Abstract
Aims
Catheter–tissue contact is critical for effective lesion creation in radiofrequency catheter ablation (RFCA). In a multicentre prospective study, we assessed the effect of direct contact force (CF) measurement on acute procedural parameters during RFCA of atrial fibrillation (AF).
Methods and results
A new open-irrigated tip catheter with CF sensing (SmartTouch™, Biosense Webster Inc.) was used. All the patients underwent the first ablation procedure for paroxysmal AF with antral pulmonary vein (PV) isolation, aiming at entry and exit conduction block in all PVs. Ninety-five patients were enroled in nine centres and successfully underwent ablation. Overall procedure time, fluoroscopy time, and ablation time were 138.0 ± 67.0, 14.3 ± 11.2, and 33.8 ± 19.4 min, respectively. The mean CF value during ablation was 12.2 ± 3.9 g. Force time integral (FTI) analysis showed that patients achieving a value below the median of 543.0gs required longer procedural (158.0 ± 74.0 vs. 117.0 ± 52.0 min, P = 0.004) and fluoroscopy (17.5 ± 13.0 vs. 11.0 ± 7.7 min, P = 0.007) times as compared with those in whom FTI was above this value. Patients in whom the mean CF during ablation was >20 g required shorter procedural time (92.0 ± 23.0 vs. 160.0 ± 67.0 min, P = 0.01) as compared with patients in whom this value was <10 g. Four groin haematomas were the only complications observed.
Conclusion
Contact force during RFCA for PV isolation affects procedural parameters, in particular procedural and fluoroscopy times, without increasing complications.
Keywords: Catheter ablation, Atrial fibrillation, Contact force
What's new?
This is the first multicentre experience on a new open-irrigated tip catheter with contact force measurement capabilities (SmartTouch™, Biosense Webster Inc.).
Contact force during radiofrequency catheter ablation for pulmonary veins (PVs) isolation affects procedural parameters reducing significantly procedure and fluoroscopy times, without increasing acute complications.
Monitoring of catheter–tissue contact during PVs isolation shows a significantly longer time of poor contact during ablation of left as compared with that of right PV ablation.
Contact force parameters were highly variable among different patients, while their variability was limited comparing different operators.
Introduction
Catheter ablation (CA) has become a well-established option for management of drug refractory atrial fibrillation (AF).1,2 Although several ablation approaches have been developed, a recent HRS/EHRA/ECAS Expert Consensus3 recommends ablation strategies that target the pulmonary veins (PVs) and/or PV antrum as the cornerstone for most AF ablation procedures, the goal being complete electrical isolation of all PVs. One of the major limitations of CA of AF is the high rate of recurrences, during the short- and long-term follow-up, mainly due to electrical reconnection of the PVs. Therefore, more durable and transmural lesions produced by radiofrequency energy (RF) are desirable to improve the procedural outcome.4,5 In this setting, optimization of electrode–tissue contact may have a two-fold benefit. First, by achieving a satisfactory contact at the electrode–tissue interface, RF delivery to the tissue is optimized with less energy dissipated into the circulating blood pool and creation of more predictable and reliable lesions.3 This may affect both the procedural parameters and long-term clinical outcome. Secondly, monitoring the electrode–tissue contact may help reduce excessive contact and with that the complications possibly related to cardiac perforation.3
The aim of the present multicentre prospective study was to evaluate the effect of contact force (CF) measurement on acute procedural parameters during CA of AF using a novel CF sensing ablation catheter.
Methods
Patients selection
This multicentre prospective and descriptive study enroled patients in nine Italian centres (see the Appendix) for a 3-month period. Patients aged between 18 and 90 years with documented symptomatic AF episodes refractory to drug therapy (Class I or III drugs) and self-terminating within 7 days were included. Exclusion criteria were: (i) long-standing persistent AF, defined as AF being the sole rhythm for >12 months before the enrolment; (ii) persistent AF, defined as non-self-terminating AF episodes, or self-terminating AF episodes lasting >7 days; (iii) left atrial (LA) anteroposterior diameter >55 mm; (iv) previous CA of AF; (v) New York Heart Association functional class >II; (vi) left ventricular ejection fraction <40%; (vii) unstable angina or acute myocardial infarction within 3 months; (viii) need for or prior cardiac surgery within 6 months; (ix) contraindication to treatment with warfarin or bleeding diathesis; and (x) severe chronic renal or hepatic impairment.
This study was approved by the institutional review committees of all centres, and all the patients signed informed consents. The principles outlined in the latest update of the Declaration of Helsinki were followed.
Ablation procedure
After transseptal catheterization, three-dimensional electroanatomical maps of the LA and PVs were reconstructed using a non-fluoroscopic navigation system (CARTO 3®, version 2, Biosense Webster Inc.) as previously described.5,6 Maps were acquired during AF or sinus rhythm using respiratory gating. Fast anatomical mapping or imaging integration with a pre-acquired computed tomography or magnetic resonance scan was used, according to the operators' preference. Radiofrequency pulses were delivered using the 3.5 mm Thermocool SmartTouch™ (Biosense Webster, Inc.) in power control mode. Radiofrequency power was set between 30 and 35 W depending on different LA sites and the catheter tip was irrigated by saline at a flow rate of 2 mL/min during mapping and of 30 mL/min during ablation. Radiofrequency was delivered up to 60 s or until local electrogram amplitude was reduced >80% to produce a circumferential lesion around the proximal part of each PV's ostium or around ipsilateral PVs according to the anatomy. The lesion around the PV ostium was created by a sequential point-by-point application of RF energy or by continuously dragging the catheter, according to the operators' preference. A circular decapolar or duodecapolar mapping catheter (LASSO®, Biosense Webster Inc.) was used to confirm PV electrical isolation with demonstration of entry and exit block. Resumption of LA to PV conduction was evaluated for 30 min after ablation. In case of reconnection, The PVs were newly isolated targeting the residual electrical breakthroughs. All centres used fixed curve sheaths (Preface, Biosense Webster, or SL0, St Jude Medical). In none of the involved centres were steerable sheaths used.
Contact force measuring
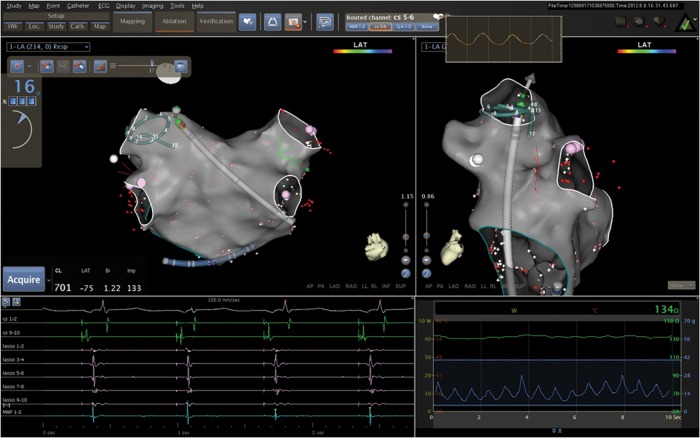
The Thermocool SmartTouch™ catheter is a 7.5 F catheter with a precision spring connecting the distal 3.5 mm irrigated electrode and the catheter shaft. The spring allows small amount of tip deflection. Sensors monitor the location signals of the transmitter coils embedded in the tip electrode and detect the micromovement of the spring. Therefore, microdeflections of the tip are translated by the system into CF values, sampled every 50 ms. Since the precision spring provides information on both lateral and axial movements in response to tissue–electrode contact, direction of CF is also given. This catheter is specifically designed for integration into the CARTO 3® system. On the system screen, direct visualization of CF values averaged on 500 ms time interval (in grams), CF direction, and instant variations of CF values over time in a real-time graphic are shown (Figure 1). After catheter insertion and initialization, the CF value was zeroed for the first time before mapping initiation, when the catheter tip was in the centre of the LA. During the procedure the CF value was re-zeroed every time it was required by the system.
Figure 1.
CARTO® 3 system screen showing a reconstruction of the LA in posteroanterior view (top left) and left lateral view (top right) with a decapolar circular mapping catheter and an ablation catheter placed at the ostium of the left superior PV. The dashboard on the left-hand side shows the value of CF (16 g), while the arrow on the catheter icon shows the CF direction. The real-time graph of the CF vs. time is also shown (blue line in the bottom right pane). Surface electrocardiogram and intracavitary electrograms from the coronary sinus catheter and circular mapping catheter before ablation are also shown (bottom left).
Operators involved in this study had previous experience in the use of Thermocool SmartTouch™ catheter in a 6-month period prior to study commencement. Since the CF value is a reliable marker of catheter contact with heart structures, the operators were encouraged to navigate the catheter with a limited use of fluoroscopy. They were allowed to view in real-time the CF values, during both the mapping and ablation phase. During RF delivery, it was recommended to make every effort to reach and maintain a displayed CF value between 10 and 40 g. According to previous studies, values in this range proved to be safe and effective.7,8
For all the ablation sites, CF data were recorded and exported after the procedure for off-line analysis. During each RF application, maximum, minimum, and mean CT values were stored and subsequently analysed. Mean CF value were calculated by averaging CF value sampled every 50 ms during each RF application. Force time integral (FTI) expressed in gram seconds and defined as the integral of the CF-time curve during the ablation period in which the catheter was located in the segment of interest was also calculated and analysed.
Statistical analysis
Normally distributed continuous variables were expressed as mean (±SD) and compared with unpaired Student's t-test. Skewed variables were expressed as median (25–75 quartiles) and compared with the rank-sum test. Normality was assessed by the Shapiro–Wilk test. Categorical variables were presented as counts and percentages, and compared with χ2 test (Pearson, Yates, or Fisher's exact test as appropriate). A P value <0.05 was considered statistically significant.
Results
Study population
Ninety-five patients were included in the study. Their clinical characteristics are shown in Table 1.
Table 1.
Clinical characteristics of the study population
| Mean age (years) | 58 ± 11 (range 24–82) |
| Male sex | 83% |
| Left atrium diameter (mm) | 40.5 ± 5 (range 33–55) |
| Left ventricle ejection fraction (%) | 58 ± 8 (range 40–65) |
| Body mass index >30 | 5% |
| Previous stroke/TIA | 4% |
| Heart disease | 51% |
| Hypertensive | 45% |
| Ischaemic | 7% |
| Valvular | 4% |
| Dilated cardiomyopathy | 4% |
| Diabetes | 6% |
Pulmonary vein isolation and contact force data
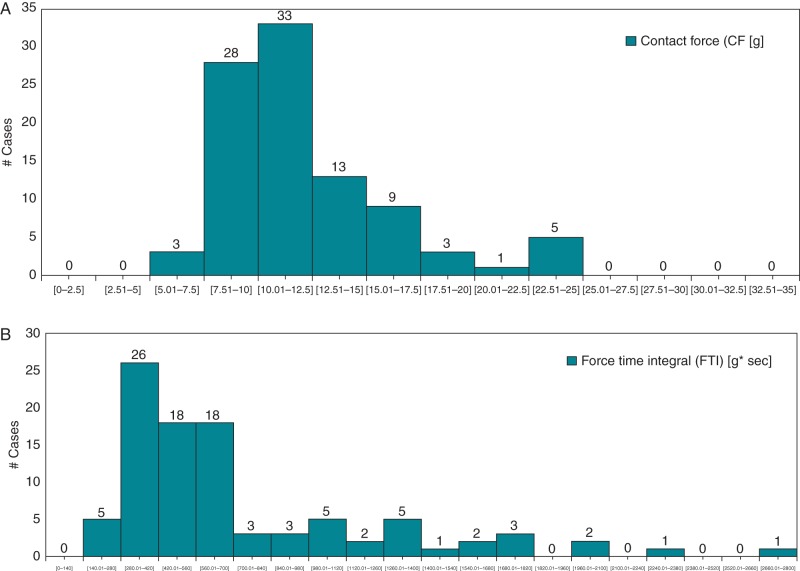
In our study population, image integration was used in 34of 95 patients without any statistically significant influence on procedural and fluoroscopy time. At the end of the procedure all the target PVs were persistently isolated. Overall procedural time was 138.0 ± 67.0 min with a fluoroscopy time of 14.3 ± 11.2 min. Ablation time was 33.8 ± 19.4 min, and mean CF values during RF applications were 12.2 ± 3.9 (range 6.7–23.7) g. A similar value of mean CF was observed during ablation of the right PVs as compared with that of the left PVs (12.5 ± 4.3 vs. 11.7 ± 4.7 g, respectively; P = 0.14). However, although the difference reaches marginal statistical significance, the percentage of time during which the CF was <5 g was lower during right as compared with left PV ablation (22 ± 14 vs. 27 ± 16%, respectively; P = 0.04). The average of the minimum and maximum CF values were 3.3 ± 2.2 (range 0.4–10.7) and 39.4 ± 13.6 (range 18–79.6) g, respectively. As shown in Figure 2A, the average CF during the entire ablation time was quite variable in different patients. However, 34.7% of the patients had a CF between 10.01 and 12.5 g and 29.5% between 7.51 and 10 g. Contact force values were well correlated with FTI (unadjusted r2 = 0.519, P < 0.001). Figure 2B shows a similarly variable distribution in different patients of FTI during the entire ablation time. Figure 3 shows the box and whiskers plot of the CF values during ablation in the nine centres. Interestingly, in seven of the nine centres, the range of the median values was minimal, within 4 g, varying from 12.48 to 8.64.
Figure 2.
Distribution of mean CF (A) and FTI (B) values during ablation in different patients.
Figure 3.
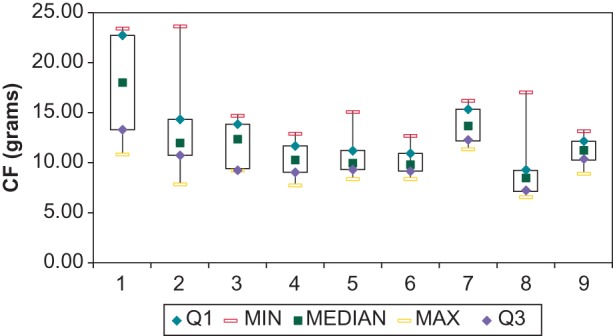
Box and whiskers plot of mean CF value during ablation in different centres.
Effect of contact force on procedural parameters
The analysis of FTI during ablation showed a median value of 543 gs: 48 patients with FTI below the median value had an average value of 394 ± 100 gs, while the 47 with FTI above had an average value of 1068 ± 520 gs. When these two groups are compared, patients with FTI below the median required significantly longer procedure (158.0 ± 74.0 vs. 117.0 ± 52.0 min, respectively; P = 0.004) and fluoroscopy (17.5 ± 13 vs. 11 ± 7.7 min, respectively; P = 0.007) times when compared with patients with FTI above the median value. Moreover, as shown in Figure 4, patients with a mean CF during ablation >20 g required significantly shorter procedural time as compared with patients in whom CF was <10 g (92.0 ± 23.0 vs. 160.0 ± 67.0 min, respectively; P = 0.01). In the former group, a non-significant trend towards shorter fluoroscopy (9.2 ± 5.1 vs. 15.3 ± 10 min, respectively; P = 0.2) and ablation (25 ± 10.1 vs. 36.3 ± 23.7 min; respectively; P = 0.26) times was noted as compared with the latter group.
Figure 4.
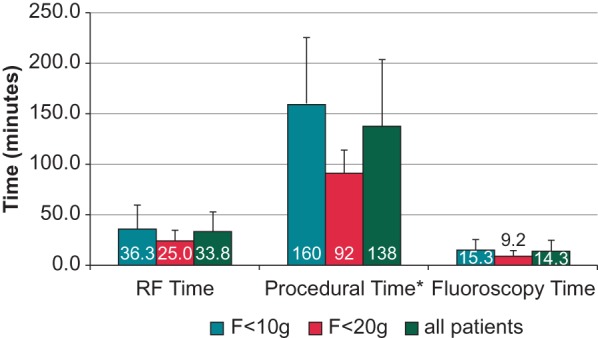
Radiofrequency (RF) time, fluoroscopy time, and procedural time in the overall population and in the two subgroups with CF < 10 g and >20 g, respectively. *P < 0.05
Complications
Four groin haematomas were observed. No stroke/transient ischaemic attack, pericardial effusion, or cardiac tamponade were reported within 30 days from the procedure.
Discussion
Main findings
Optimization of electrode–tissue contact during radiofrequency catheter ablation (RFCA) for PV isolation affects procedural parameters by significantly reducing procedural and fluoroscopy times, without increasing acute complications. Monitoring of catheter–tissue CF during PV ablation shows a wide range of values in different patients with a significantly shorter time of poor contact during right as compared with left PV ablation.
Previous studies
First data on CF measuring were reported using a catheter able to assess catheter–tissue contact by means of optical fibres.7,9 Subsequently, two other technologies were developed for clinical use, based on impedance measurement and magnetic sensors,10,11 respectively. The TOCCATA study investigators9 reported data on clinical use of a CF sensor for CA of supraventricular tachycardia and AF. They concluded that CA using real-time CF technology was safe for the treatment of supraventricular tachycardia and AF: high CF values may occur not only during catheter manipulation but also during ablation, suggesting that measuring the CF may provide additional useful information to the operator for safe catheter manipulation. With the same technology, Kumar et al.12 characterized the CF at different anatomical sites during antral PV isolation for AF. Monitoring of catheter–tissue CF during RF ablation demonstrated that there was significant variability in CF both within and between different PV anatomical sites. For left PVs, the highest CF areas were the superior and inferior quadrants, while the lowest CF were the carina and anterior quadrants. For right PVs, the areas of highest CF values were the anterior and inferior quadrants and the lowest CF was the carina. Furthermore, CF at any right PV quadrant was consistently higher than the corresponding left PV quadrant, except at the left superior quadrants where the right and left PVs had similar CF.
More importantly, data on ablation using CF sensing showed a strong linear relationship between the number of lesions with low average CF or FTI and the time to achieve acute PV isolation, confirming that catheter–tissue CF is an important determinant of procedural duration and ablation efficacy.8,13 Moreover, acute PV reconnection was strongly associated with low values of CF and FTI.12 Availability of real-time CF information during PVI was in this series associated with a significantly lower acute PV reconnection rate.12 Recently, Neuzil et al.14 showed that minimum CF and minimum FTI values are strong predictors of gap formation.
Finally, in a single-centre non-randomized study, Martinek et al.10 found that the CF sensing technology using the Thermocool SmartTouch™ catheter was able to significantly reduce ablation and procedural times in PV isolation. Energy delivery was substantially reduced by avoiding RF delivery in areas with insufficient tissue–electrode contact.
Present study
For the first time in a multicentre experience, our data demonstrates, that optimal CF values during CA of AF significantly improve the procedural parameters. In fact, during ablation, patients with an FTI above the median value had shorter procedural and fluoroscopy time and, similarly, patients with a mean CF value during ablation >20 g had shorter procedural time.
As already reported by Kumar et al.,12 optimal CF may be easier to obtain and maintain during right as compared with left PV ablation. In our study, the time with poor CF values (<5 g) was significantly shorter for right than for left PV ablation.
In our study, the mean CF value during ablation is slightly lower than the one reported in a previous study14 (12.2 ± 3.9 vs. 17.2 ± 3.5 g in our study and the previous one, respectively). The value of CF during ablation is very close to the value of 10 g, which has been considered critical for clinical success in the TOCCATA study,15 in which patients who received ablation with a mean CF below this value experienced AF recurrences. The difference between our and previous data is possibly due to different technology used. Data on follow-up will clarify if the value of 10 g is critical also using the Thermocool Smart Touch technology. In our study, we found that patients with a mean CF during ablation >20 g required significantly shorter procedural time as compared with patients in whom CF was <10 g. However, the small sample size and, above all, the lack of randomization and clinical outcome data does not, to date, allow conclusive recommendations concerning optimal CF values. Interestingly, while the mean value of CF during ablation shows wide inter-patient variability, the range of variation of this parameter is within 4 g in seven of the nine centres. This leads us to hypothesize that this value could be more patient-specific rather than operator-specific. This seems apparently in contrast with a previous report,9 which showed a wide range of CF values corresponding to the subjective feedback of ‘good contact’ defined by the operator. However, contrary to the previous study,9 in our study, the operators had already completed the learning curve of the use of the CF catheter and were not blind to the CF values.
Finally, no complication related to ablation and/or catheter manipulation was observed. Although lack of randomization does not allow a definitive conclusion, the safety profile of this CF catheter seems at least comparable with that of the conventional open-irrigated tip catheter. The low value of fluoroscopy time suggests that CF sensing could have been useful to minimize the use of fluoroscopy to manipulate the catheter without affecting procedural safety.
Limitations
First, the number of patients enroled in each centre is small and this might increase the range of data variability, mainly due to the involvement of multiple operators. However, this observational prospective study may provide a representative image of the real-life scenario in the use of CF sensing for AF ablation.
Secondly, since this was designed as a pilot study to investigate initially the effect of CF measurement on acute procedural parameters during RFCA of AF, we did not include data on long-term clinical outcome.
Thirdly, CF was computed by averaging values sampled every 50 ms during each RF application; however, bias introduced by ablation strategy (point by point vs. dragging) may not be completely excluded.
Fourthly, several parameters may affect CF: operators' attitude and expertise, use of steerable sheaths, site of transseptal puncture, individual anatomy of the LA, rotation of the heart, etc. Moreover, the direction of the force vector and the thickness of the tissue are other important variables that could play a major role in creation of a transmural and durable lesion. Although in our study we did not evaluate all these variables and their relationship with CF, our findings show that better the contact is shorter the procedural and fluoroscopy times are. This is potentially of great clinical relevance.
Conclusion
Contact force measurement is useful in CA aiming at isolation of PVs for AF. Achievement of a higher FTI and mean CF values during ablation allows reduction of both procedural and fluoroscopy time.
Acknowledgements
The authors thank Lidia Visigalli, BS, and Serena Dottori, BS, from Biosense-Webster, Italy, for their technical support.
Conflict of interest: R.D.P. is a consultant of Biosense Webster; E.B. is a consultant of Boston Scientific and Biotronik; all the other authors have no conflicts to declare. S.D. is an employee of Biosense Webster Italia.
Appendix
Participating centres (listed in alphabetical order)
Ospedale Sant'Anna, Ferrara (Matteo Bertini, Lina Marcantoni, Claudio Pratola); Ospedale Morgagni, Forlì (Alberto Bandini, Paolo Golia); Casa di Cura Montevergine, Mercogliano (Francesco Solimene, Giovanni Donnici); Casa di Cura Mediterranea, Napoli (Assunta Iuliano, Giuseppe Stabile); Policlinico Casilino, Roma (Leonardo Calò, Ermenegildo De Ruvo, Luigi Sciarra); Ospedale Sandro Pertini, Roma (Antonello Castro, Marialuisa Loricchio); Azienda Ospedaliera Santa Maria Nuova, Reggio Emilia (Nicola Bottoni, M Iori, F Quartieri); Città della Salute e della Scienza, Torino (Matteo Anselmino, Federico Ferraris, Fiorenzo Gaita); Ospedale di Circolo e Fondazione Macchi, University of Insubria, Varese (Roberto De Ponti, Raffaella Marazzi, Lorenzo A Doni).
References
- 1.Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. ESC committee for practice guidelines. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation. An update of the 2010 ESC Guidelines for the management of atrial fibrillation. Europace. 2012;14:1385–413. doi: 10.1093/europace/eus305. [DOI] [PubMed] [Google Scholar]
- 2.Wann LS, Curtis AB, January CT, Ellenbogen KA, Lowe JE, Estes NAM, III, et al. Writing on behalf of the 2006 ACC/AHA/ESC Guidelines for the Management of Patients with Atrial Fibrillation Writing Committee. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (updating the 2006 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:104–23. doi: 10.1161/CIR.0b013e3181fa3cf4. [DOI] [PubMed] [Google Scholar]
- 3.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, et al. Heart Rhythm Society Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Heart Rhythm. 2012;9:632–96. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Matsuo S, Yamane T, Date T, Inada K, Kanzaki Y, Tokuda M, et al. Reduction of AF recurrence after pulmonary vein isolation by eliminating ATP-induced transient venous re-conduction. J Cardiovasc Electrophysiol. 2007;18:704–8. doi: 10.1111/j.1540-8167.2007.00842.x. [DOI] [PubMed] [Google Scholar]
- 5.Bertaglia E, Fassini G, Anselmino M, Stabile G, Grandinetti G, Simone AD, et al. Comparison of ThermoCool(®) surround flow catheter versus ThermoCool(®) catheter in achieving persistent electrical isolation of pulmonary veins: a pilot study. J Cardiovasc Electrophysiol. 2013;24:269–73. doi: 10.1111/jce.12031. [DOI] [PubMed] [Google Scholar]
- 6.Stabile G, Scaglione M, Del Greco M, De Ponti R, Bongiorni MG, Zoppo F, et al. Reduced fluoroscopy exposure during ablation of atrial fibrillation using a novel electroanatomical navigation system: a multicentre experience. Europace. 2012;14:60–5. doi: 10.1093/europace/eur271. [DOI] [PubMed] [Google Scholar]
- 7.Yokoyama K, Nakagawa H, Shah DC, Lambert H, Leo G, Aeby N, et al. Novel contact force sensor incorporated in irrigated radiofrequency ablation catheter predicts lesion size and incidence of steam pop and thrombus. Circ Arrhythm Electrophysiol. 2008;1:354–62. doi: 10.1161/CIRCEP.108.803650. [DOI] [PubMed] [Google Scholar]
- 8.Thiagalingam A, D'Avila A, Foley L, Guerrero JL, Lambert H, Leo G, et al. Importance of catheter contact force during irrigated radiofrequency ablation: evaluation in a porcine ex vivo model using a force-sensing catheter. J Cardiovasc Electrophysiol. 2010;21:806–11. doi: 10.1111/j.1540-8167.2009.01693.x. [DOI] [PubMed] [Google Scholar]
- 9.Kuck KH, Reddy VY, Schmidt B, Natale A, Neuzil P, Saoudi N, et al. A novel radiofrequency ablation catheter using contact force sensing: TOCCATA study. Heart Rhythm. 2012;9:18–23. doi: 10.1016/j.hrthm.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 10.Martinek M, Lemes C, Sigmund E, Derdorfer M, Aichinger J, Winter S, et al. Clinical impact of a new open-irrigated radiofrequency catheter with direct force measurement on atrial fibrillation ablation. PACE. 2012;35:1312–8. doi: 10.1111/j.1540-8159.2012.03503.x. [DOI] [PubMed] [Google Scholar]
- 11.Haldar S, Jarman JW, Panikker S, Jones DG, Salukhe T, Gupta D, et al. Contact force sensing technology identifies sites of inadequate contact and reduces acute pulmonary vein reconnection: a prospective case control study. Int J Cardiol. 2012 doi: 10.1016/j.ijcard.2012.11.072. pii: S0167-5273(12)01555-0. doi: 10.1016/j.ijcard.2012.11.072 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 12.Kumar S, Morton JB, Lee J, Halloran K, Spence SJ, Gorelik A, et al. Prospective characterization of catheter-tissue contact force at different anatomic sites during antral pulmonary vein isolation. Circ Arrhythm Electrophysiol. 2012;5:1124–9. doi: 10.1161/CIRCEP.112.972208. [DOI] [PubMed] [Google Scholar]
- 13.Shah DC, Lambert H, Nakagawa H, Langenkamp A, Aeby N, Leo G. Area under the real-time contact force curve (force-time integral) predicts radiofrequency lesion size in an in vitro contractile model. J Cardiovasc Electrophysiol. 2010;21:1038–43. doi: 10.1111/j.1540-8167.2010.01750.x. [DOI] [PubMed] [Google Scholar]
- 14.Neuzil P, Reddy VY, Kautzner J, Petru J, Wichterle D, Shah D, et al. Electrical reconnection after pulmonary vein isolation is contingent in contact force during initial treatment. Results from the EFFICAS I study. Circ Arrhythm Electrophysiol. 2013;6:327–33. doi: 10.1161/CIRCEP.113.000374. [DOI] [PubMed] [Google Scholar]
- 15.Reddy VY, Shah D, Kautzner J, Schmidt B, Saoudi N, Herrera C, et al. The relationship between contact force and clinical outcome during radiofrequency catheter ablation of atrial fibrillation in the TOCCATA study. Heart Rhythm. 2012;9:1789–95. doi: 10.1016/j.hrthm.2012.07.016. [DOI] [PubMed] [Google Scholar]