Abstract
Importance
Small studies suggest low dose dopamine or low dose nesiritide may enhance decongestion and preserve renal function in patients with acute heart failure and renal dysfunction; however, neither strategy has been rigorously tested.
Objective
To test the two independent hypotheses that when compared to placebo, addition of: (1) low dose dopamine (2 ug/kg/min); or (2) low dose nesiritide (0.005 ug/kg/min without bolus) to diuretic therapy will enhance decongestion and preserve renal function in patients with acute heart failure and renal dysfunction.
Design, Setting and Participants
Multicenter, double-blind, placebo-controlled randomized clinical trial (Renal Optimization Strategies Evaluation) of 360 hospitalized participants with acute heart failure and renal dysfunction (estimated glomerular filtration rate of 15–60 ml/min/1.73m2), randomized within 24 hours of admission. Participants were randomized from September 2010 to March 2013 across 26 sites in the United States and Canada.
Interventions
Participants were randomized in an open, 1:1 allocation ratio to the dopamine or nesiritide strategies. Within each strategy, participants were randomized in a double-blind, 2:1 ratio to active treatment or placebo. The dopamine (n=122) and nesiritide (n=119) groups were independently compared to the pooled placebo group (n=119).
Main outcome measures
Co-primary endpoints included 72-hour cumulative urine volume (decongestion endpoint) and the change in serum cystatin-C from enrollment to 72 hours (renal function endpoint).
Results
Compared to placebo, low dose dopamine had no significant effect on 72-hour cumulative urine volume (8524 ml [95% CI 7917 to 9131 ml] with dopamine vs. 8296 ml [95% CI 7762 to 8830 ml] with placebo, p=0.59) or on the change in cystatin-C (0.12 mg/L [95% CI 0.06 to 0.18 mg/L] with dopamine vs. 0.11 mg/L [95% CI 0.06 to 0.16 mg/L] with placebo, p=0.72). Similarly, low dose nesiritide had no significant effect on 72-hour cumulative urine volume (8574 ml [95% CI 8014 to 9134 ml] with nesiritide vs. 8296 ml [95% CI 7762 to 8830 ml] with placebo, p=0.49) or on the change in cystatin-C (0.07 mg/L [95% CI 0.01 to 0.13 mg/L] with nesiritide vs. 0.11 mg/L [95% CI 0.06 to 0.16 mg/L] with placebo, p=0.36). Compared to placebo, there was no effect of low dose dopamine or low dose nesiritide on secondary endpoints reflective of decongestion, renal function, or clinical outcomes.
Conclusions
In participants with acute heart failure and renal dysfunction, neither low dose dopamine nor low dose nesiritide enhanced decongestion or improved renal function when added to diuretic therapy.
A primary treatment goal in acute heart failure is to achieve adequate decongestion while avoiding renal dysfunction and other side effects.1,2 Patients with acute heart failure and moderate or severe renal dysfunction are at risk for inadequate decongestion and worsening renal function—both of which are associated with worse outcomes.3 Renal adjuvant therapies that enhance decongestion and preserve renal function during treatment of acute heart failure are needed.2,4
Dopamine is an endogenous catecholamine which at low doses (≤3 μg/kg/min), may selectively activate dopamine receptors and promote renal vasodilatation.5,6 Previous studies have suggested that the addition of low dose dopamine to diuretic therapy enhances decongestion and preserves renal function during diuretic therapy in acute heart failure; however, these studies were small with variable study designs and dopamine doses.7-9
B-type natriuretic peptide (BNP) is a cardiac peptide with vasodilating, renin and aldosterone inhibiting, natriuretic and diuretic properties.10 Nesiritide is human recombinant BNP and is approved for management of acute heart failure. The recommended dose is a 2 μg/kg bolus followed by 0.01 μg/kg/min infusion. This dose lowers blood pressure and atrial pressures and produces modest improvement in dyspnea, but does not favorably impact clinical outcomes or renal function, potentially due to its hypotensive effects.11-13 Small studies using low dose nesiritide (0.005 μg/kg/min without bolus) in acute heart failure and cardiac surgery patients have demonstrated favorable effects on urine output and renal function.14,15
The Renal Optimization Strategies Evaluation (ROSE) trial utilized a novel study design to test two independent hypotheses, namely, as compared to placebo, the addition of low dose dopamine (2 μg/kg/min; hypothesis I) or low dose nesiritide (0.005 μg/kg/min without bolus; hypothesis II) to diuretic therapy will enhance decongestion and preserve renal function in patients with acute heart failure and renal dysfunction.
METHODS
Study Oversight
All study participants provided written informed consent. The National Heart, Lung, and Blood Institute (NHLBI)-sponsored Heart Failure Clinical Research Network (HFN) investigators conceived, designed, and conducted the ROSE trial. The study protocol and the statistical analysis plan are available in Appendix 1 and 2. The trial protocol was approved by an NHLBI-appointed protocol review committee and data and safety monitoring board (Appendix 3), and by the institutional review board at each participating site. The Duke Clinical Research Institute served as the data coordinating center.
Study Design
The rationale and design of the ROSE trial have been previously described.16 Briefly, patients (n=360) hospitalized for the treatment of acute heart failure who had renal dysfunction (glomerular filtration rate [GFR] of 15 to 60 mL/min/1.73m2 as estimated by the Modification of Diet in Renal Disease equation) at admission were enrolled within 24 hours of admission. The diagnosis of acute heart failure was based on at least one symptom (dyspnea, orthopnea, or edema) and one sign of heart failure (rales, edema, ascites, or pulmonary vascular congestion on chest radiography) regardless of ejection fraction. Additional entry criteria are listed in Supplemental Table 1.
To test the two independent hypotheses while minimizing the number of patients requiring central line placement for dopamine administration, participants were initially randomized 1:1 in an open fashion to the nesiritide or dopamine strategies (Figure 1). Participants randomized to the dopamine strategy were randomized in a double-blind 2:1 ratio to low dose dopamine (2 μg/kg/min for 72 hours infused via local guideline stipulated vascular access) or placebo. Participants randomized to the nesiritide strategy were subsequently randomized in a double-blind 2:1 ratio to low dose nesiritide (0.005 μg/kg/min for 72 hours infused via peripheral intravenous access without initial bolus) or placebo. A permuted block randomization scheme stratified by clinical site was performed using an automated web-based system. The placebo patients were pooled across the two strategies.
Figure 1.
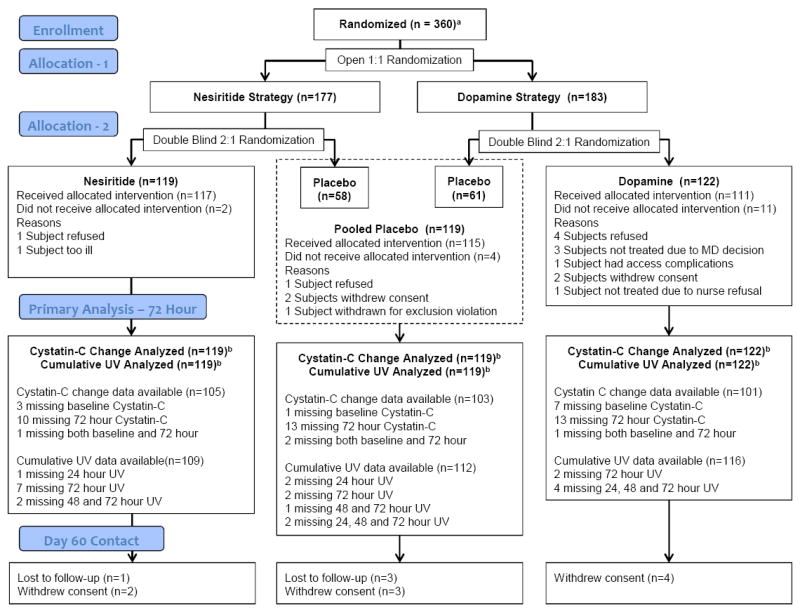
ROSE CONSORT Diagram
This figure displays the ROSE CONSORT diagram, from enrollment to randomization to final analysis population.
Abbreviations: UV, urine volume
aData on patients screened for eligibility is not available.
bThe primary endpoints were analyzed using multiple imputation techniques when data were unavailable for the endpoint.
All patients received open-label, intravenous loop diuretic treatment with a recommended total daily dose equal to 2.5x the total daily oral outpatient furosemide (or equivalent) dose at 7 days prior to admission18 up to a maximum of 600 mg/day. Patients naive to outpatient loop diuretics received 80 mg/day of intravenous furosemide. One-half of the total daily diuretic dose was administered as a bolus twice daily for at least 24 hours. Use of other medications and diuretic dosing after 24 hours were at the discretion of the clinician. All patients were placed on a 2000 mg sodium diet and 2000 cc fluid restriction in accordance to the Heart Failure Society of America 2010 comprehensive heart failure practice guideline.1 After the primary endpoint assessment at 72 hours, study drug was discontinued and subsequent treatment was at the clinician’s discretion.
As required in federally funded trials, participant self-identified race and ethnicity was recorded. After consent, participants underwent baseline assessment which included: history and physical examination for signs and symptoms of congestion, recording of cardiovascular medications, measurement of vital signs, phlebotomy for biomarkers (plasma creatinine [traceable to National Institute of Standards and Technology creatinine standard reference material], plasma cystatin-C, and plasma NT-proBNP; HFN Core Biomarker Laboratory, University of Vermont) and electrolytes (local laboratory), and completion of patient global well-being and dyspnea visual analogue scale assessments. Study assessments were repeated at 24, 48, and 72 hours. Daily 24-hour urine collections for urinary volume and urinary sodium excretion were performed for 72 hours. All patients had a telephone assessment of vital status and re-hospitalization at 60 and 180 days from randomization.
Trial Endpoints
The two co-primary endpoints were the 72-hour cumulative urinary volume as an index of decongestion efficacy and the change in cystatin-C from randomization to 72 hours as a measure of renal function preservation. A complete list of secondary endpoints is provided in Supplemental Table 2. Treatment failure was defined as the development of any one of the following during the 72 hours after randomization: development of type 1 cardio-renal syndrome as defined by an increase in serum creatinine of more than 0.3 mg/dL (26.5 μmol/L); worsening/persistent heart failure defined as the need for rescue therapy (additional intravenous vasoactive agent for heart failure treatment, ultrafiltration, or mechanical or respiratory support); or significant hypotension requiring discontinuation of study drug; significant tachycardia requiring discontinuation of study drug.
Statistical Analysis
Based on estimates of the variability in the primary outcome measures and missing data rates from previous studies,17-19 a total sample size of 360 participants would provide 90% power to detect a treatment difference of >1400 mL in cumulative urine volume at 72 hours and 88% power to detect a clinically significant difference (>0.3 mg/L) in the change in cystatin-C between active treatment versus placebo using a two-sided 0.025 level of significance. A difference of 0.3 mg/L in serum cystatin-C is considered to be clinically meaningful since a change of cystatin-C of ≥0.3 at 48 hours in patients hospitalized with acute heart failure was associated with a statistically significant; two fold increase in 180 day mortality.20 All treatment comparisons were performed according to the intention-to-treat principle. General linear models were used to examine the effect of treatment on each co-primary endpoint using a Type I error rate of 0.025 for each endpoint comparison. Treatment comparisons of change in cystatin-C were adjusted for the baseline cystatin-C. Multiple imputation was used to account for missing data. Sensitivity analyses based on complete data were also performed. For secondary endpoints, general linear models and logistic regression analysis were used with significance pre-specified at p<0.05. Time-to-event comparisons were performed using Kaplan-Meier curves, log-rank tests, and Cox proportional hazards models. The statistical analysis plan pre-specified several pertinent subgroup analyses using a conservative framework for subgroup interaction testing (interaction p-value <0.01). The current study was designed to compare both study drugs (low dose dopamine or low dose nesiritide) to placebo and not to each other, unless both treatments were superior to placebo. All statistical analyses were conducted using SAS version 9 or higher software (SAS Institute, Cary, NC).
RESULTS
Patient Population
A total of 360 participants were enrolled between September 2010 and March 2013 across 26 sites in the United States and Canada (Appendix 1). The baseline characteristics of the study groups were similar (Table 1). The median age of the study population was 70 years (interquartile range [IQR] 63.0 to 70.0 years), 73% were men and 21% were black. The median ejection fraction was 33% (IQR 22 to 50%) and 94 patients (26%) had an ejection fraction greater than 50%. A majority (67%) of patients had been hospitalized for heart failure within the previous 12 months. By design, patients had moderate to severe renal dysfunction with a median estimated GFR of 42 ml/min/1.73m2 (IQR; 31 to 54 ml/min/1.73m2). The distribution of participants among the following estimated GFR stratums: >60; 60–>45; 45–>30; ≤30 ml/min/1.73m2, was not statistically different in the dopamine, placebo, and nesiritide groups (dopamine 20%, 29%, 26%, 25%; placebo 18%, 34%, 36%, 12%; and nesiritide 17%, 38%, 34%, 11%, p=0.387).
Table 1.
Baseline Characteristics of the Study Participants According to Treatment Groupa
| Characteristic | Low dose Dopamine (n=122) | Placebo (Dopamine) (n=61) | Placebo Pooled (n=119) | Placebo (Nesiritide) (n=58) | Low dose Nesiritide (n=119) |
|---|---|---|---|---|---|
| Age, yrs | |||||
| Median | 71 | 72 | 70 | 67 | 69 |
| Interquartile range | 63-80 | 63-79 | 62-78 | 62-77 | 59-79 |
| Male sex, no. (%) | 84 (69) | 44 (72) | 89 (75) | 45 (78) | 91 (76) |
| White race, no. (%) | 90 (74) | 45 (74) | 91 (76) | 46 (79) | 91 (76) |
| Body mass index | |||||
| Median | 30.5 | 29.9 | 30.9 | 32.0 | 31.5 |
| Interquartile range | 25.4 -35.8 | 26.0-39.4 | 26.6-39.1 | 27.6-37.8 | 27.3-37.2 |
| Systolic blood pressure, mmHg | |||||
| Median | 114 | 117 | 116 | 115 | 114 |
| Interquartile range | 104-127 | 106-131 | 103-128 | 100-125 | 102-130 |
| Edema ≥2+ (scale 0-4+), no./total no. (%) | 83/120 (69) | 49/61 (80) | 89/119 (75) | 40/58 (68) | 79/118(67) |
| Orthopnea, no./total no. (%) | 103/116 (89) | 56/59 (95) | 105/116 (91) | 49/57 (86) | 99/111 (89) |
| Jugular venous pressure ≥8 cm water, no./total no. (%) | 116/117 (99) | 55/59 (93) | 105/113 (93) | 50/54 (92) | 106/113 (94) |
| Rales, no./total no. (%) | 72/120(60) | 31/61 (51) | 64/118 (54) | 33/57 (58) | 61/116(53) |
| Ejection fraction (%) | |||||
| Median | 35b | 33 | 30 | 25 | 35 |
| Interquartile range | 23-52 | 23-50 | 20-50 | 20-49 | 20-55 |
| Ejection fraction >50%, no. (%) | 30 (25) | 13 (21) | 26 (22) | 13 (22) | 38 (32) |
| Hospitalization for acute HF in previous yr, no. (%) | 79 (65) | 41(68) | 79 (67) | 38 (66) | 82 (69) |
| Ischemia as a cause of HF, no. (%) | 73 (60) | 37 (61) | 73 (61) | 36 (62) | 63(53) |
| Diabetes mellitus, no. (%) | 71 (58) | 41 (67) | 67 (56) | 26 (45) | 62 (52) |
| History of a-fib or flutter, no. (%) | 78 (64) | 38 (62) | 72 (61) | 34 (59) | 65 (55) |
| Hypertension, no. (%) | 97 (80) | 50 (82) | 101 (85) | 51 (88) | 100 (84) |
| ICD, no. (%) | 53(43) | 28 (46) | 56 (47) | 28 (48) | 48 (40) |
| Medications received before hospitalization | |||||
| ACE inhibitor or ARB, no. (%) | 53 (43) | 36 (59) | 61 (51) | 25 (43) | 65 (55) |
| Hydralazine, no. (%) | 26 (21) | 11 (18) | 23 (19) | 12 (21) | 19 (16) |
| Nitrates, no. (%) | 33 (27) | 18 (30) | 30 (25) | 12 (21) | 27 (23) |
| Beta-blocker, no. (%) | 98 (80) | 53 (87) | 103 (83) | 50 (86) | 99 (83) |
| Aldosterone antagonist, no. (%) | 34 (28) | 20 (33) | 36 (30) | 16 (28) | 39 (33) |
| Digoxin, no. (%) | 26 (21) | 18 (30) | 33 (28) | 15 (26) | 30 (25) |
| Outpatient furosemide-equivalent diuretic, no. (%) | 116 (95) | 57 (93) | 111 (93) | 54 (93) | 113(95) |
| Outpatient furosemide-equivalent dose, mg/day | |||||
| Median | 80 | 80 | 80 | 120 | 80 |
| Interquartile range | 60-140 | 60-130 | 40-160 | 40-240 | 60-160 |
| Plasma cystatin-C, mg/liter | |||||
| Median | 1.71 | 1.66 | 1.73 | 1.86 | 1.66 |
| Interquartile range | 1.33-2.16 | 1.35-2.20 | 1.43-2.16 | 1.50-2.11 | 1.48-2.13 |
| Creatinine, mg/dL | |||||
| Median | 1.59 | 1.63 | 1.64 | 1.70 | 1.65 |
| Interquartile range | 1.26-1.97 | 1.37-1.96 | 1.38-2.05 | 1.40-2.06 | 1.34-1.96 |
| eGFR, ml/min/1.73m2 | |||||
| Median | 45.5 | 45.1 | 44.5 | 34.0 | 43.8 |
| Interquartile range | 31.2-59.2 | 32.7-53.1 | 32.6-53.0 | 32.6-53.0 | 34.7-55.3 |
| Blood urea nitrogen, mg/dL | |||||
| Median | 35 | 37 | 36 | 34 | 38 |
| Interquartile range | 25-51 | 28-50 | 29-52 | 29-52 | 28-50 |
| NT-proBNP, pg/ml | |||||
| Median | 5760 | 4349 | 5046 | 6115 | 4245 |
| Interquartile range | 2958-11637 | 1687-9783 | 2319-10091 | 3172-10851 | 1847-9100 |
Abbreviations: ACE, angiotensin-converting enzyme; a-fib, atrial fibrillation; ARB, angiotensin-receptor blocker; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter defibrillator; no., number; NT-proBNP, N-terminal pro-brain natriuretic peptide; yrs, years
All p-values are greater than 0.05 for the comparisons of baseline characteristics across groups. To convert the values for blood urea nitrogen to millimoles per liter, multiply by 0.357. To convert the values for creatinine to micromoles per liter, multiply by 88.4.
n=120 with ejection fraction data
Study drug was not started in four placebo, eleven dopamine and two nesiritide patients (Consolidated Standards Of Reporting Trials [CONSORT] Diagram, Figure 1). The duration of study drug administration was not significantly different between groups (median [IQR] duration in hours; dopamine 72.0 [68.9 to 72.1]; placebo 71.6 [66.0 to 72.1]; nesiritide 72.0 [48.0 to 72.1]; p>0.05). Comparative analysis of the placebo participants from the low dose dopamine strategy versus low dose nesiritide strategy did not reveal any significant differences in baseline characteristics (Table 1) or co-primary endpoints.
Low Dose Dopamine Strategy
Co-primary Endpoints
There was no significant difference between low dose dopamine versus placebo in regards to the 72-hour cumulative urine volume (8524 ml [95% CI 7917 to 9131 ml] with dopamine vs. 8296 ml [95% CI 7762 to 8830 ml] with placebo; p=0.59) or in the change in cystatin-C from baseline to 72 hours (0.12 mg/L [95% CI 0.06 to 0.18 mg/L] with dopamine vs. 0.11 mg/L [95% CI 0.06 to 0.16 mg/L] with placebo; p=0.72; Table 2).
Table 2.
Co-Primary Endpoints: Effect of Low Dose Dopamine versus Placebo or Low Dose Nesiritide versus Placebo on Cumulative Urinary Volume over 72 Hours and Change in Cystatin-C from Baseline to 72 Hoursa
| DOPAMINE vs. PLACEBO | ||||
|---|---|---|---|---|
|
| ||||
| Placebo (n=119) | Dopamine (n=122) | Treatment Difference | p-value | |
| Cumulative urine volume from randomization to 72 hrs; ml | 8296 (7762, 8830) | 8524 (7917, 9131) | 229 (-714, 1171) | 0.59 |
| Change in cystatin-C from randomization to 72 hrs; mg/L | 0.11 (0.06, 0.16) | 0.12 (0.06, 0.18) | 0.01 (-0.08, 0.10) | 0.72 |
|
| ||||
| NESIRITIDE vs. PLACEBO | ||||
| Placebo (n=119) | Nesiritide (n=119) | Treatment Difference | p-value | |
|
| ||||
| Cumulative urine volume from randomization to 72 hrs; ml | 8296 (7762, 8830) | 8574 (8014, 9134) | 279 (-618, 1176) | 0.49 |
| Change in cystatin-C from randomization to 72 hrs; mg/L | 0.11 (0.06, 0.16) | 0.07 (0.01, 0.13) | -0.04 (-0.13, 0.05) | 0.36 |
Abbreviations: hrs, hours
Mean (95% confidence interval)
Secondary Endpoints
There were no significant between group differences in other decongestion or renal function endpoints (Table 3), the bivariate endpoint of change in serum creatinine and weight at 72 hours after randomization (p=0.89) or in the co-primary endpoints at 24 or 48 hours (Supplemental Table 3).
Table 3.
Secondary Endpoints: Low Dose Dopamine or Low Dose Nesiritide versus Placeboa
| DOPAMINE STRATEGY | Placebo (n=119) | Dopamine (n=122) | p-value | |
|---|---|---|---|---|
| Decongestion endpoints | ||||
| Cumulative urinary sodium excretion: randomization to 72 hrs, mmol | 540 (485,595) | 527 (473,581) | 0.75 | |
| Change in weight from randomization to 72 hrs, lbsb | -7.73 (-9.01, -6.44) | -7.40 (-8.83, -5.98) | 0.82 | |
| Change in NT-proBNP from randomization to 72 hrs, pg/ml | -2020 (-2724, -1316) | -2629 (-3470, -1789) | 0.43 | |
| Renal function endpoints | ||||
| Change in creatinine from randomization to 72 hrs, mg/dl | 0.02 (-0.4, 0.08) | 0.00 (-0.7, 0.08) | 0.78 | |
| Development of type 1 cardio-renal syndromec during 72 hrs, no. (%) | 24 (22) | 23 (22) | 0.88 | |
| Symptom relief endpoints | ||||
| Global wellbeing visual analog scale; AUC randomization to 72 hrs | 4704 (4442, 4965) | 4553 (4305, 4801) | 0.43 | |
| Dyspnea visual analog scale; AUC from randomization to 72 hrs | 4998 (4723, 5272) | 4936 (4660, 5211) | 0.92 | |
| Persistent or worsening HFd within 72 hrs, no. (%) | 5 (4) | 11 (9) | 0.14 | |
| Clinical outcomes | ||||
| Death from any cause within 72 hrs, no. (%) | 0 (0) | 0 (0) | N/A | |
| Treatment failuree within 72 hrs, no. (%) | 32 (28) | 35 (30) | 0.73 | |
| Study drug stopped/dose lowered due to hypotension, no./total no. (%) | 12/115 (10.4) | 1/111 (0.9) | 0.0008 | |
| Study drug stopped/dose lowered due to tachycardia, no./total no. (%) | 1/115 (0.9) | 8/111 (7.2) | 0.0007 | |
| Study drug stopped prior to 72 hours for any reason, no./total no. (%) | 29/115 (25) | 25/111 (23) | 0.72 | |
| Death through day 60, no. (%) | 12 (10) | 11 (9) | 0.78 | |
| Serious adverse event through day 60, no. (%) | 24 (20) | 30 (25) | 0.41 | |
| Days alive and free from HF hospitalization at 60 days, days | 46.6 (44.0, 49.2) | 47.3 (45.0, 49.6) | 0.68 | |
| Mortality at 180 days, % | 21.1 (14.7, 29.9) | 19.7 (13.5, 28.1) | 0.87 | |
|
| ||||
| NESIRITIDE STRATEGY | Placebo (n=119) | Nesiritide (n=119) | p-value | |
|
| ||||
| Decongestion endpoints | ||||
| Cumulative urinary sodium excretion: randomization to 72 hrs, mmol | 540 (485, 595) | 515 (468, 563) | 0.52 | |
| Change in weight from randomization to 72 hrs, lbsb | -7.73 (-9.01, -6.44) | -7.15 (-8.57, -5.73) | 0.67 | |
| Change in NT-proBNP from randomization to 72 hrs, pg/ml | -2020 (-2724, -1316) | -2273 (-3010, -1536) | 0.10 | |
| Renal function endpoints | ||||
| Change in creatinine from randomization to 72 hrs, mg/dl | 0.02 (-0.4, 0.08) | 0.02 (-0.06, 0.09) | 0.90 | |
| Development of type 1 cardio-renal syndromec during 72 hrs, no. (%) | 24 (22) | 28 (25) | 0.55 | |
| Symptom relief endpoints | ||||
| Global wellbeing visual analog scaled; AUC randomization to 72 hrs | 4704 (4442, 4965) | 4498 (4257, 4740) | 0.62 | |
| Dyspnea visual analog scale; AUC from randomization to 72 hrs | 4998 (4723, 5272) | 4831 (4592, 5070) | 0.89 | |
| Persistent or worsening HFe within 72 hrs, no. (%) | 5 (4) | 6 (5) | 0.77 | |
| Clinical outcomes | ||||
| Death from any cause within 72 hrs, no. (%) | 0 (0) | 0 (0) | N/A | |
| Treatment failuref within 72 hrs, no. (%) | 32 (28) | 48(40) | 0.04 | |
| Study drug stopped/dose lowered due to hypotension, no./total no. (%) | 12/115 (10.4) | 22/117 (18.8) | 0.07 | |
| Study drug stopped/dose lowered due to tachycardia, no./total no. (%) | 1/115 (0.9) | 0/117 (0) | 0.50 | |
| Study drug stopped prior to 72 hours for any reason, no./total no. (%) | 29/115 (25) | 29/117 (25) | 0.94 | |
| Death through day 60 | 12 (10) | 8 (7) | 0.35 | |
| Serious adverse event through day 60 | 24 (20) | 21 (18) | 0.62 | |
| Days alive and free from heart failure hospitalization at 60 days, days | 46.6 (44.0, 49.2) | 47.3 (44.9, 49.7) | 0.67 | |
| Mortality rate at 180 days, % | 21.1 (14.7, 29.9) | 19.1 (13.0, 27.6) | 0.74 | |
Abbreviations: AUC, area under the curve; CI, confidence interval; hrs, hours; All other abbreviations can be found in Table 1.
Mean (95% confidence interval).
To convert pounds to kilograms, divide by 2.2.
Type 1 cardio-renal syndrome was defined as increase in serum creatinine level of more than 0.3 mg per deciliter (26.5 μmol per liter) during the 72 hours after randomization.
Visual analog scale; Range 0-100; the lower score indicate worse score
Persistent or worsening HF was defined as need for rescue therapy (additional intravenous vasoactive agent for heart failure treatment, ultrafiltration, and mechanical circulatory or respiratory support) over 72 hours after randomization.
Treatment failure was defined as the development of any one of the following during the 72 hours after randomization: development of type 1 cardio-renal syndrome as defined above; worsening/persistent HF as defined above; significant hypotension requiring discontinuation of study drug; significant tachycardia requiring discontinuation of study drug.
There were zero deaths and no significant differences between groups in treatment failure at 72 hours. As compared to placebo, dopamine treated patients were less likely to have discontinuation or lowering of study drug dose due to hypotension and more likely to have discontinuation or lowering of study drug dose due to tachycardia. However, the overall rate of study drug discontinuation was similar between the two groups (Table 3).
At 60 days, there were no significant differences between groups in death, serious adverse events, days alive and free from heart failure hospitalization (Table 3), or the rate of the composite endpoint of death or rehospitalization or unscheduled visit for heart failure (p=0.53; Supplemental Figure 1A). At 180 days, there were no significant differences between groups in overall mortality (p=0.87; Supplemental Figure 2A).
The total dose of diuretic used during the 72 hour study period was not statistically different in the dopamine treatment group as compared to placebo group (median [IQR], furosemide equivalent dose; dopamine 460 [300–760] mg vs. placebo 600 [300–880] mg, p=0.099). Furthermore, the total fluid intake during the 72 hour study period was not statistically different between the dopamine-treated group and placebo group (median [IQR]; dopamine 3923.5 [3078.5–4687.5] ml vs. placebo 3823.5 [2982.0–4504.0] ml, p=0.448).
Subgroup Analysis
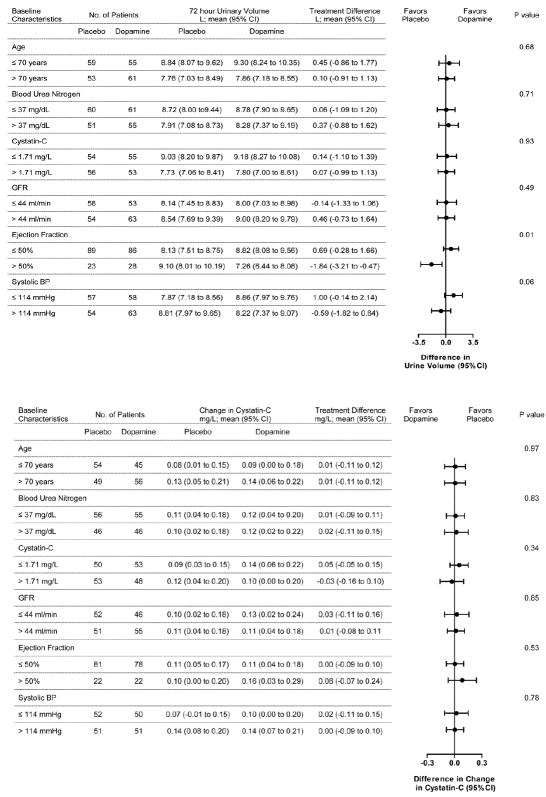
The effect of treatment group on the two co-primary endpoints was consistent across pre-specified subgroups with the exception that patients with preserved ejection fraction (>50) receiving dopamine had less urinary volume over 72 hours compared to placebo (interaction p=0.01, Figure 2).
Figure 2.
Dopamine Strategy Subgroup Analysis
This figure displays the subgroup analysis of treatment effect on: (A) cumulative urinary volume over 72 hours; and (B) change in cystatin-C from baseline to 72 hours with low dose dopamine versus placebo.
aNormalized to 1.73 m2
Low Does Nesiritide Strategy
Co-primary Endpoints
There was no significant difference between low-dose nesiritide versus placebo in the 72 hour cumulative urine volume (8574 ml [95 % CI 8014 to 9134 ml] with nesiritide vs. 8296 ml [7762 to 8830 ml] with placebo; p=0.49) or in the cystatin-C change from baseline to 72 hours (0.07 mg/L [95% CI 0.01 to 0.13] with nesiritide vs. 0.11 mg/L [0.06 to 0.16 mg/L] with placebo; p=0.36, Table 2).
Secondary Endpoints
There were no significant differences between groups in other decongestion or renal function endpoints (Table 3), the bivariate endpoint of change in serum creatinine and weight at 72 hours (p=0.77), or in the co-primary endpoints at 24 or 48 hours (Supplemental Table 3).
There were zero deaths and no significant differences between groups in symptom relief at 72 hours (Table 2). More nesiritide treated participants had treatment failure (Table 3), primarily due to a higher rate of study drug discontinuation for hypotension in nesiritide treated patients, although the incidence of study drug discontinuation for any cause was similar between treatment groups (Table 3).
At 60 days, there were no significant differences between groups in death, serious adverse events, days alive and free from heart failure hospitalization (Table 3), or the rate of the composite endpoint of death or rehospitalization or unscheduled visit for heart failure (p=0.16) (Supplemental Figure 1B). At 180 days, there were no significant differences between groups in overall mortality (p=0.74; Supplemental Figure 2B).
The total dose of diuretic used during the 72 hour study period was not statistically different in the nesiritide treatment group as compared to placebo group. (Median [IQR], furosemide equivalent dose; nesiritide 481 [280–760] mg versus placebo 600 [300–880] mg, p=0.16). Furthermore, the total fluid intake during the 72 hour study period was not statistically different between the nesiritide- and placebo-treated group (median [IQR]: nesiritide 3642.5 ml [2836.0–4751.0] vs. placebo 3823.5 ml [2982.0–4504.0], p=0.958).
Subgroup Analysis
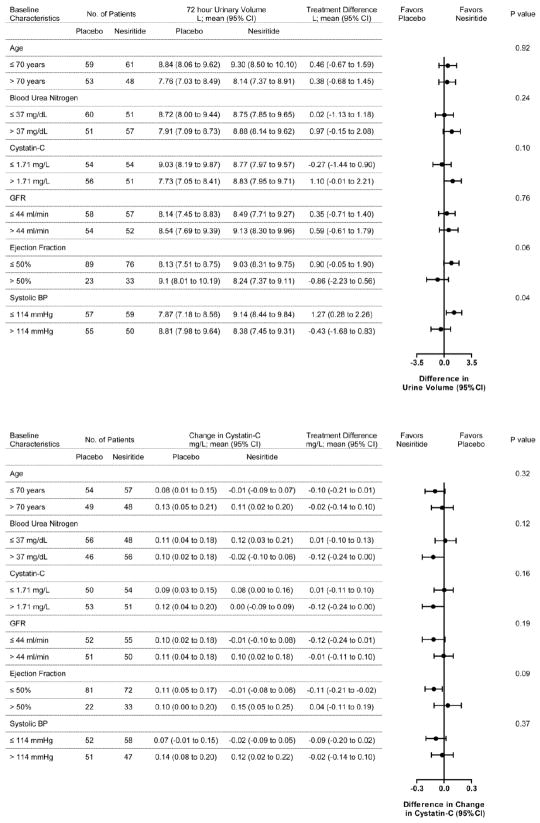
The effect of treatment group on the two co-primary endpoints was consistent across prespecified subgroups (Figure 3).
Figure 3.
Nesiritide Strategy Subgroup Analysis
This figure displays the subgroup analysis of treatment effect on: (A) cumulative urinary volume over 72 hours; and (B) change in cystatin-C from baseline to 72 hours with low dose nesiritide versus placebo.
aNormalized to 1.73 m2
COMMENT
There is an unmet need for renal-specific therapies that enhance decongestion and preserve renal function in patients with acute heart failure.2 In the current study, as compared to placebo, neither low dose dopamine nor low dose nesiritide enhanced decongestion or favorably affected renal function. The event rates of ROSE study participants are similar to other acute heart failure trials enrolling patients with renal dysfunction, suggesting that this cohort of patients is representative of contemporary acute heart failure patients with renal dysfunction.19,21,22 These findings do not support the use of low dose dopamine or low dose nesiritide as renal adjuvant therapies in patients with acute heart failure and renal dysfunction.
The current findings differ from those of previous small studies which suggested the benefits of low dose dopamine in acute heart failure.6-9 Of note, most previous studies did not target patients with renal dysfunction,6,7,9 included only patients with ejection fractions less than 40%,6-8 utilized higher dopamine doses,6,7,9 and employed variable durations of dopamine infusion.6-9 The duration of dopamine used in this study was longer than most previous studies to allow sustained effect over 72 hours. Importantly, in previous studies, different diuretic doses were used in the dopamine and placebo arms and diuretic doses were not calibrated to the patient’s outpatient diuretic requirements.7-9 The dose of dopamine used here is the most commonly used “renal-specific” dose and is postulated to reduce alpha- and beta-adrenergic mediated inotropic and pro-arrhythmic effects.5 However, the lower rates of hypotension and higher rates of tachycardia observed at this dose suggest incomplete renal specificity.
Guidelines for acute heart failure management state that use of low dose dopamine to improve diuresis and preserve renal function during diuretic therapy “may be considered,” but acknowledge the lack of data supporting efficacy.2 The current findings do not provide support for this strategy in the most clinically relevant acute heart failure population; namely, patients with renal dysfunction who are at risk for inadequate decongestion and worsening renal function.
To our knowledge, this is the first randomized trial of low dose nesiritide in patients with acute heart failure. At the recommended dose, nesiritide produces modest improvement in dyspnea, but does not favorably impact clinical outcomes, decongestion, or renal function.11,12-14,18,23,24 A case control study suggested that low dose nesiritide may provide renal-specific effects as it enhanced decongestion, spared diuretic dose and improved renal function without hypotension in acute heart failure.16 However, in ROSE, hypotension was still common in nesiritide treated patients and low dose nesiritide did not enhance decongestion or preserve renal function.
Observational studies indicate that dopamine and nesiritide are used in a significant portion of patients with acute heart failure and that their use is associated with longer length of stay, higher costs, and greater mortality.25 These data suggest that these agents are either harmful or preferentially used in high-risk patients. We tested these two agents at doses suggested to have less potential for adverse effects and to provide renal-specific actions in a high-risk acute heart failure population and found no evidence of renal specificity or clinical benefit.
Interestingly, in both the experimental strategies, there was a suggestion of differential treatment effect according to the ejection fraction or blood pressure level. The 72 hour cumulative urine volume was numerically lower with low dose dopamine as compared to placebo in subgroups of patients with higher ejection fraction or higher baseline blood pressure. With low dose nesiritide, the 72-hour cumulative urine volume was numerically higher as compared to placebo in subgroups of patients with lower ejection fraction or lower baseline blood pressure. At low dose, dopamine decreases renal and systemic vascular resistance in patients with heart failure and reduced ejection fraction.6 Similarly, even at low dose, nesiritide is a systemic vasodilator. Compared to patients with reduced ejection fraction, those with heart failure and preserved ejection fraction experience greater decreases in blood pressure and less increase in stroke volume in response to acute vasodilation, due to the unique ventricular vascular properties in this group.26 The ROSE trial was not powered to assess sub-group differences; therefore, these observations may be due to chance. However, these findings may suggest that further investigation of these or other acute heart failure therapies may need to assess the potential for differential responses in heart failure and preserved versus reduced ejection.
Our study had some important limitations. ROSE was not powered to detect differences in clinical events. Furthermore, ROSE allowed for the use of diuretics prior to randomization and adjustments in the diuretic dosing after 24 hours; these factors may have affected the co-primary endpoints. The estimation of glomerular filtration rate using the Modification of Diet in Renal Disease equation formula assumes that kidney function is stable—an assumption that may not hold in some of these study participants, as some may have experienced acute kidney injury in the context of their evolving heart failure exacerbation prior to admission.
Conclusions
In patients with acute decompensated heart failure and moderate or severe underlying renal dysfunction, neither low dose dopamine nor low dose nesiritide enhanced decongestion or improved renal function when added to diuretic therapy.
Supplementary Material
Acknowledgments
Drs. Lee and Anstrom had full access to all the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis.
Role of the Sponsor: National Heart, Lung, and Blood Institute (NHLBI) Program Officers provided advice on the trial design, analysis, and interpretation of the data, as well as the preparation, review, and approval of the manuscript and decision to submit the manuscript for publication. The NHLBI also appointed the protocol review committee and data and safety monitoring board.
Supported by grants from the NHLBI: Coordinating Center: U10 HL084904; Regional Clinical Centers: U01 HL084861, U10 HL110312, U109 HL110337, U01 HL084889, U01HL084890, U01 HL084891, U10 HL110342, U10 HL110262, U01 HL084931, U10 HL110297,U10 HL110302, U10 HL110309, U10 HL110336, U10 HL110338. This work is also supported by the National Center for Advancing Translational Sciences (NCATS): UL1TR000454, UL1 TR000135, UL1RR025008, UL1TR 000439; and the National Institute on Minority Health and Health Disparities (NIMHD): 8 U54 MD007588.
We thank the patients who participated in this study, the HFN site investigators and coordinators, the members of the HFN data and safety monitoring board and protocol review committee, and the NHLBI representatives. For a complete listing of the HFN investigators, see Appendix 1.
The authors are solely responsible for the content of this article, which does not necessarily represent the official views of the NHLBI or the National Institutes of Health.
Footnotes
Author Conflict of Interest Disclosures
HH Chen: Dr. Chen
KJ Anstrom: Dr. Anstrom
MM Givertz: Dr. Givertz
LW Stevenson: Dr. Stevenson
MJ Semigran: Dr. Semigran
SR Goldsmith: Dr. Goldsmith
BA Bart: Dr. Bart
DA Bull: Dr. Bull
J Stehlik: Dr. Stehlik
MM LeWinter: Dr. LeWinter
MA Konstam: Dr. Konstam
GS Huggins: Dr. Huggins
JL Rouleau: Dr. Rouleau
E O’Meara: Dr. O’Meara
WHW Tang: Dr. Tang
RC Starling: Dr. Starling
J Butler: Dr. Butler
A Deswal: Dr. Deswal
GM Felker: Dr. Felker
CM O’Connor: Dr. O’Connor
RE Bonita: Dr. Bonita
KB Margulies: Dr. Margulies
TP Cappola: Dr. Cappola
EO Ofili: Dr. Ofili
DL Mann: Dr. Mann
VG Davila-Roman: Dr. Davila-Roman
SE McNulty: Dr. McNulty
BA Borlaug: Dr. Borlaug
EJ Velazquez: Dr. Velazquez
KL Lee: Dr. Lee
MR Shah: Dr. Shah
AF Hernandez: Dr. Hernandez
E Braunwald: Dr. Braunwald
MM Redfield: Dr. Redfield
References
- 1.Lindenfeld J, Albert NM, Boehmer JP, et al. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16(6):e1–194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 doi: 10.1161/CIR.0b013e31829e8776. doi: 10.1161/CIR.0b013e31829e8776 3. [DOI] [PubMed] [Google Scholar]
- 3.Ronco C, McCullough P, Anker SD, et al. Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J. 2010;31(6):703–711. doi: 10.1093/eurheartj/ehp507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Givertz MM, Teerlink JR, Albert NM, et al. Acute decompensated heart failure: update on new and emerging evidence and directions for future research. J Card Fail. 2013;19(6):371–389. doi: 10.1016/j.cardfail.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Maskin CS, Ocken S, Chadwick B, LeJemtel TH. Comparative systemic and renal effects of dopamine and angiotensin-converting enzyme inhibition with enalaprilat in patients with heart failure. Circulation. 1985;72(4):846–852. doi: 10.1161/01.cir.72.4.846. [DOI] [PubMed] [Google Scholar]
- 6.Elkayam U, Ng TM, Hatamizadeh P, Janmohamed M, Mehra A. Renal vasodilatory action of dopamine in patients with heart failure: magnitude of effect and site of action. Circulation. 2008;117(2):200–205. doi: 10.1161/CIRCULATIONAHA.107.737106. [DOI] [PubMed] [Google Scholar]
- 7.Cotter G, Weissgarten J, Metzkor E, et al. Increased toxicity of high-dose furosemide versus low-dose dopamine in the treatment of refractory congestive heart failure. Clin Pharmacol Ther. 1997;62(2):187–193. doi: 10.1016/S0009-9236(97)90067-9. [DOI] [PubMed] [Google Scholar]
- 8.Varriale P, Mossavi A. The benefit of low-dose dopamine during vigorous diuresis for congestive heart failure associated with renal insufficiency: does it protect renal function? Clin Cardiol. 1997;20(7):627–630. doi: 10.1002/clc.4960200709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giamouzis G, Butler J, Starling RC, et al. Impact of dopamine infusion on renal function in hospitalized heart failure patients: results of the Dopamine in Acute Decompensated Heart Failure (DAD-HF) Trial. J Card Fail. 2010;16(2):922–930. doi: 10.1016/j.cardfail.2010.07.246. [DOI] [PubMed] [Google Scholar]
- 10.Chen HH, Burnett JC., Jr The natriuretic peptides in heart failure: diagnostic and therapeutic potentials. Proc Assoc Am Physicians. 1999;111(5):406–416. doi: 10.1111/paa.1999.111.5.406. [DOI] [PubMed] [Google Scholar]
- 11.Publication Committee for the VMAC Investigators (Vasodilatation in the Management of Acute CHF) Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;287(12):1531–1540. doi: 10.1001/jama.287.12.1531. [DOI] [PubMed] [Google Scholar]
- 12.O’Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365(1):32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 13.Chen HH, Cataliotti A, Schirger JA, Martin FL, Harstad LK, Burnett JC., Jr Local renal delivery of a natriuretic peptide a renal-enhancing strategy for B-type natriuretic peptide in overt experimental heart failure. J Am Coll Cardiol. 2009;53(15):1302–1308. doi: 10.1016/j.jacc.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen HH, Sundt TM, Cook DJ, Heublein DM, Burnett JC., Jr Low dose nesiritide and the preservation of renal function in patients with renal dysfunction undergoing cardiopulmonary-bypass surgery: a double-blind placebo-controlled pilot study. Circulation. 2007;116(11Suppl):I134–138. doi: 10.1161/CIRCULATIONAHA.106.697250. [DOI] [PubMed] [Google Scholar]
- 15.Riter HG, Redfield MM, Burnett JC, Chen HH. Nonhypotensive low-dose nesiritide has differential renal effects compared with standard-dose nesiritide in patients with acute decompensated heart failure and renal dysfunction. J Am Coll Cardiol. 2006;47(11):2334–2335. doi: 10.1016/j.jacc.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Chen HH, AbouEzzeddine OF, Anstrom KJ, et al. Targeting the kidney in acute heart failure: can old drugs provide new benefit? Renal Optimization Strategies Evaluation in Acute Heart Failure (ROSE AHF) Trial. Cir Heart Fail. 2013;6(5):1087–1094. doi: 10.1161/CIRCHEARTFAILURE.113.000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364(9):797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Owan TE, Chen HH, Frantz RP, et al. The effects of nesiritide on renal function and diuretic responsiveness in acutely decompensated heart failure patients with renal dysfunction. J Card Fail. 2008;14(4):267–275. doi: 10.1016/j.cardfail.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bart BA, Goldsmith SR, Lee KL, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. New Engl J Med. 2012;367(24):2296–2304. doi: 10.1056/NEJMoa1210357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metra M, Cotter G, Davison BA, et al. Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the Relaxin in Acute Heart Failure (RELAX-AHF) development program: correlation with outcomes. J Am Coll Cardiol. 2013;61(2):196–206. doi: 10.1016/j.jacc.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Teerlink JR, Cotter G, Davison BA, et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2013;381(9860):29–39. doi: 10.1016/S0140-6736(12)61855-8. [DOI] [PubMed] [Google Scholar]
- 22.Massie BM, O’Connor CM, Metra M, et al. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. New Engl J Med. 2010;363(15):1419–1428. doi: 10.1056/NEJMoa0912613. [DOI] [PubMed] [Google Scholar]
- 23.Gottlieb SS, Stebbins A, Voors AA, et al. Effects of Nesiritide and Predictors of Urine Output in Acute Decompensated Heart Failure: results from ASCEND-HF (Acute Study of Clinical Effectiveness of Nesiritide and Decompensated Heart Failure) J Am Coll Cardiol. 2013;62(13):1177–1183. doi: 10.1016/j.jacc.2013.04.073. [DOI] [PubMed] [Google Scholar]
- 24.Wang DJ, Dowling TC, Meadows D, et al. Nesiritide does not improve renal function in patients with chronic heart failure and worsening serum creatinine. Circulation. 2004;110(12):1620–1625. doi: 10.1161/01.CIR.0000141829.04031.25. [DOI] [PubMed] [Google Scholar]
- 25.Hauptman PJ, Swindle J, Burroughs TE, Schnitzler MA. Resource utilization in patients hospitalized with heart failure: insights from a contemporary national hospital database. Am Heart J. 2008;155(6):978–985.e1. doi: 10.1016/j.ahj.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 26.Schwartzenberg S, Redfield MM, From AM, Sorajja P, Nishimura RA, Borlaug BA. Effects of vasodilation in heart failure with preserved or reduced ejection fraction implications of distinct pathophysiologies on response to therapy. J Am Coll Cardiol. 2012;59(5):442–451. doi: 10.1016/j.jacc.2011.09.062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.