Abstract
The EURTAC trial demonstrated that the tyrosine kinase inhibitor (TKI) erlotinib was superior to chemotherapy as first-line therapy for advanced non-small cell lung cancers (NSCLC) that harbor EGFR activating mutations in a predominantly Caucasian population. Based on EURTAC and several Asian trials, anti-EGFR TKIs are standard of care for EGFR mutation-positive NSCLC. We sought to validate a rapid multiplex EGFR mutation assay as a companion diagnostic assay to select patients for this therapy. Samples from the EURTAC trial were prospectively screened for EGFR mutations using a combination of laboratory-developed tests (LDTs), and tested retrospectively with the cobas EGFR mutation test (EGFR PCR test). The EGFR PCR test results were compared to the original LDT results and to Sanger sequencing, using a subset of specimens from patients screened for the trial. Residual tissue was available from 487 (47%) of the 1044 patients screened for the trial. The EGFR PCR test showed high concordance with LDT results with a 96.3% overall agreement. The clinical outcome of patients who were EGFR-mutation detected by the EGFR PCR test was very similar to the entire EURTAC cohort. The concordance between the EGFR PCR test and Sanger sequencing was 90.6%. In 78.9% of the discordant samples, the EGFR PCR test result was confirmed by a sensitive deep sequencing assay. This retrospective study demonstrates the clinical utility of the EGFR PCR test in the accurate selection of patients for anti-EGFR TKI therapy. The EGFR PCR test demonstrated improved performance relative to Sanger sequencing.
Introduction
The efficacy of many novel targeted cancer therapies can be predicted by the detection of specific biomarkers in the tumor. The FDA has indicated that if the identification of a specific biomarker is required for the safe and efficacious administration of a drug, a well-validated FDA approved companion diagnostic assay is required for that drug. The optimal approval path for a new targeted therapy and its companion diagnostic is a parallel clinical development process that involves clinical trials for the investigational agent where the investigational diagnostic test is used to either select patients for the trials or to predict response to treatment, and ends ideally with simultaneous health authority approval of the drug and the companion diagnostic. Successful examples of this include the co-development (and co-approval) of the BRAF inhibitor vemurafenib and its companion diagnostic BRAF V600E mutation assay for BRAF-mutant metastatic melanoma[1], and the ALK inhibitor crizotinib and its companion diagnostic ALK fusion gene test in advanced ALK-fusion positive non-small cell lung cancer (NSCLC) patients.[2], [3], [4]
However, in some cases, predictive biomarkers for a targeted therapy are not recognized until after the drug is first approved. As an example, the anti-EGFR antibody cetuximab was first approved in the US for the treatment of metastatic colorectal cancer in 2004. Numerous retrospective and prospective trials subsequently revealed that tumors harboring KRAS mutations were very unlikely to respond to cetuximab. In July 2009, FDA required labeling changes for cetuximab and another anti-EGFR antibody panitumumab requiring that the indications and usage state there was no treatment benefit with the drugs for patients whose tumors had KRAS mutations in codon 12 or 13, at a time when there were no FDA-approved diagnostic assays for KRAS mutations.[5] Only later, in July 2012, did a KRAS mutation assay receive FDA approval, based on the results of a prospective randomized trial, highlighting the challenges of retrospectively validating a companion diagnostic assay after the pivotal drug trials have been completed.[6]
The anti-EGFR TKI erlotinib was initially approved for all patients with advanced NSCLC who had progressed on first-line chemotherapy. A number of subsequent studies determined that patients with EGFR-mutant NSCLC had a high likelihood of responding to these TKI, leading to trials in the first-line setting for EGFR-mutant cancer.[7], [8], [9], [10], [11], [12], [13] Four prospective randomized clinical trials studied in Asian populations demonstrated that erlotinib and gefitinib resulted in improved progression-free survival compared to chemotherapy for first line therapy in NSCLC patients with EGFR mutations.[7], [8], [9], [13] Other clinical studies in mixed ethnicity cohorts have concluded with similar results.[10],[12]
The EURTAC trial was a randomized phase 3 trial to assess the safety and efficacy of erlotinib compared with standard platinum-based chemotherapy for first-line treatment of a patient population with advanced EGFR-mutation detected NSCLC in a largely Caucasian population of European patients. Erlotinib-treated patients experienced significant improvements in median PFS (9.7 months vs. 5.2 months) compared to chemotherapy. Patients on the erlotinib arm also had a considerably higher percentage of responses (58% vs. 15%) in the intent-to-treat population.[11] This trial has been submitted for first line indication of erlotinib in EGFR mutated NSCLC patients.
The majority of activating EGFR mutations are located in exons 19 (45%) and 21 (40–45%).[14], [15], [16], [17], [18], [19], [20] Guidelines from organizations such as ASCO, CAP/AMP, and NCCN recommend the use of anti-EGFR TKIs as first-line therapy in patients with EGFR-mutant advanced NSCLC based on the results of these pivotal clinical trials. [21], [22], [23] Recent recommendations by CAP/IASLC/AMP advise the identification of EGFR mutations present at >1% of which exon 19 deletions and an exon 21 mutation (L858R) account for greater than 90% of all mutations.[24] None of the guidelines specify the testing method to be used, however the cobas EGFR Mutation test is CE-IVD approved and is recently FDA approved.[25]
Here we present the retrospective analysis of a clinical validation study of the EGFR PCR test on a subset of lung cancer specimens from patients screened for the EURTAC trial. The EGFR PCR test demonstrated improved sample workflow relative to the LDTs used in the EURTAC trial, enabling EGFR mutation screening in a single assay with a one-day turn-around time. The EGFR PCR test showed superior sensitivity and specificity compared with conventional Sanger sequencing.
Methods
The major study objectives were 1) to correlate the clinical outcomes (PFS, BORR) from the subgroup of available samples tested by the EGFR PCR test to the results from the entire EURTAC population, and 2) to compare the analytic performance of the EGFR PCR test to that of the original LDT and Sanger sequencing, using massively parallel pyrosequencing (MPP) to resolve discrepancies observed between the other 3 testing methods.
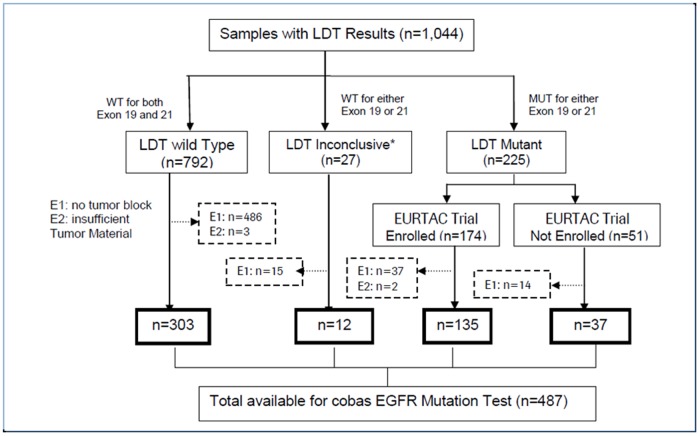
In the EURTAC trial, 1,044 patients from hospitals in France, Italy, and Spain were screened using the LDT. For this study, all samples were retrospectively analyzed under IRB approval from Copernicus IRB (00001313). Site specific IRB approval from each clinical site and written consent from all patients was obtained prior to the study conduct phase of NCT00446225.[11], [26] In 487 cases, residual specimens were available for retesting with the EGFR PCR test (Figure 1). A single 5 µm section with at least 10% tumor content from each of the 487 specimens was used for the EGFR PCR test. Genomic DNA from existing eluate or extracted from additional sections was tested on Sanger sequencing and MPP. Table 1 lists the demographics of the patients screened for the EURTAC trial by the LDT, sub-categorized by patients tested or not tested by the EGFR PCR test. Patients enrolled in the EURTAC trial were selected using a laboratory-developed test, validated by the Laboratory of Oncology (ICO-Hospital Germans Trias i Pujol, Badalona, Spain) consisting of three methodologies.[26] In this study, a single PCR-based assay for detecting EGFR mutations was used. Details of the analytical performance of this assay have been described previously.[27]
Figure 1. Flow of samples through the study.
E1 samples: tumor block not available for analysis. E2 samples: tumor material insufficient for analysis. LDT = laboratory-developed test.
Table 1. Demographics of the patient cohort screened for EURTAC trial.
| SLCG LDT MD | SLCG LDT MND | |||
| EGFR PCR tested | EGFR PCRnot tested | EGFR PCR tested | EGFR PCR not tested | |
| Total | 172 | 53 | 303 | 489 |
| Age (years), mean ± SD | 64.1±10.4 | 62.9±10.4 | 61.7±10.6 | 61.7±10.6 |
| Sex, n (%) | ||||
| Male | 41 (23.8) | 14 (26.4) | 179 (59.1) | 281 (57.5) |
| Female | 131 (76.2) | 39 (73.6) | 124 (40.9) | 208 (42.5) |
| Race/ethnicity, n (%) | ||||
| Caucasian | 168 (97.7) | 52 (98.1) | 296 (97.7) | 481 (98.4) |
| Other* | 4 (2.3) | 1 (1.9) | 7 (2.3) | 8 (1.6) |
| Smoking status, n (%) | ||||
| Never smoked | 124 (72.1) | 31 (58.5) | 74 (24.4) | 133 (27.2) |
| Past/currentsmoker | 47 (27.3) | 22 (41.5) | 219 (72.3) | 339 (69.3) |
| Unknown | 1 (0.6) | 0 (0.0) | 10 (3.3) | 17 (3.5) |
| Stage IIIB | 13 (7.6) | 2 (3.8) | 17 (5.6) | 40 (8.2) |
| Stage IV | 157 (91.3) | 50 (94.3) | 277 (91.4) | 432 (88.3) |
| Other* | 2 (1.2) | 1 (1.9) | 9 (3.0) | 17 (3.5) |
| Histology, n (%) | ||||
| Adenocarcinoma | 156 (90.7) | 47 (88.7) | 266 (87.8) | 407 (83.2) |
| BronchioalveolarCarcinoma | 1 (0.6) | 2 (3.8) | 5 (1.7) | 16 (3.3) |
| Other* | 15 (8.7) | 4 (7.5) | 32 (10.6) | 66 (13.5) |
*Other includes subjects with no information available. LDT = laboratory-developed test; MD = mutation detected; MND = mutation not detected.
SLCG inconclusive (n = 27) data not shown.
Statistical considerations
Mutation Detected (MD) was defined as the presence of either an exon 19 deletion or L858R mutation. Mutation Not Detected (MND) was defined as the absence of both exon 19 deletions and the L858R mutation. SAS/STAT® software was used for all data analysis.
Clinical outcome study statistics
Kaplan-Meier survival curves were used to assess the PFS by treatment method (chemotherapy or erlotinib) among patients who were enrolled in the EURTAC trial and screened with the LDT as well as the subset of patients who were determined to be mutation-positive by the EGFR PCR test. Nonparametric log-rank test was performed to assess PFS between patients who were randomized to chemotherapy or erlotinib. The hazard ratio (chemotherapy vs. erlotinib) relative to PFS was also calculated. Best overall response was the best response recorded from the start of treatment until disease progression and BORR (Best overall response rate) was summarized with 95% confidence limits according to Pearson-Clopper methods based on investigators assessment for each treatment arm.
Analytical performance statistics
For analytical performance, an agreement analysis was performed between the EGFR PCR test result and the LDT test. Mutation detection of exon 19 deletions and L858R mutations were analyzed in aggregate. Separately, the EGFR PCR test was also compared to Sanger sequencing and MPP by a CLIA-certified laboratory. For the agreement analyses, the positive percent agreement (PPA), negative percent agreement (NPA), and overall percent agreement (OPA) with their corresponding 95% confidence intervals (CIs) were calculated. In addition, 3-way analyses using MPP as a second reference method was performed to resolve the discrepancy results.
Mutation testing methods
EGFR PCR Test
The EGFR PCR test (cobas EGFR Mutation Test, Roche Molecular Systems, Inc, Branchburg, NJ, USA) is a CE-IVD marked multiplex allele-specific PCR-based assay designed to detect 41 mutations in exons 18, 19, 20, and 21 in FFPET specimens of human NSCLC.[28] DNA is isolated using the cobas DNA Sample Preparation Kit (Roche Molecular Systems, Branchburg, NJ). [29] A minimum of 150 ng of genomic DNA is required for PCR amplification, which can typically be isolated from a single 5 µm FFPET section. The EGFR PCR test software version used in this study was designed to detect 29 deletions in exon 19 and 2 L858R variants in exon 21. Macrodissection is only recommended if tumor content is less than 10%; laser capture microdissection is not required. The EGFR PCR test was performed per manufacturer's package insert and results were automatically analyzed and reported. The limit of detection has been validated to 5% mutant alleles. The workflow from DNA isolation to results reporting can be performed in one 8 hour period.[27]
LDT
Patients in the EURTAC study were screened using a combination of methods developed by Laboratory of Oncology, ICO-Hospital Germans Trias i Pujol, Barcelona, Spain.[11] In short, EGFR activating mutations in exons 19 and 21 were initially identified by Sanger sequencing and confirmed by fragment length analysis for exon 19 deletions (FAM-labelled primer in an ABI prism 3130 DNA analyser (Applied Biosystems, Foster City, CA, USA) and by Taqman assay for exon 21 (L858R) mutation. All tumor specimens were from the original biopsy taken prior to any treatment and before randomization. Testing was performed on ≥ 2mm2 of tissue obtained from one to three slides of 4-micron tissue sections which were subjected to laser capture microdissection to enrich for the presence of tumor cells. DNA was extracted using a standard laboratory protocol and tested at a single site in Spain in Laboratory of Oncology for EGFR activating mutations in exon 19 and 21 using a previously described method. The average turnaround time was approximately 5 days.[26]
Bi-directional Sanger sequencing
All samples tested by the EGFR PCR test were also tested by Sanger sequencing using DNA from FFPET specimens prepared by the cobas DNA Sample Preparation Kit and sequenced with 2× bidirectional Sanger sequencing by a CLIA-certified laboratory (SeqWright, Houston, TX, USA) using a validated protocol. Repeat Sanger sequencing was performed to compare the detection of EGFR mutations from adjacent sections of tissue to minimize any impact of tissue heterogeneity used for the EGFR PCR test relative to the original LDT results. Also, sequencing protocols vary by laboratory in terms of the percent tumor content/sample that requires macrodissection. DNA isolated with the cobas DNA Sample Preparation Kit and used for sequencing required ≥10% tumor content. Average turnaround time to results was 7 days. The estimated limit of detection is approximately 20% mutant alleles.[30]
Massively parallel pyrosequencing (MPP)
Samples with valid EGFR PCR test results with adequate DNA remaining from the initial extraction were tested by a MPP method (454 GS Titanium, 454 Life Sciences, Branford, CT, USA) by a CLIA-certified laboratory (SeqWright, Houston, TX, USA) using a validated protocol.[31] This method is a 5–7 day process that involves amplicon generation, pooling, ligation, emulsion PCR, amplification and massively parallel pyrosequencing with manual data analysis. The estimated limit of detection for the assay is 1.25% mutant alleles. [27] The MPP method was used to demonstrate performance of the EGFR PCR test to a more sensitive method and as an arbiter for discrepant cases observed between the LDT or the repeat Sanger sequencing. In order to preserve patient privacy associated with tested clinical samples, raw MPP sequencing results were anonymized and presented in Table S1.
Results
Specimen demographics
487 (47%) of 1,044 specimens screened for the EURTAC trial using LDTs were available for testing using the EGFR PCR test. The flow of samples through the study is shown in Figure 1. Patient demographics and baseline tumor characteristics for all patients by LDT status are shown in Table 1. There were no significant differences between subsets of patients tested and patients not tested by the EGFR PCR test (p>0.05) for each LDT status (mutation detected, mutation not detected) with the exception of country of the screening clinic.
Clinical outcomes for patients based on the EGFR PCR test results
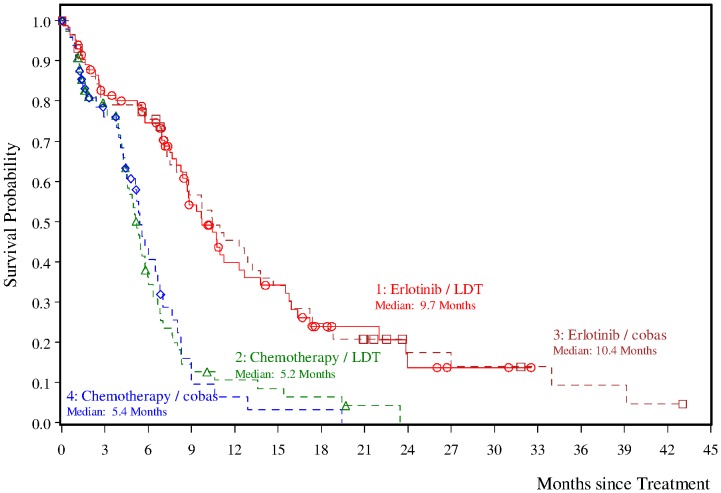
Of the 174 patients enrolled in EURTAC trial, specimens from 134 (77%) patients were available for testing using the EGFR PCR test. Excluding 11 patients with invalid EGFR PCR test results and 7 patients with a result of EGFR mutation not detected, a total of 116 (67%) patients were mutation detected by the EGFR PCR test and evaluable for clinical outcome analysis (57 patients in the chemotherapy arm and 59 in the erlotinib arm). Clinical outcomes (PFS, BORR, and OS) are presented in Table 2. Among EGFR PCR test positive patients, those treated with erlotinib had a significantly prolonged PFS when compared to patients treated with chemotherapy (p-value <0.0001, log-rank test); the median PFS was 10.4 months (95% CI: 8.0 to 13.8 months) and 5.4 months (95% CI: 4.4 to 6.8 months) for patients treated with erlotinib or chemotherapy, respectively (Figure 2). The HR based on the Cox proportional hazards model was reduced by 66% (HR 0.34; [95% CI: 0.21 to 0.54]) for patients in the erlotinib versus chemotherapy arm. One year after randomization, a higher percentage of patients in the erlotinib compared with the chemotherapy arm were event-free (45% [95% CI: 32% to 59% versus 6% [95% CI: 0% to 15%], respectively).
Table 2. Summary of Clinical Outcome Analysis among EGFR PCR test positive patients in the EURTAC trial.
| Chemotherapy (N = 57) | Erlotinib (N = 59) | ||
| PFS (Investigator) | |||
| Patients with event | 37 (64.9%) | 47 (79.7%) | |
| Patients without eventa | 20 (35.1%) | 12 (20.3%) | |
| Time to event (months) | |||
| Medianb (95%CI) | 5.4 [4.4; 6.8] | 10.4 [8.0; 13.8] | |
| p-Value (Log-Rank Test) | <0.0001 | ||
| Hazard Ratio (95% CI) | 0.34 [0.21; 0.54] | ||
| 1 year estimate | |||
| Patients remaining at risk | 2 | 24 | |
| Event-free Rateb (95%CI) | 6% [0%; 15%] | 45% [32%; 59%] | |
| Best Overall Analysis | |||
| Response rates (95% CI) | 14.0% [ 6.3%; 25.8%] | 59.3%[ 45.7%; 71.9%] | |
| Difference in Response Rates (%) | 45.29% [ 28.8%; 61.7%] | ||
| p-Value (Chi-squared Test) | <.0001 | ||
| Odds Ratio (95% CI) | 8.93 [3.59; 22.19] | ||
| OS | |||
| Patients with event | 35 (61.4%) | 36 (61.0%) | |
| Patients without eventa | 22 (38.6%) | 23 (39.0%) | |
| Time to event (months) | |||
| Medianb (95%CI) | 20.8 [17.3; 29.4] | 25.8 [16.1; 30.0] | |
| p-Value (Log-Rank Test) | 0.5381 | ||
| Hazard Ratio (95% CI) | 0.86 [0.54; 1.38] | ||
| 2 - year estimate | |||
| Patients remaining at risk | 16 | 23 | |
| Event-free Rateb (95% CI) | 43% [29%; 57%] | 51% [38%; 64%] | |
Note: All eligible patients enrolled in study ML20650 were determined as EGFR mutation detected by the LDT. Among those, patients with EGFR mutation confirmed by the EGFR PCR test were included in this table.
Event = Death or progression free, whichever comes first for PFS analysis and event = death for OS analysis.
censored.
Kaplan-Meier estimates.
including censored observations.
Figure 2. Kaplan-Meier curves of progression-free survival (PFS) for different treatments in treatment-naïve patients with non–small-cell lung cancer and EGFR mutation detected by the EGFR PCR test and LDT.
BORR were higher in patients in the erlotinib arm (59.3% [95% CI: 45.7% to 71.9%]) compared to the chemotherapy arm (14.0% [95% CI: 6.3% to 25.8%]). Patients in the erlotinib arm were much more likely to respond to therapy than patients in the chemotherapy arm (odds ratio of 8.93, [95% CI: 3.59 to 22.19]).
There was no significant difference in OS between the treatment arms (25.8 months in the erlotinib arm (95% CI: 16.1 to 30.0) and 20.8 months in the chemotherapy arm (95% CI: 17.3 to 29.4) (log-rank test p-value = 0.5381)).
PFS, BORR and OS results for EGFR PCR test positive patients did not differ significantly from those obtained in all patients enrolled in the EURTAC trial which suggests that the EGFR PCR test positive patients are representative of all EURTAC enrolled patients.
For the 7 cases where the EGFR PCR test result was mutation not detected and discrepant with the LDT, two cases resolved in favor of the LDT by MPP, three cases resolved in favor of the EGFR PCR test and one sample was invalid for both Sanger and MPP and the other was in agreement between the EGFR PCR test and Sanger but not MPP (Table S2). Anecdotally, 6 of the 7 patients were treated with erlotinib and only one patient achieved greater than or equal to median PFS based on the LDT or the EGFR PCR test.
Comparison of EGFR PCR test and LDT results
Among 432 specimens with valid results from both the EGFR PCR test and LDT, the PPA, NPA and OPA were 94.2% (146/155, CI: 89.3%, 96.9%), 97.5% (270/277, CI: 94.9%, 98.8%), and 96.3% (416/432, CI: 94.1%, 97.7%), respectively (Table 3). Thus there was a high concordance between the original LDT and EGFR PCR test results. Among sixteen specimens with discordant results, the EGFR PCR test result was confirmed by MPP in 68.8% (11/16) cases (Table S3).
Table 3. Agreement analysis between EGFR PCR test and LDT.
| SLCG LDT | Total | |||
| N = 432 | Mutation detected | Mutation not detected | ||
| EGFR PCR test | Mutation detected | 146 | 7 | 153 |
| Mutation not detected | 9 | 270 | 279 | |
| Total | 155 | 277 | 432* | |
•12 samples with inconclusive LDT results and 43 samples with invalid EGFR PCR test results were excluded.
Positive percent agreement = 94.2% (95% CI [89.3–96.9%]).
Negative percent agreement = 97.5% (95% CI [94.9–98.8%]).
Overall percent agreement = 96.3% (95% CI [94.1–97.7%]).
Comparison of the EGFR PCR test results with Sanger Sequencing
Of 487 specimens tested using the EGFR PCR test and Sanger sequencing, 406 gave valid results by both methods (38 were invalid by both methods, five were invalid by EGFR PCR test and 38 were invalid by Sanger sequencing). The PPA, NPA and OPA for EGFR PCR test compared with Sanger sequencing were 96.6% (112/116, CI: 91.7%, 98.7%), 88.3% (256/290, CI: 84.1%, 91.5%), and 90.6% (368/406, CI: 87.4%, 93.1%; Table 4), respectively. Among 38 discordant results between the EGFR PCR test and Sanger sequencing, MPP agreed with the EGFR PCR test result in 30 (78.9%) cases (Table S4). Sanger sequencing detected one L858R not detected by MPP and failed to detect 22 exon 19 deletions and 7 L858R mutations confirmed by MPP. Four MPP results were invalid, and the remaining four results agreed with Sanger. The range of percent mutant alleles of the cases missed by Sanger was 3% to 60%, with several specimens (n = 16) under the estimated limit of detection for Sanger.
Table 4. Agreement analysis between EGFR PCR test and Sanger sequencing.
| Sanger sequencing | Total | |||
| N = 406 | Mutation detected | Mutation not detected | ||
| EGFR PCR test | Mutation detected | 112 | 34 | 146 |
| Mutation not detected | 4 | 256 | 260 | |
| Total | 116 | 290 | 406 | |
*81 samples with invalid EGFR PCR test or Sanger sequencing results were excluded.
Positive percent agreement = 96.6% (95% CI [91.5–98.7%]).
Negative percent agreement = 88.3% (95% CI [84.1–91.5%]).
Overall percent agreement = 90.6% (95% CI [87.4–93.1%]).
Discussion
This study supports the feasibility of performing a retrospective clinical validation of a companion diagnostic from prospective, therapeutic clinical trials. The EGFR PCR test results were highly concordant (>96%) with the LDT results used to select patients for the EURTAC trial. As a consequence, PFS and BORR of the subset of patients with EGFR mutations detected with the EGFR PCR test were comparable to the full cohort of patients enrolled in the EURTAC trial, thus validating the use of the EGFR PCR test to select patients for treatment with anti-EGFR TKIs such as erlotinib. Median PFS survival was 9.7 versus 10.4 months for the erlotinib group and 5.2 versus 5.4 months for the LDTs and EGFR PCR test, respectively. The BORR was 58% versus 59.3% months for the erlotinib group and 15% versus 14.0% for the LDTs and EGFR PCR test, respectively. Among the 16 discordant specimens between the EGFR PCR test and LDTs, a third mutation testing method agreed with the EGFR PCR test result in 11 cases. Of seven cases that were mutation detected by the EGFR PCR test and mutation not detected by the LDT, 5 were confirmed by MPP. These patients could have potentially benefited from anti-EGFR TKI therapy. The EGFR PCR test had a number of technical advantages over the LDT used in the EURTAC trial. The LDT required laser capture microdissection of multiple tissue sections and involved 3 separate assays with a median turnaround time of 4.5 days. By comparison the EGFR PCR test required macrodissection only if the tumor content was <10% and can be performed in one day using a single 5 µm section. Furthermore the EGFR PCR test is a commercially available kit-based assay that provides an automated result, rather than a manual process subject to interpretation and which can be performed by any qualified clinical laboratory.
More than 80% of the specimens tested in this study were small biopsy specimens. The overall invalid rate for Sanger sequencing was 15.6% (76/487) compared to the EGFR PCR assay at 9% (43/487). However, the invalid rate for the subset of specimens derived from resected specimens was 0% (0/109) likely because of sufficient tissue availability. Thus the assay is extremely robust when performed on resected tumor specimens and has an approximately 90% success rate on biopsy specimens, which are often the only tumor sample available for testing in NSCLC.
Sanger sequencing has been widely used to detect EGFR mutations.[30], [32] Similar to the overall invalid rates, for the 134 EGFR mutation detected LDT samples enrolled in the EURTAC trial, Sanger sequencing had a higher invalid rate (15.7%) compared to 8.2% for the EGFR PCR test. There were also 30 mutation not detected results for Sanger sequencing (22.4%) and 7 mutation not detected results for the EGFR PCR test (5.2%). With 21 invalid results and 30 mutation not detected results, Sanger sequencing would have misclassified 38% of patients enrolled in the EURTAC trial. Similar invalid rates have been reported in three other studies, suggesting that this methodology has limitations when applied to DNA from FFPET samples.[33], [34], [35] In addition, Sanger sequencing has shown poor sensitivity in samples containing less than 20–25% mutant alleles.[35], [36], [37] When we compared the agreement between valid results for the EGFR PCR test with Sanger sequencing (n = 406), there were 38 discordant cases of which 30 were confirmed by MPP. Twenty-nine of the 30 cases resulted in mutation detected status by the EGFR PCR test and would make these patients eligible for anti-EGFR therapy. Poor sensitivity of Sanger sequencing thus explains the relatively low NPA compared to EGFR PCR test observed in this study.
Given the criticality of EGFR mutation testing in selecting specific therapies for life-threatening cancers such as advanced NSCLC, robust and accurate assays with rapid turnaround time are preferred. Recent quality assurance studies to ascertain the mutation status of a standard panel of tumors have shown that different clinical laboratories do not correctly identify the mutation status of 100% of the panel members, even when they are using the same or similar testing methodologies.[38], [39] For assays that involve mutation analysis of tumor samples, important factors contributing to the assay performance include analytic standardization, validation of reagents and methodology, laboratory experience, and the appropriate involvement of the pathologist.
In conclusion, results of the present study indicate that the cobas EGFR mutation test is a highly robust and highly accurate companion diagnostic assay to select patients for treatment with anti-EGFR therapies such as erlotinib.
Supporting Information
Listing of MPP Result.
(PDF)
Outcome from samples discrepant between the cobas EGFR PCR test and LDT that were enrolled in the clinical trial (cobas MND/LDT MD).
(PDF)
Agreement results between discordant EGFR PCR and LDT tests.
(PDF)
MPP results from resolution analysis of discordant specimens between EGFR PCR test and Sanger sequencing.
(PDF)
Acknowledgments
We would like to acknowledge Patrick O'Donnell and Karen Yu for their contributions to this study.
Funding Statement
This study was funded by Roche. The funders contributed to the study design, data collection, analysis, decision to publish and preparation of the manuscript.
References
- 1. Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, et al. (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364: 2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ou SH, Bartlett CH, Mino-Kenudson M, Cui J, Iafrate AJ (2012) Crizotinib for the treatment of ALK-rearranged non-small cell lung cancer: a success story to usher in the second decade of molecular targeted therapy in oncology. Oncologist 17: 1351–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O'Bryant CL, Wenger SD, Kim M, Thompson LA (2013) Crizotinib: a new treatment option for ALK-positive non-small cell lung cancer. Annals Pharmacotherapy 47: 189–197. [DOI] [PubMed] [Google Scholar]
- 4. Sun JM, Choi YL, Won JK, Hirsch FR, Ahn JS, et al. (2012) A dramatic response to crizotinib in a non-small-cell lung cancer patient with IHC-positive and FISH-negative ALK. J Thorac Oncol 7: e36–38. [DOI] [PubMed] [Google Scholar]
- 5.Administration USFaD (2010) Class Labeling Changes to anti-EGFR monoclonal antibodies, cetuximab (Erbitux) and panitumumab (Vectibix): KRAS Mutations.
- 6. Harbison CT, Horak CE, Ledeine JM, Mukhopadhyay P, Malone DP, et al. (2012) Validation of Companion Diagnostic for Detection of Mutations in Codons 12 and 13 of the KRAS Gene in Patients with Metastatic Colorectal Cancer: Analysis of the NCIC CTG CO.17 Trial. Arch Pathol Lab Med 137: 820–827. [DOI] [PubMed] [Google Scholar]
- 7. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, et al. (2010) Gefitinib or chemotherapy for non–small-cell lung cancer with mutated EGFR. N Engl J Med 362: 2380–2388. [DOI] [PubMed] [Google Scholar]
- 8. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, et al. (2009) Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361: 947–957. [DOI] [PubMed] [Google Scholar]
- 9. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, et al. (2011) Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 12: 735–742. [DOI] [PubMed] [Google Scholar]
- 10. Hirsch FR, Kabbinavar F, Eisen T, Martins R, Schnell FM, et al. (2011) A randomized, phase II, biomarker-selected study comparing erlotinib to erlotinib intercalated with chemotherapy in first-line therapy for advanced non-small-cell lung cancer. J Clin Oncol 29: 3567–3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, et al. (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13: 239–246. [DOI] [PubMed] [Google Scholar]
- 12. Sequist LV, Martins RG, Spigel D, Grunberg SM, Spira A, et al. (2008) First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol 26: 2442–2449. [DOI] [PubMed] [Google Scholar]
- 13. Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, et al. (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 11: 121–128. [DOI] [PubMed] [Google Scholar]
- 14. Kosaka T, Yatabe Y, Endoh H, Yoshida K, Hida T, et al. (2006) Analysis of epidermal growth factor receptor gene mutation in patients with non-small cell lung cancer and acquired resistance to gefitinib. Clin Cancer Res 12: 5764–5769. [DOI] [PubMed] [Google Scholar]
- 15. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, et al. (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350: 2129–2139. [DOI] [PubMed] [Google Scholar]
- 16. Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, et al. (2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304: 1497–1500. [DOI] [PubMed] [Google Scholar]
- 17. Pao W, Miller V, Zakowski M, Doherty J, Politi K, et al. (2004) EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 101: 13306–13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, et al. (2005) Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS 2: 225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Riely GJ, Politi KA, Miller VA, Pao W (2006) Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clin Cancer Res 12: 7232–7241. [DOI] [PubMed] [Google Scholar]
- 20. Sharma SV, Bell DW, Settleman J, Haber DA (2007) Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 7: 169–181. [DOI] [PubMed] [Google Scholar]
- 21. Garrido P, de Castro J, Concha A, Felip E, Isla D, et al. (2012) Guidelines for biomarker testing in advanced non-small-cell lung cancer. A national consensus of the Spanish Society of Medical Oncology (SEOM) and the Spanish Society of Pathology (SEAP). Clin & Transl Oncol 14: 338–349. [DOI] [PubMed] [Google Scholar]
- 22. Keedy VL, Temin S, Somerfield MR, Beasley MB, Johnson DH, et al. (2011) American Society of Clinical Oncology provisional clinical opinion: epidermal growth factor receptor (EGFR) Mutation testing for patients with advanced non-small-cell lung cancer considering first-line EGFR tyrosine kinase inhibitor therapy. J Clin Oncol 29: 2121–2127. [DOI] [PubMed] [Google Scholar]
- 23. Ettinger DS, Akerley W, Bepler G, Blum MG, Chang A, et al. (2010) Non-small cell lung cancer. J Natl Compr Canc Netw 8: 740–801. [DOI] [PubMed] [Google Scholar]
- 24. Lindeman NI, Cagle PT, Beasley MB, Chitale DA, Dacic S, et al. (2013) Molecular Testing Guideline for Selection of Lung Cancer Patients for EGFR and ALK Tyrosine Kinase Inhibitors: Guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Mol Diagn 15: 415–453. [DOI] [PubMed] [Google Scholar]
- 25.Administration USFaD (2013) FDA approves first companion diagnostic to detect gene mutation associated with a type of lung cancer.
- 26. Rosell R, Moran T, Queralt C, Porta R, Cardenal F, et al. (2009) Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 361: 958–969. [DOI] [PubMed] [Google Scholar]
- 27.O'Donnell PF, Jane; Shyu, Johnny; Current, Robert; Rehage, Taraneh; Tsai, Julie; Christensen, Mari; Tran, Ha Bich; Chien, Sean; Wei, Wen; Lawrence, H. Jeffrey; Soviero, Steven; Wu, Lin. A real-time PCR assay for detecting EGFR mutations in formalin-fixed paraffin-embedded tissue (FFPET) specimens of non-small cell lung cancer (NSCLC). 2012; Chicago, IL. [DOI] [PMC free article] [PubMed]
- 28. O'Donnell PF J, Shyu J, Current R, Rehage T, Tsai J, Christensen M., Bich Tran H, Shih-Chang C, Wei W, Lawrence HJ, Wu L, Soviero S (2013) A Real-Time PCR Assay for Detecting EGFR Mutations in Formalin-Fixed Paraffin-Embedded Tissue Specimens of Non-Small Cell Lung Cancer. BMC Cancer 13: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.(2011) cobas EGFR Mutation Test CE-IVD Package Insert, Roche Molecular Systems, Inc., USA.
- 30. Conde E, Angulo B, Tang M, Morente M, Torres-Lanzas J, et al. (2006) Molecular context of the EGFR mutations: evidence for the activation of mTOR/S6K signaling. Clin Cancer Res 12: 710–717. [DOI] [PubMed] [Google Scholar]
- 31. Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, et al. (2005) Genome sequencing in microfabricated high-density picolitre reactors. Nature 437: 376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Angulo B, Garcia-Garcia E, Martinez R, Suarez-Gauthier A, Conde E, et al. (2010) A commercial real-time PCR kit provides greater sensitivity than direct sequencing to detect KRAS mutations: a morphology-based approach in colorectal carcinoma. J Mol Diagn 12: 292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gallegos Ruiz MI, Floor K, Rijmen F, Grunberg K, Rodriguez JA, et al. (2007) EGFR and K-ras mutation analysis in non-small cell lung cancer: comparison of paraffin embedded versus frozen specimens. Cell Oncol 29: 257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ogino S, Kawasaki T, Brahmandam M, Yan L, Cantor M, et al. (2005) Sensitive sequencing method for KRAS mutation detection by Pyrosequencing. J Mol Diag 7: 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Anderson S, Bloom KJ, Vallera DU, Rueschoff J, Meldrum C, et al. (2012) Multisite Analytic Performance Studies of a Real-Time Polymerase Chain Reaction Assay for the Detection of BRAF V600E Mutations in Formalin-Fixed Paraffin-Embedded Tissue Specimens of Malignant Melanoma. Arch Pathol Lab Med 136: 1385–1391. [DOI] [PubMed] [Google Scholar]
- 36. Tan YH, Liu Y, Eu KW, Ang PW, Li WQ, et al. (2008) Detection of BRAF V600E mutation by pyrosequencing. Pathology 40: 295–298. [DOI] [PubMed] [Google Scholar]
- 37. Kotoula V, Charalambous E, Biesmans B, Malousi A, Vrettou E, et al. (2009) Targeted KRAS mutation assessment on patient tumor histologic material in real time diagnostics. PLoS One 4: e7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Beau-Faller M, Degeorges A, Rolland E, Mounawar M, Antoine M, et al. (2011) Cross-Validation Study for Epidermal Growth Factor Receptor and KRAS Mutation Detection in 74 Blinded Non-small Cell Lung Carcinoma Samples: A Total of 5550 Exons Sequenced by 15 Molecular French Laboratories. J Thorac Oncol 6: 1006–1015. [DOI] [PubMed] [Google Scholar]
- 39. Bellon E, Ligtenberg MJ, Tejpar S, Cox K, de Hertogh G, et al. (2011) External quality assessment for KRAS testing is needed: setup of a European program and report of the first joined regional quality assessment rounds. Oncologist 16: 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Listing of MPP Result.
(PDF)
Outcome from samples discrepant between the cobas EGFR PCR test and LDT that were enrolled in the clinical trial (cobas MND/LDT MD).
(PDF)
Agreement results between discordant EGFR PCR and LDT tests.
(PDF)
MPP results from resolution analysis of discordant specimens between EGFR PCR test and Sanger sequencing.
(PDF)