Abstract
A series of conformationally restricted bis-azaaromatic quaternary ammonium salts (3 and 4) have been designed and synthesized in order to investigate the possible binding conformations of N,N′-dodecane-1,12-diyl-bis-3-picolinium dibromide (bPiDDB; 2), a compound which potently inhibits neuronal nicotinic acetylcholine receptors (nAChRs) mediating nicotine-evoked dopamine release. The preliminary structure–activity relationships of these new analogues suggest that bPiDDB binds in an extended conformation at the nAChR binding site, and that flexibility of the linker may be important for its high potency in inhibiting nAChRs mediating nicotine-evoked dopamine release.
Keywords: Nicotinic acetylcholine receptor, Quaternary ammonium, Dopamine release, Nicotine addiction
Tobacco contains one of the most widely abused psychoactive substances in the world, that is, (S)-nicotine (1; Fig. 1), which is believed to be primarily responsible for tobacco dependence.1,2 Like many abused drugs, nicotine addiction has been linked to the release of the neurotransmitter, dopamine (DA).3–5 Nicotine-evoked DA release, which is thought to be responsible for reward, leading to addiction, is believed to result from activation of presynaptic neuronal nicotinic acetylcholine receptors (nAChRs).6–12 Nicotine stimulates all known nAChR subtypes13 and upon activation, these receptors modulate the release of various neurotransmitters. 14–16 The subunit composition of nAChR subtypes responsible for mediating nicotine-evoked DA release has not been elucidated conclusively.17 In this regard, subtype-selective nAChR antagonists, which inhibit nicotine-evoked DA release, may have potential as novel treatments for nicotine addiction.18–21
Figure 1.
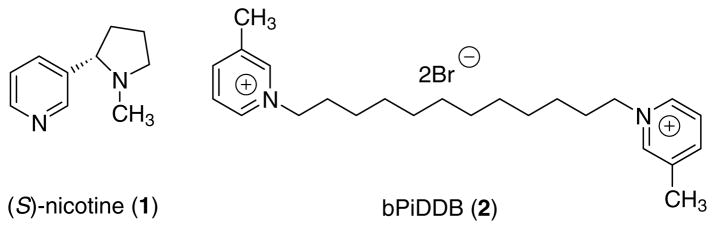
Structures of (S)-nicotine (1) and bPiDDB (2).
Previous work in our laboratories has led to the discovery of N,N′-dodecane-1,12-diyl-bis-3-picolinium dibromide (bPiDDB; 2; Fig. 1), which potently inhibited nAChR subtype(s) mediating nicotine-evoked [3H]DA release from superfused rat striatal slices in vitro (IC50 = 5 nM), and did not interact at the ligand binding site of either α4β2* or α7* nAChRs.22,23 In vivo microdialysis studies demonstrated that pretreatment with bPiDDB dose-dependently reduced the increase in extracellular DA in rat nucleus accumbens produced by acute or repeated nicotine treatment.24 In addition, we have demonstrated that despite the cationic nature and polarity of the molecule, bPiDDB is brain bioavailable, due to its facilitated transport via the blood–brain barrier choline transporter.25 Moreover, behavioral studies in rats showed that bPiDDB dose-dependently decreased intravenous nicotine self-administration, but not sucrose-maintained responding, suggesting a specific inhibition of nicotine reward.26 Taken together, bPiDDB and its analogues represent lead compounds for the development of a new class of therapeutic agents for the treatment of tobacco dependence.
bPiDDB (2) contains two 3-picolinium head groups connected via an N-N-12-methylene linker unit; thus, it is a highly flexible, di-cationic molecule. A better understanding of the potential binding conformation of bPiDDB at nAChRs responsible for mediating nicotine-evoked DA release may provide some insight into the nature of the pharmacophore requirements for optimal inhibition of this receptor. Energy minimization calculations reveal that the lowest energy conformation of bPiDDB is one in which the N-N alkyl linker is fully extended.27 However, the low energy conformations of ligand molecules may not always reflect the conformation in which the molecule binds to the receptor. Utilizing this extended or linear low energy conformation, a QSAR model utilizing a back-propagation artificial neural network approach has been constructed and has been found to afford good predictivity for inhibition of nicotine-evoked DA release.27 The hypothesis that bPiDDB binds to nAChR subtype(s) mediating nicotine-evoked DA release in a ‘linear’ or ‘extended’ conformation is supported by experimentally determined data23 showing that analogues with shorter N-N alkyl linker units have decreased inhibitory potency compared to bPiDDB. In the present study, we designed a series of model compounds which mimic different binding conformations of bPiDDB to provide further information on the active conformation of this novel nAChR antagonist.
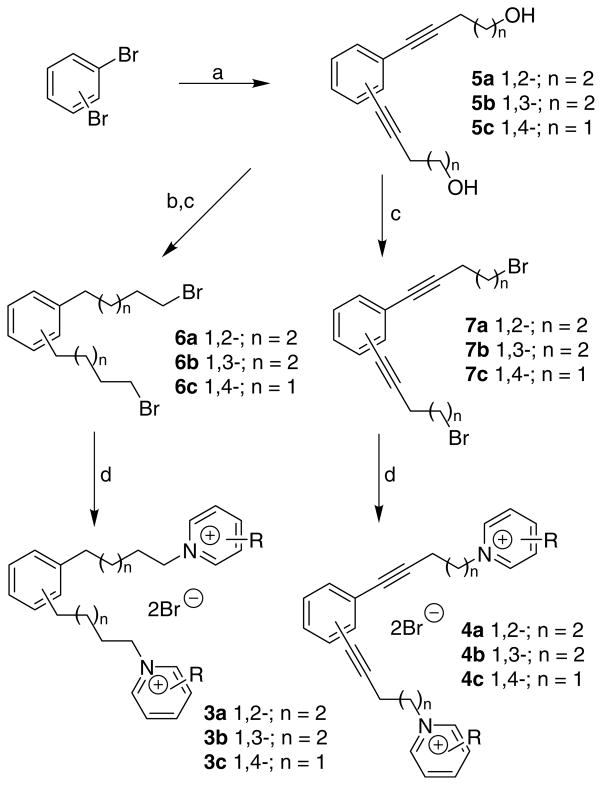
The design of the model compounds incorporated a benzene ring into the middle of the N-N linker unit, allowing a variety of arrangements of the two methylene linker units around the aromatic ring (i.e., 1,2-, 1,3-, or 1,4-positions) (see 3a, 3b, 3c, respectively, Scheme 1), and thereby, constraining these molecules into an ‘extended’ or ‘angular’ geometry. In this respect, rigidification has been used extensively in drug design to lock ligands into a desired conformation or geometry with the goal of increasing activity and selectivity of the analogues. In the current series of compounds, a triple bond also has been introduced into each of the linker units attached to the central phenyl ring to provide further restriction of conformational freedom and geometry (4a, 4b, 4c, Scheme 1).
Scheme 1.
Reagents and conditions: (a) 3-butyn-1-ol or 4-pentyn-1-ol, Pd(PPh3)2Cl2, CuI, Et3N, 80 °C; (b) H2, 10% Pd/C, MeOH, 45 psi, rt; (c) PPh3, CBr4, CH2Cl2, 0 °C; (d) azaaromatic compounds, 60 °C.
The requisite compounds of general structure 3 and 4 were prepared by the route shown in Scheme 1. The synthesis was initiated with Sonogashira coupling28 of 1,2-, 1,3-, or 1,4-dibromobenzene with 4-pentyn-1-ol (for the 1,2- and 1,3-isomers) or 3-butyn-1-ol (for 1,4-isomers) to afford compound 5a (1,2-isomer), 5b (1,3-isomer), or 5c (1,4-isomer). In the synthesis of compound 5a, the reaction required a prolonged heating time (at 80 °C) for 6 days for completion. Compounds 5a–5c and their corresponding Pd/C-catalyzed hydrogenation products were transformed into dibromides 7a–7c and 6a–6c, respectively, by bromination using PPh3/CBr4.29 Alkylation of the corresponding azaaromatic free bases, including 2-picoline, 3-picoline, 4-picoline, and nicotine, using the above obtained dibromide 6a, 6b, 6c, 7a, 7b, or 7c, afforded the corresponding bis-azaaromatic quaternary ammonium compound 3a, 3b, 3c, 4a, 4b, or 4c, respectively (Table 1). Among these analogues, the 1,2-isomers in both the 3 and 4 series (3a and 4a, respectively) and 1,3-isomers in series 4 (i.e., 4b) represent ‘angular’ conformations of bPiDDB, whereas the 1,3-isomers in series 3 (i.e., 3b, see Fig. 2 for the difference between 3b and 4b) and the 1,4-isomers in both series 3 and 4 (i.e., 3c and 4c, respectively) represent ‘extended’ conformations.
Table 1.
Inhibition of [3H]NIC binding and [3H]MLA binding to rat brain membranes, and inhibition of nicotine-evoked [3H]DA release from superfused rat striatal slices by bis-azaaromatic quaternary ammonium analogues
| Compounda | Head group | [3H]NIC binding (% inhibition at 100 nM)b | [3H]MLA binding (% inhibition at 100 nM)b | Inhibition of nicotine-evoked [3H]DA release (% inhibition at 100 nM)b |
|---|---|---|---|---|
| bPiDDB | 0 ± 0 | 0 ± 0 | 64 | |
| 1,2-Isomers (3a) |
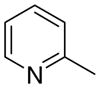
3aa
|
0 ± 0 | 7.6 ± 4.3 | 8 ± 5 |
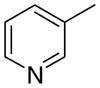
3ab
|
0 ± 0 | 5.4 ± 3.4 | 3 ± 3 | |
3ac
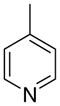
|
0 ± 0 | 3.1 ± 3.1 | 11 ± 3 | |
3ad
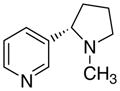
|
71 ± 2.0 | 11 ± 5.5 | 59 ± 16 | |
| 1,3-Isomers (3b) |
3ba
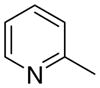
|
7.2 ± 2.8 | 0 ± 0 | 62 ± 9 |
3bb
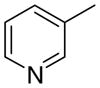
|
3.5 ± 3.5 | 6.24 ± 2.5 | 45 ± 8 | |
3bc
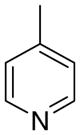
|
4.6 ± 4.0 | 3.5 ± 1.6 | 27 ± 17 | |
| 1,4-Isomers (3c) |
3ca
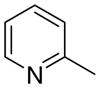
|
6.30 ± 4.21 | 0.16 ± 0.16 | 40 ± 22 |
3cb
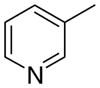
|
4.33 ± 2.19 | 7.41 ± 4.07 | 44 ± 19 | |
3cc
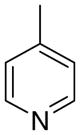
|
1.10 ± 0.89 | 7.73 ± 2.41 | 32 ± 16 | |
3cd
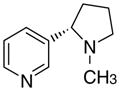
|
3.66 ± 2.39 | 5.3 ± 5.3 | 17 ± 12 | |
| 1,2-Isomers (4a) |
4aa
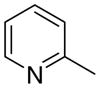
|
2.1 ± 2.1 | 1.2 ± 1.2 | 25 ± 4 |
4ab
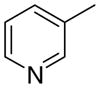
|
3.9 ± 2.8 | 0 ± 0 | 46 ± 13 | |
4ac
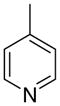
|
2.5 ± 1.5 | 5.4 ± 3.2 | 12 ± 7 | |
4ad
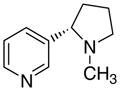
|
72 ± 3.0 | 9.2 ± 3.0 | 35 ± 20 | |
| 1,3-Isomers (4b) |
4ba
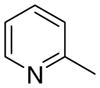
|
1.9 ± 0.7 | 0 ± 0 | 35 ± 15 |
4bb
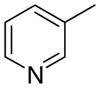
|
0.5 ± 0.4 | 8.0 ± 3.0 | 6 ± 4 | |
4bc
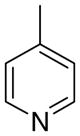
|
10 ± 5.6 | 6.4 ± 3.5 | 20 ± 12 | |
4bd
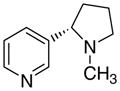
|
3.1 ± 1.7 | 2.6 ± 1.6 | 18 ± 10 | |
| 1,4-Isomers (4c) |
4cb
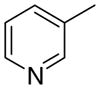
|
13.0 ± 5.4 | 3.1 ± 3.1 | 32 ± 11 |
4cc
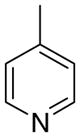
|
8.9 ± 2.5 | 2.7 ± 2.7 | 19 ± 11 |
The first letter in the compound designation indicates orientation around the central phenyl ring (i.e., a = 1,2; b = 1,3, and c = 1, 4). The second letter indicates the nature of the head group (i.e., a = 2-picolinium, b = 3-picolinium, c = 4-picolinum, and d = nicotinium).
Each value represents data from at least three independent experiments, each performed in duplicate.
Figure 2.
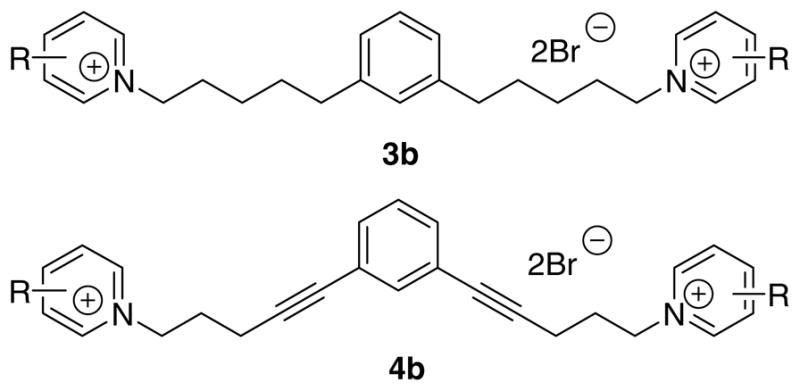
Structures of compounds 3b and 4b.
These analogues were evaluated for their ability to inhibit nicotine-evoked [3H]DA release from superfused rat striatal slices and to inhibit (S)-(−)-[3H]nicotine ([3H]NIC) binding (probing α4β2* nAChRs) and [3H]methyllycaconitine ([3H]MLA) binding (probing α7* nAChRs) to rat brain membranes. [3H]NIC and [3H]MLA binding assays were performed using 3 and 2.5 nM concentrations of radioligand, respectively, and 10 μM NIC and 10 μM MLA to assess nonspecific binding to whole brain membranes.30 Analogues were evaluated at a probe concentration of 100 nM. The amount of inhibition is presented as a percentage of radioligand binding in the absence of analogue (control, Table 1). Analogue-induced inhibition of nicotine-evoked [3H]DA release was determined using 10 μM nicotine and 100 nM analogue.30 Inhibition is presented as a percentage of the response to nicotine under control conditions (in the absence of analogue) and the values are provided in Table 1.
All of the analogues containing 2-, 3-, or 4-picolinium head groups showed little or no affinity for either α4β2* or α7* nAChRs. However, some of the analogues which contained nicotinium head groups, i.e., compounds 3ad and 4ad, displayed considerable affinity at α4β2*, which is consistent with the results obtained with our previously reported mono-nicotinium and bis-nicotinium analogues.22,31
Among the analogues of general structure 3 (Scheme 1), the 1,2-isomers (3a), including compounds 3aa, 3ab, and 3ac, exhibited significantly lower potency for inhibition of nicotine-evoked [3H]DA release than the 1,3- and 1,4-isomers (3b and 3c), including compounds 3ba, 3bb, 3bc, 3ca, 3cb, and 3cc. The 1,3- and 1,4-picolinium analogues all had similar potency. The position of the methyl group on the quaternary ammonium head group did not significantly contribute to the inhibitory potency. These results indicate that the active conformation for binding at nAChR subtypes mediating nicotine-evoked DA release is suggested to be in a more extended conformation, as reflected in the 1,3- and 1,4-isomer series, rather than in the more angular conformation in the 1,2-isomer series. Of additional interest is the observation that the 4-picolinium analogues in the 1,3- and 1,4-series were the least potent compounds.
Interestingly, in the 1,2- nicotinium compounds, 3ad and 4ad were more potent than the 1,4-compound 3cd and the 1,3-compound 4bd which is inconsistent with what was observed in the picolinium series. This suggests that the addition of a second functionality in the head group, that is, the basic pyrrolidino moiety, may promote binding of a more angular molecular conformation compared to the extended conformation in the simple picolinium molecules.
Within the more conformationally rigid analogue series (general structure 4), several differences in structure–activity relationships were observed compared to those in the more conformationally flexible 3 series. For example, compound 4bb, a 3-picolinium analogue in the 1,3-isomer series (4b), had significantly lower potency than the two structurally related 3-picolinium analogues, 4ab in the 1,2-isomer series, and 4cb in the 1,4-isomer series. Compound 4bb was also the least potent compound in the 4 series. Of the 4-picolinium analogues in the 4 series, inhibitory potency was similar between the 1,4-analogue 4cc and the 1,2- and 1,3-analogues 4ac and 4bc.
The transition from the more conformationally flexible compounds of general structure 3 to the more conformationally restricted compounds of structure 4 results generally in a decrease in inhibitory potency of nicotine-evoked [3H]DA release. This finding supports the interpretation that retaining the flexibility of the methylene linker, such as is the case in bPiDDB, is important for high inhibitory potency and optimal interaction with the nAChR protein. From the structure–activity relationship of compounds of general structure 3, it is suggested that bPiDDB binds in an ‘extended’ conformation, rather than an ‘angular’ conformation. A possible explanation for the greater potency of the more flexible analogues is that the binding site of the nAChR subtype mediating nicotine-evoked DA release is likely deep inside the channel of the receptor structure; thus, the ligands must be flexible enough to reach this binding site. This could explain why the more rigid analogues, that is, compounds 4cb and 4cc, although designed as analogues of a ‘linear’ or ‘extended’ conformation of bPiDDB, nevertheless have lower potency than their more flexible counterparts 3bb, 3bc, 3cb, and 3cc.
In summary, a series of conformationally restricted bis-azaaromatic quaternary ammonium analogues have been designed and synthesized as structural models to probe the possible binding conformation of the bPiDDB molecule. From the ability of these analogues to inhibit nicotine-evoked DA release, the results suggest that bPiDDB binds to the nAChR protein in an ‘extended’ conformation. However, flexibility of the methylene linker in the molecule is also important for high inhibitory potency. Due to the limitation of the single concentration assay utilized in the screening protocol, further studies are needed to substantiate these conclusions. Nevertheless, these findings may be of value in the further design of bPiDDB analogues as nAChR antagonists.
Acknowledgments
This research was supported by NIH Grant U19 DA17548.
References and notes
- 1.Clarke PB. Psychopharmacology. 1987;92:135. doi: 10.1007/BF00177905. [DOI] [PubMed] [Google Scholar]
- 2.Stolerman IP, Jarvis MJ. Psychopharmacology. 1995;117:2. doi: 10.1007/BF02245088. [DOI] [PubMed] [Google Scholar]
- 3.Buisson B, Bertrand D. Trends Pharmacol Sci. 2002;23:130. doi: 10.1016/S0165-6147(00)01979-9. [DOI] [PubMed] [Google Scholar]
- 4.Samaha AN, Robinson TE. Trends Pharmacol Sci. 2005;26:82. doi: 10.1016/j.tips.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Wonnacott S, Sidhpura N, Balfour DJ. Curr Opin Pharmacol. 2005;5:53. doi: 10.1016/j.coph.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Clarke PB, Pert A. Brain Res. 1985;348:355. doi: 10.1016/0006-8993(85)90456-1. [DOI] [PubMed] [Google Scholar]
- 7.Corrigall WA, Franklin KBJ, Coen KM, Clarke PBS. Psychopharmacology. 1992;107:285. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- 8.McGehee DS, Role LW. Annu Rev Physiol. 1995;57:521. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- 9.Teng L, Crooks PA, Buxton ST, Dwoskin LP. J Pharmacol Exp Ther. 1997;283:778. [PubMed] [Google Scholar]
- 10.Wonnacott S. Trends Neurosci. 1997;20:92. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- 11.Laviolette SR, van der Kooy D. Mol Psychol. 2003;8:50. doi: 10.1038/sj.mp.4001197. [DOI] [PubMed] [Google Scholar]
- 12.Rahman S, Zhang J, Corrigall WA. Neurosci Lett. 2003;48:61. doi: 10.1016/s0304-3940(03)00723-7. [DOI] [PubMed] [Google Scholar]
- 13.Parker MJ, Beck A, Luetje CW. Mol Pharmacol. 1998;54:1132. [PubMed] [Google Scholar]
- 14.McGehee DS, Role LW. Annu Rev Physiol. 1995;57:521. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- 15.Wonnacott S. Trends Neurosci. 1997;20:92. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- 16.Gotti C, Clementi F. Prog Neurobiol. 2004;74:363. doi: 10.1016/j.pneurobio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Dwoskin LP, Pivavarchyk M, Joyce BM, Neugebauer NM, Zheng G, Zhang Z, Bardo MT, Crooks PA. In: 55th Annual Nebraska Symposium on Motivation: The Motivational Impact of Nicotine and Its Role in Tobacco Use. Bevins RA, Caggiula AR, editors. Springer; in press. [Google Scholar]
- 18.Crooks PA, Ravard A, Wilkins LH, Teng LH, Buxton ST, Dwoskin LP. Drug Dev Res. 1995;36:91. [Google Scholar]
- 19.Dwoskin LP, Xu R, Ayers J, Crooks PA. Exp Opin Ther Patents. 2000;10:1561. [Google Scholar]
- 20.Dwoskin LP, Crooks PA. J Pharmacol Exp Ther. 2001;298:395. [PubMed] [Google Scholar]
- 21.Wilkins LH, Haubner A, Ayers JT, Crooks PA, Dwoskin LP. J Pharmacol Exp Ther. 2002;301:1088. doi: 10.1124/jpet.301.3.1088. [DOI] [PubMed] [Google Scholar]
- 22.Ayers JT, Dwoskin LP, Deaciuc AG, Grinevich VP, Zhu J, Crooks PA. Bioorg Med Chem Lett. 2002;12:3067. doi: 10.1016/s0960-894x(02)00687-x. [DOI] [PubMed] [Google Scholar]
- 23.Dwoskin LP, Sumithran SP, Zhu J, Deaciuc AG, Ayers JT, Crooks PA. Bioorg Med Chem Lett. 2004;14:1863. doi: 10.1016/j.bmcl.2003.10.073. [DOI] [PubMed] [Google Scholar]
- 24.Rahman S, Neugebauer NM, Zhang Z, Crooks PA, Dwoskin LP, Bardo MT. Neuropharmacology. 2007;52:755. doi: 10.1016/j.neuropharm.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Lockman PR, Manda VK, Geldenhuys WJ, Mittapalli RK, Thomas F, Albayati ZF, Crooks PA, Dwoskin LP, Allen DD. J Pharmacol Exp Ther. doi: 10.1124/jpet.107.130906. in press. [DOI] [PubMed] [Google Scholar]
- 26.Neugebauer NM, Zhang Z, Crooks PA, Dwoskin LP, Bardo MT. Psychopharmacology. 2006;184:426. doi: 10.1007/s00213-005-0163-8. [DOI] [PubMed] [Google Scholar]
- 27.Zheng F, Bayram E, Sumithran SP, Ayers JT, Zhan C, Schmitt JD, Dwoskin LP, Crooks PA. Bioorg Med Chem. 2006;14:3017. doi: 10.1016/j.bmc.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 28.Sonogashira K, Tohda Y, Hagihara N. Tetrahedron Lett. 1975;16:4467. [Google Scholar]
- 29.Nicolaou KC, Zuccarello G, Riemer C, Estevez VA, Dai WM. J Am Chem Soc. 1992;114:7360. [Google Scholar]
- 30.Dwoskin LP, Joyce BM, Zheng G, Neugebauer NM, Manda VK, Lockman P, Papke RL, Bardo MT, Crooks PA. Biochem Pharmacol. 2007;74:120. doi: 10.1016/j.bcp.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crooks PA, Ayers JT, Xu R, Sumithran SP, Grinevich VP, Wilkins LH, Deaciuc AG, Allen DD, Dwoskin LP. Bioorg Med Chem Lett. 2004;14:1869. doi: 10.1016/j.bmcl.2003.10.074. [DOI] [PubMed] [Google Scholar]