechanical or chemical damage [327], demonstrating the plasticity of the tissue. The population of β-cells can be increased by several mechanisms: replication of the existing β-cells, neogensis from pancreatic precursor ‘stem cells’ [328], and as recently discovered, trans-differentiation from fully-differentiated α-cells into β-cells [327]. Trans-differentiation was initiated in the pancreas of mice during recovery after chemical-induced destruction of β-cells, with 30–80% of regenerated β-cells derived from α-cells [327]. Besides the increase in cell number, an increase in cell size also contributes to increased β-cell mass to meet a greater insulin requirement. The mechanism by which β-cells expands is hypothesized to involve up-regulation of protein synthesis, but the exact molecular mechanism is unclear [329].
β-cell proliferation and differentiation can be influenced by certain nutrients and various growth factors. Nutrients that simulate insulin secretion and synthesis can also increase β-cell proliferation. Glucose is the most physiological relevant β-cell growth-promoting nutrient [330, 331]. Some of the growth factors that can stimulate β-cell growth, such as IGF-1 and GH, are glucose-dependent [331, 332]. However, glucose-mediated β-cell growth is largely an acute effect. Chronic exposure to high glucose will evoke β-cell apoptosis [333].
GH stimulates β-cell growth by binding to growth hormone receptor (GHR) present on β-cell which leads to JAK-mediated tyrosine phosphorylation and activation of STAT5a and 5b [334, 335]. Activated STAT5a and 5b then up-regulate cyclin D2 expression, which is an essential regulator of β-cell proliferation [336, 337]. In some types of cells, GH’s effect is mediated by increasing local IGF-1 production. But β-cell IGF-1 and GH signal transduction pathways are independent [332, 338]. IGF-1 mediated β-cell mitogenesis requires the induction of phosphatidylinositol 3-kinase (PI3K) activity, which is located downstream of insulin receptor substrate (IRS)-2 [331]. IGF-1 will activate protein kinase-B (PKB; also known as Akt) which is important for β-cell survival [339, 340]. PKB in turn can phosphorylate glycogen synthase kinase (GSK)-3 [341, 342] leading to inhibition of GKS-3[343]. Although the consequences of GSK3 inactivation are currently unclear, GSK-3 is able to control general protein synthesis and cell differentiation which contribute to β-cell hypertrophy and neogenesis [343].
THE PATHOGENESIS OF T1D
A decrease in both mass and insulin secretary function of β-cells is the common characteristic shared in both type 1 and type 2 diabetic patients. Autoimmunity plays a critical role in the development of T1D. The classical T1D is characterized by the presence of antibody (humoral) and T-cell (cellular) responses to self-islet proteins (antigens) [344–348]. Histological analysis of the pancreas from patients with T1D shows the presence of immunological activity [349]. Drugs that suppress the immune response such as cyclosporine and azathioprine can slow the progression of β-cell destruction pointing to the critical role of immune activity in development of type 1 diabetes [350, 351]. Development of T1D may be influenced by dietary factors including early infant feeding status [352], Vitamin D and omega 3 polyunsaturated fatty acid intake [353], and duration of exposure to gluten [354]. People with genetic predispositions have a higher risk to develop overt T1D. Human leukocyte antigen (HLA) encodes cell surface proteins that interact with immune cells and are an important gene family that contributes up to 40% of T1D risk. The HLA Class II region is considered to be the most influential. In Caucasians, HLA types DR3-DQA 0501-DQB1 0201 and DR4-DQA1 0301-DQB1 0302 are strongly associated with risk, while DQB1 0602 is associated with protection [355].
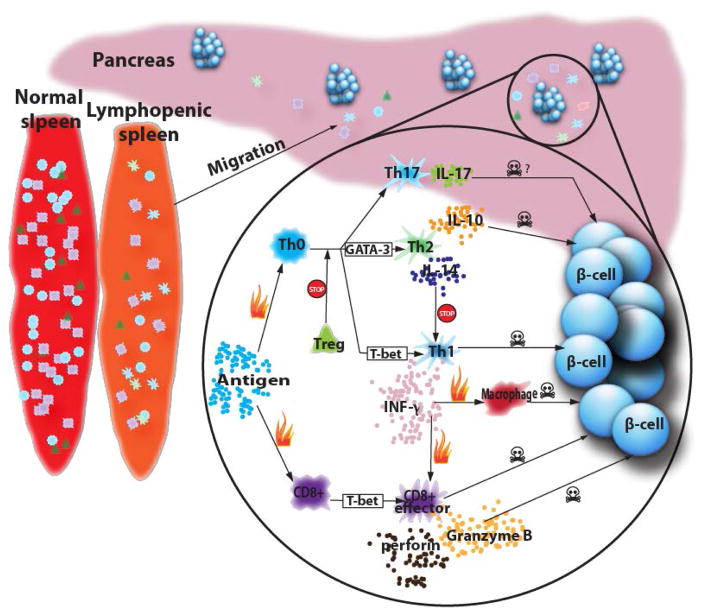
Innate immune response signaling is involved in the initiation of the autoimmune process. However, the molecular pathways of innate immunity linked to T1D development are yet to be elucidated [356]. Adaptive immunity is known to play a critical role in β-cell destruction in T1D development. Humoral and cellular immunity are the two major facets of adaptive immunity. In T1D, the appearance of multiple autoantibodies is believed to reflect progressive β-cell autoimmunity [357, 358]. Although the autoantibodies can be markers for T1D, whether they contribute to the pathogenesis is not confirmed [358]. β-cell destruction is partly mediated by the cellular immune response. A schematic illustration of immunological aspect of T1D is shown in Fig. (2).
T lymphocytes are reported to be the primary mediator in T1D progression [359], though macrophages and dendritic cells infiltrate islets before T lymphocytes [360]. The indispensable role of T lymphocytes is supported by their presence in insulitis, and detection of circulating autoreactive T lymphocytes in clinical overt T1D patients, and the observation that immunosuppressive drugs specifically against T lymphocytes delay disease progression [361]. Although T1D is T lymphocyte-dependent, paradoxically both NOD mice and human T1D patients are lymphopenic [362–365]. The decreased number of T lymphocyte drives T lymphocyte homeostatic expansion [365]. This homeostatic expansion results in increased effector/memory T lymphocytes instead of naive T lymphocytes [366]. These effector/memory T lymphocytes can generate new effector cells more efficiently, which precipitate autoimmune disease [367]. Two subsets of T lymphocytes, CD4+ and CD8+, are both involved in T1D development. The precise role of CD4+ and CD+8 T lymphocytes in β-cell destruction are controversial. It is generally accepted that CD4+ T lymphocytes contribute to provide proper homing for CD8+ effector cells as well as being effector cells themselves [368].
Naive CD4+ T lymphocytes reside as T helper (Th) 0 cells in secondary lymphoid organs before they encounter antigens. After encountering antibodies they differentiate into functional subsets namely Th1 secreting IL-2, INF-γ, and TNF-α [369, 370] and Th2 secreting IL-4, IL-5, IL-10 and IL-13 [371–374]. Studies about the correlation of diabetes and T helper cell phenotypes lead to the idea that Th1 and their cytokines promote diabetes [374, 375]. Th1 cells can either cause β-cell destruction directly [376, 377] or by secreting Th1 cytokine (INF-γ) to recruit and activate macrophages and CD8+ T lymphocytes that exert toxic functions [378]. In contrast, Th2, through secretion of the cytokine IL-4, is generally considered protective [375, 379]. Although there is evidence that Th2 plays a role in causing β-cell damage, it is through IL-10 instead of IL-4 [380–382].
Th1/Th2 differentiation is influenced by the antigen concentration, ligation of co-stimulatory molecules, and cytokine circumstance; but eventually transcription factors T-bet and GATA-3 control T helper cell differentiation [383–385]. Cells dominated by T-bet will differentiate into Th1 cells, while Th2 differentiation is directed by GATA-3 expression [385]. The activation of Th1 and Th2 cells can be suppressed by a specialized subpopulation of CD4+ T cells named regulatory T (Treg) cells. Treg cells, which are protective, contribute to immune suppression by suppressing activity of both CD4+ T lymphocytes and CD8+ cytotoxic T lymphocytes [386–388]. Treg cells comprise 5–10% of the peripheral CD4+ T lymphocyte population in mice and humans [389]. Naturally occurring Treg cells (nTreg) are generated in thymus and express surface markers CD4 and CD25, and an intracellular marker, transcription factor forkhead box P3 (Foxp3) [390]. The other Treg subpopulation named induced Treg (iTreg) is generated in response to antigen. They are not CD25+ by default. But they share features with nTreg in terms of Foxp3 expression and bystander (non-antigen-specific) immune suppression [390].
Similar to CD4+ T lymphocytes, mature CD8+ T lymphocytes reside as naive cells in secondary lymphoid organs. After encountering self-antigen [391], activated CD8+ T cell differentiate into effectors cells. Activated CD8+ T cells destroy β-cells either through a perforin-dependent pathway or alternatively by the Fas/FasL pathway [373, 392]. The pore-forming protein perforin and the granzyme B are key constituents of cytolytic granules. After conjugate formation, perforin and granzyme are released toward the target cell membrane where they synergize to cause apoptotic cell death [393]. INF-γ is reported to be crucial in activating the Fas/FasL pathway in islets. [392]. Therefore, INF-γ and granzyme B levels are markers of CD8+ T cell-induced β-cell damage. Similar to CD4+ cells, the activity of CD8+ cells can be tuned at the genetic level. T-bet, known to regulate Th1 cell differentiation, also controls the differentiation of the CD8+ cytotoxic effector cell [394].
Recently, it was reported that the activity of Th17 cell, which is a newly discovered subpopulation of CD4+ T lymphocytes secreting IL-17, is associated with autoimmune conditions in a variety of autoimmune diseases including rheumatoid arthritis [395], inflammatory bowel disease [396], and multiple sclerosis [397]. Studies have shown that Th17 cells may up-regulate IFN-γ and also extinguish IL-17 in response to IL-12 or IL-23 in the absence of TGF-β in vitro and depreciated to a Th17/1 (IL-17+IFN-γ+) or Th1 phenotype [398, 399]. Although the relative contribution of Th17 cells in T1D is not well defined, results from studies showed that high level of IL-17 transcripts were found within insulitic lesions in NOD mice [400]. Increasing levels of serum IL-17 is associated with accelerated disease progression in a T cell receptor transgenic NOD model [400]. More recently, the protective effect of therapeutic intervention with an antigen-specific agent is associated with a decrease in the Th17 population [401]. However, the specific contribution of this subpopulation of CD4+ T lymphocytes to the natural progression of T1D remains to be fully characterized.
THE PATHOGENESIS OF T2D
T2D is a result of chronic insulin resistance and loss of β-cell mass and function [284]. Both in experimental animals and people, obesity is a leading pathogenic factor for developing insulin resistance, which is always associated with impairment in energy metabolism, causing increased intracellular fat content in skeletal muscle, liver and fat, as well as pancreatic islets. Chronic insulin resistance will progress to T2D when β-cells are unable to secret adequate amounts of insulin to compensate for decreased insulin sensitivity, which is largely due to insulin secretory dysfunction and significant loss of functional β-cells [284–287, 402, 403]. Indeed, those individuals with T2D always manifest increased β-cell apoptosis and reduced β-cell mass [286, 287, 404]. Progression to full-blown T2D involves insulin resistance leading to β-cell dysfunction [405–407]. Insulin resistance is observed in a variety of patient conditions including gestational diabetes, obesity, impaired glucose tolerance (IGT) and polycystic ovarian syndrome [408, 409]. Although obesity is associated with T2D, most obese people don’t develop the disease and increased insulin secretion due to enhanced function of pre-existing β-cells or expansion of β-cell mass compensates and restores blood glucose levels [410]. Enhanced functionality involves increased nutrient signals stimulating increased growth factor signaling in β-cells [406]. In particular, increased nutrient load in the gut can enhance GLP-1 production leading to the anti-apoptotic and growth-promoting effects on β-cells[ 411, 412]. In “susceptible” individuals compensation becomes insufficient and cell dysfunction ensues. Generally, diagnosis of T2D is associated with an approximate 50 % reduction in islet function and this is thought to manifest itself at least 10–12 yr prior to diagnosis, a condition exacerbated by elevated fasting blood glucose [413]. Obese non-diabetic humans show increased relative β-cell volume in islets while obese and non-obese patients with impaired fasting glucose and T2D show at least a 40 % reduction in β-cell volume compared with respective non-diabetic patients [404]. Apoptosis of β-cells was substantially increased in all diabetic patients and was implicated as the primary mechanism underlying the decrease in β-cell mass in T2D individuals although β-cell mass is controlled by several factors including cell size, rate of cell renewal from proliferation of pre-existing cells or neogenesis (differentiation from other precursor cells) and rate of apoptosis. As the number of β-cells per islet declines in T2D patients, islet space becomes dominated by amyloid plaque deposits although the role of islet amyloid deposits in β-cell dysfunction is unclear [414]. The factors leading to a change in β-cell function (decreased insulin expression and secretion) and mass are central to the pathology of T2D.
The prevailing theories for causes of β-cell failure during the progression to T2D involve chronic exposure of the β-cell to glucose and fatty acids, also known as “glucotoxicity” and “lipotoxicity”, respectively [415–420]. It is known that transient exposure of islets to free fatty acids (FAAs)(e.g., hours) can augment GSIS whereas long-term exposure (e.g., days) decreases insulin secretion. In general, it is accepted that hyperglycemia precedes conditions for lipotoxicity while glucotoxicity can occur independently of lipotoxicity [310, 421]. Taking this idea a step further, the combination of these factors is known as “glucolipotoxicity”. We define “glucolipotoxicity” as chronic exposure of the islets to greater-than-physiological concentrations of glucose and fatty acids, leading to β-cell damage [312]. The following sections will dissect the meaning of these terms and some of the cellular and molecular mechanisms by which these phenomena alter β-cell function, with a particular emphasis on insulin synthesis and secretion. A schematic illustration of these processes is also included in Fig. (2). Bear in mind that differences in model system (in vitro vs. in vivo, primary vs. clonal cell lines (MIN6, INS1, HIT-T15, BetaTC-6), rodent vs. human, genetic vs. nongenetic), age of animal, concentrations of substrates (fatty acids, glucose), length of incubation or exposure (minutes vs. hours vs. days), etc. can all influence the outcome, making it very difficult to draw a definitive picture of β-cell pathology and diabetes mellitus. Studies described in this section involve primary cultured rat, human and mouse islet cells, as well as clonal β-cell clines and tissues harvested from humans and rodents.
Glutotoxicity -glycation and reactive oxygen species (ROS) production in the β-cells
While exact mechanisms are debatable, the general consensus is that long-term elevated levels of glucose have deleterious consequences for β-cells, which rely on signals of energy status to control metabolism [420]. Abundant expression of the low-affinity high-capacity GLUT2 in β-cells coupled to the role of glucose in stimulating insulin synthesis and secretion leads to excessive glucose concentrations in β-cells and effects on β-cell metabolic pathways. Glucotoxicity also down-regulates GLUT4 levels in insulin-responsive cells [405]. Chronic exposure to glucose leads to increases in cytosolic calcium that induce β-cell destruction [422]. It also leads to increased production of IL-1β, subsequent NF-kB activation, increases in FAS, DNA fragmentation and damaged β-cell function [423, 424].
Hyperglycemia leads to glycation reactions and production of ROS [425]. Glycation occurs non-enzymatically and can alter the function of a variety of molecules, and advanced glycation end products (AGEs) are implicated in cellular damage [426]. The antioxidant and glycation-inhibitor aminoguanidine, which prevents formation of AGEs and ROS, is able to partly ameliorate the effects of those damaging compounds on β-cell function [426], similar to the beneficial effect of the hydrogen peroxide-scavenging N-acetyl-L-cysteine on insulin expression and secretion in db/db mice or Zucker rat β-cells subjected to oxidative stress [427, 428]. The importance of ROS in β-cell pathology is supported by the observation that 8-hydroxy-2′-deoxyguanosine (8-OHdG) an oxidative stress marker, is elevated in β-cells from diabetic Goto-Kakizaki (GK) rats [429] and that the insulin [430] and glucokinase [425] promoters are sensitive to glycation and the presence of ROS (superoxide, hydrogen peroxide, nitric oxide, hydroxyl radicals). Furthermore, there are elevated levels of oxidative stress markers in the blood and urine of T2D patients, and reduced glutathione (GSH) in blood cells [312]. Fructose, D-ribose and 2-deoxy-D-ribose have a greater reducing capacity than glucose [431].
The process of aging, which involves long-term exposure to ROS, increases in body weight, a sedentary lifestyle, and reduced functionality of β-cells, partly accounts for prevalence of this disease in middle-aged to older adults [405]. Counter intuitively perhaps, the β-cell expresses relatively low levels of antioxidant enzymes, including CuZn superoxide dismutase (SOD), Mn-SOD, catalase, and glutathione peroxidase (GPx) [312, 432–435]. The Mn-SOD functions in the mitochondria, Cu/Zn-SOD in the cytosol, and both catalyze generation of hydrogen peroxide from the reaction of superoxide and hydrogen [312]. The GPx species reduce hydrogen peroxide to water with GSH, and also lipid peroxides to alcohols. Oxidized GSH (GSSG) can be converted back into GSH by GSH reductase using NADPH as a cofactor. Islet ROS levels were correlated with glucose concentration [435]. Blocking the existing GPx activity with buthioinine sulfoximine, an indirect inhibitor of GSH synthesis, hampered the beneficial effect of N-acetylcysteine, an antioxidant, on ribose-induced decreases in insulin production [435]. Isolated islets transfected with GPx exhibited a six-fold increase in enzyme activity, which negated the detrimental effects of ribose.
Hydroxyl radicals are particularly dangerous in β-cells because of their ability to cross the nuclear membrane and exert a mutatagenic effect [312]. The β-cells are particularly vulnerable to oxidative stress [312]. Oxidative phosphorylation generates ROS [436], as well as other pathways for glucose when glycolytic enzyme activity becomes saturated: glycosylation (Schiff reactions), autooxidation [437, 438], and the glucosamine pathway (O-linked glycosylation of proteins) [406, 439]. Activation of JNK and NF-kB is also stress-induced [420, 423, 440]. The activated JNK phosphorylates the Ser307 residue of IRS-1, hence blunting IRS signaling leading to decreased nuclear PDX-1 [420]. The IRS proteins are intracellular tyrosine kinase substrates that are downstream of their receptor. The IRS-1 and IRS-2 are important for β-cell function and survival; their absence leads to insulin resistance [441–443]. The insulin-insulin receptor (IR)-IRS-PI3K-Akt signaling cascade is crucial for regulating islet cell differentiation and function. Decreased Akt signaling as a result of IRS-1 phosphorylation by JNK results in an increase in Foxo-1-dependent gene expression, which plays a role in mediating PDX-1 translocation from the nucleus [444].
The mammalian target of rapomycin (mTOR) is a serine/threonine kinase that controls anabolic cellular processes in response to a variety of environmental stimuli including amino acids, glucose and oxidative stress [445, 446]. The rapamycin-sensitive complex TORC1 phosphorylates S6 kinase (S6K) and eukaryotic initiation factor 4E-binding protein-1 (4E-BP1). The rapamycin-insensitive complex TORC2 phosphorylates Ser473 of Akt and PKC [447]. Continuous activation of mTOR by glucose/fatty acids leads to IRS-2 phosphorylation, tagging it for proteasomal degradation, resulting in decreased IRS-2 levels and increased β-cell apoptosis [448]. As discussed earlier, GLP-1 has a growth-promoting effect on β-cells and enhances proliferation and protects against apoptosis [411]. The GLP-1 receptor activation in β-cells leads to IRS-2 and PKB activation mediated by CREB and transactivation of EGFR [449]
Constant entry of glucose into the β-cell leads to a state of reversible insensitivity to glucose stimulation concomitant with an exhaustion of β-cell stores. When glucotoxicity occurs, β-cell damage leads to defects in insulin production such that chronic glucose exposure-induced β-cell exhaustion becomes irreversible. Some of the explanations for glucose-induced β-cell damage include depressed rates of insulin synthesis [450], increased small heterodimer partner (SHP) nuclear receptor mRNA [451], downregulation of glucokinase mRNA [425], reduced PDX-1 mRNA, protein and insulin promoter binding activity [312, 450, 452], compromised mitochondrial function and induction of apoptosis. Upregulation of the SHP receptor is thought to act as a competitive inhibitor to prevent p300-mediated PDX-1 and BETA2 complex formation [451]. Repeated exposure to elevated glucose also reduces activity of RIPE3b1 [453, 454], while enhancing activity of insulin transcriptional repressor basic leucine zipper CCAAT/enhancer-binding protein β (C/EBPβ) [455, 456]. Changes in PDX-1 expression and activity have a profound influence on insulin transcription. Supra-physiological concentrations of glucose in the β-cell over an extended period of time are thought to affect posttranscriptional processing of PDX-1 mRNA [452]. The effect of short-term excess levels of glucose may be a reversible glucose desensitization whereas chronic, repeated exposure (long-term) causes less reversible effects on β-cell function, particularly in regards to insulin synthesis [450]. The effect on β-cells is most likely due to effects on function rather than simply apoptosis, per se [405]. For example, patients that undergo 60 % pancreatectomies do not always develop hyperglycemia and are able to compensate by enhanced functionality in remaining β-cells. Hence, initial pathology of T2D most likely involves an initial defect in β-cell responsiveness to glucose, leading to reductions in β-cell mass.
Lipotoxicity
The effect of fatty acids on β-cell apoptosis is complex and is most likely due to a multitude of factors including ceramide formation, oxidative stress, unfolded protein response, and the inflammatory response as reviewed in [415, 416]. The effects of chronic exposure to fatty acids are a bit less clear-cut but appear to involve increased lipolysis in white adipose tissue as a result of insulin resistance, a process that is amplified with body weight gain and continuous accumulation of adipose tissue [405]. This destructive cycle results in elevated circulating levels of free fatty acids that have a negative effect on GSIS and exacerbate insulin resistance in muscle and liver cells [405, 408]. Saturated fatty acids in particular, such as palmitate and oleate, have inhibitory effects on GSIS [406]. Additionally, accumulation of adipose tissue leads to increased adipocyte secretion of cytokines and adipokines such as TNF-α, IL-6, leptin, resistin and adiponectin [457]. These cytokines can be cytotoxic to β-cells, especially TNFα [458]. Although elevated levels of free fatty acids can mediate cytotoxic effects in β-cells, it is likely that in the absence of hyperglycemia and/or pre-existing β-cell functional defects, these signals may play a role in adaptation to insulin resistance without necessarily having deleterious consequences to cell viability [406].
Glucolipotoxicity -Inhibition of β-oxidation, stimulation of complex lipid formation, and mitochondrial and ER stress
The effects of glucolipotoxicity may stem from the effects of glucose on fatty acid metabolism in β-cells [421, 459]. In the context of “glucolipotoxicity”, glucose metabolism in the β-cell leads to formation of citrate, a signal for formation of malonyl-CoA in the cytosol, which then inhibits carnitine palmitoyl transferase-1 (CPT-1) activity. This has the effect of blocking fatty acid oxidation since CPT-1 plays a key role in transporting fatty acids into the mitochondria, the site of β-oxidation. This leads to accumulation of long-chain acyl-CoA esters in the cytosol. Consequently, detoxification of fat is attenuated and free fatty acids are shunted into pathways that lead to formation of cytotoxic complex lipids [145, 406, 459], through activation of AMP-activated kinase (AMPK), a metabolic sensor that drives energy metabolism [460–464]. Activity of AMPK is inversely correlated with glucose concentration and is enhanced by fatty acids in β-cells. Activation of AMPK leads to lipogenesis via transcription factor sterol-regulatory-element-binding-protein-1c (SREBP1c) [465]. Importantly, the form of lipid has a profound effect on β-cell function. Triglycerides (TGs) are relatively non-toxic, monounsaturated fatty acids are protective due to their propensity for esterification into TGs, HDLs are protective, while saturated fatty acids such as palmitate, as well as oxidized LDLs induce cell death [415, 466–468].
One of the reasons that it has been difficult to ascribe β-cell dysfunction to a single factor is that glucolipotoxic conditions lead to multiple metabolic pathways with various metabolites and intermediates, each having different effects on cellular metabolism [415]. The mechanism of action of fatty acids in β-cells is debatable due to the ability of fatty acids to either directly cross the lipid-bilayer and act intracellularly or by their ability to activate the cell-surface receptors such as GPR40 [415]. In general, repeated exposure of β-cells to fatty acids dampens GSIS, reduces nuclear translocation of PDX-1 and expression of MafA, down-regulates insulin expression and induces apoptosis [79, 415, 469–472]. Although exact mechanism by which chronic exposure of β-cells to saturated fatty acids leads to impaired insulin secretion is unclear, there are a variety of changes observed, including upregulation of the mitochondrial inner membrane protein uncoupling protein 2 (UCP2) [420, 473, 474], activation of PLC-ε [475], changes in insulin granule secretory machinery [476], and a dissociation of insulin secretory granules from voltage-gated Ca2+channels [477].
The increased flux of glucose and fatty acids places a tremendous burden on mitochondrial oxidation, leading to increased membrane potential as well as ROS production [478]. As discussed earlier, the β-cell has a limited ability to cope with oxidative stress due to inherently low expression of antioxidant enzymes. The activation of UCP2 by ROS can dissipate the membrane potential by allowing protons to leak into the mitochondrial matrix, and couple the oxidation of fuel to heat rather than ATP [478]. In islets from human donors with T2D as well as ob/ob mice, UCP2 was up-regulated [479, 480]. Although this mechanism has a protective effect against generation of ROS, the reduction in ATP production and hence decreased ATP/ADP ratio leads to reduced insulin-secretory capacity [478, 481]. This places the mitochondria in a critical position for regulating cell function since glucose sensing requires production of ATP from oxidative phosphorylation [482]. Deletion of the UCP2 gene as well as reduction of endogenously produced superoxide in the mitochondria restored islet ATP levels and enhanced GSIS [480, 483].
Since apoptotic pathways converge in the mitochondria with caspase-3 activation and cytochrome C export, function of this organelle may be key to the orchestration of events leading to cell death under conditions of glucolipotoxicity [484]. It was observed that C57BL/6J mice fed a high-fat diet for 12 wk showed a 60 % increase in mitochondria mass (despite no change in number of mitochondria) [484]. Strains of mice with a 5-exon deletion in nicotinamide nucleotide transhydrogenase (nnt) show impaired glucose clearance, and lack of GSIS [485, 486]. This enzyme is an important component of the respiratory chain, converting NADP+ and NADH into NADPH and NAD+, respectively. It is suggested that ROS/aging-associated increases in mitochondrial DNA (mtDNA) mutations could lead to increased susceptibility of the β-cell to metabolic overload [487].
ER stress and the unfolded protein response (UPR) are also implicated in β-cell dysfunction [302]. The high demand for insulin secretion as a result of chronic glucose and fatty acid-induced signaling places a tremendous metabolic burden on the ER in β-cells. The key players in the ER stress response include PERK, interferon response element (IRE)-1/X-box binding protein (XBP)-1 and activating transcription factor (ATF)-6 [488, 489]. The initial goal of the UPR is to activate chaperones such as Bip/GRP78 and GRP94 (heat shock protein 90; HSP90) and folding enzymes such as protein disulfide isomerase and peptidyl-prolyl cis-trans isomerase [489]. These proteins prevent aggregation of unfolded proteins and promote increased fidelity of protein folding. The ATF6 moves from the ER to Golgi where it is cleaved to release its bZIP domain, which then migrates into the nucleus and induces transcription of proteins involved in protein folding and ER-associated protein degradation [490]. To reduce the burden on the ER, protein translation is temporarily halted save for select proteins. The PERK, through phosphorylation of eIF2a mediates a reduction in ER load and an increase in translation of the bZIP transcription factor ATF4, leading to an increase in transcription of C/EBP homologous protein (CHOP; GADD153) and GADD34, which aid in cell recovery [491, 492]. The IRE1 is a kinase/endoribonuclease that splices XBP-1 mRNA, which is then translated into a bZIP transcription factor that induces transcription of protein-folding-related genes [491, 493]. Misfolded proteins are targeted for degradation by ubiquitination in the cytosol. In the event that this response is insufficient to attenuate the accumulation of misfolded proteins and ER function is compromised, apoptosis is induced. Thus, The PERK is responsible for the initial response of temporarily halting protein translation and entry into the ER to prevent overloading [491]. The IRE1 and ATF6 enhance transcription of genes encoding proteins that mediate protein folding, export and degradation.
To summarize the effects of glucolipotoxicity, high glucose levels prevent metabolism of fatty acids which results in funneling to pathways involving formation of toxic compounds (e.g., ceramide), which in turn down-regulate insulin, cause β-cell dysfunction, and results in apoptosis [420]. The chronic exposure of cells to glucose and fatty acids places a tremendous metabolic burden on the mitochondria and ER. Production of ROS in the mitochondria, up-regulation of UCP2 leading to decreased ATP production, and induction of the unfolded protein response may all be central to the series of events leading to apoptosis.
GENOME-WIDE ASSOCIATION STUDIES
The sequencing of the human genome, combined with advances in DNA sequencing technology allowed genome-wide association studies (GWAS) to emerge as a powerful technique for understanding the genetic basis of T2D [494]. Over the past 5 years, the field has grown exponentially, with at least 44 candidate gene loci identified that show significant associations with T2D [494]. This research has allowed for substantial progress in diabetes research because it has shed light on the physiological processes that are disrupted in the pathogenesis of diabetes, which has allowed for development of therapeutic strategies. A number of T2D candidate genes are linked to pancreatic beta cell function, providing strong evidence that alterations in insulin synthesis and secretion are central to the development of the disease [495]. The KCNJ11 (potassium inwardly-rectifying channel, subfamily J, member 11) is a common variant that was identified in 2003, and encodes a zinc transporter restricted to beta cells that is a target of sulphonylureas, drugs that bind ATP-dependent potassium channels on beta cells leading to depolarization and insulin secretion [494, 496]. Other genes with variants associated with reduced insulin secretion in diabetic patients include TCF7L2 ([497]; transcription factor 7-like 2), SLC30A8 ([498, 499]; solute carrier family 30; zinc transporter, member 8), and CDKAL1 ([500]; CDK5 regulatory subunit associated protein 1-like 1) [495]. The effect size for individual and combined loci is small, indicating that environmental and epigenetic factors play a large role in disease development in patients with genetic predisposition to the disease [494].
CONCLUSIONS AND IMPLICATIONS
In conclusion, insulin is a key metabolic hormone and defects in production, secretion and viability of pancreatic β-cells lead to diabetes mellitus, a disease of epidemic proportions in the American population. Insulin production is regulated at the transcriptional level by a number of transcription factors and post-transcriptionally by mRNA stability and factors that influence rates of protein translation. Insulin production and secretion pathways are responsive to nutrients, hormones and other environmental factors, with secretion in healthy individuals fine-tuned to match the metabolic needs of the body. Pancreatic β cell apoptosis is a hallmark of both T1D and T2D although the mechanisms leading to islet destruction are different. Auto-immune reactions leading to the destruction of insulin-producing cells play a key role in T1D. Excess levels of circulating glucose and fatty acids, termed “glucolipotoxicity”, is central to the pathogenesis of T2D, leading to pancreatic β-cell dysfunction. The metabolic changes involved are likely mediated through oxidative activity in the mitochondria and abnormal protein folding in the endoplasmic reticulum. Excess ROS and accumulation of unfolded proteins eventually lead to apoptosis. Islet destruction hinders compensatory insulin secretion in insulin resistant individuals and the disease progresses to T2D. The molecular mechanisms leading to both T1D and T2D are complex and not completely understood. Both forms of diabetes converge with loss of β cell mass. Hence, therapeutic strategies targeted at preserving and enhancing β-cell mass are likely to be important as this disease continues to grow in prevalence.
Fig. 3.
Schematic illustration of immunological aspect of T1D.
Acknowledgments
This work was supported by grants from National Center for Complementary and Alternative Medicine of National Institute of Health (1R01AT007077-01 to DL) and the American Diabetes Association research awards (7-11-BS-84 to DL).
ABBREVIATIONS
- AGEs
Aadvanced glycation end products
- AMPK
AMP-activated kinase
- ATF
Activating transcription factor
- CAMPS
cAMP sensor
- ClC-3
Chloride channel -3
- CPT-1
Carnitine palmitoyl transferase-1
- CRE
cAMP response element
- CREB
cAMP response element binding protein
- DAG
Diacylglycerol
- DHAP
Dihydroxyacetone phosphate
- DHP
Ddihydropyridines
- ER
Endoplasmic reticulum
- FFA
Free fatty acid
- FFAR-1
Free fatty acid receptor -1
- G6P
Glucose-6-phosphate
- GABA
Aminobutyric acid
- GH
Growth hormone
- GHR
Growth hormone receptor
- GIP
Insulinotropic polypeptide
- GISS
Glucose-induced insulin release
- GLP-1
Glucagon-like peptide-1
- GLUT2
Glucose transporter 2
- Gly3P
Glycerol-3-phosphate
- GPx
Glutathione peroxidase
- GS-3
Glycogen synthase kinase
- GSH
Glutathione
- GSSG
Oxidized GSH
- gSUR
Granule SUR
- GWAS
Genome -wide association studies
- HCSP
Highly Ca2+-sensitive pool
- HLH
Helix-loop-helix domain
- HVA l
High voltage-activated
- IAPP
Islet amyloid polypeptide
- IBMX
3-isobutyl-1-methylxanthine
- ICA512
Islet cell autoantigen 512
- IFN- γ
Interferon- γ
- IGF-1
Insulin-like growth factor-I
- IL-1β
Interferon-1β
- iNOS
Inducible nitric oxide synthesis
- IP3
Inositol 1,4,5-trisphosphate
- IRE
Interferon response element
- IRS-2
Receptor substrate
- JNK
c-Jun NH2-terminal kinase
- KATP channel
ATP-sensitive potassium channel
- LVA
Low voltage-activated
- MAPKs
Mitogen-activated protein kinases
- MCP-1
Monocyte chemoattractant protein-1
- NF-kB
Factor nuclear factor -kB
- NO
Nitric oxide
- OAA
Oxaloacetate
- ODNs
Oligodeoxynucleotides
- PDE
Phosphodiesterase
- PERK
Pancreatic eukaryotic translation initiation factor 2-alpha kinase
- PI3K
Phosphatidylinositol 3-kinase
- PIP2
Phosphatidylinositol 4,5-bisphosphate
- PKA
Protein kinase A
- PKB/Akt
Protein kinase-B
- PKG
cGMP/cGMP-dependent protein kinase
- PP1
Protein phosphatase 1
- PTBPs
Polypyrimidine tract binding proteins
- REM
Ras exchange motif
- rER
Rough endoplasmic reticulum
- Rim2
Rab3-interacting molecule 2
- RIPE3b
Rat insulin promoter element 3b
- ROS
Reactive oxygen species
- RRP
Readily releasable pool
- SERCA-2b
Sarcoendoplasmic reticulum Ca2+ ATPase type 2b
- SHP
Small heterodimer partner
- SNARE
Soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- SOD
Superoxide dismutase
- SREBP1c
Sterol-regulatory-element-binding-protein-1c
- SRP
Signal recognition particles
- TGs
Triglycerides
- T2D
type 2 diabetes
- TNF-α
Tumor necrosis factor-α
- TSS
Transcription start site
- UCP-2
Uncoupling protein-2
- XBP
X-box binding protein
Footnotes
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.
References
- 1.Pittman I, Philipson L, Steiner D. Insulin biosynthesis, secretion, structure, and structure-activity relationships. 2004 http://diabetesmanager.pbworks.com/w/page/17680216/Insulin%20Biosynthesis,%20Secretion,%20Structure,%20and%20Structure-Activity%20Relationships. [PubMed]
- 2.De Meyts P. Insulin and its receptor: structure, function and evolution. Bioessays. 2004;26(12):1351–62. doi: 10.1002/bies.20151. [DOI] [PubMed] [Google Scholar]
- 3.De Meyts P, Whittaker J. Structural biology of insulin and IGF1 receptors: implications for drug design. Nat Rev Drug Discov. 2002;1(10):769–83. doi: 10.1038/nrd917. [DOI] [PubMed] [Google Scholar]
- 4.Abel JJ. Crystalline insulin. Proc Natl Acad Sci. 1926;12:132–136. doi: 10.1073/pnas.12.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blundell TL, Cutfield JF, Dodson EJ, Dodson GG, Hodgkin DC, Mercola DA. The crystal structure of rhombohedral 2 zinc insulin. Cold Spring Harb Symp Quant Biol. 1972;36:233–41. doi: 10.1101/sqb.1972.036.01.031. [DOI] [PubMed] [Google Scholar]
- 6.Blundell TL, Cutfield JF, Cutfield SM, Dodson EJ, Dodson GG, Hodgkin DC, Mercola DA. Three-dimensional atomic structure of insulin and its relationship to activity. Diabetes. 1972;21(2 Suppl):492–505. doi: 10.2337/diab.21.2.s492. [DOI] [PubMed] [Google Scholar]
- 7.Blundell TL, Cutfield JF, Cutfield SM, Dodson EJ, Dodson GG, Hodgkin DC, Mercola DA, Vijayan M. Atomic positions in rhombohedral 2-zinc insulin crystals. Nature. 1971;231(5304):506–11. doi: 10.1038/231506a0. [DOI] [PubMed] [Google Scholar]
- 8.Baker EN, Blundell TL, Cutfield JF, Cutfield SM, Dodson EJ, Dodson GG, Hodgkin DC, Hubbard RE, Isaacs NW, Reynolds CD, Sakabe K, Sakabe N, Vijayan M. The structure of 2 Zn pig insulin crystals at 1.5 A resolution. Philos Trans R Soc London. 1988;B319:369–456. doi: 10.1098/rstb.1988.0058. [DOI] [PubMed] [Google Scholar]
- 9.Smith GD, Pangborn WA, Blessing RH. The structure of T6 human insulin at 1. 0 A resolution. Acta Crystallogr D Biol Crystallogr. 2003;59(Pt 3):474–82. doi: 10.1107/s0907444902023685. [DOI] [PubMed] [Google Scholar]
- 10.Dodson GG, Whittingham JL. Insulin and related proteins - structure to function and pharmacology. Springer; New York: 2002. Insulin: sequence, structure and function - a story of surprises; pp. 29–39. [Google Scholar]
- 11.Pullen RA, Lindsay DG, Wood SP, Tickle IJ, Blundell TL, Wollmer A, Krail G, Brandenburg D, Zahn H, Gliemann J, Gammeltoft S. Receptor-binding region of insulin. Nature. 1976;259(5542):369–73. doi: 10.1038/259369a0. [DOI] [PubMed] [Google Scholar]
- 12.Hua QX, Shoelson SE, Kochoyan M, Weiss MA. Receptor binding redefined by a structural switch in a mutant human insulin. Nature. 1991;354(6350):238–41. doi: 10.1038/354238a0. [DOI] [PubMed] [Google Scholar]
- 13.Ludvigsen S, Olsen HB, Kaarsholm NC. A structural switch in a mutant insulin exposes key residues for receptor binding. J Mol Biol. 1998;279(1):1–7. doi: 10.1006/jmbi.1998.1801. [DOI] [PubMed] [Google Scholar]
- 14.Kwok SC, Steiner DF, Rubenstein AH, Tager HS. Identification of a point mutation in the human insulin gene giving rise to a structurally abnormal insulin (insulin Chicago) Diabetes. 1983;32(9):872–5. doi: 10.2337/diab.32.9.872. [DOI] [PubMed] [Google Scholar]
- 15.Shoelson S, Haneda M, Blix P, Nanjo A, Sanke T, Inouye K, Steiner D, Rubenstein A, Tager H. Three mutant insulins in man. Nature. 1983;302(5908):540–3. doi: 10.1038/302540a0. [DOI] [PubMed] [Google Scholar]
- 16.Shoelson S, Fickova M, Haneda M, Nahum A, Musso G, Kaiser ET, Rubenstein AH, Tager H. Identification of a mutant human insulin predicted to contain a serine-for-phenylalanine substitution. Proc Natl Acad Sci U S A. 1983;80(24):7390–4. doi: 10.1073/pnas.80.24.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi M, Ohgaku S, Iwasaki M, Maegawa H, Shigeta Y, Inouye K. Characterization of [LeuB-24]-and [LeuB-25]-insulin analogues. Receptor binding and biological activity. Biochem J. 1982;206(3):597–603. doi: 10.1042/bj2060597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi M, Ohgaku S, Iwasaki M, Maegawa H, Shigeta Y, Inouye K. Supernormal insulin: [D-PheB24]-insulin with increased affinity for insulin receptors. Biochem Biophys Res Commun. 1982;107(1):329–36. doi: 10.1016/0006-291x(82)91708-9. [DOI] [PubMed] [Google Scholar]
- 19.Nanjo K, Sanke T, Miyano M, Okai K, Sowa R, Kondo M, Nishimura S, Iwo K, Miyamura K, Given BD. Diabetes due to secretion of a structurally abnormal insulin (insulin Wakayama). Clinical and functional characteristics of [LeuA3] insulin. J Clin Invest. 1986;77(2):514–9. doi: 10.1172/JCI112331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wollmer A, Rannefeld B, Johansen BR, Hejnaes KR, Balschmidt P, Hansen FB. Phenol-promoted structural transformation of insulin in solution. Biol Chem Hoppe Seyler. 1987;368(8):903–11. doi: 10.1515/bchm3.1987.368.2.903. [DOI] [PubMed] [Google Scholar]
- 21.Ogawa H, Burke GT, Chanley JD, Katsoyannis PG. Effect of N-methylation of selected peptide bonds on the biological activity of insulin. [2-N-methylisoleucine-A]insulin and [3-N-methylvaline-A]insulin. Int J Pept Protein Res. 1987;30(4):460–73. doi: 10.1111/j.1399-3011.1987.tb03354.x. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz G, Katsoyannis PG. Synthesis of des(tetrapeptide B(1–4)) and des(pentapeptide B(1–5)) human insulins. Two biologically active analogues. Biochemistry. 1978;17(21):4550–6. doi: 10.1021/bi00614a029. [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa SH, Tager HS. Implications of invariant residue LeuB6 in insulin-receptor interactions. J Biol Chem. 1991;266(18):11502–9. [PubMed] [Google Scholar]
- 24.Chan SJ, Seino S, Gruppuso PA, Schwartz R, Steiner DF. A mutation in the B chain coding region is associated with impaired proinsulin conversion in a family with hyperproinsulinemia. Proc Natl Acad Sci U S A. 1987;84(8):2194–7. doi: 10.1073/pnas.84.8.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gruppuso PA, Gorden P, Kahn CR, Cornblath M, Zeller WP, Schwartz R. Familial hyperproinsulinemia due to a proposed defect in conversion of proinsulin to insulin. N Engl J Med. 1984;311(10):629–34. doi: 10.1056/NEJM198409063111003. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz GP, Burke GT, Katsoyannis PG. A superactive insulin: [B10-aspartic acid]insulin(human) Proc Natl Acad Sci U S A. 1987;84(18):6408–11. doi: 10.1073/pnas.84.18.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egea PF, Stroud RM, Walter P. Targeting proteins to membranes: structure of the signal recognition particle. Curr Opin Struct Biol. 2005;15(2):213–20. doi: 10.1016/j.sbi.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Chan SJ, Keim P, Steiner DF. Cell-free synthesis of rat preproinsulins: characterization and partial amino acid sequence determination. Proc Natl Acad Sci U S A. 1976;73(6):1964–8. doi: 10.1073/pnas.73.6.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lomedico PT, Chan SJ, Steiner DF, Saunders GF. Immunological and chemical characterization of bovine preproinsulin. J Biol Chem. 1977;252(22):7971–8. [PubMed] [Google Scholar]
- 30.Patzelt C, Labrecque AD, Duguid JR, Carroll RJ, Keim PS, Heinrikson RL, Steiner DF. Detection and kinetic behavior of preproinsulin in pancreatic islets. Proc Natl Acad Sci U S A. 1978;75(3):1260–4. doi: 10.1073/pnas.75.3.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang XF, Arvan P. Intracellular transport of proinsulin in pancreatic beta-cells. Structural maturation probed by disulfide accessibility. J Biol Chem. 1995;270(35):20417–23. doi: 10.1074/jbc.270.35.20417. [DOI] [PubMed] [Google Scholar]
- 32.Munro S, Pelham HR. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- 33.Steiner DFKW, Clark JL, Oyer PE, Rubenstein A. The biosynthesis of insulin. In: Steiner DF, Freinkel N, editors. Handbook of physiology—Section 7 Endocrinology I. Williams & Wilkins; Baltimore: 1972. pp. 175–198. [Google Scholar]
- 34.Nishi M, Sanke T, Nagamatsu S, Bell GI, Steiner DF. Islet amyloid polypeptide. A new beta cell secretory product related to islet amyloid deposits. J Biol Chem. 1990;265(8):4173–6. [PubMed] [Google Scholar]
- 35.Poitout V, Hagman D, Stein R, Artner I, Robertson RP, Harmon JS. Regulation of the insulin gene by glucose and fatty acids. J Nutr. 2006;136(4):873–6. doi: 10.1093/jn/136.4.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giddings SJ, Chirgwin J, Permutt MA. Effects of glucose on proinsulin messenger RNA in rats in vivo. Diabetes. 1982;31(7):624–9. doi: 10.2337/diab.31.7.624. [DOI] [PubMed] [Google Scholar]
- 37.Welsh M, Nielsen DA, MacKrell AJ, Steiner DF. Control of insulin gene expression in pancreatic beta-cells and in an insulin-producing cell line, RIN-5F cells. II. Regulation of insulin mRNA stability. J Biol Chem. 1985;260(25):13590–4. [PubMed] [Google Scholar]
- 38.Knopp RH, Bergelin RO, Wahl PW, Walden CE. Relationships of infant birth size to maternal lipoproteins, apoproteins, fuels, hormones, clinical chemistries, and body weight at 36 weeks gestation. Diabetes. 1985;34 (Suppl 2):71–7. doi: 10.2337/diab.34.2.s71. [DOI] [PubMed] [Google Scholar]
- 39.Knoch KP, Meisterfeld R, Kersting S, Bergert H, Altkruger A, Wegbrod C, Jager M, Saeger HD, Solimena M. cAMP-dependent phosphorylation of PTB1 promotes the expression of insulin secretory granule proteins in beta cells. Cell Metab. 2006;3(2):123–34. doi: 10.1016/j.cmet.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 40.Soares MB, Schon E, Henderson A, Karathanasis SK, Cate R, Zeitlin S, Chirgwin J, Efstratiadis A. RNA-mediated gene duplication: the rat preproinsulin I gene is a functional retroposon. Mol Cell Biol. 1985;5(8):2090–103. doi: 10.1128/mcb.5.8.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanahan D. Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature. 1985;315(6015):115–22. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- 42.Bucchini D, Ripoche MA, Stinnakre MG, Desbois P, Lores P, Monthioux E, Absil J, Lepesant JA, Pictet R, Jami J. Pancreatic expression of human insulin gene in transgenic mice. Proc Natl Acad Sci U S A. 1986;83(8):2511–5. doi: 10.1073/pnas.83.8.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crowe DT, Tsai MJ. Mutagenesis of the rat insulin II 5′-flanking region defines sequences important for expression in HIT cells. Mol Cell Biol. 1989;9(4):1784–9. doi: 10.1128/mcb.9.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edlund T, Walker MD, Barr PJ, Rutter WJ. Cell-specific expression of the rat insulin gene: evidence for role of two distinct 5′ flanking elements. Science. 1985;230(4728):912–6. doi: 10.1126/science.3904002. [DOI] [PubMed] [Google Scholar]
- 45.Melloul D, Ben-Neriah Y, Cerasi E. Glucose modulates the binding of an islet-specific factor to a conserved sequence within the rat I and the human insulin promoters. Proc Natl Acad Sci U S A. 1993;90(9):3865–9. doi: 10.1073/pnas.90.9.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma A, Stein R. Glucose-induced transcription of the insulin gene is mediated by factors required for beta-cell-type-specific expression. Mol Cell Biol. 1994;14(2):871–9. doi: 10.1128/mcb.14.2.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whelan J, Poon D, Weil PA, Stein R. Pancreatic beta-cell-type-specific expression of the rat insulin II gene is controlled by positive and negative cellular transcriptional elements. Mol Cell Biol. 1989;9(8):3253–9. doi: 10.1128/mcb.9.8.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dean PM. Ultrastructural morphometry of the pancreatic-cell. Diabetologia. 1973;9(2):115–9. doi: 10.1007/BF01230690. [DOI] [PubMed] [Google Scholar]
- 49.Howell SL. The mechanism of insulin secretion. Diabetologia. 1984;26(5):319–27. doi: 10.1007/BF00266030. [DOI] [PubMed] [Google Scholar]
- 50.Hay CW, Docherty K. Comparative analysis of insulin gene promoters: implications for diabetes research. Diabetes. 2006;55(12):3201–13. doi: 10.2337/db06-0788. [DOI] [PubMed] [Google Scholar]
- 51.Steiner DF, Chan SJ, Welsh JM, Kwok SC. Structure and evolution of the insulin gene. Annu Rev Genet. 1985;19:463–84. doi: 10.1146/annurev.ge.19.120185.002335. [DOI] [PubMed] [Google Scholar]
- 52.German M, Ashcroft S, Docherty K, Edlund H, Edlund T, Goodison S, Imura H, Kennedy G, Madsen O, Melloul D. The insulin gene promoter. A simplified nomenclature. Diabetes. 1995;44(8):1002–4. doi: 10.2337/diab.44.8.1002. [DOI] [PubMed] [Google Scholar]
- 53.Gehring WJ, Affolter M, Burglin T. Homeodomain proteins. Annu Rev Biochem. 1994;63:487–526. doi: 10.1146/annurev.bi.63.070194.002415. [DOI] [PubMed] [Google Scholar]
- 54.Trainor PA, Krumlauf R. Hox genes, neural crest cells and branchial arch patterning. Curr Opin Cell Biol. 2001;13(6):698–705. doi: 10.1016/s0955-0674(00)00273-8. [DOI] [PubMed] [Google Scholar]
- 55.Ohlsson H, Karlsson K, Edlund T. IPF1, a homeodomain-containing transactivator of the insulin gene. Embo J. 1993;12(11):4251–9. doi: 10.1002/j.1460-2075.1993.tb06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leonard J, Peers B, Johnson T, Ferreri K, Lee S, Montminy MR. Characterization of somatostatin transactivating factor-1, a novel homeobox factor that stimulates somatostatin expression in pancreatic islet cells. Mol Endocrinol. 1993;7(10):1275–83. doi: 10.1210/mend.7.10.7505393. [DOI] [PubMed] [Google Scholar]
- 57.Miller CP, McGehee RE, Jr, Habener JF. IDX-1: a new homeodomain transcription factor expressed in rat pancreatic islets and duodenum that transactivates the somatostatin gene. Embo J. 1994;13(5):1145–56. doi: 10.1002/j.1460-2075.1994.tb06363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.German MS, Wang J, Chadwick RB, Rutter WJ. Synergistic activation of the insulin gene by a LIM-homeo domain protein and a basic helix-loop-helix protein: building a functional insulin minienhancer complex. Genes Dev. 1992;6(11):2165–76. doi: 10.1101/gad.6.11.2165. [DOI] [PubMed] [Google Scholar]
- 59.Karlsson O, Thor S, Norberg T, Ohlsson H, Edlund T. Insulin gene enhancer binding protein Isl-1 is a member of a novel class of proteins containing both a homeo- and a Cys-His domain. Nature. 1990;344(6269):879–82. doi: 10.1038/344879a0. [DOI] [PubMed] [Google Scholar]
- 60.Peshavaria M, Gamer L, Henderson E, Teitelman G, Wright CV, Stein R. XIHbox 8, an endoderm-specific Xenopus homeodomain protein, is closely related to a mammalian insulin gene transcription factor. Mol Endocrinol. 1994;8(6):806–16. doi: 10.1210/mend.8.6.7935494. [DOI] [PubMed] [Google Scholar]
- 61.Petersen HV, Serup P, Leonard J, Michelsen BK, Madsen OD. Transcriptional regulation of the human insulin gene is dependent on the homeodomain protein STF1/IPF1 acting through the CT boxes. Proc Natl Acad Sci U S A. 1994;91(22):10465–9. doi: 10.1073/pnas.91.22.10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peers B, Leonard J, Sharma S, Teitelman G, Montminy MR. Insulin expression in pancreatic islet cells relies on cooperative interactions between the helix loop helix factor E47 and the homeobox factor STF-1. Mol Endocrinol. 1994;8(12):1798–806. doi: 10.1210/mend.8.12.7708065. [DOI] [PubMed] [Google Scholar]
- 63.Guz Y, Montminy MR, Stein R, Leonard J, Gamer LW, Wright CV, Teitelman G. Expression of murine STF-1, a putative insulin gene transcription factor, in beta cells of pancreas, duodenal epithelium and pancreatic exocrine and endocrine progenitors during ontogeny. Development. 1995;121(1):11–8. doi: 10.1242/dev.121.1.11. [DOI] [PubMed] [Google Scholar]
- 64.Wu KL, Gannon M, Peshavaria M, Offield MF, Henderson E, Ray M, Marks A, Gamer LW, Wright CV, Stein R. Hepatocyte nuclear factor 3beta is involved in pancreatic beta-cell-specific transcription of the pdx-1 gene. Mol Cell Biol. 1997;17(10):6002–13. doi: 10.1128/mcb.17.10.6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chawengsaksophak K, James R, Hammond VE, Kontgen F, Beck F. Homeosis and intestinal tumours in Cdx2 mutant mice. Nature. 1997;386(6620):84–7. doi: 10.1038/386084a0. [DOI] [PubMed] [Google Scholar]
- 66.Thor S, Ericson J, Brannstrom T, Edlund T. The homeodomain LIM protein Isl-1 is expressed in subsets of neurons and endocrine cells in the adult rat. Neuron. 1991;7(6):881–9. doi: 10.1016/0896-6273(91)90334-v. [DOI] [PubMed] [Google Scholar]
- 67.Leonard J, Serup P, Gonzalez G, Edlund T, Montminy M. The LIM family transcription factor Isl-1 requires cAMP response element binding protein to promote somatostatin expression in pancreatic islet cells. Proc Natl Acad Sci U S A. 1992;89(14):6247–51. doi: 10.1073/pnas.89.14.6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang M, Drucker DJ. The LIM domain homeobox gene isl-1 is a positive regulator of islet cell-specific proglucagon gene transcription. J Biol Chem. 1995;270(21):12646–52. doi: 10.1074/jbc.270.21.12646. [DOI] [PubMed] [Google Scholar]
- 69.Wang M, Drucker DJ. Activation of amylin gene transcription by LIM domain homeobox gene isl-1. Mol Endocrinol. 1996;10(3):243–51. doi: 10.1210/mend.10.3.8833653. [DOI] [PubMed] [Google Scholar]
- 70.Ahlgren U, Pfaff SL, Jessell TM, Edlund T, Edlund H. Independent requirement for ISL1 in formation of pancreatic mesenchyme and islet cells. Nature. 1997;385(6613):257–60. doi: 10.1038/385257a0. [DOI] [PubMed] [Google Scholar]
- 71.Shieh SY, Tsai MJ. Cell-specific and ubiquitous factors are responsible for the enhancer activity of the rat insulin II gene. J Biol Chem. 1991;266(25):16708–14. [PubMed] [Google Scholar]
- 72.Zhao L, Cissell MA, Henderson E, Colbran R, Stein R. The RIPE3b1 activator of the insulin gene is composed of a protein(s) of approximately 43 kDa, whose DNA binding activity is inhibited by protein phosphatase treatment. J Biol Chem. 2000;275(14):10532–7. doi: 10.1074/jbc.275.14.10532. [DOI] [PubMed] [Google Scholar]
- 73.Shieh SY, Stellrecht CM, Tsai MJ. Molecular characterization of the rat insulin enhancer-binding complex 3b2. Cloning of a binding factor with putative helicase motifs. J Biol Chem. 1995;270(37):21503–8. doi: 10.1074/jbc.270.37.21503. [DOI] [PubMed] [Google Scholar]
- 74.Kataoka K, Han SI, Shioda S, Hirai M, Nishizawa M, Handa H. MafA is a glucose-regulated and pancreatic beta-cell-specific transcriptional activator for the insulin gene. J Biol Chem. 2002;277(51):49903–10. doi: 10.1074/jbc.M206796200. [DOI] [PubMed] [Google Scholar]
- 75.Matsuoka TA, Zhao L, Artner I, Jarrett HW, Friedman D, Means A, Stein R. Members of the large Maf transcription family regulate insulin gene transcription in islet beta cells. Mol Cell Biol. 2003;23(17):6049–62. doi: 10.1128/MCB.23.17.6049-6062.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Olbrot M, Rud J, Moss LG, Sharma A. Identification of beta-cell-specific insulin gene transcription factor RIPE3b1 as mammalian MafA. Proc Natl Acad Sci U S A. 2002;99(10):6737–42. doi: 10.1073/pnas.102168499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matsuoka TA, Artner I, Henderson E, Means A, Sander M, Stein R. The MafA transcription factor appears to be responsible for tissue-specific expression of insulin. Proc Natl Acad Sci U S A. 2004;101(9):2930–3. doi: 10.1073/pnas.0306233101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao L, Guo M, Matsuoka TA, Hagman DK, Parazzoli SD, Poitout V, Stein R. The islet beta cell-enriched MafA activator is a key regulator of insulin gene transcription. J Biol Chem. 2005;280(12):11887–94. doi: 10.1074/jbc.M409475200. [DOI] [PubMed] [Google Scholar]
- 79.Hagman DK, Hays LB, Parazzoli SD, Poitout V. Palmitate inhibits insulin gene expression by altering PDX-1 nuclear localization and reducing MafA expression in isolated rat islets of Langerhans. J Biol Chem. 2005;280(37):32413–8. doi: 10.1074/jbc.M506000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Harmon JS, Stein R, Robertson RP. Oxidative stress-mediated, post-translational loss of MafA protein as a contributing mechanism to loss of insulin gene expression in glucotoxic beta cells. J Biol Chem. 2005;280(12):11107–13. doi: 10.1074/jbc.M410345200. [DOI] [PubMed] [Google Scholar]
- 81.Zhang C, Moriguchi T, Kajihara M, Esaki R, Harada A, Shimohata H, Oishi H, Hamada M, Morito N, Hasegawa K, Kudo T, Engel JD, Yamamoto M, Takahashi S. MafA is a key regulator of glucose-stimulated insulin secretion. Mol Cell Biol. 2005;25(12):4969–76. doi: 10.1128/MCB.25.12.4969-4976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Knepel W, Vallejo M, Chafitz JA, Habener JF. The pancreatic islet-specific glucagon G3 transcription factors recognize control elements in the rat somatostatin and insulin-I genes. Mol Endocrinol. 1991;5(10):1457–66. doi: 10.1210/mend-5-10-1457. [DOI] [PubMed] [Google Scholar]
- 83.Sander M, Neubuser A, Kalamaras J, Ee HC, Martin GR, German MS. Genetic analysis reveals that PAX6 is required for normal transcription of pancreatic hormone genes and islet development. Genes Dev. 1997;11(13):1662–73. doi: 10.1101/gad.11.13.1662. [DOI] [PubMed] [Google Scholar]
- 84.Sosa-Pineda B, Chowdhury K, Torres M, Oliver G, Gruss P. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature. 1997;386(6623):399–402. doi: 10.1038/386399a0. [DOI] [PubMed] [Google Scholar]
- 85.Sosa-Pineda B. The gene Pax4 is an essential regulator of pancreatic beta-cell development. Mol Cells. 2004;18(3):289–94. [PubMed] [Google Scholar]
- 86.German MS, Moss LG, Wang J, Rutter WJ. The insulin and islet amyloid polypeptide genes contain similar cell-specific promoter elements that bind identical beta-cell nuclear complexes. Mol Cell Biol. 1992;12(4):1777–88. doi: 10.1128/mcb.12.4.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Whelan J, Cordle SR, Henderson E, Weil PA, Stein R. Identification of a pancreatic beta-cell insulin gene transcription factor that binds to and appears to activate cell-type-specific expression: its possible relationship to other cellular factors that bind to a common insulin gene sequence. Mol Cell Biol. 1990;10(4):1564–72. doi: 10.1128/mcb.10.4.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Buskin JN, Hauschka SD. Identification of a myocyte nuclear factor that binds to the muscle-specific enhancer of the mouse muscle creatine kinase gene. Mol Cell Biol. 1989;9(6):2627–40. doi: 10.1128/mcb.9.6.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ephrussi A, Church GM, Tonegawa S, Gilbert W. B lineage--specific interactions of an immunoglobulin enhancer with cellular factors in vivo. Science. 1985;227(4683):134–40. doi: 10.1126/science.3917574. [DOI] [PubMed] [Google Scholar]
- 90.Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- 91.Braun T, Buschhausen-Denker G, Bober E, Tannich E, Arnold HH. A novel human muscle factor related to but distinct from MyoD1 induces myogenic conversion in 10T1/2 fibroblasts. Embo J. 1989;8(3):701–9. doi: 10.1002/j.1460-2075.1989.tb03429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brennan TJ, Olson EN. Myogenin resides in the nucleus and acquires high affinity for a conserved enhancer element on heterodimerization. Genes Dev. 1990;4(4):582–95. doi: 10.1101/gad.4.4.582. [DOI] [PubMed] [Google Scholar]
- 93.Cabrera CV, Martinez-Arias A, Bate M. The expression of three members of the achaete-scute gene complex correlates with neuroblast segregation in Drosophila. Cell. 1987;50(3):425–33. doi: 10.1016/0092-8674(87)90496-x. [DOI] [PubMed] [Google Scholar]
- 94.Naya FJ, Stellrecht CM, Tsai MJ. Tissue-specific regulation of the insulin gene by a novel basic helix-loop-helix transcription factor. Genes Dev. 1995;9(8):1009–19. doi: 10.1101/gad.9.8.1009. [DOI] [PubMed] [Google Scholar]
- 95.Lee JE, Hollenberg SM, Snider L, Turner DL, Lipnick N, Weintraub H. Conversion of Xenopus ectoderm into neurons by NeuroD, a basic helix-loop-helix protein. Science. 1995;268(5212):836–44. doi: 10.1126/science.7754368. [DOI] [PubMed] [Google Scholar]
- 96.Cordle SR, Henderson E, Masuoka H, Weil PA, Stein R. Pancreatic beta-cell-type-specific transcription of the insulin gene is mediated by basic helix-loop-helix DNA-binding proteins. Mol Cell Biol. 1991;11(3):1734–8. doi: 10.1128/mcb.11.3.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.German MS, Blanar MA, Nelson C, Moss LG, Rutter WJ. Two related helix-loop-helix proteins participate in separate cell-specific complexes that bind the insulin enhancer. Mol Endocrinol. 1991;5(2):292–9. doi: 10.1210/mend-5-2-292. [DOI] [PubMed] [Google Scholar]
- 98.Peyton M, Moss LG, Tsai MJ. Two distinct class A helix-loop-helix transcription factors, E2A and BETA1, form separate DNA binding complexes on the insulin gene E box. J Biol Chem. 1994;269(41):25936–41. [PubMed] [Google Scholar]
- 99.Naya FJ, Huang HP, Qiu Y, Mutoh H, DeMayo FJ, Leiter AB, Tsai MJ. Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/neuroD-deficient mice. Genes Dev. 1997;11(18):2323–34. doi: 10.1101/gad.11.18.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang HP, Chu K, Nemoz-Gaillard E, Elberg D, Tsai MJ. Neogenesis of beta-cells in adult BETA2/NeuroD-deficient mice. Mol Endocrinol. 2002;16(3):541–51. doi: 10.1210/mend.16.3.0784. [DOI] [PubMed] [Google Scholar]
- 101.Sander M, Griffen SC, Huang J, German MS. A novel glucose-responsive element in the human insulin gene functions uniquely in primary cultured islets. Proc Natl Acad Sci U S A. 1998;95(20):11572–7. doi: 10.1073/pnas.95.20.11572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Boam DS, Clark AR, Docherty K. Positive and negative regulation of the human insulin gene by multiple trans-acting factors. J Biol Chem. 1990;265(14):8285–96. [PubMed] [Google Scholar]
- 103.Le Lay J, Stein R. Involvement of PDX-1 in activation of human insulin gene transcription. J Endocrinol. 2006;188(2):287–94. doi: 10.1677/joe.1.06510. [DOI] [PubMed] [Google Scholar]
- 104.Pino MF, Ye DZ, Linning KD, Green CD, Wicksteed B, Poitout V, Olson LK. Elevated glucose attenuates human insulin gene promoter activity in INS-1 pancreatic beta-cells via reduced nuclear factor binding to the A5/core and Z element. Mol Endocrinol. 2005;19(5):1343–60. doi: 10.1210/me.2003-0493. [DOI] [PubMed] [Google Scholar]
- 105.Inagaki N, Maekawa T, Sudo T, Ishii S, Seino Y, Imura H. c-Jun represses the human insulin promoter activity that depends on multiple cAMP response elements. Proc Natl Acad Sci U S A. 1992;89(3):1045–9. doi: 10.1073/pnas.89.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hay CW, Sinclair EM, Bermano G, Durward E, Tadayyon M, Docherty K. Glucagon-like peptide-1 stimulates human insulin promoter activity in part through cAMP-responsive elements that lie upstream and downstream of the transcription start site. J Endocrinol. 2005;186(2):353–65. doi: 10.1677/joe.1.06205. [DOI] [PubMed] [Google Scholar]
- 107.Foulkes NS, Sassone-Corsi P. Transcription factors coupled to the cAMP-signalling pathway. Biochim Biophys Acta. 1996;1288(3):F101–21. doi: 10.1016/s0304-419x(96)00025-x. [DOI] [PubMed] [Google Scholar]
- 108.Metallo SJ, Paolella DN, Schepartz A. The role of a basic amino acid cluster in target site selection and non-specific binding of bZIP peptides to DNA. Nucleic Acids Res. 1997;25(15):2967–72. doi: 10.1093/nar/25.15.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Emens LA, Landers DW, Moss LG. Hepatocyte nuclear factor 1 alpha is expressed in a hamster insulinoma line and transactivates the rat insulin I gene. Proc Natl Acad Sci U S A. 1992;89(16):7300–4. doi: 10.1073/pnas.89.16.7300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peng SY, Wang WP, Meng J, Li T, Zhang H, Li YM, Chen P, Ma KT, Zhou CY. ISL1 physically interacts with BETA2 to promote insulin gene transcriptional synergy in non-beta cells. Biochim Biophys Acta. 2005;1731(3):154–9. doi: 10.1016/j.bbaexp.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 111.Ohneda K, Mirmira RG, Wang J, Johnson JD, German MS. The homeodomain of PDX-1 mediates multiple protein-protein interactions in the formation of a transcriptional activation complex on the insulin promoter. Mol Cell Biol. 2000;20(3):900–11. doi: 10.1128/mcb.20.3.900-911.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Suckale J, Solimena M. Pancreas islets in metabolic signaling--focus on the beta-cell. Front Biosci. 2008;13:7156–71. doi: 10.2741/3218. [DOI] [PubMed] [Google Scholar]
- 113.Vander Mierde D, Scheuner D, Quintens R, Patel R, Song B, Tsukamoto K, Beullens M, Kaufman RJ, Bollen M, Schuit FC. Glucose activates a protein phosphatase-1-mediated signaling pathway to enhance overall translation in pancreatic beta-cells. Endocrinology. 2007;148(2):609–17. doi: 10.1210/en.2006-1012. [DOI] [PubMed] [Google Scholar]
- 114.Elouil H, Bensellam M, Guiot Y, Vander Mierde D, Pascal SM, Schuit FC, Jonas JC. Acute nutrient regulation of the unfolded protein response and integrated stress response in cultured rat pancreatic islets. Diabetologia. 2007;50(7):1442–52. doi: 10.1007/s00125-007-0674-4. [DOI] [PubMed] [Google Scholar]
- 115.Wicksteed B, Uchizono Y, Alarcon C, McCuaig JF, Shalev A, Rhodes CJ. A cis-element in the 5′ untranslated region of the preproinsulin mRNA (ppIGE) is required for glucose regulation of proinsulin translation. Cell Metab. 2007;5(3):221–7. doi: 10.1016/j.cmet.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 116.Shi Y, Vattem KM, Sood R, An J, Liang J, Stramm L, Wek RC. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol Cell Biol. 1998;18(12):7499–509. doi: 10.1128/mcb.18.12.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6(5):1099–108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 118.Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7(6):1153–63. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 119.Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7(6):1165–76. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 120.Delepine M, Nicolino M, Barrett T, Golamaully M, Lathrop GM, Julier C. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet. 2000;25(4):406–9. doi: 10.1038/78085. [DOI] [PubMed] [Google Scholar]
- 121.Zhang W, Feng D, Li Y, Iida K, McGrath B, Cavener DR. PERK EIF2AK3 control of pancreatic beta cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metab. 2006;4(6):491–7. doi: 10.1016/j.cmet.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 122.Fonseca SG, Fukuma M, Lipson KL, Nguyen LX, Allen JR, Oka Y, Urano F. WFS1 is a novel component of the unfolded protein response and maintains homeostasis of the endoplasmic reticulum in pancreatic beta-cells. J Biol Chem. 2005;280(47):39609–15. doi: 10.1074/jbc.M507426200. [DOI] [PubMed] [Google Scholar]
- 123.Pirot P, Naamane N, Libert F, Magnusson NE, Orntoft TF, Cardozo AK, Eizirik DL. Global profiling of genes modified by endoplasmic reticulum stress in pancreatic beta cells reveals the early degradation of insulin mRNAs. Diabetologia. 2007;50(5):1006–14. doi: 10.1007/s00125-007-0609-0. [DOI] [PubMed] [Google Scholar]
- 124.Ortsater H, Sjoholm A. A busy cell--endoplasmic reticulum stress in the pancreatic beta-cell. Mol Cell Endocrinol. 2007;277(1–2):1–5. doi: 10.1016/j.mce.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 125.Varadi A, Tsuboi T, Johnson-Cadwell LI, Allan VJ, Rutter GA. Kinesin I and cytoplasmic dynein orchestrate glucose-stimulated insulin-containing vesicle movements in clonal MIN6 beta-cells. Biochem Biophys Res Commun. 2003;311(2):272–82. doi: 10.1016/j.bbrc.2003.09.208. [DOI] [PubMed] [Google Scholar]
- 126.Ort T, Maksimova E, Dirkx R, Kachinsky AM, Berghs S, Froehner SC, Solimena M. The receptor tyrosine phosphatase-like protein ICA512 binds the PDZ domains of beta2-syntrophin and nNOS in pancreatic beta-cells. Eur J Cell Biol. 2000;79(9):621–30. doi: 10.1078/0171-9335-00095. [DOI] [PubMed] [Google Scholar]
- 127.Mziaut H, Trajkovski M, Kersting S, Ehninger A, Altkruger A, Lemaitre RP, Schmidt D, Saeger HD, Lee MS, Drechsel DN, Muller S, Solimena M. Synergy of glucose and growth hormone signalling in islet cells through ICA512 and STAT5. Nat Cell Biol. 2006;8(5):435–45. doi: 10.1038/ncb1395. [DOI] [PubMed] [Google Scholar]
- 128.Itoh N, Okamoto H. Translational control of proinsulin synthesis by glucose. Nature. 1980;283(5742):100–2. doi: 10.1038/283100a0. [DOI] [PubMed] [Google Scholar]
- 129.Permutt MA, Kipnis DM. Insulin biosynthesis. I. On the mechanism of glucose stimulation. J Biol Chem. 1972;247(4):1194–9. [PubMed] [Google Scholar]
- 130.Izquierdo JM, Majos N, Bonnal S, Martinez C, Castelo R, Guigo R, Bilbao D, Valcarcel J. Regulation of Fas alternative splicing by antagonistic effects of TIA-1 and PTB on exon definition. Mol Cell. 2005;19(4):475–84. doi: 10.1016/j.molcel.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 131.Spellman R, Smith CW. Novel modes of splicing repression by PTB. Trends Biochem Sci. 2006;31(2):73–6. doi: 10.1016/j.tibs.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 132.Wollerton MC, Gooding C, Wagner EJ, Garcia-Blanco MA, Smith CW. Autoregulation of polypyrimidine tract binding protein by alternative splicing leading to nonsense-mediated decay. Mol Cell. 2004;13(1):91–100. doi: 10.1016/s1097-2765(03)00502-1. [DOI] [PubMed] [Google Scholar]
- 133.Schmitz O, Rungby J, Edge L, Juhl CB. On high-frequency insulin oscillations. Ageing Res Rev. 2008;7(4):301–5. doi: 10.1016/j.arr.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 134.Chang TW, Goldberg AL. The metabolic fates of amino acids and the formation of glutamine in skeletal muscle. J Biol Chem. 1978;253(10):3685–93. [PubMed] [Google Scholar]
- 135.Maechler P, Wollheim CB. Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Nature. 1999;402(6762):685–9. doi: 10.1038/45280. [DOI] [PubMed] [Google Scholar]
- 136.Eto K, Tsubamoto Y, Terauchi Y, Sugiyama T, Kishimoto T, Takahashi N, Yamauchi N, Kubota N, Murayama S, Aizawa T, Akanuma Y, Aizawa S, Kasai H, Yazaki Y, Kadowaki T. Role of NADH shuttle system in glucose-induced activation of mitochondrial metabolism and insulin secretion. Science. 1999;283(5404):981–5. doi: 10.1126/science.283.5404.981. [DOI] [PubMed] [Google Scholar]
- 137.Bender K, Newsholme P, Brennan L, Maechler P. The importance of redox shuttles to pancreatic beta-cell energy metabolism and function. Biochem Soc Trans. 2006;34(Pt 5):811–4. doi: 10.1042/BST0340811. [DOI] [PubMed] [Google Scholar]
- 138.Sener A, Malaisse WJ. L-leucine and a nonmetabolized analogue activate pancreatic islet glutamate dehydrogenase. Nature. 1980;288(5787):187–9. doi: 10.1038/288187a0. [DOI] [PubMed] [Google Scholar]
- 139.Dixon G, Nolan J, McClenaghan N, Flatt PR, Newsholme P. A comparative study of amino acid consumption by rat islet cells and the clonal beta-cell line BRIN-BD11 - the functional significance of L-alanine. J Endocrinol. 2003;179(3):447–54. doi: 10.1677/joe.0.1790447. [DOI] [PubMed] [Google Scholar]
- 140.Tang-Christensen M, Larsen PJ, Thulesen J, Nielsen JR, Vrang N. Glucagon-like peptide 2, a neurotransmitter with a newly discovered role in the regulation of food ingestion. Ugeskr Laeger. 2001;163(3):287–91. [PubMed] [Google Scholar]
- 141.MacDonald PE, El-Kholy W, Riedel MJ, Salapatek AM, Light PE, Wheeler MB. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes. 2002;51 (Suppl 3):S434–42. doi: 10.2337/diabetes.51.2007.s434. [DOI] [PubMed] [Google Scholar]
- 142.MacDonald PE, Salapatek AM, Wheeler MB. Glucagon-like peptide-1 receptor activation antagonizes voltage-dependent repolarizing K(+) currents in beta-cells: a possible glucose-dependent insulinotropic mechanism. Diabetes. 2002;51 (Suppl 3):S443–7. doi: 10.2337/diabetes.51.2007.s443. [DOI] [PubMed] [Google Scholar]
- 143.McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51(1):7–18. doi: 10.2337/diabetes.51.1.7. [DOI] [PubMed] [Google Scholar]
- 144.Nolan CJ, Leahy JL, Delghingaro-Augusto V, Moibi J, Soni K, Peyot ML, Fortier M, Guay C, Lamontagne J, Barbeau A, Przybytkowski E, Joly E, Masiello P, Wang S, Mitchell GA, Prentki M. Beta cell compensation for insulin resistance in Zucker fatty rats: increased lipolysis and fatty acid signalling. Diabetologia. 2006;49(9):2120–30. doi: 10.1007/s00125-006-0305-5. [DOI] [PubMed] [Google Scholar]
- 145.Prentki M, Joly E, El-Assaad W, Roduit R. Malonyl-CoA signaling, lipid partitioning, and glucolipotoxicity: role in beta-cell adaptation and failure in the etiology of diabetes. Diabetes. 2002;51 (Suppl 3):S405–13. doi: 10.2337/diabetes.51.2007.s405. [DOI] [PubMed] [Google Scholar]
- 146.Crespin SR, Greenough WB, 3rd, Steinberg D. Stimulation of insulin secretion by infusion of free fatty acids. J Clin Invest. 1969;48(10):1934–43. doi: 10.1172/JCI106160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Roduit R, Nolan C, Alarcon C, Moore P, Barbeau A, Delghingaro-Augusto V, Przybykowski E, Morin J, Masse F, Massie B, Ruderman N, Rhodes C, Poitout V, Prentki M. A role for the malonyl-CoA/long-chain acyl-CoA pathway of lipid signaling in the regulation of insulin secretion in response to both fuel and nonfuel stimuli. Diabetes. 2004;53(4):1007–19. doi: 10.2337/diabetes.53.4.1007. [DOI] [PubMed] [Google Scholar]
- 148.Stein DT, Esser V, Stevenson BE, Lane KE, Whiteside JH, Daniels MB, Chen S, McGarry JD. Essentiality of circulating fatty acids for glucose-stimulated insulin secretion in the fasted rat. J Clin Invest. 1996;97(12):2728–35. doi: 10.1172/JCI118727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, Ellis C, Elshourbagy NA, Goetz AS, Minnick DT, Murdock PR, Sauls HR, Jr, Shabon U, Spinage LD, Strum JC, Szekeres PG, Tan KB, Way JM, Ignar DM, Wilson S, Muir AI. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem. 2003;278(13):11303–11. doi: 10.1074/jbc.M211495200. [DOI] [PubMed] [Google Scholar]
- 150.Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, Fujino M. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003;422(6928):173–6. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- 151.Prentki M. New insights into pancreatic beta-cell metabolic signaling in insulin secretion. Eur J Endocrinol. 1996;134(3):272–86. doi: 10.1530/eje.0.1340272. [DOI] [PubMed] [Google Scholar]
- 152.Chapman ER, Blasi J, An S, Brose N, Johnston PA, Sudhof TC, Jahn R. Fatty acylation of synaptotagmin in PC12 cells and synaptosomes. Biochem Biophys Res Commun. 1996;225(1):326–32. doi: 10.1006/bbrc.1996.1174. [DOI] [PubMed] [Google Scholar]
- 153.Gonzalo S, Linder ME. SNAP-25 palmitoylation and plasma membrane targeting require a functional secretory pathway. Mol Biol Cell. 1998;9(3):585–97. doi: 10.1091/mbc.9.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Prentki M, Matschinsky FM. Ca2+, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol Rev. 1987;67(4):1185–248. doi: 10.1152/physrev.1987.67.4.1185. [DOI] [PubMed] [Google Scholar]
- 155.Rhee JS, Betz A, Pyott S, Reim K, Varoqueaux F, Augustin I, Hesse D, Sudhof TC, Takahashi M, Rosenmund C, Brose N. Beta phorbol ester- and diacylglycerol-induced augmentation of transmitter release is mediated by Munc13s and not by PKCs. Cell. 2002;108(1):121–33. doi: 10.1016/s0092-8674(01)00635-3. [DOI] [PubMed] [Google Scholar]
- 156.Kashyap S, Belfort R, Gastaldelli A, Pratipanawatr T, Berria R, Pratipanawatr W, Bajaj M, Mandarino L, DeFronzo R, Cusi K. A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes. 2003;52(10):2461–74. doi: 10.2337/diabetes.52.10.2461. [DOI] [PubMed] [Google Scholar]
- 157.Salehi A, Fan BG, Ekelund M, Nordin G, Lundquist I. TPN-evoked dysfunction of islet lysosomal activity mediates impairment of glucose-stimulated insulin release. Am J Physiol Endocrinol Metab. 2001;281(1):E171–9. doi: 10.1152/ajpendo.2001.281.1.E171. [DOI] [PubMed] [Google Scholar]
- 158.Schulla V, Renstrom E, Feil R, Feil S, Franklin I, Gjinovci A, Jing XJ, Laux D, Lundquist I, Magnuson MA, Obermuller S, Olofsson CS, Salehi A, Wendt A, Klugbauer N, Wollheim CB, Rorsman P, Hofmann F. Impaired insulin secretion and glucose tolerance in beta cell-selective Ca(v)1. 2 Ca2+ channel null mice. Embo J. 2003;22(15):3844–54. doi: 10.1093/emboj/cdg389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bratanova-Tochkova TK, Cheng H, Daniel S, Gunawardana S, Liu YJ, Mulvaney-Musa J, Schermerhorn T, Straub SG, Yajima H, Sharp GW. Triggering and augmentation mechanisms, granule pools, and biphasic insulin secretion. Diabetes. 2002;51 (Suppl 1):S83–90. doi: 10.2337/diabetes.51.2007.s83. [DOI] [PubMed] [Google Scholar]
- 160.Straub SG, Sharp GW. Hypothesis: one rate-limiting step controls the magnitude of both phases of glucose-stimulated insulin secretion. Am J Physiol Cell Physiol. 2004;287(3):C565–71. doi: 10.1152/ajpcell.00079.2004. [DOI] [PubMed] [Google Scholar]
- 161.Olofsson CS, Gopel SO, Barg S, Galvanovskis J, Ma X, Salehi A, Rorsman P, Eliasson L. Fast insulin secretion reflects exocytosis of docked granules in mouse pancreatic B-cells. Pflugers Arch. 2002;444(1–2):43–51. doi: 10.1007/s00424-002-0781-5. [DOI] [PubMed] [Google Scholar]
- 162.Daniel S, Noda M, Straub SG, Sharp GW. Identification of the docked granule pool responsible for the first phase of glucose-stimulated insulin secretion. Diabetes. 1999;48(9):1686–90. doi: 10.2337/diabetes.48.9.1686. [DOI] [PubMed] [Google Scholar]
- 163.Kang L, He Z, Xu P, Fan J, Betz A, Brose N, Xu T. Munc13-1 is required for the sustained release of insulin from pancreatic beta cells. Cell Metab. 2006;3(6):463–8. doi: 10.1016/j.cmet.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 164.Kwan EP, Xie L, Sheu L, Nolan CJ, Prentki M, Betz A, Brose N, Gaisano HY. Munc13-1 deficiency reduces insulin secretion and causes abnormal glucose tolerance. Diabetes. 2006;55(5):1421–9. doi: 10.2337/db05-1263. [DOI] [PubMed] [Google Scholar]
- 165.Gerst JE. SNARE regulators: matchmakers and matchbreakers. Biochim Biophys Acta. 2003;1641(2–3):99–110. doi: 10.1016/s0167-4889(03)00096-x. [DOI] [PubMed] [Google Scholar]
- 166.Hatsuzawa K, Lang T, Fasshauer D, Bruns D, Jahn R. The R-SNARE motif of tomosyn forms SNARE core complexes with syntaxin 1 and SNAP-25 and down-regulates exocytosis. J Biol Chem. 2003;278(33):31159–66. doi: 10.1074/jbc.M305500200. [DOI] [PubMed] [Google Scholar]
- 167.Cheviet S, Bezzi P, Ivarsson R, Renstrom E, Viertl D, Kasas S, Catsicas S, Regazzi R. Tomosyn-1 is involved in a post-docking event required for pancreatic beta-cell exocytosis. J Cell Sci. 2006;119(Pt 14):2912–20. doi: 10.1242/jcs.03037. [DOI] [PubMed] [Google Scholar]
- 168.Yizhar O, Matti U, Melamed R, Hagalili Y, Bruns D, Rettig J, Ashery U. Tomosyn inhibits priming of large dense-core vesicles in a calcium-dependent manner. Proc Natl Acad Sci U S A. 2004;101(8):2578–83. doi: 10.1073/pnas.0308700100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gauthier BR, Wollheim CB. Synaptotagmins bind calcium to release insulin. Am J Physiol Endocrinol Metab. 2008;295(6):E1279–86. doi: 10.1152/ajpendo.90568.2008. [DOI] [PubMed] [Google Scholar]
- 170.Eliasson L, Ma X, Renstrom E, Barg S, Berggren PO, Galvanovskis J, Gromada J, Jing X, Lundquist I, Salehi A, Sewing S, Rorsman P. SUR1 regulates PKA-independent cAMP-induced granule priming in mouse pancreatic B-cells. J Gen Physiol. 2003;121(3):181–97. doi: 10.1085/jgp.20028707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fujimoto K, Shibasaki T, Yokoi N, Kashima Y, Matsumoto M, Sasaki T, Tajima N, Iwanaga T, Seino S. Piccolo, a Ca2+ sensor in pancreatic beta-cells. Involvement of cAMP-GEFII.Rim2. Piccolo complex in cAMP-dependent exocytosis. J Biol Chem. 2002;277(52):50497–502. doi: 10.1074/jbc.M210146200. [DOI] [PubMed] [Google Scholar]
- 172.Catterall WA. Structure and function of neuronal Ca2+ channels and their role in neurotransmitter release. Cell Calcium. 1998;24(5–6):307–23. doi: 10.1016/s0143-4160(98)90055-0. [DOI] [PubMed] [Google Scholar]
- 173.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–55. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 174.Dolphin AC. L-type calcium channel modulation. Adv Second Messenger Phosphoprotein Res. 1999;33:153–77. doi: 10.1016/s1040-7952(99)80009-3. [DOI] [PubMed] [Google Scholar]
- 175.Reid CA, Bekkers JM, Clements JD. Presynaptic Ca2+ channels: a functional patchwork. Trends Neurosci. 2003;26(12):683–7. doi: 10.1016/j.tins.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 176.Heady TN, Gomora JC, Macdonald TL, Perez-Reyes E. Molecular pharmacology of T-type Ca2+ channels. Jpn J Pharmacol. 2001;85(4):339–50. doi: 10.1254/jjp.85.339. [DOI] [PubMed] [Google Scholar]
- 177.Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83(1):117–61. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- 178.Triggle DJ. The physiological and pharmacological significance of cardiovascular T-type, voltage-gated calcium channels. Am J Hypertens. 1998;11(4 Pt 3):80S–87S. doi: 10.1016/s0895-7061(98)00004-1. [DOI] [PubMed] [Google Scholar]
- 179.Jing X, Li DQ, Olofsson CS, Salehi A, Surve VV, Caballero J, Ivarsson R, Lundquist I, Pereverzev A, Schneider T, Rorsman P, Renstrom E. CaV2. 3 calcium channels control second-phase insulin release. J Clin Invest. 2005;115(1):146–54. doi: 10.1172/JCI22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Vajna R, Klockner U, Pereverzev A, Weiergraber M, Chen X, Miljanich G, Klugbauer N, Hescheler J, Perez-Reyes E, Schneider T. Functional coupling between ‘R-type’ Ca2+ channels and insulin secretion in the insulinoma cell line INS-1. Eur J Biochem. 2001;268(4):1066–75. doi: 10.1046/j.1432-1327.2001.01969.x. [DOI] [PubMed] [Google Scholar]
- 181.Vignali S, Leiss V, Karl R, Hofmann F, Welling A. Characterization of voltage-dependent sodium and calcium channels in mouse pancreatic A- and B-cells. J Physiol. 2006;572(Pt 3):691–706. doi: 10.1113/jphysiol.2005.102368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rorsman P, Trube G. Calcium and delayed potassium currents in mouse pancreatic beta-cells under voltage-clamp conditions. J Physiol. 1986;374:531–50. doi: 10.1113/jphysiol.1986.sp016096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gromada J, Hoy M, Renstrom E, Bokvist K, Eliasson L, Gopel S, Rorsman P. CaM kinase II-dependent mobilization of secretory granules underlies acetylcholine-induced stimulation of exocytosis in mouse pancreatic B-cells. J Physiol. 1999;518 ( Pt 3):745–59. doi: 10.1111/j.1469-7793.1999.0745p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Henquin JC. The interplay between cyclic AMP and ions in the stimulus-secretion coupling in pancreatic B-cells. Arch Int Physiol Biochim. 1985;93(1):37–48. doi: 10.3109/13813458509104514. [DOI] [PubMed] [Google Scholar]
- 185.Malaisse WJ, Malaisse-Lagae F. The role of cyclic AMP in insulin release. Experientia. 1984;40(10):1068–74. doi: 10.1007/BF01971453. [DOI] [PubMed] [Google Scholar]
- 186.Sutherland EW, Robison GA. The role of cyclic AMP in the control of carbohydrate metabolism. Diabetes. 1969;18(12):797–819. doi: 10.2337/diab.18.12.797. [DOI] [PubMed] [Google Scholar]
- 187.Charles MA, Fanska R, Schmid FG, Forsham PH, Grodsky GM. Adenosine 3′,5′-monophosphate in pancreatic islets: glucose-induced insulin release. Science. 1973;179(73):569–71. doi: 10.1126/science.179.4073.569. [DOI] [PubMed] [Google Scholar]
- 188.Drucker DJ. Minireview: the glucagon-like peptides. Endocrinology. 2001;142(2):521–7. doi: 10.1210/endo.142.2.7983. [DOI] [PubMed] [Google Scholar]
- 189.Meier JJ, Nauck MA, Schmidt WE, Gallwitz B. Gastric inhibitory polypeptide: the neglected incretin revisited. Regul Pept. 2002;107(1–3):1–13. doi: 10.1016/s0167-0115(02)00039-3. [DOI] [PubMed] [Google Scholar]
- 190.Jones PM, Persaud SJ. Protein kinases, protein phosphorylation, and the regulation of insulin secretion from pancreatic beta-cells. Endocr Rev. 1998;19(4):429–61. doi: 10.1210/edrv.19.4.0339. [DOI] [PubMed] [Google Scholar]
- 191.Renstrom E, Eliasson L, Rorsman P. Protein kinase A-dependent and -independent stimulation of exocytosis by cAMP in mouse pancreatic B-cells. J Physiol. 1997;502 ( Pt 1):105–18. doi: 10.1111/j.1469-7793.1997.105bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ozaki N, Shibasaki T, Kashima Y, Miki T, Takahashi K, Ueno H, Sunaga Y, Yano H, Matsuura Y, Iwanaga T, Takai Y, Seino S. cAMP-GEFII is a direct target of cAMP in regulated exocytosis. Nat Cell Biol. 2000;2(11):805–11. doi: 10.1038/35041046. [DOI] [PubMed] [Google Scholar]
- 193.de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396(6710):474–7. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 194.Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998;282(5397):2275–9. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- 195.Kang G, Chepurny OG, Malester B, Rindler MJ, Rehmann H, Bos JL, Schwede F, Coetzee WA, Holz GG. cAMP sensor Epac as a determinant of ATP-sensitive potassium channel activity in human pancreatic beta cells and rat INS-1 cells. J Physiol. 2006;573(Pt 3):595–609. doi: 10.1113/jphysiol.2006.107391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Fujimoto K, Shibasaki T, Yokoi N, Kashima Y, Matsumoto M, Sasaki T, Tajima N, Iwanaga T, Seino S. Piccolo, a Ca2+ sensor in pancreatic beta-cells. Involvement of cAMP-GEFII.Rim2. Piccolo complex in cAMP-dependent exocytosis. J Biol Chem. 2002;277(52):50497–502. doi: 10.1074/jbc.M210146200. [DOI] [PubMed] [Google Scholar]
- 197.Wang Y, Okamoto M, Schmitz F, Hofmann K, Sudhof TC. Rim is a putative Rab3 effector in regulating synaptic-vesicle fusion. Nature. 1997;388(6642):593–8. doi: 10.1038/41580. [DOI] [PubMed] [Google Scholar]
- 198.Gundelfinger ED, Kessels MM, Qualmann B. Temporal and spatial coordination of exocytosis and endocytosis. Nat Rev Mol Cell Biol. 2003;4(2):127–39. doi: 10.1038/nrm1016. [DOI] [PubMed] [Google Scholar]
- 199.de Rooij J, Rehmann H, van Triest M, Cool RH, Wittinghofer A, Bos JL. Mechanism of regulation of the Epac family of cAMP-dependent RapGEFs. J Biol Chem. 2000;275(27):20829–36. doi: 10.1074/jbc.M001113200. [DOI] [PubMed] [Google Scholar]
- 200.Kuno T, Shuntoh H, Sakaue M, Saijoh K, Takeda T, Fukuda K, Tanaka C. Site-directed mutagenesis of the cAMP-binding sites of the recombinant type I regulatory subunit of cAMP-dependentprotein kinase. Biochem Biophys Res Commun. 1988;153(3):1244–50. doi: 10.1016/s0006-291x(88)81361-5. [DOI] [PubMed] [Google Scholar]
- 201.Ringheim GE, Taylor SS. Effects of cAMP-binding site mutations on intradomain cross-communication in the regulatory subunit of cAMP-dependent protein kinase I. J Biol Chem. 1990;265(32):19472–8. [PubMed] [Google Scholar]
- 202.Stark JM, Jasin M. Extensive loss of heterozygosity is suppressed during homologous repair of chromosomal breaks. Mol Cell Biol. 2003;23(2):733–43. doi: 10.1128/MCB.23.2.733-743.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kashima Y, Miki T, Shibasaki T, Ozaki N, Miyazaki M, Yano H, Seino S. Critical role of cAMP-GEFII--Rim2 complex in incretin-potentiated insulin secretion. J Biol Chem. 2001;276(49):46046–53. doi: 10.1074/jbc.M108378200. [DOI] [PubMed] [Google Scholar]
- 204.Nakazaki M, Crane A, Hu M, Seghers V, Ullrich S, Aguilar-Bryan L, Bryan J. cAMP-activated protein kinase-independent potentiation of insulin secretion by cAMP is impaired in SUR1 null islets. Diabetes. 2002;51(12):3440–9. doi: 10.2337/diabetes.51.12.3440. [DOI] [PubMed] [Google Scholar]
- 205.Shiota C, Larsson O, Shelton KD, Shiota M, Efanov AM, Hoy M, Lindner J, Kooptiwut S, Juntti-Berggren L, Gromada J, Berggren PO, Magnuson MA. Sulfonylurea receptor type 1 knock-out mice have intact feeding-stimulated insulin secretion despite marked impairment in their response to glucose. J Biol Chem. 2002;277(40):37176–83. doi: 10.1074/jbc.M206757200. [DOI] [PubMed] [Google Scholar]
- 206.Schmidt M, Evellin S, Weernink PA, von Dorp F, Rehmann H, Lomasney JW, Jakobs KH. A new phospholipase-C-calcium signalling pathway mediated by cyclic AMP and a Rap GTPase. Nat Cell Biol. 2001;3(11):1020–4. doi: 10.1038/ncb1101-1020. [DOI] [PubMed] [Google Scholar]
- 207.Shibasaki T, Sunaga Y, Seino S. Integration of ATP, cAMP, and Ca2+ signals in insulin granule exocytosis. Diabetes. 2004;53 (Suppl 3):S59–62. doi: 10.2337/diabetes.53.suppl_3.s59. [DOI] [PubMed] [Google Scholar]
- 208.Barg S, Huang P, Eliasson L, Nelson DJ, Obermuller S, Rorsman P, Thevenod F, Renstrom E. Priming of insulin granules for exocytosis by granular Cl(−) uptake and acidification. J Cell Sci. 2001;114(Pt 11):2145–54. doi: 10.1242/jcs.114.11.2145. [DOI] [PubMed] [Google Scholar]
- 209.Kang G, Chepurny OG, Holz GG. cAMP-regulated guanine nucleotide exchange factor II (Epac2) mediates Ca2+-induced Ca2+ release in INS-1 pancreatic beta-cells. J Physiol. 2001;536(Pt 2):375–85. doi: 10.1111/j.1469-7793.2001.0375c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kang G, Joseph JW, Chepurny OG, Monaco M, Wheeler MB, Bos JL, Schwede F, Genieser HG, Holz GG. Epac-selective cAMP analog 8-pCPT-2′-O-Me-cAMP as a stimulus for Ca2+-induced Ca2+ release and exocytosis in pancreatic beta-cells. J Biol Chem. 2003;278(10):8279–85. doi: 10.1074/jbc.M211682200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bode HP, Moormann B, Dabew R, Goke B. Glucagon-like peptide 1 elevates cytosolic calcium in pancreatic beta-cells independently of protein kinase A. Endocrinology. 1999;140(9):3919–27. doi: 10.1210/endo.140.9.6947. [DOI] [PubMed] [Google Scholar]
- 212.Kang G, Chepurny OG, Rindler MJ, Collis L, Chepurny Z, Li WH, Harbeck M, Roe MW, Holz GG. A cAMP and Ca2+ coincidence detector in support of Ca2+-induced Ca2+ release in mouse pancreatic beta cells. J Physiol. 2005;566(Pt 1):173–88. doi: 10.1113/jphysiol.2005.087510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Holz GG, Kang G, Harbeck M, Roe MW, Chepurny OG. Cell physiology of cAMP sensor Epac. J Physiol. 2006;577(Pt 1):5–15. doi: 10.1113/jphysiol.2006.119644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Nadal A, Ropero AB, Laribi O, Maillet M, Fuentes E, Soria B. Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor alpha and estrogen receptor beta. Proc Natl Acad Sci U S A. 2000;97(21):11603–8. doi: 10.1073/pnas.97.21.11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sutter-Dub MT. Rapid non-genomic and genomic responses to progestogens, estrogens, and glucocorticoids in the endocrine pancreatic B cell, the adipocyte and other cell types. Steroids. 2002;67(2):77–93. doi: 10.1016/s0039-128x(01)00142-8. [DOI] [PubMed] [Google Scholar]
- 216.Nadal A, Rovira JM, Laribi O, Leon-quinto T, Andreu E, Ripoll C, Soria B. Rapid insulinotropic effect of 17beta-estradiol via a plasma membrane receptor. FASEB J. 1998;12(13):1341–8. doi: 10.1096/fasebj.12.13.1341. [DOI] [PubMed] [Google Scholar]
- 217.Stevenson JC, Crook D, Godsland IF, Collins P, Whitehead MI. Hormone replacement therapy and the cardiovascular system. Nonlipid effects. Drugs. 1994;47 (Suppl 2):35–41. doi: 10.2165/00003495-199400472-00007. [DOI] [PubMed] [Google Scholar]
- 218.Brussaard HE, Gevers Leuven JA, Frolich M, Kluft C, Krans HM. Short-term oestrogen replacement therapy improves insulin resistance, lipids and fibrinolysis in postmenopausal women with NIDDM. Diabetologia. 1997;40(7):843–9. doi: 10.1007/s001250050758. [DOI] [PubMed] [Google Scholar]
- 219.Ropero AB, Soria B, Nadal A. A nonclassical estrogen membrane receptor triggers rapid differential actions in the endocrine pancreas. Mol Endocrinol. 2002;16(3):497–505. doi: 10.1210/mend.16.3.0794. [DOI] [PubMed] [Google Scholar]
- 220.Hawkins MB, Thornton JW, Crews D, Skipper JK, Dotte A, Thomas P. Identification of a third distinct estrogen receptor and reclassification of estrogen receptors in teleosts. Proc Natl Acad Sci U S A. 2000;97(20):10751–6. doi: 10.1073/pnas.97.20.10751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ropero AB, Fuentes E, Rovira JM, Ripoll C, Soria B, Nadal A. Non-genomic actions of 17beta-oestradiol in mouse pancreatic beta-cells are mediated by a cGMP-dependent protein kinase. J Physiol. 1999;521(Pt 2):397–407. doi: 10.1111/j.1469-7793.1999.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sheng M, McFadden G, Greenberg ME. Membrane depolarization and calcium induce c-fos transcription via phosphorylation of transcription factor CREB. Neuron. 1990;4(4):571–82. doi: 10.1016/0896-6273(90)90115-v. [DOI] [PubMed] [Google Scholar]
- 223.Cartin L, Lounsbury KM, Nelson MT. Coupling of Ca(2+) to CREB activation and gene expression in intact cerebral arteries from mouse : roles of ryanodine receptors and voltage-dependent Ca(2+) channels. CircRes. 2000;86(7):760–7. doi: 10.1161/01.res.86.7.760. [DOI] [PubMed] [Google Scholar]
- 224.Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–61. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- 225.Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2(8):599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- 226.Arendt J. Melatonin and the mammalian pineal gland. Chapman and Hall; London: 1994. [Google Scholar]
- 227.Peschke E, Muhlbauer E, Musshoff U, Csernus VJ, Chankiewitz E, Peschke D. Receptor (MT(1)) mediated influence of melatonin on cAMP concentration and insulin secretion of rat insulinoma cells INS-1. J Pineal Res. 2002;33(2):63–71. doi: 10.1034/j.1600-079x.2002.02919.x. [DOI] [PubMed] [Google Scholar]
- 228.Kemp DM, Ubeda M, Habener JF. Identification and functional characterization of melatonin Mel 1a receptors in pancreatic beta cells: potential role in incretin-mediated cell function by sensitization of cAMP signaling. Mol Cell Endocrinol. 2002;191(2):157–66. doi: 10.1016/s0303-7207(02)00064-3. [DOI] [PubMed] [Google Scholar]
- 229.Ramracheya RD, Muller DS, Squires PE, Brereton H, Sugden D, Huang GC, Amiel SA, Jones PM, Persaud SJ. Function and expression of melatonin receptors on human pancreatic islets. J Pineal Res. 2008;44(3):273–9. doi: 10.1111/j.1600-079X.2007.00523.x. [DOI] [PubMed] [Google Scholar]
- 230.Peschke E. Melatonin, endocrine pancreas and diabetes. J Pineal Res. 2008;44(1):26–40. doi: 10.1111/j.1600-079X.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 231.Bailey CJ, Atkins TW, Matty AJ. Melatonin inhibition of insulin secretion in the rat and mouse. Horm Res. 1974;5(1):21–8. doi: 10.1159/000178615. [DOI] [PubMed] [Google Scholar]
- 232.Frankel BJ, Strandberg MJ. Insulin release from isolated mouse islets in vitro: no effect of physiological levels of melatonin or arginine vasotocin. J Pineal Res. 1991;11(3–4):145–8. doi: 10.1111/j.1600-079x.1991.tb00470.x. [DOI] [PubMed] [Google Scholar]
- 233.Peschke E, Bach AG, Muhlbauer E. Parallel signaling pathways of melatonin in the pancreatic beta-cell. J Pineal Res. 2006;40(2):184–91. doi: 10.1111/j.1600-079X.2005.00297.x. [DOI] [PubMed] [Google Scholar]
- 234.Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spegel P, Bugliani M, Saxena R, Fex M, Pulizzi N, Isomaa B, Tuomi T, Nilsson P, Kuusisto J, Tuomilehto J, Boehnke M, Altshuler D, Sundler F, Eriksson JG, Jackson AU, Laakso M, Marchetti P, Watanabe RM, Mulder H, Groop L. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41(1):82–8. doi: 10.1038/ng.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Peschke E, Peschke D, Hammer T, Csernus V. Influence of melatonin and serotonin on glucose-stimulated insulin release from perifused rat pancreatic islets in vitro. J Pineal Res. 1997;23(3):156–63. doi: 10.1111/j.1600-079x.1997.tb00349.x. [DOI] [PubMed] [Google Scholar]
- 236.Picinato MC, Haber EP, Cipolla-Neto J, Curi R, de Oliveira Carvalho CR, Carpinelli AR. Melatonin inhibits insulin secretion and decreases PKA levels without interfering with glucose metabolism in rat pancreatic islets. J Pineal Res. 2002;33(3):156–60. doi: 10.1034/j.1600-079x.2002.02903.x. [DOI] [PubMed] [Google Scholar]
- 237.Nishida S, Segawa T, Murai I, Nakagawa S. Long-term melatonin administration reduces hyperinsulinemia and improves the altered fatty-acid compositions in type 2 diabetic rats via the restoration of Delta-5 desaturase activity. J Pineal Res. 2002;32(1):26–33. doi: 10.1034/j.1600-079x.2002.10797.x. [DOI] [PubMed] [Google Scholar]
- 238.von Gall C, Stehle JH, Weaver DR. Mammalian melatonin receptors: molecular biology and signal transduction. Cell Tissue Res. 2002;309(1):151–62. doi: 10.1007/s00441-002-0581-4. [DOI] [PubMed] [Google Scholar]
- 239.Petit L, Lacroix I, de Coppet P, Strosberg AD, Jockers R. Differential signaling of human Mel1a and Mel1b melatonin receptors through the cyclic guanosine 3′-5′-monophosphate pathway. Biochem Pharmacol. 1999;58(4):633–9. doi: 10.1016/s0006-2952(99)00134-3. [DOI] [PubMed] [Google Scholar]
- 240.Bach AG, Wolgast S, Muhlbauer E, Peschke E. Melatonin stimulates inositol-1,4,5-trisphosphate and Ca2+ release from INS1 insulinoma cells. J Pineal Res. 2005;39(3):316–23. doi: 10.1111/j.1600-079X.2005.00253.x. [DOI] [PubMed] [Google Scholar]
- 241.Godson C, Reppert SM. The Mel1a melatonin receptor is coupled to parallel signal transduction pathways. Endocrinology. 1997;138(1):397–404. doi: 10.1210/endo.138.1.4824. [DOI] [PubMed] [Google Scholar]
- 242.Orskov C. Glucagon-like peptide-1, a new hormone of the entero-insular axis. Diabetologia. 1992;35(8):701–11. [PubMed] [Google Scholar]
- 243.Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101(3):515–20. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Nauck MA, Bartels E, Orskov C, Ebert R, Creutzfeldt W. Additive insulinotropic effects of exogenous synthetic human gastric inhibitory polypeptide and glucagon-like peptide-1-(7–36) amide infused at near-physiological insulinotropic hormone and glucose concentrations. JClin Endocrinol Metab. 1993;76(4):912–7. doi: 10.1210/jcem.76.4.8473405. [DOI] [PubMed] [Google Scholar]
- 245.Ahren B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov. 2009;8(5):369–85. doi: 10.1038/nrd2782. [DOI] [PubMed] [Google Scholar]
- 246.Doyle ME, Egan JM. Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol Ther. 2007;113(3):546–93. doi: 10.1016/j.pharmthera.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 248.Rossetti L, Massillon D, Barzilai N, Vuguin P, Chen W, Hawkins M, Wu J, Wang J. Short term effects of leptin on hepatic gluconeogenesis and in vivo insulin action. J Biol Chem. 1997;272(44):27758–63. doi: 10.1074/jbc.272.44.27758. [DOI] [PubMed] [Google Scholar]
- 249.Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, Cheetham CH, Earley AR, Barnett AH, Prins JB, O’Rahilly S. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387(6636):903–8. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 250.Fehmann HC, Peiser C, Bode HP, Stamm M, Staats P, Hedetoft C, Lang RE, Goke B. Leptin: a potent inhibitor of insulin secretion. Peptides. 1997;18(8):1267–73. doi: 10.1016/s0196-9781(97)00135-6. [DOI] [PubMed] [Google Scholar]
- 251.Kulkarni RN, Wang ZL, Wang RM, Hurley JD, Smith DM, Ghatei MA, Withers DJ, Gardiner JV, Bailey CJ, Bloom SR. Leptin rapidly suppresses insulin release from insulinoma cells, rat and human islets and, in vivo, in mice. J Clin Invest. 1997;100(11):2729–36. doi: 10.1172/JCI119818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Zhao AZ, Bornfeldt KE, Beavo JA. Leptin inhibits insulin secretion by activation of phosphodiesterase 3B. J Clin Invest. 1998;102(5):869–73. doi: 10.1172/JCI3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Chen NG, Swick AG, Romsos DR. Leptin constrains acetylcholine-induced insulin secretion from pancreatic islets of ob/ob mice. J Clin Invest. 1997;100(5):1174–9. doi: 10.1172/JCI119629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Emilsson V, Liu YL, Cawthorne MA, Morton NM, Davenport M. Expression of the functional leptin receptor mRNA in pancreatic islets and direct inhibitory action of leptin on insulin secretion. Diabetes. 1997;46(2):313–6. doi: 10.2337/diab.46.2.313. [DOI] [PubMed] [Google Scholar]
- 255.Ishida K, Murakami T, Mizuno A, Iida M, Kuwajima M, Shima K. Leptin suppresses basal insulin secretion from rat pancreatic islets. Regul Pept. 1997;70(2–3):179–82. doi: 10.1016/s0167-0115(97)01002-1. [DOI] [PubMed] [Google Scholar]
- 256.Kieffer TJ, Heller RS, Leech CA, Holz GG, Habener JF. Leptin suppression of insulin secretion by the activation of ATP-sensitive K+ channels in pancreatic beta-cells. Diabetes. 1997;46(6):1087–93. doi: 10.2337/diab.46.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ookuma M, Ookuma K, York DA. Effects of leptin on insulin secretion from isolated rat pancreatic islets. Diabetes. 1998;47(2):219–23. doi: 10.2337/diab.47.2.219. [DOI] [PubMed] [Google Scholar]
- 258.Poitout V, Rouault C, Guerre-Millo M, Briaud I, Reach G. Inhibition of insulin secretion by leptin in normal rodent islets of Langerhans. Endocrinology. 1998;139(3):822–6. doi: 10.1210/endo.139.3.5812. [DOI] [PubMed] [Google Scholar]
- 259.Seufert J, Kieffer TJ, Habener JF. Leptin inhibits insulin gene transcription and reverses hyperinsulinemia in leptin-deficient ob/ob mice. Proc Natl Acad Sci U S A. 1999;96(2):674–9. doi: 10.1073/pnas.96.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Fehmann HC, Berghofer P, Brandhorst D, Brandhorst H, Hering B, Bretzel RG, Goke B. Leptin inhibition of insulin secretion from isolated human islets. Acta Diabetol. 1997;34(4):249–52. doi: 10.1007/s005920050083. [DOI] [PubMed] [Google Scholar]
- 261.Lupi R, Marchetti P, Maffei M, Del Guerra S, Benzi L, Marselli L, Bertacca A, Navalesi R. Effects of acute or prolonged exposure to human leptin on isolated human islet function. Biochem Biophys Res Commun. 1999;256(3):637–41. doi: 10.1006/bbrc.1999.0384. [DOI] [PubMed] [Google Scholar]
- 262.Fehmann HC, Bode HP, Ebert T, Karl A, Goke B. Interaction of GLP-I and leptin at rat pancreatic B-cells: effects on insulin secretion and signal transduction. Horm Metab Res. 1997;29(11):572–6. doi: 10.1055/s-2007-979103. [DOI] [PubMed] [Google Scholar]
- 263.Ahren B, Havel PJ. Leptin inhibits insulin secretion induced by cellular cAMP in a pancreatic B cell line (INS-1 cells) Am J Physiol. 1999;277(4 Pt 2):R959–66. doi: 10.1152/ajpregu.1999.277.4.R959. [DOI] [PubMed] [Google Scholar]
- 264.Sonksen PH. Insulin, growth hormone and sport. J Endocrinol. 2001;170(1):13–25. doi: 10.1677/joe.0.1700013. [DOI] [PubMed] [Google Scholar]
- 265.Guler HP, Schmid C, Zapf J, Froesch ER. Effects of recombinant insulin-like growth factor I on insulin secretion and renal function in normal human subjects. Proc Natl Acad Sci U S A. 1989;86(8):2868–72. doi: 10.1073/pnas.86.8.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Van Schravendijk CF, Heylen L, Van den Brande JL, Pipeleers DG. Direct effect of insulin and insulin-like growth factor-I on the secretory activity of rat pancreatic beta cells. Diabetologia. 1990;33(11):649–53. doi: 10.1007/BF00400565. [DOI] [PubMed] [Google Scholar]
- 267.Zhang F, Sjoholm K, Zhang Q. Attenuation of insulin secretion by insulin-like growth factor binding protein-1 in pancreatic beta-cells. Biochem Biophys Res Commun. 2007;362(1):152–7. doi: 10.1016/j.bbrc.2007.07.160. [DOI] [PubMed] [Google Scholar]
- 268.Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54 (Suppl 2):S97–107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- 269.Sparre T, Larsen MR, Heding PE, Karlsen AE, Jensen ON, Pociot F. Unraveling the pathogenesis of type 1 diabetes with proteomics: present and future directions. Mol Cell Proteomics. 2005;4(4):441–57. doi: 10.1074/mcp.R500002-MCP200. [DOI] [PubMed] [Google Scholar]
- 270.von Herrath M, Sanda S, Herold K. Type 1 diabetes as a relapsing-remitting disease? Nat Rev Immunol. 2007;7(12):988–94. doi: 10.1038/nri2192. [DOI] [PubMed] [Google Scholar]
- 271.Tisch R, Wang B. Dysrulation of T cell peripheral tolerance in type 1 diabetes. Adv Immunol. 2008;100:125–49. doi: 10.1016/S0065-2776(08)00805-5. [DOI] [PubMed] [Google Scholar]
- 272.Tsai S, Shameli A, Santamaria P. CD8+ T cells in type 1 diabetes. Adv Immunol. 2008;100:79–124. doi: 10.1016/S0065-2776(08)00804-3. [DOI] [PubMed] [Google Scholar]
- 273.Mandrup-Poulsen T, Helqvist S, Molvig J, Wogensen LD, Nerup J. Cytokines as immune effector molecules in autoimmune endocrine diseases with special reference to insulin-dependent diabetes mellitus. Autoimmunity. 1989;4(3):191–218. doi: 10.3109/08916938909003049. discussion 219–34. [DOI] [PubMed] [Google Scholar]
- 274.Pankewycz OG, Guan JX, Benedict JF. Cytokines as mediators of autoimmune diabetes and diabetic complications. Endocr Rev. 1995;16(2):164–76. doi: 10.1210/edrv-16-2-164. [DOI] [PubMed] [Google Scholar]
- 275.Rabinovitch A, Suarez-Pinzon WL. Cytokines and their roles in pancreatic islet beta-cell destruction and insulin-dependent diabetes mellitus. Biochem Pharmacol. 1998;55(8):1139–49. doi: 10.1016/s0006-2952(97)00492-9. [DOI] [PubMed] [Google Scholar]
- 276.Cardozo AK, Proost P, Gysemans C, Chen MC, Mathieu C, Eizirik DL. IL-1beta and IFN-gamma induce the expression of diverse chemokines and IL-15 in human and rat pancreatic islet cells, and in islets from pre-diabetic NOD mice. Diabetologia. 2003;46(2):255–66. doi: 10.1007/s00125-002-1017-0. [DOI] [PubMed] [Google Scholar]
- 277.Li L, El-Kholy W, Rhodes CJ, Brubaker PL. Glucagon-like peptide-1 protects beta cells from cytokine-induced apoptosis and necrosis: role of protein kinase B. Diabetologia. 2005;48(7):1339–49. doi: 10.1007/s00125-005-1787-2. [DOI] [PubMed] [Google Scholar]
- 278.Thomas HE, Darwiche R, Corbett JA, Kay TW. Interleukin-1 plus gamma-interferon-induced pancreatic beta-cell dysfunction is mediated by beta-cell nitric oxide production. Diabetes. 2002;51(2):311–6. doi: 10.2337/diabetes.51.2.311. [DOI] [PubMed] [Google Scholar]
- 279.Kwon G, Corbett JA, Rodi CP, Sullivan P, McDaniel ML. Interleukin-1 beta-induced nitric oxide synthase expression by rat pancreatic beta-cells: evidence for the involvement of nuclear factor kappa B in the signaling mechanism. Endocrinology. 1995;136(11):4790–5. doi: 10.1210/endo.136.11.7588208. [DOI] [PubMed] [Google Scholar]
- 280.Takamura T, Kato I, Kimura N, Nakazawa T, Yonekura H, Takasawa S, Okamoto H. Transgenic mice overexpressing type 2 nitric-oxide synthase in pancreatic beta cells develop insulin-dependent diabetes without insulitis. J Biol Chem. 1998;273(5):2493–6. doi: 10.1074/jbc.273.5.2493. [DOI] [PubMed] [Google Scholar]
- 281.Lindsay RM, Smith W, Rossiter SP, McIntyre MA, Williams BC, Baird JD. N omega-nitro-L-arginine methyl ester reduces the incidence of IDDM in BB/E rats. Diabetes. 1995;44(3):365–8. doi: 10.2337/diab.44.3.365. [DOI] [PubMed] [Google Scholar]
- 282.Heitmeier MR, Scarim AL, Corbett JA. Interferon-gamma increases the sensitivity of islets of Langerhans for inducible nitric-oxide synthase expression induced by interleukin 1. J Biol Chem. 1997;272(21):13697–704. doi: 10.1074/jbc.272.21.13697. [DOI] [PubMed] [Google Scholar]
- 283.Flodstrom M, Tyrberg B, Eizirik DL, Sandler S. Reduced sensitivity of inducible nitric oxide synthase-deficient mice to multiple low-dose streptozotocin-induced diabetes. Diabetes. 1999;48(4):706–13. doi: 10.2337/diabetes.48.4.706. [DOI] [PubMed] [Google Scholar]
- 284.Stoffers DA. The development of beta-cell mass: recent progress and potential role of GLP-1. Horm Metab Res. 2004;36(11–12):811–21. doi: 10.1055/s-2004-826168. [DOI] [PubMed] [Google Scholar]
- 285.Tourrel C, Bailbe D, Lacorne M, Meile MJ, Kergoat M, Portha B. Persistent improvement of type 2 diabetes in the Goto-Kakizaki rat model by expansion of the beta-cell mass during the prediabetic period with glucagon-like peptide-1 or exendin-4. Diabetes. 2002;51(5):1443–52. doi: 10.2337/diabetes.51.5.1443. [DOI] [PubMed] [Google Scholar]
- 286.Sakuraba H, Mizukami H, Yagihashi N, Wada R, Hanyu C, Yagihashi S. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia. 2002;45(1):85–96. doi: 10.1007/s125-002-8248-z. [DOI] [PubMed] [Google Scholar]
- 287.Marchetti P, Del Guerra S, Marselli L, Lupi R, Masini M, Pollera M, Bugliani M, Boggi U, Vistoli F, Mosca F, Del Prato S. Pancreatic islets from type 2 diabetic patients have functional defects and increased apoptosis that are ameliorated by metformin. J Clin Endocrinol Metab. 2004;89(11):5535–41. doi: 10.1210/jc.2004-0150. [DOI] [PubMed] [Google Scholar]
- 288.Sreenan S, Pick AJ, Levisetti M, Baldwin AC, Pugh W, Polonsky KS. Increased beta-cell proliferation and reduced mass before diabetes onset in the nonobese diabetic mouse. Diabetes. 1999;48(5):989–96. doi: 10.2337/diabetes.48.5.989. [DOI] [PubMed] [Google Scholar]
- 289.Suarez-Pinzon WL, Yan Y, Power R, Brand SJ, Rabinovitch A. Combination therapy with epidermal growth factor and gastrin increases beta-cell mass and reverses hyperglycemia in diabetic NOD mice. Diabetes. 2005;54(9):2596–601. doi: 10.2337/diabetes.54.9.2596. [DOI] [PubMed] [Google Scholar]
- 290.Ogawa N, List JF, Habener JF, Maki T. Cure of overt diabetes in NOD mice by transient treatment with anti-lymphocyte serum and exendin-4. Diabetes. 2004;53(7):1700–5. doi: 10.2337/diabetes.53.7.1700. [DOI] [PubMed] [Google Scholar]
- 291.Suarez-Pinzon WL, Power RF, Yan Y, Wasserfall C, Atkinson M, Rabinovitch A. Combination therapy with glucagon-like peptide-1 and gastrin restores normoglycemia in diabetic NOD mice. Diabetes. 2008;57(12):3281–8. doi: 10.2337/db08-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Eizirik DL, Mandrup-Poulsen T. A choice of death--the signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia. 2001;44(12):2115–33. doi: 10.1007/s001250100021. [DOI] [PubMed] [Google Scholar]
- 293.Hostens K, Pavlovic D, Zambre Y, Ling Z, Van Schravendijk C, Eizirik DL, Pipeleers DG. Exposure of human islets to cytokines can result in disproportionately elevated proinsulin release. J Clin Invest. 1999;104(1):67–72. doi: 10.1172/JCI6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Eizirik DL, Kutlu B, Rasschaert J, Darville M, Cardozo AK. Use of microarray analysis to unveil transcription factor and gene networks contributing to Beta cell dysfunction and apoptosis. Ann N Y Acad Sci. 2003;1005:55–74. doi: 10.1196/annals.1288.007. [DOI] [PubMed] [Google Scholar]
- 295.Norlin S, Ahlgren U, Edlund H. Nuclear factor-{kappa}B activity in {beta}-cells is required for glucose-stimulated insulin secretion. Diabetes. 2005;54(1):125–32. doi: 10.2337/diabetes.54.1.125. [DOI] [PubMed] [Google Scholar]
- 296.Darville MI, Eizirik DL. Regulation by cytokines of the inducible nitric oxide synthase promoter in insulin-producing cells. Diabetologia. 1998;41(9):1101–8. doi: 10.1007/s001250051036. [DOI] [PubMed] [Google Scholar]
- 297.Andersson AK, Borjesson A, Sandgren J, Sandler S. Cytokines affect PDX-1 expression, insulin and proinsulin secretion from iNOS deficient murine islets. Mol Cell Endocrinol. 2005;240(1–2):50–7. doi: 10.1016/j.mce.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 298.Cardozo AK, Kruhoffer M, Leeman R, Orntoft T, Eizirik DL. Identification of novel cytokine-induced genes in pancreatic beta-cells by high-density oligonucleotide arrays. Diabetes. 2001;50(5):909–20. doi: 10.2337/diabetes.50.5.909. [DOI] [PubMed] [Google Scholar]
- 299.Chen MC, Proost P, Gysemans C, Mathieu C, Eizirik DL. Monocyte chemoattractant protein-1 is expressed in pancreatic islets from prediabetic NOD mice and in interleukin-1 beta-exposed human and rat islet cells. Diabetologia. 2001;44(3):325–32. doi: 10.1007/s001250051622. [DOI] [PubMed] [Google Scholar]
- 300.Kutlu B, Darville MI, Cardozo AK, Eizirik DL. Molecular regulation of monocyte chemoattractant protein-1 expression in pancreatic beta-cells. Diabetes. 2003;52(2):348–55. doi: 10.2337/diabetes.52.2.348. [DOI] [PubMed] [Google Scholar]
- 301.Cardozo AK, Heimberg H, Heremans Y, Leeman R, Kutlu B, Kruhoffer M, Orntoft T, Eizirik DL. A comprehensive analysis of cytokine-induced and nuclear factor-kappa B-dependent genes in primary rat pancreatic beta-cells. J Biol Chem. 2001;276(52):48879–86. doi: 10.1074/jbc.M108658200. [DOI] [PubMed] [Google Scholar]
- 302.Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29(1):42–61. doi: 10.1210/er.2007-0015. [DOI] [PubMed] [Google Scholar]
- 303.Oyadomari S, Takeda K, Takiguchi M, Gotoh T, Matsumoto M, Wada I, Akira S, Araki E, Mori M. Nitric oxide-induced apoptosis in pancreatic beta cells is mediated by the endoplasmic reticulum stress pathway. Proc Natl Acad Sci U S A. 2001;98(19):10845–50. doi: 10.1073/pnas.191207498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Oyadomari S, Araki E, Mori M. Endoplasmic reticulum stress-mediated apoptosis in pancreatic beta-cells. Apoptosis. 2002;7(4):335–45. doi: 10.1023/a:1016175429877. [DOI] [PubMed] [Google Scholar]
- 305.Ammendrup A, Maillard A, Nielsen K, Aabenhus Andersen N, Serup P, Dragsbaek Madsen O, Mandrup-Poulsen T, Bonny C. The c-Jun amino-terminal kinase pathway is preferentially activated by interleukin-1 and controls apoptosis in differentiating pancreatic beta-cells. Diabetes. 2000;49(9):1468–76. doi: 10.2337/diabetes.49.9.1468. [DOI] [PubMed] [Google Scholar]
- 306.Negri S, Oberson A, Steinmann M, Sauser C, Nicod P, Waeber G, Schorderet DF, Bonny C. cDNA cloning and mapping of a novel islet-brain/JNK-interacting protein. Genomics. 2000;64(3):324–30. doi: 10.1006/geno.2000.6129. [DOI] [PubMed] [Google Scholar]
- 307.Ferdaoussi M, Abdelli S, Yang JY, Cornu M, Niederhauser G, Favre D, Widmann C, Regazzi R, Thorens B, Waeber G, Abderrahmani A. Exendin-4 protects beta-cells from interleukin-1 beta-induced apoptosis by interfering with the c-Jun NH2-terminal kinase pathway. Diabetes. 2008;57(5):1205–15. doi: 10.2337/db07-1214. [DOI] [PubMed] [Google Scholar]
- 308.Bonny C, Oberson A, Negri S, Sauser C, Schorderet DF. Cell-permeable peptide inhibitors of JNK: novel blockers of beta-cell death. Diabetes. 2001;50(1):77–82. doi: 10.2337/diabetes.50.1.77. [DOI] [PubMed] [Google Scholar]
- 309.Donath MY, Halban PA. Decreased beta-cell mass in diabetes: significance, mechanisms and therapeutic implications. Diabetologia. 2004;47(3):581–9. doi: 10.1007/s00125-004-1336-4. [DOI] [PubMed] [Google Scholar]
- 310.Weir GC, Laybutt DR, Kaneto H, Bonner-Weir S, Sharma A. Beta-cell adaptation and decompensation during the progression of diabetes. Diabetes. 2001;50 (Suppl 1):S154–9. doi: 10.2337/diabetes.50.2007.s154. [DOI] [PubMed] [Google Scholar]
- 311.Zhou YP, Marlen K, Palma JF, Schweitzer A, Reilly L, Gregoire FM, Xu GG, Blume JE, Johnson JD. Overexpression of repressive cAMP response element modulators in high glucose and fatty acid-treated rat islets. A common mechanism for glucose toxicity and lipotoxicity? J Biol Chem. 2003;278(51):51316–23. doi: 10.1074/jbc.M307972200. [DOI] [PubMed] [Google Scholar]
- 312.Robertson RP, Harmon J, Tran PO, Tanaka Y, Takahashi H. Glucose toxicity in beta-cells: type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes. 2003;52(3):581–7. doi: 10.2337/diabetes.52.3.581. [DOI] [PubMed] [Google Scholar]
- 313.Elouil H, Cardozo AK, Eizirik DL, Henquin JC, Jonas JC. High glucose and hydrogen peroxide increase c-Myc and haeme-oxygenase 1 mRNA levels in rat pancreatic islets without activating NFkappaB. Diabetologia. 2005;48(3):496–505. doi: 10.1007/s00125-004-1664-4. [DOI] [PubMed] [Google Scholar]
- 314.Gurgul E, Lortz S, Tiedge M, Jorns A, Lenzen S. Mitochondrial catalase overexpression protects insulin-producing cells against toxicity of reactive oxygen species and proinflammatory cytokines. Diabetes. 2004;53(9):2271–80. doi: 10.2337/diabetes.53.9.2271. [DOI] [PubMed] [Google Scholar]
- 315.Fridlyand LE, Philipson LH. Does the glucose-dependent insulin secretion mechanism itself cause oxidative stress in pancreatic beta-cells? Diabetes. 2004;53(8):1942–8. doi: 10.2337/diabetes.53.8.1942. [DOI] [PubMed] [Google Scholar]
- 316.Grill V, Bjorklund A. Overstimulation and beta-cell function. Diabetes. 2001;50 (Suppl 1):S122–4. doi: 10.2337/diabetes.50.2007.s122. [DOI] [PubMed] [Google Scholar]
- 317.Maedler K, Oberholzer J, Bucher P, Spinas GA, Donath MY. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic beta-cell turnover and function. Diabetes. 2003;52(3):726–33. doi: 10.2337/diabetes.52.3.726. [DOI] [PubMed] [Google Scholar]
- 318.Maedler K, Spinas GA, Dyntar D, Moritz W, Kaiser N, Donath MY. Distinct effects of saturated and monounsaturated fatty acids on beta-cell turnover and function. Diabetes. 2001;50(1):69–76. doi: 10.2337/diabetes.50.1.69. [DOI] [PubMed] [Google Scholar]
- 319.Kharroubi I, Ladriere L, Cardozo AK, Dogusan Z, Cnop M, Eizirik DL. Free fatty acids and cytokines induce pancreatic beta-cell apoptosis by different mechanisms: role of nuclear factor-kappaB and endoplasmic reticulum stress. Endocrinology. 2004;145(11):5087–96. doi: 10.1210/en.2004-0478. [DOI] [PubMed] [Google Scholar]
- 320.Cnop M, Hannaert JC, Hoorens A, Eizirik DL, Pipeleers DG. Inverse relationship between cytotoxicity of free fatty acids in pancreatic islet cells and cellular triglyceride accumulation. Diabetes. 2001;50(8):1771–7. doi: 10.2337/diabetes.50.8.1771. [DOI] [PubMed] [Google Scholar]
- 321.Rys-Sikora KE, Gill DL. Fatty acid-mediated calcium sequestration within intracellular calcium pools. J Biol Chem. 1998;273(49):32627–35. doi: 10.1074/jbc.273.49.32627. [DOI] [PubMed] [Google Scholar]
- 322.Bonner-Weir S. beta-cell turnover: its assessment and implications. Diabetes. 2001;50 (Suppl 1):S20–4. doi: 10.2337/diabetes.50.2007.s20. [DOI] [PubMed] [Google Scholar]
- 323.Pick A, Clark J, Kubstrup C, Levisetti M, Pugh W, Bonner-Weir S, Polonsky KS. Role of apoptosis in failure of beta-cell mass compensation for insulin resistance and beta-cell defects in the male Zucker diabetic fatty rat. Diabetes. 1998;47(3):358–64. doi: 10.2337/diabetes.47.3.358. [DOI] [PubMed] [Google Scholar]
- 324.Kloppel G, Lohr M, Habich K, Oberholzer M, Heitz PU. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Pathol Res. 1985;4(2):110–25. doi: 10.1159/000156969. [DOI] [PubMed] [Google Scholar]
- 325.Buettner R, Newgard CB, Rhodes CJ, O’Doherty RM. Correction of diet-induced hyperglycemia, hyperinsulinemia, and skeletal muscle insulin resistance by moderate hyperleptinemia. Am J Physiol Endocrinol Metab. 2000;278(3):E563–9. doi: 10.1152/ajpendo.2000.278.3.E563. [DOI] [PubMed] [Google Scholar]
- 326.Sherry NA, Kushner JA, Glandt M, Kitamura T, Brillantes AM, Herold KC. Effects of autoimmunity and immune therapy on beta-cell turnover in type 1 diabetes. Diabetes. 2006;55(12):3238–45. doi: 10.2337/db05-1034. [DOI] [PubMed] [Google Scholar]
- 327.Thorel F, Nepote V, Avril I, Kohno K, Desgraz R, Chera S, Herrera PL. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464(7292):1149–54. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Bonner-Weir S. Islet growth and development in the adult. J Mol Endocrinol. 2000;24(3):297–302. doi: 10.1677/jme.0.0240297. [DOI] [PubMed] [Google Scholar]
- 329.Pende M, Kozma SC, Jaquet M, Oorschot V, Burcelin R, Le Marchand-Brustel Y, Klumperman J, Thorens B, Thomas G. Hypoinsulinaemia, glucose intolerance and diminished beta-cell size in S6K1-deficient mice. Nature. 2000;408(6815):994–7. doi: 10.1038/35050135. [DOI] [PubMed] [Google Scholar]
- 330.Rhodes CJ, White MF. Molecular insights into insulin action and secretion. Eur J Clin Invest. 2002;32 (Suppl 3):3–13. doi: 10.1046/j.1365-2362.32.s3.2.x. [DOI] [PubMed] [Google Scholar]
- 331.Hugl SR, White MF, Rhodes CJ. Insulin-like growth factor I (IGF-I)-stimulated pancreatic beta-cell growth is glucose-dependent. Synergistic activation of insulin receptor substrate-mediated signal transduction pathways by glucose and IGF-I in INS-1 cells. J Biol Chem. 1998;273(28):17771–9. doi: 10.1074/jbc.273.28.17771. [DOI] [PubMed] [Google Scholar]
- 332.Cousin SP, Hugl SR, Myers MG, Jr, White MF, Reifel-Miller A, Rhodes CJ. Stimulation of pancreatic beta-cell proliferation by growth hormone is glucose-dependent: signal transduction via janus kinase 2 (JAK2)/signal transducer and activator of transcription 5 (STAT5) with no crosstalk to insulin receptor substrate-mediated mitogenic signalling. Biochem J. 1999;344(Pt 3):649–58. [PMC free article] [PubMed] [Google Scholar]
- 333.Donath MY, Gross DJ, Cerasi E, Kaiser N. Hyperglycemia-induced beta-cell apoptosis in pancreatic islets of Psammomys obesus during development of diabetes. Diabetes. 1999;48(4):738–44. doi: 10.2337/diabetes.48.4.738. [DOI] [PubMed] [Google Scholar]
- 334.Galsgaard ED, Gouilleux F, Groner B, Serup P, Nielsen JH, Billestrup N. Identification of a growth hormone-responsive STAT5-binding element in the rat insulin 1 gene. Mol Endocrinol. 1996;10(6):652–60. doi: 10.1210/mend.10.6.8776725. [DOI] [PubMed] [Google Scholar]
- 335.Brelje TC, Stout LE, Bhagroo NV, Sorenson RL. Distinctive roles for prolactin and growth hormone in the activation of signal transducer and activator of transcription 5 in pancreatic islets of langerhans. Endocrinology. 2004;145(9):4162–75. doi: 10.1210/en.2004-0201. [DOI] [PubMed] [Google Scholar]
- 336.Friedrichsen BN, Richter HE, Hansen JA, Rhodes CJ, Nielsen JH, Billestrup N, Moldrup A. Signal transducer and activator of transcription 5 activation is sufficient to drive transcriptional induction of cyclin D2 gene and proliferation of rat pancreatic beta-cells. Mol Endocrinol. 2003;17(5):945–58. doi: 10.1210/me.2002-0356. [DOI] [PubMed] [Google Scholar]
- 337.Rulifson IC, Karnik SK, Heiser PW, ten Berge D, Chen H, Gu X, Taketo MM, Nusse R, Hebrok M, Kim SK. Wnt signaling regulates pancreatic beta cell proliferation. Proc Natl Acad Sci U S A. 2007;104(15):6247–52. doi: 10.1073/pnas.0701509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Nielsen JH, Galsgaard ED, Moldrup A, Friedrichsen BN, Billestrup N, Hansen JA, Lee YC, Carlsson C. Regulation of beta-cell mass by hormones and growth factors. Diabetes. 2001;50 (Suppl 1):S25–9. doi: 10.2337/diabetes.50.2007.s25. [DOI] [PubMed] [Google Scholar]
- 339.Bruning JC, Winnay J, Bonner-Weir S, Taylor SI, Accili D, Kahn CR. Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell. 1997;88(4):561–72. doi: 10.1016/s0092-8674(00)81896-6. [DOI] [PubMed] [Google Scholar]
- 340.Tuttle RL, Gill NS, Pugh W, Lee JP, Koeberlein B, Furth EE, Polonsky KS, Naji A, Birnbaum MJ. Regulation of pancreatic beta-cell growth and survival by the serine/threonine protein kinase Akt1/PKBalpha. Nat Med. 2001;7(10):1133–7. doi: 10.1038/nm1001-1133. [DOI] [PubMed] [Google Scholar]
- 341.Frystyk J, Skjaerbaek C, Vestbo E, Fisker S, Orskov H. Circulating levels of free insulin-like growth factors in obese subjects: the impact of type 2 diabetes. Diabetes Metab Res Rev. 1999;15(5):314–22. doi: 10.1002/(sici)1520-7560(199909/10)15:5<314::aid-dmrr56>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 342.Dickson LM, Lingohr MK, McCuaig J, Hugl SR, Snow L, Kahn BB, Myers MG, Jr, Rhodes CJ. Differential activation of protein kinase B and p70(S6)K by glucose and insulin-like growth factor 1 in pancreatic beta-cells (INS-1) J Biol Chem. 2001;276(24):21110–20. doi: 10.1074/jbc.M101257200. [DOI] [PubMed] [Google Scholar]
- 343.Chan TO, Rittenhouse SE, Tsichlis PN. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- 344.Bottazzo GF, Florin-Christensen A, Doniach D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet. 1974;2(7892):1279–83. doi: 10.1016/s0140-6736(74)90140-8. [DOI] [PubMed] [Google Scholar]
- 345.Palmer JP, Asplin CM, Clemons P, Lyen K, Tatpati O, Raghu PK, Paquette TL. Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science. 1983;222(4630):1337–9. doi: 10.1126/science.6362005. [DOI] [PubMed] [Google Scholar]
- 346.Kuglin B, Gries FA, Kolb H. Evidence of IgG autoantibodies against human proinsulin in patients with IDDM before insulin treatment. Diabetes. 1988;37(1):130–2. doi: 10.2337/diab.37.1.130. [DOI] [PubMed] [Google Scholar]
- 347.Baekkeskov S, Aanstoot HJ, Christgau S, Reetz A, Solimena M, Cascalho M, Folli F, Richter-Olesen H, De Camilli P. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990;347(6289):151–6. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- 348.Christie MR, Tun RY, Lo SS, Cassidy D, Brown TJ, Hollands J, Shattock M, Bottazzo GF, Leslie RD. Antibodies to GAD and tryptic fragments of islet 64K antigen as distinct markers for development of IDDM. Studies with identical twins. Diabetes. 1992;41(7):782–7. doi: 10.2337/diab.41.7.782. [DOI] [PubMed] [Google Scholar]
- 349.Foulis AK, Farquharson MA, Hardman R. Aberrant expression of class II major histocompatibility complex molecules by B cells and hyperexpression of class I major histocompatibility complex molecules by insulin containing islets in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1987;30(5):333–43. doi: 10.1007/BF00299027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Root EJ, Combs GF., Jr Disruption of endoplasmic reticulum is the primary ultrastructural lesion of the pancreas in the selenium-deficient chick. Proc Soc Exp Biol Med. 1988;187(4):513–21. doi: 10.3181/00379727-187-42697. [DOI] [PubMed] [Google Scholar]
- 351.Feutren G, Papoz L, Assan R, Vialettes B, Karsenty G, Vexiau P, Du Rostu H, Rodier M, Sirmai J, Lallemand A. Cyclosporin increases the rate and length of remissions in insulin-dependent diabetes of recent onset. Results of a multicentre double-blind trial. Lancet. 1986;2(8499):119–24. doi: 10.1016/s0140-6736(86)91943-4. [DOI] [PubMed] [Google Scholar]
- 352.Ziegler AG, Schmid S, Huber D, Hummel M, Bonifacio E. Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. JAMA. 2003;290(13):1721–8. doi: 10.1001/jama.290.13.1721. [DOI] [PubMed] [Google Scholar]
- 353.Littorin B, Blom P, Scholin A, Arnqvist HJ, Blohme G, Bolinder J, Ekbom-Schnell A, Eriksson JW, Gudbjornsdottir S, Nystrom L, Ostman J, Sundkvist G. Lower levels of plasma 25-hydroxyvitamin D among young adults at diagnosis of autoimmune type 1 diabetes compared with control subjects: results from the nationwide Diabetes Incidence Study in Sweden (DISS) Diabetologia. 2006;49(12):2847–52. doi: 10.1007/s00125-006-0426-x. [DOI] [PubMed] [Google Scholar]
- 354.Frisk G, Hansson T, Dahlbom I, Tuvemo T. A unifying hypothesis on the development of type 1 diabetes and celiac disease: gluten consumption may be a shared causative factor. Med Hypotheses. 2008;70(6):1207–9. doi: 10.1016/j.mehy.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 355.Pugliese A. Genetics of type 1 diabetes. Endocrinol Metab Clin North Am. 2004;33(1):1–16. vii. doi: 10.1016/S0889-8529(03)00082-3. [DOI] [PubMed] [Google Scholar]
- 356.Pino SC, Kruger AJ, Bortell R. The role of innate immune pathways in type 1 diabetes pathogenesis. Curr Opin Endocrinol Diabetes Obes. 2010 doi: 10.1097/MED.0b013e3283372819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Bingley PJ, Christie MR, Bonifacio E, Bonfanti R, Shattock M, Fonte MT, Bottazzo GF, Gale EA. Combined analysis of autoantibodies improves prediction of IDDM in islet cell antibody-positive relatives. Diabetes. 1994;43(11):1304–10. doi: 10.2337/diab.43.11.1304. [DOI] [PubMed] [Google Scholar]
- 358.Notkins AL, Lernmark A. Autoimmune type 1 diabetes: resolved and unresolved issues. J Clin Invest. 2001;108(9):1247–52. doi: 10.1172/JCI14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Wallet MA, Tisch R. Type 1 diabetes, inflammation and dendritic cells. Drug Discovery Today. 2006;3(3):373–379. [Google Scholar]
- 360.Knip M, Siljander H. Autoimmune mechanisms in type 1 diabetes. Autoimmun Rev. 2008;7(7):550–7. doi: 10.1016/j.autrev.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 361.Roep BO. The role of T-cells in the pathogenesis of Type 1 diabetes: from cause to cure. Diabetologia. 2003;46(3):305–21. doi: 10.1007/s00125-003-1089-5. [DOI] [PubMed] [Google Scholar]
- 362.Guberski DL, Butler L, Kastern W, Like AA. Genetic studies in inbred BB/Wor rats. Analysis of progeny produced by crossing lymphopenic diabetes-prone rats with nonlymphopenic diabetic rats. Diabetes. 1989;38(7):887–93. doi: 10.2337/diab.38.7.887. [DOI] [PubMed] [Google Scholar]
- 363.MacMurray AJ, Moralejo DH, Kwitek AE, Rutledge EA, Van Yserloo B, Gohlke P, Speros SJ, Snyder B, Schaefer J, Bieg S, Jiang J, Ettinger RA, Fuller J, Daniels TL, Pettersson A, Orlebeke K, Birren B, Jacob HJ, Lander ES, Lernmark A. Lymphopenia in the BB rat model of type 1 diabetes is due to a mutation in a novel immune-associated nucleotide (Ian)-related gene. Genome Res. 2002;12(7):1029–39. doi: 10.1101/gr.412702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Schulze-Koops H. Lymphopenia and autoimmune diseases. Arthritis Res Ther. 2004;6(4):178–80. doi: 10.1186/ar1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117(2):265–77. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 366.Cho BK, Rao VP, Ge Q, Eisen HN, Chen J. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J Exp Med. 2000;192(4):549–56. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Isaacs JD, Greer S, Sharma S, Symmons D, Smith M, Johnston J, Waldmann H, Hale G, Hazleman BL. Morbidity and mortality in rheumatoid arthritis patients with prolonged and profound therapy-induced lymphopenia. Arthritis Rheum. 2001;44(9):1998–2008. doi: 10.1002/1529-0131(200109)44:9<1998::AID-ART348>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 368.Thivolet C, Bendelac A, Bedossa P, Bach JF, Carnaud C. CD8+ T cell homing to the pancreas in the nonobese diabetic mouse is CD4+ T cell-dependent. J Immunol. 1991;146(1):85–8. [PubMed] [Google Scholar]
- 369.Romagnani S. Biology of human TH1 and TH2 cells. J Clin Immunol. 1995;15(3):121–9. doi: 10.1007/BF01543103. [DOI] [PubMed] [Google Scholar]
- 370.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 371.Almawi WY, Tamim H, Azar ST. Clinical review 103: T helper type 1 and 2 cytokines mediate the onset and progression of type I (insulin-dependent) diabetes. J Clin Endocrinol Metab. 1999;84(5):1497–502. doi: 10.1210/jcem.84.5.5699. [DOI] [PubMed] [Google Scholar]
- 372.Bucy RP, Karr L, Huang GQ, Li J, Carter D, Honjo K, Lemons JA, Murphy KM, Weaver CT. Single cell analysis of cytokine gene coexpression during CD4+ T-cell phenotype development. Proc Natl Acad Sci U S A. 1995;92(16):7565–9. doi: 10.1073/pnas.92.16.7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4(9):835–42. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 374.Katz JD, Benoist C, Mathis D. T helper cell subsets in insulin-dependent diabetes. Science. 1995;268(5214):1185–8. doi: 10.1126/science.7761837. [DOI] [PubMed] [Google Scholar]
- 375.Liblau RS, Singer SM, McDevitt HO. Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol Today. 1995;16(1):34–8. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 376.Pilstrom B, Bjork L, Bohme J. Monokine-producing cells predominate in the recruitment phase of NOD insulitis while cells producing Th1-type cytokines characterize the effector phase. J Autoimmun. 1997;10(2):147–55. doi: 10.1006/jaut.1996.0115. [DOI] [PubMed] [Google Scholar]
- 377.Faust A, Rothe H, Schade U, Lampeter E, Kolb H. Primary nonfunction of islet grafts in autoimmune diabetic nonobese diabetic mice is prevented by treatment with interleukin-4 and interleukin-10. Transplantation. 1996;62(5):648–52. doi: 10.1097/00007890-199609150-00019. [DOI] [PubMed] [Google Scholar]
- 378.Held W, MacDonald HR, Weissman IL, Hess MW, Mueller C. Genes encoding tumor necrosis factor alpha and granzyme A are expressed during development of autoimmune diabetes. Proc Natl Acad Sci U S A. 1990;87(6):2239–43. doi: 10.1073/pnas.87.6.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Berman MA, Sandborg CI, Wang Z, Imfeld KL, Zaldivar F, Jr, Dadufalza V, Buckingham BA. Decreased IL-4 production in new onset type I insulin-dependent diabetes mellitus. J Immunol. 1996;157(10):4690–6. [PubMed] [Google Scholar]
- 380.Lee MS, Wogensen L, Shizuru J, Oldstone MB, Sarvetnick N. Pancreatic islet production of murine interleukin-10 does not inhibit immune-mediated tissue destruction. J Clin Invest. 1994;93(3):1332–8. doi: 10.1172/JCI117092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Wick M, Dubey P, Koeppen H, Siegel CT, Fields PE, Chen L, Bluestone JA, Schreiber H. Antigenic cancer cells grow progressively in immune hosts without evidence for T cell exhaustion or systemic anergy. J Exp Med. 1997;186(2):229–38. doi: 10.1084/jem.186.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Moritani M, Yoshimoto K, Tashiro F, Hashimoto C, Miyazaki J, Ii S, Kudo E, Iwahana H, Hayashi Y, Sano T. Transgenic expression of IL-10 in pancreatic islet A cells accelerates autoimmune insulitis and diabetes in non-obese diabetic mice. Int Immunol. 1994;6(12):1927–36. doi: 10.1093/intimm/6.12.1927. [DOI] [PubMed] [Google Scholar]
- 383.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100(6):655–69. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 384.Neurath MF, Weigmann B, Finotto S, Glickman J, Nieuwenhuis E, Iijima H, Mizoguchi A, Mizoguchi E, Mudter J, Galle PR, Bhan A, Autschbach F, Sullivan BM, Szabo SJ, Glimcher LH, Blumberg RS. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn’s disease. J Exp Med. 2002;195(9):1129–43. doi: 10.1084/jem.20011956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Chakir H, Wang H, Lefebvre DE, Webb J, Scott FW. T-bet/GATA-3 ratio as a measure of the Th1/Th2 cytokine profile in mixed cell populations: predominant role of GATA-3. J Immunol Methods. 2003;278(1–2):157–69. doi: 10.1016/s0022-1759(03)00200-x. [DOI] [PubMed] [Google Scholar]
- 386.Piccirillo CA, Shevach EM. Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J Immunol. 2001;167(3):1137–40. doi: 10.4049/jimmunol.167.3.1137. [DOI] [PubMed] [Google Scholar]
- 387.Randolph DA, Fathman CG. Cd4+Cd25+ regulatory T cells and their therapeutic potential. Annu Rev Med. 2006;57:381–402. doi: 10.1146/annurev.med.57.121304.131337. [DOI] [PubMed] [Google Scholar]
- 388.Chen Z, Herman AE, Matos M, Mathis D, Benoist C. Where CD4+CD25+ T reg cells impinge on autoimmune diabetes. J Exp Med. 2005;202(10):1387–97. doi: 10.1084/jem.20051409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Brusko T, Atkinson M. Treg in type 1 diabetes. Cell Biochem Biophys. 2007;48(2–3):165–75. doi: 10.1007/s12013-007-0018-5. [DOI] [PubMed] [Google Scholar]
- 390.Filippi C, Bresson D, von Herrath M. Antigen-specific induction of regulatory T cells for type 1 diabetes therapy. Int Rev Immunol. 2005;24(5–6):341–60. doi: 10.1080/08830180500371116. [DOI] [PubMed] [Google Scholar]
- 391.Gagnerault MC, Luan JJ, Lotton C, Lepault F. Pancreatic lymph nodes are required for priming of beta cell reactive T cells in NOD mice. J Exp Med. 2002;196(3):369–77. doi: 10.1084/jem.20011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.McKenzie MD, Dudek NL, Mariana L, Chong MM, Trapani JA, Kay TW, Thomas HE. Perforin and Fas induced by IFNgamma and TNFalpha mediate beta cell death by OT-I CTL. Int Immunol. 2006;18(6):837–46. doi: 10.1093/intimm/dxl020. [DOI] [PubMed] [Google Scholar]
- 393.Shi L, Kraut RP, Aebersold R, Greenberg AH. A natural killer cell granule protein that induces DNA fragmentation and apoptosis. J Exp Med. 1992;175(2):553–66. doi: 10.1084/jem.175.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Sullivan BM, Juedes A, Szabo SJ, von Herrath M, Glimcher LH. Antigen-driven effector CD8 T cell function regulated by T-bet. Proc Natl Acad Sci U S A. 2003;100(26):15818–23. doi: 10.1073/pnas.2636938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Chabaud M, Garnero P, Dayer JM, Guerne PA, Fossiez F, Miossec P. Contribution of interleukin 17 to synovium matrix destruction in rheumatoid arthritis. Cytokine. 2000;12(7):1092–9. doi: 10.1006/cyto.2000.0681. [DOI] [PubMed] [Google Scholar]
- 396.Suzuki Y, Kuroda Y, Morita A, Fujino Y, Tanioka Y, Kawamura T, Saitoh Y. Fibrin glue sealing for the prevention of pancreatic fistulas following distal pancreatectomy. Arch Surg. 1995;130(9):952–5. doi: 10.1001/archsurg.1995.01430090038015. [DOI] [PubMed] [Google Scholar]
- 397.Graber JJ, Allie SR, Mullen KM, Jones MV, Wang T, Krishnan C, Kaplin AI, Nath A, Kerr DA, Calabresi PA. Interleukin-17 in transverse myelitis and multiple sclerosis. J Neuroimmunol. 2008;196(1–2):124–32. doi: 10.1016/j.jneuroim.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 398.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, Romagnani S. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204(8):1849–61. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30(1):92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Vukkadapu SS, Belli JM, Ishii K, Jegga AG, Hutton JJ, Aronow BJ, Katz JD. Dynamic interaction between T cell-mediated beta-cell damage and beta-cell repair in the run up to autoimmune diabetes of the NOD mouse. Physiol Genomics. 2005;21(2):201–11. doi: 10.1152/physiolgenomics.00173.2004. [DOI] [PubMed] [Google Scholar]
- 401.Jain R, Tartar DM, Gregg RK, Divekar RD, Bell JJ, Lee HH, Yu P, Ellis JS, Hoeman CM, Franklin CL, Zaghouani H. Innocuous IFNgamma induced by adjuvant-free antigen restores normoglycemia in NOD mice through inhibition of IL-17 production. J Exp Med. 2008;205(1):207–18. doi: 10.1084/jem.20071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Cozar-Castellano I, Fiaschi-Taesch N, Bigatel TA, Takane KK, Garcia-Ocana A, Vasavada R, Stewart AF. Molecular control of cell cycle progression in the pancreatic beta-cell. Endocr Rev. 2006;27(4):356–70. doi: 10.1210/er.2006-0004. [DOI] [PubMed] [Google Scholar]
- 403.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–6. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 404.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–10. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 405.Pietropaolo M, Le Roith D. Pathogenesis of diabetes: our current understanding. Clin Cornerstone. 2001;4(2):1–16. doi: 10.1016/s1098-3597(01)90025-0. [DOI] [PubMed] [Google Scholar]
- 406.Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest. 2006;116(7):1802–12. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Rhodes CJ. Type 2 diabetes-a matter of beta-cell life and death? Science. 2005;307(5708):380–4. doi: 10.1126/science.1104345. [DOI] [PubMed] [Google Scholar]
- 408.Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46(1):3–10. [PubMed] [Google Scholar]
- 409.Polonsky KS. Dynamics of insulin secretion in obesity and diabetes. Int J Obes Relat Metab Disord. 2000;24 (Suppl 2):S29–31. doi: 10.1038/sj.ijo.0801273. [DOI] [PubMed] [Google Scholar]
- 410.Flier SN, Kulkarni RN, Kahn CR. Evidence for a circulating islet cell growth factor in insulin-resistant states. Proc Natl Acad Sci U S A. 2001;98(13):7475–80. doi: 10.1073/pnas.131192998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3(3):153–65. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 412.Van Citters GW, Kabir M, Kim SP, Mittelman SD, Dea MK, Brubaker PL, Bergman RN. Elevated glucagon-like peptide-1-(7–36)-amide, but not glucose, associated with hyperinsulinemic compensation for fat feeding. J Clin Endocrinol Metab. 2002;87(11):5191–5198. doi: 10.1210/jc.2002-020002. [DOI] [PubMed] [Google Scholar]
- 413.Holman RR. Assessing the potential for alpha-glucosidase inhibitors in prediabetic states. Diabetes Res Clin Pract. 1998;40 (Suppl):S21–5. doi: 10.1016/s0168-8227(98)00038-2. [DOI] [PubMed] [Google Scholar]
- 414.Jaikaran ET, Clark A. Islet amyloid and type 2 diabetes: from molecular misfolding to islet pathophysiology. Biochim Biophys Acta. 2001;1537(3):179–203. doi: 10.1016/s0925-4439(01)00078-3. [DOI] [PubMed] [Google Scholar]
- 415.Poitout V, Amyot J, Semache M, Zarrouki B, Hagman D, Fontes G. Glucolipotoxicity of the pancreatic beta cell. Biochim BiophysActa. 2010;1801(3):289–298. doi: 10.1016/j.bbalip.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Poitout V. Glucolipotoxicity of the pancreatic beta-cell: myth or reality? Biochem Soc Trans. 2008;36(Pt 5):901–4. doi: 10.1042/BST0360901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29(3):351–66. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Zhao NQ, Yu YR, Tan HW, Deng G, Zhang XX. Role of apoptosis and mitochondrial apoptotic pathway in glucolipotoxicity-induced islet beta-cell dysfunction. Nan Fang Yi Ke Da Xue Xue Bao. 2008;28(11):2009–13. [PubMed] [Google Scholar]
- 419.Wajchenberg BL. beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev. 2007;28(2):187–218. doi: 10.1210/10.1210/er.2006-0038. [DOI] [PubMed] [Google Scholar]
- 420.Chang-Chen KJ, Mullur R, Bernal-Mizrachi E. beta-cell failure as a complication of diabetes. Rev Endocr Metab Disord. 2008;9(4):329–343. doi: 10.1007/s11154-008-9101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Poitout V, Robertson RP. Minireview: Secondary beta-cell failure in type 2 diabetes--a convergence of glucotoxicity and lipotoxicity. Endocrinology. 2002;143(2):339–42. doi: 10.1210/endo.143.2.8623. [DOI] [PubMed] [Google Scholar]
- 422.Khaldi MZ, Guiot Y, Gilon P, Henquin JC, Jonas JC. Increased glucose sensitivity of both triggering and amplifying pathways of insulin secretion in rat islets cultured for 1 wk in high glucose. Am J Physiol Endocrinol Metab. 2004;287(2):E207–E217. doi: 10.1152/ajpendo.00426.2003. [DOI] [PubMed] [Google Scholar]
- 423.Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002;110(6):851–60. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Ohara-Imaizumi M, Cardozo AK, Kikuta T, Eizirik DL, Nagamatsu S. The cytokine interleukin-1 beta reduces the docking and fusion of insulin granules in pancreatic beta-cells, preferentially decreasing the first phase of exocytosis. J Biol Chem. 2004;279(40):41271–41274. doi: 10.1074/jbc.C400360200. [DOI] [PubMed] [Google Scholar]
- 425.Kajimoto Y, Matsuoka T, Kaneto H, Watada H, Fujitani Y, Kishimoto M, Sakamoto K, Matsuhisa M, Kawamori R, Yamasaki Y, Hori M. Induction of glycation suppresses glucokinase gene expression in HIT-T15 cells. Diabetologia. 1999;42(12):1417–24. doi: 10.1007/s001250051313. [DOI] [PubMed] [Google Scholar]
- 426.Tajiri Y, Moller C, Grill V. Long-term effects of aminoguanidine on insulin release and biosynthesis: evidence that the formation of advanced glycosylation end products inhibits B cell function. Endocrinology. 1997;138(1):273–80. doi: 10.1210/endo.138.1.4851. [DOI] [PubMed] [Google Scholar]
- 427.Tanaka Y, Gleason CE, Tran PO, Harmon JS, Robertson RP. Prevention of glucose toxicity in HIT-T15 cells and Zucker diabetic fatty rats by antioxidants. Proc Natl Acad Sci U S A. 1999;96(19):10857–62. doi: 10.1073/pnas.96.19.10857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Kaneto H, Kajimoto Y, Miyagawa J, Matsuoka T, Fujitani Y, Umayahara Y, Hanafusa T, Matsuzawa Y, Yamasaki K, Hori M. Beneficial effects of antioxidants in diabetes - Possible protection of pancreatic beta-cells against glucose toxicity. Diabetes. 1999;48(12):2398–2406. doi: 10.2337/diabetes.48.12.2398. [DOI] [PubMed] [Google Scholar]
- 429.Ihara Y, Toyokuni S, Uchida K, Odaka H, Tanaka T, Ikeda H, Hiai H, Seino Y, Yamada Y. Hyperglycemia causes oxidative stress in pancreatic beta-cells of GK rats, a model of type 2 diabetes. Diabetes. 1999;48(4):927–32. doi: 10.2337/diabetes.48.4.927. [DOI] [PubMed] [Google Scholar]
- 430.Matsuoka T, Kajimoto Y, Watada H, Kaneto H, Kishimoto M, Umayahara Y, Fujitani Y, Kamada T, Kawamori R, Yamasaki Y. Glycation-dependent, reactive oxygen species-mediated suppression of the insulin gene promoter activity in HIT cells. J Clin Invest. 1997;99(1):144–50. doi: 10.1172/JCI119126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Kaneto H, Fujii J, Myint T, Miyazawa N, Islam KN, Kawasaki Y, Suzuki K, Nakamura M, Tatsumi H, Yamasaki Y, Taniguchi N. Reducing sugars trigger oxidative modification and apoptosis in pancreatic beta-cells by provoking oxidative stress through the glycation reaction. Biochem J. 1996;320 ( Pt 3):855–63. doi: 10.1042/bj3200855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Grankvist K, Marklund SL, Taljedal IB. CuZn-superoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse. Biochem J. 1981;199(2):393–8. doi: 10.1042/bj1990393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Malaisse WJ, Malaisse-Lagae F, Sener A, Pipeleers DG. Determinants of the selective toxicity of alloxan to the pancreatic B cell. Proc Natl Acad Sci U S A. 1982;79(3):927–30. doi: 10.1073/pnas.79.3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Grankvist K, Marklund S, Taljedal IB. Superoxide dismutase is a prophylactic against alloxan diabetes. Nature. 1981;294(5837):158–60. doi: 10.1038/294158a0. [DOI] [PubMed] [Google Scholar]
- 435.Tanaka Y, Tran PO, Harmon J, Robertson RP. A role for glutathione peroxidase in protecting pancreatic beta cells against oxidative stress in a model of glucose toxicity. Proc Natl Acad Sci U S A. 2002;99(19):12363–8. doi: 10.1073/pnas.192445199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40(4):405–12. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 437.Wolff SP, Dean RT. Glucose autoxidation and protein modification. The potential role of ‘autoxidative glycosylation’ in diabetes. Biochem J. 1987;245(1):243–50. doi: 10.1042/bj2450243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Hunt JV, Dean RT, Wolff SP. Hydroxyl radical production and autoxidative glycosylation. Glucose autoxidation as the cause of protein damage in the experimental glycation model of diabetes mellitus and ageing. Biochem J. 1988;256(1):205–12. doi: 10.1042/bj2560205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Kaneto H, Xu G, Song KH, Suzuma K, Bonner-Weir S, Sharma A, Weir GC. Activation of the hexosamine pathway leads to deterioration of pancreatic beta-cell function through the induction of oxidative stress. J Biol Chem. 2001;276(33):31099–104. doi: 10.1074/jbc.M104115200. [DOI] [PubMed] [Google Scholar]
- 440.Kaneto H, Xu G, Fujii N, Kim S, Bonner-Weir S, Weir GC. Involvement of c-Jun N-terminal kinase in oxidative stress-mediated suppression of insulin gene expression. J Biol Chem. 2002;277(33):30010–30018. doi: 10.1074/jbc.M202066200. [DOI] [PubMed] [Google Scholar]
- 441.Withers DJ, Gutierrez JS, Towery H, Burks DJ, Ren JM, Previs S, Zhang Y, Bernal D, Pons S, Shulman GI, Bonner-Weir S, White MF. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391(6670):900–4. doi: 10.1038/36116. [DOI] [PubMed] [Google Scholar]
- 442.Kubota N, Terauchi Y, Tobe K, Yano W, Suzuki R, Ueki K, Takamoto I, Satoh H, Maki T, Kubota T, Moroi M, Okada-Iwabu M, Ezaki O, Nagai R, Ueta Y, Kadowaki T, Noda T. Insulin receptor substrate 2 plays a crucial role in beta cells and the hypothalamus. J Clin Invest. 2004;114(7):917–27. doi: 10.1172/JCI21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Bruning JC, Winnay J, Cheatham B, Kahn CR. Differential signaling by insulin receptor substrate 1 (IRS-1) and IRS-2 in IRS-1-deficient cells. Mol Cell Biol. 1997;17(3):1513–21. doi: 10.1128/mcb.17.3.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Martinez SC, Tanabe K, Cras-Meneur C, Abumrad NA, Bernal-Mizrachi E, Permutt MA. Inhibition of Foxol protects pancreatic islet beta-cells against fatty acid and endoplasmic reticulum stress-induced apoptosis. Diabetes. 2008;57(4):846–859. doi: 10.2337/db07-0595. [DOI] [PubMed] [Google Scholar]
- 445.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124(3):471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 446.Mori H, Inoki K, Opland D, Muenzberg H, Villanueva EC, Faouzi M, Ikenoue T, Kwiatkowski D, Macdougald OA, Myers MG, Jr, Guan KL. Critical roles for the TSC-mTOR pathway in {beta}-cell function. Am J Physiol Endocrinol Metab. 2009 doi: 10.1152/ajpendo.00262.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10(3):457–468. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- 448.Briaud IM, Lingohr MK, Dickson LM, McCuaig JF, Lawrence JC, Rhodes CJ. IRS-2 proteasomal degradation mediated by a mTOR-induced negative feedback downregulates PKB-mediated signalling pathway in beta-cells. Diabetologia. 2004;47:A26–A26. doi: 10.1074/jbc.M412179200. [DOI] [PubMed] [Google Scholar]
- 449.Jhala US, Canettieri G, Screaton RA, Kulkarni RN, Krajewski S, Reed J, Walker J, Lin X, White M, Montminy M. cAMP promotes pancreatic beta-cell survival via CREB-mediated induction of IRS2. Genes Dev. 2003;17(13):1575–80. doi: 10.1101/gad.1097103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Olson LK, Redmon JB, Towle HC, Robertson RP. Chronic Exposure of Hit Cells to High Glucose-Concentrations Paradoxically Decreases Insulin Gene-Transcription and Alters Binding of Insulin Gene Regulatory Protein. J Clin Invest. 1993;92(1):514–519. doi: 10.1172/JCI116596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Park KG, Lee KM, Seo HY, Suh JH, Kim HS, Wang L, Won KC, Lee HW, Park JY, Lee KU, Kim JG, Kim BW, Choi HS, Lee IK. Glucotoxicity in the INS-1 rat insulinoma cell line is mediated by the orphan nuclear receptor small heterodimer partner. Diabetes. 2007;56(2):431–7. doi: 10.2337/db06-0753. [DOI] [PubMed] [Google Scholar]
- 452.Olson LK, Sharma A, Peshavaria M, Wright CVE, Towle HC, Robertson RP, Stein R. Reduction of Insulin Gene-Transcription in Hit-T15 Beta-Cells Chronically Exposed to a Supraphysiological Glucose-Concentration Is Associated with Loss of Stf-1 Transcription Factor Expression (Vol 92, Pg 9127, 1995) Proc Natl Acad Sci U S A. 1995;92(24):11322–11322. doi: 10.1073/pnas.92.20.9127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Sharma A, Olson LK, Robertson RP, Stein R. The Reduction of Insulin Gene-Transcription in Hit-T15 Beta-Cells Chronically Exposed to High Glucose-Concentration Is Associated with the Loss of Ripe3b1 and Stf-1 Transcription Factor Expression. Mol Endocrinol. 1995;9(9):1127–1134. doi: 10.1210/mend.9.9.7491105. [DOI] [PubMed] [Google Scholar]
- 454.Poitout V, Olson LK, Robertson RP. Chronic exposure of beta TC-6 cells to supraphysiologic concentrations of glucose decreases binding of the RIPE3b1 insulin gene transcription activator. J Clin Invest. 1996;97(4):1041–1046. doi: 10.1172/JCI118496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Lu M, Seufert J, Habener JF. Pancreatic beta-cell-specific repression of insulin gene transcription by CCAAT enhancer-binding protein beta - Inhibitory interactions with basic helix-loop-helix transcription factor E47. J Biol Chem. 1997;272(45):28349–28359. doi: 10.1074/jbc.272.45.28349. [DOI] [PubMed] [Google Scholar]
- 456.Seufert J, Weir GC, Habener JF. Differential expression of the insulin gene transcriptional repressor CCAAT/enhancer-binding protein beta and transactivator islet duodenum homeobox-1 in rat pancreatic beta cells during the development of diabetes mellitus. J Clin Invest. 1998;101(11):2528–2539. doi: 10.1172/JCI2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Kharroubi I, Ladriere L, Cardozo AK, Cnop M, Eizirik DL. Free fatty acids and cytokines induce pancreatic beta cell apoptosis by different mechanisms: role of NF-kappa B and endoplasmic reticulum stress. Diabetologia. 2004;47:A176–A176. doi: 10.1210/en.2004-0478. [DOI] [PubMed] [Google Scholar]
- 458.Eizirik DL. Interleukin-1 Induced Impairment in Pancreatic-Islet Oxidative-Metabolism of Glucose Is Potentiated by Tumor Necrosis Factor. Acta Endocrinologica. 1988;119(3):321–325. doi: 10.1530/acta.0.1190321. [DOI] [PubMed] [Google Scholar]
- 459.Prentki M, Corkey BE. Are the beta-cell signaling molecules malonyl-CoA and cystolic long-chain acyl-CoA implicated in multiple tissue defects of obesity and NIDDM? Diabetes. 1996;45(3):273–83. doi: 10.2337/diab.45.3.273. [DOI] [PubMed] [Google Scholar]
- 460.Roche E, Farfari S, Witters LA, Assimacopoulos-Jeannet F, Thumelin S, Brun T, Corkey BE, Saha AK, Prentki M. Long-term exposure of beta-INS cells to high glucose concentrations increases anaplerosis, lipogenesis, and lipogenic gene expression. Diabetes. 1998;47(7):1086–94. doi: 10.2337/diabetes.47.7.1086. [DOI] [PubMed] [Google Scholar]
- 461.Ruderman N, Prentki M. AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat Rev Drug Discov. 2004;3(4):340–51. doi: 10.1038/nrd1344. [DOI] [PubMed] [Google Scholar]
- 462.Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003;144(12):5179–83. doi: 10.1210/en.2003-0982. [DOI] [PubMed] [Google Scholar]
- 463.Salt IP, Johnson G, Ashcroft SJ, Hardie DG. AMP-activated protein kinase is activated by low glucose in cell lines derived from pancreatic beta cells, and may regulate insulin release. Biochem J. 1998;335 ( Pt 3):533–9. doi: 10.1042/bj3350533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Wang X, Zhou L, Li G, Luo T, Gu Y, Qian L, Fu X, Li F, Li J, Luo M. Palmitate activates AMP-activated protein kinase and regulates insulin secretion from beta cells. Biochem Biophys Res Commun. 2007;352(2):463–8. doi: 10.1016/j.bbrc.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 465.Foufelle F, Ferre P. New perspectives in the regulation of hepatic glycolytic and lipogenic genes by insulin and glucose: a role for the transcription factor sterol regulatory element binding protein-1c. Biochem J. 2002;366(Pt 2):377–91. doi: 10.1042/BJ20020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Shimabukuro M, Higa M, Zhou YT, Wang MY, Newgard CB, Unger RH. Lipoapoptosis in beta-cells of obese prediabetic fa/fa rats. Role of serine palmitoyltransferase overexpression. J Biol Chem. 1998;273(49):32487–90. doi: 10.1074/jbc.273.49.32487. [DOI] [PubMed] [Google Scholar]
- 467.Moore PC, Ugas MA, Hagman DK, Parazzoli SD, Poitout V. Evidence against the involvement of oxidative stress in fatty acid inhibition of insulin secretion. Diabetes. 2004;53(10):2610–6. doi: 10.2337/diabetes.53.10.2610. [DOI] [PubMed] [Google Scholar]
- 468.Cnop M, Hannaert JC, Grupping AY, Pipeleers DG. Low density lipoprotein can cause death of islet beta-cells by its cellular uptake and oxidative modification. Endocrinology. 2002;143(9):3449–53. doi: 10.1210/en.2002-220273. [DOI] [PubMed] [Google Scholar]
- 469.Sako Y, Grill VE. A 48-Hour Lipid Infusion in the Rat Time-Dependently Inhibits Glucose-Induced Insulin-Secretion and B-Cell Oxidation through a Process Likely Coupled to Fatty-Acid Oxidation. Endocrinology. 1990;127(4):1580–1589. doi: 10.1210/endo-127-4-1580. [DOI] [PubMed] [Google Scholar]
- 470.Elks ML. Chronic perifusion of rat islets with palmitate suppresses glucose-stimulated insulin release. Endocrinology. 1993;133(1):208–14. doi: 10.1210/endo.133.1.8319569. [DOI] [PubMed] [Google Scholar]
- 471.Zhou YP, Grill V. Long term exposure to fatty acids and ketones inhibits B-cell functions in human pancreatic islets of Langerhans. J Clin Endocrinol Metab. 1995;80(5):1584–90. doi: 10.1210/jcem.80.5.7745004. [DOI] [PubMed] [Google Scholar]
- 472.Zhou YP, Grill VE. Long-Term Exposure of Rat Pancreatic-Islets to Fatty-Acids Inhibits Glucose-Induced Insulin-Secretion and Biosynthesis through a Glucose Fatty-Acid Cycle. J Clin Invest. 1994;93(2):870–876. doi: 10.1172/JCI117042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Briaud I, Kelpe CL, Johnson LM, Tran PO, Poitout V. Differential effects of hyperlipidemia on insulin secretion in islets of langerhans from hyperglycemic versus normoglycemic rats. Diabetes. 2002;51(3):662–8. doi: 10.2337/diabetes.51.3.662. [DOI] [PubMed] [Google Scholar]
- 474.Chan CB, De Leo D, Joseph JW, McQuaid TS, Ha XF, Xu F, Tsushima RG, Pennefather PS, Salapatek AM, Wheeler MB. Increased uncoupling protein-2 levels in beta-cells are associated with impaired glucose-stimulated insulin secretion: mechanism of action. Diabetes. 2001;50(6):1302–10. doi: 10.2337/diabetes.50.6.1302. [DOI] [PubMed] [Google Scholar]
- 475.Schmitz-Peiffer C, Laybutt DR, Burchfield JG, Gurisik E, Narasimhan S, Mitchell CJ, Pedersen DJ, Braun U, Cooney GJ, Leitges M, Biden TJ. Inhibition of PKCepsilon improves glucose-stimulated insulin secretion and reduces insulin clearance. Cell Metab. 2007;6(4):320–8. doi: 10.1016/j.cmet.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 476.Olofsson CS, Collins S, Bengtsson M, Eliasson L, Salehi A, Shimomura K, Tarasov A, Holm C, Ashcroft F, Rorsman P. Long-term exposure to glucose and lipids inhibits glucose-induced insulin secretion downstream of granule fusion with plasma membrane. Diabetes. 2007;56(7):1888–97. doi: 10.2337/db06-1150. [DOI] [PubMed] [Google Scholar]
- 477.Hoppa MB, Collins S, Ramracheya R, Hodson L, Amisten S, Zhang Q, Johnson P, Ashcroft FM, Rorsman P. Chronic palmitate exposure inhibits insulin secretion by dissociation of Ca(2+) channels from secretory granules. Cell Metab. 2009;10(6):455–65. doi: 10.1016/j.cmet.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307(5708):384–7. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 479.Anello M, Lupi R, Spampinato D, Piro S, Masini M, Boggi U, Del Prato S, Rabuazzo AM, Purrello F, Marchetti P. Functional and morphological alterations of mitochondria in pancreatic beta cells from type 2 diabetic patients. Diabetologia. 2005;48(2):282–9. doi: 10.1007/s00125-004-1627-9. [DOI] [PubMed] [Google Scholar]
- 480.Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, Hagen T, Vidal-Puig AJ, Boss O, Kim YB, Zheng XX, Wheeler MB, Shulman GI, Chan CB, Lowell BB. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001;105(6):745–755. doi: 10.1016/s0092-8674(01)00378-6. [DOI] [PubMed] [Google Scholar]
- 481.Schiff M, Loublier S, Coulibaly A, Benit P, de Baulny HO, Rustin P. Mitochondria and diabetes mellitus: untangling a conflictive relationship? J Inherit Metab Dis. 2009;32(6):684–98. doi: 10.1007/s10545-009-1263-0. [DOI] [PubMed] [Google Scholar]
- 482.Maechler P, Wollheim CB. Mitochondrial function in normal and diabetic beta-cells. Nature. 2001;414(6865):807–12. doi: 10.1038/414807a. [DOI] [PubMed] [Google Scholar]
- 483.Krauss S, Zhang CY, Scorrano L, Dalgaard LT, St-Pierre J, Grey ST, Lowell BB. Superoxide-mediated activation of uncoupling protein 2 causes pancreatic beta cell dysfunction. J Clin Invest. 2003;112(12):1831–42. doi: 10.1172/JCI19774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Mulder H, Ling C. Mitochondrial dysfunction in pancreatic beta-cells in Type 2 diabetes. Mol Cell Endocrinol. 2009;297(1–2):34–40. doi: 10.1016/j.mce.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 485.Toye AA, Lippiat JD, Proks P, Shimomura K, Bentley L, Hugill A, Mijat V, Goldsworthy M, Moir L, Haynes A, Quarterman J, Freeman HC, Ashcroft FM, Cox RD. A genetic and physiological study of impaired glucose homeostasis control in C57BL/6J mice. Diabetologia. 2005;48(4):675–86. doi: 10.1007/s00125-005-1680-z. [DOI] [PubMed] [Google Scholar]
- 486.Freeman H, Shimomura K, Horner E, Cox RD, Ashcroft FM. Nicotinamide nucleotide transhydrogenase: a key role in insulin secretion. Cell Metab. 2006;3(1):35–45. doi: 10.1016/j.cmet.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 487.Trifunovic A, Larsson NG. Mitochondrial dysfunction as a cause of ageing. J Intern Med. 2008;263(2):167–178. doi: 10.1111/j.1365-2796.2007.01905.x. [DOI] [PubMed] [Google Scholar]
- 488.Seo HY, Kim YD, Lee KM, Min AK, Kim MK, Kim HS, Won KC, Park JY, Lee KU, Choi HS, Park KG, Lee IK. Endoplasmic reticulum stress-induced activation of activating transcription factor 6 decreases insulin gene expression via up-regulation of orphan nuclear receptor small heterodimer partner. Endocrinology. 2008;149(8):3832–41. doi: 10.1210/en.2008-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Mori K. Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell. 2000;101(5):451–4. doi: 10.1016/s0092-8674(00)80855-7. [DOI] [PubMed] [Google Scholar]
- 490.Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell. 1999;10(11):3787–3799. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Shen X, Zhang K, Kaufman RJ. The unfolded protein response--a stress signaling pathway of the endoplasmic reticulum. J Chem Neuroanat. 2004;28(1–2):79–92. doi: 10.1016/j.jchemneu.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 492.Barone MV, Crozat A, Tabaee A, Philipson L, Ron D. Chop (Gadd153) and Its Oncogenic Variant, Tls-Chop, Have Opposing Effects on the Induction of G(1)/S Arrest. Genes & Development. 1994;8(4):453–464. doi: 10.1101/gad.8.4.453. [DOI] [PubMed] [Google Scholar]
- 493.Korennykh AV, Egea PF, Korostelev AA, Finer-Moore J, Zhang C, Shokat KM, Stroud RM, Walter P. The unfolded protein response signals through high-order assembly of Ire1. Nature. 2009;457(7230):687–U2. doi: 10.1038/nature07661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Wheeler E, Barroso I. Genome-wide association studies and type 2 diabetes. Brief Funct Genomics. 2011;10(2):52–60. doi: 10.1093/bfgp/elr008. [DOI] [PubMed] [Google Scholar]
- 495.Frayling TM. Genome-wide association studies provide new insights into type 2 diabetes aetiology. Nat Rev Genet. 2007;8(9):657–62. doi: 10.1038/nrg2178. [DOI] [PubMed] [Google Scholar]
- 496.Gloyn AL, Weedon MN, Owen KR, Turner MJ, Knight BA, Hitman G, Walker M, Levy JC, Sampson M, Halford S, McCarthy MI, Hattersley AT, Frayling TM. Large-scale association studies of variants in genes encoding the pancreatic beta-cell KATP channel subunits Kir6. 2 (KCNJ11) and SUR1 (ABCC8) confirm that the KCNJ11 E23K variant is associated with type 2 diabetes. Diabetes. 2003;52(2):568–72. doi: 10.2337/diabetes.52.2.568. [DOI] [PubMed] [Google Scholar]
- 497.Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, Helgason A, Stefansson H, Emilsson V, Helgadottir A, Styrkarsdottir U, Magnusson KP, Walters GB, Palsdottir E, Jonsdottir T, Gudmundsdottir T, Gylfason A, Saemundsdottir J, Wilensky RL, Reilly MP, Rader DJ, Bagger Y, Christiansen C, Gudnason V, Sigurdsson G, Thorsteinsdottir U, Gulcher JR, Kong A, Stefansson K. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38(3):320–3. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- 498.Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445(7130):881–5. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 499.Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU, Prokunina-Olsson L, Ding CJ, Swift AJ, Narisu N, Hu T, Pruim R, Xiao R, Li XY, Conneely KN, Riebow NL, Sprau AG, Tong M, White PP, Hetrick KN, Barnhart MW, Bark CW, Goldstein JL, Watkins L, Xiang F, Saramies J, Buchanan TA, Watanabe RM, Valle TT, Kinnunen L, Abecasis GR, Pugh EW, Doheny KF, Bergman RN, Tuomilehto J, Collins FS, Boehnke M. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316(5829):1341–5. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Steinthorsdottir V, Thorleifsson G, Reynisdottir I, Benediktsson R, Jonsdottir T, Walters GB, Styrkarsdottir U, Gretarsdottir S, Emilsson V, Ghosh S, Baker A, Snorradottir S, Bjarnason H, Ng MC, Hansen T, Bagger Y, Wilensky RL, Reilly MP, Adeyemo A, Chen Y, Zhou J, Gudnason V, Chen G, Huang H, Lashley K, Doumatey A, So WY, Ma RC, Andersen G, Borch-Johnsen K, Jorgensen T, van Vliet-Ostaptchouk JV, Hofker MH, Wijmenga C, Christiansen C, Rader DJ, Rotimi C, Gurney M, Chan JC, Pedersen O, Sigurdsson G, Gulcher JR, Thorsteinsdottir U, Kong A, Stefansson K. A variant in CDKAL1 influences insulin response and risk of type 2 diabetes. Nat Genet. 2007;39(6):770–5. doi: 10.1038/ng2043. [DOI] [PubMed] [Google Scholar]