Abstract
Purpose
Gastric cancer is a leading cause of death, particularly in the developing world. The literature reports individual socioeconomic status (SES) or neighborhood SES as related to survival, but the effect of both has not been studied. This study investigated the effect of individual and neighborhood SES simultaneously on mortality in gastric cancer patients in Taiwan.
Materials and Methods
A study was conducted of 3,396 patients diagnosed with gastric cancer between 2002 and 2006. Each patient was followed for five years or until death. Individual SES was defined by income-related insurance premium (low, moderate, and high). Neighborhood SES was based on household income dichotomized into advantaged and disadvantaged areas. Multilevel logistic regression model was used to compare survival rates by SES group after adjusting for possible confounding factors.
Results
In patients younger than 65 years, 5-year overall survival rates were lowest for those with low individual SES. After adjusting for patient characteristics (age, gender, Charlson Comorbidity Index Score), gastric cancer patients with high individual SES had 68% risk reduction of mortality (adjusted odds ratio [OR] of mortality, 0.32; 95% confidence interval [CI], 0.17–0.61). Patients aged 65 and above had no statistically significant difference in mortality rates by individual SES group. Different neighborhood SES did not statistically differ in the survival rates.
Conclusion
Gastric cancer patients aged less than 65 years old with low individual SES have higher risk of mortality, even under an universal healthcare system. Public health strategies, education and welfare policies should seek to correct the inequality in gastric cancer survival, especially in those with lower individual SES.
Introduction
Gastric cancer is a leading cause of death worldwide, with the 989,600 new cases in 2008 accounting for 8% of cancer cases. The 738,000 gastric cancer-related deaths worldwide in 2008 represented 10% of cancer deaths. In developing countries, more than 70% of new diagnoses and deaths from cancer are in gastric cancer patients [1]. In Taiwan and other Asian countries, gastric cancer remains an important cancer with high mortality. Gastric cancer ranks as the sixth-highest cause of cancer-related deaths in Taiwan, with a mortality rate of 6.8 per 100 000 [2].
According to the literature, gastric cancer incidence and survival are related to many risk factors, including individual and social risk factors. Individual factors include lymph node status, sex, race, genetics, individual socioeconomic status (SES) and diet; social factors include public health policies, availability of refrigeration and neighborhood SES [3]–[5]. The survival of patients with gastric cancer is related to individual SES [6], [7]. Patients in neighborhoods with the highest levels of SES may also enjoy better long-term survival [4].
Chang et al. pointed out that cancer patients with low individual SES who lived in disadvantaged neighborhoods had a higher risk of mortality than those in more favorable circumstances [8], including those with lung cancer, colorectal cancer, breast cancer, cervical cancer, prostate cancer, head and neck cancer, and pancreas cancer. We will consider the effect of neighborhood and individual SES on gastric cancer survival simultaneously in patients covered under the Taiwan National Health Insurance (NHI) system.
Materials and Methods
Ethics statement
This study was approved by the Institutional Review Board of Buddhist Dalin Tzu Chi General Hospital, Taiwan. Review board requirements for written informed consent were waived because all personal identifying information was removed from the dataset prior to analysis.
Database
In March 1995, the Taiwan Department of Health integrated 13 health insurance schemes into a universal insurance program. As a compulsory social insurance program, the program covers approximately 99% of the residents of Taiwan and has contracts with 97% of medical providers [9]. Taiwan's NHI has the unique characteristics of universal insurance coverage and a single-payer system with the government as sole insurer. Patients have free access to seek care with any physician or hospital they choose. The insurance premium is calculated by the insurant's individual monthly income reported to the Bureau. The data for this study were collected from Taiwan's National Health Insurance Research Database (NHIRD) for the years 2002 to 2006. This dataset is organized and managed by Taiwan's National Health Research Institutes but collected by Taiwan's NHI Program. These databases were monitored for completeness and accuracy by Taiwan's Department of Health. To verify accuracy of diagnosis, Taiwan's NHI Bureau randomly reviews the charts of one per 100 ambulatory and one per 20 inpatient claims and conducts interviews of patients [10], [11].
Our study cohort consisted of Taiwan's incidental gastric cancer patients (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes 147.9) who received some combination of surgery, adjuvant chemotherapy and chemoradiotherapy for their disease between 2002 and 2006. In Taiwan, the stage was not available in NHIRD. We selected the patients who received gastric resection and lymph nodes dissection as curative intent resection and tried to research the outcome of the gastric cancer patients.
Measurement
The key dependent variable of interest was 5-year overall survival rate. We did not attempt to determine the cause-specific survival rate because the registry data we used did not contain this information. The use of overall survival data should not interfere significantly with our results because, as Roohan et al. have shown in a study adapting a clinical morbidity index for use with ICD-9-CM administrative databases, the survival models for all-cause mortality and cancer-specific mortality do not differ significantly [12].
The key independent variables of the current study were the effects on survival of individual SES and neighborhood SES. Survival of each gastric cancer patient was determined by linking that patient's 2002-2006 mortality data with claims data for the first curative treatment up to five years prior to death. Patient characteristics included age, gender, geographic location, treatment modality, comorbiditye and monthly income. Comorbidity was based on the modified Charlson Comorbidity Index Score (CCIS), a widely accepted measure for risk adjustment in administrative claims data sets [13].
Individual-level measures
National health insurance in Taiwan identified each person's income and definite the level of insurance premium. We used the income-related insurance premium as a proxy for individual SES, an important prognostic factor for cancer [14]–[16]. The gastric cancer patients were classified into three groups: (1) low SES, lower than US$528 per month (New Taiwan Dollars (NT) $0 to $15,840), (2) moderate SES, between US$528 to $833 per month (NT $15,841 to $25,000), and (3) high SES, US$833 per month (NT $25,001) or more [17], [18]. We selected NT$15,840 as the low income level cutoff point because this was the government-stipulated minimum wage for full-time employees in Taiwan in 2006.
Neighborhood-level socioeconomic status
Low neighborhood income was associated with health disparity [19]. We use neighborhood income as a proxy of neighborhood SES was based on the average neighborhood household income reported in Taiwan's 2001 Census. In that census, neighborhood household income was measured by township using per capita income (in New Taiwan dollars, NT$) based on 2001 tax statistics released by Taiwan's Ministry of Finance (http://www.fdc.gov.tw/dp.asp?mp=5). The categorization into advantaged or disadvantaged neighborhoods was based on the median values, with advantaged neighborhoods having a higher-than-median neighborhood household income and disadvantaged neighborhoods having a lower-than-median household income [20], [21].
Other variables
We used population density, percentage of residents with college level or more education, percentage of residents ≥65 years old, percentage of residents who were agriculture workers, and the number of physicians per 100,000 persons to categorize residences into one of seven levels of urbanization, as previously described [22]. Urban residences were categorized as level 1, suburban residences were categorized as levels 2 and 3 and rural residences were categorized into levels 4 to 7. Hospitals were categorized by hospital accreditation level (medical center, regional hospital or district hospital). The geographic regions were recorded as northern, central, southern and eastern Taiwan.
Statistical analysis
All statistical operations were performed using SPSS (version 15, SPSS Inc., Chicago, IL). Pearson's chi-square test was used for categorical variables such as gender, level of urbanization, geographic region of residence, CCIS, treatment modality, tumor extent and hospital characteristics (teaching level, ownership and caseload). Continuous variables were analyzed by one-way analysis of variance. The mortality rates between different SES was compared using Pearson's chi-square test.
The multilevel logistic regression model was used to analyze the relationship between the main outcomes of the different SES groups and those of the reference group after adjusting for hospital, and patient demographics age, gender, CCIS, urbanization and area of residence, adjuvant treatment modality (radiotherapy, chemotherapy, chemoradiotherapy) and hospital characteristics. In this study, the multilevel logistic regression method was used because of concern for the potential clustering effect in a hospital. A hospital-level random effect might account for possible correlations between hospitalization costs within a hospital's panel simply because of hospital policies, procedures, or physician compensation mechanisms that were unique to that hospital. A two-sided P-value (P<0.05) was considered significant.
Results
Demographic data and clinical characteristics
A total of 3396 gastric cancer patients who received curative-intent surgery with or without adjuvant therapy were included in this study (Table 1). Compared to those with high individual SES, gastric cancer patients with low individual SES were more likely to be older, to live in rural areas. There was no statistically significant difference in the comorbidities, geographic regions and treatment in regional and district hospitals between each individual SES groups.
Table 1. Baseline characteristics (gastric cancer with surgery, n = 3396).
| Variables | Age <65 years (n = 1498) | Age ≧65 years (n = 1898) | ||||||||||||
| High SES | Moderate SES | Low SES | P value | High SES | Moderate SES | Low SES | P value | |||||||
| (n = 515) | (n = 509) | (n = 474) | (n = 59) | (n = 660) | (n = 1179) | |||||||||
| Mean age, years (±SD) | 50.2±8.3 | 51.9±8.9 | 53.9±9.1 | <0.001 | 73.2±6.7 | 74.2±5.5 | 75.2±5.9 | <0.001 | ||||||
| Gender | <0.001 | <0.001 | ||||||||||||
| Male (%) | 361 | (70.1) | 292 | (57.4) | 244 | (51.5) | 10 | (16.9) | 244 | (37.0) | 355 | (30.1) | ||
| Female (%)Comorbidities | 154 | (29.9) | 217 | (42.6) | 230 | (48.5) | 49 | (83.1) | 416 | (63.0) | 824 | (69.9) | ||
| Chronic renal failure | 2 | (0.4) | 7 | (1.4) | 3 | (0.6) | 0.184 | 0 | (0.0) | 9 | (1.4) | 10 | (0.8) | 0.417 |
| Chronic obstructive pulmonary disease | 3 | (0.6) | 3 | (0.6) | 1 | (0.2) | 0.613 | 2 | (3.4) | 20 | (3.0) | 42 | (3.6) | 0.832 |
| Heart disease | 6 | (1.2) | 1 | (0.2) | 2 | (0.4) | 0.111 | 4 | (6.8) | 24 | (3.6) | 58 | (4.9) | 0.313 |
| Hypertensive | 47 | (9.1) | 46 | (9.0) | 45 | (9.5) | 0.967 | 18 | (30.5) | 112 | (17.0) | 299 | (25.4) | <0.001 |
| Stroke | 0 | (0.0) | 0 | (0.0) | 1 | (0.2) | 0.339 | 0 | (0.0) | 0 | (0.0) | 1 | (0.1) | 0.737 |
| Diabetes mellitus | 30 | (5.8) | 43 | (8.4) | 39 | (8.2) | 0.211 | 14 | (23.7) | 79 | (12.0) | 169 | (14.3) | 0.030 |
| Adjuvant Therapy | 0.221 | 0.952 | ||||||||||||
| Nil (%) | 328 | (63.7) | 313 | (61.5) | 286 | (60.3) | 50 | (84.7) | 535 | (81.1) | 969 | (82.2) | ||
| Radiotherapy (%) | 9 | (1.7) | 8 | (1.6) | 15 | (3.2) | 1 | (1.7) | 13 | (2.0) | 25 | (2.1) | ||
| Chemotherapy (%) | 118 | (22.9) | 139 | (27.3) | 113 | (23.8) | 6 | (10.2) | 93 | (14.1) | 147 | (12.5) | ||
| Chemoradiotherapy (%) | 60 | (11.7) | 49 | (9.6) | 60 | 12.7() | 2 | (3.4) | 19 | (2.9) | 38 | (3.2) | ||
| Hospital characteristics | 0.051 | 0.123 | ||||||||||||
| Teaching level | ||||||||||||||
| Medical center (%) | 374 | (72.6) | 334 | (65.6) | 331 | (69.8) | 45 | (76.3) | 419 | (63.5) | 808 | (68.5) | ||
| Regional (%) | 137 | (26.6) | 169 | (33.2) | 133 | (28.1) | 13 | (22.0) | 223 | (33.8) | 344 | (29.2) | ||
| District (%) | 4 | (0.8) | 6 | (1.2) | 10 | (2.1) | 1 | (1.7) | 18 | (2.7) | 27 | (2.3) | ||
| Urbanization | <0.001 | <0.001 | ||||||||||||
| Urban (%) | 200 | (38.8) | 111 | (21.8) | 146 | (30.8) | 25 | (42.4) | 34 | (5.2) | 427 | (36.2) | ||
| Suburban (%) | 233 | (45.2) | 206 | (40.5) | 221 | (46.6) | 27 | (45.8) | 161 | (24.4) | 558 | (47.3) | ||
| Rural (%) | 82 | (15.9) | 192 | (37.7) | 107 | (22.6) | 7 | (11.9) | 465 | (70.5) | 194 | (16.5) | ||
| Geographic Region | <0.001 | <0.001 | ||||||||||||
| Northern (%) | 302 | (58.6) | 217 | (42.6) | 287 | (60.5) | 32 | (54.2) | 215 | (32.6) | 757 | (64.2) | ||
| Central (%) | 56 | (10.9) | 68 | (13.4) | 65 | (13.7) | 7 | (11.9) | 115 | (17.4) | 104 | (8.8) | ||
| Southern/ Eastern (%) | 157 | (30.5) | 224 | (44.0) | 122 | (25.7) | 20 | (33.9) | 330 | (50.0) | 318 | (27.0) | ||
Abbreviation: SES, socioeconomic status.
Univariate survival analysis
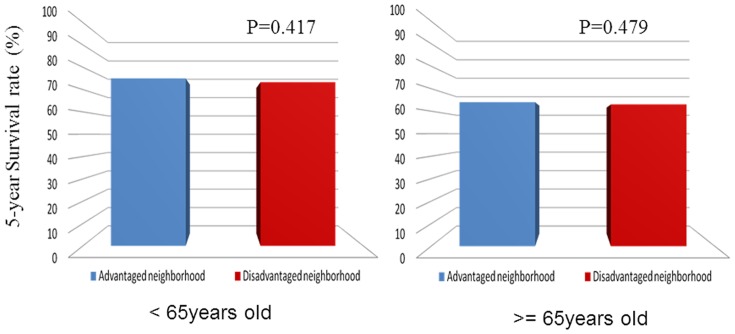
As can be seen in Table 2, among gastric cancer patients younger than 65 years, those categorized as high individual SES had significantly better survival rates than all comparison groups (P<0.001) (Fig 1a). For those 65 years and above, individual SES was not statistically associated to gastric cancer survival in those who received curative-intent treatment (Fig 1b). There was no statistically significant difference in gastric cancer survival between different neighborhood SES for both age groups (Fig 2a and 2b).
Table 2. 5-year survival rate in different SES groups (Gastric cancer with curative surgery, n = 3396).
| Age <65 years (n = 1498) | Age ≧65 years (n = 1898) | |||||
| n | Alive (%) | P value | n | Alive (%) | P value | |
| Individual socioeconomic status. | <0.001 | 0.816 | ||||
| High | 515 | 414(80.4) | 59 | 37(62.7) | ||
| Moderate | 509 | 373(73.3) | 660 | 424(64.2) | ||
| Low | 474 | 330(69.6) | 1179 | 740(62.8) | ||
| Neighborhood socioeconomic status | 0.417 | 0.479 | ||||
| Advantaged | 907 | 683(75.3) | 957 | 613(64.1) | ||
| Disadvantaged | 591 | 434(73.4) | 941 | 588(62.5) | ||
Figure 1. The effect of individual SES on survival rates in gastric cancer patients aged less than 65 years (1a) and aged 65 and above (1b).
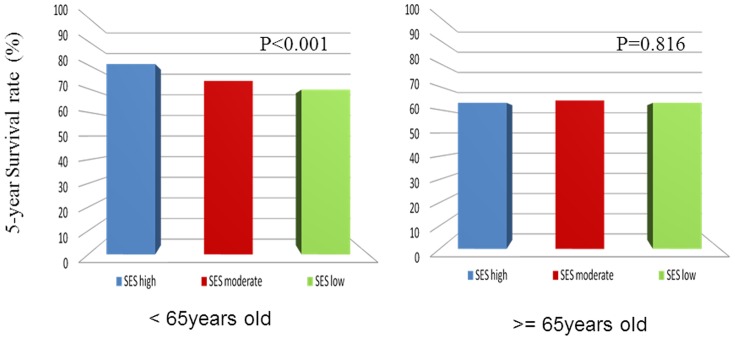
Figure 2. The effect of neighborhood SES on survival rates in gastric cancer patients aged less than 65 years old (2a) and aged 65 and above (2b).
Multilevel logistic regression model
The different medical provider was managed as a random intercept due to those patients might be treated in different providers with variant medical resources, capabilities, policies and physicians. A multilevel random intercept logistic regression model with the random effect of hospital showed that in high individual SES patients less than 65 years old, there was 68% risk reduction compared with those with lowest individual SES (adjusted odds ratio [OR]: 0.32; 95% CI: 0.17–0.61). Different neighborhood didn't seem to have significant effect on survival (Table 3). In aged patients, there was no statistical difference between those with different individual SES or those lived in different neighborhood (Table 3).
Table 3. Adjusted odds ratios of individual SES and neighborhood SES for mortality (gastric cancer with curative surgery, n = 3396).
| Variable | Age <65 years | Age≧65years | ||||
| Adjusted OR** | 95%CI* | P value | Adjusted OR** | 95%CI* | P value | |
| Individual socioeconomic status | ||||||
| Low | 1 | 1 | ||||
| Moderate | 0.72 | 0.40–1.29 | 0.278 | 0.90 | 0.61–1.33 | 0.624 |
| High | 0.32 | 0.17–0.61 | <0.001 | 1.17 | 0.49–2.80 | 0.713 |
| Neighborhood socioeconomic status | ||||||
| Disadvantaged | 1 | 1 | ||||
| Advantaged | 0.61 | 0.33–1.10 | 0.101 | 0.71 | 0.48–1.05 | 0.089 |
*Abbreviation: 95% CI, 95% confidence interval.
**Adjust for the patients' age, gender, adjuvant therapy, urbanization, geographic region, comorbidities, and hospital characteristics.
Discussion
This study found that, among gastric cancer patients in Taiwan younger than 65 years, those with high individual SES had a 68% lower risk of mortality than those with low SES, after adjusting for age at diagnosis, gender, comorbidities and hospital characteristics. The effect of SES was less evident in those 65 years and older. This study shows that the high individual SES lead to better survival of gastric cancer patients, even under a national health welfare and insurance system.
Previous studies analyzed SES status at either the individual or neighborhood level. Neighborhood socioeconomic context may affect health outcomes, after adjusting for individual SES [23]. Individual SES was reported to be related to survival independently of other factors, although the association was small [16], [24]. Kuwahara et al. reported a disparity in survival by occupation among gastric cancer patients in Japan, largely due to more advanced disease among the unemployed and manual laborers [25]. Patients with higher individual SES and therefore higher income may receive organized and opportunistic screening more often than those in lower income groups, thus permitting earlier detection [26]. In this study, we found that gastric cancer patients aged less than 65 years with low SES leads to the worst outcome, even in the patients under curative-intent therapy.
Deprived neighborhoods may indicate fewer medical resources, a more polluted environment, less social support and a poorer attitude toward health. In 1956, Torgersen et al. found that the prognosis for gastric cancer was related to the region of Oslo in which patients lived. Gastric cancer patients who lived in substandard housing areas had higher mortality rates [27], [28]. Fifty-six years later, Siemerink et al. used postal codes in The Netherlands to determine that neighborhood SES is an independent prognostic factor for gastric cancer survival [29]. In England, gastric cancer patients with lower neighborhood SES received gastrectomy, with no obvious association between survival and neighborhood SES [7], a hint that adequate treatment leads to similar survival rates in all patients. At the population level, disadvantaged SES neighborhoods may indicate inequities of medical resources, such as fewer hospitals and surgeons, which has been reported to impair disease treatment outcomes [30], [31].
Early diagnosis and multimodal treatment of gastric cancer improves outcomes, but overall mortality differs between rich and poor neighborhood [6]. Socioeconomic inequality is an independent factor influencing the prognosis of gastric cancer patients. Boyd reported that in Canada, the magnitude of the association between community income and survival would be weaker in Canada than in the U.S., because Ontario's universal, comprehensive, provincial health system might mitigate the adverse impact of poverty on cancer outcome by removing barriers to care for the poor [32]. To compare with neighborhood and individual effect in our study, the patients aged less than 65 years with high individual SES had the best survival. We also found that the difference was not statistically significant in community-income represented neighborhood SES. In patients older than 65 years, neither individual SES nor neighborhood SES indicates to different survival outcomes. Such observations indicate that, even under an universal health-insurance system, the patient with low individual SES has the worst survival rate of all patients. Gastric cancer patients need early detection and multimodal treatment to improve their outcomes. Patients with higher SES communicate more effectively with medical profession during the receipt of health care [33]. Patients living in disadvantaged neighborhoods also tend to have higher levels of social isolation, depression and occasional stress than patients living in neighborhoods with high SES [34].
This study has several limitations. First, the diagnosis of gastric cancer, as well as other comorbidities in this study, was garnered from ICD-9-CM codes on NHI claims. While this method of identification is not ideal, the NHI Bureau in Taiwan does randomly review the charts and interview patients to spot verify the accuracy of diagnosis. Another limitation was our lack of access to detailed information on gastric cancer stage, pattern of relapse and other risk factors, such as tobacco use and dietary habits. Curative and palliative treatment were also a limitation. Although we selected the patients with resection of stomach and lymph nodes dissection, exact extensiveness and type of dissection was not clear. However, given the robustness of the evidence, statistical analysis, and sensitivity analysis in this study, these limitations are unlikely to compromise our results.
Gastric cancer patients aged less than 65 years with low SES have poorer outcomes than those with high SES. For such patients, greater accessibility, education and information will likely improve their gastric cancer outcomes. Although the system of social welfare and national health insurance broke the health inequality between different neighborhoods SES, and provide for medical service to these low SES patients, the health gap associated with personal poverty remains a challenge.
Acknowledgments
This study is based in part on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health and managed by the National Health Research Institutes (registration number 101115). The interpretation and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Department of Health, or the National Health Research Institutes.
Funding Statement
The authors have no support or funding to report.
References
- 1. Jemal A, Bray F (2011) Center MM, Ferlay J, Ward E, et al (2011) Global cancer statistics. CA Cancer J Clin 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 2.(2012) 2011 Statistics of Causes of Death. In: Department of Health EY, R.O.C.(Taiwan), editor.
- 3. Kelley JR, Duggan JM (2003) Gastric cancer epidemiology and risk factors. J Clin Epidemiol 56: 1–9. [DOI] [PubMed] [Google Scholar]
- 4. Kunz PL, Gubens M, Fisher GA, Ford JM, Lichtensztajn DY, et al. (2012) Long-term survivors of gastric cancer: a California population-based study. J Clin Oncol 30: 3507–3515. [DOI] [PubMed] [Google Scholar]
- 5. Pereira L, Zamudio R, Soares-Souza G, Herrera P, Cabrera L, et al. (2012) Socioeconomic and nutritional factors account for the association of gastric cancer with Amerindian ancestry in a Latin American admixed population. PLoS One 7: e41200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coleman MP, Rachet B, Woods LM, Mitry E, Riga M, et al. (2004) Trends and socioeconomic inequalities in cancer survival in England and Wales up to 2001. Br J Cancer 90: 1367–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leigh Y, Seagroatt V, Goldacre M, McCulloch P (2006) Impact of socio-economic deprivation on death rates after surgery for upper gastrointestinal tract cancer. Br J Cancer 95: 940–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang CM, Su YC, Lai NS, Huang KY, Chien SH, et al. (2012) The combined effect of individual and neighborhood socioeconomic status on cancer survival rates. PLoS One 7: e44325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NHI (2008) NHI profile (updated 7 july 2008). http://www.nhi.gov.tw/english/webdata.asp?menu=11&menu_id=290&webdata_id=1884.
- 10. Tseng CH (2004) Mortality and causes of death in a national sample of diabetic patients in Taiwan. Diabetes Care 27: 1605–1609. [DOI] [PubMed] [Google Scholar]
- 11.Website BoNHI (2006) http://www.nhi.gov.tw/information/bulletin_file/421_0890036465-19.doc.
- 12. Roohan PJ, Bickell NA, Baptiste MS, Therriault GD, Ferrara EP, et al. (1998) Hospital volume differences and five-year survival from breast cancer. Am J Public Health 88: 454–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deyo RA, Cherkin DC, Ciol MA (1992) Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of Clinical Epidemiology 45: 613–619. [DOI] [PubMed] [Google Scholar]
- 14. Braaten T, Weiderpass E, Lund E (2009) Socioeconomic differences in cancer survival: the Norwegian Women and Cancer Study. BMC Public Health 9: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kwok J, Langevin SM, Argiris A, Grandis JR, Gooding WE, et al. (2010) The impact of health insurance status on the survival of patients with head and neck cancer. Cancer 116: 476–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cella DF, Orav EJ, Kornblith AB, Holland JC, Silberfarb PM, et al. (1991) Socioeconomic status and cancer survival. J Clin Oncol 9: 1500–1509. [DOI] [PubMed] [Google Scholar]
- 17. Lin HC, Chao PZ, Lee HC (2008) Sudden sensorineural hearing loss increases the risk of stroke: a 5-year follow-up study. Stroke 39: 2744–2748. [DOI] [PubMed] [Google Scholar]
- 18. Chou FH-C, Tsai K-Y, Su C-Y, Lee C-C (2011) The incidence and relative risk factors for developing cancer among patients with schizophrenia: A nine-year follow-up study. Schizophrenia research 129: 97–103. [DOI] [PubMed] [Google Scholar]
- 19. Lemstra M, Neudorf C, Opondo J (2006) Health disparity by neighbourhood income. Can J Public Health 97: 435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nakash O, Gerber Y, Goldbourt U, Benyamini Y, Drory Y, et al. (2013) Ethnicity and long-term prognosis after myocardial infarction: a population-based cohort study. Med Care 51: 137–143. [DOI] [PubMed] [Google Scholar]
- 21. Lee CC, Chien SH, Hung SK, Yang WZ, Su YC (2012) Effect of individual and neighborhood socioeconomic status on oral cancer survival. Oral Oncol 48: 253–261. [DOI] [PubMed] [Google Scholar]
- 22.Liu CYHY, Chung YL, Chen YJ, Weng WS, Liu JS, et al.. (2006) Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey (in Chinese). J Health Manage 1–22.
- 23. Pickett KE, Pearl M (2001) Multilevel analyses of neighbourhood socioeconomic context and health outcomes: a critical review. J Epidemiol Community Health 55: 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fontana V, Decensi A, Orengo MA, Parodi S, Torrisi R, et al. (1998) Socioeconomic status and survival of gastric cancer patients. Eur J Cancer 34: 537–542. [DOI] [PubMed] [Google Scholar]
- 25. Kuwahara A, Takachi R, Tsubono Y, Sasazuki S, Inoue M, et al. (2010) Socioeconomic status and gastric cancer survival in Japan. Gastric Cancer 13: 222–230. [DOI] [PubMed] [Google Scholar]
- 26. Lee HY, Park EC, Jun JK, Hahm MI, Jung KW, et al. (2010) Trends in socioeconomic disparities in organized and opportunistic gastric cancer screening in Korea (2005-2009). Cancer Epidemiol Biomarkers Prev 19: 1919–1926. [DOI] [PubMed] [Google Scholar]
- 27.Torgersen O (1956) The geographic distribution of gastric cancer in Oslo. Acta Pathol Microbiol Scand Suppl 39: 86. [DOI] [PubMed]
- 28. Petersen M, Torgersen O (1956) The epidemiology of gastric cancer in Oslo: cartographic analysis of census tracts and mortality rates of sub-standard housing areas. Br J Cancer 10: 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Siemerink EJ, Hospers GA, Mulder NH, Siesling S, van der Aa MA (2011) Disparities in survival of stomach cancer among different socioeconomic groups in North-East Netherlands. Cancer Epidemiol 35: 413–416. [DOI] [PubMed] [Google Scholar]
- 30. Chang CM, Huang KY, Hsu TW, Su YC, Yang WZ, et al. (2012) Multivariate analyses to assess the effects of surgeon and hospital volume on cancer survival rates: a nationwide population-based study in Taiwan. PLoS One 7: e40590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McCavit TL, Lin H, Zhang S, Ahn C, Quinn CT, et al. (2011) Hospital volume, hospital teaching status, patient socioeconomic status, and outcomes in patients hospitalized with sickle cell disease. Am J Hematol 86: 377–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boyd C, Zhang-Salomons JY, Groome PA, Mackillop WJ (1999) Associations between community income and cancer survival in Ontario, Canada, and the United States. J Clin Oncol 17: 2244–2255. [DOI] [PubMed] [Google Scholar]
- 33.Dixon A HJ, Murray R, Poteliakhoff E (2003) Is the NHS Equitable? A Review of Evidence. LSE Health and Social Care Discussion Paper.
- 34. Wang JJ, Snyder M, Kaas M (2001) Stress, loneliness, and depression in Taiwanese rural community-dwelling elders. Int J Nurs Stud 38: 339–347. [DOI] [PubMed] [Google Scholar]


