Abstract
Aging leads to accumulation of irreversible advanced glycation end-products (AGEs), contributing to vascular stiffening and endothelial dysfunction. When combined with the AGE-crosslink breaker Alagebrium, exercise training reverses cardiovascular aging in experimental animals. This study is the first to examine the effect of Alagebrium, with and without exercise training, on endothelial function, arterial stiffness and cardiovascular risk in older individuals. Forty-eight non-exercising individuals (mean age 70±4 years) without manifest diseases or use of medication were allocated into 4 groups for a 1-year intervention: Exercise training & Alagebrium (200mg/day); Exercise training & placebo; No exercise training & Alagebrium (200mg/day); No exercise training & placebo. We performed a maximal exercise test (VO2max) and measured endothelial function using venous occlusion plethysmography and intra-arterial infusion of acetylcholine, sodium nitroprusside and NG-monomethyl-L-arginine. Arterial stiffness was measured using pulse wave velocity. Cardiovascular risk was calculated using the Lifetime Risk Score (LRS). In the exercise training groups, LRS and VO2max improved significantly (23.9±4.5 to 27.2±4.6mlO2/min/kg, p<0.001). Endothelial response to the vasoactive substances did not change, nor did arterial stiffness in any of the four groups. In conclusion, one year of exercise training significantly improved physical fitness and lifetime risk for cardiovascular disease without affecting endothelial function or arterial stiffness. The use of the AGE-crosslink breaker Alagebrium had no independent effect on vascular function, nor did it potentiate the effect of exercise training. Despite the clinical benefits of exercise training for older individuals, neither exercise training nor Alagebrium (alone or in combination) was able to reverse the vascular effects of decades of sedentary aging.
Keywords: Advanced Glycation End-products, Aging, Alagebrium, Arterial stiffness, Endothelial function, Exercise training
1. Introduction
Advanced age is associated with an increased risk for cardiovascular diseases, at least partly because of age-related changes in vessel characteristics that lead to arterial stiffening and endothelial dysfunction (Lakatta and Levy, 2003; Seals et al., 2011). Another detrimental age-related impact on arterial vessels is the accumulation of Advanced Glycation End-products (AGEs) in the arterial wall (Bakris et al., 2004; Brownlee, 1995; Zieman and Kass, 2004). AGEs are the end-product of a non-enzymatic reaction with sugar derivatives that leads to irreversible protein-protein crosslinks. This process occurs continuously and ultimately results in an accumulation of complex arrangements of cross-linked proteins and AGEs (Bakris et al., 2004; Brownlee, 1995; Zieman and Kass, 2004). When AGEs link to long-lived proteins, such as collagen in the arterial wall, they contribute to arterial stiffening (Bakris et al., 2004; Brownlee, 1995; Zieman and Kass, 2004). Furthermore, AGEs bind to specific AGE-binding receptors on endothelial cells and quenches nitric oxide, thereby leading to endothelial dysfunction (Brownlee, 1995; Smit and Lutgers, 2004; Zieman and Kass, 2004).
Based on the potential role of AGEs in the development of endothelial dysfunction and arterial stiffness, i.e. characteristic vascular adaptations that relate to the increased cardiovascular risk in the older population, therapeutic strategies that reverse the process of AGE formation and accumulation may have beneficial potential. A pharmacologic agent has been created to specifically break already formed AGE-crosslinks (Vasan et al., 1996). This drug, a thiazolium-derivative known as Alagebrium, breaks established AGE-crosslinks between proteins (Kass et al., 2001). Previous animal studies and initial phase I and II patient studies demonstrated a reduced vascular stiffness and improved endothelial function (Bakris et al., 2004; Kass et al., 2001; Zieman et al., 2007). Whether older individuals, who typically demonstrate endothelial dysfunction and stiffer arteries, also benefit from an AGE-crosslink breaker is currently unknown.
Physical exercise training is a potent stimulus to reduce cardiovascular risk, but also improve endothelial function and arterial stiffness (DeSouza et al., 2000; Taddei et al., 2000; Tanaka et al., 2000). Preliminary work in rats suggested that the combination of exercise training and an AGE-crosslink breaker reverses cardiovascular adaptations to advanced age in rats (Steppan et al., 2012). Whether AGE-crosslink breakers enhance the cardiovascular benefits from exercise training in humans is currently unknown. Therefore, the aim of our study was to examine the effects of a 1-year treatment with the AGE-crosslink breaker Alagebrium on endothelial function, arterial stiffness and cardiovascular risk in healthy older individuals, and combine this intervention in a factorial design with exercise training. The primary hypothesis was that both 1-year AGE-crosslink breakers and aerobic exercise training improve endothelial function and arterial stiffness. An additional hypothesis was the presence of a superior effect on endothelial function and arterial stiffness when both interventions were combined.
2. Methods
2.1 Ethics Statement
The study was performed according to Good Clinical Practice standards, and approved by the Medical Ethics Committee (Arnhem/Nijmegen, the Netherlands). All participants gave written informed consent. This study is registered at Clinical Trial.gov (NCT01417663).
2.2 Participants
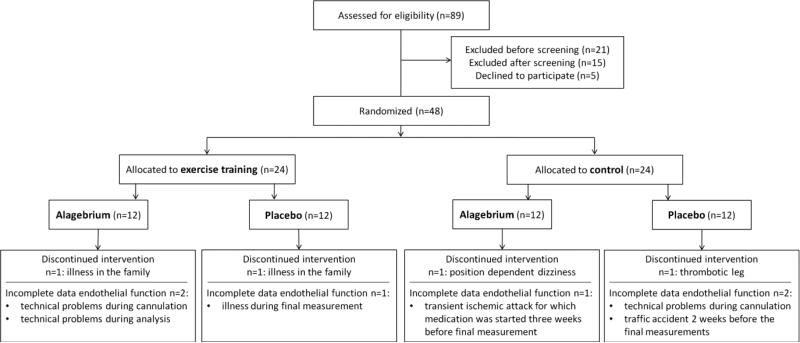
Forty-eight older individuals (age 70±4years), who did not perform regular (≤1h/week) exercise for the last 5-10 years, were recruited from the local community (Figure 1). All participants were non-smoking subjects, aged ≥65 years, without diseases or disorders that could compromise physical activity, were not regularly seeing a general practitioner or medical specialist and were not using medication known to interfere with the cardiovascular system or hormone replacement therapy. None of our participants have (a history of) cardiovascular disease. Furthermore, participants with hypertension (>160/90mmHg), diabetes mellitus, hypercholesterolemia (total cholesterol >7.5mmol/l) and body mass index >32.5kg/m2 were excluded from participation. Because of our assessment of endothelial function in the lower limbs, we also excluded participants with the presence of significant atherosclerotic lesions in the lower limbs found with physical examination indicated by murmers over the femoral artery or the absence of peripheral pulsations of the dorsalis pedis artery and/or posterior tibial artery.
Figure 1.
Consort-style flowchart regarding inclusion, allocation and drop-out of participants.
2.3 Experimental Design
After an extensive medical screening, participants were allocated to 4 different intervention groups according to a factorial design for a 1-year intervention: 1) Exercise training & Alagebrium; 2) Exercise training & placebo; 3) No exercise training (control) & Alagebrium; 4) No exercise training (control) & placebo.
Measurements were performed before, during (6 months) and after one year. First, participants were clinically evaluated to be healthy. Then, we performed non-invasive vascular measurements of the common femoral artery diameter and central pulse wave velocity (i.e. arterial stiffness). On a separate day, participants performed an incremental maximal bicycle exercise stress test. Before and after the 1-year intervention, we also assessed lower limb endothelial function using venous occlusion plethysmography and intra-arterial infusion of vasoactive substances. This measurement was performed on a separate day.
2.4 One year intervention
2.4.1 Exercise training
The randomization procedure for exercise training was performed with 48 envelopes. Exercise training was performed 3 times/week for 12 months on a cycle ergometer (Medgraphics, Corival Cycle Ergometer, St Paul, MN, USA). Each exercise session consisted of 10 minutes warm up, followed by 30 minutes of cycling exercise at 70–85% of the individual heart rate reserve and ended with 5 minutes cool down. Heart rate was continuously monitored (Polar RS800; Polar Electro Oy, Kempele, Finland). Workload was individually adjusted throughout the training to enhance physical fitness. The researchers were blinded during data analysis.
2.4.2 Alagebrium vs. placebo
Participants were randomized to AGE-crosslink breaker Alagebrium 100mg twice a day or placebo (twice a day). This dose of Alagebrium, produced by Synvista Therapeutics, was selected as the lowest dose that was effective in both animal (Asif et al., 2000; Vaitkevicius et al., 2001) and human (Bakris et al., 2004; Kass et al., 2001) studies to maximize efficacy and minimize toxicity. Double-blind randomization was controlled by the university hospital pharmacist and was kept strictly confidential during the study. Compliance with the study drug was controlled by asking participants to keep a journal and sign the time of each ingestion. Every 3 months, this journal was compared with the (empty) strips of tablets.
2.5 Measurements
2.5.1 General characteristics
All participants underwent clinical evaluations including measurements of body composition. Blood pressure measurements were performed three times in the supine position using a manual sphygmomanometer around the left arm after a 10 minute rest. Venous blood samples were taken after an overnight fast to measure lipid levels, glucose and glycosylated haemoglobin (HbA1c). In addition, high sensitivity C-reactive protein (hs-CRP) was examined as a measure of inflammation.
2.5.2 Incremental maximal bicycle exercise stress test
An incremental maximal exercise stress test on a bicycle ergometer (Lode, Excalibur Sport, Groningen, the Netherlands) was performed to measure maximal oxygen uptake (VO2max) (Balady et al., 2010). After 3 minutes rest, participants started cycling at a workload of 50Watt, which was increased by 10Watt/minute. Continuous measurement of oxygen uptake (VO2) was performed using an automatic gas analyzer (Oxycon alpha, Jaeger, Breda, the Netherlands). Peak oxygen uptake (mlO2/min/kg) was calculated as the average oxygen uptake during the last minute of the test and then scaled for body weight and lean body mass.
2.5.3 Cardiovascular risk
The Lifetime Risk Score (LRS) is based on an algorithm that incorporates gender, age, systolic blood pressure, diabetes mellitus, total cholesterol, smoking, body mass index, and physical fitness (Berry et al., 2012; Berry et al., 2011). The LRS has a strong predictive capacity for future cardiovascular mortality (Berry et al., 2012; Berry et al., 2011).
2.5.4 Vascular measurements
Vascular measurements were performed under standardized conditions. Participants were asked to refrain from coffee, tea, alcohol, chocolate, vitamin C supplements or fruit 14 hours prior and fasting overnight prior to the examinations. Room temperature was set at 22±1°C.
Common femoral artery diameter
Using high-resolution echo ultrasonography with a 7.5 MHz linear array transducer (Picus, Pie Medical Benelux, Maastricht, the Netherlands) we measured the right common femoral artery (CFA) diameter 2cm proximal to the bifurcation (Thijssen et al., 2007). The percentage change in arterial diameter was calculated and used for analysis.
Arterial stiffness
We measured systemic arterial stiffness using central pulse wave velocity (PWV) (Vlachopoulos et al., 2010). A three lead electrocardiogram (ECG) was used for R-wave detection. The pulse wave was measured by echo-Doppler ultrasound (WakiLoki Doppler, 4MHz, Atys) at the left carotid artery and right CFA.
Lower limb endothelial function
Endothelial function of the lower limb was measured from resistance artery blood flow responses using venous occlusion plethysmography during intra-arterial infusion of vasoactive substances (Joyner et al., 2001; Wilkinson and Webb, 2001) in the upper leg, previously described in detail by Kooijman et al. (Kooijman et al., 2003). In short, an intra-arterial cannula was introduced into the right CFA at the level of the inguinal ligament. This cannula was used for intra-arterial administration of vasoactive substances and for intra-arterial blood pressure monitoring. Bilateral blood flow in the upper legs was measured by ECG-triggered venous occlusion plethysmography. Mercury-in-silastic strain gauges were placed at mid thigh to quantify changes in leg volume from changes in upper leg blood flow.
After instrumentation and at least 45 minutes after cannulation of the femoral artery, infusion of the vasoactive substances started. We infused the endothelium dependent vasodilator acetylcholine (ACh), the endothelium independent vasodilator sodium nitroprusside (SNP), and the nitric oxide synthase inhibitor NG-monomethyl-L-arginine (L-NMMA). Acetylcholine was administered at 1, 4, 16, 32 and 64 μg/mL/100mL leg volume, SNP 0.06, 0.20 and 0.60 μg/mL/100mL leg volume, and L-NMMA 0.05, 0.10, 0.20 and 0.40 mg/mL/100mL leg volume. The order of infusion was fixed and each substance was infused for 5 minutes. During each substance infusion, the calf circulation was occluded by inflating cuffs directly below the knee to suprasystolic values (≥220 mmHg) to avoid the use of high dosages with subsequent possible systemic effects of the vasoactive substances (Kooijman et al., 2003). Two consecutive infusions were performed before deflating the lower leg cuffs to restore normal blood flow for 5 minutes. During these 5 minutes of recovery, 0.9% saline (or 5% glucose during SNP measurements) was infused to maintain a constant flow rate. Between administration of different substances a 20 minute rest period was inserted with continuous flow of 0.9% saline.
Besides changes in blood flow, the blood flow ratio between the infusion and control leg was also calculated to correct for possible systemic effects.
2.6 Statistical Analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS, Chicago, IL, USA). Data are presented as mean ± standard deviation (SD), except for the figures in which mean ± standard error of the mean (SEM) are used. At baseline, a one-way ANOVA (Analysis of variance) comparison was performed between the 4 groups with Bonferroni correction. Differences between the 4 groups in response to the 1-year intervention were analyzed using the Linear Mixed Model. The interaction between exercise training and Alagebrium was analyzed (Time*Training*Medication), and both individual effects of exercise training vs. no exercise training and Alagebrium vs. placebo were analyzed (Time*Training and Time*Medication, respectively). Changes in blood flow and blood flow ratio responses to increasing dosages of vasoactive substances were also analyzed using the Linear Mixed Model at baseline and after one year. Statistical significance was set at a (2-sided) p-value <0.05. Sample size was calculated for an effect of Alagebrium and exercise training on endothelial function. Based on previous studies (DeSouza et al., 2000; Kooijman et al., 2003; Taddei et al., 2000) that examined the influence of physical activity on endothelial function using intra-arterial infusion of ACh, SNP, and L-NMMA, a group size of 10 subjects is sufficient to detect relevant changes in endothelial function of 3.9ml/min/dl with exercise training with a power of >0.95, given a standard deviation of 4.7ml/min/dl and an α-error of 0.05. Moreover, based on previous work from our lab (Thijssen et al., 2007), 8 subjects would allow for a power of >0.95 to detect a change in arterial compliance with exercise training of 0.015mm2/mmHg given a standard deviation of 0.01mm2/mmHg and an alpha error of 0.05. The magnitude of both of these changes would be considered clinically and physiologically significant. Due to the long intervention period and possibility for drop-outs, group sizes of 12 participants were created.
3. Results
Four individuals, 1 per intervention arm, did not finish the intervention due to reasons unrelated to the intervention (Figure 1). At baseline, we found no differences between the 4 groups in gender, age, and physical fitness (Table 1). Also, no differences were observed in cardiovascular risk factors, e.g. body composition, blood pressure, total cholesterol, triglycerides, glucose, HbA1c, and hs-CRP among the 4 groups at baseline (Table 1). Drug compliance was high with >95% of the drug taken.
Table 1.
Characteristics and cardiovascular risk factors of the 4 groups at baseline and after one year intervention.
| Characteristics Baseline & after one year intervention | Linear Mixed Model: changes due to the 1-year interventions | ||||||
|---|---|---|---|---|---|---|---|
| Exercise training | No exercise training | Time*Training | Time*Medication | Time*Training Medication | |||
| Alagebrium | placebo | Alagebrium | placebo | p-value | p-value | p-value | |
| Male : Female (n) | 6:5 | 8:3 | 8:3 | 3:8 | |||
| Age (years) | 69±3 | 68±3 | 70±3 | 71±5 | |||
| VO2max (mlO2/min/kg) | <0.001 | 0.969 | 0.757 | ||||
| Baseline | 23.2±4.2 | 24.5±4.9 | 25.6±4.3 | 24.1±4.2 | |||
| 12 months | 26.7±4.0 | 27.7±5.2 | 25.0±4.7 | 23.9±3.6 | |||
| VO2max per lean body mass (mlO2/min/kg) | <0.001 | 0.740 | 0.864 | ||||
| Baseline | 36.4±6.7 | 35.5±5.7 | 36.0±5.1 | 35.5±5.9 | |||
| 12 months | 40.2±4.9 | 40.3±5.7 | 34.5±5.5 | 34.9±5.1 | |||
| Body Mass Index (kg/m2) | 0.812 | 0.209 | 0.706 | ||||
| Baseline | 26.9±3.5 | 27.0±2.6 | 26.6±3.0 | 24.3±3.3 | |||
| 12 months | 26.2±4.0 | 26.8±2.9 | 26.2±3.1 | 24.3±3.1 | |||
| Lean Body Mass (kg) | 0.225 | 0.610 | 0.777 | ||||
| Baseline | 53.8±13.3 | 57.1±9.6 | 55.2±8.5 | 46.6±8.7 | |||
| 12 months | 53.2±11.5 | 56.6±9.5 | 55.4±8.8 | 47.1±8.7 | |||
| Waist circumference (cm) | 0.790 | 0.366 | 0.763 | ||||
| Baseline | 97.7±11.5 | 96.4±7.5 | 93.3±9.6 | 88.2±11.0 | |||
| 12 months | 96.8±11.9 | 96.3±7.5 | 92.8±8.5 | 89.0±10.4 | |||
| Hip circumference (cm) | 0.491 | 0.735 | 0.772 | ||||
| Baseline | 103.4±5.9 | 103.2±5.8 | 101.9±6.0 | 99.6±7.1 | |||
| 12 months | 102.5±7.2 | 102.9±6.1 | 100.6±5.9 | 98.6±6.3 | |||
| Waist-to-Hip ratio | 0.228 | 0.597 | 0.869 | ||||
| Baseline | 0.94±0.06 | 0.94±0.04 | 0.92±0.08 | 0.88±0.07 | |||
| 12 months | 0.94±0.06 | 0.94±0.03 | 0.92±0.08 | 0.90±0.07 | |||
| Systolic blood pressure (mmHg) | 0.230 | 0.275 | 0.189 | ||||
| Baseline | 128±10 | 131±9 | 137±15 | 134±15 | |||
| 12 months | 128±14 | 128±8 | 138±17 | 135±16 | |||
| Diastolic blood pressure (mmHg) | 0.869 | 0.376 | 0.703 | ||||
| Baseline | 80±9 | 76±6 | 79±8 | 79±12 | |||
| 12 months | 80±9 | 74±3 | 80±10 | 76±10 | |||
| Pulse Pressure (mmHg) | 0.155 | 0.629 | 0.150 | ||||
| Baseline | 47±7 | 56±8 | 58±14 | 56±9 | |||
| 12 months | 49±9 | 53±9 | 58±16 | 59±9 | |||
| Total cholesterol (mmol/l) | 0.467 | 0.162 | 0.422 | ||||
| Baseline | 5.3±1.0 | 5.7±0.9 | 5.5±0.6 | 5.2±0.9 | |||
| 12 months | 5.1±0.8 | 5.9±0.9 | 5.2±0.7 | 5.1±0.5 | |||
| HDL cholesterol (mmol/l) | 0.763 | 0.662 | 0.918 | ||||
| Baseline | 1.4±0.3 | 1.2±0.2 | 1.2±0.3 | 1.6±0.2* | |||
| 12 months | 1.4±0.4 | 1.3±0.3 | 1.2±0.3 | 1.6±0.3 | |||
| LDL cholesterol (mmol/l) | 0.789 | 0.204 | 0.437 | ||||
| Baseline | 3.4±1.0 | 4.1±0.7 | 3.7±0.5 | 3.2±0.7* | |||
| 12 months | 3.1±0.7 | 4.1±0.8 | 3.5±0.5 | 3.1±0.5 | |||
| Triglycerides (mmol/l) | 0.125 | 0.401 | 0.673 | ||||
| Baseline | 1.2±0.4 | 1.0±0.3 | 1.2±0.4 | 1.0±0.4 | |||
| 12 months | 1.3±0.5 | 1.2±0.3 | 1.2±0.4 | 1.0±0.3 | |||
| Glucose (mmol/l) | 0.831 | 0.390 | 0.502 | ||||
| Baseline | 5.2±0.6 | 4.9±0.5 | 5.1±0.4 | 4.9±0.3 | |||
| 12 months | 5.1±0.8 | 5.2±0.6 | 5.1±0.5 | 4.9±0.3 | |||
| HbA1c (%) | 0.358 | 0.244 | 0.728 | ||||
| Baseline | 5.6±0.4 | 5.8±0.3 | 5.7±0.4 | 5.6±0.2 | |||
| 12 months | 5.6±0.4 | 5.8±0.4 | 5.5±0.6 | 5.5±0.2 | |||
| Hs-CRP (mg/l) | 0.194 | 0.987 | 0.048 | ||||
| Baseline | 1.4±1.0 | 2.3±1.3 | 2.1±1.7 | 1.4±1.3 | |||
| 12 months | 1.9±1.4 | 1.4±1.0 | 2.0±1.3 | 1.2±0.9 | |||
Data are presented as mean ± standard deviation. Abbreviations: HDL high density lipoprotein, LDL low density lipoprotein, HbA1c glycosylated hemoglobin, hs-CRP high sensitivity C-reactive protein.
At baseline (one-way ANOVA) HDL cholesterol was higher in the no exercise training & placebo group compared with the exercise training & placebo group (p=0.011) and no exercise training & Alagebrium group (p=0.012). LDL cholesterol was lower in the no exercise training & placebo group compared with the exercise training & placebo group (p=0.049).
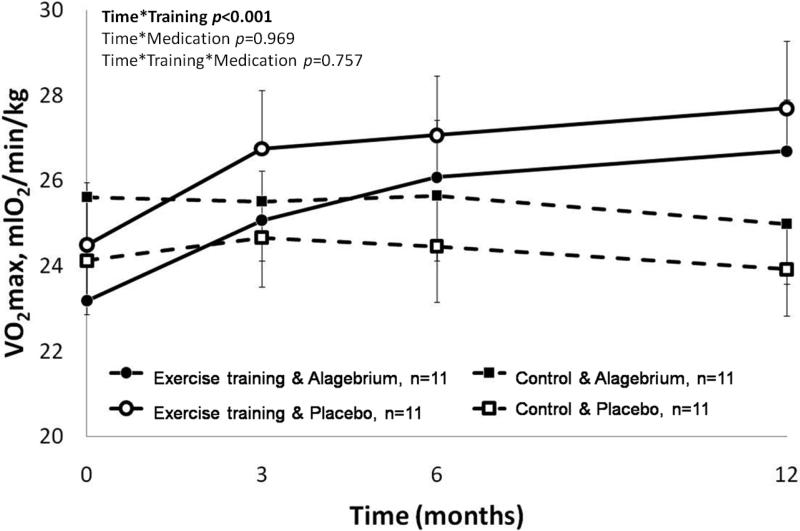
One year of exercise training significantly improved physical fitness by 15% (Time*Training p<0.001, Figure 2). Alagebrium did not influence physical fitness (Time*Medication p=0.969 and Time*Training*Medication p=0.757).
Figure 2. Significant increase in physical fitness after one year of exercise training.
Exercise training groups (solid lines: with Alagebrium ●; with placebo ○) significantly improved their physical fitness, while the control groups (dashed lines: with Alagebrium ■; with placebo □) showed a small, non-significant, decline in VO2max.
The 1-year intervention did not alter body composition, blood pressure, lipid levels, glucose, and HbA1c in any of the 4 groups (all p>0.10, Table 1). A small interaction effect was seen in hs-CRP when combining the 4 groups (Time*Training*Medication p=0.048). However, nor the individual effect of exercise training, nor the individual effect of medication had a significant influence on hs-CRP levels (Time*Training p=0.194 and Time*Medication p=0.987, respectively). Furthermore, no overall difference was seen in hs-CRP before and after one year (n=44, pre 1.83±1.38mg/l vs. post 1.60±1.14mg/l, paired t-test p=0.166).
3.1 Cardiovascular risk prediction
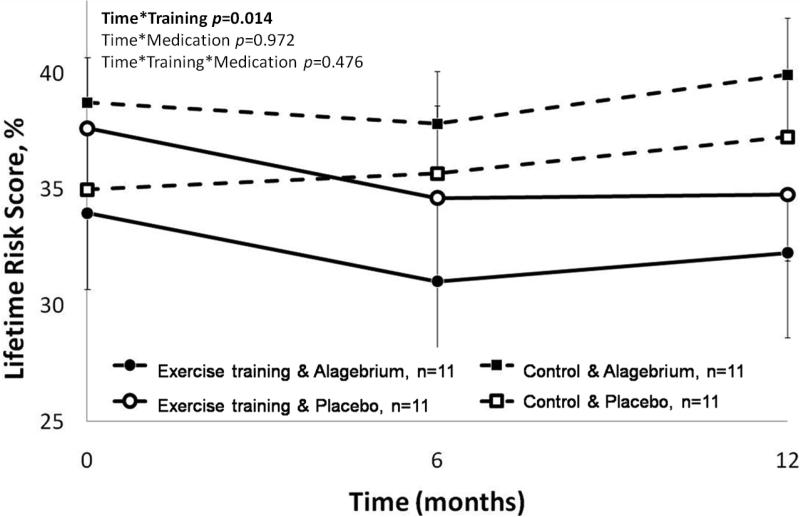
Lifetime Risk Score significantly improved after exercise training (Time*Training p=0.014), with an 8% decrease in 30-year risk prediction. Alagebrium did not change LRS or alter the impact of exercise training on Lifetime Risk Score (Time*Medication p=0.972 and Time*Training*Medication p=0.476, respectively, Figure 3).
Figure 3. Improvement in Lifetime Risk Score with one year intervention of exercise training.
Lifetime Risk Score improved significantly in the exercise training groups (solid lines: with Alagebrium ●; with placebo ○) while the control groups (dashed lines: with Alagebrium ■; with placebo □) did not change significantly. Alagebrium had no influence on the Lifetime Risk Score.
3.2 Vascular measurements
Common femoral artery diameter
Due to technical problems, we had to exclude 7 participants from the echo Doppler measurements. Exercise training was associated with an increase in CFA diameter of 6% (Time*Training p=0.043). Use of the AGE-crosslink breaker Alagebrium did not change CFA diameter or alter the magnitude of effect observed with exercise training (Time*Medication p=0.948 and Time*Training*Medication p=0.837, respectively, Table 2).
Table 2.
Vascular measurements with common femoral artery diameter and arterial stiffness.
| Vascular measurements | Linear Mixed Model: changes due to the 1-year interventions | ||||||
|---|---|---|---|---|---|---|---|
| Exercise training | No exercise training | Time*Training | Time*Medication | Time*Training Medication | |||
| Alagebrium | placebo | Alagebrium | placebo | p-value | p-value | p-value | |
| Common femoral artery diameter (mm) | 0.043 | 0.948 | 0.837 | ||||
| Baseline | 9.5±0.9 | 10.1±1.3 | 10.7±1.8 | 9.8±1.6 | |||
| 6 months | 10.0±1.0 | 10.5±1.4 | 10.9±1.5 | 9.9±1.6 | |||
| 12 months | 10.1±1.0 | 10.6±1.3 | 10.9±1.5 | 10.0±1.6 | |||
| Arterial stiffness, pulse wave velocity (m/s) | 0.425 | 0.106 | 0.886 | ||||
| Baseline | 13.3±5.4 | 11.0±3.2 | 13.2±4.3 | 11.5±3.0 | |||
| 6 months | 11.7±3.7 | 10.8±2.4 | 12.7 ±2.8 | 12.4±3.4 | |||
| 12 months | 12.3±4.1 | 11.4±2.6 | 12.2±1.7 | 12.7±3.9 | |||
Data are presented as mean ± standard deviation.
Arterial stiffness
We did not observe changes in arterial stiffness measured with pulse wave velocity after any of the four 1-year interventions (Table 2).
Endothelial function
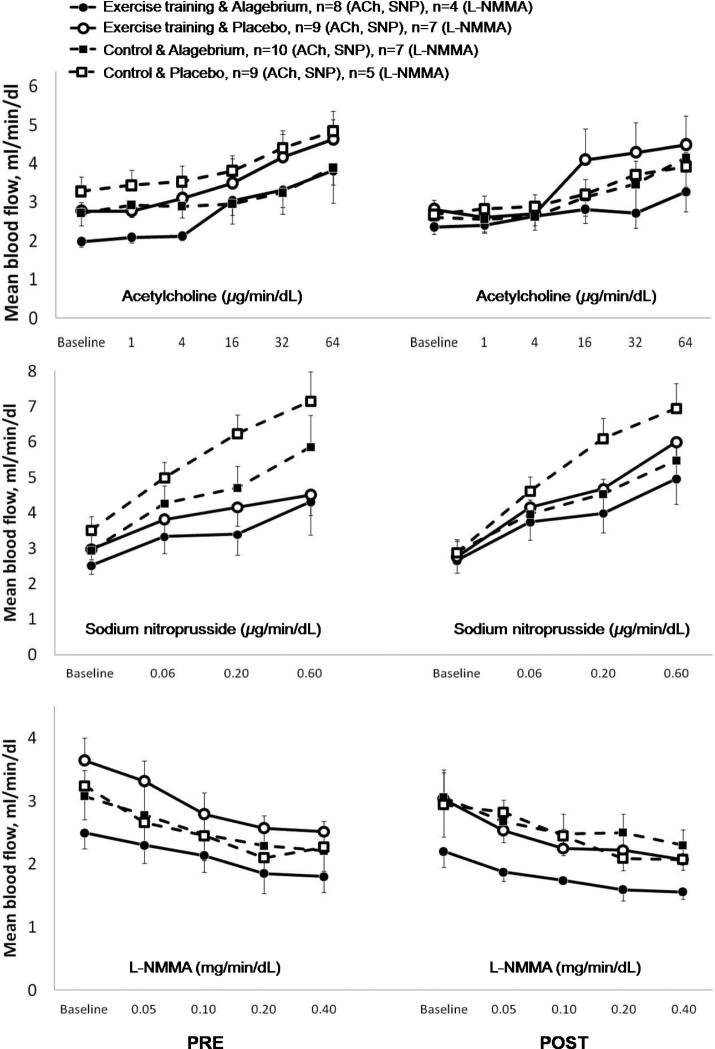
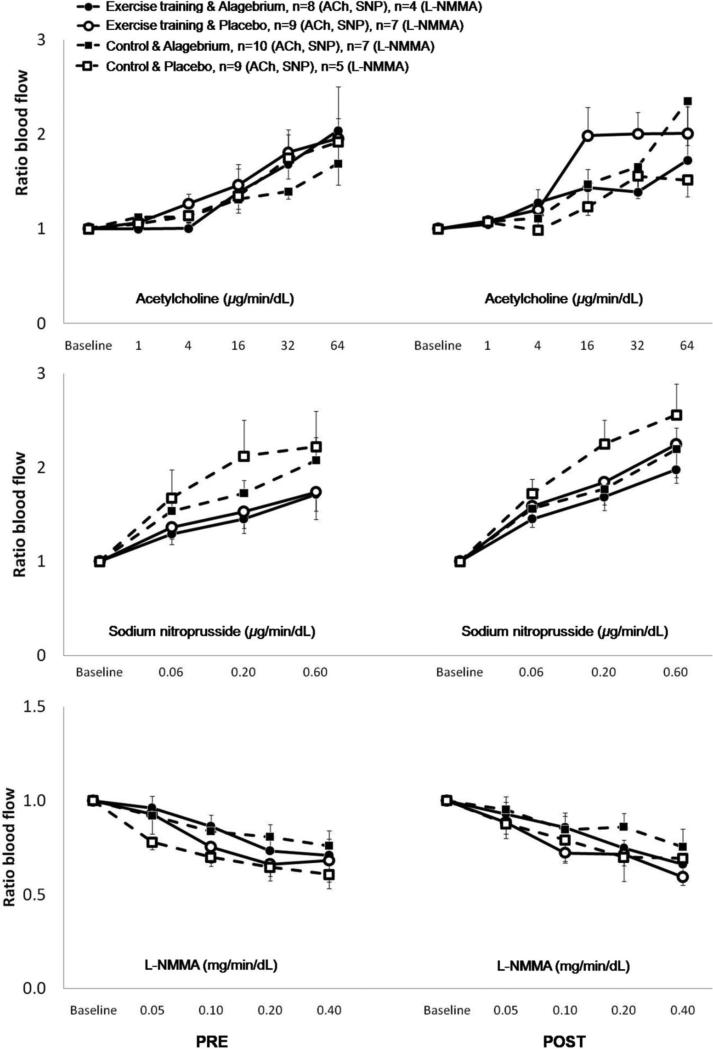
All participants received ACh and SNP, and by design 24 participants were given L-NMMA. Due to technical problems, 8 participants were excluded from analyses (Figure 1). We found no significant effect of the interventions (exercise training, Alagebrium, or both) on blood flow or blood flow ratio responses to the incremental doses of ACh, SNP or L-NMMA (all p>0.10, Figures 4 and 5). In addition, no differences were observed amongst the four groups in blood flow or blood flow ratio responses to the pharmacological stimuli at baseline as well as after one year of intervention (Figures 4 and 5).
Figure 4. Mean blood flow response to intra-arterial infusion of vasoactive substances.
No changes were observed in mean blood flow response to increasing dosage of vasoactive substances between any of the four groups during the year intervention: exercise training & Alagebrium (solid line + ●), exercise training & placebo (solid line + ○), control & Alagebrium (dashed line + ■), and control & placebo (dashed line + □). Pre = baseline measurements, post = after 1-year interventions.
Figure 5. Blood flow ratio response to intra-arterial infusion of vasoactive substances.
No changes were observed in blood flow ratio (infusion/control leg) response to increasing dosage of vasoactive substances between any of the four groups during the year intervention: exercise training & Alagebrium (solid line + ●), exercise training & placebo (solid line + ○), control & Alagebrium (dashed line + ■), and control & placebo (dashed line + □). Pre = baseline measurements, post = after 1-year interventions.
4. Discussion
We examined the effects of the AGE-crosslink breaker Alagebrium, alone and in combination with aerobic exercise training on endothelial function, arterial stiffness and cardiovascular risk in sedentary older individuals. Although our interventions were performed successfully (i.e. improvement in physical fitness of 15% and drug intake compliance of >95%), none of the interventions improved endothelial function or arterial stiffness. Nonetheless, the Lifetime Risk Score improved significantly in the exercise training groups, whilst the AGE-crosslink breaker was not associated with any further improvement. Therefore, the cardioprotective effects of 1-year exercise training in previously sedentary older subjects cannot be potentiated by the AGE-crosslink breaker Alagebrium, nor be explained by improvements in endothelial function or arterial stiffness.
Our primary outcome measure, the endothelial function, was measured using intra-arterial infusion of (incremental doses of) vasoactive drugs, which is widely considered to be the “gold standard” (Joyner et al., 2001; Wilkinson and Webb, 2001). Despite a 15% improvement in physical fitness and >95% compliance in drug intake, neither endothelium-dependent or - independent vasodilation, nor the contribution of nitric oxide to basal vascular tone, changed in any group over the intervention period. While most studies investigating Alagebrium have focused on arterial and ventricular stiffness, only one study examined the influence of short term (8 weeks) Alagebrium on endothelial function (Zieman et al., 2007). They reported an improvement in brachial artery endothelial function. However, this outcome was measured in 10 patients with isolated systolic hypertension (versus our healthy older individuals), in a different vascular bed (i.e. a conduit artery in the upper arm versus resistance vessels in the lower limbs in the present study), and using the widely used method of flow mediated dilation; i.e. an indirect method to measure endothelial function.
Even though there is consistency in the literature about the positive effects of exercise training on improving endothelial function in patient groups in whom endothelial function is initially depressed, the effect of exercise training on endothelial function of subjects with marginal endothelial dysfunction are less obvious (Bergholm et al., 1999; Thijssen et al., 2007; Thijssen et al., 2010). In contrast to several exercise training studies that reported improved endothelial function in the brachial artery after training (DeSouza et al., 2000; Seals et al., 2011; Taddei et al., 2000), the endothelial function in the lower limbs did not improve in our study groups. A first explanation may relate to differences in vascular responses between the arms and legs, with lower limbs being less responsive to exercise training as the lower limbs already demonstrate a higher activity level than the upper limbs during daily living (e.g. during walking, cycling, climbing the stairs) (Rowley et al., 2011; Thijssen et al., 2011). Another explanation for this discrepancy may be the long duration of exercise training in our study. Indeed, previous studies found that exercise training initially leads to functional adaptations (improved endothelium), followed by structural adaptations (increase in vascular diameter) when exercise training continues, allowing endothelial function to return towards baseline levels (Thijssen et al., 2010). Interestingly, we observed an increase in CFA diameter in the exercise training groups, most likely as a result of the repeated exposure of elevation in shear stress on the arterial wall during exercise (Thijssen et al., 2010). Subsequently, functional endothelial responses might have returned to baseline near the end of our study. However, this remains speculative since we did not perform repeated measures to assess the time course of endothelial function.
We found no change in arterial stiffness after our interventions. The observation of unaltered stiffness after training is in line with a recent study that found that intrinsic structural characteristics of the arterial wall remained unaffected in previously sedentary elderly after one year of exercise training (Shibata and Levine, 2012). However, both animal and human studies found that Alagebrium decreased arterial stiffness (Kass et al., 2001; Little et al., 2005; Vaitkevicius et al., 2001; Zieman et al., 2007). In our study, we found a small, albeit non-significant, trend of Alagebrium to improve central PWV. More recently, the combination of Alagebrium with exercise training, in a design similar to the present study but performed in rats, reversed the effects of cardiovascular aging in older sedentary rats (Steppan et al., 2012). Differences between studies in doses of Alagebrium and exercise, but also in species examined may explain these results.
An important difference between our study and previous human studies that examined AGE-crosslink breakers is the inclusion of patient populations versus non-diseased participants in our study. Cardiovascular patients demonstrate a priori endothelial dysfunction and arterial stiffening that likely exceeds that of non-diseased, sedentary older individuals. Therefore, interventions in such populations are more amenable to an improvement. Nonetheless, the rationale of our study to examine older individuals is strong, since it is known that this population is associated with an increased cardiovascular risk, has decreased endothelial function and reports stiffer arteries compared with young individuals (DeSouza et al., 2000; Lakatta and Levy, 2003; Seals et al., 2011; Taddei et al., 2000; Tanaka et al., 2000).
This study represents the longest duration of therapy with an AGE-crosslink breaker in humans reported in the literature, whilst we are the first to combine AGE-crosslink breakers with exercise training in humans. Some short duration (open-label) AGE-crosslink breaker studies with patient groups found an improvement in endothelial function (Zieman et al., 2007) and a decrease in arterial and possibly myocardial stiffness (Kass et al., 2001; Little et al., 2005; Zieman et al., 2007), while a longer 9-month study with stable heart failure patients showed no improvements in cardiac function with Alagebrium (Hartog et al., 2011).
Whilst the lack of improvement in endothelial function and arterial stiffness after a 1-year intervention is somewhat disappointing, our results strongly reinforce previous suggestions that vascular changes induced by biological aging and physical inactivity cannot be easily reversed. According to Byberg et al. (Byberg et al., 2009), it takes several years for middle-aged men to achieve a decrease in (all cause) mortality after becoming physically active after years of physical inactivity. Also in our study, we included older subjects who have not performed (regular) exercise for the last several years. Thus, despite the strong rationale for direct effects of AGE-crosslink breaker Alagebrium and exercise training on the arterial wall, our 1-year intervention may be insufficient to undo the negative effects of decades of sedentary aging.
Despite the absence of a direct effect on the vasculature, significant improvement in physical fitness has important health benefits (Byberg et al., 2009; Löllgen et al., 2009). Physical fitness has recently been demonstrated to have the strongest predictive capacity for future cardiovascular diseases and all-cause mortality (Blair et al., 1989; Kodama et al., 2009). Indeed, the improvement in physical fitness in the exercise training groups importantly contributed to the significant improvement in Lifetime Risk Score, whilst Alagebrium did not have any (additional) effects. Therefore, it must be emphasized that, despite the absence of a direct vascular effects, the performance of a 1-year exercise training program in previously sedentary elderly resulted in a significant and clinically meaningful reduction in risk for future cardiovascular disease.
4.1 Limitations and strengths
The use of invasive and highly valid measures of endothelial function, the long intervention period with a unique combination of novel interventions, and the high compliance with drug-intake and exercise training represent unique aspects of our study. Nonetheless, our study has a number of potential limitations. For example, power analysis supported a sample size of 8-10 participants per group to detect relevant differences with a small chance of type II error. Even though not all participants could be included in the analysis of the endothelial function, even the Control & Alagebrium group (n=10) failed to show differences after the one year intervention. Nor were there trends in any of the other groups (n=9).Thus, even if all data from the venous occlusion plethysmography could have been used, it is unlikely that a physiologically meaningful improvement in endothelial function was missed. Also, arterial stiffness, with presumably sufficient participants per group, did not improve with these interventions. This suggests that the beneficial effects of exercise training on cardiovascular risk are not explained by improvements in endothelial function or arterial stiffness. Another potential limitation is that we did not measure blood levels and pharmacokinetics of Alagebrium. However, we were meticulous at ensuring compliance with the study medication. Moreover, the doses used in this study were comparable to the doses used in other pre-clinical and clinical trials where an effect of Alagebrium to break AGE-crosslinks was evident (Asif et al., 2000; Bakris et al., 2004; Kass et al., 2001; Vaitkevicius et al., 2001).
5. Conclusion
One year of exercise training in older individuals significantly improved physical fitness and lifetime risk for cardiovascular disease without affecting endothelial function or arterial stiffness. The use of the AGE-crosslink breaker Alagebrium had no independent effect on vascular function, nor did it potentiate the effect of endurance training. Despite the benefits and cardioprotective effects of exercise training for older individuals, neither exercise nor Alagebrium (either alone or in combination) was able to reverse the effects of decades of sedentary aging on the vasculature.
Study highlights.
Aging is accompanied by the formation of advanced glycation end-products (AGEs).
AGE-crosslink breaker Alagebrium is thought to reduce cardiovascular risk.
Unique one year intervention with Alagebrium and/or exercise training in elderly.
Alagebrium had no additional effect on the cardioprotective effects of exercise.
Exercise training and Alagebrium were unable to reverse the effects of sedentary aging.
Acknowledgements
We thank the participants for their devotion and participation. We also thank Ms. L. Pardoel and Mr. J. Evers for their assistance during testing. Furthermore, we thank Mrs. Dr. N. Peer for the statistical advice.
Funding: This work was supported by the Netherlands Heart Foundation (grant 2006B235 to Mrs. Oudegeest-Sander and 2009T064 to Dr. Thijssen). The overall study was also supported by National Institutes of Health (grant R01AG017479 to Dr. Levine from the National Institute of Aging). The authors declare that no competing interests are associated with this study.
Sponsor's Role: Since it was an investigator initiated study, Synvista Therapeutics had no role in the study design, data collection, analysis, interpretation, writing or submitting papers for publication. Synvista Therapeutics only provided the Alagebrium and placebo tablets.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
MHOS, MGMOR, PS, APJD, BDL, MTEH conceived and planned the study. MHOS performed the research, MHOS, DHJT, BDL, MTEH performed the data analyses and interpreted the data. All authors contributed to drafting the manuscript and approved the final version of the manuscript.
Conflict of interest: None declared.
References
- Asif M, Egan J, Vasan S, Jyothirmayi GN, Masurekar MR, Lopez S, Williams C, Torres RL, Wagle D, Ulrich P, Cerami A, Brines M, Regan TJ. An advanced glycation endproduct cross-link breaker can reverse age-related increases in myocardial stiffness. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:2809–2813. doi: 10.1073/pnas.040558497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakris GL, Bank AJ, Kass DA, Neutel JM, Preston RA, Oparil S. Advanced glycation end-product cross-link breakers. A novel approach to cardiovascular pathologies related to the aging process. American journal of hypertension. 2004;17:23S–30S. doi: 10.1016/j.amjhyper.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, Forman D, Franklin B, Guazzi M, Gulati M, Keteyian SJ, Lavie CJ, Macko R, Mancini D, Milani RV. Clinician's Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- Bergholm R, Makimattila S, Valkonen M, Liu ML, Lahdenpera S, Taskinen MR, Sovijarvi A, Malmberg P, Yki-Jarvinen H. Intense physical training decreases circulating antioxidants and endothelium-dependent vasodilatation in vivo. Atherosclerosis. 1999;145:341–349. doi: 10.1016/s0021-9150(99)00089-1. [DOI] [PubMed] [Google Scholar]
- Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, Greenland P, Van Horn L, Tracy RP, Lloyd-Jones DM. Lifetime risks of cardiovascular disease. The New England journal of medicine. 2012;366:321–329. doi: 10.1056/NEJMoa1012848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry JD, Willis B, Gupta S, Barlow CE, Lakoski SG, Khera A, Rohatgi A, de Lemos JA, Haskell W, Lloyd-Jones DM. Lifetime risks for cardiovascular disease mortality by cardiorespiratory fitness levels measured at ages 45, 55, and 65 years in men. The Cooper Center Longitudinal Study. Journal of the American College of Cardiology. 2011;57:1604–1610. doi: 10.1016/j.jacc.2010.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr., Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. Jama. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Advanced protein glycosylation in diabetes and aging. Annual review of medicine. 1995;46:223–234. doi: 10.1146/annurev.med.46.1.223. [DOI] [PubMed] [Google Scholar]
- Byberg L, Melhus H, Gedeborg R, Sundstrom J, Ahlbom A, Zethelius B, Berglund LG, Wolk A, Michaelsson K. Total mortality after changes in leisure time physical activity in 50 year old men: 35 year follow-up of population based cohort. BMJ (Clinical research ed. 2009;338:b688. doi: 10.1136/bmj.b688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- Hartog JW, Willemsen S, van Veldhuisen DJ, Posma JL, van Wijk LM, Hummel YM, Hillege HL, Voors AA. Effects of alagebrium, an advanced glycation endproduct breaker, on exercise tolerance and cardiac function in patients with chronic heart failure. European journal of heart failure. 2011;13:899–908. doi: 10.1093/eurjhf/hfr067. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Dietz NM, Shepherd JT. From Belfast to Mayo and beyond: the use and future of plethysmography to study blood flow in human limbs. J Appl Physiol. 2001;91:2431–2441. doi: 10.1152/jappl.2001.91.6.2431. [DOI] [PubMed] [Google Scholar]
- Kass DA, Shapiro EP, Kawaguchi M, Capriotti AR, Scuteri A, deGroof RC, Lakatta EG. Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation. 2001;104:1464–1470. doi: 10.1161/hc3801.097806. [DOI] [PubMed] [Google Scholar]
- Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. Jama. 2009;301:2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- Kooijman M, Rongen GA, Smits P, Hopman MT. Preserved alpha-adrenergic tone in the leg vascular bed of spinal cord-injured individuals. Circulation. 2003;108:2361–2367. doi: 10.1161/01.CIR.0000096480.55857.3C. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Little WC, Zile MR, Kitzman DW, Hundley WG, O'Brien TX, Degroof RC. The effect of alagebrium chloride (ALT-711), a novel glucose cross-link breaker, in the treatment of elderly patients with diastolic heart failure. Journal of cardiac failure. 2005;11:191–195. doi: 10.1016/j.cardfail.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Löllgen H, Böckenhoff A, Knapp G. Physical activity and all-cause mortality: an updated meta-analysis with different intensity categories. International journal of sports medicine. 2009;30:213–224. doi: 10.1055/s-0028-1128150. [DOI] [PubMed] [Google Scholar]
- Rowley NJ, Dawson EA, Birk GK, Cable NT, George K, Whyte G, Thijssen DH, Green DJ. Exercise and arterial adaptation in humans: uncoupling localized and systemic effects. J Appl Physiol. 2011;110:1190–1195. doi: 10.1152/japplphysiol.01371.2010. [DOI] [PubMed] [Google Scholar]
- Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 2011;120:357–375. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Levine BD. Effect of exercise training on biologic vascular age in healthy seniors. American journal of physiology. 2012;302:H1340–1346. doi: 10.1152/ajpheart.00511.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit AJ, Lutgers HL. The clinical relevance of advanced glycation endproducts (AGE) and recent developments in pharmaceutics to reduce AGE accumulation. Curr Med Chem. 2004;11:2767–2784. doi: 10.2174/0929867043364342. [DOI] [PubMed] [Google Scholar]
- Steppan J, Tran H, Benjo AM, Pellakuru L, Barodka V, Ryoo S, Nyhan SM, Lussman C, Gupta G, White AR, Daher JP, Shoukas AA, Levine BD, Berkowitz DE. Alagebrium in combination with exercise ameliorates age-associated ventricular and vascular stiffness. Exp Gerontol. 2012;47:565–572. doi: 10.1016/j.exger.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation. 2000;101:2896–2901. doi: 10.1161/01.cir.101.25.2896. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102:1270–1275. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- Thijssen DH, de Groot PC, Smits P, Hopman MT. Vascular adaptations to 8-week cycling training in older men. Acta physiologica (Oxford, England) 2007;190:221–228. doi: 10.1111/j.1748-1716.2007.01685.x. [DOI] [PubMed] [Google Scholar]
- Thijssen DH, Maiorana AJ, O'Driscoll G, Cable NT, Hopman MT, Green DJ. Impact of inactivity and exercise on the vasculature in humans. European journal of applied physiology. 2010;108:845–875. doi: 10.1007/s00421-009-1260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen DH, Rowley N, Padilla J, Simmons GH, Laughlin MH, Whyte G, Cable NT, Green DJ. Relationship between upper and lower limb conduit artery vasodilator function in humans. J Appl Physiol. 2011;111:244–250. doi: 10.1152/japplphysiol.00290.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaitkevicius PV, Lane M, Spurgeon H, Ingram DK, Roth GS, Egan JJ, Vasan S, Wagle DR, Ulrich P, Brines M, Wuerth JP, Cerami A, Lakatta EG. A cross-link breaker has sustained effects on arterial and ventricular properties in older rhesus monkeys. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:1171–1175. doi: 10.1073/pnas.98.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasan S, Zhang X, Zhang X, Kapurniotu A, Bernhagen J, Teichberg S, Basgen J, Wagle D, Shih D, Terlecky I, Bucala R, Cerami A, Egan J, Ulrich P. An agent cleaving glucose-derived protein crosslinks in vitro and in vivo. Nature. 1996;382:275–278. doi: 10.1038/382275a0. [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. Journal of the American College of Cardiology. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- Wilkinson IB, Webb DJ. Venous occlusion plethysmography in cardiovascular research: methodology and clinical applications. British journal of clinical pharmacology. 2001;52:631–646. doi: 10.1046/j.1365-2125.2001.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieman SJ, Kass DA. Advanced glycation endproduct crosslinking in the cardiovascular system: potential therapeutic target for cardiovascular disease. Drugs. 2004;64:459–470. doi: 10.2165/00003495-200464050-00001. [DOI] [PubMed] [Google Scholar]
- Zieman SJ, Melenovsky V, Clattenburg L, Corretti MC, Capriotti A, Gerstenblith G, Kass DA. Advanced glycation endproduct crosslink breaker (alagebrium) improves endothelial function in patients with isolated systolic hypertension. Journal of hypertension. 2007;25:577–583. doi: 10.1097/HJH.0b013e328013e7dd. [DOI] [PubMed] [Google Scholar]