Abstract
Objective
To determine if the antioxidant N-acetylcysteine is able to alter peripheral and central redox capabilities in patients with Parkinson’s or Gaucher disease.
Methods
The study included non-demented adult subjects: 3 with Parkinson’s disease, 3 with Gaucher disease and 3 healthy controls. Baseline brain glutathione concentrations were measured using 7 Tesla magnetic resonance spectroscopy. Baseline blood reduced-to-oxidized glutathione ratios were determined for each subject. Brain glutathione concentrations and blood redox ratios were then determined during and at specified time points after a single, 150mg/kg N-acetylcysteine infusion.
Results
N-acetylcysteine increased blood glutathione redox ratios in those with Parkinson’s and Gaucher disease and healthy controls, which was followed by an increase in brain glutathione concentrations in all subjects.
Conclusions
This is the first demonstration that with magnetic resonance spectroscopy, it is possible to directly measure and monitor increases in brain glutathione levels in the human brain in response to a single, intravenous administration of N-acetylcysteine. This work shows the potential utility of magnetic resonance spectroscopy monitoring which could assist in determining dosing regimens for clinical trials of this potentially useful antioxidant therapy for Parkinson’s disease, Gaucher disease and other neurodegenerative disorders.
Keywords: Magnetic resonance spectroscopy, N-acetylcysteine, glutathione, redox ratio
Introduction
Decreased nigral glutathione (GSH) in Parkinson’s disease1 (PD) and increased reactive oxygen species in blood and skin fibroblasts in Gaucher disease2, 3(GD) support the presence of oxidative stress in PD and GD. This study was undertaken to determine if the antioxidant N-acetylcysteine (NAC) is able to alter peripheral and central redox capabilities in these patients. We show for the first time that a single intravenous dose of NAC increases whole blood GSH redox ratio (GSH/GSSG) in PD, GD and healthy controls, which is then followed by an increase in brain [GSH] as measured by 7 Tesla magnetic resonance spectroscopy (MRS).
Multiple pathogenic factors including oxidative stress, mitochondrial dysfunction, lysosomal or proteasomal dysfunction, and glutathione depletion are thought to contribute to the pathogenesis of PD.4–7 The significance of GSH depletion is not established, however a reduction in GSH or alteration in GSH redox status may occur early in PD progression and could be a result of oxidative stress.7
GD is a lysosomal disorder that is caused by deficient glucocerebrosidase (GBA) enzyme activity. A connection has been made between GD and PD, as those with homozygous and heterozygous mutations of GBA are at greater risk for developing PD.8 Altered GBA enzyme activity appears to contribute to increased alpha-synuclein misfolding and aggregation, which may also be enhanced by altered redox status within neurons.9
Since GSH depletion in PD and an impaired adaptive response to oxidative stress in GD have both been observed, antioxidants that increase GSH may have a protective effect in these disorders. Unfortunately, past PD research using intravenously administered GSH failed to provide a definitive benefit.10 This failure may be related to a suboptimal dosing regimen, but more likely is due to the saturable mechanism for GSH passage across the blood-brain barrier as well as a lack of uptake into neurons.11–13
A possible way to boost CNS GSH levels while avoiding the above is to employ NAC, a membrane-permeable cysteine precursor which serves as the rate limiting substrate for GSH biosynthesis. NAC increases intracellular GSH in human erythrocytes,14 however its effect on human brain GSH concentrations is unknown. Despite in vivo studies of NAC in animal models of neurodegenerative disease,15, 16 and a small clinical trial of oral NAC in patients with late stage Alzheimer’s disease, 17 it has yet to be shown that NAC can in fact alter GSH concentrations in the human brain. We hypothesized that intravenously administered NAC increases brain GSH concentrations and blood GSH/GSSG ratios. We utilized ultra-high field MRS methodology18 to measure the effect of NAC administration on cerebral GSH levels and mass spectrometry to measure its effects on blood redox status.
Methods
Subjects
This study enrolled 3 people with PD, 3 people with GD, and 3 healthy controls (HC). The study protocol was approved by the University of Minnesota Human Research Protection Program, the National Institutes of Neurological Disorders and Stroke (Protocol #6721), and was listed on ClinicalTrials.gov: NCT01427517. All participants gave written informed consent before enrollment. Non-demented individuals with mild to moderate PD (Hoehn and Yahr stage 2) were recruited from the University of Minnesota’s Movement Disorders Center. Patients with genetically and metabolically confirmed GD type 1 were recruited from the University of Minnesota and the Lysosomal Disease Network/NIH. The study included adult male (N=4) and female subjects (N=5), age (years), gender and classification: PD: 52F, 53F, 58F; GD: 18M, 50M, 64M; and HC: 58F, 58M, 58F. All subjects were on stable treatment regimens for 1 month prior to enrollment. Use of antioxidant supplements, including coenzyme Q-10 and vitamin E, was not allowed within 3 weeks of enrollment. Individuals with a history of asthma or bronchospasm were excluded from the study.
Clinical Assessments & Study Scheme
Age, ethnicity, gender, disease duration, weight, smoking status, use of alcohol and illicit drugs, Unified Parkinson’s Disease Rating Scale score (I–III), Hoehn & Yahr staging, Montreal Cognitive Assessment Score, medications, supplements and vital signs were recorded at baseline. Following collection of a baseline blood sample, the subject was placed into the MR scanner to determine the baseline brain GSH concentration. The subject was then removed from the scanner and NAC (150 mg/kg) was administered intravenously over 1 hour. The subject was placed back into the scanner ~30min following the start of infusion and brain GSH measurements and blood samples were collected at 15 minute intervals until 1 hour after the end of the infusion.
MRS Protocol
Brain MR scans were performed using a 7T, 90-cm horizontal bore magnet (Siemens MAGNETOM) and a quadrature surface coil, as described previously.18 Images acquired with a 2 × 1 × 2 mm resolution MPRAGE sequence were used for the selection of the volume-of-interest (VOI). All first- and second-order shims were adjusted using FASTMAP with echo-planar imaging readout.19 Spectra were acquired from the occipital cortex (22 × 22 × 22 mm3) with a modified semi-LASER sequence (echo time TE = 26 ms, repetition time TR = 5 s).20 Unsuppressed water spectra acquired from the same VOI were used to remove residual eddy current effects and as a quantification reference. Single-shot data were saved during acquisition; individual FIDs were frequency and phase corrected prior to summation.
Metabolite Quantification
Metabolites were quantified using LCModel21 as described before.18,20 The metabolite model spectra were generated based on previously reported chemical shifts and coupling constants.22,23 Macromolecule spectra were acquired from the occipital cortex using an inversion recovery sequence (TR = 2 s, inversion time TI = 0.680 s).24 Metabolites quantified with Cramér-Rao lower bounds (CRLB, estimated error of the metabolite quantification) > 50% or correlation coefficient < −0.5 were classified as not detected.25 Concentrations were not corrected for T1 and T2 effects or cerebrospinal fluid contribution to the VOI.
GSH/GSSG Redox Ratio
Sample Collection and Analysis
Blood samples were collected via a catheter that was placed in the subject’s lower leg into a tube containing EDTA. To prevent GSH oxidation, 1 mL of whole blood was immediately mixed with 0.5 ml of 3% meta-phosphoric acid (Sigma). The blood samples were stored at 4°C and analyzed the same day as blood collection. Fifty microliters of blood were extracted with 2 mL of methanol (Fisher). The supernatant was evaporated and reconstituted in 300 μL of mobile phase and filtered into high performance liquid chromatography vials using Acrodisc® nylon syringe filters. The chromatographic system used consisted of a Hewlett-Packard 1100 series (Agilent Technologies) with a quadrupole mass spectrometer (model G1946A). The analytes were separated using a Zorbax Eclipse XDB C18 column (150 mm × 3.0 mm, 3.5μ particle size) and eluted isocratically with a mobile phase consisting of 20 mM ammonium formate (Fluka) buffer (pH 3.5) and acetonitrile (Sigma) (98:2, v/v). The analytes were detected using an electrospray ionization-MS system in the positive ion mode (quantitation ions: m/z 122, m/z 164, m/z 308, and m/z 613 for cysteine, NAC, GSH, and oxidized glutathione (GSSG), respectively). Each sample was analyzed in triplicate. The GSH/GSSG redox ratio was determined using the peak area response ratio for GSH and GSSG. GSH/GSSG redox ratio was reported as the mean of the triplicate values with all values within 50 percent of the mean.
Data Analysis
Percent change from baseline was calculated for both blood GSH/GSSG ratios and brain GSH levels for each subject at each time point. Maximal percent change for each subject was determined.
Results
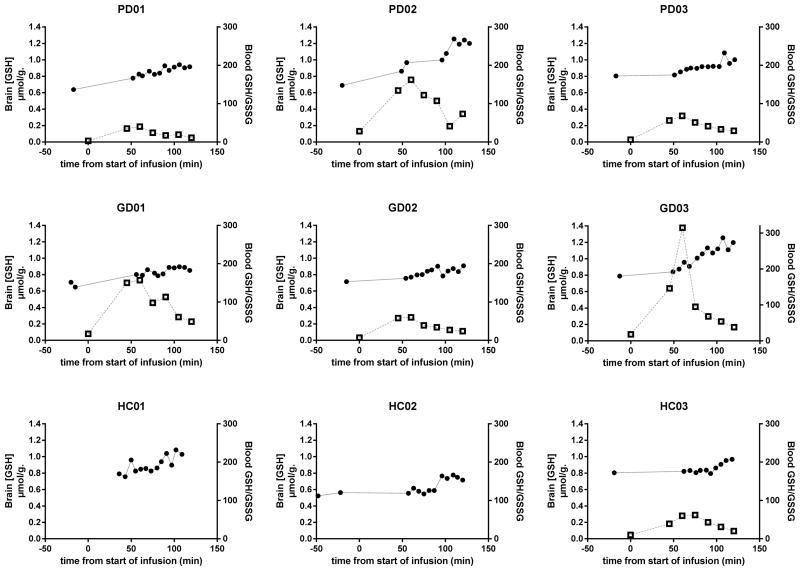
Blood GSH/GSSG ratios increased following the start of NAC infusion, reaching a maximum at approximately 60–75 minutes. Brain GSH also increased with maximal values observed at approximately 90–110 minutes after the start of infusion. Subjects with the greatest percent change in blood GSH/GSSG ratio after NAC infusion also had the greatest percent change in brain GSH, with the exception of subject PD03. (Figure 1) The average maximal percent change from baseline in brain [GSH] was 55%, 41% and 34% in the PD, GD and HC groups, respectively. Although brain [GSH] reached maximal percent change from baseline at 90–110 minutes and then began to decline in all subjects, none of the subjects had returned to their baseline brain [GSH] at 120 minutes post NAC infusion.
Figure 1.
Blood GSH redox ratio (open squares) and brain GSH concentrations (closed circles) in PD, GD and healthy control subjects. Blood GSH redox ratios for HC 01 and HC02 not shown, due to difficulty with GSH/GSSG stabilization prior to bioanalysis.
Discussion
In this proof-of-concept study, we show that brain GSH levels and blood GSH redox ratios increase following intravenous NAC administration and thereby demonstrate the feasibility of altering and monitoring changes in peripheral and central antioxidant status. These results support the hypothesis that NAC enhances GSH synthesis in the brain. While the ultimate question as to whether altering brain GSH will affect the course of PD or GD remains unanswered, this represents the first demonstration that MRS can measure pharmacodynamic changes in response to intravenous NAC administration in PD, GD and controls. The [GSH] measured by MRS in the occipital cortex VOI also includes the contribution of blood GSH. However, blood makes up only ~3 percent of the volume in brain.26 Thus, the change detected in brain [GSH] in this study could not be due primarily to the contribution of blood GSH because the mean maximal increase in blood GSH of 30% measured in this study would only lead to a MRS voxel [GSH] increase of 0.9%. In addition, the maximal change in the brain [GSH] occurred with a ~30 min delay relative to the redox change in blood (Figure. 1), further substantiating the limited effect of blood on the brain measurement.
Although the average maximal percent change from baseline in brain GSH appeared to differ among the subject groups, the number of subjects in this study is too small to draw any definitive conclusions. A study involving a larger number of subjects is required to determine if individuals with PD or GD have altered baseline GSH levels that may be due to their subtype or severity of condition, e.g., cortical Lewy pathology in advanced PD, and whether there are differences in the change from baseline in brain GSH between subject types. In this study we focused on the occipital cortex rather than the substantia nigra, where a GSH deficit has been detected postmortem in PD.1 Due to challenges associated with MRS in the substantia nigra in humans, including its location, very small size (limiting signal-to-noise ratio), and high iron content (giving rise to broad intrinsic linewidths, thereby limiting spectral resolution),27,28 we chose the occipital cortex to increase our chances of reliably detecting a NAC effect on GSH. Our results suggest that the effect of NAC on [GSH] is more global and that NAC is able to increase brain [GSH] even in controls.
Following a single intravenous NAC dose, we observed a transient increase in blood GSH redox ratio which was attributed to an increase in GSH as well as a decrease in GSSG in all subjects. GSSG disassociation by NAC, resulting in a NAC-GS complex and free GSH, could contribute to the transient nature of this change. Despite a rapid decrease in whole blood GSH redox ratios following the end of the NAC infusion, [GSH] in the brain remained elevated at 2 hours after the start of infusion. It is unknown whether the pharmacologic actions of NAC will be the same for oral administration. There are reports of positive effects of oral NAC in disorders with CNS involvement.29,30 Future studies are necessary to determine if oral NAC affects brain [GSH] and if so, to establish optimal dosage regimens to attain sustained elevation of brain [GSH] with repeated dosing.
Acknowledgments
This work was supported by a Lysosomal Disease Network subaward and the Minnesota Medical Foundation. The Lysosomal Disease Network is a part of NIH Rare Diseases Clinical Research Network (RDCRN). Funding for this project (LDN protocol #6721) has been provided by U54NS065768 from the NINDS and the NIH Office of Rare Diseases Research (ORDR). The views expressed in written materials or publications do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention by trade names, commercial practices, or organizations imply endorsement by the U.S. Government. The Center for MR Research is supported by the National Center for Research Resources (NCRR) biotechnology research resource grant P41 RR008079, National Institute of Biomedical Imaging and Bioengineering (NIBIB) grant P41 EB015894, the Institutional Center Cores for Advanced Neuroimaging award P30 NS076408, NCRR grant S10 RR026783 and WM Keck Foundation.
References
- 1.Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoy-Agid F, Jenner P, Marsden CD. Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann Neurol. 1994;36:348–355. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- 2.DeGanuto M, Pittis MG, Pines A, Dominissini S, Kelley MR, Garcia R, Quadrifoglio F, Bembi B, Tell G. Altered Intracellular Redox Status in Gaucher Disease Fibroblasts and Impairment of Adaptive Response Against Oxidative Stress. J Cell Physiol. 2007;21:223–235. doi: 10.1002/jcp.21023. [DOI] [PubMed] [Google Scholar]
- 3.Roversi FM, Galdieri LC, Grego BHC, et al. Blood oxidative stress markers in Gaucher disease patients. Clinica Chimica Acta. 2006;364:316–320. doi: 10.1016/j.cca.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 4.Beal M. F Oxidatively modified proteins in aging and disease. Free Radic Biol Med. 2002;32 (9):797. doi: 10.1016/s0891-5849(02)00780-3. [DOI] [PubMed] [Google Scholar]
- 5.Vila M, Ramonet D, Perier C. Mitochondrial Alterations in Parkinson’s disease: new clues. J Neurochem. 2008;107:317–328. doi: 10.1111/j.1471-4159.2008.05604.x. [DOI] [PubMed] [Google Scholar]
- 6.McNaught KS, Jackson T, JnoBaptiste R, Kapustin A, Olanow CW. Proteasomal dysfunction in sporadic Parkinson’s disease. Neurology. 2006;66(10):S37. doi: 10.1212/wnl.66.10_suppl_4.s37. [DOI] [PubMed] [Google Scholar]
- 7.Jenner P. Oxidative Stress in Parkinson’s Disease. Ann Neurol. 2003:S26–S38. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- 8.Sidranski E, Nalls MA, Aasly JO, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med. 2009;361:1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westbroek W, Gustafson AM, Sidransky E. Exploring the link between glucocereborsidase mutations and parkinsonism. 2011 Sep;17(9):485–93. doi: 10.1016/j.molmed.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hauser RA, Lyons KE, McClain T, Carter S, Perlmutter D. Randomized. Double-Blind, Pilot Evaluation of Intravenous Glutathione in Parkinson’s Disease. Movement Disorders. 2009;24 (7):979–983. doi: 10.1002/mds.22401. [DOI] [PubMed] [Google Scholar]
- 11.Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62(6):649–71. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- 12.Aoyama K, Watabe M, Nakaki T. Regulation of Glutathione Synthesis. J Pharmacol Sci. 2008;108:227–238. doi: 10.1254/jphs.08r01cr. [DOI] [PubMed] [Google Scholar]
- 13.Aoyama K, et al. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci. 2006;9(1):119–26. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- 14.Whillier S, Raftos JE, Chapman B, Kuchel P. Role of N-acetylcysteine in glutathione synthesis in human erythrocytes. Redox Report. 2009;14(3):115–124. doi: 10.1179/135100009X392539. [DOI] [PubMed] [Google Scholar]
- 15.Berman AE, Chan WY, Brennan AM, Reyes RC, Adler BL, et al. N-acetylcysteine Prevents Loss of Dopaminergic Neurons in the EAAC1−/− Mouse. Ann Neurol. 2011;69:509–520. doi: 10.1002/ana.22162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandhir R, Sood A, Mehrotra A, Kamboj SS. N-acetylcysteiene Reverses Mitochondrial Dysfunctions and Behavioral Abnormalisites in 3-Nitroproprionic Ace-Induced Huntington’s Disease. Neurodegenerative Dis. 2012;9:145–157. doi: 10.1159/000334273. [DOI] [PubMed] [Google Scholar]
- 17.Adair JC, Knoefel JE, Morgan N. Controlled trial of N-acetylcysteine for patients with probable Alzheimer’s disease. Neurology. 2001;57:1515–1517. doi: 10.1212/wnl.57.8.1515. [DOI] [PubMed] [Google Scholar]
- 18.Tkáč I, Öz G, Adriany G, Ugurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at high magnetic fields: Metabolite quantification at 4T vs. 7T. Magn Reson Med. 2009 Jul 9;62(4):868–79. doi: 10.1002/mrm.22086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gruetter R, Tká I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med. 2000;43(2):319–23. doi: 10.1002/(sici)1522-2594(200002)43:2<319::aid-mrm22>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Öz G, Tká I. Short-echo, single-shot, full-intensity proton magnetic resonance spectroscopy for neurochemical profiling at 4 T: Validation in the cerebellum and brainstem. Magn Reson Med. 2011 Apr;65(4):901–10. doi: 10.1002/mrm.22708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001 Jun;14(4):260–4. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- 22.Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000 May;13(3):129–53. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 23.Tká I. Refinement of simulated basis set for LCModel analysis. Proceedings 16th Scientific Meeting, International Society for Magnetic Resonance in Medicine; Toronto. 2008. p. 1624. [Google Scholar]
- 24.Behar KL, Rothman DL, Spencer DD, Petroff OA. Analysis of macromolecule resonances in 1H NMR spectra of human brain. Magn Reson Med. 1994;32(3):294–302. doi: 10.1002/mrm.1910320304. [DOI] [PubMed] [Google Scholar]
- 25.Provencher SW. LCModel & LCMgui User’s Manual. 2001. [Google Scholar]
- 26.Sabatini U, Celsis P, Viallard G, Rascol, Marc-Vergnes JP. Quantitative Assessment of Cerebral Blood Volume by Single-Photon Emission Computed Tomography. Stroke. 1991;22(3):324–330. doi: 10.1161/01.str.22.3.324. [DOI] [PubMed] [Google Scholar]
- 27.Emir UE, Tuite PJ, Öz G. Elevated Pontine and Putamenal GABA Levels in Mild-Moderate Parkinson Disease Detected by 7 Tesla Proton MRS. PlosOne. 2012;7(1):1–8. doi: 10.1371/journal.pone.0030918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Öz G, Terpstra M, Tká I, Aia P, Lowary J, Tuite PJ, Gruetter R. Proton MRS of the unilateral substantia nigra in the human brain at 4 tesla: detection of high GABA concentrations. Magn Reson Med. 2006 Feb;55(2):296–301. doi: 10.1002/mrm.20761. [DOI] [PubMed] [Google Scholar]
- 29.Grant JE, Odlaug BL, Kim SW. N-acetylcysteine, a glutamate modulator, in the treatment of trichotillomania: a double-blind, placebo-controlled study. Arch Gen Psychiatry. 2009;66(7):756–63. doi: 10.1001/archgenpsychiatry.2009.60. [DOI] [PubMed] [Google Scholar]
- 30.Hurd RW, Wilder BJ, Helveston WR, Uthman BM. Treatment of four siblings with progressive myoclonus epilepsy of the Unverricht-Lundborg type with N-acetylcysteine. Neurology. 1996;47:1264–1268. doi: 10.1212/wnl.47.5.1264. [DOI] [PubMed] [Google Scholar]