Abstract
Organisms, like cells, maintain tight control of iron. In humans as well as other mammals, control is achieved through the regulation of iron uptake into the body rather than through the excretion of iron. The mechanisms by which humans and mice regulate both iron uptake and the distribution of iron within the body and cells are reviewed. Special emphasis is given to the iron transporters involved in this process.
1. Introduction
Iron is an essential nutrient. Iron deficiency results in anemia, while overload leads to formation of reactive oxygen species, which causes cell and tissue damage. In a balanced state, about 0.5–2 mg of dietary iron is absorbed through intestinal enterocytes every day, and the same amount is lost in the urine, feces, sweat and sloughed cells (Andrews, 2000a; Smith, 1965). To keep the balance, iron homeostasis needs to be tightly controlled. How cells and organisms regulate their iron content, how various tissues coordinate iron distribution, and how dysregulated iron homeostasis leads to metabolic, hematological and neurodegenerative diseases are the hot topics of study in the field of iron biology (Hentze, Muckenthaler, & Andrews, 2004). In general, approximately 65% of the iron in the body is incorporated into hemoglobin (Hb) of red blood cells (RBCs) and 10% is present in myoglobin, other enzymes and cytochromes. The remaining body iron is stored in the liver, macrophages of the reticuloendothelial system and bone marrow (Andrews, 1999a; Munoz, Villar, & Garcia-Erce, 2009). The ability of iron to accept and donate electrons makes it an essential component of oxygen-binding molecules (e.g. Hb and myoglobin), cytochromes in the electron transport chain and as a cofactor in a variety of enzymes. Excess iron can be toxic by catalyzing the Fenton reaction, in which H2O2 is converted to the highly reactive hydroxyl radical (OH•). Hydroxyl radicals oxidize proteins, fatty acids, and nucleic acids, leading to cellular dysfunction (Aruoma, Halliwell, Laughton, Quinlan, & Gutteridge, 1989). To avoid the harmful effects of free iron during oxidative stress, iron is usually bound to specific proteins, such as intracellular ferritin for storage and circulating transferrin (Tf) for transport or utilization.
2. Iron Import across the Plasma Membrane
2.1. Tf and Transferrin Receptors
Tf is the major iron carrier protein in the blood. It is produced primarily in the liver as a glycoprotein. Two high-affinity Fe3+ binding sites are located in the amino- and carboxy-terminus (Crichton & Charloteaux-Wauters, 1987). The binding of Tf to iron atoms is associated with binding of an anion, typically bicarbonate (Aisen & Listowsky, 1980), and the Tf-bound iron (TBI) can be released by acidification (Nunez, Gaete, Watkins, & Glass, 1990). Mammalian cells take up TBI through receptor-mediated endocytosis.
There are two known transferrin receptors (TfR) in human body, TfR1 and TfR2. TfR1 is ubiquitously expressed. It functions as a glycosylated homodimer with the N-terminal 61 amino acids forming the cytoplasmic domain (Andrews, 2000b; Jing & Trowbridge, 1987; Kuhn, McClelland, & Ruddle, 1984). The cytoplasmic domain directs the rapid internalization of Tf–TfR1 complex through clathrin-coated vesicles (Fig. 1). Once internalized, the vesicles are acidified by proton pumps (Yamashiro, Tycko, Fluss, Maxfield, 1984), decreasing the binding of Fe3+ to Tf. The resulting changes in both Tf and TfR1 facilitate the dissociation of iron from Tf (Sipe Murphy, 1991). Fe3+ released from Tf is reduced by Steap (six-transmembrane epithelial antigen of the prostate) 3, the major ferrireductase in the erythroid Tf/TfR1 cycle (Ohgami et al., 2005; Sendamarai, Ohgami, Fleming, & Lawrence, 2008). After reduction, Fe2+ is transported into the cytosol by DMT1 (divalent metal transporter 1) or ZIP14 (RT, IRT_like protein) (Zhao, Gao, Enns, & Knutson, 2010). Both Tf and TfR1 return to the cell surface, where the iron-depleted Tf is released allowing TfR1 to bind the iron-loaded Tf for another round of internalization. This Tf/TfR1 cycle is the major pathway for iron uptake by erythrocytes.
Figure 1. Model for the Tf/TfR1 cycle and overview of receptor-mediated pathways for endocytosis of extracellular heme and Hb.
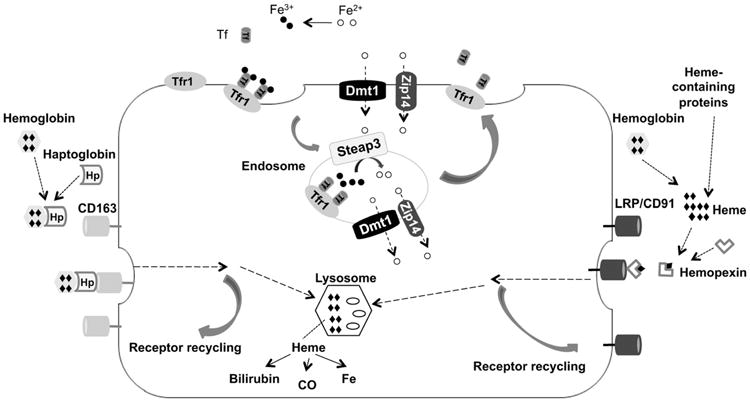
Holo-Tf binds TfR1 on the cell surface; the complex internalizes in endosomes, where acidification promotes iron release from Tf. Fe3+ is reduced to Fe2+ by Steap3, and then transported out of endosomes into the cytosol through DMT1 and Zip14. Both Tf and TfR1 return to the cell surface, where at neutral pH iron-depleted Tf is released from TfR1 and TfR1 is ready to bind more iron-laden Tf for another round of endocytosis. CD163 is a receptor for uptake of extracellular Hb bound to Hp. LRP/CD91 represents the pathway for uptake of heme–Hx complexes. Entocytosed heme can be further metabolized into bilirubin, Fe and carbon monoxide.
Erythrocytes require iron incorporation into the heme of Hb to carry oxygen. Without adequate iron, the maturation of erythrocytes is impaired, leading to microcytic hypochromic anemia. In normal individuals, two-thirds of the total body iron is in developing erythroid precursors and mature RBCs. About 20–25 mg of iron is needed every day for Hb synthesis (Andrews & Schmidt, 2007). In the plasma, nearly all circulating iron is bound to Tf. The uptake of TBI into cells is dependent on the number of TfRs. The expression of TfR1 during erythroid maturation correlates with the changing rates of Hb production (Chan & Gerhardt, 1992). The importance of the Tf/TfR1-mediated iron uptake for erythropoiesis is demonstrated in the murine models. Hypotransferrinemic (Trfhpx/hpx) mice carry a spontaneous mutation in the Tf gene (Bernstein, 1987). These mice have only about 1% of normal circulating Tf. They develop severe microcytic hypochromic anemia, indicating an essential role of Tf in iron delivery to developing erythroid precursors (Trenor, Campagna, Sellers, Andrews, & Fleming, 2000). TfR1−/− mice die in utero between embryonic day 9.5 and 11.5 as a result of severe anemia (Levy, Jin, Fujiwara, Kuo, & Andrews, 1999). The heterozygous mice lacking one copy of the TfR1 gene also exhibit iron-deficient erythropoiesis.
TfR2 is a member of a TfR-like family. The amino acid sequence of the transmembrane and extracellular domains in TfR2 has 45% identity and 65% similarity to that of TfR1. Unlike the ubiquitously expressed TfR1, TfR2 is highly expressed in hepatocytes, the primary site of iron accumulation in the iron overload disease, hereditary hemochromatosis (HH). In untreated HH patients, the expression of TfR1 is undetectable in hepatocytes, but their livers accumulate iron, suggesting the existence of iron uptake pathways other than Tf/TfR1. HH type 3, is due to mutations in TfR2 gene (Camaschella et al., 2000; Roetto et al., 2001). The function of TfR2 in iron metabolism is not clear. TfR2 is stabilized by diferric Tf in cell cultures (Johnson & Enns, 2004; Robb & Wessling-Resnick, 2004) and the cytoplasmic domain of TfR2 is responsible for its stabilization by diferric Tf (Chen & Enns, 2007). TfR2 is capable of binding and internalizing diferric Tf in vitro (Graham et al., 2008; Kawabata et al., 1999), However, cellular iron uptake may not be the major function of TfR2, because both human and mouse mutations, as well as the mouse knockout, lead to increased rather than decreased iron deposition in the liver (Camaschella et al., 2000; Fleming et al., 2002). Moreover, in mice lacking functional TfR2, Tf-mediated iron uptake by hepatocytes was only slightly decreased compared to wild-type mice with a similar amount of iron loading. Rather than playing a role in cellular iron uptake, TfR2 is more likely to be a modulator of hepcidin transcription. Hepcidin is a hormone secreted by the liver and binds to ferroportin1 (FPN1). FPN1 is the only known iron exporter on the surface of absorptive enterocytes and tissue macrophages, functioning in releasing dietary iron or iron recycled from RBCs into the blood circulation (Donovan et al., 2000; McKie et al., 2000). Upon hepcidin binding, FPN1 is internalized and degraded (Nemeth et al., 2004). HH causing mutations in TfR2 reduce hepcidin expression, resulting in increased FPN and increased uptake of iron into the body.
2.2. Heme and Hb Receptors
The major source of plasma iron comes from macrophages that recycle iron from senescent or damaged erythrocytes rather than from intestinal iron absorption (Knutson & Wessling-Resnick, 2003). However, erythrophagocytosis is not the only way for macrophages to acquire iron. They can also obtain iron from circulating Tf via the Tf/TfR1 cycle and from the uptake of heme and Hb (Wilkins, Williams, & Cavill, 1977). Hb is the most abundant and functionally important protein in erythrocytes. But once released from RBCs, it becomes highly toxic because of the oxidative properties of heme (protoporphyrin IX and iron) via the Fenton reaction to produce reactive hydroxyl radical causing cell damage (Tolosano et al., 2002).
Normally, only a small amount of extravascular hemolysis occurs due to demolition of senescent erythrocytes, causing Hb release into plasma. Under various intravascular hemolysis-linked conditions, such as hemorrhage, hemoglobinopathies, ischemia reperfusion, sickle cell disease or malaria, large amounts of free Hb are released (Tolosano et al., 2002). Released plasma Hb is readily bound stoichiometrically by the liver-derived plasma protein haptoglobin (Hp), forming an Hb–Hp complex (Nielsen & Moestrup, 2009). CD163, an Hb scavenger receptor present on the surface of monocytes and macrophages in the liver and other tissues, mediates the endocytosis and subsequent degradation of Hb–Hp complex (Kristiansen et al., 2001). The Hb–Hp complex is degraded in lysosomes to release heme and other proteolytic products within the cells. The heme is then degraded by heme oxygenase 1 to release iron, carbon monoxide, and biliverdin. The free iron enters the same intracellular pool as iron taken up from other sources. Mice lacking functional Hp do not have obvious disorders in iron metabolism, suggesting that Hp does not play a major role in normal iron metabolism (Lim et al., 1998). When the binding capacity of Hp is exceeded, plasma Hb is quickly oxidized to ferrihemoglobin (Tolosano et al., 2002). Ferrihemoglobin dissociates into globin and ferriheme. Ferriheme binds to hemopexin (Hx), forming heme–Hx complex, which is internalized by receptor-mediated endocytosis into macrophages. The receptor for heme–Hx complex is LDL receptor-related protein (LRP)/CD91 (Hvidberg et al., 2005). Mice in which the Hx gene is inactivated do not exhibit disturbed iron metabolism, suggesting that the role of Hx in iron homeostasis is important only under pathological conditions (Tolosano et al., 1999). Hx levels in serum correlates with heme levels in the blood. A high Hx level indicates significant degradation of heme-containing compounds. Low Hx levels are one of the diagnostic features of hemolytic anemia. Extracellular heme can be cleared through these two receptor-mediated endocytic pathways, thus avoiding the strong oxidative features and proinflammatory effects of free heme.
2.3. Iron Transporters and their Implications in Human Diseases
2.3.1. Divalent Metal Transporter 1
DMT1 is the most extensively characterized iron transport protein. It was identified in 1995 from a screen for murine homologs of Nramp1 (natural resistance-associated macrophage protein 1), a protein involved in host immune defense. Therefore, DMT1 was first named Nramp2. Nramp1 is an integral membrane glycoprotein expressed in lysosomes of macrophages. It is targeted to the membrane of the phagosome after phagocytosis (Gruenheid, Pinner, Desjardins, & Gros, 1997). In contrast, DMT1 is ubiquitously expressed and does not seem particularly associated with organs or cells implicated in host defense. The DMT1 gene is on chromosome 15, which is away from known host defense genes (Gruenheid, Cellier, Vidal, & Gros, 1995).
The DMT1 gene encodes four different protein isoforms. The upstream 5′ exon, termed exon 1A, adds an in-frame translation initiation codon and extends the open reading frame (ORF) of the protein by 29–31 amino acids in different species (Hubert & Hentze, 2002). The sequences of the variants with or without exon 1A are identical after reaching exon 2 and until reaching the C-terminal sequences of the ORF. Two other DMT1 messenger RNA (mRNA) variants are produced by alternative splicing at the 3′ end. One contains the stem-loop structure, iron response element (IRE), and the other does not (Lee, Gelbart, West, Halloran, & Beutler, 1998). Four isoforms are produced by alternative splicing of the 5 end exon 1A and the 3′ end IRE, yielding DMT-1A(+IRE), DMT-1B(+IRE), DMT-1A(—IRE), and DMT-1B(—IRE) isoforms (Hubert & Hentze, 2002). Like the IREs in the 3′ UTR of the TfR1 mRNA, iron regulatory protein (IRP) binds under low iron conditions and stabilizes the DMT1 mRNA, leading to increased DMT1 protein levels (Andrews, 1999b). However, in Caco2 cells, the 3′ end IRE is not necessary for iron regulation and the exon 1A itself is associated with iron regulation (Hubert & Hentze, 2002). The tissue distribution profile indicates that the exon 1B isoform is ubiquitously expressed, while the expression of exon 1A isoform is tissue specific and most abundant in the duodenum and the kidney. The same study also found that, in mice, DMT1 regulation in the kidney is associated with the presence of an IRE in the 3′ UTR, whereas in the duodenum, iron regulation is most strongly associated with the presence of exon 1A.
In Belgrade (b) rats and mk mice, rodent models of DMT1 dysfunction, a single nucleotide change in the DMT1 gene results in a substitution of arginine for glycine at position 185 (G185R), leading to iron deficiency and anemia (Fleming et al., 1997, 1998). The b rat suffers from anemia accompanied by elevated plasma iron and iron-binding capacity, decreased stainable iron in tissues and decreased growth rate. (Oates and Morgan 1996) showed that the reduced uptake of both Fe3+ and Fe2+ iron in the b rat most likely involves a defective iron carrier associated with the apical membrane of the duodenum.
Using a positional cloning approach, (Fleming et al. 1998, 1997) identified DMT1 as the defective gene for both b rats and mk mice. Injection of RNA synthesized from DMT1 complementary DNA in Xenopus oocytes promotes the uptake of iron as well as manganese, cobalt, and zinc (Gunshin et al., 1997). In Caco2 cells, DMT1 was shown to transport iron preferentially over other divalent cations (Tandy et al., 2000). Studies in other cultured mammalian cells have also demonstrated that DMT1 can transport a variety of divalent cations at the plasma membrane, including iron (Fleming et al., 1998; Zhang, Lee, Wang, & Soong, 2000). Uptake of metals by DMT1 is pH dependent. Iron uptake is most efficient in COS-7 and HEK 293 cells transfected with the DMT1(−IRE) isoform at pH 5.5–6.5, whereas iron uptake decreases to almost baseline level at pH 7.5 or above (Ludwiczek et al., 2007).
DMT1 expression is significantly induced in duodenal epithelial cells in rats fed iron-deficient diet (Gunshin et al., 1997). Dietary iron deficiency results in a dramatic upregulation of the DMT1(+IRE) form in proximal duodenum (Canonne-Hergaux, Gruenheid, Ponka, & Gros, 1999; Gunshin et al., 1997). In situ hybridization indicates that DMT1 is highly expressed in enterocyte villus. An immunohistochemical study of human duodenum shows that DMT1 localizes to enterocytes, especially at the microvillus brush border membrane (Griffiths, Kelly, Smith, & Cox, 2000). Localization of DMT1 is in agreement with the known physiological site for iron absorption in the intestine, which is mostly restricted to the brush border of the proximal intestine (Muir & Hopfer, 1985). In Caco2 cells, preincubation with the DMT1-specific antibody significantly inhibits iron uptake at pH 5.5 (Tandy et al., 2000). Mice with specific inactivation of DMT1 gene in the intestine are born alive, but they rapidly develop iron-deficiency anemia (Gunshin, Fujiwara, et al., 2005). Taken together, these results suggest that DMT1 is the major iron transporter in the small intestine.
In b rats, iron uptake from Tf by erythropoietic cells is reduced and globin synthesis is defective (Edwards, Sullivan, & Hoke, 1980; Sladic-Simic et al., 1966). A small decrease in endocytosis of Tf, associated with diminished iron uptake and increased iron release by exocytosis, indicates that the defect of iron uptake in b rats is after the Tf/TfR1 endocytosis (Bowen & Morgan, 1987). In fact, even though holo-Tf is taken up into reticulocytes, iron is poorly retained, and much is recycled to the extracellular space along with Tf in b rats, suggesting that their reticulocytes are unable to transport iron out of the vesicle after endocytosis (Garrick, Gniecko, Liu, Cohan, & Garrick, 1993). Given the evidence that both b rats and mk mice have the same G185R mutation in DMT1 and endosomal iron release is not efficient in the b rat, DMT1 is hypothesized to function in iron transport out of endosomes (Fleming et al., 1998).
Several studies report cases of DMT1 mutations in humans. (Mims et al., 2005) report the first case of a female with severe hypochromic microcytic anemia and iron overload. A G-to-C mutation in exon 12 (DMT1 1285 G → C) was found in this patient, resulting in a glutamic acid to aspartic acid (E399D) substitution. However, the predominant effect of this mutation is exon 12 skipping during processing of the mRNA present in erythroid cells. Removal of exon 12 deletes transmembrane domain 8, which may interfere with proper protein insertion into the membrane. E399D resides in the fourth predicted intracellular loop of DMT1 and forms part of a highly conserved transport signature motif among species (Lam-Yuk-Tseung, Mathieu, & Gros, 2005). The mutants E399D, E399Q, and E399A expressed in LLC-PK1 kidney cells are fully functional in terms of stability and targeting to the membrane as well as being transport competent, indicating that DMT1G1285C is not a complete loss of function. In healthy individuals, exon 12 skipping risk is 10%. However, G1285C mutation greatly increases the exon 12 skipping risk to 90%. Consequently, the amount of functional E399D produced in this patient is limited, and it may be adequate for iron absorption, but not sufficient for iron utilization in the erythrocytes. Therefore, the clinical phenotype appears to be due to exon 12 skipping rather than E399D substitution. Interestingly, a liver biopsy indicated that this patient has severe iron loading in both hepatocytes and Kupffer cells, suggesting the existence of another iron uptake pathway functioning in the liver.
Iolascon et al., (2006) reported an infant with hypochromic, microcytic anemia, and hepatic iron overload with increased serum iron, Tf saturation, and serum ferritin levels before treatment with recombinant erythropoietin (rEpo). Screening for DMT1 identified two novel mutations: a 3-bp deletion in intron 4, causing a splicing abnormality, and a C to T transition in exon 13, resulting in the substitution of arginine with cysteine (R416C). This patient displayed severe iron loading in the liver despite low serum ferritin levels at age 5. The high iron stores were disproportionate to the iron from transfusions or bone marrow redistribution, indicating a significant increase of intestinal iron absorption. Administration of rEpo resulted in rapid improvement of anemia, suggesting the existence of a DMT1-independent pathway for iron utilization by the Tf/TfR1 cycle in erythrocytes.
(Beaumont et al., 2006) reported a case of congenital microcytic hypochromic anemia due to DMT1 mutations in a 6-year-old French girl. This patient had two mutations in DMT1, including a deletion of a GTG codon in exon 5 and a glycine to valine (G212V) mutation in exon 8, resulting in the in-frame deletion of V114 (delV114) in TM2 and a G212V substitution in TM5, respectively. Different from two previous reported DMT1 mutations, the anemia in this case was less severe, indicating partial loss of function.
(Bardou-Jacquet et al., 2011) reported a 27-year-old woman with hypochromic microcytic anemia and hepatic iron overload. This patient also had two mutations in DMT1, including the known G212V mutation and a novel N491S substitution. Overexpression of the mutant forms in Huh7 cells revealed that the N491S mutation led to disturbed protein trafficking with more DMT1 accumulating in endoplasmic reticulum.
(Barrios et al., 2012) and (Blanco, Kannengiesser, Grandchamp, Tasso, and Beaumont 2009) reported the same homozygous mutation in DMT1 in a 7-year-old boy and a 10-year-old boy, respectively. The mutation is located in the first transmembrane domain and leads to a glycine to arginine substitution (G75R). Both patients had hypochromic microcytic anemia. The functional consequence of this mutation is unknown.
In summary, the identical mutation in DMT1 of Belgrade rats and mk mice, together with reported cases of DMT1 mutations in humans, emphasizes the role of DMT1 in erythropoiesis (Beaumont et al., 2006). In addition, DMT1 is expressed in bone marrow and optimally functions at acidic pH, such as in endosomes (Gunshin et al., 1997). These observations support the rationale that DMT1 is an endosomal iron transport protein, especially in erythroid cells.
2.3.2. Natural Resistance-Associated Macrophage Protein 1
Nramp1 was first identified as a gene responsible for intracellular protection against pathogens. It encodes a phagocyte-specific membrane protein (Malo et al., 1994; Soe-Lin, Sheftel, Wasyluk, & Ponka, 2008; Vidal, Malo, Vogan, Skamene, & Gros, 1993). Expression of Nramp1 is highest in the reticuloendothelial organs (primarily the spleen and liver). Unlike Nramp2, Nramp1 mutations have not been found to cause severe anemia in mice (Soe-Lin et al., 2009), but result in increased susceptibility to infection. Intracellular localization studies indicate that Nramp1 is localized to late endosomal/lysosomal compartments rather than plasma membrane of macrophages. After completion of phagocytosis, Nramp1 is rapidly recruited to the membrane of phagosomes (Gruenheid et al., 1997; Searle et al., 1998). By overexpressing an epitope-tagged Nramp1 in CHO cells and measuring radioisotope activities, (Forbes and Gros 2003) showed that Nramp1 can transport both Fe2+ and Mn2+. Nramp1 transports divalent metals down the proton gradient, acting as a phagosomal efflux pump (Jabado et al., 2000). Thus, the antimicrobial effect of Nramp1 that leads to its identification appears to be due to the ability of Nramp1 to deplete bacteria-containing phagosomes of essential metal nutrients (Forbes & Gros, 2001).
A study by (Soe-Lin et al. 2009) demonstrated that Nramp1−/− mice have a higher splenic iron content and increased splenic macrophage iron deposits compared to wild-type animals, consistent with inefficient recycling of erythrophagocytosed iron and retention of iron within reticuloendothelial cells. The knockout mouse has increased Tf saturation, most likely due to compensatory increase in dietary iron absorption. Under the erythropoietic stress conditions induced by phenylhydrazine, Nramp1−/− mice show significantly decreased Tf saturation and elevated levels of nonheme iron in both the liver and the spleen compared to wild-type mice under the same conditions, indicating that Nramp1−/− mice is unable to use stored iron for efficient erythropoiesis. By using radiolabeled iron, they showed that iron could not be appropriately released from macrophages in Nramp1−/− mice. Overexpression of Nramp1 in RAW264.7 macrophages, which lack functional Nramp1, also results in an increased cellular iron release following phagocytosis of59 Fe-labeled RBCs (Soe-Lin et al., 2008). Taken together, these results indicate that Nramp1 is involved in macrophage recycling of iron from the phagocytosis of senescent erythrocytes.
2.3.3. ZRT, IRT-Like Protein 14
ZIP14 (also known as SLC39A14, solute carrier 39 family, member 14) is member of the ZIP1 metal ion transporter superfamily of proteins. The ZIP family proteins take the name from ZRT, IRT-like protein, where ZRT represents zinc-regulated transporter and IRT stands for iron-regulated transporter (Guerinot, 2000). (Nomura et al., 1994) first cloned Zip14, which they named KIAA0062. Northern blot analysis detected ubiquitous expression of Zip14, with the highest expression in the liver and the lowest expression in the spleen, thymus, and peripheral blood leukocytes. A multiple tissue expression array also showed that Zip14 was ubiquitously expressed with high expression in the liver, pancreas and heart (Taylor, Morgan, Johnson, & Nicholson, 2005).
(Taylor et al., 2005) first characterized the metal transport activity of ZIP14. They showed that ZIP14 overexpression in CHO cells stimulated the uptake of zinc. In 2005, (Liuzzi et al., 2005) also found that over-expression of mouse Zip14 in HEK 293 cells increased zinc uptake. In 2006, (Liuzzi, Aydemir, Nam, Knutson, and Cousins 2006) reported that over-expression of Zip14 in HEK 293 cells and Sf9 insect cells enhanced not only the uptake of zinc but also iron. The iron was presented to the cells as ferric citrate, the major form of non-Tf-bound iron (NTBI) that appears in plasma in conditions of iron overload (Grootveld et al., 1989). Zinc can inhibit iron uptake, suggesting that these two metals share the same transporter. Knockdown of endogenous Zip14 in AML12 cells (a mouse hepatocyte cell line) by small interfering RNA (siRNA) resulted in decreased uptake of iron from ferric citrate. Similarly, overexpression of Zip14 in Xenopus oocytes stimulates the uptake of ferrous iron into cells (Pinilla-Tenas et al., 2011). In 2008, Gao, Zhao, Knutson, and Enns (2008) showed that ZIP14 over-expression stimulated NTBI uptake in HeLa cells and that suppression of endogenous ZIP14 in the HepG2 cells decreased NTBI uptake. Moreover, Zip14 can transport iron efficiently at pH 7.4 (Liuzzi et al., 2006), the pH at the plasma membrane surface of hepatocytes. In contrast, DMT1, the first identified iron importer, transports iron optimally at pH 5.5 (Garrick et al., 2006; Gunshin et al., 1997) and is readily detected in endosomes but not on the plasma membrane of hepatocytes (Shindo et al., 2006). These observations provide strong support that ZIP14, rather than DMT1, mediates NTBI uptake in hepatocytes.
The majority of iron in the plasma is bound to Tf When the amount of iron in the plasma exceeds the capacity of Tf, the level of NTBI increases, contributing to tissue iron loading. In iron overload disorders, such as HH, excess iron is mainly found in the liver, pancreas and heart (Hentze et al., 2004), where ZIP14 is highly expressed (Taylor et al., 2005). In HFE-associated HH, the most prevalent form of hemochromatosis (Olynyk et al., 1999), mutation of a single amino acid (C282Y) in the HFE gene leads to iron overload (Feder et al., 1996). Hepatocytes from Hfe−/− mice can take up more NTBI compared to wild-type animals (Chua, Olynyk, Leedman, & Trinder, 2004). The role of HFE in iron metabolism remains to be determined. Interestingly, overexpression of HFE in HepG2 cells decreases ZIP14 levels by decreasing the stability of ZIP14 (Gao et al., 2008). The reduced ZIP14 levels are associated with decreased uptake of both NTBI and TBI, suggesting that ZIP14 participates in both pathways of iron acquisition. Moreover, Zip14 localizes to the plasma membrane, as well as the Tf-containing endosomes (Zhao et al., 2010). Overexpression of Zip14 in HEK 293 cells increases TBI uptake, whereas depletion of endogenous ZIP14 in HepG2 cells decreases TBI uptake. These results indicate that ZIP14 also participates in the iron uptake from Tf.
2.3.4. Transient Receptor Potential Cation Channel, Mucolipin Subfamily, Member 1
Most cells take up iron through receptor-mediated endocytosis. The endolysosome system consists of early endosomes, recycling endosomes, late endosomes, and lysosomes. DMT1 and Zip14 are candidates for endosomal iron release. How iron is released from lysosomes into the cytosol remains elusive. By measuring radioactive iron uptake, (Dong et al., 2008) showed that TRPML1 (transient receptor potential cation channel, mucolipin subfamily, member 1, also known as mucolipin1 MCOLN1or ML1) functions as a Fe2+ -permeable channel in late endosomes and lysosomes. In 2000, three groups cloned TRPML1, which is mutated in mucolipidosis type IV (ML IV) patients (Bargal et al., 2000; Bassi et al., 2000; Sun et al., 2000). TRPML1 belongs to the transient receptor potential (TRP) ion channel family and is primarily localized in late endosomes and lysosomes (Nilius, Owsianik, Voets, & Peters, 2007; Pryor, Reimann, Gribble, & Luzio, 2006). TRPML1 is ubiquitously expressed with the highest expression levels in the brain, kidney, spleen, liver, and heart (Sun et al., 2000). By comparing the amino acid sequence with known protein motifs, a TRP cation channel domain and an internal calcium and sodium channel pore region were identified.
Overexpression ML IV mutant forms of TRPML1 in HEK 293 cells demonstrated that ML IV mutations significantly impair the Fe2+ permeability of TRPML1 to various degrees, correlating well with disease severity. The TRPML1−/− ML IV human skin fibroblasts show an increase in lysosomal Fe2+ levels and a reduction in cytosolic Fe2+ levels compared to control cells. Moreover, some ML IV patients were reported to have iron deficiency or anemia (Altarescu et al., 2002). Based on these results, TRPML1 is proposed to mediate Fe2+ released from late endosomes and lysosomes. The hematological symptoms of ML IV patients may be due to impaired iron transport and the intralysosomal iron accumulation may also contribute to the neurodegenerative symptoms observed in these patients.
2.4. Ferrireductases
2.4.1. Ferrireductases for Iron Absorption
Dietary iron is found in two forms, either as heme iron, found in meat and meat products, or non heme iron, present in vegetables, fruits and beans. Non-heme iron predominates in most diets, comprising 90–95% of total daily iron intake. The absorption of dietary iron occurs at the apical membrane of duodenal enterocytes. The primary form of nonheme iron in food is Fe3+, which is insoluble and must be reduced to Fe2+ form before transporting across enterocytes (Fig. 2). The responsible enzyme is thought to be cytochrome b-like ferrireductase (Dcytb) (McKie et al., 2001). However, Gunshin, Starr, et al., (2005) demonstrated that the absence of Dcytb did not impair body iron stores when mice were fed normal chow diet, indicating that there was no major effect on intestinal absorption. They concluded that Dcytb is dispensable for intestinal iron absorption in mice. However, mice are capable of synthesizing ascorbic acid and may have less need for a duodenal surface ferric reductase (Sharp & Srai, 2007). In addition, direct iron absorption was not measured in the knockout study. The role of Dcytb in intestinal iron absorption remains unclear. The recently identified Steap2 protein might be another candidate for the ferrireductase role in the small intestine (Ohgami, Campagna, McDonald, & Fleming, 2006). Other dietary components, such as ascorbic acid, histidine and cysteine, are also able to reduce Fe3+ to Fe2+ (Glahn & Van Campen, 1997; Han, Failla, Hill, Morris, & Smith, 1995; Swain, Tabatabai, & Reddy, 2002).
Figure 2. Schematic representation of intestinal iron absorption.
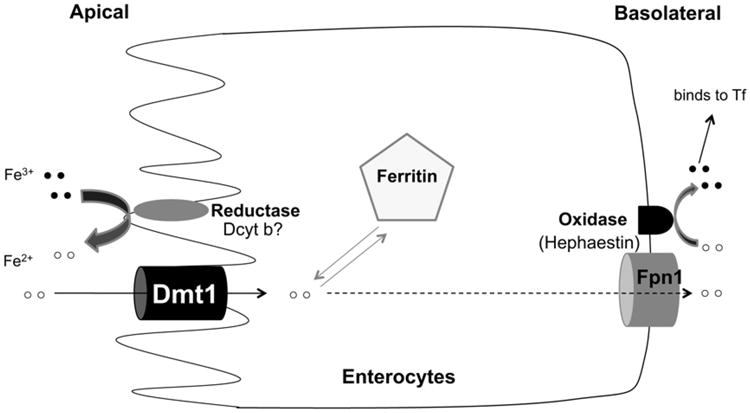
The absorption of iron takes place predominantly in the duodenum. Dietary nonheme iron (mostly Fe3+) is reduced to Fe2+ form by a ferric reductase. Fe2+ then enters enterocytes through DMT1. Depending upon the body's iron status, enterocyte iron can be stored in ferritin or exported into circulation through (FPN1). The exiting iron is reoxidized to Fe3+ through a ferrous oxidase (hephaestin) before loading onto Tf.
2.4.2. Ferrireductase in Tf/TfR1 Cycle
Most of the iron in blood is bound to Tf. Monoferric or diferric Tf is taken up into cells through Tf/TfR1 cycle (Fig. 1). This cycle begins with the binding of iron-bound Tf to TfR1 on the cell surface. This complex localizes to clathrin-coated pits, whose invagination initiates endocytosis. Once internalized, Fe3+ is dissociated from Tf due to endosomal acidification. An essential step before iron is exported into cytosol is the reduction of Fe3+ to Fe2+. RBC precursors take up iron mostly through Tf cycle. A deficiency in iron acquisition by RBCs leads to hypochromic, microcytic anemia phenotype of the mutant mouse nm1054. By using positional cloning, (Ohgami et al., 2005) identified that Steap3 gene is responsible for the iron deficiency anemia in these animals. They showed that Steap3 is highly expressed in hematopoietic tissues and colocalizes with the endosomal markers. Overexpression of Steap3 in HEK 293 cells stimulated the reduction of iron and facilitates TBI uptake. Moreover, by target deletion of Steap3, they found that mice lacking Steap3 were deficient in erythroid ferrireductase activity. Recently, a heterozygous nonsense mutation in exon 3 of the Steap3 gene was identified in a family with three affected siblings (Grandchamp et al., 2011). Those individuals all have transfusion-dependent hypochromic anemia and iron overload. The mutation leads to a Cys 100 to stop codon substitution and causes very low expression level of this protein. Taken together, these results indicate that Steap3 is a ferrireductase required for efficient Tf-dependent iron uptake in erythrocytes.
3. Iron Exporters and their Implications in Human Diseases
3.1. Ferroportin 1
FPN1 also known as MTP1 and IREG1 (Abboud & Haile, 2000; Donovan et al., 2000; McKie et al., 2000) exports iron out of cells (Canonne-Hergaux, Donovan, Delaby, Wang, & Gros, 2006). Being the only known Fe2+ exporter in mammals, its regulation is key to both iron homeostasis as well as iron distribution in the body. Dietary iron absorbed by the intestine is exported out of enterocytes by FPN1 to be bound by Tf and transported through the blood. TBI is ultimately taken up into tissues expressing TfRs. Senescent RBCs are taken up by macrophages in the liver and spleen where the RBCs are broken down. Iron is scavenged from the Hb and the iron that is released by heme is transported out of the macrophage by FPN1.
FPN1 (SLC40A1) is controlled at the levels of transcription, translation, and posttranslation making it able to accommodate a large range of effectors. It is regulated transcriptionally by iron (Abboud & Haile, 2000; Donovan et al., 2000; Knutson & Wessling-Resnick, 2003; McKie et al., 2000). In addition FPN1 can transport both zinc and cadmium and is controlled transcriptionally by metal transcription factor-1 (MTF-1) through the ability of MTF-1 to bind zinc and cadmium (Troadec, Ward, Lo, Kaplan, & De Domenico, 2010). The binding of cytosolic zinc or cadmium to MTF-1 causes the translocation of MTF-1 from the cytoplasm to the nucleus where it activates FPN1 transcription thus increasing the transport of these metals out of the cell.
Most studies have concentrated on the translational and posttranslational mechanisms of FPN1 regulation. The iron-labile pool within the cell positively regulates the translation of FPN1 using a stem-loop, IRE, upstream of the translational start site (Abboud & Haile, 2000; McKie et al., 2000). Under conditions of low intracellular iron, iron-responsive proteins, IRP1 and IRP2, bind to the IRE and block the translation of FPN1 preventing further loss of iron from the cell. Under high iron conditions, the IRPs no longer bind to the IRE resulting in increased FPN1 translation. Increased levels of FPN1 help to lower intracellular iron levels. Tissue-specific alternate splicing of FPN1 that eliminates the IRE and creates an alternate transcriptional initiation site eliminates the control of FPN1 by intracellular iron (Zhang, Hughes, Ollivierre-Wilson, Ghosh, & Rouault, 2009). This form of FPN1 occurs in intestinal enterocytes and erythroid precursor cells. In the case of intestinal enterocytes, lack of intracellular iron regulation is desirable because otherwise low dietary iron would inhibit iron export from the enterocytes and high dietary iron would maximize iron export.
FPN1 levels are also regulated by hepcidin, iron transport, and ceruloplasmin through posttranslational mechanisms (De Domenico et al., 2007, 2011; Nemeth et al., 2004). The binding of hepcidin to FPN1 induces its phosphorylation by JAK2 kinase, internalization, ubiquitination, and subsequent degradation in lysosomes (De Domenico, Lo, Ward, & Kaplan, 2009, 2010). Thus, high levels of iron in the body result in the upregulation of hepcidin and the downregulation of FPN to limit further dietary iron uptake. Hepcidin expression is also positively regulated by inflammation (Nicolas et al., 2002). Limited dietary iron uptake and sequestration of iron in macrophages are part of an innate immune responses to lower circulation iron in the plasma that could be utilized by bacteria or contribute to inflammatory processes. These responses explain the anemia of chronic disease.
3.2. Feline Leukemia Virus-C Receptor and Heme Export
The feline leukemia virus-C receptor (FLVCR) was first identified by functional cloning and was predicted to be an organic anion carrier based on its sequence similarity to other members of this transporter family (Tailor, Willett, & Kabat, 1999). The observation that cats viremic with feline leukemia virus subgroup C were profoundly anemic and had defects in erythropoiesis leads (Quigley et al., 2000) to identify FLVCR as a heme exporter that is essential to prevent apoptosis of differentiating erythroid cells (Keel et al., 2008). Flvcr null mice die in midgestation from a lack of ability to undergo definitive erythropoiesis (Quigley et al., 2004). Mice with conditional deletions of Flvcr develop severe hyperchromic macrocytic anemia with a block in erythroid maturation at the proerythroblastic stage in addition to cardiomegaly and splenomegaly (Keel et al., 2008). Macrophages from Flvcr-deficient mice accumulate more intracellular iron in the form of ferritin than control mice. Further studies indicate that Flvcr facilitates the export of heme from macrophages. These studies lead to the proposal that FLVCR serves to function as a heme overflow valve if heme synthesis exceeds Hb synthesis. In addition, hepatocytes and duodenal enterocytes accumulate iron when Flvcr is downregulated implying a role for Flvcr in heme export in these cell types.
4. Intracellular Iron Transport and Iron Chaperones
Whereas the uptake of iron via the Tf/TfR1, DMT1 pathway has been extensively studied, much less is known as to how iron is transported within the cell. Intracellular chaperones for both copper and zinc transport have been identified and reviewed (Robinson & Winge, 2010; Rosenzweig, 2002); however, the identification of iron chaperones was lacking until recently with the discovery that poly r(C)-binding proteins (PCBP) 1 and 2 facilitate the loading of iron into ferritin (Shi, Bencze, Stemmler, & Philpott, 2008). PCBP1 and 2 were originally identified as RNA-binding proteins. Further studies indicate that they also play a role in the transfer of iron to the iron-dependent prolyl hydroxylase 2 (PHD2) that negatively regulates the stability of hypoxia-inducible factor 1 α. Depletion of PCBP1 or PCBP2 in cells results in the loss of iron incorporation in PHD2 and loss of activity of this enzyme (Nandal et al., 2011).
Another iron chaperone protein, frataxin, is proposed to transfer iron to the Fe–S complex within mitochondria (Gerber, Muhlenhoff, & Lill, 2003; Muhlenhoff et al., 2003). Frataxin will be discussed further in the mitochondrial iron transport section.
5. Mitochondrial Iron Import and Export
Extensive iron metabolism occurs in the mitochondrion. The incorporation of iron into protophorin IX to generate heme occurs in this organelle as well as most iron sulfur (Fe–S) biogenesis. Both heme and Fe– S-containing proteins are involved in the transfer of electrons in the oxidative phosphorylation pathway and in key enzymes in the tricarboxylic acid cycle within mitochondria. Mutations in the proteins involved in the transport of iron into and out of mitochondria result in neurodegenerative diseases, cancer, anemias and a host of other disorders.
Most of the information concerning mitochondrial iron transport is derived from mutations in the proteins involved in this process. The form of iron that is transported into mitochondria is unknown. Iron can be transferred directly from the endosome through DMT1 to the mitochondrion in erythroid precursors. How this is accomplished and the mitochondrial acceptor has not been identified (Ponka, Sheftel, & Zhang, 2002; Zhang, Sheftel, & Ponka, 2005) (Fig. 3). The cloning of the frascati mutation in zebra fish that causes anemia resulted in the identification of mitoferrin1 (SLS25A37), a transporter in the inner mitochondrial membrane. Mitoferrin1 is expressed in erythroid cells (Shaw et al., 2006). A paralog of mitoferrin1, mitoferrin2 (SLC25A28), is expressed ubiquitously (Shaw et al., 2006). Mitoferrin interacts with Abcb10, which also participates in this process (Chen et al., 2009). Both mitoferrin and Abcb10 are required for the synthesis of heme and Fe–S clusters suggesting that they may play a role in mitochondrial iron uptake (Chen et al., 2009). Frataxin is an iron-binding protein located in the mitochondria and plays an important part in Fe–S assembly by binding to scaffold proteins Isu1 and Isu2. Isu1 and 2 interact with Nfs1 and IsdII as part of the Fe–S assembly complex (Leidgens, De Smet, & Foury, 2010). Ferrochelatase is responsible for the final step of heme synthesis by loading protoporphyrin IX with iron. Ferrochelatase is an FeS cluster-containing protein, thereby linking heme and FeS biogenesis in the mitochondrion. Excess iron in the mitochondria of erythroid cells and testes is stored in mitoferritin, an H-ferritin-like protein (Levi et al., 2001).
Figure 3. Schematic of mitochondrial iron transport.
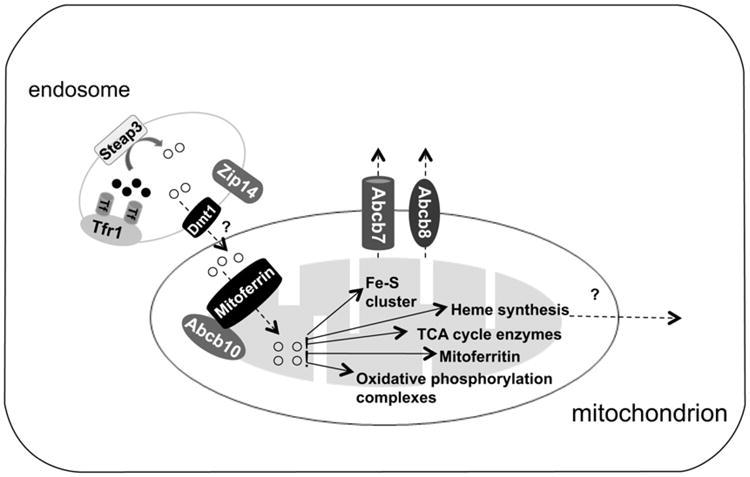
How iron crosses the outer membrane of mitochondrion is not clear. Evidence from erythroid precursors suggests that internalized Tf-containing endosomes are dock to mitochondria and iron is transported directly from the endosome into the mitochondrion. Mitoferrin and Abcb10 facilitate the transport of iron across the inner mitochondria. Iron in the mitochondrion can then be used for different metabolic processes, including heme synthesis and Fe/S cluster biosynthesis. Abcb7/8 facilitates the export of a component for the FeS assembly. The transporter responsible for the exit of heme from mitochondria remains to be determined.
Defects in heme biosynthesis or Fe–S clusters result in iron accumulation in mitochondria implying the involvement of specific transporters in the export of these molecules. Mutations in the ATP-binding cassette protein B7 (ABCB7) are associated with sideroblastic anemia and cerebellar ataxia (Allikmets et al., 1999; Bekri et al., 2000; Csere, Lill, & Kispal, 1998). In sideroblastic anemias, iron accumulates in mitochondria. Both ABCB7 and more recently ABCB8 appear to be involved in the export of iron out of mitochondria (Ichikawa et al., 2012). Whether these two proteins directly transport iron or stimulate another transporter remains unanswered.
6. Future Directions
With the recent discovery of a host of new membrane proteins that affect iron transport within the body, many issues remain to be resolved.
How are the newly identified transporters affecting iron uptake and iron distribution in cells regulated at the transcriptional and post-transcriptional levels?
Are there additional iron chaperones involved in the delivery of iron to different organelles and in the transcytosis of iron across epithelial and endothelial barriers?
What forms of iron are transported across the mitochondrial membrane and what are their transporters?
How is iron homeostasis achieved in individual organs such as the heart, brain, skeletal muscle, kidney, and pancreas?
What are the best targets for altering iron homeostasis in the body in the case of severe iron overload in diseases like HH and β-thalassemia?
Acknowledgments
We would like to thank our colleague, Dr An-Sheng Zhang, for his helpful comments and suggestions. This work was supported by NIH grants DK072166 and DK054488 (C.A.E.) and a Tartar Trust fellowship grant (N.Z.).
Footnotes
Capital letters denote the human gene and lower case letters denote the the mouse gene.
References
- Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. Journal of Biological Chemistry. 2000;275:19906–19912. doi: 10.1074/jbc.M000713200. [DOI] [PubMed] [Google Scholar]
- Aisen P, Listowsky I. Iron transport and storage proteins. Annual Review of Biochemistry. 1980;49:357–393. doi: 10.1146/annurev.bi.49.070180.002041. [DOI] [PubMed] [Google Scholar]
- Allikmets R, Raskind WH, Hutchinson A, Schueck ND, Dean M, Koeller DM. Mutation of a putative mitochondrial iron transporter gene (ABC7) in X-linked sideroblastic anemia and ataxia (XLSA/A) Human Molecular Genetics. 1999;8:743–749. doi: 10.1093/hmg/8.5.743. [DOI] [PubMed] [Google Scholar]
- Altarescu G, Sun M, Moore DF, Smith JA, Wiggs EA, Solomon BI, et al. The neurogenetics of mucolipidosis type IV. Neurology. 2002;59:306–313. doi: 10.1212/wnl.59.3.306. [DOI] [PubMed] [Google Scholar]
- Andrews NC. Disorders of iron metabolism. The New England Journal of Medicine. 1999a;341:1986–1995. doi: 10.1056/NEJM199912233412607. [DOI] [PubMed] [Google Scholar]
- Andrews NC. The iron transporter DMT1. International Journal of Biochemistry and Cell Biology. 1999b;31:991–994. doi: 10.1016/s1357-2725(99)00065-5. [DOI] [PubMed] [Google Scholar]
- Andrews NC. Iron homeostasis: insights from genetics and animal models. Nature Reviews Genetics. 2000a;1:208–217. doi: 10.1038/35042073. [DOI] [PubMed] [Google Scholar]
- Andrews NC. Iron metabolism: iron deficiency and iron overload. Annual Review of Genomics and Human Genetics. 2000b;1:75–98. doi: 10.1146/annurev.genom.1.1.75. [DOI] [PubMed] [Google Scholar]
- Andrews NC, Schmidt PJ. Iron homeostasis. Annual Review of Physiology. 2007;69:69–85. doi: 10.1146/annurev.physiol.69.031905.164337. [DOI] [PubMed] [Google Scholar]
- Aruoma OI, Halliwell B, Laughton MJ, Quinlan GJ, Gutteridge JM. The mechanism of initiation of lipid peroxidation. Evidence against a requirement for an iron(II)-iron(III) complex. Biochemical Journal. 1989;258:617–620. doi: 10.1042/bj2580617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardou-Jacquet E, Island ML, Jouanolle AM, Detivaud L, Fatih N, Ropert M, et al. A novel N491S mutation in the human SLC11A2 gene impairs protein trafficking and in association with the G212V mutation leads to microcytic anemia and liver iron overload. Blood Cells, Molecules, and Diseases. 2011;47:243–248. doi: 10.1016/j.bcmd.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Bargal R, Avidan N, Ben-Asher E, Olender Z, Zeigler M, Frumkin A, et al. Identification of the gene causing mucolipidosis type IV. Nature Genetics. 2000;26:118–123. doi: 10.1038/79095. [DOI] [PubMed] [Google Scholar]
- Barrios M, Moreno-Carralero MI, Cuadrado-Grande N, Baro M, Vivanco JL, Moran-Jimenez MJ. The homozygous mutation G75R in the human SLC11A2 gene leads to microcytic anaemia and iron overload. British Journal of Haematology. 2012;157:514–516. doi: 10.1111/j.1365-2141.2012.09043.x. [DOI] [PubMed] [Google Scholar]
- Bassi MT, Manzoni M, Monti E, Pizzo MT, Ballabio A, Borsani G. Cloning of the gene encoding a novel integral membrane protein, mucolipidin- and identification of the two major founder mutations causing mucolipidosis type IV. American Journal of Human Genetics. 2000;67:1110–1120. doi: 10.1016/s0002-9297(07)62941-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont C, Delaunay J, Hetet G, Grandchamp B, de Montalembert M, Tchernia G. Two new human DMT1 gene mutations in a patient with microcytic anemia, low ferritinemia, and liver iron overload. Blood. 2006;107:4168–4170. doi: 10.1182/blood-2005-10-4269. [DOI] [PubMed] [Google Scholar]
- Bekri S, Kispal G, Lange H, Fitzsimons E, Tolmie J, Lill R, et al. Human ABC7 transporter: gene structure and mutation causing X-linked sideroblastic anemia with ataxia with disruption of cytosolic iron-sulfur protein maturation. Blood. 2000;96:3256–3264. [PubMed] [Google Scholar]
- Bernstein SE. Hereditary hypotransferrinemia with hemosiderosis, a murine disorder resembling human atransferrinemia. The Journal of Laboratory and Clinical Medicine. 1987;110:690–705. [PubMed] [Google Scholar]
- Blanco E, Kannengiesser C, Grandchamp B, Tasso M, Beaumont C. Not all DMT1 mutations lead to iron overload. Blood Cells, Molecules, and Diseases. 2009;43:199–201. doi: 10.1016/j.bcmd.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Bowen BJ, Morgan EH. Anemia of the Belgrade rat: evidence for defective membrane transport of iron. Blood. 1987;70:38–44. [PubMed] [Google Scholar]
- Camaschella C, Roetto A, Cali A, De Gobbi M, Garozzo G, Carella M, et al. The gene TFR2 is mutated in a new type of haemochromatosis mapping to 7q22. Nature Genetics. 2000;25:14–15. doi: 10.1038/75534. [DOI] [PubMed] [Google Scholar]
- Canonne-Hergaux F, Donovan A, Delaby C, Wang HJ, Gros P. Comparative studies of duodenal and macrophage ferroportin proteins. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2006;290:G156–G163. doi: 10.1152/ajpgi.00227.2005. [DOI] [PubMed] [Google Scholar]
- Canonne-Hergaux F, Gruenheid S, Ponka P, Gros P. Cellular and subcellular localization of the Nramp2 iron transporter in the intestinal brush border and regulation by dietary iron. Blood. 1999;93:4406–4417. [PubMed] [Google Scholar]
- Chan LN, Gerhardt EM. Transferrin receptor gene is hyperexpressed and transcriptionally regulated in differentiating erythroid cells. Journal of Biological Chemistry. 1992;267:8254–8259. [PubMed] [Google Scholar]
- Chen J, Enns CA. The cytoplasmic domain of transferrin receptor 2 dictates its stability and response to holo-transferrin in Hep3B cells. Journal of Biological Chemistry. 2007;282:6201–6209. doi: 10.1074/jbc.M610127200. [DOI] [PubMed] [Google Scholar]
- Chen W, Paradkar PN, Li L, Pierce EL, Langer NB, Takahashi-Makise N, et al. Abcb10 physically interacts with mitoferrin-1 (Slc25a37) to enhance its stability and function in the erythroid mitochondria. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:16263–16268. doi: 10.1073/pnas.0904519106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua AC, Olynyk JK, Leedman PJ, Trinder D. Nontransferrin-bound iron uptake by hepatocytes is increased in the Hfe knockout mouse model of hereditary hemochromatosis. Blood. 2004;104:1519–1525. doi: 10.1182/blood-2003-11-3872. [DOI] [PubMed] [Google Scholar]
- Crichton RR, Charloteaux-Wauters M. Iron transport and storage. European Journal of Biochemistry. 1987;164:485–506. doi: 10.1111/j.1432-1033.1987.tb11155.x. [DOI] [PubMed] [Google Scholar]
- Csere P, Lill R, Kispal G. Identification of a human mitochondrial ABC transporter, the functional orthologue of yeast Atm1p. FEBS Letters. 1998;441:266–270. doi: 10.1016/s0014-5793(98)01560-9. [DOI] [PubMed] [Google Scholar]
- De Domenico I, Lo E, Ward DM, Kaplan J. Hepcidin-induced internalization of ferroportin requires binding and cooperative interaction with Jak2. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3800–3805. doi: 10.1073/pnas.0900453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Domenico I, Lo E, Ward DM, Kaplan J. Human mutation D157G in ferroportin leads to hepcidin-independent binding of Jak2 and ferroportin down-regulation. Blood. 2010;115:2956–2959. doi: 10.1182/blood-2009-10-251306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Domenico I, Lo E, Yang B, Korolnek T, Hamza I, Ward DM, et al. The role of ubiquitination in hepcidin-independent and hepcidin-dependent degradation of ferroportin. Cell Metabolism. 2011;14:635–646. doi: 10.1016/j.cmet.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- De Domenico I, Ward DM, di Patti MC, Jeong SY, David S, Musci G, et al. Ferroxidase activity is required for the stability of cell surface ferroportin in cells expressing GPI-ceruloplasmin. The EMBO Journal. 2007;26:2823–2831. doi: 10.1038/sj.emboj.7601735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XP, Cheng X, Mills E, Delling M, Wang F, Kurz T, et al. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature. 2008;455:992–996. doi: 10.1038/nature07311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403:776–781. doi: 10.1038/35001596. [DOI] [PubMed] [Google Scholar]
- Edwards JA, Sullivan AL, Hoke JE. Defective delivery of iron to the developing red cell of the Belgrade laboratory rat. Blood. 1980;55:645–648. [PubMed] [Google Scholar]
- Feder JN, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy DA, Basava A, et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nature Genetics. 1996;13:399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- Fleming MD, Romano MA, Su MA, Garrick LM, Garrick MD, Andrews NC. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:1148–1153. doi: 10.1073/pnas.95.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming MD, Trenor CC, 3rd, Su MA, Foernzler D, Beier DR, Dietrich WF, et al. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nature Genetics. 1997;16:383–386. doi: 10.1038/ng0897-383. [DOI] [PubMed] [Google Scholar]
- Fleming RE, Ahmann JR, Migas MC, Waheed A, Koeffler HP, Kawabata H, et al. Targeted mutagenesis of the murine transferrin receptor-2 gene produces hemochromatosis. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10653–10658. doi: 10.1073/pnas.162360699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes JR, Gros P. Divalent-metal transport by NRAMP proteins at the interface of host-pathogen interactions. Trends in Microbiology. 2001;9:397–403. doi: 10.1016/s0966-842x(01)02098-4. [DOI] [PubMed] [Google Scholar]
- Forbes JR, Gros P. Iron, manganese, and cobalt transport by Nramp1 (Slc11a1) and Nramp2 (Slc11a2) expressed at the plasma membrane. Blood. 2003;102:1884–1892. doi: 10.1182/blood-2003-02-0425. [DOI] [PubMed] [Google Scholar]
- Gao J, Zhao N, Knutson MD, Enns CA. The hereditary hemochromatosis protein, HFE, inhibits iron uptake via down-regulation of Zip14 in HepG2 cells. Journal of Biological Chemistry. 2008;283:21462–21468. doi: 10.1074/jbc.M803150200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrick MD, Gniecko K, Liu Y, Cohan DS, Garrick LM. Transferrin and the transferrin cycle in Belgrade rat reticulocytes. Journal of Biological Chemistry. 1993;268:14867–14874. [PubMed] [Google Scholar]
- Garrick MD, Kuo HC, Vargas F, Singleton S, Zhao L, Smith JJ, et al. Comparison of mammalian cell lines expressing distinct isoforms of divalent metal transporter 1 in a tetracycline-regulated fashion. Biochemical Journal. 2006;398:539–546. doi: 10.1042/BJ20051987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber J, Muhlenhoff U, Lill R. An interaction between frataxin and Isu1/Nfs1 that is crucial for Fe/S cluster synthesis on Isu1. EMBO Reports. 2003;4:906–911. doi: 10.1038/sj.embor.embor918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn RP, Van Campen DR. Iron uptake is enhanced in Caco-2 cell monolayers by cysteine and reduced cysteinyl glycine. Journal of Nutrition. 1997;127:642–647. doi: 10.1093/jn/127.4.642. [DOI] [PubMed] [Google Scholar]
- Graham RM, Reutens GM, Herbison CE, Delima RD, Chua AC, Olynyk JK, et al. Transferrin receptor 2 mediates uptake of transferrin-bound and non-transferrin-bound iron. Journal of Hepatology. 2008;48:327–334. doi: 10.1016/j.jhep.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Grandchamp B, Hetet G, Kannengiesser C, Oudin C, Beaumont C, Rodrigues-Ferreira S, et al. A novel type of congenital hypochromic anemia associated with a nonsense mutation in the STEAP3/TSAP6 gene. Blood. 2011;118:6660–6666. doi: 10.1182/blood-2011-01-329011. [DOI] [PubMed] [Google Scholar]
- Griffiths WJ, Kelly AL, Smith SJ, Cox TM. Localization of iron transport and regulatory proteins in human cells. The Quarterly Journal of Medicine. 2000;93:575–587. doi: 10.1093/qjmed/93.9.575. [DOI] [PubMed] [Google Scholar]
- Grootveld M, Bell JD, Halliwell B, Aruoma OI, Bomford A, Sadler PJ. Non-transferrin-bound iron in plasma or serum from patients with idiopathic hemochromatosis. Characterization by high performance liquid chromatography and nuclear magnetic resonance spectroscopy. Journal of Biological Chemistry. 1989;264:4417–4422. [PubMed] [Google Scholar]
- Gruenheid S, Cellier M, Vidal S, Gros P. Identification and characterization of a second mouse Nramp gene. Genomics. 1995;25:514–525. doi: 10.1016/0888-7543(95)80053-o. [DOI] [PubMed] [Google Scholar]
- Gruenheid S, Pinner E, Desjardins M, Gros P. Natural resistance to infection with intracellular pathogens: the Nramp1 protein is recruited to the membrane of the phagosome. Journal of Experimental Medicine. 1997;185:717–730. doi: 10.1084/jem.185.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerinot ML. The ZIP family of metal transporters. Biochimica et Biophysica Acta. 2000;1465:190–198. doi: 10.1016/s0005-2736(00)00138-3. [DOI] [PubMed] [Google Scholar]
- Gunshin H, Fujiwara Y, Custodio AO, Direnzo C, Robine S, Andrews NC. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. Journal of Clinical Investigation. 2005;115:1258–1266. doi: 10.1172/JCI24356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- Gunshin H, Starr CN, Direnzo C, Fleming MD, Jin J, Greer EL, et al. Cybrd1 (duodenal cytochrome b) is not necessary for dietary iron absorption in mice. Blood. 2005;106:2879–2883. doi: 10.1182/blood-2005-02-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han O, Failla ML, Hill AD, Morris ER, Smith JC., Jr Reduction of Fe(III) is required for uptake of nonheme iron by Caco-2 cells. Journal of Nutrition. 1995;125:1291–1299. doi: 10.1093/jn/125.5.1291. [DOI] [PubMed] [Google Scholar]
- Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117:285–297. doi: 10.1016/s0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- Hubert N, Hentze MW. Previously uncharacterized isoforms of divalent metal transporter (DMT)-1: implications for regulation and cellular function. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:12345–12350. doi: 10.1073/pnas.192423399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvidberg V, Maniecki MB, Jacobsen C, Hojrup P, Moller HJ, Moestrup SK. Identification of the receptor scavenging hemopexin-heme complexes. Blood. 2005;106:2572–2579. doi: 10.1182/blood-2005-03-1185. [DOI] [PubMed] [Google Scholar]
- Ichikawa Y, Bayeva M, Ghanefar M, Potini V, Sun L, Mutharasan RK, et al. Disruption of ATP-binding cassette B8 in mice leads to cardiomyopathy through a decrease in mitochondrial iron export. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:4152–4157. doi: 10.1073/pnas.1119338109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iolascon A, d'Apolito M, Servedio V, Cimmino F, Piga A, Camaschella C. Microcytic anemia and hepatic iron overload in a child with compound heterozygous mutations in DMT1 (SCL11A2) Blood. 2006;107:349–354. doi: 10.1182/blood-2005-06-2477. [DOI] [PubMed] [Google Scholar]
- Jabado N, Jankowski A, Dougaparsad S, Picard V, Grinstein S, Gros P. Natural resistance to intracellular infections: natural resistance-associated macrophage protein 1 (Nramp1) functions as a pH-dependent manganese transporter at the phagosomal membrane. Journal of Experimental Medicine. 2000;192:1237–1248. doi: 10.1084/jem.192.9.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing SQ, Trowbridge IS. Identification of the intermolecular disulfide bonds of the human transferrin receptor and its lipid-attachment site. The EMBO Journal. 1987;6:327–331. doi: 10.1002/j.1460-2075.1987.tb04758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MB, Enns CA. Diferric transferrin regulates transferrin receptor 2 protein stability. Blood. 2004;104:4287–4293. doi: 10.1182/blood-2004-06-2477. [DOI] [PubMed] [Google Scholar]
- Kawabata H, Yang R, Hirama T, Vuong PT, Kawano S, Gombart AF, et al. Molecular cloning of transferrin receptor 2. A new member of the transferrin receptor-like family. Journal of Biological Chemistry. 1999;274:20826–20832. doi: 10.1074/jbc.274.30.20826. [DOI] [PubMed] [Google Scholar]
- Keel SB, Doty RT, Yang Z, Quigley JG, Chen J, Knoblaugh S, et al. A heme export protein is required for red blood cell differentiation and iron homeostasis. Science. 2008;319:825–828. doi: 10.1126/science.1151133. [DOI] [PubMed] [Google Scholar]
- Knutson M, Wessling-Resnick M. Iron metabolism in the reticuloendothelial system. Critical Reviews in Biochemistry and Molecular Biology. 2003;38:61–88. doi: 10.1080/713609210. [DOI] [PubMed] [Google Scholar]
- Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, et al. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- Kuhn LC, McClelland A, Ruddle FH. Gene transfer, expression, and molecular cloning of the human transferrin receptor gene. Cell. 1984;37:95–103. doi: 10.1016/0092-8674(84)90304-0. [DOI] [PubMed] [Google Scholar]
- Lam-Yuk-Tseung S, Mathieu M, Gros P. Functional characterization of the E399D DMT1/NRAMP2/SLC11A2 protein produced by an exon 12 mutation in a patient with microcytic anemia and iron overload. Blood Cells, Molecules and Diseases. 2005;35:212–216. doi: 10.1016/j.bcmd.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Lee PL, Gelbart T, West C, Halloran C, Beutler E. The human Nramp2 gene: characterization of the gene structure, alternative splicing, promoter region and polymorphisms. Blood Cells, Molecules and Diseases. 1998;24:199–215. doi: 10.1006/bcmd.1998.0186. [DOI] [PubMed] [Google Scholar]
- Leidgens S, De Smet S, Foury F. Frataxin interacts with Isu1 through a conserved tryptophan in its beta-sheet. Human Molecular Genetics. 2010;19:276–286. doi: 10.1093/hmg/ddp495. [DOI] [PubMed] [Google Scholar]
- Levi S, Corsi B, Bosisio M, Invernizzi R, Volz A, Sanford D, et al. A human mitochondrial ferritin encoded by an intronless gene. Journal of Biological Chemistry. 2001;276:24437–24440. doi: 10.1074/jbc.C100141200. [DOI] [PubMed] [Google Scholar]
- Levy JE, Jin O, Fujiwara Y, Kuo F, Andrews NC. Transferrin receptor is necessary for development of erythrocytes and the nervous system. Nature Genetics. 1999;21:396–399. doi: 10.1038/7727. [DOI] [PubMed] [Google Scholar]
- Lim SK, Kim H, bin Ali A, Lim YK, Wang Y, Chong SM, et al. Increased susceptibility in Hp knockout mice during acute hemolysis. Blood. 1998;92:1870–1877. [PubMed] [Google Scholar]
- Liuzzi JP, Aydemir F, Nam H, Knutson MD, Cousins RJ. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13612–13617. doi: 10.1073/pnas.0606424103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzi JP, Lichten LA, Rivera S, Blanchard RK, Aydemir TB, Knutson MD, et al. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:6843–6848. doi: 10.1073/pnas.0502257102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwiczek S, Theurl I, Muckenthaler MU, Jakab M, Mair SM, Theurl M, et al. Ca2+ channel blockers reverse iron overload by a new mechanism via divalent metal transporter-1. Nature Medicine. 2007;13:448–454. doi: 10.1038/nm1542. [DOI] [PubMed] [Google Scholar]
- Malo D, Vogan K, Vidal S, Hu J, Cellier M, Schurr E, et al. Haplotype mapping and sequence analysis of the mouse Nramp gene predict susceptibility to infection with intracellular parasites. Genomics. 1994;23:51–61. doi: 10.1006/geno.1994.1458. [DOI] [PubMed] [Google Scholar]
- McKie AT, Barrow D, Latunde-Dada GO, Rolfs A, Sager G, Mudaly E, et al. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science. 2001;291:1755–1759. doi: 10.1126/science.1057206. [DOI] [PubMed] [Google Scholar]
- McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, et al. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Molecular Cell. 2000;5:299–309. doi: 10.1016/s1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- Mims MP, Guan Y, Pospisilova D, Priwitzerova M, Indrak K, Ponka P, et al. Identification of a human mutation of DMT1 in a patient with microcytic anemia and iron overload. Blood. 2005;105:1337–1342. doi: 10.1182/blood-2004-07-2966. [DOI] [PubMed] [Google Scholar]
- Muhlenhoff U, Stadler JA, Richhardt N, Seubert A, Eickhorst T, Schweyen RJ, et al. A specific role of the yeast mitochondrial carriers MRS3/4p in mitochondrial iron acquisition under iron-limiting conditions. The Journal of Biological Chemistry. 2003;278:40612–40620. doi: 10.1074/jbc.M307847200. [DOI] [PubMed] [Google Scholar]
- Muir A, Hopfer U. Regional specificity of iron uptake by small intestinal brushborder membranes from normal and iron-deficient mice. American Journal of Physiology. 1985;248:G376–G379. doi: 10.1152/ajpgi.1985.248.3.G376. [DOI] [PubMed] [Google Scholar]
- Munoz M, Villar I, Garcia-Erce JA. An update on iron physiology. World Journal of Gastroenterology. 2009;15:4617–4626. doi: 10.3748/wjg.15.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandal A, Ruiz JC, Subramanian P, Ghimire-Rijal S, Sinnamon RA, Stemmler TL, et al. Activation of the HIF prolyl hydroxylase by the iron chaperones PCBP1 and PCBP2. Cell Metabolism. 2011;14:647–657. doi: 10.1016/j.cmet.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. The Journal of Clinical Investigation. 2002;110:1037–1044. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen MJ, Moestrup SK. Receptor targeting of hemoglobin mediated by the haptoglobins: roles beyond heme scavenging. Blood. 2009;114:764–771. doi: 10.1182/blood-2009-01-198309. [DOI] [PubMed] [Google Scholar]
- Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiological Reviews. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- Nomura N, Nagase T, Miyajima N, Sazuka T, Tanaka A, Sato S, et al. Prediction of the coding sequences of unidentified human genes. II. The coding sequences of 40 new genes (KIAA0041-KIAA0080) deduced by analysis of cDNA clones from human cell line KG-1. DNA Research. 1994;1:223–229. doi: 10.1093/dnares/1.5.223. [DOI] [PubMed] [Google Scholar]
- Nunez MT, Gaete V, Watkins JA, Glass J. Mobilization of iron from endocytic vesicles. The effects of acidification and reduction. Journal of Biological Chemistry. 1990;265:6688–6692. [PubMed] [Google Scholar]
- Oates PS, Morgan EH. Defective iron uptake by the duodenum of Belgrade rats fed diets of different iron contents. The American Journal of Physiology. 1996;270:G826–G832. doi: 10.1152/ajpgi.1996.270.5.G826. [DOI] [PubMed] [Google Scholar]
- Ohgami RS, Campagna DR, Greer EL, Antiochos B, McDonald A, Chen J, et al. Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nature Genetics. 2005;37:1264–1269. doi: 10.1038/ng1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgami RS, Campagna DR, McDonald A, Fleming MD. The Steap proteins are metalloreductases. Blood. 2006;108:1388–1394. doi: 10.1182/blood-2006-02-003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olynyk JK, Cullen DJ, Aquilia S, Rossi E, Summerville L, Powell LW. A population-based study of the clinical expression of the hemochromatosis gene. New England Journal of Medicine. 1999;341:718–724. doi: 10.1056/NEJM199909023411002. [DOI] [PubMed] [Google Scholar]
- Pinilla-Tenas JJ, Sparkman BK, Shawki A, Illing AC, Mitchell CJ, Zhao N, et al. Zip14 is a complex broad-scope metal-ion transporter whose functional properties support roles in the cellular uptake of zinc and nontransferrin-bound iron. American Journal of Physiology. Cell Physiology. 2011;301:C862–C871. doi: 10.1152/ajpcell.00479.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponka P, Sheftel AD, Zhang AS. Iron targeting to mitochondria in erythroid cells. Biochemical Society Transactions. 2002;30:735–738. doi: 10.1042/bst0300735. [DOI] [PubMed] [Google Scholar]
- Pryor PR, Reimann F, Gribble FM, Luzio JP. Mucolipin-1 is a lysosomal membrane protein required for intracellular lactosylceramide traffic. Traffic. 2006;7:1388–1398. doi: 10.1111/j.1600-0854.2006.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley JG, Burns CC, Anderson MM, Lynch ED, Sabo KM, Overbaugh J, et al. Cloning of the cellular receptor for feline leukemia virus subgroup C (FeLV-C), a retrovirus that induces red cell aplasia. Blood. 2000;95:1093–1099. [PubMed] [Google Scholar]
- Quigley JG, Yang Z, Worthington MT, Phillips JD, Sabo KM, Sabath DE, et al. Identification of a human heme exporter that is essential for erythropoiesis. Cell. 2004;118:757–766. doi: 10.1016/j.cell.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Robb A, Wessling-Resnick M. Regulation of transferrin receptor 2 protein levels by transferrin. Blood. 2004;104:4294–4299. doi: 10.1182/blood-2004-06-2481. [DOI] [PubMed] [Google Scholar]
- Robinson NJ, Winge DR. Copper metallochaperones. Annual Review of Biochemistry. 2010;79:537–562. doi: 10.1146/annurev-biochem-030409-143539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roetto A, Totaro A, Piperno A, Piga A, Longo F, Garozzo G, et al. New mutations inactivating transferrin receptor 2 in hemochromatosis type 3. Blood. 2001;97:2555–2560. doi: 10.1182/blood.v97.9.2555. [DOI] [PubMed] [Google Scholar]
- Rosenzweig AC. Metallochaperones: bind and deliver. Chemistry and Biology. 2002;9:673–677. doi: 10.1016/s1074-5521(02)00156-4. [DOI] [PubMed] [Google Scholar]
- Searle S, Bright NA, Roach TI, Atkinson PG, Barton CH, Meloen RH, et al. Localisation of Nramp1 in macrophages: modulation with activation and infection. Journal of Cell Science. 1998;111(Pt 19):2855–2866. doi: 10.1242/jcs.111.19.2855. [DOI] [PubMed] [Google Scholar]
- Sendamarai AK, Ohgami RS, Fleming MD, Lawrence CM. Structure of the membrane proximal oxidoreductase domain of human Steap3, the dominant ferrireductase of the erythroid transferrin cycle. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7410–7415. doi: 10.1073/pnas.0801318105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P, Srai SK. Molecular mechanisms involved in intestinal iron absorption. World Journal of Gastroenterology. 2007;13:4716–4724. doi: 10.3748/wjg.v13.i35.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw GC, Cope JJ, Li L, Corson K, Hersey C, Ackermann GE, et al. Mitoferrin is essential for erythroid iron assimilation. Nature. 2006;440:96–100. doi: 10.1038/nature04512. [DOI] [PubMed] [Google Scholar]
- Shi H, Bencze KZ, Stemmler TL, Philpott CC. A cytosolic iron chaperone that delivers iron to ferritin. Science. 2008;320:1207–1210. doi: 10.1126/science.1157643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo M, Torimoto Y, Saito H, Motomura W, Ikuta K, Sato K, et al. Functional role of DMT1 in transferrin-independent iron uptake by human hepatocyte and hepatocellular carcinoma cell, HLF. Hepatology Research. 2006;35:152–162. doi: 10.1016/j.hepres.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Sipe DM, Murphy RF. Binding to cellular receptors results in increased iron release from transferrin at mildly acidic pH. Journal of Biological Chemistry. 1991;266:8002–8007. [PubMed] [Google Scholar]
- Sladic-Simic D, Zivkovic N, Pavic D, Marinkovic D, Martinovic J, Martinovitch PN. Hereditary hypochromic microcytic anemia in the laboratory rat. Genetics. 1966;53:1079–1089. doi: 10.1093/genetics/53.6.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RS. Iron deficiency and iron overload. Archives of Disease in Childhood. 1965;40:343–363. doi: 10.1136/adc.40.212.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soe-Lin S, Apte SS, Andriopoulos B, Jr, Andrews MC, Schranzhofer M, Kahawita T, et al. Nramp1 promotes efficient macrophage recycling of iron following erythrophagocytosis in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:5960–5965. doi: 10.1073/pnas.0900808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soe-Lin S, Sheftel AD, Wasyluk B, Ponka P. Nramp1 equips macrophages for efficient iron recycling. Experimental Hematology. 2008;36:929–937. doi: 10.1016/j.exphem.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Sun M, Goldin E, Stahl S, Falardeau JL, Kennedy JC, Acierno JS, Jr, et al. Mucolipidosis type IV is caused by mutations in a gene encoding a novel transient receptor potential channel. Human Molecular Genetics. 2000;9:2471–2478. doi: 10.1093/hmg/9.17.2471. [DOI] [PubMed] [Google Scholar]
- Swain JH, Tabatabai LB, Reddy MB. Histidine content of low-molecularweight beef proteins influences nonheme iron bioavailability in Caco-2 cells. Journal of Nutrition. 2002;132:245–251. doi: 10.1093/jn/132.2.245. [DOI] [PubMed] [Google Scholar]
- Tailor CS, Willett BJ, Kabat D. A putative cell surface receptor for anemia-inducing feline leukemia virus subgroup C is a member of a transporter superfamily. Journal of Virology. 1999;73:6500–6505. doi: 10.1128/jvi.73.8.6500-6505.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandy S, Williams M, Leggett A, Lopez-Jimenez M, Dedes M, Ramesh B, et al. Nramp2 expression is associated with pH-dependent iron uptake across the apical membrane of human intestinal Caco-2 cells. Journal of Biological Chemistry. 2000;275:1023–1029. doi: 10.1074/jbc.275.2.1023. [DOI] [PubMed] [Google Scholar]
- Taylor KM, Morgan HE, Johnson A, Nicholson RI. Structure-function analysis of a novel member of the LIV-1 subfamily of zinc transporters, ZIP14. FEBS Letters. 2005;579:427–432. doi: 10.1016/j.febslet.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Tolosano E, Fagoonee S, Hirsch E, Berger FG, Baumann H, Silengo L, et al. Enhanced splenomegaly and severe liver inflammation in haptoglobin/hemopexin double-null mice after acute hemolysis. Blood. 2002;100:4201–4208. doi: 10.1182/blood-2002-04-1270. [DOI] [PubMed] [Google Scholar]
- Tolosano E, Hirsch E, Patrucco E, Camaschella C, Navone R, Silengo L, et al. Defective recovery and severe renal damage after acute hemolysis in hemopexin-deficient mice. Blood. 1999;94:3906–3914. [PubMed] [Google Scholar]
- Trenor CC, 3rd, Campagna DR, Sellers VM, Andrews NC, Fleming MD. The molecular defect in hypotransferrinemic mice. Blood. 2000;96:1113–1118. [PubMed] [Google Scholar]
- Troadec MB, Ward DM, Lo E, Kaplan J, De Domenico I. Induction of FPN1 transcription by MTF-1 reveals a role for ferroportin in transition metal efflux. Blood. 2010;116:4657–4664. doi: 10.1182/blood-2010-04-278614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal SM, Malo D, Vogan K, Skamene E, Gros P. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell. 1993;73:469–485. doi: 10.1016/0092-8674(93)90135-d. [DOI] [PubMed] [Google Scholar]
- Wilkins M, Williams P, Cavill I. Transferrin iron uptake by human synovium. Annals of the Rheumatic Diseases. 1977;36:474–475. doi: 10.1136/ard.36.5.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro DJ, Tycko B, Fluss SR, Maxfield FR. Segregation of transferrin to a mildly acidic (pH 6.5) para-Golgi compartment in the recycling pathway. Cell. 1984;37:789–800. doi: 10.1016/0092-8674(84)90414-8. [DOI] [PubMed] [Google Scholar]
- Zhang AS, Sheftel AD, Ponka P. Intracellular kinetics of iron in reticulocytes: evidence for endosome involvement in iron targeting to mitochondria. Blood. 2005;105:368–375. doi: 10.1182/blood-2004-06-2226. [DOI] [PubMed] [Google Scholar]
- Zhang DL, Hughes RM, Ollivierre-Wilson H, Ghosh MC, Rouault TA. A ferroportin transcript that lacks an iron-responsive element enables duodenal and erythroid precursor cells to evade translational repression. Cell Metabolism. 2009;9:461–473. doi: 10.1016/j.cmet.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Lee T, Wang Y, Soong TW. Heterologous expression, functional characterization and localization of two isoforms of the monkey iron transporter Nramp2. Biochemical Journal. 2000;349:289–297. doi: 10.1042/0264-6021:3490289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N, Gao J, Enns CA, Knutson MD. ZRT/IRT-like protein 14 (ZIP14) promotes the cellular assimilation of iron from transferrin. Journal of Biological Chemistry. 2010;285:32141–32150. doi: 10.1074/jbc.M110.143248. [DOI] [PMC free article] [PubMed] [Google Scholar]


