Abstract
Chemical modification of proteins is a common theme in their regulation. Nitrosylation of protein sulfhydryl groups has been shown to confer nitric oxide (NO)-like biological activities and to regulate protein functions. Several other nucleophilic side chains -- including those with hydroxyls, amines, and aromatic carbons -- are also potentially susceptible to nitrosative attack. Therefore, we examined the reactivity and functional consequences of nitros(yl)ation at a variety of nucleophilic centers in biological molecules. Chemical analysis and spectroscopic studies show that nitrosation reactions are sustained at sulfur, oxygen, nitrogen, and aromatic carbon centers, with thiols being the most reactive functionality. The exemplary protein, BSA, in the presence of a 1-, 20-, 100-, or 200-fold excess of nitrosating equivalents removes 0.6 +/- 0.2, 3.2 +/- 0.4, 18 +/- 4, and 38 +/- 10, respectively, moles of NO equivalents per mole of BSA from the reaction medium; spectroscopic evidence shows the proportionate formation of a polynitrosylated protein. Analogous reaction of tissue-type plasminogen activator yields comparable NO protein stoichiometries. Disruption of protein tertiary structure by reduction results in the preferential nitrosylation of up to 20 thus-exposed thiol groups. The polynitrosylated proteins exhibit antiplatelet and vasodilator activity that increases with the degree of nitrosation, but S-nitroso derivatives show the greatest NO-related bioactivity. Studies on enzymatic activity of tissue-type plasminogen activator show that polynitrosylation may lead to attenuated function. Moreover, the reactivity of tyrosine residues in proteins raises the possibility that NO could disrupt processes regulated by phosphorylation. Polynitrosylated proteins were found in reaction mixtures containing interferon-gamma/lipopolysaccharide-stimulated macrophages and in tracheal secretions of subjects treated with NO gas, thus suggesting their physiological relevance. In conclusion, multiple sites on proteins are susceptible to attack by nitrogen oxides. Thiol groups are preferentially modified, supporting the notion that S-nitrosylation can serve to regulate protein function. Nitrosation reactions sustained at additional nucleophilic centers may have (patho)physiological significance and suggest a facile route by which abundant NO bioactivity can be delivered to a biological system, with specificity dictated by protein substrate.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
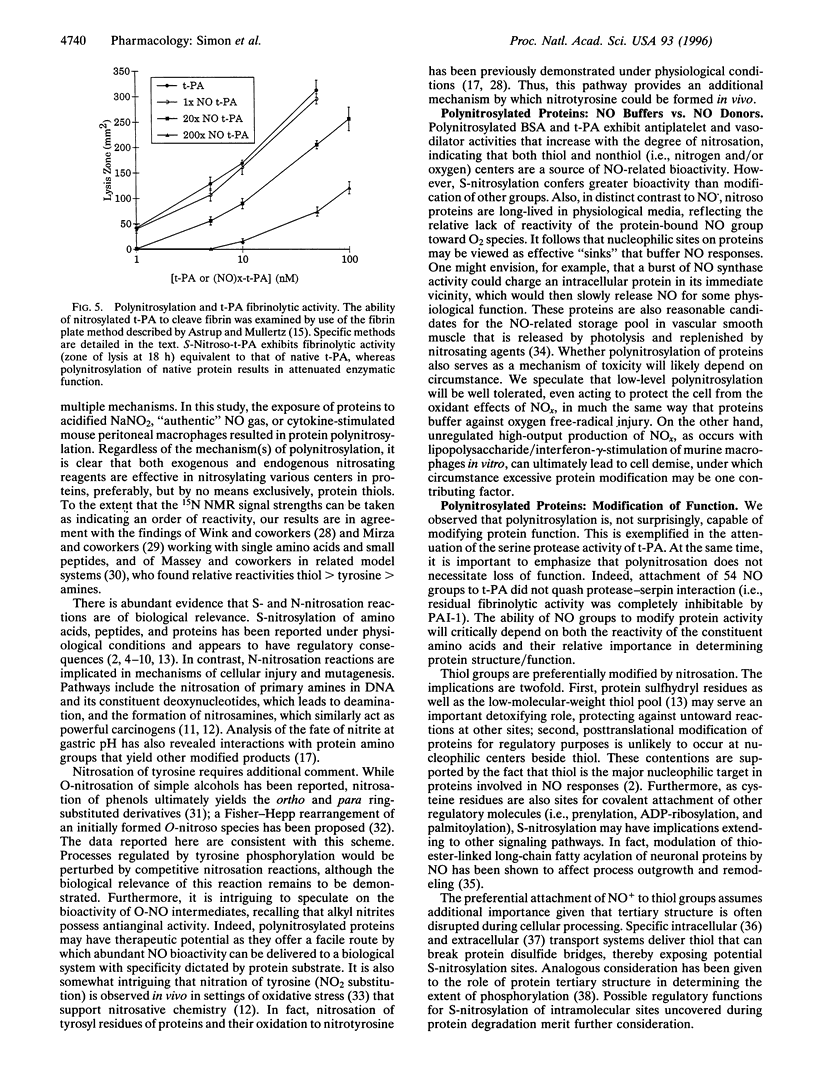
- ASTRUP T., MULLERTZ S. The fibrin plate method for estimating fibrinolytic activity. Arch Biochem Biophys. 1952 Oct;40(2):346–351. doi: 10.1016/0003-9861(52)90121-5. [DOI] [PubMed] [Google Scholar]
- Azuma H., Ishikawa M., Sekizaki S. Endothelium-dependent inhibition of platelet aggregation. Br J Pharmacol. 1986 Jun;88(2):411–415. doi: 10.1111/j.1476-5381.1986.tb10218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolotina V. M., Najibi S., Palacino J. J., Pagano P. J., Cohen R. A. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994 Apr 28;368(6474):850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- Bonnett R., Holleyhead R., Johnson B. L., Randall E. W. Reaction of acidified nitrite solutions with peptide derivatives: evidence for nitrosamine and thionitrite formation from 15N N.m.r. studies. J Chem Soc Perkin 1. 1975;(22):2261–2241. doi: 10.1039/p19750002261. [DOI] [PubMed] [Google Scholar]
- Challis B. C. Rapid nitrosation of phenols and its implications for health hazards from dietary nitrites. Nature. 1973 Aug 17;244(5416):466–466. doi: 10.1038/244466a0. [DOI] [PubMed] [Google Scholar]
- Chen S. L., Kim K. H. Effect of sulfhydryl-disulfide state on protein phosphorylation: phosphorylation of bovine serum albumin. Arch Biochem Biophys. 1985 May 15;239(1):163–171. doi: 10.1016/0003-9861(85)90823-9. [DOI] [PubMed] [Google Scholar]
- Gaston B., Reilly J., Drazen J. M., Fackler J., Ramdev P., Arnelle D., Mullins M. E., Sugarbaker D. J., Chee C., Singel D. J. Endogenous nitrogen oxides and bronchodilator S-nitrosothiols in human airways. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):10957–10961. doi: 10.1073/pnas.90.23.10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert H. F. Redox control of enzyme activities by thiol/disulfide exchange. Methods Enzymol. 1984;107:330–351. doi: 10.1016/0076-6879(84)07022-1. [DOI] [PubMed] [Google Scholar]
- Hess D. T., Patterson S. I., Smith D. S., Skene J. H. Neuronal growth cone collapse and inhibition of protein fatty acylation by nitric oxide. Nature. 1993 Dec 9;366(6455):562–565. doi: 10.1038/366562a0. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Lippton H., Edwards J. C., Baricos W. H., Hyman A. L., Kadowitz P. J., Gruetter C. A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981 Sep;218(3):739–749. [PubMed] [Google Scholar]
- Ischiropoulos H., Zhu L., Chen J., Tsai M., Martin J. C., Smith C. D., Beckman J. S. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys. 1992 Nov 1;298(2):431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- Jókay I., Kelemenics K., Földes I. Sulfhydryl groups generated by macrophages into the culture medium. Immunol Lett. 1988 Mar;17(3):217–222. doi: 10.1016/0165-2478(88)90032-6. [DOI] [PubMed] [Google Scholar]
- Knowles M. E., McWeeny D. J., Couchman L., Thorogood M. Interaction of nitrite with proteins at gastric pH. Nature. 1974 Feb 1;247(5439):288–289. doi: 10.1038/247288a0. [DOI] [PubMed] [Google Scholar]
- Lander H. M., Ogiste J. S., Pearce S. F., Levi R., Novogrodsky A. Nitric oxide-stimulated guanine nucleotide exchange on p21ras. J Biol Chem. 1995 Mar 31;270(13):7017–7020. doi: 10.1074/jbc.270.13.7017. [DOI] [PubMed] [Google Scholar]
- Laval F., Wink D. A. Inhibition by nitric oxide of the repair protein, O6-methylguanine-DNA-methyltransferase. Carcinogenesis. 1994 Mar;15(3):443–447. doi: 10.1093/carcin/15.3.443. [DOI] [PubMed] [Google Scholar]
- Lipton S. A., Choi Y. B., Pan Z. H., Lei S. Z., Chen H. S., Sucher N. J., Loscalzo J., Singel D. J., Stamler J. S. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993 Aug 12;364(6438):626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- Mellion B. T., Ignarro L. J., Myers C. B., Ohlstein E. H., Ballot B. A., Hyman A. L., Kadowitz P. J. Inhibition of human platelet aggregation by S-nitrosothiols. Heme-dependent activation of soluble guanylate cyclase and stimulation of cyclic GMP accumulation. Mol Pharmacol. 1983 May;23(3):653–664. [PubMed] [Google Scholar]
- Mirza U. A., Chait B. T., Lander H. M. Monitoring reactions of nitric oxide with peptides and proteins by electrospray ionization-mass spectrometry. J Biol Chem. 1995 Jul 21;270(29):17185–17188. doi: 10.1074/jbc.270.29.17185. [DOI] [PubMed] [Google Scholar]
- Pisoni R. L., Acker T. L., Lisowski K. M., Lemons R. M., Thoene J. G. A cysteine-specific lysosomal transport system provides a major route for the delivery of thiol to human fibroblast lysosomes: possible role in supporting lysosomal proteolysis. J Cell Biol. 1990 Feb;110(2):327–335. doi: 10.1083/jcb.110.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br J Pharmacol. 1987 Sep;92(1):181–187. doi: 10.1111/j.1476-5381.1987.tb11310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D. I., Ezratty A. M., Loscalzo J. The fibrin(ogen)olytic properties of cathepsin D. Biochemistry. 1994 May 31;33(21):6555–6563. doi: 10.1021/bi00187a024. [DOI] [PubMed] [Google Scholar]
- Simon D. I., Stamler J. S., Jaraki O., Keaney J. F., Osborne J. A., Francis S. A., Singel D. J., Loscalzo J. Antiplatelet properties of protein S-nitrosothiols derived from nitric oxide and endothelium-derived relaxing factor. Arterioscler Thromb. 1993 Jun;13(6):791–799. doi: 10.1161/01.atv.13.6.791. [DOI] [PubMed] [Google Scholar]
- Skawinski W. J., Adebodun F., Cheng J. T., Jordan F., Mendelsohn R. Labeling of tyrosines in proteins with [15N]tetranitromethane, a new NMR reporter for nitrotyrosines. Biochim Biophys Acta. 1993 Mar 26;1162(3):297–308. doi: 10.1016/0167-4838(93)90294-2. [DOI] [PubMed] [Google Scholar]
- Stamler J. S. Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell. 1994 Sep 23;78(6):931–936. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Stamler J. S., Simon D. I., Jaraki O., Osborne J. A., Francis S., Mullins M., Singel D., Loscalzo J. S-nitrosylation of tissue-type plasminogen activator confers vasodilatory and antiplatelet properties on the enzyme. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8087–8091. doi: 10.1073/pnas.89.17.8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler J. S., Simon D. I., Osborne J. A., Mullins M. E., Jaraki O., Michel T., Singel D. J., Loscalzo J. S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):444–448. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler J. S., Singel D. J., Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992 Dec 18;258(5090):1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- Stamler J., Mendelsohn M. E., Amarante P., Smick D., Andon N., Davies P. F., Cooke J. P., Loscalzo J. N-acetylcysteine potentiates platelet inhibition by endothelium-derived relaxing factor. Circ Res. 1989 Sep;65(3):789–795. doi: 10.1161/01.res.65.3.789. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7738–7742. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturini C. M., Palmer R. M., Moncada S. Vascular smooth muscle contains a depletable store of a vasodilator which is light-activated and restored by donors of nitric oxide. J Pharmacol Exp Ther. 1993 Sep;266(3):1497–1500. [PubMed] [Google Scholar]
- Wink D. A., Nims R. W., Darbyshire J. F., Christodoulou D., Hanbauer I., Cox G. W., Laval F., Laval J., Cook J. A., Krishna M. C. Reaction kinetics for nitrosation of cysteine and glutathione in aerobic nitric oxide solutions at neutral pH. Insights into the fate and physiological effects of intermediates generated in the NO/O2 reaction. Chem Res Toxicol. 1994 Jul-Aug;7(4):519–525. doi: 10.1021/tx00040a007. [DOI] [PubMed] [Google Scholar]