Abstract
A series of des-keto lobeline analogs has been synthesized and evaluated for their ability to inhibit the dopamine transporter (DAT) and serotonin transporter (SERT) function and for their affinity for the synaptic vesicle monoamine transporter (VMAT2), as well as for α4β2* and α7* neuronal nicotinic acetylcholine receptors (nAChRs). The enantiomers 8R-hydroxylobel-9-ene (3a) and 10S-hydroxylobel-7-ene (3c) exhibited high potency and selectivity at SERT and DAT, respectively.
Keywords: Lobeline, Dopamine transporter, Serotonin transporter, Vesicular monoamine transporter
Monoamine neurotransmitter transporters such as the dopamine transporter (DAT), the serotonin transporter (SERT), the norepinephrine transporter (NET), and the vesicular monoamine transporter (VMAT2) are considered valid targets for the development of therapeutic agents aimed at treating a variety of neurological and psychiatric diseases. For example, several antidepressant drugs, such as fluoxetine, bupropion, and reboxetine, act as SERT, DAT, and NET inhibitors, respectively. These antidepressants increase the extracellular concentration of the respective neurotransmitter by inhibiting transporter function.1–7 Additionally, tetrabenazine, an inhibitor of VMAT2 function, is used to treat Huntington’s Chorea.8,9 Recently, DAT has also been considered as a primary target for the development of medications to treat cocaine abuse.10–12
(−)-Lobeline (the 2R,6S,10S-stereoisomer, 1; Scheme 1), the major alkaloid of Lobelia inflata, has high affinity for several neuronal nicotinic acetylcholine receptor (nAChR) subtypes,13–16 and interacts nonselectively with monoamine transporters (DAT, SERT, NET, and VMAT2).15–18 Structural modification of lobeline revealed that the des-keto analog, 8R-hydroxylobel-9-ene (3a; Scheme 1), has high potency and selectivity for inhibition of [3H]5-hydroxytryptamine ([3H]5-HT) uptake over [3H]dopamine ([3H]DA) uptake, and also has increased selectivity for these transporters as a result of reduced affinity for nAChRs.15 This intriguing result prompted us to carry out a more detailed investigation of the structure–activity relationships of various stereoisomeric forms of 3a and the double bond reduced analog 4a. The pharmacological profile of these compounds was expected to provide information on the importance of the C-8/C-10 stereochemistry on the interaction with DAT, SERT, and VMAT2. Thus, the present study investigated the synthesis and pharmacological activities of isomeric 8- and 10-hydroxy lobelenes, that is 8R-hydroxylobel-9-ene (3a), 8S-hydroxylobel-9-ene (3b), 10S-hydroxylobel-7-ene (3c), and 10R-hydroxylobel-7-ene (3d), as well as the isomeric 8- and 10-hydroxylobelanes, that is 8R-hydroxylobelane (4a), 8S-hydroxylobelane (4b), 10S-hydroxylobelane (4c), and 10R-hydroxylobelane (4d). These compounds were evaluated for their ability to inhibit [3H]nicotine ([3H]NIC) binding (probing α4β2* nAChRs) and [3H]methyllycaconitine ([3H]MLA) binding (probing α7* nAChRs) to rat brain membranes, to inhibit [3H]5-HT and [3H]DA uptake into rat hippocampal and striatal synaptosomes, respectively, and to inhibit [3H]dihydrotetrabenazine ([3H]DTBZ) binding to rat synaptic vesicle membranes.
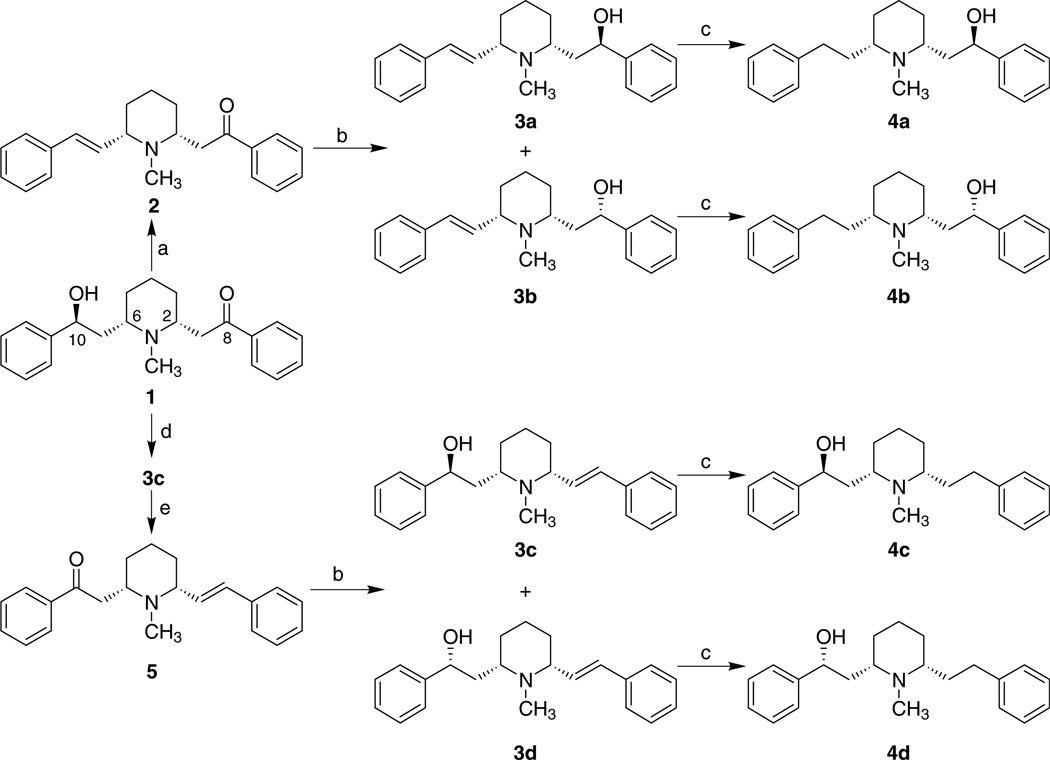
Scheme 1.
Reagents and conditions: (a) 85% H3PO4, 60 °C; (b) NaBH4, EtOH, rt; (c) H2, 10% Pd/C, MeOH, 45 psi, rt; (d) Zn/Hg, HCl (5%), reflux; (e) CrO3, H2SO4, acetone, 0 °C.
The synthetic routes to compounds 3a–3d19 and 4a–4d19 are illustrated in Scheme 1. Compound 2 was prepared by dehydration of lobeline (1) with 85% H3PO4, to afford the E-isomer exclusively, according to a previously reported method.20,21 Reduction of 2 gave a mixture of two isomers, 3a and 3b, in a ratio of 9:20 (determined by GC–MS). The pure form of 3a was obtained by fractional recrystallization of this isomeric mixture. Compound 3b was obtained by silica gel chromatography of the mother liquors from the crystallization of 3a. Compound 3c, which was prepared by Clemmensen reduction of lobeline, as previously reported,20 was converted into compound 5 by Jones oxidation. Compound 3d was obtained along with 3c, from 5, utilizing the same procedure as that employed in the synthesis of 3a and 3b from 2 (vide supra). Catalytic hydrogenation of the unsaturated compounds 3a, 3b, 3c, and 3d afforded the corresponding reduced compounds 4a, 4b, 4c, and 4d, respectively.
The above lobeline analogs were evaluated as inhibitors of [3H]NIC binding and [3H]MLA binding to rat brain membranes, as inhibitors of [3H]DA uptake into rat striatal synaptosomes to assess DAT function, as inhibitors of [3H]5-HT uptake into rat hippocampal synaptosomes to assess SERT function, and as inhibitors of [3H]DTBZ binding to rat synaptic vesicle membranes to assess interaction with VMAT2 (Table 1).17 Analog-induced inhibition was compared with that induced by lobeline and the selective DAT, SERT, and VMAT2 transporter inhibitors GBR-12909, fluoxetine, and Ro 4–1284, respectively.11,22,23 Lobeline potently inhibited [3H]NIC binding with a Ki value of 4 nM, and had low affinity (Ki = 6.26 µM) for α7* nAChRs.24 des-Keto lobeline analogs exhibited diminished affinity at α4β2* and α7* nAChRs, except for compounds 3a and 4a, which had slightly higher potency than lobeline at α7* nAChRs. These results indicate the importance of the keto group in lobeline for α4β2* binding. Lobeline exhibited moderate selectivity for VMAT2 (Ki = 2.76 µM) over DAT (Ki = 28.2 µM) and SERT (Ki = 46.8 µM), and had relatively low affinity for the latter two transporters. However, most of the des-keto analogs exhibited higher potency as well as selectivity for DAT or SERT when compared to lobeline. None of these analogs exhibited high affinity and selectivity for VMAT2. All the des-keto analogs were generally equipotent with lobeline (within one order of magnitude of each other), and compound 3d exhibited the highest affinity (Ki = 0.59 µM). These results are consistent with earlier results obtained with previously reported defunctionalized lobeline analogs.15 In the current des-keto series, all analogs exhibited increased potency for inhibition of DAT and SERT compared to lobeline. Within this series, compound 3c exhibited the highest affinity for DAT (Ki = 0.11 µM) and compound 3a, the enantiomer of 3c, exhibited the highest affinity for SERT (Ki = 0.044 µM).
Table 1.
Inhibition of [3H]NIC binding (probing α4β2** nAChRs) and the [3H]MLA binding (probing α7** nAChRs) on rat brain membranes, [3H]DTBZ binding (probing VMAT2) on rat synaptic vesicle membranes, [3H]DA uptake into rat striatal synaptosomes, and [3H]5-HT uptake into rat hippocampal synaptosomes by lobeline and its des-keto analogs
|
Ki, µM, ±SEMa |
Ki ratiob | |||||
|---|---|---|---|---|---|---|
| [3H]NIC (α4β2*) | [3H]MLA (α7*) | [3H]DA (DAT) | [3H]5-HT (SERT) | [3H]DTBZ (VMAT2) | DAT/SERT/VMAT2 | |
| Fluoxetine | — | — | — | 0.041c | — | — |
| GBR-12909 | — | — | 0.018c | — | — | — |
| Ro 4–1284 | — | — | — | — | 0.028 ± 0.03 | — |
| Lobeline | 0.004 ± 0.000 | 6.26 ± 1.30 | 28.2 ± 6.73 | 46.8 ± 3.7 | 2.76 ± 0.64 | 10.2/17.0/1 |
| 3a | 4.19 ± 0.80 | 1.70 ± 0.32 | 0.86c | 0.044c | 5.16 ± 0.30 | 19.5/1/117.3 |
| 3b | >100 | >100 | 0.96 ± 0.11 | 3.75 ± 0.75 | 6.06 ± 0.45 | 1/3.9/6.3 |
| 3c | 9.75 ± 0.91 | >100 | 0.11 ± 0.003 | 19.0 ± 3.9 | 6.44 ± 0.54 | 1/173/58.5 |
| 3d | >100 | >100 | 0.29 ± 0.02 | 7.50 ± 1.80 | 0.59 ± 0.15 | 1/26/2.0 |
| 4a | 2.36 ± 0.18 | 1.21 ± 0.09 | 1.88 ± 0.12 | 0.15 ± 0.02 | 1.98 ± 0.31 | 13/1/13.2 |
| 4b | 33.6 ± 8.54 | > 100 | 1.26 ± 0.17 | 2.22 ± 0.36 | 3.01 ± 0.44 | 1/1.8/2.4 |
| 4c | 1.77 ± 0.61 | 39.3 ± 12.9 | 1.39 ± 0.08 | 4.27 ± 1.00 | 3.09 ± 0.41 | 1/3.1/2.2 |
| 4d | >100 | >100 | 0.57 ± 0.04 | 7.30 ± 0.50 | 6.60 ± 2.96 | 1/12.8/11.6 |
GBR-12909 (a specific DAT inhibitor), fluoxetine (a specific SERT inhibitor), and Ro 4–1284 (a specific VMAT2 inhibitor) were used as standards for comparison.
Each Ki value represents data from at least four independent experiments, each performed in duplicate.
For ratios between three monoamine transporters (DAT, SERT, and VMAT2), the highest affinity value was taken as 1.
Data as reported in Ref. 15.
Compound 3a was 20-fold more selective in inhibiting SERT over DAT15 and was 117-fold more selective for SERT over VMAT2. Compound 3b, which has the antipodal chirality at the C8-hydroxyl group compared to compound 3a, showed similar affinity for DAT and VMAT2 as 3a, but was 2 orders of magnitude less potent than 3a for SERT. Interestingly, compound 3c, the enantiomer of 3a, exhibited 220-fold greater selectivity in inhibiting DAT over SERT, which is the reverse of the selectivity observed with 3a. However, this reversal of selectivity did not occur in other pairs of enantiomers, that is, compounds 3b and 3d, both of which showed greater potency in inhibiting DAT over SERT (4- and 25-fold, respectively), and 3d was more selective than 3b at DAT. All four of these compounds inhibited DAT with Ki values all within one order of magnitude of each other. Thus, the binding site on SERT is more sensitive to stereochemical changes at the C-8/C-10 hydroxyl group than is the binding site on DAT.
Compounds 4a–4d generally exhibited a similar potency and selectivity profile as their corresponding double bond unsaturated congeners, 3a–3d. The four compounds 4a–4d were slightly less potent than their corresponding precursors (3a–3d) in inhibiting DAT function. Moreover, compounds 4a–4d exhibited similar potency as their corresponding precursors (3a–3d) in inhibiting SERT function. Thus, compounds 4a–4d are less selective for DAT and SERT, compared to their corresponding double bond analogs. This indicates the double bond in these compounds is more important for the binding at DAT than at SERT.
In summary, a series of des-keto lobeline analogs has been synthesized, in which the oxygen of the keto group of lobeline has been eliminated. Pharmacological evaluation shows that all the analogs have diminished affinity at α4β2* nAChRs and most of them also have diminished affinity at α7* nAChRs. In addition, all the analogs are equipotent with lobeline at VMAT2. Moreover, some of these analogs have high potency and selectivity at either DAT or SERT. The current study indicates that the stereochemistry at C-8/C-10 in these molecules is important for inhibition of SERT, but not for inhibition of DAT. In contrast, the double bond in these analogs is more important for inhibition of DAT than for inhibition of SERT function. Further structural modification based on this series of analogs may reveal important information about the DAT and SERT pharmacophores.
Acknowledgment
This research was supported by NIH Grant DA 13519.
References and notes
- 1.Hyttel J. Int. Clin. Psychopharmacol. 1994;1:19. doi: 10.1097/00004850-199403001-00004. [DOI] [PubMed] [Google Scholar]
- 2.Schloss P, Williams DC. J. Psychopharmacol. 1998;12:115. doi: 10.1177/026988119801200201. [DOI] [PubMed] [Google Scholar]
- 3.Owens MJ, Nemeroff CB. Depression Anxiety. 1998;8:5. [PubMed] [Google Scholar]
- 4.White KJ, Walline CC, Barker EL. AAPS J. 2005;7:E421. doi: 10.1208/aapsj070242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ascher JA, Cole JO, Colin JN, Feighner JP, Ferris RM, Fibiger HC, Golden RN, Martin P, Potter WZ, Richelson E, Sulser FJ. Clin. Psychiatry. 1995;56:395. [PubMed] [Google Scholar]
- 6.Frazer AJ. Clin. Psychiatry. 2000;61:25. [Google Scholar]
- 7.Massana J, Moller HJ, Burrows GD, Montenegro RM. Int. Clin. Psychopharmacol. 1999;14:73. doi: 10.1097/00004850-199903000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Jankovic J, Beach J. Neurology. 1997;48:358. doi: 10.1212/wnl.48.2.358. [DOI] [PubMed] [Google Scholar]
- 9.Kenney C, Jankovic J. Expert Rev. Neurother. 2006;6:7. doi: 10.1586/14737175.6.1.7. [DOI] [PubMed] [Google Scholar]
- 10.Newman AH. Expert Opin. Ther. Pat. 2000;10:1095. [Google Scholar]
- 11.Carroll FI, Lewin AH, Marscarella SW. In: Neurotransmitter Transporters: Structure, Function and Regulation. Reith ME, editor. Totowa, NJ: Humana Press; 2002. p. 381. [Google Scholar]
- 12.Carroll FI. J. Med. Chem. 2003;46:1775. doi: 10.1021/jm030092d. [DOI] [PubMed] [Google Scholar]
- 13.Miller DK, Crooks PA, Dwoskin LP. Neuropharmacology. 2000;39:2654. doi: 10.1016/s0028-3908(00)00140-4. [DOI] [PubMed] [Google Scholar]
- 14.Briggs CA, McKenna DG. Mol. Pharmacol. 1998;54:1095. [Google Scholar]
- 15.Miller DK, Crooks PA, Zheng G, Grinevich VP, Norrholm S, Dwoskin LP. J. Pharmacol. Exp. Ther. 2004;310:1035. doi: 10.1124/jpet.104.068098. [DOI] [PubMed] [Google Scholar]
- 16.Dwoskin LP, Crooks PA. Biochem. Pharmacol. 2002;63:89. doi: 10.1016/s0006-2952(01)00899-1. [DOI] [PubMed] [Google Scholar]
- 17.Teng L, Crooks PA, Sonsalla PK, Dwoskin LP. J. Pharmacol. Exp. Ther. 1997;280:1432. [PubMed] [Google Scholar]
- 18.Teng L, Crooks PA, Dwoskin LP. J. Neurochem. 1998;71:258. doi: 10.1046/j.1471-4159.1998.71010258.x. [DOI] [PubMed] [Google Scholar]
- 19.Compound 3a: 41.5° (c 1.0, CHCl3); mp 104–105 °C; 1H NMR (300 MHz, CDCl3) δ 1.42–1.72 (m, 6H), 1.87 (m, 1H), 2.04 (m, 1H), 2.32 (s, 3H), 2.93 (m, 1H), 3.28 (m, 1H), 4.98 (dd, J = 10.5, 3.3 Hz, 1H), 6.23 (dd, J = 16.2, 6.3 Hz, 1H), 6.45 (d, J = 16.2 Hz, 1H), 7.20–7.42 (m, 10H); 13C NMR (75 MHz, CDCl3) δ 24.6, 26.7, 33.0, 41.5, 63.4, 65.5, 74.6, 125.6, 126.3, 127.2, 127.5, 128.4, 128.7, 130.4, 133.1, 137.2, 145.4 ppm; MS m/z 321 (M+); Anal. Calcd for C22H27NO·HCl·1.0H2O: C, 70.29; H, 8.04; N, 3.73. Found: C, 70.20; H,8.28; N, 4.01.Compound 3b: –131.0° (c 1.0, CHCl3); mp 117– 118 °C; 1H NMR (300 MHz, CDCl3) δ 1.35–1.64 (m, 3H), 1.66–1.80 (m, 2H), 1.87 (m, 1H), 2.02 (m, 1H), 2.45 (m, 1H), 2.47 (s, 3H), 2.65 (m, 1H), 5.20 (dd, J = 11.1, 3.3 Hz, 1H), 6.13 (dd, J = 16.2, 8.4 Hz, 1H), 6.47 (d, J = 16.2 Hz, 1H), 7.20–7.42 (m, 10H); 13C NMR (75 MHz, CDCl3) δ 24.0, 30.0, 33.7, 40.2, 41.6, 63.3, 68.6, 72.1, 125.6, 126.3, 127.0, 127.5, 128.3, 128.7, 130.7, 134.1, 137.0, 145.4 ppm; MS m/z 321 (M+); Anal. Calcd for C22H27NO·HCl·1/3H2O: C, 72.61; H, 7.94; N, 3.85. Found: C, 72.43; H, 8.02; N, 3.97.Compound 3c: –44.8° (c 1.0, CHCl3); mp 107–108 °C; 1H, 13C NMR and MS are same as those of compound 3a; Anal. Calcd for C22H27NO·HCl·0.5H2O: C, 72.01; H, 7.97; N, 3.82. Found: C, 71.96; H, 8.12; N, 3.77.Compound 3d: 128.8° (c 1.0, CHCl3); mp 118–119 °C; 1H, 13C NMR and MS are same as those of compound 3b; Anal. Calcd for C22H27NO·HCl·0.1H2O: C, 73.46; H, 7.90; N, 3.89. Found: C, 73.47; H, 7.78; N, 4.24.Compound 4a: 75.5 °(c 1.0, CHCl3); mp 83–84 °C; 1H NMR (300 MHz, CDCl3) δ 1.14 (m, 1H), 1.25 (m, 1H), 1.45–1.70 (m, 5H), 1.76–2.04 (m, 3H), 2.30 (s, 3H), 2.57– 2.79 (m, 2H), 2.87 (m, 1H), 3.20 (m, 1H), 5.01 (dd,J = 10.8, 3.0 Hz, 1H), 7.15–7.43 (m, 10H); 13C NMR (75 MHz, CDCl3) δ 23.7, 23.9, 25.4, 25.7, 32.6, 36.0, 40.2, 62.0, 65.0, 76.4, 125.6, 125.9, 127.1, 128.4, 128.5, 128.6, 142.3, 145.2 ppm; MS m/z 323 (M+); Anal. Calcd for C22H29NO·HCl·2/3H2O: C, 71.04; H, 8.49; N, 3.77. Found: C, 71.00; H, 8.62; N, 4.09.Compound 4b: –75.4° (c 1.0, CHCl3); 1H NMR (300 MHz, CDCl3) δ 1.26–1.44 (m, 2H), 1.44–1.57 (m, 2H), 1.67–2.00 (m, 6H), 2.36 (m, 1H), 2.39 (s, 3H), 2.60– 2.72 (m, 3H), 5.14 (t, J = 6.0 Hz, 1H), 7.15–7.40 (m, 10H); 13C NMR (75 MHz, CDCl3) δ 24.7, 26.7, 31.5, 33.7, 36.1, 39.1, 61.8, 62.8, 72.5, 125.7, 125.9, 126.8, 128.3, 128.5, 142.4, 145.7 ppm; MS m/z 323 (M+); Anal. Calcd for C22H29NO·HCl·2/3H2O: C, 71.04; H, 8.49; N, 3.77. Found: C, 70.84; H, 8.27; N, 4.06.Compound 4c: –77.9° (c 1.0, CHCl3); mp 83–84 °C; 1H, 13C NMR and MS are same as those of compound 4a; Anal. Calcd for C22H29NO·HCl·1.0H2O: C, 69.91; H, 8.53; N, 3.71. Found: C, 69.63; H, 8.36; N, 3.88.Compound 4d: 74.4° (c 1.0, CHCl3); 1H, 13C NMR and MS are same as those of compound 4b; Anal. Calcd for C22H29NO·HCl·1/3H2O: C, 72.21; H, 8.45; N, 3.83. Found: C, 72.05; H, 8.71; N, 4.06.
- 20.Flammia D, Malgorzata D, Damaj MI, Martin B, Glennon RA. J. Med. Chem. 1999;42:3726. doi: 10.1021/jm990286m. [DOI] [PubMed] [Google Scholar]
- 21.Ebnöther A. Helv. Chim. Acta. 1958;41:386. doi: 10.1002/hlca.19760590721. [DOI] [PubMed] [Google Scholar]
- 22.Fuller RW, Wong DT, Robertson DW. Med. Res. Rev. 1991;11:17. doi: 10.1002/med.2610110103. [DOI] [PubMed] [Google Scholar]
- 23.Lee LC, Vander Borght T, Sherman PS, Frey KA, Kilbourn MR. J. Med. Chem. 1996;39:191. doi: 10.1021/jm950117b. [DOI] [PubMed] [Google Scholar]
- 24.Zheng G, Dwoskin LP, Deaciuc AG, Norrholm SD, Crooks PA. J. Med. Chem. 2005;48:5551. doi: 10.1021/jm0501228. [DOI] [PMC free article] [PubMed] [Google Scholar]