Abstract
Background
Delivery of effective treatment for pediatric solid tumors poses a particular challenge to centers in middle-income countries (MIC) already vigorously addressing pediatric cancer. This study aimed to improve our understanding of barriers to effective treatment of pediatric solid tumors in MICs.
Methods
An ecological model centering in pediatric sarcoma and expanding to country as the environment were used as benchmark for studying delivery of solid tumor care in MICs. Data on resources was gathered from seven centers members of the Central American Association of Pediatric Hematologists and Oncologists (AHOPCA) using an infrastructure assessment tool. Pediatric sarcoma outcomes data was available and retrieved from hospital-based cancer registries for six of the seven centers and analyzed by country. Patients diagnosed from 1/1/2000-12/31/2009 with osteosarcoma, Ewing sarcoma, rhabdomyosarcoma, and other soft tissue sarcomas were included in the analysis. In order to explore correlations between resources and outcomes a pilot performance-index was created.
Findings
Results identified specific human resources, communication, quality and infrastructure deficits. Treatment abandonment rate, metastatic disease at diagnosis, relapse rate and 4-year abandonment-sensitive overall survival (AOS) varied considerably by country (1-38%, 15-54%, 24-52%, 21-51%, respectively). Treatment abandonment rate correlated inversely with health economic expenditure per capita (r= −0.86, p=0.03) and life expectancy at birth (r = −0.93, p=0.007). Four-year AOS correlated inversely with under-5 mortality rate (r= −0.80, p=0.05) and directly with the pilot performance-index (r =0.98, p=0.005).
Interpretation
Initiatives to improve treatment effectiveness of pediatric solid tumor care in MIC and pediatric sarcoma in particular are warranted. Building capacity and infrastructure, improving supportive care and communication, and fostering comprehensive multidisciplinary teams are identified as keystones in Central America. A measure that meaningfully describes performance in delivering pediatric cancer care is feasible and needed to advance comparative prospective analysis of pediatric cancer care and define resource-clusters internationally.
Keywords: Pediatric sarcoma, Childhood cancer, Developing countries, Middle-income countries, Treatment abandonment, Survival analysis, Outcomes research, Composite indicator, Health services research
INTRODUCTION
There is growing interest in improving our understanding of cancer disparities around the world1-3 and addressing the high burden of cancer-related mortality faced by low- and middle-income countries (LMC)4. Pediatric cancer is no exception, since 80% of children with cancer live in LMC5. In many countries, as standards of living improve and millennium development goals are achieved, the burden of cancer becomes more tangible6. However, the purchasing capacity and the allocation of needed technologies and clinical skills may be lagging.
Delivery of effective treatment for pediatric solid tumors poses a particular challenge to middle-income countries (MIC) already vigorously addressing pediatric cancer. Successful frameworks for improving outcomes for patients with leukemia in resource-poor settings have been developed7; however, these models may not be directly applicable to solid tumors. Experience from development of pediatric brain tumor programs in MICs has demonstrated the importance of multidisciplinary care, empowering the care team, adhering to protocols, telemedicine and the twinning model8-12. Similar to brain tumors, pediatric extra-cranial solid neoplasms are a heterogeneous group of malignancies with very specific therapeutic principles by disease and risk group, which share an inherent need for meticulous comprehensive multidisciplinary care.
The Central American Association of Pediatric Hematologists and Oncologists (AHOPCA) offers a unique opportunity to look at the interface between resource-availability and pediatric solid tumor outcomes. AHOPCA has a successful track-record in delivery of protocol-based treatment13-18 and has prospectively collected outcomes in POND (Pediatric Oncology Networked Database)19, 20. However, despite parallel development of pediatric leukemia and solid tumor programs, improvements in outcomes for children with solid tumors have lagged behind those seen in leukemia13, 14, 21. Infrastructure shortfalls and difficulty with implementation of multidisciplinary care are thought to be highly influential.
This study aimed to improve our understanding of barriers to effective treatment of pediatric solid tumors in MICs. An ecologic conceptual framework was used as benchmark for studying delivery of solid tumor care, identifying non-biologic factors of interest, and illustrating key components of comprehensive multidisciplinary care. In the absence of an established measure, associations between resources and outcomes were explored in a “proof-of-principle” exercise through a pilot performance-index. Results aimed to inform development of targeted strategies to address identified system-level barriers to pediatric cancer care in MIC and set the stage for further studies on the interface between non-biologic factors and outcomes.
METHODS
Conceptual framework
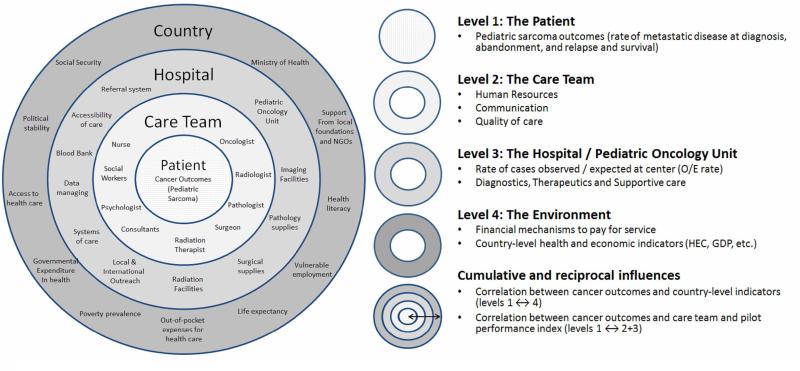
A four-level ecological model was used to guide data collection and analysis. The model ascends from patient to country and seeks to illustrate influences between the levels (see Figure 1).
Figure 1. Conceptual Framework.
Ecological model of pediatric solid tumor care, non-biological factors of interest, and components of the analysis at each level of the framework
Level 1, The Patients
Shortage of national cancer registries in LMC documenting survival has limited the study of regional differences in pediatric cancer outcomes22. We recently reported on pediatric sarcoma outcomes diagnosed between Jan/1/2000-Dec/31/2009 for six of the seven AHOPCA-member countries (all except Dominican Republic)23. Data had been previously collected and stored in hospital-based cancer registries using POND19, 20; a web-based, password protected, data management tool provided free of charge to centers in the developing world and managed by St. Jude Children's Research Hospital International Outreach Program. The diagnoses included were osteosarcoma (VIIIa), Ewing sarcoma (VIIIc), rhabdomyosarcoma (IXa) and other soft-tissue sarcomas (VIIIb, IXa, IXd). Number in parenthesis represents the International Classification of Childhood Cancer diagnostic group for each diagnosis, based on its third edition (ICCC-3)24. International Classification of Disease (ICD) codes were not available in the database. Primary outcomes included rate of metastatic disease at presentation, treatment abandonment, and relapse, and survival23. This same population was selected for this study, but data were analyzed by country and survival analysis was updated to reflect follow-up through January 1, 2013 (see Table I).
Table I.
Pediatric sarcoma outcomes of interest (Level 1) by country.
| UMIC | LMIC | |||||||
|---|---|---|---|---|---|---|---|---|
| HNN, Costa Rica |
HNP, Panama |
HRRC, Dominican Republic |
HNNBB, El Salvador |
UNOP, Guatemala |
HMI, Honduras |
HILM, Nicaragua |
Summary statistic* |
|
| Level 1: Pediatric Sarcoma Outcomes | ||||||||
| Number of cases reported (2000-2009) | 115 | 64 | NA | 102 | 259 | 94 | 151 | 785 |
| Number cases in children <15 y/o | 113 | 64 | NA | 98 | 217 | 77 | 133 | 702 |
| Age median (range) in years) | 9.5 (0.1-17) | 9.2 (0.1-15) | NA | 8.4 (0.3-17) | 10.8 (0.3-18) | 10.8 (0.3-18)** | 11.7 (0.3-19) | 10.2 (0.1-24) |
| Metastatic disease at diagnosis (for cohort) | 15% | 54% | NA | 19% | 38% | 36% | 42% | 37% |
| 2000-2004 (n=304) | 19% | 56% | NA | 20% | 41% | 30% | 41% | 36%# |
| 2005-2009 (n=418) | 13% | 53% | NA | 19% | 34% | 38% | 43% | 32%# |
| Treatment abandonment rate (as first event) | 1% | 11% | NA | 28% | 22% | 38% | 21% | 21% |
| 2000-2004 (n=323) | 2% | 14% | NA | 25% | 21% | 71%# | 24% | 23%# |
| 2005-2009 (n=462) | 0% | 9% | NA | 30% | 22% | 23%# | 19% | 19%# |
| Relapse/Progressive disease (as first event, excluding abandonment) | 52% | 40% | NA | 36% | 50% | 24% | 36% | 38% |
| 2000-2004 (n=248) | 66%# | 42% | NA | 67%# | 63% | 38% | 50% | 58.5%# |
| 2005-2009 (n=373) | 43%# | 48% | NA | 41%# | 64% | 39% | 44% | 49.1%# |
| 4-year Overall Survival (OS) | 51% | 22% | NA | 49% | 30% | 51% | 34% | 40% |
| 4-year Abandonment-sensitive OS (AOS) | 51% | 22% | NA | 37% | 25% | 21% | 29% | 32% |
| 2000-2004 (n=323) | 43% | 25% | NA | 36% | 28% | 15% | 26% | 30% |
| 2005-2009 (n=462) | 57% | 19% | NA | 37% | 23% | 24% | 30% | 33% |
| 4-year AOS for localized disease | 54% | 45% | NA | 39% | 39% | 33% | 42% | 38.0% |
| 4-year Event Free Survival (EFS) | 42% | 20% | NA | 36% | 25% | 41% | 31% | 33% |
| 4-year Abandonment-sensitive EFS (AEFS) | 42% | 20% | NA | 28% | 21% | 18% | 27% | 26% |
Sum for cases count, median for age and overall result for rates and survival estimates.
Two patients in Honduras with osteosarcoma were treated at at 21 and 23 years of age, range otherwise as reported in table.
Denotes difference is significant (p≤0.05).
Level 2, The Care Team
Data for levels 2-3 (the care team and the centers) was obtained using an exploratory tool, first during January-June 2010, then updated January-June 2011. Pediatric oncologists members of AHOPCA and involved in treatment of solid tumors, served as liaisons for content validity and data collection at their centers. Items were asked by discipline, but analyzed by level and component in the framework. Regarding level 2 (The Care Team), the tool addressed human resources (32 questions), communication (8), and perceived quality of care (17). Free-text comments were allowed and are reported along with objective data for each section.
Level 3, The Centers
Seven AHOPCA-member centers were analyzed: Hospital Nacional de Niños (HNN), Costa Rica, Hospital Nacional de Niños Benjamin Bloom (HNNBB), El Salvador, Unidad Nacional de Oncologia Pediatrica (UNOP), Guatemala, Hospital Materno Infantil (HMI), Honduras, Hospital Infantil “La Mascota”(HILM), Nicaragua, Hospital del Niño de Panama (HNP), Panama, and Hospital Roberto Reid Cabral (HRRC), Dominican Republic. These centers are designated public national referral centers for pediatric oncology care. However, in some countries patients may alternatively access care at satellite centers, private institutions, or social security hospitals. Under-diagnosis, under-referral, and efflux to other countries may also occur. This is the case, for example, in the Dominican Republic. An oncology institute and private hospitals (which primarily sees adults, but accept children) exist, as well as a second public pediatric oncology unit in Santiago (second largest city in the country, north-west of Santo Domingo). The expected number of children with cancer who are registered in POND in the AHOPCA-member countries has been estimated by other authors to range from 25 to 100% using the observed registrations of patients under 15 years of age from 2004 to 2007 and the expected incidence of pediatric cancer of 100 cases per million under 15 years of age (25% in Dominican Republic, 35% in Panama, 50% in Guatemala, 62% in Honduras, 77% in El Salvador, and 100% in Costa Rica).25
As designated public national referral centers, the seven AHOPCA-centers included were deemed representative and therefore appropriate for the analysis of resources in the public sector in each country. Infrastructure data from the Dominican Republic was analyzed despite lack of matching outcomes data because it was felt to enrich and balance the granularity of comparative data between upper-middle-income countries (UMIC) and lower-middle-income countries (LMIC). Finally, in the absence of national cancer registries in all countries except Costa Rica, data in POND from the remaining six AHOPCA-centers was considered representative and appropriate for the analysis of outcomes in the public sector in each country. Regarding level 3, infrastructure-availability was considered of interest and the tool addressed diagnostics (8 questions), therapeutics (15), and supportive care (27).
Case capture rate was evaluated using rate of cases observed vs. expected (O/E rate). Costa Rica is the only AHOPCA-member country with a national cancer registry26. Therefore, O/E rates for pediatric sarcoma were calculated using average of observed cases between 2005-2009, population statistics for 2009, and reported incidence of pediatric sarcoma in Costa Rica (1984-1992 collection period: 9.7 cases/million)26; all based on data for children 0-15 years. Costa Rica has about 4,000,000 inhabitants, of whom about 28% are under 15 years of age (about 1,200,000). O/E rate for childhood cancer was similarly calculated using incidence data from Costa Rica (136 cases/million)26. These incidences are lower than in high-income countries26, but higher than in other Latin American countries5. Therefore, they may overestimate or underestimate the true O/E rate for other countries in the region. Stability in the incidence of childhood cancers in Costa Rica for 1984-1992 (collection period with data available by ICCC-3 diagnosis group), compared to 1998-2002 (the most recent collection period), was confirmed by searching the Cancer Incidence in Five Continents (CI5) online database for males and females 0-14 years of age, for “all sites” (C00-96) and “bone” (C40-41)27. However, incidence rates from the CI5's most recent collection period could not be used to obtain the expected incidence for males and females with pediatric sarcoma needed for this study because cancers are reported exclusively by topography, rather than by diagnostic group. Bone topography (C40-41) can be selected to represent “bone tumors”, but rhabdomyosarcomas, soft tissue sarcomas and nonosseous Ewing sarcomas, which can occur in a multitude of topographical sites can't be selected and therefore their observed incidence can't be retrieved.
Level 4, The Environment
Country-level health and economic indicators and financial mechanisms used to pay for services were considered of interest to evaluate the environment AHOPCA-member centers face while delivering care. Established indicators of interest were obtained from the World Bank online database28. Applying the World Bank classification by country income-group29, Costa Rica, Panama and Dominican Republic are classified as upper middle-income countries (UMIC) and El Salvador, Guatemala, Honduras and Nicaragua into lower middle-income countries (LMIC). The center's reliance on foundations, the public health care system, and out-of-pocket expenditures was qualitatively assessed in the assessment tool.
Correlations and development of the pilot performance index
An indicator reflecting hospital- or country-level performance in delivery of cancer care does not exist. Among surrogate indicators previously analyzed, annual governmental health care expenditure per capita (HEC)30 has shown the best correlation with pediatric cancer survival22. This and other country-level indicators were tested for correlation with pediatric sarcoma outcomes.
In order to evaluate performance, 40 items from levels 2 and 3 were selected and aggregated into a pilot performance-index. This was a proof-of-principle exercise, done to see if the incorporated multitude of non-biological factors could be aggregated and analyzed in a meaningful way. Composite indicators have become more popular and accepted for the assessment of global health and guidelines for their development published31, 32. However, a cancer-oriented composite indicator has not been developed32. In the case of pediatric extra-cranial solid tumors, such an indicator could help define the resources of interest, allow for comparative research between countries, and allow for longitudinal monitoring of acquisition of better infrastructure and its impact on survival. In the composite indicator literature, item selection and valuation (weighting) is perhaps most controversial31, 33, 34.
As expected, the proportion between level 2-3 items (108) and centers (7) limited the use of regression or factor analysis to identify significant items. Although alternative approaches for establishment of priorities and weights for composite indicators have been published35, 36, addressing the above-mentioned controversy was beyond the scope of his study. Therefore, items were selected by two investigators (PF and CR-G) based on probable sensitivity as a screening question and the list revised and approved by all other investigators. Items were selected using the investigators’ best judgment, based on their individual experience practicing pediatric oncology in high- and well as lower-income settings. Furthermore, in the absence of quantitative methods to back-up a gradation strategy, all items were weighted equally. This somewhat ad-hoc process has been successfully applied in the early stages of development of other composite indicators37, 38. With regards to the items, no AHOPCA-member center reported access to all items selected as “of interest”, but all the incorporated items were present in at least one center. -Items selected to be included in the pilot performance-index are marked with asterisk in Figure 3. Cross-sectional correlation of survival for 2000-2009 and index scores for 2009 was considered adequate because pediatric sarcoma outcomes failed to show significant improvement within the study period (see Table I). Therefore, cross-sectional correlation presumably offers the best-powered survival estimate and the best-case scenario for each country.
Figure 3. Resource-availability assessment by country for Level 2 - The Care Team, left, and Level 3 - The Centers, right.
Assessment of the care team focused on A) Human resources, B) Communication, and C) Quality of care. Assessment of the centers involved evaluation of infrastructure available with focus on D) Diagnostics, E) Therapeutics, and F) Supportive care. Counts represent the number of countries with affirmative answer, among 7 countries analyzed. Items with asterix (*) were incorporated into the pilot performance index. Dom. Rep= Dominican Republic. RTx = Radiation therapist. XRT = Radiation therapy. Ø= Not available in any country.
Data analysis
Survival analysis included standard as well as abandonment-sensitive overall (OS and AOS) and event-free survival (EFS and AEFS)23. Refusal and abandonment were analyzed as one event (“abandonment”) as recommended by the International Society of Pediatric Oncology Working Group on Treatment Abandonment.39 Four survival analyses were performed: 1) Event-free survival (EFS) with events defined as relapse, progressive disease, secondary malignancy, or death; 2) Abandonment-sensitive event-free survival (AEFS) with events defined as abandonment of therapy, relapse, progressive disease, secondary malignancy, or death; 3) Overall survival (OS) with event defined as death, and 4) Abandonment-sensitive overall survival (AOS) with events defined as abandonment and death. Time to event was calculated from the time of diagnosis until the first event or until last contact if no event occurred. The Kaplan-Meier method was used to obtain 4-year survival estimates and the Log Rank test to assess statistical significance. Descriptive methods summarized the care and infrastructure assessment. Categorical and ordinal items were converted to binary variables and analyzed by country's income-group using the World Bank classification29. The Pearson or Spearman method was used to test for correlation between variables depending on evaluation of the outcome on scatter plots. A p-value<0.05 was considered significant.
RESULTS
Level 1, The Patients
Center-level results for 785 cases of pediatric sarcoma diagnosed from 2000-2009 are presented in Table I and Figure 1. Rate of treatment abandonment ranged from 1% to 38%, metastatic disease at diagnosis ranged from 15% to 54%, and relapse/progressive disease as first event (which excludes patients who abandon therapy from the denominator) ranged from 24% to 52%. Rates for these outcomes decreased significantly by era in the region, but not at each center. Four-year AOS estimate for the region was 32%, but ranged from 21% to 51%. Standard methods overestimated four-year survival by up to 34% compared to abandonment-sensitive methods. Survival showed some improvement by era in all countries except in Panama and Guatemala; however, differences did not achieve statistical significance. Difference between AOS and AEFS was minimal.
Level 2, The Care Team
Human Resources
As seen in Table II and Figure 3A, all centers had fully trained pediatric oncologists, as well as other providers of interest. However, subspecialty training and/or pediatric experience was limited among pathology, surgical and radiation therapy providers. Pediatric oncology staff had large patient panels, particularly in LMIC (>50 new cancer cases/oncologist/year), and was not able to work exclusively at the public institution in 4 of 7 countries. Income inequalities between the private and public health sector were offered by providers as an explanation in the free-text comments. Pain and palliative care providers were available in five and three centers respectively. Legal or child protection teams were available in four centers, but reported to be only infrequently used in free-text comments.
Table II.
Characteristics of interest from the care team, centers and environment by country (levels 2-4)
| UMIC | LMIC | |||||||
|---|---|---|---|---|---|---|---|---|
| HNN, Costa Rica |
HNP, Panama |
HRRC, Dominican Republic |
HNNBB, El Salvador |
UNOP, Guatemala |
HMI, Honduras |
HILM, Nicaragua |
Summary statistic* |
|
| Total Population 0-14 years old (millions) | 1.15 | 1.00 | 3.04 | 2.03 | 5.89 | 2.76 | 2.00 | 17.87* |
| Level 2: Human resources (2010) | ||||||||
| Pediatric oncologists (ratio)** | 3 (1:33) | 2 (1:40) | 2 (1:32) | 3 (1:63) | 4(1:100) | 3 (1:102) | 3 (1:64) | 3 (1:72) |
| Social workers dedicated to oncology (ratio) | 2 (1:50) | 1 (1:80) | 1 (1:65) | 2 (1:95) | 2 (1:200) | 1 (1:305) | 1 (1:192) | 1 (1:165) |
| Psychology providers dedicated to oncology (ratio) | 2 (1:50) | 2 (1:40) | 1 (1:65) | 3 (1:63) | 4 (1:100) | 1 (1:305) | 1 (1:192) | 2 (1:135) |
| Pathology providers (ratio)** | 4 (1:25) | 2 (1:40) | 2 (1:32) | 3 (1:63) | 1 (1:400) | 5 (1:61) | 1 (1:192) | 2 (1:135) |
| Daytime nurse to patient ratio | 1:8 | 1:6 | NA | 1:7 | 1:5 | 1:7 | 1:10 | 1:7 |
| Nighttime nurse to patient ratio | 1:15 | 1:20 | NA | 1:7 | 1:5 | 1:10 | 1:10 | 1:9 |
| Level 3: Centers (O/E rate) | ||||||||
| Maximum age accepted at center (years) | 13 | 15 | 18 | 12 | 18 | 18 | 15 | 15 |
| O/E rate for all childhood cancers# | ||||||||
| Expected based on CR incidence rate | 156 | 136 | 413 | 276 | 801 | 375 | 272 | 2430* |
| Observed cases in 2009 | 100 | 80 | 65 | 190 | 300 | 305 | 192 | 1232* |
| O/E rate (vs. CR incidence) | 64% | 59% | 16% | 69% | 37% | 81% | 71% | 57% |
| O/E rate for pediatric sarcoma# | ||||||||
| Expected based on CR incidence rate | 11 | 10 | 29 | 20 | 57 | 26 | 19 | 173* |
| Observed cases** | 13 | 7 | NA | 12 | 28 | 13 | 18 | 92* |
| O/E rate (vs. CR incidence) | 120% | 73% | NA | 64% | 42% | 41% | 81% | 70% |
| Level 4: Countries | ||||||||
| Health expenditure per capita (current US$) | 668 | 591 | 271 | 229 | 186 | 117 | 105 | 229 |
| Gross national income, per capita (PPP int. $) | 6180 | 6550 | 4690 | 3310 | 2660 | 1790 | 1040 | 3310 |
| Life expectancy at birth, total (years) | 79 | 76 | 73 | 72 | 71 | 73 | 73 | 73 |
| Literacy rate, adult total (% of people≥15y) | 96 | 94 | 88 | 84 | 74 | 84 | 78 | 84 |
| Human development index (HDI) value | 0.738 | 0.76 | 0.68 | 0.669 | 0.569 | 0.619 | 0.582 | 0.669 |
| Mortality rate, under 5-years (per 1,000) | 10 | 21 | 28 | 18 | 33 | 25 | 28 | 25 |
| Prev. undernourishment (% of population) | 5 | 15 | 24 | 9 | 22 | 12 | 19 | 15 |
| Deaths among <5y from diarrheal illness (%)$ | 3.0 | 10.7 | 12.0 | 12.4 | 13.1 | 12.2 | 12.2 | 12 |
| Prevalence of HIV, total (% of pop. ages 15-49) | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| Poverty gap at $2 a day (PPP) (%) | 1 | 2 | 4 | 4 | 9 | 18 | 12 | 4 |
| Vulnerable employment, total (% of total) | 20 | 27 | 42 | 37 | 50 | 50 | 45 | 42 |
| Out-of-pocket health expenditure (% of total) | 29 | 24 | 39 | 35 | 56 | 36 | 40 | 36 |
| Economically active children, total (% of 7-14y) | 6 | 9 | 6 | 7 | 18 | 9 | 31 | 9 |
Column reports the median value, except for incidence and ‘Population aged 0-14 years’ rows, which are flagged with (*) and for which summary statistic reports the sum.
Ratios are for providers to new cancer patients at the centers. Pathologists were not exclusive for oncology.
Based on country's population in 2009 and incidence in Costa Rica (CR) of childhood cancer (136/million <15 years old) and pediatric sarcoma (9.6/million <15 years old).
**Due to low incidence and variability year by year, determined using average from 2005-2009.
Indicator from 2000.
Communication
Formal multidisciplinary care meetings and direct communication was pursued (Figure 3B). However, patients were infrequently assigned to a primary oncologist, communication was not always considered effective and meeting with radiation therapists was considered most challenging. Distance between pediatric oncology and radiation therapy centers was offered as main reason for the later in free-text comments.
Quality of care
Treatment delays were reported to occur more frequently due to bed-availability than chemotherapy-availability (Figure 3C). In most centers, detailed reporting of surgical and pathologic margins, urgent access to radiation services and proper timing of radiation services were reported to be inconsistently achieved.
Level 3, The Centers
O/E Rate
Based on population estimates, 2430 new cases of childhood cancer and 173 new cases of pediatric sarcoma would be expected in children <15 years old in the region each year (Table II). Variability in capture rates between centers was evidenced by the O/E rate range for all cancers and sarcomas. Pediatric sarcomas appeared overrepresented in Costa Rica only (120%) and markedly underrepresented in Guatemala and Honduras (42 and 41% respectively).
Diagnostics
Computed tomography and magnetic resonance imaging were available in all centers, but time-sensitive or urgent access was limited (Figure 3D). Regarding radiology, nuclear medicine services were limited the most and positron emission tomography (PET scan) was uniformly unavailable. Pathology services were available in all centers, but quality of slides, access to full panel of immunohistochemistry, and molecular studies were reported to be limited and of concern. Due to these limitations, all centers sought help from international collaborators in the evaluation of rare or complex cases.
Therapeutics
All centers had basic chemotherapy drugs and uniform treatment guidelines available (Figure 3E). Treatment of bone sarcomas was limited by inability to monitor methotrexate levels in real-time and cost. Access to limb sparing procedures (LSP) through international collaborations had recently increased, but was only consistently available in Costa Rica. Radiation therapy was available in all countries, but cobalt-based radiation was the only option in three of four LMIC.
Supportive care
Although pediatric intensive care units were uniformly available (Figure 3F), several centers expressed access was inadequate for oncology patients, in part due to bed availability, but also due to a sense of “fatalism” around the oncologic diagnosis. Permanent vascular devices were infrequently available and anesthesia services for imaging or radiation sometimes required the physician travel with the patient and provide the service. Prostheses were also infrequently available after amputation. Finally, limitations in access to blood products during therapy and surgery were reported, particularly regarding platelets.
Level 4, The Environment
The countries’ performance on established indicators
Guatemala had the largest pediatric population and ranked lowest for most indicators, except health care expenditure per capita, gross national income per capita, and rate of economically active children (Nicaragua lowest) and proportion of population living under $2/day (Honduras lowest). Dominican Republic had the highest percent of undernourishment (as % of population) and El Salvador the highest percent of deaths due to diarrheal illness. Costa Rica scored best in all health and economic indicators considered, except for gross national income per capita and human development index, for which Panama took the lead. Furthermore, Panama had lowest % out-of-pocket health expenditure. Of note, HIV prevalence was <1% in all countries analyzed and therefore HIV/AIDS was unlikely significantly burden the health care system.
Financing of care
All centers had support from strong local foundations. Through advocacy, local ministries of health and foundations had secured coverage of chemotherapy, supportive care medications, and surgery in all countries; with infrequent need for patient out-of-pocket contribution. However, a contribution by the patient was frequently required to cover cost of radiology services, radiation therapy, bone grafts, external prosthesis and rehabilitation services.
Correlations
Pilot performance index
Centers varied in terms of total score (range 35-80%), but most drastically by domains. Figure 4 shows distribution of index and domain scores and how they could be interpreted in meaningful ways. For example, in response to acknowledgement of deficits in human resources and communication, the AHOPCA-center in Guatemala initiated efforts to incorporate radiation therapists, surgeons, and other specialists with interest in pediatrics formally into their staff and implemented multidisciplinary care meetings with these subspecialties present. The total score for LMIC vs. UMIC was equal, but UMIC tended to have better infrastructure sub-scores and LMIC better communication sub-scores.
Figure 4.
Pilot performance-index scores by domain for each center and for lower vs. upper middle-income countries.
Correlation of patient outcomes with established indicators
Treatment abandonment rate correlated inversely with health economic expenditure per capita (r= −0.86, p=0.03) and life expectancy at birth (r =0.93, p=0.007) (see Table III). Rate of vulnerable employment showed strong correlation (r>0.75), but failed to achieve significance. O/E rate correlated significantly with several established indicators associated with better standards of living. AOS at 4-years correlated inversely with under-5 mortality rate (r=−0.80, p=0.05).
Table III.
Correlation of established indicators and pilot performance indicator with pediatric sarcoma outcomes in the six countries with outcomes data (analysis excludes Dominican Republic)
| Treatment abandonment* Pearson | Obs./Exp. Rate for sarcomas Spearman | Abandonment-sensitive OS* Spearman | ||||
|---|---|---|---|---|---|---|
| r | p | R | p | r | p | |
| Health expenditure per capita (current US$) | −0.86 | 0.03 | 0.94 | 0.005 | 0.55 | 0.25 |
| Gross national income, per capita | −0.74 | 0.09 | 0.89 | 0.02 | 0.20 | 0.70 |
| Mortality rate, under-5 (per 1,000) | 0.56 | 0.20 | 0.89 | 0.02 | −0.80 | 0.05 |
| Life expectancy at birth, total (years) | −0.93 | 0.007 | 0.67 | 0.15 | 0.62 | 0.19 |
| Literacy rate, adult total (% of people ages 15 and above)* | −0.74 | 0.09 | 0.93 | 0.008 | 0.49 | 0.33 |
| Human Development Index | −0.66 | 0.15 | 0.89 | 0.02 | 0.44 | 0.38 |
| Prevalence of undernourishment (% of population) | 0.35 | 0.50 | −0.77 | 0.07 | −0.72 | 0.10 |
| Deaths among <5y due to diarrheal illness (%) | 0.45 | 0.37 | −0.77 | 0.07 | −0.67 | 0.13 |
| Poverty gap at $2 a day (PPP) (%) | 0.53 | 0.28 | −0.83 | 0.04 | −0.63 | 0.18 |
| Vulnerable employment, total (% of total employment) | 0.78 | 0.07 | −0.81 | 0.05 | −0.69 | 0.12 |
| Out-of-pocket health expenditure (% of total expenditure on health) | 0.52 | 0.29 | −0.88 | 0.02 | −0.29 | 0.57 |
| Economically active children, total (% of children 7-14y) | 0.21 | 0.69 | −0.89 | 0.02 | −0.32 | 0.53 |
| Pilot Performance Index score (%) | −0.20 | 0.70 | 0.37 | 0.48 | 0.94 | 0.005 |
| Human Resources score | NA | NA | NA | NA | 0.98 | 0.0003 |
| Communication score | NA | NA | NA | NA | 0.41 | 0.42 |
| Quality of care score | NA | NA | NA | NA | 0.62 | 0.19 |
| Supportive care score | NA | NA | NA | NA | 0.93 | 0.008 |
| Diagnostics score | NA | NA | NA | NA | 0.78 | 0.07 |
| Therapeutics score | NA | NA | NA | NA | 0.35 | 0.49 |
Treatment abandonment rates improved over time, therefore rates from 2005-2009 were used in correlations. AOS did not improve over time and therefore rates for 2000-2009 were used for analysis (see methods).
Correlation of patient outcomes with the pilot performance-index
As seen in Table III, AOS at 4-years correlated most strongly with the pilot performance-index (r =0.98, p=0.005) and among the index's six subscales, human resources and supportive care scores showed independent significant correlation with AOS. Diagnostic capacities had strong correlation (r>0.75), but did not achieve significance. Figure 5 shows the comparison between the pilot index's total score, health expenditure per capita (HEC) and AOS survival, which reveals:
Some LMIC performed better than expected based on their HEC as in the case of El Salvador and Guatemala.
HEC and the pilot performance-index followed a similar trend, but correlation between HEC and AOS was not significant (r=0.55, p=0.25), likely due to large discrepancy between HEC, pilot score and AOS estimate in Panama.
Variability in outcomes was smaller than variability in index scores, particularly among lowest scores, therefore unaccounted factors may be limiting the center's capacity to compensate for their context.
Figure 5.
Pilot performance-index scores by center and their correlation with health care expenditure per capita (HEC, r=0.55, p=0.25) and pediatric sarcoma abandonment-sensitive overall survival (AOS, r=0.95, p=0.005) for the six countries in Central America with outcomes data (analysis excluded Dominican Republic).
DISCUSSION
Treatment of childhood cancer requires multidisciplinary care of high complexity; improved outcomes seen over the last decades in high-income countries (HIC) can only be interpreted under this premise, and the same principles must guide the development of pediatric cancer programs in countries with limited resources. As centers in MIC advance care in the management of children with cancer, we must understand unique features, identify strengths and limitations, and develop step-wise initiatives for rational capacity-building and improved outcomes.
In this study we aimed to improve our understanding of barriers to effective treatment of pediatric solid tumors in MIC. We selected children with sarcomas as the study population because outcomes for this group of patients reflect the complexity of multidisciplinary care and could thus be used as a surrogate to test the strengths and weaknesses of the health-care system. We assumed the impact of system-level barriers to care would be similar (or at least consonant) between pediatric sarcoma and other extra-cranial solid tumors because the effective treatment of most extra-cranial solid tumors requires meticulous comprehensive multidisciplinary care and a similar pool of human and infrastructure resources.
Our results point to specific human resources, communication, quality, and infrastructure deficits that could explain the observed low survival for children with sarcoma in AHOPCA-member countries, reveal areas to work on, and serve as a starting point for the development of a more meaningful indicator of pediatric cancer center performance. Importantly, we evaluated resources and capacities at public referral centers caring for patients from the general population (not selected centers or patients with means, insurance, etc.) and practicing within heterogeneous health-care systems. This analysis did not take into account cultural barriers to cancer care, which would be more likely to vary from country to country and region to region. Therefore, we believe that our findings are consonant to what would be observed in other MIC, generalizable and applicable to other MIC, and could be used to develop similar regional initiatives worldwide.
Pediatric sarcomas collectively represent 6-16% of childhood cancers and in HIC estimated survival has improved to reach about 70%40, 41. In AHOPCA-member countries, abandonment-sensitive overall survival (AOS) ranged from 23.5% to 47.1% at 4-years. The importance of accounting for treatment abandonment in survival analysis in LMC was illustrated in Figure 1; analysis based on standard definition of overall survival would noticeably overestimate survival in countries with treatment abandonment rate >20%. Also, AOS was almost identical to abandonment-sensitive event-free survival (AEFS) in LMICs, suggesting that survival after relapse was greatly compromised.
Previously identified reasons for observed worse pediatric sarcoma outcomes in AHOPCA-centers include late presentation, high relapse rate, and high treatment abandonment rate23. The current study suggests performance factors at the level of the care team and centers are also important. These non-biological factors are likely most influential and actionable in resource-limited settings and deserve to be prioritized. Our analysis revealed specific concerns with human resources, clinical space, communication, diagnostic imaging, specialized pathology, specialized surgery, blood banking, coordination of multidisciplinary care, and access to radiation services. Targeted strategies are underway, which may positively impact patient care, extend the benefits diagonally to the health care system and other disciplines, and will be monitored prospectively.
The impact of specific socioeconomic, contextual or infrastructure factors on outcomes can be difficult to assess using single measures. Therefore, composite measures have become more popular and accepted for assessment of global health strategies31, 32. In our study, some established health and economic indicators reflected the relationship between resources and outcomes well, as was the case for treatment abandonment and O/E rate. However, with regards to decision-making, the pilot performance index correlated with AOS best and its sub-scales were meaningful for determining priorities. Our results suggest the complex variety of factors involved in delivery of pediatric cancer care can be conceptualized in discrete components, analyzed using transparent methods, interpreted in ways meaningful to leadership at a pediatric cancer center, and promote positive change.
We acknowledge the limitations of our study, including possible ecologic fallacy, the use of grouped data, scarcity of population-based cancer registries, and its retrospective and cross-sectional nature. However, lessons learned will guide next steps in the goal of developing a measure that meaningfully describes performance in delivering pediatric cancer care and defines resource-clustering internationally.
Figure 2.
Panel A. Overall survival (OS) and Panel B. Abandonment-sensitive overall survival (AOS) for new cases of pediatric sarcoma diagnosed 2000-2009.
Acknowledgements
The authors gratefully acknowledge support from the Dana-Farber/Boston Children's Cancer and Blood Disorders Center Global Health Initiative and St. Jude Children's Research Hospital International Outreach Program and would like to thank AHOPCA's data managers and clinical staff for their inspiring work.
Funding Sources: First author's effort was supported by Pathophysiology of Human Blood Cells (T32 HL 7574) and Program in Cancer Outcomes Research Training (R25 CA092203); both National Institutes of Health training grants.
Footnotes
Financial disclosures: No disclosures.
REFERENCES
- 1.Farmer P, Frenk J, Knaul FM, Shulman LN, Alleyne G, Armstrong L, et al. Expansion of cancer care and control in countries of low and middle income: a call to action. Lancet. 2010;376(9747):1186–93. doi: 10.1016/S0140-6736(10)61152-X. [DOI] [PubMed] [Google Scholar]
- 2.Ribeiro RC, Pui CH. Saving the children--improving childhood cancer treatment in developing countries. N Engl J Med. 2005;352(21):2158–60. doi: 10.1056/NEJMp048313. [DOI] [PubMed] [Google Scholar]
- 3.Masera G, Eden T, Schrappe M, Nachman J, Gadner H, Gaynon P, et al. Statement by members of the Ponte di Legno Group on the right of children to have full access to essential treatment for acute lymphoblastic leukemia. Pediatric blood & cancer. 2004;43(2):103–4. doi: 10.1002/pbc.20135. [DOI] [PubMed] [Google Scholar]
- 4.Knaul FM. FJaL, S, for the Global Task Force on Expanded Access to Cancer Care and Control in Developing Countries. Closing the Cancer Divide: A Blueprint to Expand Access in Low and Middle Income Countries. . : Harvard Global Equity Initiative. Boston, MA: Nov, 2011. 2011. [Google Scholar]
- 5.Howard SC, Metzger ML, Wilimas JA, Quintana Y, Pui CH, Robison LL, et al. Childhood cancer epidemiology in low-income countries. Cancer. 2008;112(3):461–72. doi: 10.1002/cncr.23205. [DOI] [PubMed] [Google Scholar]
- 6.2008-2013 Action Plan for the global Strategy for the Prevention and Control of Noncommunicable Diseases. WHO Press, Geneva Switzerland. Printed by WHO Document Production Services; Geneva, Switzerland: [1/30/2012]. available online: http://www.who.int/cancer/en/ [Google Scholar]
- 7.Howard SC, Pedrosa M, Lins M, Pedrosa A, Pui CH, Ribeiro RC, et al. Establishment of a pediatric oncology program and outcomes of childhood acute lymphoblastic leukemia in a resource-poor area. JAMA. 2004;291(20):2471–5. doi: 10.1001/jama.291.20.2471. [DOI] [PubMed] [Google Scholar]
- 8.Messing-Junger AM, Janssen G, Pape H, Bock WJ, Gobel U, Lenard HG, et al. Interdisciplinary treatment in pediatric patients with malignant CNS tumors. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2000;16(10-11):742–50. doi: 10.1007/s003810000335. [DOI] [PubMed] [Google Scholar]
- 9.Al-Qudimat MR, Day S, Almomani T, Odeh D, Qaddoumi I. Clinical nurse coordinators: a new generation of highly specialized oncology nursing in Jordan. J Pediatr Hematol Oncol. 2009;31(1):38–41. doi: 10.1097/MPH.0b013e31818b3536. [DOI] [PubMed] [Google Scholar]
- 10.Albright AL, Sposto R, Holmes E, Zeltzer PM, Finlay JL, Wisoff JH, et al. Correlation of neurosurgical subspecialization with outcomes in children with malignant brain tumors. Neurosurgery. 2000;47(4):879–85. doi: 10.1097/00006123-200010000-00018. discussion 85-7. [DOI] [PubMed] [Google Scholar]
- 11.Qaddoumi I, Musharbash A, Elayyan M, Mansour A, Al-Hussaini M, Drake J, et al. Closing the survival gap: implementation of medulloblastoma protocols in a low-income country through a twinning program. Int J Cancer. 2008;122(6):1203–6. doi: 10.1002/ijc.23160. [DOI] [PubMed] [Google Scholar]
- 12.Qaddoumi I, Mansour A, Musharbash A, Drake J, Swaidan M, Tihan T, et al. Impact of telemedicine on pediatric neuro-oncology in a developing country: the Jordanian-Canadian experience. Pediatric blood & cancer. 2007;48(1):39–43. doi: 10.1002/pbc.21085. [DOI] [PubMed] [Google Scholar]
- 13.Antillon F, Castellanos M, Valverde P, Luna-Fineman S, Garrido C, Serrato T, et al. Treating Pediatric soft tissue sarcomas in a country with limited resources: the experience of the Unidad Nacional de Oncologia Pediatrica in Guatemala. Pediatric blood & cancer. 2008;51(6):760–4. doi: 10.1002/pbc.21699. [DOI] [PubMed] [Google Scholar]
- 14.Luna-Fineman S, Barnoya M, Bonilla M, Fu L, Baez F, Rodriguez-Galindo C. Retinoblastoma in Central America: Report from the Central American Association of Pediatric Hematology Oncology (AHOPCA). Pediatric blood & cancer. 2012;58(4):545–50. doi: 10.1002/pbc.23307. [DOI] [PubMed] [Google Scholar]
- 15.Bonilla M, Moreno N, Marina N, deReyes G, Shurtleff SA, Downing JR, et al. Acute lymphoblastic leukemia in a developing country: preliminary results of a nonrandomized clinical trial in El Salvador. J Pediatr Hematol Oncol. 2000;22(6):495–501. doi: 10.1097/00043426-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Gupta S, Bonilla M, Fuentes SL, Caniza M, Howard SC, Barr R, et al. Incidence and predictors of treatment-related mortality in paediatric acute leukaemia in El Salvador. British journal of cancer. 2009;100(7):1026–31. doi: 10.1038/sj.bjc.6604895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta S, Bonilla M, Valverde P, Fu L, Howard SC, Ribeiro RC, et al. Treatment-related mortality in children with acute myeloid leukaemia in Central America: Incidence, timing and predictors. Eur J Cancer. 2012;48(9):1363–9. doi: 10.1016/j.ejca.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Metzger ML, Howard SC, Fu LC, Pena A, Stefan R, Hancock ML, et al. Outcome of childhood acute lymphoblastic leukaemia in resource-poor countries. Lancet. 2003;362(9385):706–8. doi: 10.1016/S0140-6736(03)14228-6. [DOI] [PubMed] [Google Scholar]
- 19.Quintana Y, Patel AN, Naidu PE, Howard SC, Antillon FA, Ribeiro RC. POND4Kids: A Web-based Pediatric Cancer Database for Hospital-based Cancer Registration and Clinical Collaboration. Stud Health Technol Inform. 2011;164:227–31. [PubMed] [Google Scholar]
- 20.Ayoub L, Fu L, Pena A, Sierra JM, Dominguez PC, Pui CH, et al. Implementation of a data management program in a pediatric cancer unit in a low income country. Pediatric blood & cancer. 2007;49(1):23–7. doi: 10.1002/pbc.20966. [DOI] [PubMed] [Google Scholar]
- 21.Friedrich P, Ortiz R, Strait K, Fuentes S, Gamboa Y, Arambu I, et al. Pediatric sarcoma in Central America: outcomes, challenges, and plans for improvement. Cancer. 2013;119(4):871–9. doi: 10.1002/cncr.27816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribeiro RC, Steliarova-Foucher E, Magrath I, Lemerle J, Eden T, Forget C, et al. Baseline status of paediatric oncology care in ten low-income or mid-income countries receiving My Child Matters support: a descriptive study. Lancet Oncol. 2008;9(8):721–9. doi: 10.1016/S1470-2045(08)70194-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedrich P, Ortiz R, Strait K, Fuentes S, Gamboa Y, Arambu I, et al. Pediatric sarcoma in Central America: Outcomes, challenges, and plans for improvement. Cancer. 2012 doi: 10.1002/cncr.27816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International Classification of Childhood Cancer. Cancer. (third edition) 2005;103(7):1457–67. doi: 10.1002/cncr.20910. [DOI] [PubMed] [Google Scholar]
- 25.Sala A, Rossi E, Antillon F, Molina AL, de Maselli T, Bonilla M, et al. Nutritional status at diagnosis is related to clinical outcomes in children and adolescents with cancer: a perspective from Central America. Eur J Cancer. 2012;48(2):243–52. doi: 10.1016/j.ejca.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Parkin DM, International Agency for Research on Cancer . International incidence of childhood cancer. II. International Agency for Research on Cancer; Distributed in the USA by Oxford University Press; Lyon New York: 1998. [Google Scholar]
- 27. [July 10, 2013];Cancer Incidence in Five Continents Volumes I to IX. International Agency for Research on Cancer. Online analysis by cancer for Costa Rica, Volume IX (1998-2002), males and females from 0-14 years of age. Webpage: http://ci5.iarc.fr/CI5i-ix/ci5i-ix.htm. Last update: 02-03-2011. 2007 [cited; Available from. [Google Scholar]
- 28.World Development Indicators [January 25, 2012];The World Bank. Available online at: http://data.worldbank.org/data-catalog/world-development-indicators. [cited; Available from.
- 29.How we Classify Countries [1/30/12];The World Bank Group. Data. 2012 Available online: http://data.worldbank.org/about/country-classifications. [cited; Available from.
- 30.Health expenditure per capita (current US$) [1/30/12];The World Bank Group. Data. 2012 Available online: http://data.worldbank.org/indicator/SH.XPD.PCAP.
- 31.European Commission. Joint Research Centre. Organization for Economic Co-operation and Development. SourceOECD (Online service) Handbook on constructing composite indicators : methodology and user guide. OECD; Paris: 2008. [Google Scholar]
- 32.Bandura R. A Survey of Composite Indices Measuring Country Performance: 2008 Update.. A UNDP/ODS Working Paper; Office of Development Studies United Nations Developmental Programme; 2008. [Google Scholar]
- 33.Cherchye LWNPT. An Introduction to ‘Benefit of the Doubt’ Composite Indicators. Social indicators research. 2007;82(1):111–45. [Google Scholar]
- 34.Saltelli A. Composite Indicators between Analysis and Advocacy. Social indicators research. 2007;81(1):65–77. [Google Scholar]
- 35.Jurado A-MJ. Construction and Evolution of a Multidimensional Well-Being Index for the Spanish Regions. Social indicators research. 2012;107(2):259–79. [Google Scholar]
- 36.Zhou PABZD. Weighting and Aggregation in Composite Indicator Construction: a Multiplicative Optimization Approach. Social indicators research. 2010;96(1):169–81. [Google Scholar]
- 37.The Level of Effort in the National Response to HIV/AIDS . The AIDS Program Effort Index (API) 2003 Round. USAID, UNAIDS, WHO, and the Policy Project; 2003. [Google Scholar]
- 38.2001 Environmental Sustainability Index. An Initiative of the Global Leaders for Tomorrow Environment Task Force, World Economic Forum.. Annual Meeting in Davos, Switzerland. Yale Center for Environmental Law and Policy and Center for International Earth Science Information Network.; 2001. [Google Scholar]
- 39.Mostert S, Arora RS, Arreola M, Bagai P, Friedrich P, Gupta S, et al. Abandonment of treatment for childhood cancer: position statement of a SIOP PODC Working Group. Lancet Oncol. 2011;12(8):719–20. doi: 10.1016/S1470-2045(11)70128-0. [DOI] [PubMed] [Google Scholar]
- 40.Smith MA, Seibel NL, Altekruse SF, Ries LA, Melbert DL, O'Leary M, et al. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(15):2625–34. doi: 10.1200/JCO.2009.27.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaatsch P. Epidemiology of childhood cancer. Cancer treatment reviews. 2010;36(4):277–85. doi: 10.1016/j.ctrv.2010.02.003. [DOI] [PubMed] [Google Scholar]