Abstract
Hematopoiesis gives rise to blood cells of different lineages throughout normal life. Abnormalities in this developmental program lead to blood cell diseases including leukemia. The establishment of a cell culture system for the clonal development of hematopoietic cells made it possible to discover proteins that regulate cell viability, multiplication and differentiation of different hematopoietic cell lineages, and the molecular basis of normal and abnormal blood cell development. These regulators include cytokines now called colony-stimulating factors (CSFs) and interleukins (ILs). There is a network of cytokine interactions, which has positive regulators such as CSFs and ILs and negative regulators such as transforming growth factor beta and tumor necrosis factor (TNF). This multigene cytokine network provides flexibility depending on which part of the network is activated and allows amplification of response to a particular stimulus. Malignancy can be suppressed in certain types of leukemic cells by inducing differentiation with cytokines that regulate normal hematopoiesis or with other compounds that use alternative differentiation pathways. This created the basis for the clinical use of differentiation therapy. The suppression of malignancy by inducing differentiation can bypass genetic abnormalities that give rise to malignancy. Different CSFs and ILs suppress programmed cell death (apoptosis) and induce cell multiplication and differentiation, and these processes of development are separately regulated. The same cytokines suppress apoptosis in normal and leukemic cells, including apoptosis induced by irradiation and cytotoxic cancer chemotherapeutic compounds. An excess of cytokines can increase leukemic cell resistance to cytotoxic therapy. The tumor suppressor gene wild-type p53 induces apoptosis that can also be suppressed by cytokines. The oncogene mutant p53 suppresses apoptosis. Hematopoietic cytokines such as granulocyte CSF are now used clinically to correct defects in hematopoiesis, including repair of chemotherapy-associated suppression of normal hematopoiesis in cancer patients, stimulation of normal granulocyte development in patients with infantile congenital agranulocytosis, and increase of hematopoietic precursors for blood cell transplantation. Treatments that decrease the level of apoptosis-suppressing cytokines and downregulate expression of mutant p53 and other apoptosis suppressing genes in cancer cells could improve cytotoxic cancer therapy. The basic studies on hematopoiesis and leukemia have thus provided new approaches to therapy.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arends M. J., Wyllie A. H. Apoptosis: mechanisms and roles in pathology. Int Rev Exp Pathol. 1991;32:223–254. doi: 10.1016/b978-0-12-364932-4.50010-1. [DOI] [PubMed] [Google Scholar]
- Askew D. S., Ashmun R. A., Simmons B. C., Cleveland J. L. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991 Oct;6(10):1915–1922. [PubMed] [Google Scholar]
- Azumi J. I., Sachs L. Chromosome mapping of the genes that control differentiation and malignancy in myeloid leukemic cells. Proc Natl Acad Sci U S A. 1977 Jan;74(1):253–257. doi: 10.1073/pnas.74.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak Y., Paran M., Levin S., Sachs L. In vitro induction of myeloid proliferation and maturation in infantile genetic agranulocytosis. Blood. 1971 Jul;38(1):74–80. [PubMed] [Google Scholar]
- Bissonnette R. P., Echeverri F., Mahboubi A., Green D. R. Apoptotic cell death induced by c-myc is inhibited by bcl-2. Nature. 1992 Oct 8;359(6395):552–554. doi: 10.1038/359552a0. [DOI] [PubMed] [Google Scholar]
- Blatt C., Aberdam D., Schwartz R., Sachs L. DNA rearrangement of a homeobox gene in myeloid leukaemic cells. EMBO J. 1988 Dec 20;7(13):4283–4290. doi: 10.1002/j.1460-2075.1988.tb03326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt C., Lotem J., Sachs L. Inhibition of specific pathways of myeloid cell differentiation by an activated Hox-2.4 homeobox gene. Cell Growth Differ. 1992 Oct;3(10):671–676. [PubMed] [Google Scholar]
- Blatt C., Sachs L. Deletion of a homeobox gene in myeloid leukemias with a deletion in chromosome 2. Biochem Biophys Res Commun. 1988 Nov 15;156(3):1265–1270. doi: 10.1016/s0006-291x(88)80769-1. [DOI] [PubMed] [Google Scholar]
- Bonilla M. A., Gillio A. P., Ruggeiro M., Kernan N. A., Brochstein J. A., Abboud M., Fumagalli L., Vincent M., Gabrilove J. L., Welte K. Effects of recombinant human granulocyte colony-stimulating factor on neutropenia in patients with congenital agranulocytosis. N Engl J Med. 1989 Jun 15;320(24):1574–1580. doi: 10.1056/NEJM198906153202402. [DOI] [PubMed] [Google Scholar]
- Bradbury D., Zhu Y. M., Russell N. Regulation of Bcl-2 expression and apoptosis in acute myeloblastic leukaemia cells by granulocyte-macrophage colony-stimulating factor. Leukemia. 1994 May;8(5):786–791. [PubMed] [Google Scholar]
- Bradley T. R., Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966 Jun;44(3):287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- Burgess A. W., Camakaris J., Metcalf D. Purification and properties of colony-stimulating factor from mouse lung-conditioned medium. J Biol Chem. 1977 Mar 25;252(6):1998–2003. [PubMed] [Google Scholar]
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- Clarke A. R., Gledhill S., Hooper M. L., Bird C. C., Wyllie A. H. p53 dependence of early apoptotic and proliferative responses within the mouse intestinal epithelium following gamma-irradiation. Oncogene. 1994 Jun;9(6):1767–1773. [PubMed] [Google Scholar]
- Clarke A. R., Purdie C. A., Harrison D. J., Morris R. G., Bird C. C., Hooper M. L., Wyllie A. H. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993 Apr 29;362(6423):849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- Cohen L., Sachs L. Constitutive gene expression in myeloid leukemia and cell competence for induction of differentiation by the steroid dexamethasone. Proc Natl Acad Sci U S A. 1981 Jan;78(1):353–357. doi: 10.1073/pnas.78.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debbas M., White E. Wild-type p53 mediates apoptosis by E1A, which is inhibited by E1B. Genes Dev. 1993 Apr;7(4):546–554. doi: 10.1101/gad.7.4.546. [DOI] [PubMed] [Google Scholar]
- Degos L. All-trans-retinoic acid treatment and retinoic acid receptor alpha gene rearrangement in acute promyelocytic leukemia: a model for differentiation therapy. Int J Cell Cloning. 1992 Mar;10(2):63–69. doi: 10.1002/stem.5530100202. [DOI] [PubMed] [Google Scholar]
- Demetri G. D., Griffin J. D. Granulocyte colony-stimulating factor and its receptor. Blood. 1991 Dec 1;78(11):2791–2808. [PubMed] [Google Scholar]
- Donehower L. A., Harvey M., Slagle B. L., McArthur M. J., Montgomery C. A., Jr, Butel J. S., Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992 Mar 19;356(6366):215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Eschbach J. W., Egrie J. C., Downing M. R., Browne J. K., Adamson J. W. Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. N Engl J Med. 1987 Jan 8;316(2):73–78. doi: 10.1056/NEJM198701083160203. [DOI] [PubMed] [Google Scholar]
- Evan G. I., Wyllie A. H., Gilbert C. S., Littlewood T. D., Land H., Brooks M., Waters C. M., Penn L. Z., Hancock D. C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992 Apr 3;69(1):119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Fanidi A., Harrington E. A., Evan G. I. Cooperative interaction between c-myc and bcl-2 proto-oncogenes. Nature. 1992 Oct 8;359(6395):554–556. doi: 10.1038/359554a0. [DOI] [PubMed] [Google Scholar]
- Fibach E., Hayashi M., Sachs L. Control of normal differentiation of myeloid leukemic cells to macrophages and granulocytes. Proc Natl Acad Sci U S A. 1973 Feb;70(2):343–346. doi: 10.1073/pnas.70.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fibach E., Landau T., Sachs L. Normal differentiation of myeloid leukaemic cells induced by a differentiation-inducing protein. Nat New Biol. 1972 Jun 28;237(78):276–278. doi: 10.1038/newbio237276a0. [DOI] [PubMed] [Google Scholar]
- Fibach E., Sachs L. Control of normal differentiation of myeloid leukemic cells. IV. Induction of differentiation by serum from endotoxin treated mice. J Cell Physiol. 1974 Apr;83(2):177–185. doi: 10.1002/jcp.1040830203. [DOI] [PubMed] [Google Scholar]
- Fibach E., Sachs L. Control of normal differentiation of myeloid leukemic cells. VIII. Induction of differentiation to mature granulocytes in mass culture. J Cell Physiol. 1975 Oct;86(2 Pt 1):221–230. doi: 10.1002/jcp.1040860205. [DOI] [PubMed] [Google Scholar]
- Fibach E., Sachs L. Control of normal differentiation of myeloid leukemic cells. XI. Induction of a specific requirement for cell viability and growth during the differentiation of myeloid leukemic cells. J Cell Physiol. 1976 Oct;89(2):259–266. doi: 10.1002/jcp.1040890209. [DOI] [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Mosmann T. R., Howard M., O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991 Dec 1;147(11):3815–3822. [PubMed] [Google Scholar]
- Fujiwara T., Grimm E. A., Mukhopadhyay T., Cai D. W., Owen-Schaub L. B., Roth J. A. A retroviral wild-type p53 expression vector penetrates human lung cancer spheroids and inhibits growth by inducing apoptosis. Cancer Res. 1993 Sep 15;53(18):4129–4133. [PubMed] [Google Scholar]
- GINSBURG H., SACHS L. FORMATION OF PURE SUSPENSIONS OF MAST CELLS IN TISSUE CULTURE BY DIFFERENTIATION OF LYMPHOID CELLS FROM THE MOUSE THYMUS. J Natl Cancer Inst. 1963 Jul;31:1–39. [PubMed] [Google Scholar]
- Gerassi E., Sachs L. Regulation of the induction of colonies in vitro by normal human lymphocytes. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4546–4550. doi: 10.1073/pnas.73.12.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootwine E., Webb C. G., Sachs L. Participation of myeloid leukaemic cells injected into embryos in haematopoietic differentiation in adult mice. Nature. 1982 Sep 2;299(5878):63–65. doi: 10.1038/299063a0. [DOI] [PubMed] [Google Scholar]
- Griffin J. D., Löwenberg B. Clonogenic cells in acute myeloblastic leukemia. Blood. 1986 Dec;68(6):1185–1195. [PubMed] [Google Scholar]
- Hirano T., Akira S., Taga T., Kishimoto T. Biological and clinical aspects of interleukin 6. Immunol Today. 1990 Dec;11(12):443–449. doi: 10.1016/0167-5699(90)90173-7. [DOI] [PubMed] [Google Scholar]
- Hoffman-Liebermann B., Sachs L. Regulation of actin and other proteins in the differentiation of myeloid leukemic cells. Cell. 1978 Aug;14(4):825–834. doi: 10.1016/0092-8674(78)90338-0. [DOI] [PubMed] [Google Scholar]
- Ichikawa Y., Pluznik D. H., Sachs L. Feedback inhibition of the development of macrophage and granulocyte colonies. I. Inhibition by macrophage. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1480–1486. doi: 10.1073/pnas.58.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa Y., Pluznik D. H., Sachs L. In vitro control of the development of macrophage and granulocyte colonies. Proc Natl Acad Sci U S A. 1966 Aug;56(2):488–495. doi: 10.1073/pnas.56.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Henderson L., Klein F., Palaszynski E. Procedures for the purification of interleukin 3 to homogeneity. J Immunol. 1982 Dec;129(6):2431–2436. [PubMed] [Google Scholar]
- Jacobsen S. E., Ruscetti F. W., Dubois C. M., Lee J., Boone T. C., Keller J. R. Transforming growth factor-beta trans-modulates the expression of colony stimulating factor receptors on murine hematopoietic progenitor cell lines. Blood. 1991 Apr 15;77(8):1706–1716. [PubMed] [Google Scholar]
- Kaplinsky C., Lotem J., Sachs L. Protection of human myeloid leukemic cells against doxorubicin-induced apoptosis by granulocyte-macrophage colony-stimulating factor and interleukin 3. Leukemia. 1996 Mar;10(3):460–465. [PubMed] [Google Scholar]
- Kawano M., Hirano T., Matsuda T., Taga T., Horii Y., Iwato K., Asaoku H., Tang B., Tanabe O., Tanaka H. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988 Mar 3;332(6159):83–85. doi: 10.1038/332083a0. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Taga T., Akira S. Cytokine signal transduction. Cell. 1994 Jan 28;76(2):253–262. doi: 10.1016/0092-8674(94)90333-6. [DOI] [PubMed] [Google Scholar]
- Korsmeyer S. J. Bcl-2 initiates a new category of oncogenes: regulators of cell death. Blood. 1992 Aug 15;80(4):879–886. [PubMed] [Google Scholar]
- Krystosek A., Sachs L. Control of lysozyme induction in the differentiation of myeloid leukemic cells. Cell. 1976 Dec;9(4 Pt 2):675–684. doi: 10.1016/0092-8674(76)90131-8. [DOI] [PubMed] [Google Scholar]
- Landau T., Sachs L. Characterization of the inducer required for the development of macrophage and granulocyte colonies. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2540–2544. doi: 10.1073/pnas.68.10.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. M., Bernstein A. p53 mutations increase resistance to ionizing radiation. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5742–5746. doi: 10.1073/pnas.90.12.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. J., Momand J., Finlay C. A. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- Liebermann D., Hoffman-Liebermann B., Sachs L. Molecular dissection of differentiation in normal and leukemic myeloblasts: separately programmed pathways of gene expression. Dev Biol. 1980 Sep;79(1):46–63. doi: 10.1016/0012-1606(80)90072-x. [DOI] [PubMed] [Google Scholar]
- Liebermann D., Sachs L. Co-regulation of type C RNA virus production and cell differentiation in myeloid leukemic cells. Cell. 1978 Nov;15(3):823–835. doi: 10.1016/0092-8674(78)90267-2. [DOI] [PubMed] [Google Scholar]
- Lipton J. H., Sachs L. Characterization of macrophage- and granulocyte-inducing proteins for normal and leukemic myeloid cells produced by the Krebs ascites tumor. Biochim Biophys Acta. 1981 Apr 3;673(4):552–569. doi: 10.1016/0304-4165(81)90486-4. [DOI] [PubMed] [Google Scholar]
- Lotem J., Cragoe E. J., Jr, Sachs L. Rescue from programmed cell death in leukemic and normal myeloid cells. Blood. 1991 Aug 15;78(4):953–960. [PubMed] [Google Scholar]
- Lotem J., Sachs L. A mutant p53 antagonizes the deregulated c-myc-mediated enhancement of apoptosis and decrease in leukemogenicity. Proc Natl Acad Sci U S A. 1995 Oct 10;92(21):9672–9676. doi: 10.1073/pnas.92.21.9672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotem J., Sachs L. Control of in vivo differentiation of myeloid leukemic cells. IV. Inhibition of leukemia development by myeloid differentiation-inducing protein. Int J Cancer. 1984 Jan 15;33(1):147–154. doi: 10.1002/ijc.2910330122. [DOI] [PubMed] [Google Scholar]
- Lotem J., Sachs L. Control of normal differentiation of myeloid leukemic cells. XII. Isolation of normal myeloid colony-forming cells from bone marrow and the sequence of differentiation to mature granulocytes in normal and D+ myeloid leukemic cells. J Cell Physiol. 1977 Jul;92(1):97–108. doi: 10.1002/jcp.1040920112. [DOI] [PubMed] [Google Scholar]
- Lotem J., Sachs L. Control of sensitivity to induction of apoptosis in myeloid leukemic cells by differentiation and bcl-2 dependent and independent pathways. Cell Growth Differ. 1994 Mar;5(3):321–327. [PubMed] [Google Scholar]
- Lotem J., Sachs L. Different blocks in the differentiation of myeloid leukemic cells. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3507–3511. doi: 10.1073/pnas.71.9.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotem J., Sachs L. Genetic dissection of the control of normal differentiation in myeloid leukemic cells. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5554–5558. doi: 10.1073/pnas.74.12.5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotem J., Sachs L. Hematopoietic cells from mice deficient in wild-type p53 are more resistant to induction of apoptosis by some agents. Blood. 1993 Aug 15;82(4):1092–1096. [PubMed] [Google Scholar]
- Lotem J., Sachs L. Hematopoietic cytokines inhibit apoptosis induced by transforming growth factor beta 1 and cancer chemotherapy compounds in myeloid leukemic cells. Blood. 1992 Oct 1;80(7):1750–1757. [PubMed] [Google Scholar]
- Lotem J., Sachs L. In vivo control of differentiation of myeloid leukemic cells by recombinant granulocyte-macrophage colony-stimulating factor and interleukin 3. Blood. 1988 Feb;71(2):375–382. [PubMed] [Google Scholar]
- Lotem J., Sachs L. In vivo induction of normal differentiation in myeloid leukemia cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3781–3785. doi: 10.1073/pnas.75.8.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotem J., Sachs L. In vivo inhibition of the development of myeloid leukemia by injection of macrophage- and granulocyte-inducing protein. Int J Cancer. 1981 Sep 15;28(3):375–386. doi: 10.1002/ijc.2910280318. [DOI] [PubMed] [Google Scholar]
- Lotem J., Sachs L. Induction of dependence on hematopoietic proteins for viability and receptor upregulation in differentiating myeloid leukemic cells. Blood. 1989 Aug 1;74(2):579–585. [PubMed] [Google Scholar]
- Lotem J., Sachs L. Interferon-gamma inhibits apoptosis induced by wild-type p53, cytotoxic anti-cancer agents and viability factor deprivation in myeloid cells. Leukemia. 1995 Apr;9(4):685–692. [PubMed] [Google Scholar]
- Lotem J., Sachs L. Mechanisms that uncouple growth and differentiation in myeloid leukemia cells: restoration of requirement for normal growth-inducing protein without restoring induction of differentiation-inducing protein. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4347–4351. doi: 10.1073/pnas.79.14.4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotem J., Sachs L. Regulation by bcl-2, c-myc, and p53 of susceptibility to induction of apoptosis by heat shock and cancer chemotherapy compounds in differentiation-competent and -defective myeloid leukemic cells. Cell Growth Differ. 1993 Jan;4(1):41–47. [PubMed] [Google Scholar]
- Lotem J., Sachs L. Regulation of bcl-2, bcl-XL and bax in the control of apoptosis by hematopoietic cytokines and dexamethasone. Cell Growth Differ. 1995 Jun;6(6):647–653. [PubMed] [Google Scholar]
- Lotem J., Sachs L. Regulation of cell-surface receptors for hematopoietic differentiation-inducing protein MGI-2 on normal and leukemic myeloid cells. Int J Cancer. 1987 Oct 15;40(4):532–539. doi: 10.1002/ijc.2910400417. [DOI] [PubMed] [Google Scholar]
- Lotem J., Sachs L. Selective regulation by hydrocortisone of induction of in vivo differentiation of myeloid leukemic cells with granulocyte-macrophage colony-stimulating factor, interleukin 6 and interleukin 1 alpha. Leukemia. 1992 May;6(5):426–431. [PubMed] [Google Scholar]
- Lotem J., Sachs L. Selective regulation of the activity of different hematopoietic regulatory proteins by transforming growth factor beta 1 in normal and leukemic myeloid cells. Blood. 1990 Oct 1;76(7):1315–1322. [PubMed] [Google Scholar]
- Lotem J., Shabo Y., Sachs L. The network of hemopoietic regulatory proteins in myeloid cell differentiation. Cell Growth Differ. 1991 Sep;2(9):421–427. [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- Maeda S., Sachs L. Control of normal differentiation of myeloid leukemic cells. XIII. Inducibility for some stages of differentiation by dimethylsulfoxide and its disassociation from inducibility by MGI. J Cell Physiol. 1978 Feb;94(2):181–185. doi: 10.1002/jcp.1040940207. [DOI] [PubMed] [Google Scholar]
- McCarthy S. A., Symonds H. S., Van Dyke T. Regulation of apoptosis in transgenic mice by simian virus 40 T antigen-mediated inactivation of p53. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3979–3983. doi: 10.1073/pnas.91.9.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D., Nossal G. J., Warner N. L., Miller J. F., Mandel T. E., Layton J. E., Gutman G. A. Growth of B-lymphocyte colonies in vitro. J Exp Med. 1975 Dec 1;142(6):1534–1549. doi: 10.1084/jem.142.6.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. The granulocyte-macrophage colony-stimulating factors. Science. 1985 Jul 5;229(4708):16–22. doi: 10.1126/science.2990035. [DOI] [PubMed] [Google Scholar]
- Mier J. W., Gallo R. C. Purification and some characteristics of human T-cell growth factor from phytohemagglutinin-stimulated lymphocyte-conditioned media. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6134–6138. doi: 10.1073/pnas.77.10.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motro B., Itin A., Sachs L., Keshet E. Pattern of interleukin 6 gene expression in vivo suggests a role for this cytokine in angiogenesis. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3092–3096. doi: 10.1073/pnas.87.8.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson I., Gullberg U., Lantz M., Richter J. The receptors for regulatory molecules of hematopoiesis. Eur J Haematol. 1992 Jan;48(1):1–9. doi: 10.1111/j.1600-0609.1992.tb01786.x. [DOI] [PubMed] [Google Scholar]
- Oltvai Z. N., Korsmeyer S. J. Checkpoints of dueling dimers foil death wishes. Cell. 1994 Oct 21;79(2):189–192. doi: 10.1016/0092-8674(94)90188-0. [DOI] [PubMed] [Google Scholar]
- Oster W., Schulz G. Interleukin 3: biological and clinical effects. Int J Cell Cloning. 1991 Jan;9(1):5–23. doi: 10.1002/stem.5530090105. [DOI] [PubMed] [Google Scholar]
- Paran M., Ichikawa Y., Sachs L. Production of the inducer for macrophage and granulocyte colonies by leukemic cells. J Cell Physiol. 1968 Dec;72(3):251–254. doi: 10.1002/jcp.1040720313. [DOI] [PubMed] [Google Scholar]
- Paran M., Sachs L., Barak Y., Resnitzky P. In vitro induction of granulocyte differentiation in hematopoietic cells from leukemic and non-leukemic patients. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1542–1549. doi: 10.1073/pnas.67.3.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paran M., Sachs L. The continued requirement for inducer for the development of macrophage and granulocyte colonies. J Cell Physiol. 1968 Dec;72(3):247–250. doi: 10.1002/jcp.1040720312. [DOI] [PubMed] [Google Scholar]
- Paran M., Sachs L. The single cell origin of normal granulocyte colonies in vitro. J Cell Physiol. 1969 Feb;73(1):91–92. doi: 10.1002/jcp.1040730113. [DOI] [PubMed] [Google Scholar]
- Pike B. L., Robinson W. A. Human bone marrow colony growth in agar-gel. J Cell Physiol. 1970 Aug;76(1):77–84. doi: 10.1002/jcp.1040760111. [DOI] [PubMed] [Google Scholar]
- Pluznik D. H., Sachs L. The cloning of normal "mast" cells in tissue culture. J Cell Physiol. 1965 Dec;66(3):319–324. doi: 10.1002/jcp.1030660309. [DOI] [PubMed] [Google Scholar]
- Pluznik D. H., Sachs L. The induction of clones of normal mast cells by a substance from conditioned medium. Exp Cell Res. 1966 Oct;43(3):553–563. doi: 10.1016/0014-4827(66)90026-7. [DOI] [PubMed] [Google Scholar]
- Ruhl S., Pluznik D. H. Dissociation of early and late markers of murine myeloid differentiation by interferon-gamma and interleukin-6. J Cell Physiol. 1993 Apr;155(1):130–138. doi: 10.1002/jcp.1041550117. [DOI] [PubMed] [Google Scholar]
- Sachs L. Cell differentiation and bypassing of genetic defects in the suppression of malignancy. Cancer Res. 1987 Apr 15;47(8):1981–1986. [PubMed] [Google Scholar]
- Sachs L. Control of normal cell differentiation and the phenotypic reversion of malignancy in myeloid leukaemia. Nature. 1978 Aug 10;274(5671):535–539. doi: 10.1038/274535a0. [DOI] [PubMed] [Google Scholar]
- Sachs L. Growth, differentiation and the reversal of malignancy. Sci Am. 1986 Jan;254(1):40–47. doi: 10.1038/scientificamerican0186-40. [DOI] [PubMed] [Google Scholar]
- Sachs L., Lotem J. Control of programmed cell death in normal and leukemic cells: new implications for therapy. Blood. 1993 Jul 1;82(1):15–21. [PubMed] [Google Scholar]
- Sachs L., Lotem J. The network of hematopoietic cytokines. Proc Soc Exp Biol Med. 1994 Jul;206(3):170–175. doi: 10.3181/00379727-206-43736. [DOI] [PubMed] [Google Scholar]
- Sachs L. Regulation of membrane changes, differentiation, and malignancy in carcinogenesis. Harvey Lect. 1974;68:1–35. [PubMed] [Google Scholar]
- Sachs L. The Wellcome Foundation lecture, 1986. The molecular regulators of normal and leukaemic blood cells. Proc R Soc Lond B Biol Sci. 1987 Aug 21;231(1264):289–312. doi: 10.1098/rspb.1987.0045. [DOI] [PubMed] [Google Scholar]
- Sachs L. The adventures of a biologist: prenatal diagnosis, hematopoiesis, leukemia, carcinogenesis, and tumor suppression. Adv Cancer Res. 1995;66:1–40. [PubMed] [Google Scholar]
- Sachs L. The control of growth and differentiation in normal and leukemic blood cells. Cancer. 1990 May 15;65(10):2196–2206. doi: 10.1002/1097-0142(19900515)65:10<2196::aid-cncr2820651006>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Sachs L. The molecular control of blood cell development. Science. 1987 Dec 4;238(4832):1374–1379. doi: 10.1126/science.3317831. [DOI] [PubMed] [Google Scholar]
- Sachs L. The molecular control of hematopoiesis: from clonal development in culture to therapy in the clinic. Int J Cell Cloning. 1992 Jul;10(4):196–204. doi: 10.1002/stem.5530100402. [DOI] [PubMed] [Google Scholar]
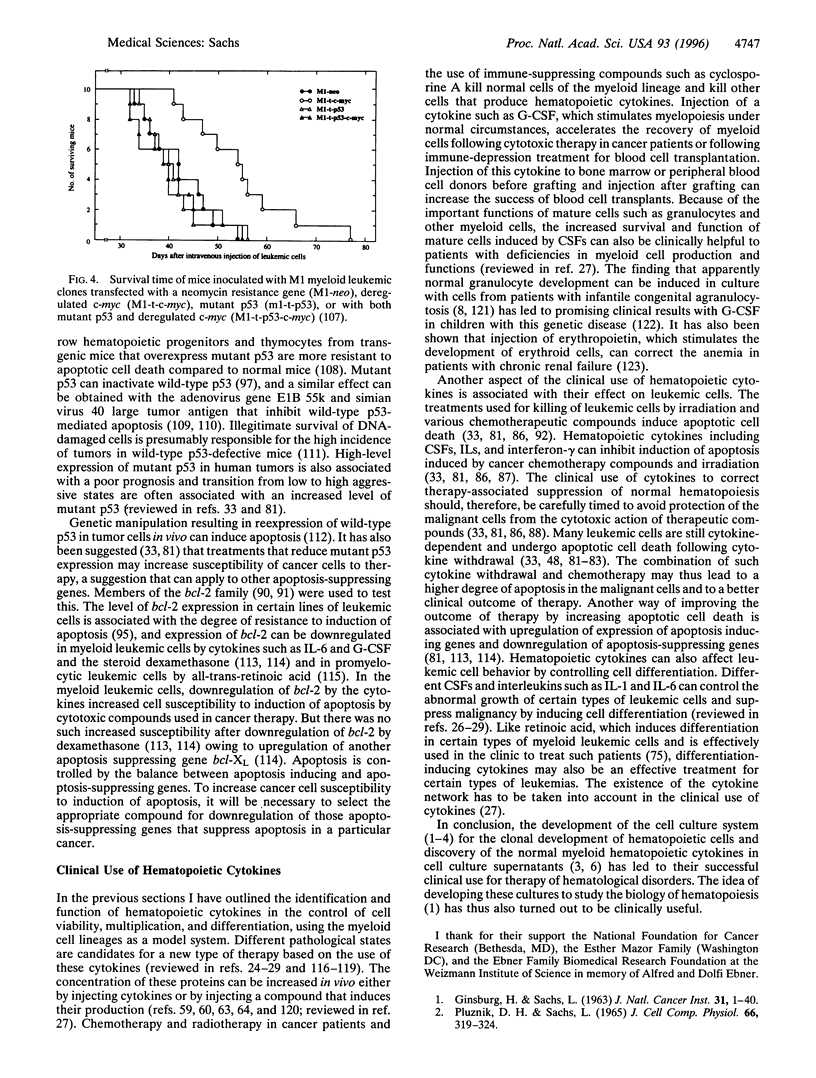
- Sachs L. The molecular control of hemopoiesis and leukemia. C R Acad Sci III. 1993 Sep;316(9):871–891. [PubMed] [Google Scholar]
- Sakamoto K. M., Gasson J. C. Clinical applications of human granulocyte-macrophage colony-stimulating factor. Int J Cell Cloning. 1991 Nov;9(6):531–541. doi: 10.1002/stem.5530090603. [DOI] [PubMed] [Google Scholar]
- Shabo Y., Lotem J., Rubinstein M., Revel M., Clark S. C., Wolf S. F., Kamen R., Sachs L. The myeloid blood cell differentiation-inducing protein MGI-2A is interleukin-6. Blood. 1988 Dec;72(6):2070–2073. [PubMed] [Google Scholar]
- Shabo Y., Lotem J., Sachs L. Autoregulation of interleukin 6 and granulocyte-macrophage colony-stimulating factor in the differentiation of myeloid leukemic cells. Mol Cell Biol. 1989 Sep;9(9):4109–4112. doi: 10.1128/mcb.9.9.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabo Y., Lotem J., Sachs L. Induction of genes for transcription factors by normal hematopoietic regulatory proteins in the differentiation of myeloid leukemic cells. Leukemia. 1990 Dec;4(12):797–801. [PubMed] [Google Scholar]
- Stanley E. R., Heard P. M. Factors regulating macrophage production and growth. Purification and some properties of the colony stimulating factor from medium conditioned by mouse L cells. J Biol Chem. 1977 Jun 25;252(12):4305–4312. [PubMed] [Google Scholar]
- Stephenson J. R., Axelrad A. A., McLeod D. L., Shreeve M. M. Induction of colonies of hemoglobin-synthesizing cells by erythropoietin in vitro. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1542–1546. doi: 10.1073/pnas.68.7.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A., Harris A. W., Bath M. L., Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 1990 Nov 22;348(6299):331–333. doi: 10.1038/348331a0. [DOI] [PubMed] [Google Scholar]
- Thompson C. B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995 Mar 10;267(5203):1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- Vaux D. L., Cory S., Adams J. M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988 Sep 29;335(6189):440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Vink A., Coulie P., Warnier G., Renauld J. C., Stevens M., Donckers D., Van Snick J. Mouse plasmacytoma growth in vivo: enhancement by interleukin 6 (IL-6) and inhibition by antibodies directed against IL-6 or its receptor. J Exp Med. 1990 Sep 1;172(3):997–1000. doi: 10.1084/jem.172.3.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb C. G., Gootwine E., Sachs L. Developmental potential of myeloid leukemia cells injected into midgestation embryos. Dev Biol. 1984 Jan;101(1):221–224. doi: 10.1016/0012-1606(84)90132-5. [DOI] [PubMed] [Google Scholar]
- White E. Life, death, and the pursuit of apoptosis. Genes Dev. 1996 Jan 1;10(1):1–15. doi: 10.1101/gad.10.1.1. [DOI] [PubMed] [Google Scholar]
- Williams G. T., Smith C. A., Spooncer E., Dexter T. M., Taylor D. R. Haemopoietic colony stimulating factors promote cell survival by suppressing apoptosis. Nature. 1990 Jan 4;343(6253):76–79. doi: 10.1038/343076a0. [DOI] [PubMed] [Google Scholar]
- Witte O. N. Steel locus defines new multipotent growth factor. Cell. 1990 Oct 5;63(1):5–6. doi: 10.1016/0092-8674(90)90280-r. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Suda T., Suda J., Eguchi M., Miura Y., Harada N., Tominaga A., Takatsu K. Purified interleukin 5 supports the terminal differentiation and proliferation of murine eosinophilic precursors. J Exp Med. 1988 Jan 1;167(1):43–56. doi: 10.1084/jem.167.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonish-Rouach E., Resnitzky D., Lotem J., Sachs L., Kimchi A., Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991 Jul 25;352(6333):345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]