Abstract
Objective
Although the relation between lifetime depression and smoking cessation outcome has been well-studied, the proposition that different symptomatic expressions of depression exert disparate predictive effects on risk of smoking cessation failure has largely gone uninvestigated. This study analyzed the individual contributions of depression's two hallmark affective symptoms, anhedonia (i.e., diminished interest in normally enjoyable activities) and depressed mood (i.e., elevated sadness), to the prediction of smoking cessation outcome.
Method
Participants were adult daily smokers (N=1469; Mean age = 45 years, 58% Female, 84% White) enrolled in a smoking cessation treatment study. Lifetime history of anhedonia and depressed mood were classified via structured interview prior to quit day. Seven-day point prevalence smoking abstinence was assessed at 8-weeks and 6-months post-quit.
Results
When examined separately, both lifetime anhedonia, OR(95% CI)=1.42(1.16-1.73), p=.004, and depressed mood, OR(95% CI)=1.35(1.11-1.63), p=.002, predicted increased odds of relapse. These relations remained after adjusting for covariates, including lifetime depressive disorder, which did not predict outcome. After controlling for the covariation between lifetime anhedonia and depressed mood, anhedonia predicted cessation outcome, OR(95% CI)=1.31(1.05-1.62), p=.02, while depressed mood did not, p=.19. Symptom duration (>2 weeks), treatment, and substance use disorder did not modify relations of lifetime anhedonia and depressed mood with cessation outcome.
Conclusions
Results suggest that: (1) symptoms of affective disturbance capture depression-relevant risk of cessation failure, which is not adequately demarcated by the lifetime depressive disorder diagnosis; and (2) anhedonia is a more sensitive index of this affective disturbance than depressed mood per se. Clinical attention to anhedonia may facilitate smoking cessation.
Keywords: Anhedonia, Depressed Mood, Smoking, Smoking Cessation, Depression
The relation between lifetime depression and smoking cessation outcome has been extensively studied, but remains poorly understood. One important barrier to understanding this association is that important sources of variation within depression may alter how depression confers risk of smoking relapse. Indeed, a recent meta-analytic study showed a modest but statistically significant overall relation between pre-quit lifetime depression status and post-quit relapse risk (Hitsman et al., 2013), but also identified several moderators of this relation, including recency of depression, type of depression measure, and other factors (Hitsman et al., 2013). Other research that indicates that recurrence is another important source of heterogeneity, as recurrent (vs. single episode) forms of lifetime depression predict poorer cessation outcome (Brown et al., 2001; Haas, Munoz, Humfleet, Reus, & Hall, 2004).
One important, yet often overlooked, issue is depression's symptomatic heterogeneity. Depression comprises a diverse array of symptoms spanning affective (e.g., sadness), behavioral (e.g., psychomotor changes), cognitive (e.g., concentration problems), and vegetative (e.g., sleep and appetite disruption) features that loosely cluster together (Zimmerman, McGlinchey, Young, & Chelminski, 2006). Given the diversity of depressive symptoms, is possible that only certain symptomatic expressions of depression increase risk of relapse, whereas others are relatively benign with regard to smoking cessation. Thus, combining all symptoms of depression into a single diagnostic category may increase error and obscure important variability in relapse risk within the population of smokers with lifetime depressive symptoms and syndromes.
Anhedonia (i.e., diminished interest or pleasure in normally enjoyable activities) and depressed mood (i.e., elevated sadness) constitute the two hallmark features of depression (APA, 1994). Either anhedonia or depressed mood is required (in addition to at least four other symptoms) to qualify for a DSM-IV major depression diagnosis (APA, 1994). Although they are both key symptoms of the same syndrome (i.e., depression), anhedonia and depressed mood are empirically distinct (Zimmerman et al., 2006; i.e., anhedonia commonly occurs without concurrent depressed mood and depresssed mood occurs without concurrent anhedoina), have unique neural correlates (Wacker, Dillon, & Pizzagalli, 2009), and are putatively distinct depressive endophenotypes (Hasler, Drevets, Manji, & Charney, 2004).
Research suggests that these two facets of depression are particularly relevant for smoking cessation because they both appear to impact smoking motivation and do so via discrete mechanisms. There is copious evidence that negative affect states, including sadness, influences smoking motivation (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004; Falcone et al., 2012; Leventhal et al., 2013; Litvin & Brandon, 2010). Further, trait depressed mood and negative affect predict greater exacerbations in state negative affect and urge to smoke for negative affect relief upon tobacco abstinence (Gilbert et al., 1998; Leventhal et al., 2013). Anhedonia is also associated with smoking motivation and some research suggests that anhedonic individuals smoke in order to enhance the ability to enjoy activities and experience pleasure (Cook, Spring, & McChargue, 2007; Leventhal, Waters, Kahler, Ray, & Sussman, 2009). Furthermore, anhedonia predicts declines in state positive affect as well as increases in urge to smoke for pleasure upon tobacco abstinence (Cook, Spring, McChargue, & Hedeker, 2004; Leventhal, Ameringer, Osborne, Zvolensky, & Langdon, in press; Leventhal et al., 2009). Thus, the overarching depression construct could heighten risk of cessation failure via two distinct contingencies: (1) omission training whereby abstinence produces deficits in positive affect in anhedonic individuals, which results in a “time out” from reward and strong drive to re-attain smoking-mediated reward, and/or (2) negative reinforcement in which smokers with depressed mood become hyper motivated to escape the distress (negative affect) of withdrawal.
Despite the putatively important roles of anhedonia and depressed mood in smoking, empirical data on the relative risk of smoking relapse conferred by lifetime anhedonia and depressed mood is lacking. Yet, distilling the elements of depression that most powerfully predict smoking cessation failure could: (1) elucidate the motivational processes that maintain addiction, (2) meaningfully increase the prediction of cessation failure by reducing error in the predictor, and (3) suggest new relapse prevention interventions that address the core elements of depression-related vulnerability.
The current study addresses two critical questions in an effort to distill the depression phenotype as it is related to risk of cessation failure. Does lifetime anhedonia or depressed mood (irrespective of whether they occur in conjunction with a clinical depressive disorder) predict cessation outcomes? Do both types of affective symptoms make independent, additive contributions to prediction, or is one symptom prepotent in accounting for the relation? We focuses on affective as opposed to other types of depressive symptoms because prior cessation research using paper-and-pencil symptom indices illustrate that anhedonia and depressed mood predict poor cessation outcomes (Cook, Spring, McChargue, & Doran, 2010; Leventhal, Ramsey, Brown, LaChance, & Kahler, 2008; Niaura et al., 2001; c.f., Schnoll, Leone, & Hitsman, 2013), whereas non-affective dimensions of depression (e.g., somatic features manifested as sleep problems, appetite changes, concentration problems, and psychomotor slowing) do not directly or incrementally augment such predictions (Leventhal et al., 2008; Schnoll et al., 2013). Similarly, tobacco withdrawal research demonstrates that affective withdrawal symptoms predict cessation failure more consistently than non-affective withdrawal symptoms (McCarthy, Piasecki, Fiore, & Baker, 2006; Piasecki et al., 2000). Thus, affective features may perhaps capture relatively pure facets of depression that directly magnify smoking motivation during a quit attempt.
We hypothesized that lifetime history of anhedonia and depressed mood would predict poorer cessation outcomes over and above lifetime DSM-IV classified depressive disorder, which represents an amalgam of cognitive, behavioral, and vegetative features. Given that anhedonia and depressed mood might impede smoking cessation through distinct affective mechanisms (i.e., reward enhancement vs. distress relief), we further hypothesized that these two features would yield additive predictive effects. This research will address these hypotheses using DSM-based interview assessment of anhedonia and depressed mood (Hitsman et al., 2011). Because DSM-based indices are commonly used in treatment settings, their use in this research permits broader and more immediate application of the knowledge derived from them.
Method
Participants and Procedure
Residents of the southeastern Wisconsin region were recruited via community flyers and TV, radio, and newspaper advertisements from January 2005 to June 2007 to participate in a smoking cessation clinical trial (Piper et al., 2009).1 Inclusion criteria were: (1) ≥ 10 cigarettes per day for the past 6 months; (2) carbon monoxide (CO) level > 9 parts per million (ppm); and (3) desire to quit. Exclusion criteria were: (a) current use of non-cigarette forms of tobacco; (b) medical contraindications to study medications; (c) psychiatric disorders contraindicated for medications (e.g., psychosis, eating disorder); (c) currently drink ≥ 6 alcoholic beverages daily; (d) pregnant or breast-feeding; and (e) medical condition precluding study completion.
Following a phone screen, eligible smokers first attended an information session and provided informed consent. Participants then completed more in-depth screening, including a medical history, vital signs measurements, and a carbon monoxide (CO) breath test. Participants also completed questionnaires assessing demographic, smoking history, and tobacco dependence at that visit. Eligible participants then attended three baseline assessment visits: (1) a psychiatric interview and questionnaire assessment session; (2) a medical assessment session (e.g., lipid profiles, diabetes screen), and (3) a pre-quit session involving setting a quit day for the following week, cessation counseling, and randomization into one of six treatment conditions (Bupropion SR [n=264; 150 mg twice per day for one week pre-quit and 8 weeks post-quit]; Nicotine lozenge [n=260; 2 or 4 mg lozenges, dose based on package instructions for 12 weeks post-quit]; Nicotine patch [n=262; 24-hour patch of 21, 14, and 7 mg titrated over 8 weeks post-quit]; Nicotine patch + Nicotine lozenge [n=267]; Bupropion SR + Nicotine lozenge [n=262]; or Placebo [n=189; five conditions that matched the five active conditions]). After the pre-quit visits, study visits occurred on quit day and 1, 2, 4, and 8 weeks after quitting. All participants received individual counseling at the pre-quit visit, quit day, and every subsequent study visit up to 8 weeks post-quit day. Following U.S. Public Health Service Guidelines (USDHHS, 2008), the manualized counseling sessions lasted 10 to 20 minutes each and provided social support as well as problem-solving training. Counselors were bachelor's-level case managers supervised by a licensed clinical psychologist. Randomization was double-blind and blocked on gender and race.
Of the 5269 individuals who completed a phone screen, 2120 did not meet eligibility criteria for various reasons (e.g., insufficient motivation to quit [26%], smoked fewer than 10 cig/day [13%], psychosis/severe mental illness [4.5%], drinking ≥ 6 alcoholic beverages daily [2%], use of exclusionary medications [15%]). Among the eligible participants, 1504 were randomized into one of the treatment arms and 1470 completed the baseline psychiatric interview. One participant refused to answer the anhedonia item, resulting in a final N of 1469 (see Figure 1). Participants with versus without complete data did not significantly differ on any study variable. This study was approved by the University of Wisconsin Internal Review Board.
Figure 1.
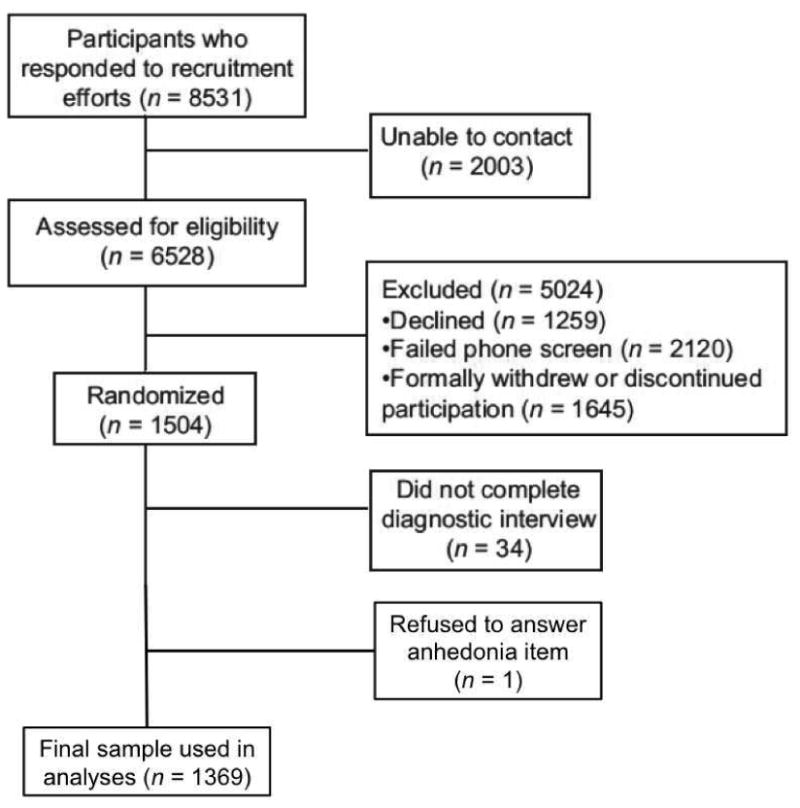
Study flow diagram.
Measures
Fagerström Test of Nicotine Dependence (FTND)
The FTND is a well-validated six item measure of dependence severity (Heatherton, Kozlowski, Frecker, & Fagerström, 1991).
World Mental Health Survey Initiative Version of the Composite International Diagnostic Interview (CIDI)
The CIDI is a well-validated structured interview, which provided information for diagnosing lifetime DSM-IV psychiatric disorders (Kessler & Ustun, 2004). Trained interviewers administered modules for only key disorders to reduce participant burden (i.e., lifetime social phobia [diagnostic prevalence in the current sample: n = 199, 13.6%], panic attacks [n = 455, 31.0%], generalized anxiety disorder [n = 99, 6.7%], major depressive episode [n = 259, 17.6%], dysthymic disorder [n = 33, 23.3%], mania/hypomania [n = 47, 3.2 %,], alcohol abuse [n = 714, 48.6%], alcohol dependence [n = 142, 9.7%], drug abuse [n = 372, 25.2%], and drug dependence [n = 87, 5.9%]).
Lifetime anhedonia and depressed mood were classified based on the CIDI Major Depressive Episode DSM-IV-based screener items: “Have you ever had a period lasting several days or longer when you lost interest in most things you usually enjoy like work, hobbies, and personal relationships?” (yes/no); and “Have you ever in your life had a period lasting several days or longer when most of the day you felt sad, empty or depressed?” (yes/no).2 For supplemental analyses, we further categorized participants who endorsed anhedonia and depressed mood on symptom chronicity based on the follow up query, “Did you ever have a period of [depressed mood/anhedonia] that lasted most of the day nearly every day for two weeks or longer?” (yes/no). We also coded lifetime diagnoses for depressive disorders (major depressive episode and/or dysthymic disorder) and other disorders (i.e., all other psychiatric disorders assessed) as covariates. For supplemental analyses, we also distinguished recurrent (i.e., two or more depressive episodes) versus single episode depression among participants with lifetime depression.
Smoking Outcomes
Seven-day point-prevalence abstinence (“Have you smoked at all, even a puff, in the last seven days?” yes/no) was assessed at 8 weeks and 6 months following the target quit day. Self-reports were confirmed by a CO < 10 ppm assessed using a Bedfont Smokerlyzer. Participants who did not provide outcome data were coded as non-abstinent.
Analytic Plan
Preliminary analyses involved computing descriptive statistics and intercorrelations amongst anhedonia, depressed mood, psychiatric disorders, demographics, and FTND score. As an initial primary analysis, we compared abstinence rates by anhedonia and depressed mood status at each follow-up point with separate Chi-squared tests. The chief primary analysis involved repeated measures analyses for binomial outcomes using generalized estimating equations (GEE; Zeger & Liang, 1986) in which 7-day point prevalence smoking abstinence at 8 weeks and 6 months after quit date served as the dependent variable. We first tested a set of individual models in which separate GEEs for lifetime anhedonia and depressed mood were conducted. We then tested a set of combined GEE models in which lifetime anhedonia and depressed mood were simultaneously included as predictors to illustrate their incremental effects after controlling for their covariance with one another. To analyze how anhedonia and depressed mood predicted cessation outcome incrementally with regard to other relevant variables, we tested the models in three stages: (1) baseline models that included only lifetime anhedonia and/or depressed mood, in addition to time, and treatment condition; (2) an adjusted model that added FTND and demographic variables that were significantly associated with anhedonia or depressed mood (only gender was associated; see Table 1); and (3) a further adjusted model that added lifetime history of depressive mood disorder and any other non-depressive disorder (i.e., substance use or anxiety disorder). For comparative purposes, we tested additional GEE models examining lifetime depressive disorder3 as a predictor of outcome with varying levels of covariate adjustment as described above. Several supplemental individual GEEs were also conducted to determine how symptom chronicity, depression recurrence, and substance use disorder (SUD), affected the relations of depressive symptoms with cessation outcomes. These controlled only for treatment and time and were conducted to further elucidate the nature and generalizability of the relations of lifetime anhedonia and depressed mood with cessation outcomes. Results are presented as odds ratios (ORs) with 95% confidence intervals (CIs).
Table 1. Descriptive Statistics and Intercorrelation of Lifetime, Anhedonia, Depressed Mood, and Baseline Characteristics.
| Variable | M(SD) or % in entire sample | Intercorrelationsc | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | ||
| 1. Anhedonia | 43.3% | - | |||||||
| 2. Depressed Mood | 54.7% | .54† | - | ||||||
| 3. Depressive Disorder | 17.7% | .41† | .37† | - | |||||
| 4. Other Disorder | 70.6% | .25† | .18† | .15† | - | ||||
| 5. Age | 44.7 (11.1) | .03 | .01 | .02 | -.07** | - | |||
| 6. Femalea | 58.4% | .08** | .14† | .12† | -.10*** | -.03 | - | ||
| 7. Caucasianb | 83.7% | -.03 | -.01 | .001 | -.04 | -.03 | -.02 | - | |
| 8. FTND | 5.4 (2.1) | .06* | .06* | .06* | .04 | .16† | -.06* | -.01 | - |
Note. N = 1469. FTND = Fagerström Test of Nicotine Dependence.
Female coded as 1, Male coded as 0.
Caucasian coded as 1, Non-Caucasian coded as 0.
Correlations between two binary variables are represented as φ coefficients, correlations between two continuous variables are represented as Pearson r coefficients, and correlations between a continuous and binary variables are Point-biserial r coefficients.
p < .05,
p < .01,
p < .001,
p < .0001
Results
Preliminary Analyses
Descriptive information and intercorrelations among demographics, FTND scores, and lifetime anhedonia, depressed mood, and psychopathology are provided in Table 1. Lifetime anhedonia and depressed mood were moderately intercorrelated (ϕ = .54) and were similarly correlated with lifetime depressive disorders, other lifetime psychiatric disorders, demographics, and nicotine dependence (Table 1). Lifetime anhedonia and depressed mood did not significantly differ across the treatment conditions.
Primary Analyses
Bivariate analyses illustrated that lifetime anhedonia and depressed mood were both associated with lower abstinence rates at 8-week and 6-month follow ups (Table 2). In individual models that tested the effects of lifetime anhedonia and depressed mood in separate GEE analyses, each symptom indicator predicted lower odds of abstinence across varying levels of covariate adjustment (Table 3). In combined GEE models that tested the effects of lifetime anhedonia and depressed mood as simultaneous predictors, anhedonia retained a significant effect even after covariate adjustment while depressed mood did not (Table 3). Additional GEEs illustrated that lifetime depressive mood disorder did not predict relapse either when controlling for only treatment condition and time (not adjusting for lifetime anhedonia or depressed mood), OR (95% CI) = 1.21 (0.94-1.56), p = .15, or after additionally adjusting for FTND, gender, and non-depressive disorders, OR (95% CI) = 1.09 (0.83-1.42), p = .53.
Table 2. Smoking Abstinence Rates by Lifetime Anhedonia and Depressed Mood.
| Follow Up Time | Anhedonia | Depressed Mood | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| No (n = 833) |
Yes (n = 636) |
χ2 | p | No (n = 666) |
Yes (n = 803) |
χ2 | p | |
| 8-Weeks Post Quit | 47.5% | 39.2% | 10.3 | .0001 | 47.5% | 41.0% | 6.2 | .01 |
| 6-Months Post Quit | 36.0% | 29.3% | 7.5 | .006 | 37.2% | 29.6% | 9.5 | .002 |
Note. N = 1469.
Table 3. Lifetime Anhedonia and Depressed Mood as Predictors of Smoking Relapse.
| Covariates in Model | Anhedoniac | Depressed Moodd | ||
|---|---|---|---|---|
|
|
|
|||
| OR (95% CI) | p | OR (95% CI) | p | |
| Individual Modelsa | ||||
| Treatment and Time Onlyb | 1.42 (1.16-1.73) | .0004 | 1.35 (1.11-1.63) | .002 |
| + FTND + Genderc | 1.36 (1.11-1.65) | .002 | 1.27 (1.04-1.54) | .02 |
| + Depressive disorder + Other disorderd | 1.35 (1.09-1.69) | .007 | 1.24 (1.01-1.53) | .04 |
|
| ||||
| Combined Modelse | ||||
| Treatment and Time Onlyb | 1.31 (1.04-1.65) | .02 | 1.17 (0.93-1.47) | .19 |
| + FTND + Genderc | 1.29 (1.00-1.63) | .04 | 1.11 (0.88-1.40) | .39 |
| + Depressive disorder + Other disorderd | 1.29 (1.00-1.66) | .046 | 1.12 (0.88-1.42) | .37 |
Note. N = 1469. Results from generalized estimating equations of anhedonia and depressed mood predicting 7-day point-prevalence abstinence (relapsed = 1, abstinent = 0) across the 8-week and 6-month post-quit follow ups.
For each outcome, separate models were conducted for anhedonia and depressed mood.
Adjusted for treatment condition and follow up timepoint.
Adjusted for treatment condition, follow up timepoint, FTND, and gender.
Adjusted for treatment condition, follow up timepoint, FTND, gender, lifetime history of depressive mood disorder, and lifetime history of other disorder (i.e., anxiety or substance use disorder).
For each outcome, combined models were conducted including both anhedonia and depressed mood as simultaneous predictors to control for their covariance with one another. FTND = Fagerström Test of Nicotine Dependence score. OR = Odds Ratio. CI = Confidence Interval.
Supplemental analyses
Supplemental GEE analyses predicting cessation outcomes demonstrated that lifetime anhedonia and depressed mood did not significantly interact with time, treatment condition (active vs. placebo), or each other. To examine the role of symptom chronicity, we distinguished participants who reported lifetime anhedonia or depressed mood most of the day nearly every day for two weeks or longer versus those who reported those symptoms for shorter periods of time. In participants who reported lifetime anhedonia, those who experienced symptoms for at least 2 weeks (n=323) vs. less than 2 weeks (n=312) did not differ in cessation outcomes in GEEs controlling for treatment and time, OR (95% CI) = 1.05 (0.78-1.41), p = .74. Similarly, in those with lifetime depressed mood, smokers with symptoms ≥ 2-weeks (n=362) vs. < 2-weeks (n=440) did not differ in cessation outcomes, OR (95% CI) = 1.07 (0.81-1.39), p = .63.
To address the role of depression recurrence, we tested GEE models controlling for treatment and time which showed that participants who reported 2 or more lifetime depressive episodes (recurrent depression; n = 188) had greater odds of relapse relative to the rest of the sample (n =1281), OR (95% CI) = 1.50 (1.16-2.02), p = .007, and relative to the subgroup of smokers with single-episode major depression (n = 71), OR (95% CI) = 2.01 (1.19-3.41), p = .009. We then tested combined GEEs including recurrent depression and lifetime anhedonia as simultaneous predictors controlling for treatment and time, and found a significant effect of anhedonia, OR (95% CI) = 1.31 (1.06-1.61), p = .01, but not recurrent depression, OR (95% CI) = 1.29 (0.94-1.77), p = .12. A second combined model including lifetime depressed mood and recurrent depression illustrated a significant effect of depressed mood, OR (95% CI) = 1.27 (1.04-1.55), p = .01, and a marginal effect for recurrent depression, OR (95% CI) = 1.35 (0.99-1.84), p = .06. Finally, when all three were simultaneously included, each predictor was reduced to non-significance (anhedonia: p = .11, depressed mood: p = .21; recurrent depression: p = .17).
To examine whether relations of lifetime anhedonia and depressed mood to cessation outcome were dependent upon SUD, we examined whether lifetime SUD status moderated the relations of anhedonia and depressed mood to cessation outcomes in GEE models. Neither lifetime anhedonia nor depressed mood interacted with lifetime SUD to predict cessation outcome (ps ≥ .36).
Discussion
Lifetime symptoms of anhedonia and depressed mood both predicted greater likelihood of smoking cessation failure in this study while lifetime depressive disorder did not. When these two predictors were analyzed separately, anhedonia and depressed mood both predicted cessation failure after controlling for pre-treatment nicotine dependence severity, gender, and presence of a comorbid non-depressive disorder (i.e., an anxiety or SUD). Thus, anhedonia and depressed mood are not merely proxies for other indicators of relapse risk, such as nicotine dependence (Japuntich et al., 2011), and may reflect vulnerability processes that uniquely contribute to cessation failure. Furthermore, because the psychiatric exclusion criteria in this study were minimal and there was a sizable prevalence of psychiatric comorbidity in this sample, these results provide evidence that lifetime anhedonia and depressed mood independently predict cessation failure over and above several psychiatric diagnoses (i.e., depressive, anxiety, and substance use disorders). This is notable, given prior evidence implicating some anxiety and substance use disorders as risk factors for smoking relapse (Breslau, Peterson, Schultz, Andreski, & Chilcoat, 1996; Piper, Cook, Schlam, Jorenby, & Baker, 2011; Zvolensky et al., 2008).
After accounting for the covariance between lifetime anhedonia and depressed mood, only anhedonia incrementally predicted cessation outcomes. Moreover, the two types of symptoms did not interact in predicting cessation outcome. Thus, the data suggest that among the three depression predictors we studied (i.e., lifetime anhedonia, depressed mood, and depressive disorder), anhedonia per se provides significant, unique information about an individual's ability to achieve long-term abstinence from tobacco. Both anhedonia and depressed mood had similar rates of occurrence in the sample, indicating that base rates did not differentially influence power. Further, both factors were similarly related to lifetime depressive disorder, suggesting equivalent validity as depression indices. These observations suggest that the relative predictive validity of anhedonia is not artifactual, which raises questions about why anhedonia might be a superior predictor of cessation failure.
The divergence of results for anhedonia and depressed mood mirrors some past results (Cook et al., 2010; Leventhal et al., 2008) and may indicate that the impact of depression's hallmark features on cessation outcome could be explained by two processes: (1) a nonspecific affective disturbance common to both anhedonia and depressed mood; and (2) a specific effect of anhedonia unique from nonspecific affective disturbance. Prior work indicates that anhedonia predicts larger declines in positive affect upon smoking abstinence (Cook et al., 2004). There is also evidence that abstinence-induced reductions in positive affect increase risk of smoking relapse (Strong et al., 2009). We therefore speculate that to the extent that tobacco abstinence reduces positive affect and expectations of pleasure, anhedonic individuals will face increased motivation to return to smoking. This account is consistent with evidence that nicotine modulates both reward and the incentive value of reward-associated stimuli (Caggiula et al., 2009). On the other hand, the relative lack of incremental predictive value of depressed mood in explaining smoking cessation outcome suggests that low-arousal forms of negative affect common to depression (e.g., sad, empty, depressed) may not be a clear and unique marker of lifetime depression-related risk of cessation failure.
Supplemental analyses helped to clarify several features of the relations examined herein. First, symptom chronicity (i.e., experiencing symptoms for greater vs. less than 2 weeks) did not differentiate cessation outcomes among smokers who had reported experiencing anhedonia and depressed mood at some point in their lifetimes. This finding concords with prior work showing no differences in nicotine dependence and cigarettes smoked per day between smokers who endorse these symptoms for < 2 weeks versus ≥ 2 weeks (Hitsman et al., 2011), and suggests that the DSM-based diagnostic threshold for depressive symptom duration may not differentiate the level of risk for cessation failure carried by lifetime affective symptoms. Second, we replicated prior findings illustrating that recurrent depression is associated with greater risk of cessation failure than singe episode depression (Brown et al., 2001; Haas et al., 2004). We further found that lifetime anhedonia incrementally predicted odds of cessation failure over and above recurrent depression as did lifetime depressed mood. Hence, anhedonia, depressed mood, and recurrent depression may each be important markers of relapse risk that are not adequately captured by the standard lifetime depression diagnosis variable. Third, the relations of lifetime anhedonia and depressed mood with cessation failure did not significantly differ among those with versus without a SUD. Thus, it is unlikely that the risk of relapse conferred by anhedonia and depressed mood was due to substance-induced affective disturbance in SUD-positive smokers. Furthermore, these results suggest that relapse risk due to anhedonia and depressed mood may generalize across smokers with and without a SUD.
Clearly, the reliance on single-item binary measures is a psychometric concern that affects interpretation of the findings, though such brief measures might be highly feasible for use in real world clinical settings. In addition, this study focused on anhedonia and depressed mood and did not explore the predictive power of individual non-affective symptoms; hence, distilling how other depressive phenotypes relate to cessation outcome could be an informative future research direction. Also, it should be noted that the 95% CIs for anhedonia, depressed mood, and depressive disorder overlapped with one another in their prediction of cessation outcomes. Therefore, none of these predictors was significantly superior to the others in predicting cessation outcomes in head-to-head comparisons; nevertheless, only anhedonia possessed significant orthogonal variance in such predictions. Furthermore, because the base rates for lifetime depressive disorder and recurrent depression were lower than for lifetime anhedonia and depressed mood, there was somewhat less statistical power for comparisons involving the former. In addition, the CIDI measure used in this study did not identify current or on-going episodes of anhedonia or depressed mood. Rather, it merely assessed occurrence at any point in the participants' lifetime. Hence, it is unclear whether these findings reflect enduring vulnerabilities somewhat independently of current symptom level, or reflect a heightened tendency toward symptom exacerbations in response to a quit attempt. Regardless of the mechanism, demonstrating that single-symptom lifetime history indices of anhedonia and depressed mood predict cessation outcome has clinical applications and sheds light on sources of variation in the risk of smoking relapse that is carried by lifetime depression.
The finding that lifetime anhedonia incrementally predicts smoking cessation outcome over and above lifetime depressed mood, history of depressive disorder, and other factors has several implications. First, standard depression diagnostic measures may obscure important heterogeneity within the depression diagnosis, masking depression subtypes or subdimensions that may exert more meaningful influences on cessation outcome. Hence, a depression diagnosis, comprising a combination of cognitive, vegetative, behavioral, and affective features, may not optimally index vulnerability for cessation failure. This conclusion is consistent with findings regarding the non-uniformity of depression symptoms within a broader literature, which demonstrates that different individual symptoms have distinct patterns of covariation with a variety of clinical characteristics, including comorbid psychiatric disorders, personality, familial psychopathology, and adverse life events (Keller, Neale, & Kendler, 2007; Lux & Kendler, 2010). Second, anhedonia may closely index motivational processes that spur cessation failure, whereas depressed mood may be a more peripheral marker of these motivational processes. In other words, it may not be feeling sad that directly leads to cessation failure, but rather a pervasive inability to experience pleasure and incentive motivation (pleasurable anticipation) for diverse life activities. Third, it may be worthwhile to incorporate measures that isolate anhedonia in assessment batteries that are designed to assess risk of cessation failure (e.g., Bolt et al., 2009). Fourth, anhedonia-related features are present in several psychopathologies (e.g., PTSD, schizophrenia; APA, 1994). Hence, it may be informative to determine whether anhedonia mediates the relation of other psychiatric syndromes to cessation failure; i.e., it may constitute a core common mechanism (of final common pathway) linking multiple manifestations of psychological disturbance with difficulty quitting smoking. Finally, incorporating smoking cessation treatments that boost interest in and pleasure from enjoyable activities (e.g., behavioral activation treatment; MacPherson et al., 2010) may be helpful for mitigating relapse risk in smokers with mood disturbance, and perhaps for smokers in general.
Acknowledgments
This research was supported by grants P50DA019706, K08DA021311, K08DA02504, and 1K05CA139871 from National Institutes of Health (NIH), 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources (NCRR), and an Institutional Clinical and Translational Science Award (UW-Madison; KL2 Grant # 1KL2RR025012-01). GlaxoSmithKline provided medication to patients at no cost.
Footnotes
As reported in a previous analysis (Piper et al., 2009), all five medication conditions decreased rates of abstinence relative to placebo, and nicotine patch + lozenge condition produced higher abstinence rates compared to the monotherapies.
Because a DSM major depression diagnosis requires the presence of anhedonia or depressed mood and the CIDI was designed for the purpose of making DSM diagnoses, the CIDI interviewers assess other depressive symptoms only among individuals who endorse anhedonia or depressed mood for two weeks or longer. Therefore, individual symptom data on the depressive symptoms outside of anhedonia and depressed mood were only available for those who endorsed two-weeks of anhedonia or depressed mood in this study, which precluded analyses of the corresponding predictive effects of other individual depressive symptoms on cessation outcome.
All findings were unchanged when history of major depressive disorder was included as a predictor instead of history of depressive disorder (i.e., major depression and/or dysthymic disorder)
References
- APA. Diagnostic and statistical manual of mental disorders (fourth edition) American Psychiatric Association; 1994. [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychological Review. 2004;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Bolt DM, Piper ME, McCarthy DE, Japuntich SJ, Fiore MC, Smith SS, Baker TB. The Wisconsin Predicting Patients' Relapse questionnaire. Nicotine and Tobacco Research. 2009;11(5):481–492. doi: 10.1093/ntr/ntp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Peterson E, Schultz L, Andreski P, Chilcoat H. Are smokers with alcohol disorders less likely to quit? Am J Public Health. 1996;86(7):985–990. doi: 10.2105/ajph.86.7.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Kahler CW, Niaura R, Abrams DB, Sales SD, Ramsey SE, Miller IW. Cognitive-behavioral treatment for depression in smoking cessation. Journal of consulting and clinical psychology. 2001;69(3):471–480. doi: 10.1037//0022-006x.69.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: a dual-reinforcement model. Nebr Symp Motiv. 2009;55:91–109. doi: 10.1007/978-0-387-78748-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J, Spring B, McChargue D, Doran N. Effects of anhedonia on days to relapse among smokers with a history of depression: a brief report. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2010;12(9):978–982. doi: 10.1093/ntr/ntq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue D. Influence of nicotine on positive affect in anhedonic smokers. Psychopharmacology. 2007;192(1):87–95. doi: 10.1007/s00213-006-0688-5. [DOI] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue D, Hedeker D. Hedonic capacity, cigarette craving, and diminished positive mood. Nicotine and Tobacco Research. 2004;6(1):39–47. doi: 10.1080/14622200310001656849. [DOI] [PubMed] [Google Scholar]
- Falcone M, Gold AB, Wileyto EP, Ray R, Ruparel K, Newberg A, Lerman C. mu-Opioid receptor availability in the amygdala is associated with smoking for negative affect relief. Psychopharmacology. 2012;222(4):701–708. doi: 10.1007/s00213-012-2673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert DG, McClernon FJ, Rabinovich NE, Plath LC, Jensen RA, Meliska CJ. Effects of smoking abstinence on mood and craving in men: influences of negative-affect-related personality traits, habitual nicotine intake and repeated measurement. Personality and Individual Differences. 1998;25:399–423. [Google Scholar]
- Haas AL, Munoz RF, Humfleet GL, Reus VI, Hall SM. Influences of mood, depression history, and treatment modality on outcomes in smoking cessation. Journal of consulting and clinical psychology. 2004;72(4):563–570. doi: 10.1037/0022-006X.72.4.563. [DOI] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29(10):1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hitsman B, Buka SL, Veluz-Wilkins AK, Mohr DC, Niaura R, Gilman SE. Accuracy of a brief screening scale for lifetime major depression in cigarette smokers. Psychol Addict Behav. 2011;25(3):559–564. doi: 10.1037/a0022772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitsman B, Papandonatos GD, McChargue DE, Demott A, Herrera MJ, Spring B, Niaura R. Past major depression and smoking cessation outcome: a systematic review and meta-analysis update. Addiction. 2013;108(2):294–306. doi: 10.1111/add.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japuntich SJ, Leventhal AM, Piper ME, Bolt DM, Roberts LJ, Fiore MC, Baker TB. Smoker characteristics and smoking-cessation milestones. Am J Prev Med. 2011;40(3):286–294. doi: 10.1016/j.amepre.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MC, Neale MC, Kendler KS. Association of different adverse life events with distinct patterns of depressive symptoms. Am J Psychiatry. 2007;164(10):1521–1529. doi: 10.1176/appi.ajp.2007.06091564. quiz 1622. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Ustun TB. The World Mental Health (WMH) Survey Iniative Version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI) The International Journal of Methods in Psychiatric Research. 2004;13:93–121. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Ameringer KJ, Osborne E, Zvolensky MJ, Langdon KJ. Anxiety and depressive symptoms and affective patterns of tobacco withdrawal. Drug and Alcohol Dependence. doi: 10.1016/j.drugalcdep.2013.06.015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Greenberg JB, Trujillo MA, Ameringer KJ, Lisha NE, Pang RD, Monterosso J. Positive and negative affect as predictors of urge to smoke: temporal factors and mediational pathways. Psychol Addict Behav. 2013;27(1):262–267. doi: 10.1037/a0031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Ramsey SE, Brown RA, LaChance HR, Kahler CW. Dimensions of depressive symptoms and smoking cessation. Nicotine Tob Res. 2008;10(3):507–517. doi: 10.1080/14622200801901971. 791307090 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Kahler CW, Ray LA, Sussman S. Relations between anhedonia and smoking motivation. Nicotine and Tobacco Research. 2009;11(9):1047–1054. doi: 10.1093/ntr/ntp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvin EB, Brandon TH. Testing the influence of external and internal cues on smoking motivation using a community sample. Exp Clin Psychopharmacol. 2010;18(1):61–70. doi: 10.1037/a0017414. [DOI] [PubMed] [Google Scholar]
- Lux V, Kendler KS. Deconstructing major depression: a validation study of the DSM-IV symptomatic criteria. Psychol Med. 2010;40(10):1679–1690. doi: 10.1017/S0033291709992157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson L, Tull MT, Matusiewicz AK, Rodman S, Strong DR, Kahler CW, Lejuez CW. Randomized controlled trial of behavioral activation smoking cessation treatment for smokers with elevated depressive symptoms. Journal of consulting and clinical psychology. 2010;78(1):55–61. doi: 10.1037/a0017939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DE, Piasecki TM, Fiore MC, Baker TB. Life before and after quitting smoking: an electronic diary study. Journal of abnormal psychology. 2006;115(3):454–466. doi: 10.1037/0021-843X.115.3.454. [DOI] [PubMed] [Google Scholar]
- Niaura R, Britt DM, Shadel WG, Goldstein M, Abrams D, Brown R. Symptoms of depression and survival experience among three samples of smokers trying to quit. Psychol Addict Behav. 2001;15(1):13–17. doi: 10.1037/0893-164x.15.1.13. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Niaura R, Shadel WG, Abrams D, Goldstein M, Fiore MC, Baker TB. Smoking withdrawal dynamics in unaided quitters. Journal of abnormal psychology. 2000;109(1):74–86. doi: 10.1037//0021-843x.109.1.74. [DOI] [PubMed] [Google Scholar]
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Baker TB. Anxiety diagnoses in smokers seeking cessation treatment: relations with tobacco dependence, withdrawal, outcome and response to treatment. Addiction. 2011;106(2):418–427. doi: 10.1111/j.1360-0443.2010.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Smith SS, Schlam TR, Fiore MC, Jorenby DE, Fraser D, Baker TB. A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Arch Gen Psychiatry. 2009;66(11):1253–1262. doi: 10.1001/archgenpsychiatry.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Leone FT, Hitsman B. Symptoms of depression and smoking behaviors following treatment with transdermal nicotine patch. J Addict Dis. 2013;32(1):46–52. doi: 10.1080/10550887.2012.759870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong DR, Kahler CW, Leventhal AM, Abrantes AM, Lloyd-Richardson E, Niaura R, Brown RA. Impact of bupropion and cognitive-behavioral treatment for depression on positive affect, negative affect, and urges to smoke during cessation treatment. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2009;11(10):1142–1153. doi: 10.1093/ntr/ntp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDHHS. Treating tobacco use and dependence: 2008 update Clinical practice guideline. Rockville, MD: U.S. Public Health Service; 2008. [Google Scholar]
- Wacker J, Dillon DG, Pizzagalli DA. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, fMRI, and volumetric techniques. Neuroimage. 2009;46(1):327–337. doi: 10.1016/j.neuroimage.2009.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- Zimmerman M, McGlinchey JB, Young D, Chelminski I. Diagnosing major depressive disorder: II: is there justification for compound symptom criteria? J Nerv Ment Dis. 2006;194(4):235–240. doi: 10.1097/01.nmd.0000207423.36765.89. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Gibson LE, Vujanovic AA, Gregor K, Bernstein A, Kahler C, Feldner MT. Impact of Posttraumatic Stress Disorder on early smoking lapse and relapse during a self-guided quit attempt among community-recruited daily smokers. Nicotine Tob Res. 2008;10(8):1415–1427. doi: 10.1080/14622200802238951. [DOI] [PubMed] [Google Scholar]


