Abstract
Background
The course of human immunodeficiency virus type-1 (HIV-1) infection is influenced by a complex interplay between viral and host factors. HIV infection stimulates several proinflammatory genes, such as cyclooxigense-2 (COX-2), which leads to an increase in prostaglandin (PG) levels in the plasma of HIV-1-infected patients. These genes play an indeterminate role in HIV replication and pathogenesis. The effect of prostaglandin E2 (PGE2) on HIV infection is quite controversial and even contradictory, so we sought to determine the role of PGE2 and the signal transduction pathways involved in HIV infection to elucidate possible new targets for antiretrovirals.
Results
Our results suggest that PGE2 post-infection treatment acts in the late stages of the viral cycle to reduce HIV replication. Interestingly, viral protein synthesis was not affected, but a loss of progeny virus production was observed. No modulation of CD4 CXCR4 and CCR5 receptor expression, cell proliferation, or activation after PGE2 treatment was detected. Moreover, PGE2 induced an increase in intracellular cAMP (cyclic AMP) levels through the EP2/EP4 receptors. PGE2 effects were mimicked by dbcAMP and by a specific Epac (exchange protein directly activated by cyclic AMP) agonist, 8-Cpt-cAMP. Treatment with PGE2 increased Rap1 activity, decreased RhoA activity and subsequently reduced the polymerization of actin by approximately 30% compared with untreated cells. In connection with this finding, polarized viral assembly platforms enriched in Gag were disrupted, altering HIV cell-to-cell transfer and the infectivity of new virions.
Conclusions
Our results demonstrate that PGE2, through Epac and Rap activation, alters the transport of newly synthesized HIV-1 components to the assembly site, reducing the release and infectivity of new cell-free virions and cell-to-cell HIV-1 transfer.
Background
The course of human immunodeficiency virus type-1 (HIV-1) infection is influenced by a complex interplay between viral and host factors. With the aim of controlling HIV-1 infection, the immune system triggers an inflammatory response comprising both cellular effectors and soluble factors, such as interleukin (IL)-1 and IL-6, TNF-α and interferons (IFN) α, β and γ [1]–[3]. Moreover, HIV-1 infection stimulates proinflammatory genes, including inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) and, thus, their products NO and PGE2, respectively. In the immune system, PGE2 is mainly produced by monocytes. Several studies have reported an increase in PGE2 serum levels in HIV-infected patients as a consequence of viral infection and oxidative stress [4]–[6]. This lipid mediator has been shown to participate in the regulation of virus replication at different levels in a cell-type selective manner [7]. Moreover, PGE2 is a potent mediator of immune responses [8]–[13] that plays crucial roles in several biological events, such as T-cell proliferation and differentiation, the expression of membrane receptors, and the secretion of diverse cytokines in cellular immune reactions [14]. Therefore, PGE2 effects are pleiotropic, and it has been postulated that this heterogeneity depends on different pathways of PGE2 receptors.
PGE2 exerts its effects through a family of four different G protein-coupled receptors, EP1-4 [15]. Among these, the EP2 and EP4 receptors increase cyclic AMP (cAMP) via activation of adenylate cyclase (AC) [16]. To date, most cAMP-mediated effects of PGE2 via EP2/4 have been explained by the classic downstream target, protein kinase A (PKA), which phosphorylates downstream targets such as the cAMP response element binding protein (CREB). However some actions of cAMP have been reported to be independent of PKA [17], [18], including activation of the small GTPase Rap1. cAMP activates Rap1 through the Epac (exchange protein directly activated by cyclic AMP) protein family, which is a recently discovered family of cyclic nucleotide exchange factors (or cyclic AMP GEFs) that catalyze GTP exchange on Rap1. Epac-Rap1 signaling regulates integrin-mediated cell adhesion and chemotaxis. Thus, some reports have related the Epac-Rap1 pathway to inflammation processes and actin cytoskeleton reorganization [19], [20].
All viruses are obligate intracellular parasites without an independent metabolism that therefore strictly depend on their target cell for replication. Among other host factors, cortical actin is an important and common structure that is imperative for entry and intracellular transport of most viruses [21], [22].
HIV-1 assembly and budding take place within a lipid raft-rich platform that appears to be constrained and/or maintained by the actin and tubulin cytoskeleton. Disruption of either actin or tubulin remodeling disperses this platform, resulting in reduced cell-to-cell HIV-1 spread, and disruption of the actin cytoskeleton results in reduced cell-free virion release and viral infectivity [23]. Furthermore, some studies have reported that HIV-1 release from infected cells can be blocked by disturbing the actin network with specific toxins as cytochalasin D (cytoD) or mycalolyde B [24], [25].
In addition to infection with cell-free virions, the importance of cell-associated spread across connecting membrane bridges and close cell-cell contacts referred to as virological synapses (VSs) for HIV-1 propagation is increasingly being recognized, and it is thought to constitute the predominant mechanism of HIV-1 propagation in T lymphocyte cultures [26]–[29]. HIV-1 transmission across the VS depends on cell polarization, including dynamic reorganization of the actin cytoskeleton and recruitment of virion components to cell-cell contacts [30].
Our aim was to study the overall effect of PGE2 in an in vitro HIV-1-infected T cell system. Surprisingly, we showed that PGE2, a pro-inflammatory molecule, reduced HIV-1 replication in human PBL cultures. We ruled out the possibility that this effect was due to cytotoxicity, changes in proliferation or cell receptor expression differences. We demonstrated that this effect was mediated through the EP2 and EP4 receptors and that it was mainly dependent on the Epac-Rap1 pathway. Rap1 decreased RhoA activity, resulting in actin depolymerization. As a consequence, polarized viral assembly platforms enriched in Gag were disrupted along with virion release, infectivity and cell-to-cell transfer.
Materials and Methods
Cell lines
The indicator cell line TZM-bl was obtained from the NIH AIDS Research and Reference Reagent Program (Division of AIDS, NIAID, NIH: TZM-bl courtesy of Dr John C Kappes, DrXiaoyun Wu and Tranzyme, Inc.). This indicator cell line is a HeLa cell line derivative that expresses high levels of CD4 and CCR5 with endogenously expressed CXCR4. TZM-bl cells contain HIV long terminal repeat (LTR)-driven β-galactosidase and luciferase reporter cassettes that are activated by HIV tat expression. TZM-bl cells were routinely subcultured every 3 to 4 d by trypsinization and were maintained in Dulbecco's modified Eagle's medium (DMEM) (Gibco, Rockville, MD, USA) supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin, and 2 mM L-glutamine at 37°C and 5% CO2. We obtained 293T cells from the American Type Culture Collection (Manassas, VA), and maintained them in DMEM supplemented with 10% FBS, 1% penicillin/streptomycin, and 2 mM L-glutamine at 37°C and 5% CO2. The CEM-T cell line was obtained from the NIH AIDS Research and Reference Reagent Program (Division of AIDS, NIAID NIH: CEM-SS (Cat 776) from Peter L. Nara). CEM-T cells and 8E5 cells, a lymphocytic leukemia line which carries one copy of the HIV genome defective for reverse transcriptase activity and produces noninfectious viral particles, were cultured in RPMI with 10% FBS, 1% penicillin/streptomycin, and 2 mM L-glutamine at 37°C and 5% CO2.
Human peripheral blood lymphocytes (PBL) were isolated from buffy coats from the transfusion centers of Madrid following national guidelines. Ficoll-Hypaque density gradient centrifugation (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom)following the current procedures of Spanish HIV HGM BioBank [31]. PBL were activated over 3 d with 1 µg/ml phytohemaglutinin (PHA) and then cultured with IL-2 (60 U/ml).
Primary CD4 T-cells were isolated by immunomagnetic negative selection (MiltenyiBiotec) of PBL obtained from healthy donors. Final preparations were >95% CD4-positive T cells as assessed by flow cytometry.
Reagents
We obtained 6-Bnz-cAMP, 8-CPT-2Me-cAMP, H89, KT5720 and AZT (3′-azido-2′,3′-dideoxythymidine) from Sigma (St Louis, MO, USA). T20 (enfuvirtide) was obtained from Roche (Palo Alto, CA). Butaprost, Misoprostol, and Sulprostone were obtained from Cayman Chemical (Ann Arbor, MI, USA). Prostaglandin E2 (PGE2), and the MEK1 kinase inhibitor PD98059 were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
HIV-1 preparation and infection
Preparation of X4-HIV-1NL4.3 and R5-HIV-1Bal and measurement of viral replication were performed as described (Valenzuela-Fernandez et al. 2005). Highly infectious preparations were generated by several consecutive passages of the original HIV-1 isolates in PBL. Briefly, PBL cells were infected with one synchronous dose of X4-HIV-1NL4.3 or R5-HIV-1Bal, and culture supernatants were recovered 3 d later and stored at -80°C. Freshly thawed aliquots were filtered through 0.22-µm filters before use.
HIV-1 infection was assayed in PHA-activated PBL or CEM-T cells for 2 h at concentrations of 10-30 ng X4-HIV-1NL4.3 p24Gag/106 cells. Cells were washed with fresh medium to remove viral input. Infected cells were kept in culture, and p24 gag antigen was measured using a HIV-p24 ELISA kit (Innotest HIV-1 antigen mAb; Innogenetic, Ghent, Belgium) at the indicated times in each experiment.
Production of VSV-pseudotyped and HIV-1recombinant viruses
High-titer VSV-pseudotyped recombinant virus stocks were produced in 293T cells by cotransfection of pNL4-3.Luc.R-E- together with the pcDNA3-VSV plasmid encoding the vesicular stomatitis virus G-protein using the calcium phosphate transfection system (Promega, Madison, WI, USA). Supernatants containing virus stocks were harvested 48 h post-transfection, centrifuged to remove cell debris, and stored at -80°C until use. Cell-free viral stock was tested using an enzyme-linked immunoassay for antigen HIV-p24 detection (Innotest HIV-1 antigen mAb; Innogenetic, Ghent, Belgium).
VSV-pseudotyped HIV-1 infection assay
Cells were plated on a 24-well plate and were inoculated with virus stocks (100 ng HIV-1NL4.3 p24Gag/106 cells). After 16 h, the cells were stimulated with PGE2 (0.1 µM) and maintained for 48 h before lysis. Then, the lysates were spun down, and the supernatant was used to measure luciferase activity with a luminometer (1450 MicrobetaLuminiscence Counter) following the instructions of the luciferase assay kit (Promega Corporation, WI, USA).
Western blot analysis
Cells were lysed in Tris-buffered saline (TBS) containing 0.1% sodium dodecyl sulfate (SDS). The protein contents were measured using bicinchoninic acid method (BCA protein assay) according to the manufacturer's instructions (Pierce, Rockford, IL, USA). The samples were separated on a 10–15% SDS polyacrylamide gel and blotted onto a polyvinylidene fluoride membrane (Millipore, Bedford, MA, USA) by semidry transference blotting. After blocking the membranes in 10 mM TBS containing 0.1% Tween-20 (TBS-T) containing 5% skim milk, the membranes were probed with the respective primary antibodies. After washing, the membranes were incubated with HRP-conjugated secondary antibody (1∶5000 in TBS-T containing 5% skim milk) for 1 h. Proteins were detected using the Immun-Star Western C Kit (Bio-Rad Laboratories, Hercules, CA, USA). In all cases, equal amounts of total protein were analyzed across all groups. We used α-tubulin (Sigma, St. Louis, MO) as an internal control to validate the amount of protein loaded onto the gels. For quantitation, the pixel intensity for each band was determined using the Image/J program and then normalized to the amount of α-tubulin.
TZM-bl cell line infectivity assay
TZM-bl indicator cells (2×104 cells per well) were added to 96-well microtiter plate wells (Falcon, Lincoln Park, NJ) in 100 µl of complete medium and allowed to adhere for 18 h at 37°C. An equivalent amount of each virus stock (100 ng HIV-1NL4.3 p24Gag/106 cells) was added to the cell monolayers in DMEM in a final volume of 100 µl. Viral infection was allowed to proceed for 2 h at 37°C, after which the cells were washed and 200 µl of complete DMEM was added. Briefly, the supernatants were removed, and the cells were lysed with a Steady Glo luciferase assay system (Promega Corporation, WI, USA). Luciferase activity was measured after 16 h. The light intensity of each well was measured on a luminometer(1450 MicrobetaLuminiscence Counter) following the instructions of the luciferase assay kit (Promega Corporation, WI, USA). Mock-infected cells were used to determine background luminescence. All of the infectivity assays were performed at least in duplicate.
cAMP measurement
Activated and infected PBL were rinsed twice with pre-warmed serum-free medium and then pre-incubated for the indicated times with PGE2 (0.1 µM) at 37°C. Stimulation of the cells was stopped by the addition of 65% ice-cold ethanol. The supernatants were collected and concentrated in a Speed Vac evaporator. Intracellular cAMP concentrations were measured by commercial ELISA (cAMP Direct Biotrack™ EIA Amersham). The assay was performed according to the manufacturer's recommendation.
Rap activation assays
Infected human CEM-T cellswere stimulated with PGE2 (0.1 µM) at different time points: 0, 1, 5, 15, and 30 min. Next, a Rap-1 pull-down assay was performed according to the manufacturer's recommendation (Rap1 Activation Assay Kit, Upstate Biotechnology, Lake Placid NY). The samples were subjected to SDS-PAGE and Western blot analysis using an anti-Rap1 antibody (Upstate Biotechnology, Lake Placid, NY).
Rho assay
Rho-pull down assays using a GST-Rhotekin fusion protein were performed according to the manufacturer's recommendation (Thermo Scientific, Pierce). CEM-T cells were lysed, cell lysates were clarified by centrifugation at 18 000×g for 15 min at 4°C, and equal volumes of lysates were incubated with GST-RBD beads at 4°C for 45 min. The beads were washed with RIPA buffer, and the proteins associated with GST-RBD bound to beads were boiled and separated by SDS–PAGE. Immunoblotting was carried out using a specific anti-RhoA monoclonal antibody.
Confocal analysis
Infected CEM-T cells were plated on type I collagen (PuroCol®; Inamed Biomaterials, Fremont, CA)-coated glass coverslips at 37°C for 30 min and immediately fixed in 4% paraformaldehyde and 0.025% glutaraldehyde for 10 min. The fixed cells were permeabilized with Saponine 0.1% for 10 min. After two washes in PBS, the cells were incubated with 1% BSA for 30 min and stained for intracellular Gag CA p24 using an RD1-labeled mouse anti-CA p24 monoclonal antibody (1∶100) (Beckman Coulter) and FITC-labeled Phalloidin (Invitrogen, Carlsbad, CA, USA). DAPI (1 µg/ml; Alexis Biochemicals) was applied to label nuclei. Confocal laser scanning microscopy was performed with a LEICA AOBS-TCS-SP2system. Separate images were taken in the corresponding channels, and merged images were composed. Image acquisition and data processing for all of the samples were performed under the same conditions.
Cytotoxicity assay
Cell viability was determined using a thiazolyl blue tetrazolium bromide (MTT) (Sigma, St. Louis, MO, USA) assay following the manufacturer's instructions. Each experiment was performed in triplicate. The percentage of cell viability was calculated as a percentage of the untreated controls.
Cell proliferation
Human PBL cells were grown in the absence or presence of PGE2 at different concentrations for 1 and 3 d in a 37°C and 5% CO2 atmosphere. Cell proliferation was measured by quantifying the bromodeoxyuridine (BrdU) incorporated into the newly synthesized DNA of the replicating cells. The assay was performed according to the manufacturer's instructions (BrdU Cell Proliferation Assay, Chemicon International).
F-actin quantification
CEM-T cells werestimulated with PGE2 (0.1 µM) for different times, fixed with 4% paraformaldehyde at different time points, permeabilized with 0.5% Triton X-100 (5 min) and stained with FITC-labeled Phalloidin (Invitrogen, Carlsbad, CA, USA). The percentage of positive cells was analyzed in a FACScalibur cytometer (BD Biosciences, CA).
Flow cytometry
Human PBL were treated with PGE2 for 3 d. After this, cells were harvested, washed in PBS buffer, and then stained with the following monoclonal antibodies (mAbs): CD4-PC7, CCR5-PE, CXCR4-FITC, CD69-PC5, CD38-FITC (all from BD Biosciences). After incubation with the mAbs for 30 min at 4°C in the dark, the cells were washed and fixed in 2% paraformaldehyde solution for flow cytometric analysis using a FACScalibur cytometer (BD Biosciences, CA). Live cells were gated according to their forward- and side-scatter profiles. These experiments were repeated at least three times using cells from different donors.
Analysis of cell-free viral infections and cell-to-cell transfer by flow cytometry
To measure cell-cell spread by flow cytometry, an adaptation of the assay of Sourisseau el al. was used. Donor cells were infected with HIV-1NL4.3 and used a few days later when 20–40% of the cells were Gag+. Cell-to-cell HIV transfer was performed as described elsewhere[29].
To investigate the impact of PGE2 on cell-to-cell transfer and transmission, effector cells were treated with PGE2 (0.1 µM) 1 d prior to setting up co-culture. Target cells were stained with the cell trace dye CFSE Far red (CFSE) (Invitrogen, Carlsbad, CA, USA)at 37°C for 25 min, 24 h prior to starting the co-cultivation. Effector and target cells were seeded at a 2∶1 ratio to a final concentration of 0.9×106 cells/ml in a final volume of 2 ml in 12-well plates, either in mixed co-culture or separated in transwell chambers with a virus-permeable membrane (0.4 µm pore size) (NUNC). An absence of CMSF-positive cells in the effector cell population confirmed the integrity of the transwell membrane. Virus transfer was assessed by flow cytometry for intracellular Gag CA p24 in target cells at 6 h after the start of co-culture.
All of the samples were analyzed using a FACS Scalibur cytometer (BD Biosciences, CA) and Kaluza software (Tree Star). The anti-CD4 mAb Leu3a (0.25 µg/ml, BD Biosciences) was used as an inhibition control.
Results
PGE2 inhibits HIV-1 replication
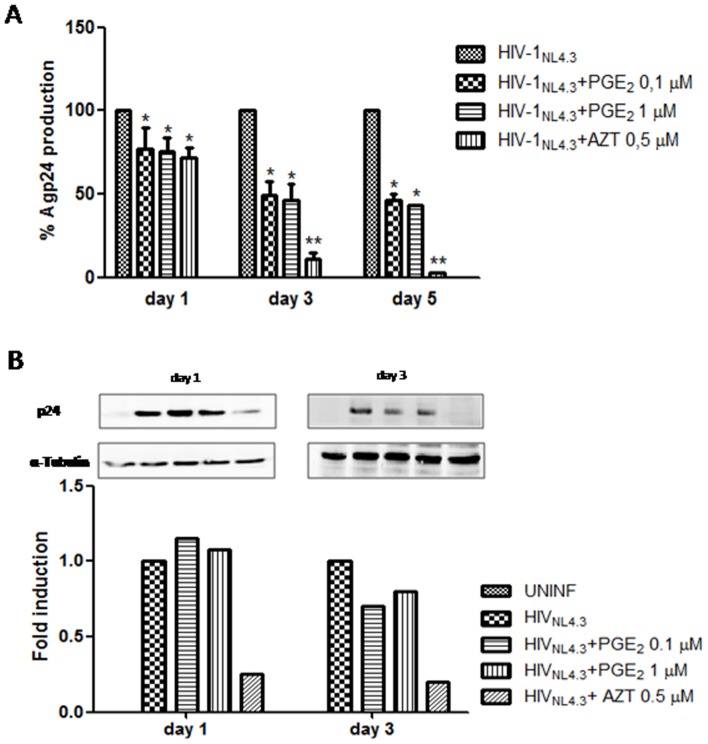
We studied the role of PGE2 on HIV-1 infection in PHA-activated PBL. Activated PBL were infected with X4-HIV-1NL4.3 (20 ng X4-HIV-1NL4.3 p24/106 cells) for 2 h at 37°C. Cells were washed 3 times with PBS and cultured at 37°C in 5% CO2. PGE2 has a very short half-life: 9 min in human plasma and approximately 1 d in culture medium, which depends largely on the pH of the medium [32]. Therefore, the culture was supplemented with PGE2 every 2 d. Release of HIV p24 antigen in the supernatant of the cultures was quantified every 2 d using a p24gag ELISA kit starting at 1 d post-infection (dpi). Fig. 1A shows that the release of p24gag antigen by infected cells was inhibited by approximately 35% at 1 dpi at both doses tested, rising to 45–50% inhibition 3 d later. Similar levels of inhibition were observed at 5 dpi. Moreover, similar results were obtained with the R5-HIV-1Bal isolate (Fig. S1), indicating that the effect of PGE2 on HIV-1 replication was independent of which HIV-1 co-receptor was used by HIV.
Figure 1. Inhibition of HIV-1 replication in human PBL by post-treatment with PGE2.
A) Human PBL were infected with X4 strain HIV-1NL4.3 (20 ng HIV-1NL4.3 p24Gag/106 cells) for 2 h and treated with PGE2 (0.1 and 1 µM) each 2 d. AZT (0.5 µM) was used as a positive control of inhibition. The culture supernatants were removed each 2 days and soluble p24 viral protein was monitored by ELISA at the indicted times. Results are shown as means ± SEM and expressed as a percentage of the value of the utreated control cultures. B) Representative WB for intracellular p24 protein determination in uninfected PBL or infected untreated and treated with PGE2 (0.1, 1 µM) or AZT (0.5 µM) at the indicated times. Antibody directed against α-tubulin was used as a protein loading control. Bottom, graph depicting the results obtained after performing a densitometer analysis of the blots. For quantification, the pixel intensity of each band was normalized to the amount of tubulin. Statistical differences in comparison to HIV-1-infected cells *:p<0.05.; **:p<0.01.
To determine whether the reduction of p24 gag antigen in the PBL supernatants was due to a decrease at the transcriptional or translational levels or to a deficiency in the output of new HIV, we performed Western blot experiments to quantify intracellular levels of p24 protein. As shown in Fig. 1B, at 1 dpi, no differences were found in the intracellular p24 levels between the treated and untreated PBL; but at 3dpi, the levels of viral protein were significantly lower in PGE2-stimulated cultures (approximately 30%) compared with controls. These results argue against the treatment with PGE2 affecting the transcription and translation of viral mRNA and indicate that it is necessary to observe more than one round of replication to detect changes in intracellular levels of p24 protein.
To confirm that the inhibition of HIV-1 production was not due to cytotoxic effects of PGE2, cytotoxicity assays were always performed in parallel with the anti-viral activity assays. The results demonstrated minimal cytotoxicity associated with PGE2 treatment of PBL within the concentration range of 0.1 to 1 µM at different studied times (Fig. S2A). Moreover, when we studied the proliferation of PHA-stimulated PBL treated with PGE2, no changes in cell proliferation were found after 3 d of culture at any PGE2 dose tested (Fig. S2B). However, T-cell activation involves the induction of several cell surface markers. We ruled out that the PGE2 effects were due to changes in cell proliferation (Fig. S2B) or alterations in the activation of cell surface markers (Fig. S2C).
PGE2 does not affect viral entry, retrotranscriptase or integrase activity, or -LTR transcription
A previous study of the effect of PGE2 treatment on HIV-1 replication in human monocyte-derived macrophages reported that the cell surface expression of CCR5 was substantially decreased in response to treatment with 10−5 M PGE2 for 48 h [33]. Therefore, cell surface expression of CD4, CXCR4 and CCR5 in PHA-activated PBL treated or not with PGE2 was studied by flow cytometry after different times, and no changes were found (Fig. S2D).
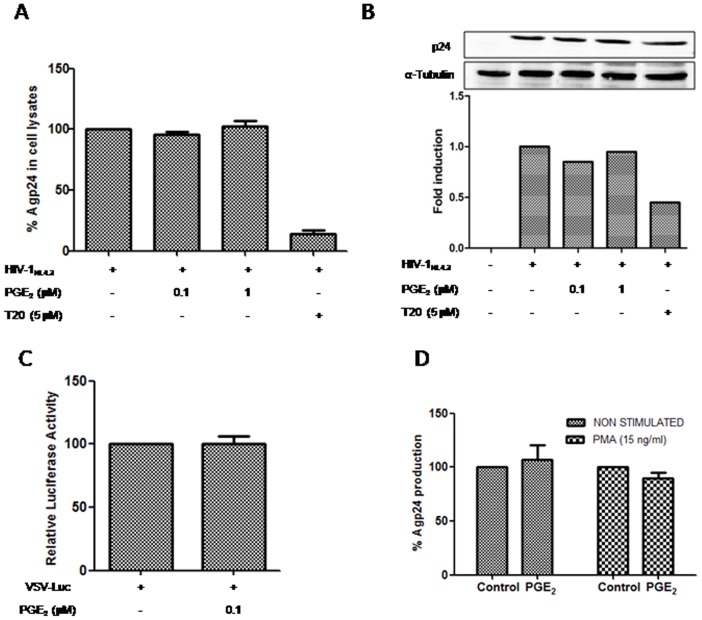
To further analyze the involvement of PGE2 in viral entry, PBL were pre-incubated with PGE2 (0.1 or 1 µM) for 16 h and infected with HIV (X4-HIV-1NL4.3) for 2 h. Then, cells were washed and lysed, and p24 levels were measured using a p24gag antigen ELISA kit and by Western blotting. Analysis of the intracellular viral protein levels failed to reveal any gross changes in p24 expression between treated and non-treated HIV-1-infected PBL (Figs. 2A, 2B). Shortly after the viral capsid enters a cell, the reverse transcriptase enzyme (RT) liberates the single-stranded (+) RNA genome from the attached viral proteins and copies it into a cDNA molecule [34]. Subsequently, the DNA is transported to the nucleus, where it is spliced into the human DNA by the HIV enzyme integrase [35]. To address the role of PGE2 in RT activity and viral DNA integration, we used a single virus infection cycle with HIV-1 VSV-Luc, which enters cells via endocytosis and thereby bypasses CD4/CXCR4 and the cortical actin. We infected the PBL with a VSV-pseudotyped HIV-1 (100 ng X4-HIV-1NL4.3 p24/106 cells). High luciferase activity levels were detected 2 d after PBL infection with the VSV-pseudotyped HIV-1 clone, and treatment with 0.1 µM of PGE2 did not alter luciferase activity (Fig. 2C). Summing up, PGE2 did not affect the RT enzyme or the level of viral DNA integration into the cell genome.
Figure 2. PGE2 does not affect initial phases of HIV-1 viral cycle.
PBL were treated with PGE2 (0.1, 1 µM) for 16 hours, then cells were infected with HIVNL4.3 insolate (15 ng/106 cells) for 2 h and immediately afterwards cells were lysated and p24 protein was quantified by ELISA A) and western blot B). Data represents p24 concentration relative to infected and non-treated cells (mean ± SEM) of three experiments performed in triplicate in each case. T20 (5 µM) was used as a positive control of entry inhibition. To convert band intensity into a quantitative measurement, the Western blot was analyzed densitometrically. Data present the fold induction relative to control cells C) PGE2 effect in post-entry steps were analyzed by infection with one-cycle viral particles VSV-pseudotyped-pNL4-3.Luc (100 ng HIV-1NL4.3 p24Gag/106 cells). Data are shown as percentage of non treated cells. Results are from three independent experiments performed in triplicate. D) To study PGE2 effect in LTR-transactivation, 8E5/LAV were used. Cells were activated or not with PMA (15 ng/ml) and treated with PGE2 for 3 days. The culture supernatants were removed and p24 viral protein was monitored by ELISA. All data are shown as percentage of non treated cells.
Previous reports have shown that PGE2 treatment of T-cell cultures increases viral LTR transcriptional activity through c/EBP and CREB [36] or NF-κB [37]. To determine the role of PGE2 in LTR transcriptional activity, we used the 8E5 cell line containing a single integrated defective copy of HIV-1 [38]. The 8E5 cells were exposed or not to PMA (15 ng/ml) alone or in combination with PGE2, and the supernatants were assayed for HIV p24 core antigen at 3 d. As previously reported [39], a 10-fold increase in p24 gag antigen was observed in PMA 8E5 treated cells (Fig. S3). The 8E5 cells stimulated or not with PMA and treated with PGE2 did not modify p24 gag levels compared to non-PGE2-treated cells (Fig. S2D). Summing up, PGE2 did not modify LTR transcriptional activity in 8E5 cells.
Repression of HIV-1 production, viral infectivity and cell-to-cell spread by PGE2
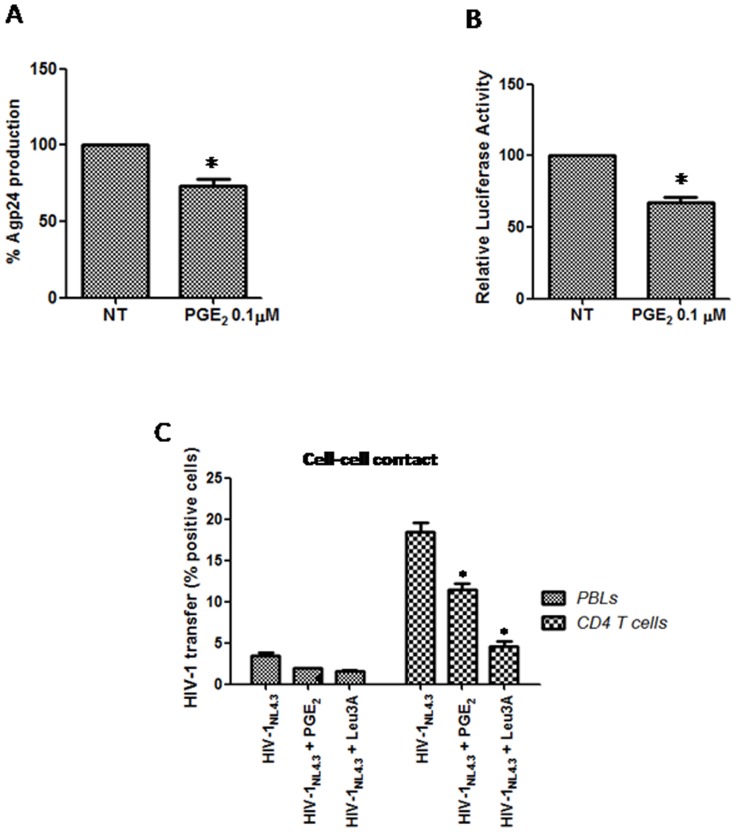
All of the results pointed to a role for PGE2 in the late steps of the HIV-1 life cycle. To study the biological relevance of the repressive action of PGE2 on HIV-1 release, we researched the effect of PGE2 on HIV-1 production. We cotransfected 293T cells with pNL4-3.Luc.R-E- (60 µg; NIH-AIDS reagent program 6070013) and a CXCR4-tropic env glycoprotein vector (30 µg; HXB2-env; NIH-AIDS reagent program 5040154), and the cells were stimulated or not with PGE2. The virus production was quantified after 2 d using a p24gag antigen ELISA kit. The viral production in 293T cells treated with PGE2 was significantly lower than production in untreated cells (Fig. 3A). Our result was consistent with PGE2 exerting its effect late in the HIV-1 life cycle, reducing virus release into the culture supernatants.
Figure 3. Effects of PGE2 on HIV-1 release and viral infectivity.
A) HEK 293T cell line was transfected with pNL4.3-Luc-R-E- and CXCR4-tropic env glycoprotein vector, and treated with PGE2 (0.1 µM) 2d. Agp24 levels in supernatants were quantified by ELISA. B) To study the infectivity of new HIV virus the TZM-bl cells were incubated with the supernatants from PBL infected and treated or not with PGE2 (0.1 µM) as detailed in Materials and Methods. After 16 h, the cells were lysed and luciferase activity was determined. Results are shown as percentage of non treated. C) PBL or purified primary CD4 T cells were infected with HIV-1NL4-3 isolate and cultured during 2 d. Bellow cells were treated with PGE2 1 d more, and then co-cultured with the CMSF labeled target cells in presence or not of PGE2. Effectors and target cells were seeded at a 2∶1 ratio in mixed co-culture. Anti-CD4 antibody Leu3a (0.25 µg/ml) was used as positive control. Virus transfer was assessed by flow cytometry for intracellular Gag CA p24 in target cells at 6 h after the start of co-culture. Results represent the means of 3 individual experiments. Error bars indicate standard error values. Statistical differences in comparison to control values (A, B) or shown as a percentage of positive cells for HIV-1 transfer ± SEM, *:p<0.05.
To further probe the inhibitory mechanism of PGE2, we used a virus-cell infection system that utilizes an indicator cell line, TZM-bl, which has been engineered to express CD4 and CCR5. Because CXCR4 is endogenously expressed on TZM-bl cells, it is susceptible to infection by diverse HIV-1 isolates. For the infectivity assay, supernatants from PBL were normalized for p24gag content (100 ng X4-HIV-1NL4.3 p24/106 cells) and tested on TZM-bl cells. Interestingly, supernatants from HIV-1-infected PBL treated with PGE2 produced a decrease in luciferase activity of approximately 30% compared with supernatants from untreated cells (Fig. 3B). To summarize, our results indicate that PGE2 decreases both the viral release and the infectivity of cell-free viral particles.
Because cell-cell virion transfer constitutes the predominant mechanism of HIV-1 propagation in T lymphocyte cultures, we directly compared the efficiency of cell-cell transfer in the presence or absence of PGE2. Thus, we performed a flow cytometry assay to quantify the appearance of Gag+target T cells following co-culture with infected T cells. PBL or purified primary CD4+ T cells were infected with 400 ng/106 cells and cultured for 48 h, resulting in 10-12% infection rate as determined by measurement of the intracellular p24 expression using flow cytometry. Thereafter, the cells were treated with PGE2 for 24 h more, and then co-cultured with uninfected cells that were previously incubated with CMSF (see M&M) at a 2∶1 infected: uninfected cell ratio, which has been reported to increase viral spread [29]. The cells were then co-cultured to allow cell-cell contact or separated by a small pore-size transwell membrane (0.4 µm) that excluded cells while permitting free viral diffusion. After 6 h, viral CA p24 was detected in the target cells using flow cytometry, indicating viral transfer. The results showed that when PGE2 was present in the mixed co-cultures, the transfer was decreased when using PBL or purified primary CD4+ T cells (Fig. 3D). Addition of the blocking anti-CD4 antibody Leu3a abrogated the capture of p24 antigen. Nevertheless, no differences in CA p24 were detected after 6 h in any of the potential target cell populations in the transwell co-culture experiments, where direct cell-cell contact was blocked, confirming that PGE2does not affect the transmission of free virus, but rather it is more involved in cell-cell viral transmission.
PGE2 decreases actin polymerization
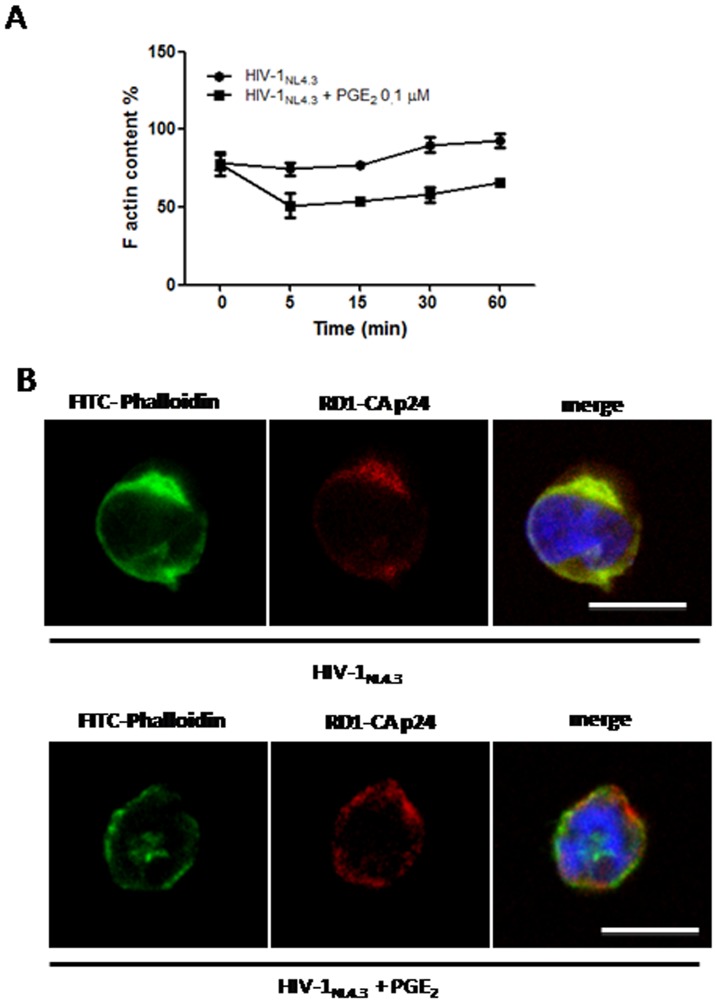
Our results suggest that PGE2 acts in the late stages of the HIV life cycle. Moreover, some studies have indicated that actin may be involved in these final stages such as the viral assembly and budding processes [40]–[43] and in HIV cell-cell transmission [44]–[46]. Thus, we researched whether reorganization of the actin cytoskeleton was affected after PGE2 treatment in T cells. Infected CEM cells were stimulated with PGE2 (0.1 µM) at the indicated times, and polymerized actin was evaluated using flow cytometry (see M&M). As shown in Fig. 4A, PGE2 treatment reduced actin polymerization in HIV-infected cells compared with untreated cells at all of the times indicated. Moreover,virion budding and release can be affected by disruption of the HIV-1 polarized assembly platform. Therefore, in addition to actin polymerization, we investigated the intracellular distribution of Gag under PGE2 treatment. In infected CEM cells that were fixed, permeabilized, and stained, Gag was localized at one pole of the cell. As expected, treatment of CEM cells with the actin-depolymerizing agent cytochalasin D (10 µM) significantly reduced the percentage of cells with capped Gag (data not shown). Surprisingly, PGE2 treatment resulted in a loss of polarized Gag staining in CEM cells (Fig. 4B).
Figure 4. Effects of PGE2 treatment on actin poymerization and Gag distribution in T cell.
Actin polymerization triggered by PGE2 (0.1 µM) for the indicated times in HIV-1 infected cells. Data are from three independent experiments, presented as mean ±SEM. D) Actin polimerization and Gag distribution in a T cell line treated or not with PGE2. Infected CEM-T cells treated or not with PGE2 (0.1 µM) for 16 h, were stained with FITC-labeled Phalloidin, and RD1-labeled mouse anti-CA p24 monoclonal antibody, in order to visualize actin organization and Gag distribution, respectively. Scale bar: 5 µm.
PGE2 decreases Agp24 production via the EP2 and EP4 receptors
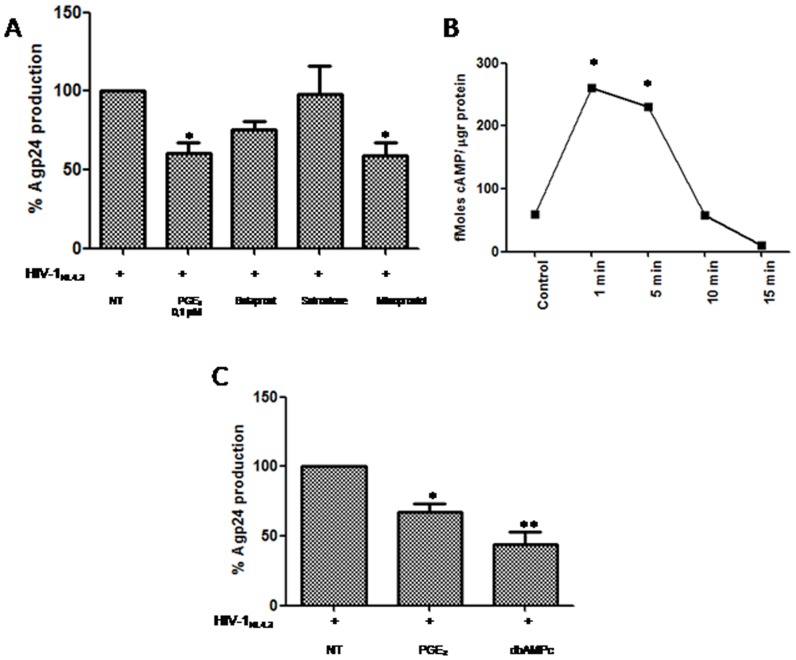
To determine which PGE2 (EP) receptors were involved in the decrease of HIV-1 replication in human PBL, we employed synthetic analogues of PGE2 that act as selective EP receptor agonists. We used three EP receptor agonists: butaprost, an EP2-specific agonist; misoprostol, a preferential EP3/EP2 agonist that also binds EP4 at high concentrations; and sulprostone, an EP1/EP3 agonist. Misoprostol and butaprost induced a decrease in p24gag antigen release in comparison to PGE2, whereas sulprostone did not produce this effect (Fig. 5A). These results indicated that the inhibitory effect of PGE2 on HIV-1 replication in PBL cultures was mediated through EP2 and possibly EP4 receptors. Because EP2 receptors are coupled to Gα resulting in the activation of adenylatecyclase, we analyzed whether PGE2 induced cAMP liberation in PBL. As shown in Fig. 5B, PGE2 treatment produced a rapid increase in cAMP as early as 1 min after stimulation followed by a gradual decrease. In addition, the effects of PGE2 on viral production were mimicked by dbcAMP, suggesting that cAMP is an important mediator of PGE2 effects (Fig. 5C).
Figure 5. Role of different EP receptors and cAMP on HIV-1 repression.
A) Infected PBL were treated with different agonists: butaprost (EP2) (10 µM), sulprostone (EP1/EP3) (10 µM) or misoprostol (EP4, EP3>EP2) (35 µM), or. Agp24 levels were quantified by ELISA 3 d later. B) PGE2 interaction with EP2/EP4 receptors induces cAMP liberation. PBL were treated with PGE2 (0.1 µM) for the indicated times, and intracellular cAMP was quantified as described under “Material and Methods”. C) Effect of dbcAMP (200 µM) on Agp24 production in human PBL after 3 days of infection. Statistical differences in comparison to HIV-1-infected (A,C) or control cells (B) *:p<0.05.; **:p<0.01.
PGE2 effect on HIV-1 replication involves Epac/Rap/RhoA signaling
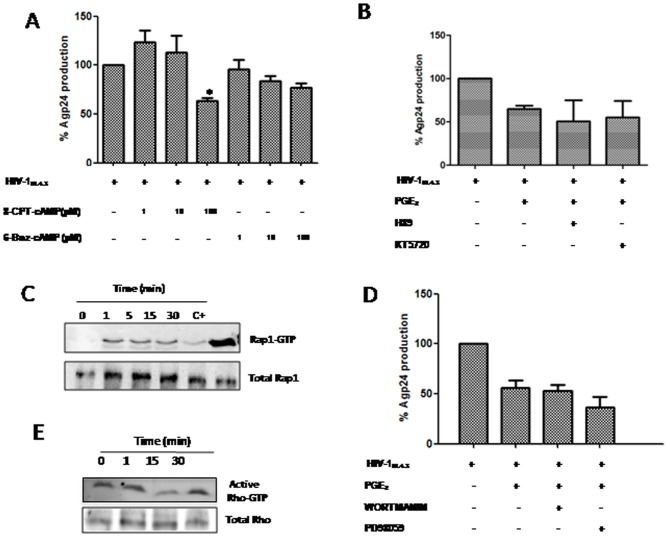
Intracellular cAMP regulates diverse cellular functions, mainly through two downstream effectors: Epac and PKA. To evaluate the effects of both effectors on cAMP-induced HIV-1 inhibition, we treated PBL cultures with different concentrations of two highly selective cAMP analogs to activate PKA or Epac, 6-Bnz-cAMP and 8-pCPT-2-O-Me-cAMP, respectively. The results demonstrated that 6-Bnz did not significantly reduce p24gag antigen release; however, higher doses of 8-CPT (100 µM) decreased viral replication approximately 40%, confirming the main role of Epac protein on the effects of PGE2 as detailed above (Fig. 6A). To rule out the involvement of PKA on PGE2 actions in our model, the effects of PKA inhibitors such as H89 and KT5720 were researched. Surprisingly, no inhibitor affected p24 gag antigen production in PGE2-stimulated PBL (Fig. 6B), confirming again the main role of Epac. To confirm the partial involvement of this signaling on PGE2-mediated effects, we performed new western blot assays with longer incubation times, which resulted in a moderate increase in p-CREB activity in treated cells at the indicated times (Fig. S5A).
Figure 6. PGE2 regulates T cell actin polymerization throughout Epac/Rap1 and RhoA signaling.
A) PBL were infected and cultured in the presence of different concentrations of 8-CPT-2-OMe-cAMP (1, 10, 100 µM) or 6-Bnz-cAMP (1, 10, 100 µM) for 3 d. Supernatants were collected and Agp24 levels were determined by ELISA. B) Effect of PKA inhibitors (H89 5 µM, KT5720 200 nM) on PBL treated with PGE2 3d post-infection. C) Pull-down assay of active Rap on infected CEM-T cells for the indicated times. Western blots were probed for Rap1. D) Effect of PI3k and ERK1 inhibitors, wortmanin (100 nM) and PD98059 (10 µM) respectively, on HIV-1 replication in PGE2-stimulated PBL. E) Immunoblot analysis of pull-down assay for Rho-GTP. Rho-A antibody was used to detect activated Rho-GTP from a pull-down assay using beads covalently bound to Rhotekin and also total Rho-A in cell lysates. Error bars indicate standard error values. Statistical differences in comparison to non-treated HIV-1-infected cells *:p<0.05.
In summary, Epac activation by cAMP is the main pathway responsible for the inhibition of HIV-1 production.Epac proteins mediate cAMP-dependent activation of Rap. Therefore, the requirement for Rap activation in this process was evaluated. Rap activation was measured in PBL treated with PGE2 for different times. PGE2 treatment resulted in a rapid Rap activation as confirmed by pull-down experiments (Fig. 6C), indicating that this protein could also be involved in the repression of HIV-1 replication. Alternatively, ERK signaling is initiated by small GTPases such as Rap, which is a member of the Ras family. Several studies have indicated that cAMP-dependent signaling is closely interwoven with the phosphatidyl inositol 3-kinase (PI3K)/PKB/Akt pathway [47], [48]. When assessing the involvement of ERK in PGE2-mediated HIV-1 inhibition, we did not observe an overall statistically significant effect of the inhibitor PD98059 on p24gag antigen production (Fig. 6D). Furthermore, to check the involvement of PI3K signaling in PGE2-mediated HIV-1 inhibition, we tested the PI3K inhibitor, wortmannin, which is a highly potent and selective PI3K inhibitor [49]. As expected, this drug did not show a significant effect on HIV-1 infection (Fig. 6D). In addition, pAkt levels did not increase after treatment at the indicated times, indicating that there was no involvement of these pathways in the observed effects of PGE2 on HIV-1 replication (Fig. S5B).
We have previously shown a reduction in actin polymerization in HIV-1- infected CEM cells treated with PGE2 (Fig. 4A). Moreover, it has previously been reportedthat Rap1 negatively regulates Rhoin other systems[50], and it is clear that Rho controls actin stability. Because we found that PGE2 induces Rap1 activation and inhibition of actin polymerization, we sought to study Rho activation. We observed that PGE2 treatment of CEM cells produced decreased RhoA activity (Fig. 6E).
Discussion
Virus infections often cause acute inflammatory responses that are mediated by several cellular effectors and soluble factors. Although these responses have an important protective role, they may also have deleterious effects on the host. The balance between these protective and deleterious effects may ultimately determine the course of disease after viral infection. PGs are important regulators of this inflammatory reaction.
PGs serve as second messengers that elicit a wide range of physiological responses in cells and tissues. In particular, the PGs of the E series are known to have immunomodulatory properties. In addition to mediating inflammatory symptoms, PGs may exert anti-inflammatory effects. For example, PGE2 inhibits the secretion of IFN-γ, a cytokine that has antiviral activity [51], and switches the immune response toward a Th2-type cytokine profile (IL-4 and IL-5), which are less effective in developing an antiviral response [9]. In addition, a direct role for COX and PGs in controlling viral replication has been described for a wide range of virus infections, but their actions appear to be dependent on both the virus and cell type [52]. For instance, COX and/or PG is required for efficient replication of herpes viruses [53], [54], bovine leukemia virus [55], and rotavirus [56]. In the case of HIV-1, PGE2 has been shown to stimulate virus replication by activating viral promoters [36]. It has been described that PG negatively affects HIV replication in macrophages [7]. However, the mechanisms by which PGs regulate viral replication are largely unclear.
We have examined how PGE2 treatment affects HIV infection. We have found a decrease (35–40%) in p24 Ag levels after a single post-infection PGE2 treatment (data no shown).Repetitive dosing every 2 d increased the inhibition values of p24 Ag obtained 3 dpi from an initial 35% to 55% or even 60% in the case of the highest PGE2 dose (1 µM). As described above, PGE2 has a very short half-life [32]. Therefore, it is logical to find an increase of the inhibitory effect on p24Ag production after repetitive dosing. However, the results obtained after 5 d of infection were very similar to those found 3 d post-infection, indicating that the maximum inhibition was reached after 3 d of treatment. Moreover, the inhibitory effect of PGE2 on viral replication was independent of the viral isolate used, indicating a viral co-receptor type independency. One of the most interesting aspects was the discrepancy between the results of p24 Ag quantification in the culture supernatant of PBL and in cell lysates at 1 dpi. These data indicated that PGE2 affected HIV production and not “de novo” synthesis of p24 Ag.
PGE2 is well known to regulate cell functions through cAMP. It has been shown that cAMP inhibits cell proliferation and reduces the effector functions in T-cells [57]. Our results show that HIV-1 inhibition mediated by PGE2 is not due to a change in cell proliferation. However, it has been reported that cAMP may stimulate cellular proliferation by acting synergistically with other growth factors. The mitogenic effects of cAMP are PKA and Epac-dependent and involve modulation of downstream signaling networks essential for proliferation [58], [59]. The differences between the results of different studies might be due to different cell models used as well as the time of treatment.
A previous study of the effect of PGE2 treatment on HIV-1 replication in human PBMCs reported that the cell surface expression of CCR5 was substantially decreased in response to treatment with 10−5 M PGE2 for 2 d. The observed decrease in HIV-1 replication in that study was attributed to the decrease in the CCR5 co-receptor expression and was interpreted to mean that HIV-1 entry into the cells was decreased [33]. Here, we ruled out any similar effect because we did not find any modulation of surface CD4, CXCR4 or CCR5 levels after PGE2 treatment.
Initially, the effects of cAMP were solely attributed to activation of PKA and cAMP-gated ion channels, but the contribution of the alternative cAMP target Epac has become increasingly appreciated (reviewed in [60]. Although some cellular responses associated with intracellular cAMP increase are regulated exclusively by the PKA [61]or Epac [62] signaling pathways, in many processes, they are frequently interconnected [63] or can even play opposing roles [64]. The synthesis and characterization of cAMP analogs that selectively bind and activate either PKA or Epac have now made it possible to discriminate between the two signaling pathways [65].
Epac proteins were initially characterized as guanine nucleotide exchange factors (GEF) for both Rap1 and Rap2 [66]. Rap belongs to the Ras family of small G proteins, which cycle between an inactive guanosine diphosphate (GDP)-bound state and an active guanosine triphosphate (GTP)-bound state. Many effectors proteins, including adaptor proteins implicated in the modulation of the actin cytoskeleton, regulators of G proteins of the Rho family and phospholipases (reviewed in [60]) to relay signaling downstream from Rap. Moreover, Rap1A positively regulates T-cell signaling, and these effects have been reported to be mediated via the activation of integrins [67], [68]. We have demonstrated that PGE2 is able to increase cAMP levels. Our findings are consistent with the fact that the effects of PGE2 are predominantly mediated through Epac/Rap1 rather than through PKA. Our evidence that the observed effect of PGE2 is independent of PKA is based on our findings that the specific PKA inhibitors used did not produce any increase inHIV-1 replication. Moreover, the specific analog 6-Bnz-1 at 100 µM was indeed unable to reduce Agp24 production to the same level as 8-CPT did. PGE2 can exert its actions through one of several signaling pathways, depending on the receptor subtype through which the cellular response is mediated. Our results show that ERK and PI3K inhibitors are unable to reverse the inhibitory PGE2 effectof HIV-1 replicationin human T cells.
Within the HIV life cycle, we can differentiate several stages: entry, reverse transcription of the viral genome, integration of proviral DNA, genome transcription to give rise to new copies of RNA and new proteins for viral assembly and exit of new viral particles [69]. In recent years, a great variety of proteins and cellular factors involved in each of the stages of the cycle have been described that are therefore capable of altering HIV-1 replication [70]. PGE2 is one of the host factors that has been described as a modulator of the HIV cycle. However, there is little data about PGE2 related to a decrease of viral replication [7], [33] given that our results are the first to elicit this effect in T cells. In our study, the screening of the viral cycle stages discounted PGE2 involvement in the processes of entry, reverse transcription, integration, viral transcription and translation. All of our results pointed to a role for PGE2 in the late stages of the HIV-1 viral cycle such as viral assembly or budding. To elucidate the effect of PGE2 on viral assembly, we performed confocal microscopy experiments to analyze the Gag distribution in CEM-T cells. Microscopic examination of Gag in untreated cells revealed co-localization to a polarized F-actin distribution. PGE2 treatment (0.1 µM) changed F-actin and Gag delivery patterns; both staining patterns were redistributed and dispersed around the inner leaflet of the plasma membrane and within the cytoplasm. These results indicated a possible role of PGE2 in actin polymerization and distribution that was confirmed by our flow cytometry experiments, which showed a decrease in the number of F-actin-positive cells in PGE2-treated CEM-T cells. Some studies have shown the involvement of actin in the assembly and output of new HIV progeny by the use of actin polymerization inhibitors [24], [25]. The mechanism of transport of HIV-1 Gag and Env to the site of virion assembly is a directed process that is currently under intense scrutiny. It is known that F- actin is associated with the nucleocapsid (NC) domain of the polyprotein Gag, thus allowing its transport to assembly areas [40]–[43]. A recent article reported that the HIV maturation process takes place next to the plasma membrane where there is the greatest concentration of Gag and Gag/Pol polyprotein. Thus, the changes in Gag location and differences in the Gag/Gag-Pol ratio modify the infectivity of the new viral particles [71]. Our data showed a significant reduction in the output process of new viral particles (30%) and a significant decrease in the infectivity of the new viral particles (40%). Taken together, and using all of our data, we could attribute the decrease in infectivity to a diminished Gag concentration during viral assembly and at the maturation sites, leading to fewer p24-gag molecules per virus; in further studies, experiments will be performed to confirm this hypothesis.
In addition to infection with cell-free virions, the importance of cell-to-cell transmission in HIV-1 propagation, referred to as VS, is increasingly recognized and it is thought to constitute the predominant mechanism of HIV-1 propagation in T-lymphocyte cultures [26]–[29], [72], [73]. HIV-1 transmission across the VS depends on cell polarization, including the dynamic reorganization of the actin cytoskeleton and recruitment of virion components to cell-cell contacts [30], [41], [74]. Thus, we researched the impact of PGE2 treatment on HIV cell-to-cell transfer, obtaining a significant decrease in the viral transfer of PGE2-treated cells compared with untreated cells. Our data described above show a decrease in actin polymerization in the presence ofPGE2; these data are in agreement with the data published by Clare Jolly, who showed that cell treatment with actin polymerization inhibitors decreased the number of VS as a consequence of the inhibition of Gag and Env capping.
In summary, we demonstrated that PGE2 triggered the inhibition of HIV through Epac and Rap-1. The activation of these proteins altered the actin-dependent transport of newly synthesized HIV-1 components to the assembly platform site, and this disruption reduced HIV-1 transmission, cell-free virion release and viral infectivity. Several studies have reported an increase in PGE2 serum levels in HIV-infected patients as a consequence of viral infection and oxidative stress [4]-[6]. Although in patients HIV-1 viral load depends on multiple factors, it would be required to study differences in HIV-1 viral load as consequence of their PGE2 levels in serum.
Supporting Information
Inhibition of HIV-1Bal replication in human PBL by post-treatment with PGE2. Human PBL were infected with R5 strain HIVBaL (15 ng/106 cells) for 2 h and treated with PGE2 (0.1, 1 µM) for 3 days. AZT (0.5 µM) was used as a positive control of inhibition. HIV-1 infection was monitored by measuring Agp24 production in supernatants by ELISA at 3 d. Results are shown as mean ± SEM and expressed as a percentage of the value of the untreated control cultures. Statistical differences in comparison to HIV-1-infected cells *:p<0.05.; **:p<0.01.
(TIF)
PGE2 effects are not due to changes in cell viability, proliferation, activation or cell receptors. A) Human activated PBL were treated with PGE2 at doses of 0.1, 1 µM, and cell viability was measured by MTT assay 3, 5, and 7 d later. B) Human activated PBL were exposed to 0.1, 1 µM of PGE2, and 3 d later cell proliferation was measured by incorporation of BrdU. C) Expression of CD69, and CD38 after PGE2 treatment at the indicated times. D) Activated PBL were treated with PGE2 (0.1 µM), and CD4, CXCR4 and CCR5 surface expression was evaluated by flow cytometry at the indicated times. The experiments showed are the mean of three independent experiments. Live cells were gated according to forward and side scatter profiles. Results represent the means of 3 individual experiments. Error bars indicate standard error values.
(TIF)
Effect of PMA in LTR-promoter expression. PMA treatment for 3 d increase p24 core protein levels in 8E5 cell line culture supernatants about 10 times.
(TIF)
HIV free-viral particle. Purified primary CD4 T cells were infected during 3 d with HIV-1NL4-3 isolate, treated with PGE2, and then co-cultured either with the CMSF labeled target cells. Effector and target cells were seeded at a 2∶1 ratio separated in transwell chambers with a virus-permeable membrane (0.4 µm pore size). Virus transfer was assessed by flow cytometry for intracellular Gag CA p24 in target cells at 6 h after the start of co-culture. Results are shown as a percentage of positive cells for HIV-1 transfer ± SEM of 3 independent experiments.
(TIF)
Western blot of A) p-CREB and B) p-AKT in PGE2-stimulated CEM-T cells at the indicated times. Bottom, the graph depicting the results obtained after performing a densitometer analysis of the blots. Western blot representative of three is shown.
(TIF)
Acknowledgments
The authors thank the Spanish HIV HGM BioBank supported by ISC III (Grant n° RD09/0076/00103). Also, we thank Dr. Laura Díaz for her technical assistance of the Flow Cytometry Unit (CA11/00290) and Rafael Samaniego for his technical assistance in the Confocal Microscopy unit.
Funding Statement
This work was supported by grants provided by Fondo de Investigacion Sanitaria en España (FIS) [grant numbers INTRASALUD PS09/02029, RD09/0076/00103]; the Fundación Eugenio Rodríguez Pascual; Red Española de Investigación en SIDA (RIS) [grant numbers RETIC RD06/0006/0035, RD12-0017-0037]; “Fundación para la Investigación y la Prevención del Sida en España” (FIPSE); and Comunidad de Madrid [INDISNET S-2010/BMD-2332]. MIC is supported by Fondo de Investigación Sanitaria (CA10/01274). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hilkens CM, Vermeulen H, van Neerven RJ, Snijdewint FG, Wierenga EA, et al. (1995) Differential modulation of T helper type 1 (Th1) and T helper type 2 (Th2) cytokine secretion by prostaglandin E2 critically depends on interleukin-2. Eur J Immunol 25: 59–63. [DOI] [PubMed] [Google Scholar]
- 2. Graziosi C, Gantt KR, Vaccarezza M, Demarest JF, Daucher M, et al. (1996) Kinetics of cytokine expression during primary human immunodeficiency virus type 1 infection. Proc Natl Acad Sci U S A 93: 4386–4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Breen EC, McDonald M, Fan J, Boscardin J, Fahey JL (2000) Cytokine gene expression occurs more rapidly in stimulated peripheral blood mononuclear cells from human immunodeficiency virus-infected persons. Clin Diagn Lab Immunol 7: 769–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Elbim C, Pillet S, Prevost MH, Preira A, Girard PM, et al. (2001) The role of phagocytes in HIV-related oxidative stress. J Clin Virol 20: 99–109. [DOI] [PubMed] [Google Scholar]
- 5. Lu Y, Wahl LM (2005) Oxidative stress augments the production of matrix metalloproteinase-1, cyclooxygenase-2, and prostaglandin E2 through enhancement of NF-kappa B activity in lipopolysaccharide-activated human primary monocytes. J Immunol 175: 5423–5429. [DOI] [PubMed] [Google Scholar]
- 6. Pereira CF, Boven LA, Middel J, Verhoef J, Nottet HS (2000) Induction of cyclooxygenase-2 expression during HIV-1-infected monocyte-derived macrophage and human brain microvascular endothelial cell interactions. J Leukoc Biol 68: 423–428. [PubMed] [Google Scholar]
- 7. Hayes MM, Lane BR, King SR, Markovitz DM, Coffey MJ (2002) Prostaglandin E(2) inhibits replication of HIV-1 in macrophages through activation of protein kinase A. Cell Immunol 215: 61–71. [DOI] [PubMed] [Google Scholar]
- 8. Snijdewint FG, Kalinski P, Wierenga EA, Bos JD, Kapsenberg ML (1993) Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J Immunol 150: 5321–5329. [PubMed] [Google Scholar]
- 9. Betz M, Fox BS (1991) Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J Immunol 146: 108–113. [PubMed] [Google Scholar]
- 10. Roper RL, Phipps RP (1994) Prostaglandin E2 regulation of the immune response. Adv Prostaglandin Thromboxane Leukot Res 22: 101–111. [PubMed] [Google Scholar]
- 11. Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, et al. (1999) Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med 5: 698–701. [DOI] [PubMed] [Google Scholar]
- 12. van der Pouw Kraan TC, van Lier RA, Aarden LA (1995) PGE2 and the immune response. A central role for prostaglandin E2 in downregulating the inflammatory immune response. Mol Med Today 1: 61. [PubMed] [Google Scholar]
- 13. Trebino CE, Stock JL, Gibbons CP, Naiman BM, Wachtmann TS, et al. (2003) Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc Natl Acad Sci U S A 100: 9044–9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goetzl EJ, An S, Zeng L (1995) Specific suppression by prostaglandin E2 of activation-induced apoptosis of human CD4+CD8+ T lymphoblasts. J Immunol 154: 1041–1047. [PubMed] [Google Scholar]
- 15. Narumiya S, Sugimoto Y, Ushikubi F (1999) Prostanoid receptors: structures, properties, and functions. Physiol Rev 79: 1193–1226. [DOI] [PubMed] [Google Scholar]
- 16. Regan JW (2003) EP2 and EP4 prostanoid receptor signaling. Life Sci 74: 143–153. [DOI] [PubMed] [Google Scholar]
- 17. Aronoff DM, Oates JA, Boutaud O (2006) New insights into the mechanism of action of acetaminophen: Its clinical pharmacologic characteristics reflect its inhibition of the two prostaglandin H2 synthases. Clin Pharmacol Ther 79: 9–19. [DOI] [PubMed] [Google Scholar]
- 18. Aronoff DM, Canetti C, Serezani CH, Luo M, Peters-Golden M (2005) Cutting edge: macrophage inhibition by cyclic AMP (cAMP): differential roles of protein kinase A and exchange protein directly activated by cAMP-1. J Immunol 174: 595–599. [DOI] [PubMed] [Google Scholar]
- 19. Noda S, Yashiro M, Nshii T, Hirakawa K (2010) Hypoxia upregulates adhesion ability to peritoneum through a transforming growth factor-beta-dependent mechanism in diffuse-type gastric cancer cells. Eur J Cancer 46: 995–1005. [DOI] [PubMed] [Google Scholar]
- 20. Grandoch M, Roscioni SS, Schmidt M (2010) The role of Epac proteins, novel cAMP mediators, in the regulation of immune, lung and neuronal function. Br J Pharmacol 159: 265–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ploubidou A, Way M (2001) Viral transport and the cytoskeleton. Curr Opin Cell Biol 13: 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Naghavi MH, Goff SP (2007) Retroviral proteins that interact with the host cell cytoskeleton. Curr Opin Immunol 19: 402–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jolly C, Mitar I, Sattentau QJ (2007) Adhesion molecule interactions facilitate human immunodeficiency virus type 1-induced virological synapse formation between T cells. J Virol 81: 13916–13921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sasaki H, Nakamura M, Ohno T, Matsuda Y, Yuda Y, et al. (1995) Myosin-actin interaction plays an important role in human immunodeficiency virus type 1 release from host cells. Proc Natl Acad Sci U S A 92: 2026–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sasaki H, Ozaki H, Karaki H, Nonomura Y (2004) Actin filaments play an essential role for transport of nascent HIV-1 proteins in host cells. Biochem Biophys Res Commun 316: 588–593. [DOI] [PubMed] [Google Scholar]
- 26. Mothes W, Sherer NM, Jin J, Zhong P (2010) Virus cell-to-cell transmission. J Virol 84: 8360–8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sol-Foulon N, Sourisseau M, Porrot F, Thoulouze MI, Trouillet C, et al. (2007) ZAP-70 kinase regulates HIV cell-to-cell spread and virological synapse formation. EMBO J 26: 516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rudnicka D, Feldmann J, Porrot F, Wietgrefe S, Guadagnini S, et al. (2009) Simultaneous cell-to-cell transmission of human immunodeficiency virus to multiple targets through polysynapses. J Virol 83: 6234–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sourisseau M, Sol-Foulon N, Porrot F, Blanchet F, Schwartz O (2007) Inefficient human immunodeficiency virus replication in mobile lymphocytes. J Virol 81: 1000–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hubner W, McNerney GP, Chen P, Dale BM, Gordon RE, et al. (2009) Quantitative 3D video microscopy of HIV transfer across T cell virological synapses. Science 323: 1743–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Garcia-Merino I, de Las Cuevas N, Jimenez JL, Gallego J, Gomez C, et al. (2009) The Spanish HIV BioBank: a model of cooperative HIV research. Retrovirology 6: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Watzer B, Zehbe R, Halstenberg S, James Kirkpatrick C, Brochhausen C (2009) Stability of prostaglandin E(2) (PGE (2)) embedded in poly-D,L: -lactide-co-glycolide microspheres: a pre-conditioning approach for tissue engineering applications. J Mater Sci Mater Med 20: 1357–1365. [DOI] [PubMed] [Google Scholar]
- 33. Thivierge M, Le Gouill C, Tremblay MJ, Stankova J, Rola-Pleszczynski M (1998) Prostaglandin E2 induces resistance to human immunodeficiency virus-1 infection in monocyte-derived macrophages: downregulation of CCR5 expression by cyclic adenosine monophosphate. Blood 92: 40–45. [PubMed] [Google Scholar]
- 34. Greene WC, Peterlin BM (2002) Charting HIV's remarkable voyage through the cell: Basic science as a passport to future therapy. Nat Med 8: 673–680. [DOI] [PubMed] [Google Scholar]
- 35. Bukrinsky M (2004) A hard way to the nucleus. Mol Med 10: 1–5. [PMC free article] [PubMed] [Google Scholar]
- 36. Dumais N, Barbeau B, Olivier M, Tremblay MJ (1998) Prostaglandin E2 Up-regulates HIV-1 long terminal repeat-driven gene activity in T cells via NF-kappaB-dependent and -independent signaling pathways. J Biol Chem 273: 27306–27314. [DOI] [PubMed] [Google Scholar]
- 37. Dumais N, Bounou S, Olivier M, Tremblay MJ (2002) Prostaglandin E(2)-mediated activation of HIV-1 long terminal repeat transcription in human T cells necessitates CCAAT/enhancer binding protein (C/EBP) binding sites in addition to cooperative interactions between C/EBPbeta and cyclic adenosine 5′-monophosphate response element binding protein. J Immunol 168: 274–282. [DOI] [PubMed] [Google Scholar]
- 38. Gendelman HE, Theodore TS, Willey R, McCoy J, Adachi A, et al. (1987) Molecular characterization of a polymerase mutant human immunodeficiency virus. Virology 160: 323–329. [DOI] [PubMed] [Google Scholar]
- 39. Laurence J, Cooke H, Sikder SK (1990) Effect of tamoxifen on regulation of viral replication and human immunodeficiency virus (HIV) long terminal repeat-directed transcription in cells chronically infected with HIV-1. Blood 75: 696–703. [PubMed] [Google Scholar]
- 40. Graziano F, Elia C, Laudanna C, Poli G, Alfano M (2011) Urokinase plasminogen activator inhibits HIV virion release from macrophage-differentiated chronically infected cells via activation of RhoA and PKCepsilon. PLoS One 6: e23674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jolly C, Mitar I, Sattentau QJ (2007) Requirement for an intact T-cell actin and tubulin cytoskeleton for efficient assembly and spread of human immunodeficiency virus type 1. J Virol 81: 5547–5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gladnikoff M, Shimoni E, Gov NS, Rousso I (2009) Retroviral assembly and budding occur through an actin-driven mechanism. Biophys J 97: 2419–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cooper J, Liu L, Woodruff EA, Taylor HE, Goodwin JS, et al. (2011) Filamin A protein interacts with human immunodeficiency virus type 1 Gag protein and contributes to productive particle assembly. J Biol Chem 286: 28498–28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lehmann M, Nikolic DS, Piguet V (2011) How HIV-1 takes advantage of the cytoskeleton during replication and cell-to-cell transmission. Viruses 3: 1757–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vasiliver-Shamis G, Cho MW, Hioe CE, Dustin ML (2009) Human immunodeficiency virus type 1 envelope gp120-induced partial T-cell receptor signaling creates an F-actin-depleted zone in the virological synapse. J Virol 83: 11341–11355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jolly C, Kashefi K, Hollinshead M, Sattentau QJ (2004) HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J Exp Med 199: 283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cui Q, So KF (2004) Involvement of cAMP in neuronal survival and axonal regeneration. Anat Sci Int 79: 209–212. [DOI] [PubMed] [Google Scholar]
- 48. Nijholt IM, Dolga AM, Ostroveanu A, Luiten PG, Schmidt M, et al. (2008) Neuronal AKAP150 coordinates PKA and Epac-mediated PKB/Akt phosphorylation. Cell Signal 20: 1715–1724. [DOI] [PubMed] [Google Scholar]
- 49. Davies SP, Reddy H, Caivano M, Cohen P (2000) Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351: 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, et al. (2005) Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood 105: 1950–1955. [DOI] [PubMed] [Google Scholar]
- 51. Hasler F, Bluestein HG, Zvaifler NJ, Epstein LB (1983) Analysis of the defects responsible for the impaired regulation of EBV-induced B cell proliferation by rheumatoid arthritis lymphocytes. II. Role of monocytes and the increased sensitivity of rheumatoid arthritis lymphocytes to prostaglandin E. J Immunol 131: 768–772. [PubMed] [Google Scholar]
- 52. Steer SA, Corbett JA (2003) The role and regulation of COX-2 during viral infection. Viral Immunol 16: 447–460. [DOI] [PubMed] [Google Scholar]
- 53. Janelle ME, Gravel A, Gosselin J, Tremblay MJ, Flamand L (2002) Activation of monocyte cyclooxygenase-2 gene expression by human herpesvirus 6. Role for cyclic AMP-responsive element-binding protein and activator protein-1. J Biol Chem 277: 30665–30674. [DOI] [PubMed] [Google Scholar]
- 54. Symensma TL, Martinez-Guzman D, Jia Q, Bortz E, Wu TT, et al. (2003) COX-2 induction during murine gammaherpesvirus 68 infection leads to enhancement of viral gene expression. J Virol 77: 12753–12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pyeon D, Diaz FJ, Splitter GA (2000) Prostaglandin E(2) increases bovine leukemia virus tax and pol mRNA levels via cyclooxygenase 2: regulation by interleukin-2, interleukin-10, and bovine leukemia virus. J Virol 74: 5740–5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rossen JW, Bouma J, Raatgeep RH, Buller HA, Einerhand AW (2004) Inhibition of cyclooxygenase activity reduces rotavirus infection at a postbinding step. J Virol 78: 9721–9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. van Oirschot BA, Stahl M, Lens SM, Medema RH (2001) Protein kinase A regulates expression of p27(kip1) and cyclin D3 to suppress proliferation of leukemic T cell lines. J Biol Chem 276: 33854–33860. [DOI] [PubMed] [Google Scholar]
- 58. Holz GG, Chepurny OG, Schwede F (2008) Epac-selective cAMP analogs: new tools with which to evaluate the signal transduction properties of cAMP-regulated guanine nucleotide exchange factors. Cell Signal 20: 10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Roscioni SS, Elzinga CR, Schmidt M (2008) Epac: effectors and biological functions. Naunyn Schmiedebergs Arch Pharmacol 377: 345–357. [DOI] [PubMed] [Google Scholar]
- 60. Gloerich M, Bos JL (2010) Epac: defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol 50: 355–375. [DOI] [PubMed] [Google Scholar]
- 61. Huang S, Wettlaufer SH, Hogaboam C, Aronoff DM, Peters-Golden M (2007) Prostaglandin E(2) inhibits collagen expression and proliferation in patient-derived normal lung fibroblasts via E prostanoid 2 receptor and cAMP signaling. Am J Physiol Lung Cell Mol Physiol 292: L405–413. [DOI] [PubMed] [Google Scholar]
- 62. Lorenowicz MJ, van Gils J, de Boer M, Hordijk PL, Fernandez-Borja M (2006) Epac1-Rap1 signaling regulates monocyte adhesion and chemotaxis. J Leukoc Biol 80: 1542–1552. [DOI] [PubMed] [Google Scholar]
- 63. Bos JL (2006) Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci 31: 680–686. [DOI] [PubMed] [Google Scholar]
- 64. Mei FC, Qiao J, Tsygankova OM, Meinkoth JL, Quilliam LA, et al. (2002) Differential signaling of cyclic AMP: opposing effects of exchange protein directly activated by cyclic AMP and cAMP-dependent protein kinase on protein kinase B activation. J Biol Chem 277: 11497–11504. [DOI] [PubMed] [Google Scholar]
- 65. Enserink JM, Christensen AE, de Rooij J, van Triest M, Schwede F, et al. (2002) A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol 4: 901–906. [DOI] [PubMed] [Google Scholar]
- 66. Kawasaki H, Springett GM, Toki S, Canales JJ, Harlan P, et al. (1998) A Rap guanine nucleotide exchange factor enriched highly in the basal ganglia. Proc Natl Acad Sci U S A 95: 13278–13283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bivona TG, Wiener HH, Ahearn IM, Silletti J, Chiu VK, et al. (2004) Rap1 up-regulation and activation on plasma membrane regulates T cell adhesion. J Cell Biol 164: 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dustin ML, Bivona TG, Philips MR (2004) Membranes as messengers in T cell adhesion signaling. Nat Immunol 5: 363–372. [DOI] [PubMed] [Google Scholar]
- 69. Nisole S, Saib A (2004) Early steps of retrovirus replicative cycle. Retrovirology 1: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Goff SP (2007) Host factors exploited by retroviruses. Nat Rev Microbiol 5: 253–263. [DOI] [PubMed] [Google Scholar]
- 71. Haraguchi H, Sudo S, Noda T, Momose F, Kawaoka Y, et al. (2010) Intracellular localization of human immunodeficiency virus type 1 Gag and GagPol products and virus particle release: relationship with the Gag-to-GagPol ratio. Microbiol Immunol 54: 734–746. [DOI] [PubMed] [Google Scholar]
- 72. Blanco J, Bosch B, Fernandez-Figueras MT, Barretina J, Clotet B, et al. (2004) High level of coreceptor-independent HIV transfer induced by contacts between primary CD4 T cells. J Biol Chem 279: 51305–51314. [DOI] [PubMed] [Google Scholar]
- 73. Sattentau Q (2008) Avoiding the void: cell-to-cell spread of human viruses. Nat Rev Microbiol 6: 815–826. [DOI] [PubMed] [Google Scholar]
- 74. Jolly C, Sattentau QJ (2007) Human immunodeficiency virus type 1 assembly, budding, and cell-cell spread in T cells take place in tetraspanin-enriched plasma membrane domains. J Virol 81: 7873–7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Inhibition of HIV-1Bal replication in human PBL by post-treatment with PGE2. Human PBL were infected with R5 strain HIVBaL (15 ng/106 cells) for 2 h and treated with PGE2 (0.1, 1 µM) for 3 days. AZT (0.5 µM) was used as a positive control of inhibition. HIV-1 infection was monitored by measuring Agp24 production in supernatants by ELISA at 3 d. Results are shown as mean ± SEM and expressed as a percentage of the value of the untreated control cultures. Statistical differences in comparison to HIV-1-infected cells *:p<0.05.; **:p<0.01.
(TIF)
PGE2 effects are not due to changes in cell viability, proliferation, activation or cell receptors. A) Human activated PBL were treated with PGE2 at doses of 0.1, 1 µM, and cell viability was measured by MTT assay 3, 5, and 7 d later. B) Human activated PBL were exposed to 0.1, 1 µM of PGE2, and 3 d later cell proliferation was measured by incorporation of BrdU. C) Expression of CD69, and CD38 after PGE2 treatment at the indicated times. D) Activated PBL were treated with PGE2 (0.1 µM), and CD4, CXCR4 and CCR5 surface expression was evaluated by flow cytometry at the indicated times. The experiments showed are the mean of three independent experiments. Live cells were gated according to forward and side scatter profiles. Results represent the means of 3 individual experiments. Error bars indicate standard error values.
(TIF)
Effect of PMA in LTR-promoter expression. PMA treatment for 3 d increase p24 core protein levels in 8E5 cell line culture supernatants about 10 times.
(TIF)
HIV free-viral particle. Purified primary CD4 T cells were infected during 3 d with HIV-1NL4-3 isolate, treated with PGE2, and then co-cultured either with the CMSF labeled target cells. Effector and target cells were seeded at a 2∶1 ratio separated in transwell chambers with a virus-permeable membrane (0.4 µm pore size). Virus transfer was assessed by flow cytometry for intracellular Gag CA p24 in target cells at 6 h after the start of co-culture. Results are shown as a percentage of positive cells for HIV-1 transfer ± SEM of 3 independent experiments.
(TIF)
Western blot of A) p-CREB and B) p-AKT in PGE2-stimulated CEM-T cells at the indicated times. Bottom, the graph depicting the results obtained after performing a densitometer analysis of the blots. Western blot representative of three is shown.
(TIF)