Abstract
Cyclic AMP (cAMP) is an important intracellular signaling molecule for many G protein-mediated signaling pathways but the specificity of cAMP signaling in cells with multiple signaling pathways is not well-understood. In Dictyostelium, at least two different G protein signaling pathways, mediated by the Gα2 and Gα4 subunits, are involved with cAMP accumulation, spore production, and chemotaxis and the stimulation of these pathways results in the activation of ERK2, a mitogen-activated protein kinase that can down regulate the cAMP-specific phosphodiesterase RegA. The regA gene was disrupted in gα2− and gα4− cells to determine if the absence of this phosphodiesterase rescues the development of these G protein mutants as it does for erk2− mutants. The regA− mutation had no major effects on developmental morphology but enriched the distribution of the Gα mutant cells to the prespore/prestalk border in chimeric aggregates. The loss of RegA function had no effect on Gα4-mediated folate chemotaxis. However, the regA gene disruption in gα4− cells, but not in gα2− cells, resulted in a substantial rescue and acceleration of spore production. This rescue in sporulation required cell autonomous signaling because the precocious sporulation could not be induced through intercellular signaling in chimeric aggregates. However, intercellular signals from regA− strains increased the expression of the prestalk gene ecmB and accelerated the vacuolization of stalk cells. Intercellular signaling from gα4−regA− strain did not induce ecmA gene expression indicating cell-type specificity in the promotion of prestalk cell development. regA gene disruption in a Gα4HC (Gα4 overexpression) strain did not result in precocious sporulation or stalk cell development indicating that elevated Gα4 subunit expression can mask regA− associated phenotypes even when provided with wild-type intercellular signaling. These finding indicate that the Gα2 and Gα4-mediated pathways provide different contributions to the development of spores and stalk cells and that the absence of RegA function can bypass some but not all defects in G protein regulated spore development.
Keywords: Phosphodiesterase, G protein, Dictyostelium, Development, Gene expression
Introduction
Many G protein-mediated signal transduction pathways modulate cellular cAMP levels through the direct or indirect regulation of adenylyl cyclases and phosphodiesterases and the resulting changes in cAMP levels can affect protein kinase A (PKA) function [1–4]. The binding of cAMP to the regulatory subunit of PKA (PAK-R) releases the catalytic subunit (PKA-C) that can phosphorylate proteins in both the cytoplasm and nucleus [5]. In the soil amoebae Dictyostelium discoideum, cAMP can also be released from cells where it functions as an external stimulator of certain G protein-coupled receptors that regulate a variety of developmental processes [6–8]. Defining the many roles of cAMP in cellular signaling can be challenging particularly when cAMP levels are regulated through multiple G protein signaling pathways that might operate within a single cell. However, the use of genetic analysis in simple eukaryotes such as Dictyostelium can help determine the roles of cAMP-specific signal transduction pathways and provide insight into the role of cAMP in cellular functions and cell fate [9].
When starved, Dictyostelium use G protein signaling pathways to either forage for bacterial food sources or undergo multicellular development to form a fruiting body that consists of a mass of spores on top of a stalk [10, 11]. Extracellular folate can trigger the foraging response at the onset of starvation whereas extracellular cAMP directs the aggregation of cells a few hours after nutrient deprivation [12, 13]. Both of these external signals generate transient increases in cAMP but through different G protein-coupled receptors and G protein Gα subunits [14–16]. The Gα2 subunit couples to cAMP receptors and is required for cAMP chemotaxis, the expression of some developmentally regulated genes, and sporulation [16, 17]. The Gα4 subunit is required for responses to folate and possibly to other signals that regulate developmental processes such as slug and fruiting body morphogenesis and sporulation [15, 18]. The Gα4 subunit is required for the activation of the mitogen activated protein kinase (MAPK) ERK2 in response to folate whereas activation of this kinase in response to external cAMP requires cAMP receptors but not G protein function [19, 20]. ERK2 function is required for generating the intercellular cAMP signal required for cell aggregation but not for cAMP chemotaxis and so erk2− cells can only aggregate in the presence of cells producing sufficient cAMP [21]. The aggregation defect of erk2− cells can be suppressed by the loss of a cAMP-specific phosphodiesterase RegA and the alteration of a putative MAPK phosphorylation site in RegA prevents the inactivation of the phosphodiesterase suggesting ERK2 phosphorylates and down regulates RegA [22]. Loss of RegA also results in accelerated spore development similar to that observed for either the overexpression of the PKA catalytic subunit (PKA-C) or the loss of the PKA regulatory subunit (PKA-R) suggesting that elevated levels of cAMP result in precocious development through increased PKA activity [23–25].
The activation of ERK2 in response to either extracellular cAMP or folate suggests that the Gα2- and Gα4-mediated signaling pathways might elevate cAMP levels through the down regulation of RegA. Therefore we investigated the ability of the regA gene disruption to suppress the mutant phenotypes associated with the loss of Gα2 or Gα4 function. Developmental morphogenesis, cell movement within aggregates, cell type specific gene expression and spore production were characterized for gα2− and gα4− cells with or without the regA gene disruption to determine what developmental processes can be suppressed from the loss of RegA function. A chimera analysis was used to examine the importance of cell autonomous and intercellular signaling in the suppression of G protein signaling defects. The results of these phenotypic analyses indicate that the loss of RegA function can efficiently suppress sporulation defects in gα4− but not gα2− cells suggesting that decreased cAMP turnover can overcome some but not all of the defects in developmental G protein-mediated signaling.
Materials and methods
Strains and cell culturing
All strains were isogenic to the wild-type strain, KAx-3, except where noted. The creation of gα2−, gα4−, and Gα4HC (Gα4 subunit overexpression) strains has been previously described [16, 18]. The regA−, gα2−regA−, and gα4−regA− mutants gene disruptions were created using a regA gene disruption construct previously described by others [26]. Several clones were analyzed for each regA− mutant created and in all cases these clones exhibited identical developmental phenotypes. The regA gene disruptions were verified using PCR amplification analysis of the genomic DNA with the oligonucleotides (sense strand 5′-GGATTTGGAGACAAATTGAACGACCAACC-3′) and (antisense strand 5′-GGGTTAAATATTGAGCGGCATTGAAAGAGG-3′). The GFP expression vector was previously described [27]. Cell type-specific reporter genes ecmA:lacZ, ecmB:lacZ, and pspA:GFP (V/PsA-I-S65T-GFP) were obtained from the Dicty stock center or from original sources [28–31]. Cells were grown in axenic HL5 medium or on bacterial lawns of Klebsiella aerogenes [32]. Electroporation of Dictyostelium was conducted as previously reported [33]. Transformed cells were selected and maintained in medium containing 2–10 μg/ml of the drug G418 or 3 μg/ml of the drug Blasticidin S and drug selection was removed several hours prior to analysis. Folate solutions were prepared by neutralizing folic acid with 100 mM NaHCO3.
Development, chemotaxis, and spore assays
Cells were grown to mid-log phase (approximately 2–3 × 106 cells/ml), washed twice in phosphate buffer (12mM NaH2PO4 adjusted to pH 6.1 with KOH), and suspended in phosphate buffer (1 × 108 cells/ml), before spotting on nonnutrient plates (phosphate buffer, 1.5% agar) for development or chemotaxis assays [34]. Cell development was analyzed using a dissecting microscope or a fluorescence microscope. The staining of cells with lacZ reporter constructs in developing mounds, slugs, and fruiting bodies was conducted as previously described [35]. Quantitative β-galactosidase enzymatic assays were conducted as previously described [36]. Chimeric cell suspensions of 1 × 108 cells/ml were spotted onto prewetted Whatmann No. 50 paper filters and then overlayed on nonnutrient agar plates for development. β-galactosidase activity of cell lysates was assayed using 0-nitrophenyl β-D-galactopyranoside (ONPG) as the substrate. Chemotaxis assays were performed by spotting droplets (~ 0.5 μl) of cell suspension (107 to 108 cells/ml) on nonnutrient plates followed by the spotting of 1μl of folate solutions (10−2 to 10−4 M) approximately 2–3 mm away from the cell droplet. Cell movement was monitored with a dissecting microscope and a digital camera as previously described [33]. For the quantitative sporulation analysis, cells were placed on Whatmann 50 filters saturated in phosphate buffer that were later overlayed on nonnutrient agar. Developing aggregates were vigorously washed from filters using phosphate buffer and microscopically examined for spores. Fluorescence microscopy was used to identify spores stained with Fluorescent Brightener 28 (Sigma-Aldrich) or spores expressing GFP. Chimeras were created by mixing cells at the ratios indicated before plating on nonnutrient agar plates or paper filters.
Results
Loss of RegA has a subtle affect on developmental morphology
Dictyostelium G protein-mediated signaling pathways can regulate cAMP levels in response to vegetative and developmental signals and in some cases this regulation might involve the down regulation of the RegA phosphodiesterase. To determine if the loss of RegA function can suppress developmental defects associated with G protein mutations a regA gene disruption created in strains that lacked either the Gα2 or Gα4 subunit gene (Supplementary Fig. 1). When starved, most gα2−regA− cells failed to aggregate but some cells were capable of forming very small mounds that became raised from the substratum after 48 hours (Fig. 1). These small aggregates were also observed for gα2−regA− cells grown on bacterial lawns (data not shown). As expected from previous studies, the starvation of gα2− cells did not result in multicellular aggregates. Disruption of the regA gene did not substantially rescue the morphological development of gα4− cells that typically terminate development as multicellular aggregates with extended tip structures. The gα4−regA− aggregates terminated development as slug-like structures but without the slug migration associated with wild-type aggregates. As previously described by others, the disruption of the regA gene in wild-type cells resulted in accelerated development to form fruiting bodies with small spore heads and stalks with a thick base [26]. These developmental phenotypes indicated that the loss of RegA function does not substantially change the morphological development of wild-type or these G protein mutant strains.
Figure 1.
Morphological developmental of clonal wild-type, gα2−, and gα4− strains with or without the regA gene disruption (regA−). Wild-type (WT) and mutant strains were plated for development as described in the materials and methods and photographs were taken after 14 hours of starvation. All images were photographed at the same magnification except for insets of individual aggregates that are magnified 4X to show greater detail of morphology. The anterior side of magnified individual aggregates is shown at the right of the image.
Localization of regA− strains in developing chimeras
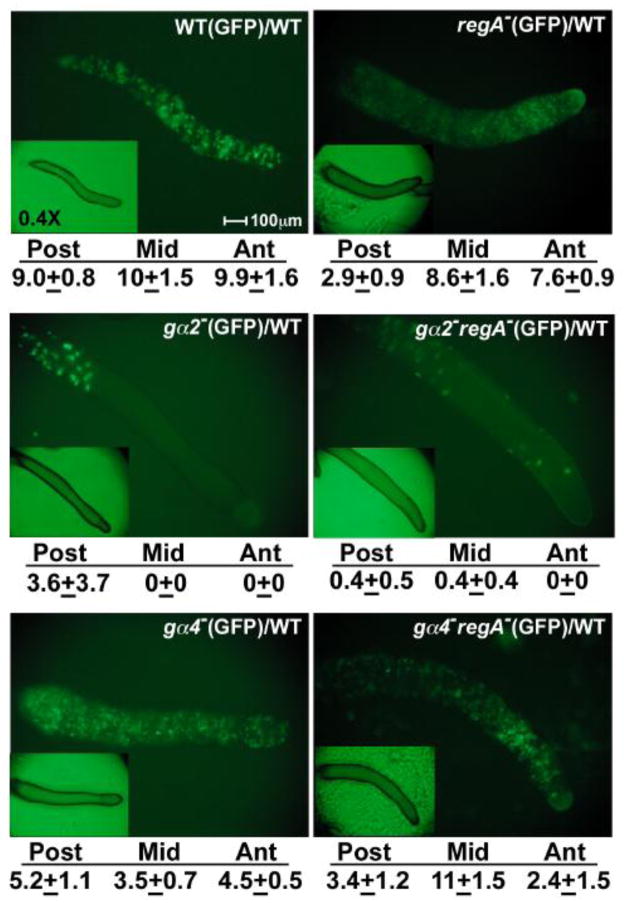
Both Gα2 and Gα4 subunits can mediate chemotactic responses and so gα2−regA− and gα4−regA− cells were compared to gα2− and gα4− cells, respectively, to determine if the loss of RegA function alters the localization of cells within a developing aggregate. The distribution of mutant cells was analyzed in chimeras consisting primarily of wild-type cells to ensure proper intercellular signaling within the aggregate and the mutant cells were distinguished from the wild-type cells using a GFP expression vector (Fig 2). Very few gα2−regA− and gα2− cells were associated with the chimeric aggregates supporting the idea these cells are defective with respect to cAMP chemotaxis during aggregation. Most of the gα2− and gα2−regA− cells retained in the chimeric slugs were detected near the posterior region but some gα2−regA− cells were localized to a region near the anterior but not at the anterior tip. Both gα4−regA− and gα4− cells co-aggregated efficiently with wild-type cells and both mutant strains were distributed throughout the entire length of the chimeric slugs. However, the gα4−regA− cells were enriched in a region near the anterior, similar to that observed for gα2−regA− and regA− cells. In contrast, gα4− cells were most abundant near the posterior of chimeric slugs. These observations suggest that the loss of RegA function can promote the localization of cells near the anterior of developing aggregates regardless of whether the Gα2 or Gα4 subunit is present but the contributions of cell migration and cell-cell adhesion to the distribution of cells in this region remain to be determined.
Figure 2.
Chimeras of mutant strains expressing a GFP vector and wild-type cells with no GFP vector. Strains carrying the GFP vector were harvested, mixed at a ratio 1:10 with wild-type cells with no GFP vector, and plated for development as described in the materials and methods. The distribution of GFP expressing cells was recorded after 16 hours after starvation. Insets show bright-field microscopy images of slugs at 0.4X magnification. All slugs are positioned with the anterior to the right. Development of clonal strains expressing GFP was indistinguishable from parental strains with no GFP. Relative levels of GFP fluorescence for the anterior (Ant, 10% of slug), middle (Mid, 20% of slug) and posterior region (Post, 70% of slug) was measured from the images of three different slugs using ImageJ software (NIH, Bethesda, MD). The values listed below the images represent the mean and standard deviation of measurements from three different slugs.
Folate chemotaxis is not affected by the loss of RegA
The altered localization of regA− mutants in chimeras supports a possible role for RegA in the regulation of cell movement and previous studies have shown that the loss of RegA function partially impairs cAMP chemotaxis due to the reduced ability to suppress lateral pseudopod formation [37]. To examine the possible impact of the regA gene disruption on folate chemotaxis, wild-type, gα4− and gα2− cells with or without regA gene disruption were assayed for folate chemotaxis in an above agar assay (Fig. 3). The loss of RegA function had very little affect on the ability of wild-type or gα2− mutants to chemotax to folate indicating RegA function is not required for folate chemotaxis. The importance of RegA function in cAMP but not folate chemotaxis suggests the role of RegA function can be variable with respect to chemotactic responses. Perhaps cAMP chemotaxis is more sensitive to the loss of RegA because RegA directly regulates the level of endogenously produced chemoattractant. The loss of RegA function did not suppress the defect of folate chemotaxis in gα4− cells indicating that the requirement of Gα4 in folate chemotaxis cannot be bypassed through the elevation of cAMP levels.
Figure 3.
Chemotaxis of wild-type and mutant cells to folate. Wild-type (WT), regA−, gα2−, gα2−regA−, gα4−, gα4−regA− cells were assayed for chemotactic movement to folate as described in the materials and methods. Measurements are the maximum distance of cell migration from the edge of the original cell droplet to the leading edge of cells migrating toward the source of folate. Chemotaxis data are the mean of six individual assays and error bar is the standard deviation. Unpaired Student’s t-test P value is listed for comparison of strains with or without regA gene disruption.
Loss of RegA rescues sporulation in gα4− cells but not gα2− cells
The terminal developmental morphology of gα4−regA− aggregates resembles a slug rather than a fruiting body but spores could be readily detected in these structures as early as 12 hours after starvation (data not shown). Precocious spore development was observed in regA− aggregates as previously reported but the onset of spore formation for this mutant typically occured about 14 hours after starvation suggesting gα4−regA− aggregates have a slightly more accelerated development of spores. A quantitative sporulation assay revealed that gα4−regA− produce nearly half as many spores as wild-type aggregates by 24 hours of starvation whereas gα4− aggregates produced a 600-fold lower level of spores than wild-type aggregates (Table 1). Sporulation in regA− aggregates was slightly higher than that of wild-type aggregates. Spores were also detected in some of the small aggregates that developed in clonal populations of gα2−regA− cells but these spores were extremely rare and therefore not detectable by the quantitative spore assay. No aggregates or spores were detected in starved clonal populations of gα2− cells. These observations suggest that loss of RegA function effectively rescues sporulation for gα4− but not gα2− cells.
Table 1.
Spore yield at 24 hours of development
| Strain % | Sporesa |
|---|---|
| WT | 100 ± 17 |
| regA- | 121 ± 10 |
| g〈4- | 0.15 ± 0.13 |
| g〈4- | regA- 41.3 ± 10.2 |
| g〈2- | NDb |
| g〈2-regA- | NDb |
Spore yield normalized to WT spore yield.
ND - none detected.
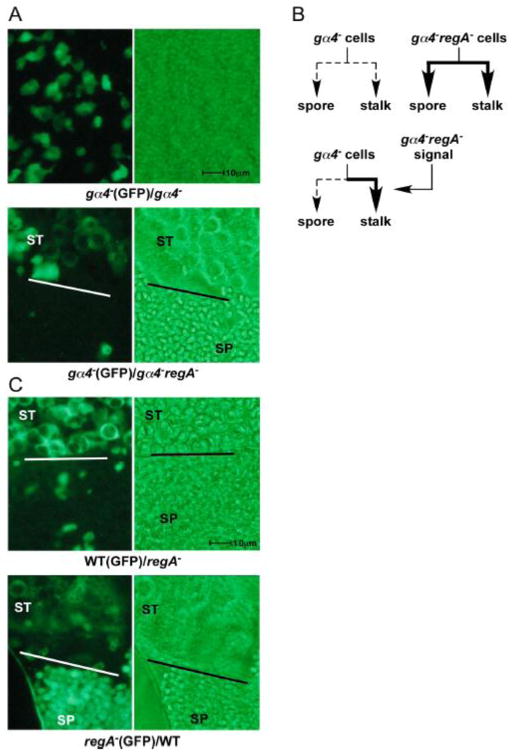
The dramatic rescue of spore production in gα4−regA− aggregates suggests that the loss of phosphodiesterase activity and presumed elevation of cAMP levels can promote sporulation in this mutant. However, cAMP can function as both an intracellular and extracellular signal during multicellular development and so the rescue of spore production could potentially result from extracellular cAMP or some other extracellular signal dependent on cAMP. To examine this possibility, gα4− cells expressing a GFP reporter construct were developed as chimeras in a 1 to 3 ratio with gα4− cells or gα4−regA− cells. After 18 hours of development spores were easily detected in the gα4−(GFP)/gα4−regA− chimeras (5.1 ± 1.3 spores/103 cells) but none of the spores in the gα4−(GFP)/gα4−regA− chimeras expressed GFP suggesting that all spores were from gα4−regA− cells (Fig. 4A). As expected no spores were observed in the gα4−(GFP)/gα4− chimeras at this stage of development. Therefore intercellular signaling was not sufficient to correct the sporulation defect of gα4− cells. The gα4−(GFP) cells in the gα4−(GFP)/gα4−regA− chimeras did produce vacuolated cells that are typically associated with stalk cell development. These vacuolated gα4−(GFP) cells were observed as early as 12 hours after starvation in the presence of gα4−regA− cells whereas these vacuolated cells were not observed until nearly 20 hours in chimeras with just gα4− cells suggesting gα4−regA− cells provide intercellular signals that promote precocious prestalk cell maturation. This chimera analysis demonstrates that gα4−regA− intercellular signaling can accelerate gα4− prestalk cell development but that precocious sporulation requires a cell autonomous signal (Fig. 4B). The precociously vacuolated gα4− stalk cells were found in close proximity to the gα4−regA− spores suggesting that intercellular signaling from prespore or spore cells might regulate the maturation of stalk cells.
Figure 4.
Spore and stalk cell differentiation in wild-type, gα4− and regA− strains developed as clonal or chimeric aggregates. (A) gα4− cells carrying a GFP expression vector were mixed at a 1:3 ratio with gα4− or gα4−regA− cells with no expression vector and developed on nonnutrient agar plates for 18 hours. Chimeric aggregates were transferred to phosphate buffer on microscope slides and squashed with coverslips and visualized for the presence of spores with or without GFP expression and vacuolated cells. Images display a region of a representative aggregate. Bars in the middle of some images indicate the separation of vacuolated stalk cell (ST) and spore cell (SP) enriched regions of the aggregates. Vacuolated stalk cells appear as large rounded cells with the GFP restricted from the center due to the large vacuole. Spores appear as small rod-shaped cells and undifferentiated cells appear as irregular shaped cells with no large vacuoles. The image of the gα4−(GFP)/gα4− chimeric aggregate (upper panels) contained no stalk or spore cells. (B) Cell fate of gα4− and gα4−regA− strains. Thin dashed lines represent weak spore and stalk cell differentiation and thick lines represent precocious differentiation. Signal produced by gα4−regA− cells only induces gα4− precocious stalk cell development (C) Wild-type (WT) and regA− cells carrying a GFP expression vector with mixed at a 1:3 ratio with regA− and wild-type cells, respectively, with no GFP vector and analyzed as described for chimeras in (A). Brightfield images are shown as the right side panel. All images are shown at the same magnification.
The requirement of a cell autonomous signal in precocious spore development was also investigated in chimeras with wild-type cells. Spore development was examined in wild-type(GFP)/regA− and regA−(GFP)/wild-type chimeras with GFP expressing cells at a 1 to 3 ratio with cells not expressing GFP. After 18 hours of development, only regA− spores were observed suggesting that the rapid spore production required cell autonomous signaling associated with the loss of RegA (Fig. 4C). The regA− spores were highly concentrated and in close proximity to vacuolated cells as previously observed for the gα4− chimeras. Vacuolated stalk cells were observed for both wild-type and regA− cells in these chimeras by 18 hours of development indicating that intercellular signals from regA− cells are capable of accelerating stalk cell development. Stalk and spore cells were not observed in clonal aggregates of wild-type cells at 18 hours of development (data not shown).
Precocious prestalk gene expression in regA− strains
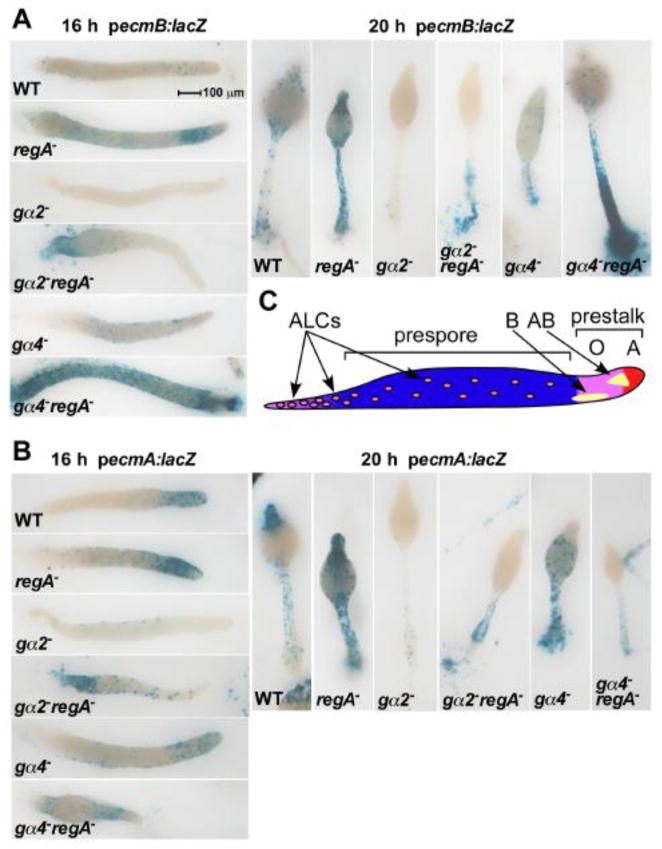
Different anterior prestalk cell regions and anterior-like cells (ALCs) within a developing aggregate are defined in part by the expression of prestalk specific reporter genes ecmA:lacZ (prestalk O and prestalk A anterior regions) and ecmB:lacZ (prestalk B and prestalk AB regions) [10]. To examine the spatial distribution and relative abundance of these prestalk cells in Gα subunit and regA− mutants, the mutant strains containing the prestalk-specific reporter genes were mixed with wild-type cells at a 1:10 ratio to create chimeras. The higher proportion of wild-type cells corrected for defects in morphological development and intercellular signaling but also allowed for better detection of individual stained cells within the aggregates. Although reporter gene copy number can vary between different transformants, the expression of both ecmA and ecmB reporter genes was consistently greater and detected earlier in the regA− mutants compared to strains that have RegA suggesting the loss of RegA can enhance the onset of prestalk gene expression (Fig. 5). The proportion of cells expressing the ecmA or ecmB reporter genes was elevated in chimeras with the gα2−regA− cells as compared to gα2− cells and this difference might be in part affected by better retention of gα2−regA− cells in the chimeric slugs. The gα2−regA− cells were also detected closer to the anterior than the gα2− cells. The proportion of gα4−regA− cells expressing the ecmB reporter gene was much greater than that of gα4− cells and the localization of gα4−regA− cells to the prestalk AB region was precocious. The prestalk AB region is not typically detected until late in the slug phase just prior to the fruiting body phase. The detection of ecmB expression in the prestalk AB region at 16 hours of development is in agreement with the accelerated development of gα4−regA− cells. Clonal gα4−regA− aggregates also displayed elevated ecmB expression levels (data not shown). Expression of the ecmA reporter gene in gα4−regA− cells was more intense in the prestalk O region compared to the prestalk A region of slugs compared to gα4− cells consistent with the enhanced localization of gα4−regA− to the prespore/prestalk border. Interestingly, neither gα4−regA− nor gα4− cells expressed the ecmA reporter gene in the anterior region of fruiting bodies suggesting this phenotype is specific to the loss of Gα4 function.
Figure 5.
Prestalk gene expression in wild-type, gα2−, and gα4− cells with or without the regA gene disruption. Wild-type and mutants strains carrying the ecmA (A) or ecmB (B) reporter gene vectors (ecmA:lacZ and ecmB:lacZ, respectively) were mixed at a ratio of 1:10 with wild-type cells and developed on filters supported by nonnutrient agar. Chimeras were fixed and stained for β-galactosidase activity at 12,16, 20, and 24 hours after starvation. Slug stage (16 hours) and early culminant (20 hours) images are shown with the anterior side at the right and on top, respectively (12 and 24 hours stages not shown). (C) Model displaying the distribution of specific tissue types at the slug stage. Prestalk AB cells form a funnel-shaped region within the core of the anterior region that begins the formation of the stalk. Prestalk B cells are detected near the surface of the prestalk O region.
Loss of RegA produces an intercellular signal that enhances ecmB but not ecmA expression
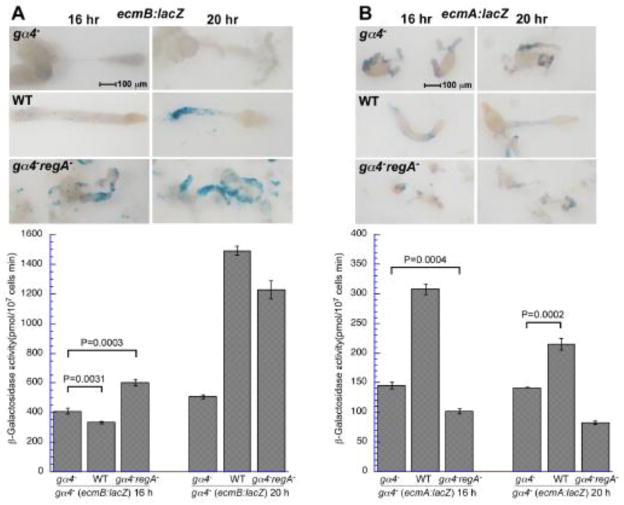
The enhanced ecmB expression due to the loss of RegA function in gα4− cells could possibly result from intercellular signals released by the mutant cells. To examine this possibility, gα4− cells expressing the ecmB reporter gene were developed as chimeras with wild-type, gα4− and gα4−regA− strains and the extent of ecmB reporter gene expression was compared between the different chimeras. The ratio of the gα4− cells carrying the ecmB reporter gene was kept the same among the different chimeras so that the extent of ecmB expression could be directly compared between the different chimeras. By 20 h of development, chimeras with either wild-type or gα4−regA− cells had a 2 to 3-fold greater ecmB expression compared to chimeras with only gα4− cells. The lack of fruiting body formation in the gα4−(ecmB:lacZ)/gα4−regA− chimeras indicates that formation of a stalk tube is not required for this intercellular induction of ecmB. A similar analysis of the ecmA:lacZ reporter gene expression gα4− cells demonstrated that only wild-type and not gα4−regA− cells could produce an intercellular signal for the induction of ecmA expression (Fig 6B). These observations indicate that Gα4 function is important for the production of intercellular signals that promote prestalk gene expression but that only the signal inducing ecmB expression requires a reduction in RegA function.
Figure 6.
Prestalk reporter gene expression in gα4− cells developed as chimeras with wild-type, gα4−, or gα4−regA− cells. gα4− cells carrying a prestalk lacZ expression vector were harvested, mixed at a ratio of 1:10 with wild-type (WT), gα4−, and gα4−regA− cells and developed on filters as described in the materials and methods. All chimeras were developed and stained in side-by-side experiments to allow comparison of reporter gene expression without variability in staining duration. (A) gα4− cells carrying the ecmB:lacZ expression vector (ecmB:lacZ). (B) gα4− cells carrying the ecmA:lacZ expression vector (ecmB:lacZ). Chimeras were fixed and stained for β-galactosidase activity at 12,16, 20, and 24 hours after starvation (only 16 and 20 hours images are shown). The slug and early culminant in the top panels are shown with anterior at the right. Quantitative measurements of β-galactosidase activity using ONPG assay described in the materials and methods are indicated in lower panel graphs (16 and 20 h time points only). β-galactosidase activity is the mean of three assays and error bars indicate standard deviation of the error. Data shown represent one of multiple assays with similar results. Student’s unpaired t-test was used to calculate the statistical significance of differences between the control chimera (contains gα4− cells) and the chimeras containing WT or gα4−regA− cells. In all cases, P < 0.0001 except where indicated on the graph.
Gα4 subunit overexpression delays regA− sporulation
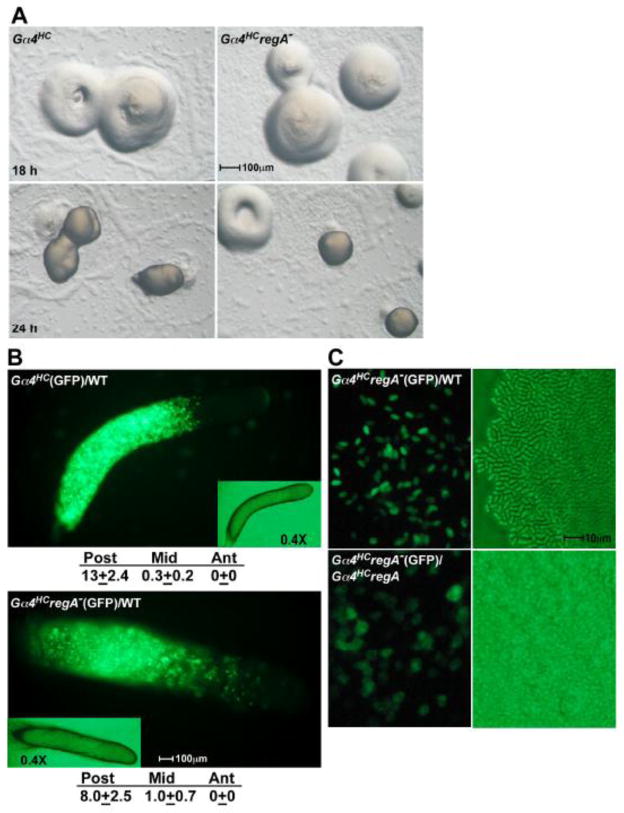
Overexpression of the Gα4 subunit (Gα4HC) enhances folate chemotaxis and blocks multicellular development after cells aggregate to form a mound. Clonal Gα4HC aggregates typically terminate development as a mound without spore or stalk cell differentiation [15, 18]. Gα4HC cells localize to the prespore region and form spores in chimeras with wild-type cells or when anterior regions of wild-type slugs are transplanted onto Gα4HC aggregates [36]. Therefore the intercellular signaling required for Gα4HC sporulation is presumed to originate or be mediated by prestalk or stalk cells. To determine if the loss of RegA can rescue spore development, the RegA gene was disrupted in Gα4HC cells. The developmental morphogenesis of Gα4HCregA− cells was like that of Gα4HC cells and resulted in no sporulation within 24 hours (Fig. 7A). This phenotype was also observed when a Gα4 expression vector was transformed into regA− cells (data not shown). As previously reported, the developmental block associated with Gα4 overexpression can be leaky with some aggregates forming fruiting bodies by 48 hours of starvation [18, 36]. However, no differences were observed between Gα4HC and Gα4HCregA− aggregates with respect to the leakiness of the developmental block. When mixed with an excess of wild-type cells, GFP-labeled Gα4HC and Gα4HCregA− cells were found primarily in the prespore region (Fig. 7B). A small subset of Gα4HCregA− cells was found in the prestalk O region but these cells might have reduced Gα4 subunit expression due to lost copies of the expression vector. Mixed with wild-type cells, the Gα4HCregA− cells were capable of robust sporulation after 24 hours of development but precocious sporulation was not observed (Fig. 7C). Gα4HCregA− cells also did not exhibit precocious sporulation when developed with an excess of regA− cells even though regA− spores were observed by 15 hours of development in these chimeras (data not shown). These results indicate the loss of RegA function does not facilitate sporulation or stalk cell development in Gα4HC cells through cell autonomous or intercellular signaling but does not prevent Gα4HC cells from responding to a sporulation signal provided by wild-type cells.
Figure 7.
Development of Gα4HC and Gα4HCregA− strains. (A) Gα4HC and Gα4HCregA− cells were grown and plated for development as described in Materials and methods. Developing aggregates were photographed at 18 and 24 hours of development. (B) Gα4HC (upper panel) and Gα4HCregA− (lower panel) cells carrying the GFP vector were harvested, mixed at a ratio 1:8 with wild-type cells with no GFP vector and developed for 18 hours. Insets shows bright-field microscopy images of slugs at 0.4X magnification. The slugs are positioned with the anterior to the right. Relative levels of GFP fluorescence were determined for the anterior (Ant), middle (Mid), and posterior (Post) regions of three slugs using the analysis described in Fig. 2. (C) Spores from Gα4HCregA−(GFP)/WT fruiting body (upper panels) and cells from Gα4HCregA−(GFP)/Gα4HCregA− aggregate (lower panel) at 26 hours of development with fluorescence (left panels) and brightfield (right panels) images. Gα4HCregA−(GFP) chimeras were mixed at a ratio 1:8 with cells not expressing GFP.
Discussion
The treatment of submerged Dictyostelium monolayers with the membrane-permeable cAMP analog 8-bromo-cAMP (8-Br-cAMP) results in sporulation and the loss of RegA or PKA-R can also promote precocious sporulation [24, 38]. These earlier studies have firmly established the importance of intracellular cAMP and PKA in sporulation but do not provide insight into how external signals work down stream of G protein-mediated signaling pathways to regulate sporulation. Our findings suggest that signaling through multiple G protein signaling pathways is necessary for the regulation of this process and that these pathways are not redundant in their contribution to sporulation. The rescue of sporulation when the regA gene is disrupted in gα4− cells suggests the Gα4 subunit mediates a signal transduction pathway that down regulates RegA activity as part of a developmental mechanism involved with spore development. This down regulation of RegA function is in part cell autonomous and likely to occur in cells destined to become spores because intercellular signaling from gα4−regA− cells is not sufficient to stimulate sporulation of gα4− cells in developing chimeras. Gα4-mediated signaling is thought to function early in spore development because cells expressing spore coat proteins do not display strong Gα4 expression [15, 18]. The ability of gα4−regA− cells to readily form spores without fruiting body formation indicates that sporulation does not require Gα4-mediated cell movement or morphology.
We propose that the Gα4-mediated activation of ERK2 results in the phosphorylation and down regulation of RegA activity, allowing a rise in cAMP and the activation of PKA (Fig. 8). PKA is the primary effector of cAMP signaling in many eukaryotic pathways and others have already demonstrated that PKA plays an important role in Dictyostelium sporulation [39, 40]. Sporulation of regA− cells can be significantly reduced by the expression of a mutant PKA-R subunit that constitutively inhibits the release of active PKA-C, implying that regA− sporulation requires PKA activity [24]. Earlier studies have indicated that prespore-specific overexpression of the PKA-C subunit does not rescue sporulation in gα4− aggregates even though the expression of some late prespore genes (e.g., pspA) can be enhanced [41]. However, a possible requirement for Gα4-mediated PKA activation prior to prespore-specific gene expression might explain the inability of the prespore-specific PKA-C to suppress gα4− sporulation defects. Alternatively, the rescue of sporulation in gα4−regA− aggregates might be accomplished through cAMP effectors other than PKA (e.g., cyclic nucleotide regulated ion channels or EPACs) but thus far no other effectors have been identified that regulate Dictyostelium sporulation. The external signal for Gα4-mediated sporulation remains to be determined but the signal is not likely to be folate because responsiveness to folate decreases during cellular aggregation [42]. Others have proposed that the Gα4 subunit couples with the GrlA receptor and mediates the release of GABA (γ-aminobutyric acid) in response to spore differentiation factor 3, SDF-3 [43]. However, the involvement of SDF-3 in the down regulation of RegA function has not been examined. The inhibited sporulation in the Gα4HC and Gα4HCregA− strains implies that Gα4 subunit overexpression blocks a developmental mechanism, such as prestalk cell differentiation, that cannot be rescued through the loss of RegA function. It is possible that the absence of RegA only accelerates the maturation of prestalk cells once this cell type has been established. Excessive Gα4 subunit could possibly affect stoichiometric interactions with other signaling components such as G protein-coupled receptors, Gβγ dimers, or ERK2 and therefore alter the Gα4 or other Gα subunit-mediated signaling pathways.
Figure 8.
Model of Gα2- and Gα4-mediated regulation of cAMP and sporulation. Gα4 subunit-coupled receptors require Gα4 subunit for ERK2 activation. The specific signal (S) and receptor (R) for the sporulation response remain to be determined. Stimulation of cAMP receptors (cARs) does not require Gα2 subunit for ERK2 activation but rather an undefined G protein-independent mechanism (X?). ERK2 phosphorylates and down regulates RegA phosphodiesterase function leading to increased levels of cAMP that can function inside or outside the cell. Intracellular cAMP can bind the PKA regulatory subunit (PKA-R) and release the active catalytic subunit (PKA-C). Active PKA-C phosphorylates target proteins to mediate sporulation and possibly contribute to other signaling mechanisms. Gα4-mediated down regulation of RegA function produces an intercellular signal (Y?) that enhances ecmB expression and stalk cell development. The identity of this intercellular signal has not yet been determined but could potentially be cAMP or other signals dependent on cAMP signaling (e.g., activation of PKA). Potential Gα2-mediated sporulation signaling that does not directly depend on the down regulation of RegA is shown with dashed lines.
The sporulation of gα2−regA− cells was extremely limited indicating that some developmental defect associated with the sporulation of gα2− cells is not readily suppressed by the regA gene disruption. One such defect might be the loss of chemotactic movement to cAMP, an essential mechanism for proper aggregation and cell movement within a multicellular aggregate. This idea is supported by low representation of gα2−regA− cells in the prespore region in chimeric aggregates where intercellular signaling is likely to be optimal for spore development. However, multicellular development is not essential for the sporulation of wild-type cell monolayers induced by the 8-Br-cAMP but this induction does not exclude the importance of extracellular cAMP or other intercellular signals in sporulation. Gα2 function is necessary for the transient rise in cAMP in response to extracellular cAMP and so perhaps the Gα2 function is biased for adenylyl cyclase activation rather than regulating cAMP levels through RegA inactivation (Fig. 8). Unlike the mammalian Gαs subunit, the Dictyostelium Gα2 subunit is not a direct activator of adenylyl cyclase and other studies have indicated the importance of other signaling proteins in the activation of adenylyl cyclase [44]. The Gα2 subunit might also contribute to spore development through the regulation of developmental gene expression or other cellular responses [16].
In addition to accelerating spore development, the regA− mutation also promotes the rapid development of prestalk cells, in particular those expressing the ecmB gene. The enrichment of regA− mutants cells near the prespore/prestalk O border of chimeric aggregates is consistent with the development of prestalk cells that express ecmB in the prestalk B region. EcmB expression can also be elevated in other areas of the slugs as indicated by the distribution of ecmB expressing gα4−regA− cells in chimeras. The increased ecmB expression and precocious stalk cell vacuolization can be mediated through intercellular signaling provided by cells that lack RegA function. However, the overexpression of the Gα4 subunit blocks the production and reception of the signal that promotes precocious stalk development in a cell autonomous manner. The inability of this strain to make prestalk cells probably accounts for the lack of signal reception but how Gα4 subunit overexpression blocks signal production in the absence of RegA is not clear. The enhancement of this prestalk subtype specific signal in regA− strains suggests that the intercellular signal could be cAMP or perhaps the production of other morphogens that are regulated by cAMP levels (Fig. 8). A variety of signals such as differentiation inducing factors (DIFs), GABA, and spore development factors (SDFs) have been identified as regulators of prestalk and/or prespore cell maturation and so possibly one of these signals could be directly or indirectly involved with the precocious prestalk cell development [43, 45, 46]. Future studies might identify if these or other external signals are responsible for the accelerated stalk development.
While multiple signaling pathways might down regulate RegA activity, the results of this study indicate that the loss of RegA function is not sufficient to overcome the sporulation defects of all G protein signaling pathways that regulate sporulation. Therefore these G protein-mediated signaling pathways must have specific responses necessary for sporulation that either do not involve cAMP signaling or involve cAMP signaling mechanisms specific to a particular pathway. Given the ability of cAMP to diffuse throughout a cell, pathway specific cAMP signaling could be achieved by separating the pathways to different cell types or stages of development. If multiple pathways operated within a common cell then pathway specific signaling complexes (signalsomes) could allow for pathway specific cAMP signaling. Support for cAMP regulated signalsomes in other eukaryotes has been provided by specific subcellular distribution of different cAMP specific-phosphodiesterase isoforms and the localization of PKAs through A-kinase anchoring proteins (AKAPs) [3, 47, 48]. An association between RegA and PKA-R has been discovered in Dictyostelium and this observation opens the possibility that selective down regulation of RegA could allow for the activation of PKA within the same location or signalsome [24]. While often considered a simple eukaryote, Dictyostelium has similar signaling proteins and possibly similar signaling mechanisms that are found in more complex eukaryotes.
Conclusions
The Gα4-mediated down regulation of RegA is an important step in spore development and the loss of RegA function can correct for the defective sporulation associated with the loss of Gα4 function. However, loss of RegA function cannot rescue sporulation defects associated with the Gα2-mediated signaling pathway or accelerate spore development in cells that overexpress the Gα4 subunit. Therefore the convergence of signaling pathways to down regulate of RegA is likely only one of the signaling mechanisms to control sporulation. The down regulation of RegA generates an intercellular signal responsible for the induction of the ecmB, but not ecmA, gene expression and the maturation of prestalk cells. The precocious development of spore and stalk cells associated with regA− strains suggests that down regulation of RegA is needed for the coordinated development of these different cell types.
Supplementary Material
Supplementary Figure. 1. PCR amplification of regA gene disruptions. Representative clones of wild-type (WT), regA−, gα2−regA−, gα4−regA−, and Gα4HCregA− cells were lysed and extracts were subjected to PCR amplification as described in the material and methods section. Disruption of the regA gene with the blasticidin S resistance gene results in a 3.5 kb segment whereas the regA gene with no disruption results in a 2 kb segment. A 1 kb molecular weight marker (M) was used to determine the size of PCR products. Some lanes from the same gel image were spliced together to eliminate unnecessary lanes.
Acknowledgments
The authors thank the laboratories of R. Kay, G. Shaulsky, W. Loomis, T. Egelhoff and the Dicty Stock Center for providing gene expression vectors. This work was supported by the grant R15 GM097717-01 provided to JAH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sunahara RK, Dessauer CW, Gilman AG. Annual review of pharmacology and toxicology. 1996;36:461–80. doi: 10.1146/annurev.pa.36.040196.002333. [DOI] [PubMed] [Google Scholar]
- 2.Edwards HV, Christian F, Baillie GS. Seminars in cell & developmental biology. 2012;23(2):181–90. doi: 10.1016/j.semcdb.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Lynch MJ, Hill EV, Houslay MD. Current topics in developmental biology. 2006;75:225–59. doi: 10.1016/S0070-2153(06)75007-4. [DOI] [PubMed] [Google Scholar]
- 4.Bos JL. Trends in biochemical sciences. 2006;31(12):680–6. doi: 10.1016/j.tibs.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Taylor SS, et al. Biochimica et biophysica acta. 2008;1784(1):16–26. doi: 10.1016/j.bbapap.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aubry L, Firtel R. Annu Rev Cell Dev Biol. 1999;15:469–517. doi: 10.1146/annurev.cellbio.15.1.469. [DOI] [PubMed] [Google Scholar]
- 7.Soderbom F, Loomis WF. Trends in microbiology. 1998;6(10):402–6. doi: 10.1016/s0966-842x(98)01348-1. [DOI] [PubMed] [Google Scholar]
- 8.Manahan CL, et al. Annual review of cell and developmental biology. 2004;20:223–53. doi: 10.1146/annurev.cellbio.20.011303.132633. [DOI] [PubMed] [Google Scholar]
- 9.Williams JG. Genetics. 2010;185(3):717–26. doi: 10.1534/genetics.110.119297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams JG. EMBO reports. 2006;7(7):694–8. doi: 10.1038/sj.embor.7400714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loomis WF, editor. The Development of Dictyostelium discoideum. Academic Press; New York: 1982. [Google Scholar]
- 12.De Wit RJ, et al. Differentiation; research in biological diversity. 1986;32(3):192–9. doi: 10.1111/j.1432-0436.1986.tb00573.x. [DOI] [PubMed] [Google Scholar]
- 13.Klein PS, et al. Science. 1988;241(4872):1467–72. doi: 10.1126/science.3047871. [DOI] [PubMed] [Google Scholar]
- 14.van Haastert PJ. Biochimica et biophysica acta. 1985;846(3):324–33. doi: 10.1016/0167-4889(85)90002-3. [DOI] [PubMed] [Google Scholar]
- 15.Hadwiger JA, Lee S, Firtel RA. Proc Natl Acad Sci U S A. 1994;91(22):10566–70. doi: 10.1073/pnas.91.22.10566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumagai A, et al. J Biol Chem. 1991;266(2):1220–8. [PubMed] [Google Scholar]
- 17.Kumagai A, et al. Cell. 1989;57(2):265–75. doi: 10.1016/0092-8674(89)90964-1. [DOI] [PubMed] [Google Scholar]
- 18.Hadwiger JA, Firtel RA. Genes Dev. 1992;6(1):38–49. doi: 10.1101/gad.6.1.38. [DOI] [PubMed] [Google Scholar]
- 19.Maeda M, et al. J Biol Chem. 1996;271:3351–3354. doi: 10.1074/jbc.271.7.3351. [DOI] [PubMed] [Google Scholar]
- 20.Maeda M, Firtel RA. J Biol Chem. 1997;272(38):23690–5. doi: 10.1074/jbc.272.38.23690. [DOI] [PubMed] [Google Scholar]
- 21.Segall JE, et al. J Cell Biol. 1995;128(3):405–13. doi: 10.1083/jcb.128.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maeda M, et al. Science (New York, NY. 2004;304(5672):875–8. doi: 10.1126/science.1094647. [DOI] [PubMed] [Google Scholar]
- 23.Shaulsky G, Escalante R, Loomis WF. Proc Natl Acad Sci U S A. 1996;93(26):15260–5. doi: 10.1073/pnas.93.26.15260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaulsky G, Fuller D, Loomis WF. Development. 1998;125(4):691–9. doi: 10.1242/dev.125.4.691. [DOI] [PubMed] [Google Scholar]
- 25.Simon MN, et al. Nature. 1992;356(6365):171–2. doi: 10.1038/356171a0. [DOI] [PubMed] [Google Scholar]
- 26.Thomason PA, et al. The EMBO journal. 1998;17(10):2838–45. doi: 10.1093/emboj/17.10.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levi S, Polyakov M, Egelhoff TT. Plasmid. 2000;44(3):231–8. doi: 10.1006/plas.2000.1487. [DOI] [PubMed] [Google Scholar]
- 28.Early A, Abe T, Williams J. Cell. 1995;83(1):91–9. doi: 10.1016/0092-8674(95)90237-6. [DOI] [PubMed] [Google Scholar]
- 29.Jermyn K, Traynor D, Williams J. Development. 1996;122(3):753–60. doi: 10.1242/dev.122.3.753. [DOI] [PubMed] [Google Scholar]
- 30.Fey P, et al. Methods in molecular biology. 2006;346:51–74. doi: 10.1385/1-59745-144-4:51. [DOI] [PubMed] [Google Scholar]
- 31.Anjard C, et al. Development. 1992;115(3):785–90. doi: 10.1242/dev.115.3.785. [DOI] [PubMed] [Google Scholar]
- 32.Watts DJ, Ashworth JM. The Biochemical journal. 1970;119(2):171–4. doi: 10.1042/bj1190171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hadwiger JA. Developmental biology. 2007;312(1):1–12. doi: 10.1016/j.ydbio.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hadwiger JH, Natarajan K, Firtel RA. Development. 1996;122:1215–1224. doi: 10.1242/dev.122.4.1215. [DOI] [PubMed] [Google Scholar]
- 35.Haberstroh L, Firtel RA. Genes & development. 1990;4(4):596–612. doi: 10.1101/gad.4.4.596. [DOI] [PubMed] [Google Scholar]
- 36.Hadwiger JA, Srinivasan J. Differentiation. 1999;64(4):195–204. doi: 10.1046/j.1432-0436.1999.6440195.x. [DOI] [PubMed] [Google Scholar]
- 37.Wessels DJ, et al. Molecular biology of the cell. 2000;11(8):2803–20. doi: 10.1091/mbc.11.8.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kay RR. Development. 1989;105:753–759. [Google Scholar]
- 39.Anjard C, et al. Differentiation; research in biological diversity. 1997;62(1):43–9. doi: 10.1046/j.1432-0436.1997.6210043.x. [DOI] [PubMed] [Google Scholar]
- 40.Loomis WF. Microbiology and molecular biology reviews: MMBR. 1998;62(3):684–94. doi: 10.1128/mmbr.62.3.684-694.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mann SK, et al. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(22):10561–5. doi: 10.1073/pnas.91.22.10561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Wit RJW, De Wit TFR. Developmental biology. 1986;118(2):385–391. [Google Scholar]
- 43.Anjard C, Su Y, Loomis WF. Development (Cambridge, England) 2009;136(5):803–12. doi: 10.1242/dev.032607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kriebel PW, Parent CA. IUBMB life. 2004;56(9):541–6. doi: 10.1080/15216540400013887. [DOI] [PubMed] [Google Scholar]
- 45.Kay RR, Berks M, Traynor D. Development. 1989;107(Suppl):81–90. doi: 10.1242/dev.107.Supplement.81. [DOI] [PubMed] [Google Scholar]
- 46.Anjard C, Loomis WF. Development. 2006;133(11):2253–61. doi: 10.1242/dev.02399. [DOI] [PubMed] [Google Scholar]
- 47.Stefan E, et al. Journal of the American Society of Nephrology: JASN. 2007;18(1):199–212. doi: 10.1681/ASN.2006020132. [DOI] [PubMed] [Google Scholar]
- 48.McConnachie G, Langeberg LK, Scott JD. Trends in molecular medicine. 2006;12(7):317–23. doi: 10.1016/j.molmed.2006.05.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure. 1. PCR amplification of regA gene disruptions. Representative clones of wild-type (WT), regA−, gα2−regA−, gα4−regA−, and Gα4HCregA− cells were lysed and extracts were subjected to PCR amplification as described in the material and methods section. Disruption of the regA gene with the blasticidin S resistance gene results in a 3.5 kb segment whereas the regA gene with no disruption results in a 2 kb segment. A 1 kb molecular weight marker (M) was used to determine the size of PCR products. Some lanes from the same gel image were spliced together to eliminate unnecessary lanes.