Abstract
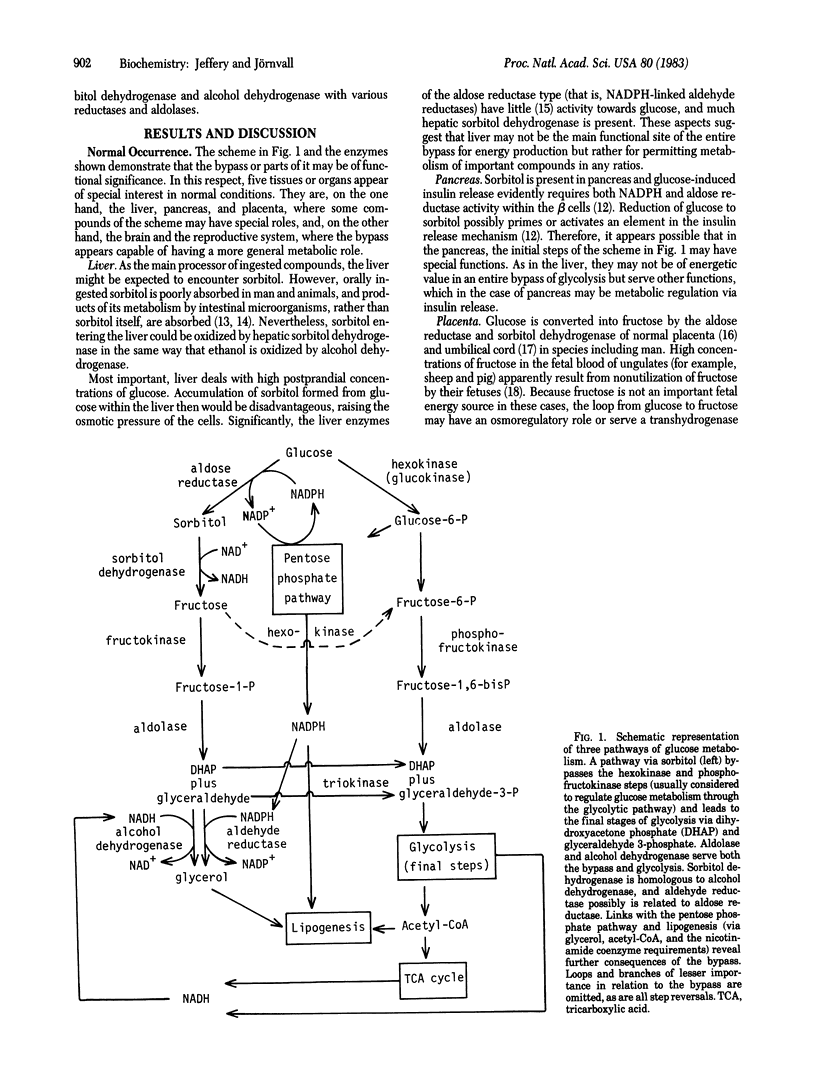
A pathway from glucose via sorbitol bypasses the control points of hexokinase and phosphofructokinase in glucose metabolism. It also may produce glycerol, linking the bypass to lipid synthesis. Utilization of this bypass is favored by a plentiful supply of glucose--hence, conditions under which glycolysis also is active. The bypass further involves oxidation of NADPH, so the pentose phosphate pathway and the bypass are mutually facilitative. Possible consequences in different organs under normal and pathological, especially diabetic, conditions are detailed. Enzymes with related structures (for example, sorbitol dehydrogenase and alcohol dehydrogenase, and possibly, aldehyde reductase and aldose reductase, respectively) are linked functionally by this scheme. Some enzymes of the bypass also feature in glycolysis (aldolase and alcohol dehydrogenase), and these enzymes, with the reductases involved, are proteins known to occur in different classes or multiple isozyme forms. Two of the enzymes (aldolase and alcohol dehydrogenase) both involve classes with and without a catalytic metal (zinc). The existence of parallel pathways and the occurrence of similar enzymic steps in one pathway may help to explain the abundance and multiplicity of enzymes such as reductases, aldolases, and alcohol dehydrogenases.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry M. N., Kun E. Ethanol oxidation by isolated rat-liver cells. Stimulatory effects of fructose. Eur J Biochem. 1978 Aug 15;89(1):237–241. doi: 10.1111/j.1432-1033.1978.tb20918.x. [DOI] [PubMed] [Google Scholar]
- Bosron W. F., Li T. K., Dafeldecker W. P., Vallee B. L. Human liver pi-alcohol dehydrogenase: kinetic and molecular properties. Biochemistry. 1979 Mar 20;18(6):1101–1105. doi: 10.1021/bi00573a026. [DOI] [PubMed] [Google Scholar]
- Bosron W. F., Li T. K., Vallee B. L. New molecular forms of human liver alcohol dehydrogenase: isolation and characterization of ADHIndianapolis. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5784–5788. doi: 10.1073/pnas.77.10.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachet E. A. Presence of the complete sorbitol pathway in the human normal umbilical cord tissue. Biol Neonate. 1973;23(3):314–323. doi: 10.1159/000240609. [DOI] [PubMed] [Google Scholar]
- Branlant G., Biellmann J. F. Purification and some properties of aldehyde reductases from pig liver. Eur J Biochem. 1980 Apr;105(3):611–621. doi: 10.1111/j.1432-1033.1980.tb04539.x. [DOI] [PubMed] [Google Scholar]
- Britton H. G., Huggett A. S., Nixon D. A. Carbohydrate metabolism in the sheep placenta. Biochim Biophys Acta. 1967 Apr 25;136(3):426–440. doi: 10.1016/0304-4165(67)90002-5. [DOI] [PubMed] [Google Scholar]
- Christensen U., Tüchsen E., Andersen B. Initial velocity and product inhibition studies on L-iditol:NAD oxidoreductase. Acta Chem Scand B. 1975;29(1):81–87. doi: 10.3891/acta.chem.scand.29b-0081. [DOI] [PubMed] [Google Scholar]
- Chylack L. T., Jr, Kinoshita J. H. A biochemical evaluation of a cataract induced in a high-glucose medium. Invest Ophthalmol. 1969 Aug;8(4):401–412. [PubMed] [Google Scholar]
- Clements R. S., Jr, Winegrad A. I. Modulation of mammalian polyol: NADP oxidoreductase activity by ADP and ATP. Biochem Biophys Res Commun. 1969 Sep 10;36(6):1006–1012. doi: 10.1016/0006-291x(69)90304-0. [DOI] [PubMed] [Google Scholar]
- Corder C. N., Braughler J. M., Culp P. A. Quantitative histochemistry of the sorbitol pathway in glomeruli and small arteries of human diabetic kidney. Folia Histochem Cytochem (Krakow) 1979;17(2):137–145. [PubMed] [Google Scholar]
- Gabbay K. H. Hyperglycemia, polyol metabolism, and complications of diabetes mellitus. Annu Rev Med. 1975;26:521–536. doi: 10.1146/annurev.me.26.020175.002513. [DOI] [PubMed] [Google Scholar]
- Gabbay K. H., O'Sullivan J. B. The sorbitol pathway. Enzyme localization and content in normal and diabetic nerve and cord. Diabetes. 1968 May;17(5):239–243. doi: 10.2337/diab.17.5.239. [DOI] [PubMed] [Google Scholar]
- Gabbay K. H., Tze W. J. Inhibition of glucose-induced release of insulin by aldose reductase inhibitors. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1435–1439. doi: 10.1073/pnas.69.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensel R., Mayr U., Stetter K. O., Kandler O. Comparative studies of lactic acid dehydrogenases in lactic acid bacteria. I. Purification and kinetics of the allosteric L-lactic acid dehydrogenase from Lactobacillus casei ssp. casei and Lactobacillus curvatus. Arch Microbiol. 1977 Feb 4;112(1):81–93. doi: 10.1007/BF00446658. [DOI] [PubMed] [Google Scholar]
- Hoffee P., Snyder P., Sushak C., Jargiello P. Deoxyribose-5-P aldolase: subunit structure and composition of active site lysine region. Arch Biochem Biophys. 1974 Oct;164(2):736–742. doi: 10.1016/0003-9861(74)90087-3. [DOI] [PubMed] [Google Scholar]
- Jedziniak J. A., Chylack L. T., Jr, Cheng H. M., Gillis M. K., Kalustian A. A., Tung W. H. The sorbitol pathway in the human lens: aldose reductase and polyol dehydrogenase. Invest Ophthalmol Vis Sci. 1981 Mar;20(3):314–326. [PubMed] [Google Scholar]
- Jörnvall H. Differences between alcohol dehydrogenases. Structural properties and evolutionary aspects. Eur J Biochem. 1977 Feb;72(3):443–452. doi: 10.1111/j.1432-1033.1977.tb11268.x. [DOI] [PubMed] [Google Scholar]
- Jörnvall H., Persson M., Jeffery J. Alcohol and polyol dehydrogenases are both divided into two protein types, and structural properties cross-relate the different enzyme activities within each type. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4226–4230. doi: 10.1073/pnas.78.7.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavolas H. J., Orr J. C., Engel L. L. Human placental 17 beta-estradiol dehydrogenase. IV. Differentiation of 17 beta-estradiol-activated transhydrogenase from the transhydrogenase function of 17 beta-estradiol dehydrogenase. J Biol Chem. 1969 Aug 25;244(16):4413–4421. [PubMed] [Google Scholar]
- Levy H. R. Glucose-6-phosphate dehydrogenases. Adv Enzymol Relat Areas Mol Biol. 1979;48:97–192. doi: 10.1002/9780470122938.ch3. [DOI] [PubMed] [Google Scholar]
- McClain C. J., Kromhout J. P., Zieve L., Duane W. C. Effect of sorbitol on psychomotor function: its use in alcoholic cirrhosis. Arch Intern Med. 1981 Jun;141(7):901–903. [PubMed] [Google Scholar]
- Moonsammy G. I., Stewart M. A. Purification and properties of brain aldose reductase and L-hexonate dehydrogenase. J Neurochem. 1967 Dec;14(12):1187–1193. doi: 10.1111/j.1471-4159.1967.tb06166.x. [DOI] [PubMed] [Google Scholar]
- Parés X., Vallee B. L. New human liver alcohol dehydrogenase forms with unique kinetic characteristics. Biochem Biophys Res Commun. 1981 Jan 15;98(1):122–130. doi: 10.1016/0006-291x(81)91878-7. [DOI] [PubMed] [Google Scholar]
- Penhoet E., Rajkumar T., Rutter W. J. Multiple forms of fructose diphosphate aldolase in mammalian tissues. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1275–1282. doi: 10.1073/pnas.56.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDS O. C., RUTTER W. J. Preparation and properties of yeast aldolase. J Biol Chem. 1961 Dec;236:3177–3184. [PubMed] [Google Scholar]
- Randall G. C., L'Ecuyer C. Tissue glycogen and blood glucose and fructose levels in the pig fetus during the second half of gestation. Biol Neonate. 1976;28(1-2):74–82. doi: 10.1159/000240806. [DOI] [PubMed] [Google Scholar]
- Rehg J. E., Torack R. M. Partial purification and characterization of sorbitol dehydrogenase from rat brain. J Neurochem. 1977 Mar;28(3):655–660. doi: 10.1111/j.1471-4159.1977.tb10438.x. [DOI] [PubMed] [Google Scholar]
- Ris M. M., von Wartburg J. P. Heterogeneity of NADPH-dependent aldehyde reductase from human and rat brain. Eur J Biochem. 1973 Aug 1;37(1):69–77. doi: 10.1111/j.1432-1033.1973.tb02958.x. [DOI] [PubMed] [Google Scholar]
- SHIRASU H. EFFECT OF SYNTHETIC ESTROGENS ON NAD(P) TRANSHYDROGENASE OF RAT PLACENTA. J Biochem. 1964 Apr;55:462–463. doi: 10.1093/oxfordjournals.jbchem.a127908. [DOI] [PubMed] [Google Scholar]
- Schell Dompert E., Siebert G. Metabolism of Sorbitol in the intact organism. Hoppe Seylers Z Physiol Chem. 1980 Jul;361(7):1069–1075. doi: 10.1515/bchm2.1980.361.2.1069. [DOI] [PubMed] [Google Scholar]
- Servo C., Pitkänen E. Variation in polyol levels in cerebrospinal fluid and serum in diabetic patients. Diabetologia. 1975 Dec;11(6):575–580. doi: 10.1007/BF01222109. [DOI] [PubMed] [Google Scholar]
- Sheaff C. M., Doughty C. C. Physical and kinetic properties of homogenous bovine lens aldose reductase. J Biol Chem. 1976 May 10;251(9):2696–2702. [PubMed] [Google Scholar]
- Takazakura E., Nakamoto Y., Hayakawa H., Kawai K., Muramoto S. Onset and progression of diabetic glomerulosclerosis; a prospective study based on serial renal biopsies. Diabetes. 1975 Jan;24(1):1–9. doi: 10.2337/diab.24.1.1. [DOI] [PubMed] [Google Scholar]
- Tulsiani D. R., Touster Resolution and partial characterization of two aldehyde reductases of mammalian liver. J Biol Chem. 1977 Apr 25;252(8):2545–2550. [PubMed] [Google Scholar]
- Van Schaftingen E., Jett M. F., Hue L., Hers H. G. Control of liver 6-phosphofructokinase by fructose 2,6-bisphosphate and other effectors. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3483–3486. doi: 10.1073/pnas.78.6.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma S. D., Schocket S. S., Richards R. D. Implications of aldose reductase in cataracts in human diabetes. Invest Ophthalmol Vis Sci. 1979 Mar;18(3):237–241. [PubMed] [Google Scholar]
- Ward J. D., Baker R. W., Davis B. H. Effect of blood sugar control on the accumulation of sorbitol and fructose in nervous tissues. Diabetes. 1972 Dec;21(12):1173–1178. doi: 10.2337/diab.21.12.1173. [DOI] [PubMed] [Google Scholar]
- Wermuth B. Purification and properties of an NADPH-dependent carbonyl reductase from human brain. Relationship to prostaglandin 9-ketoreductase and xenobiotic ketone reductase. J Biol Chem. 1981 Feb 10;256(3):1206–1213. [PubMed] [Google Scholar]
- Yoshida A., Impraim C. C., Huang I. Y. Enzymatic and structural differences between usual and atypical human liver alcohol dehydrogenases. J Biol Chem. 1981 Dec 10;256(23):12430–12436. [PubMed] [Google Scholar]


