Abstract
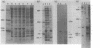
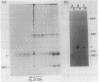
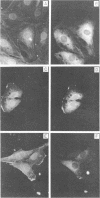
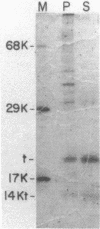
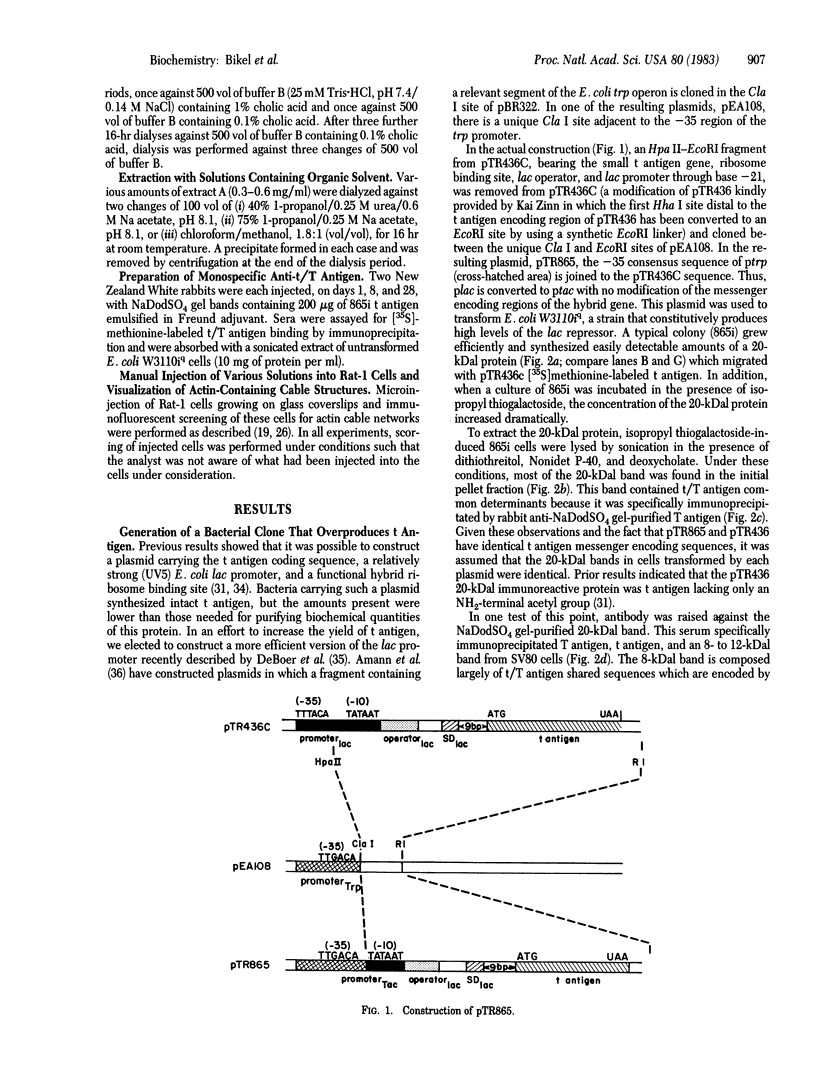
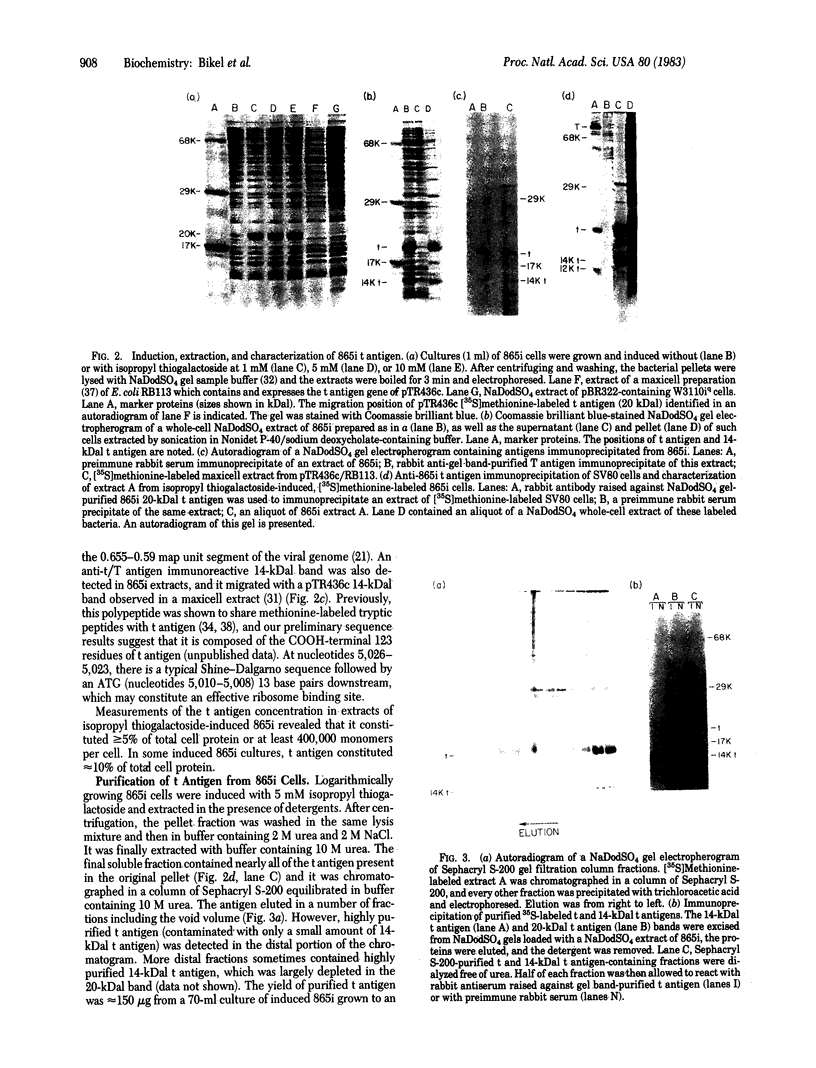
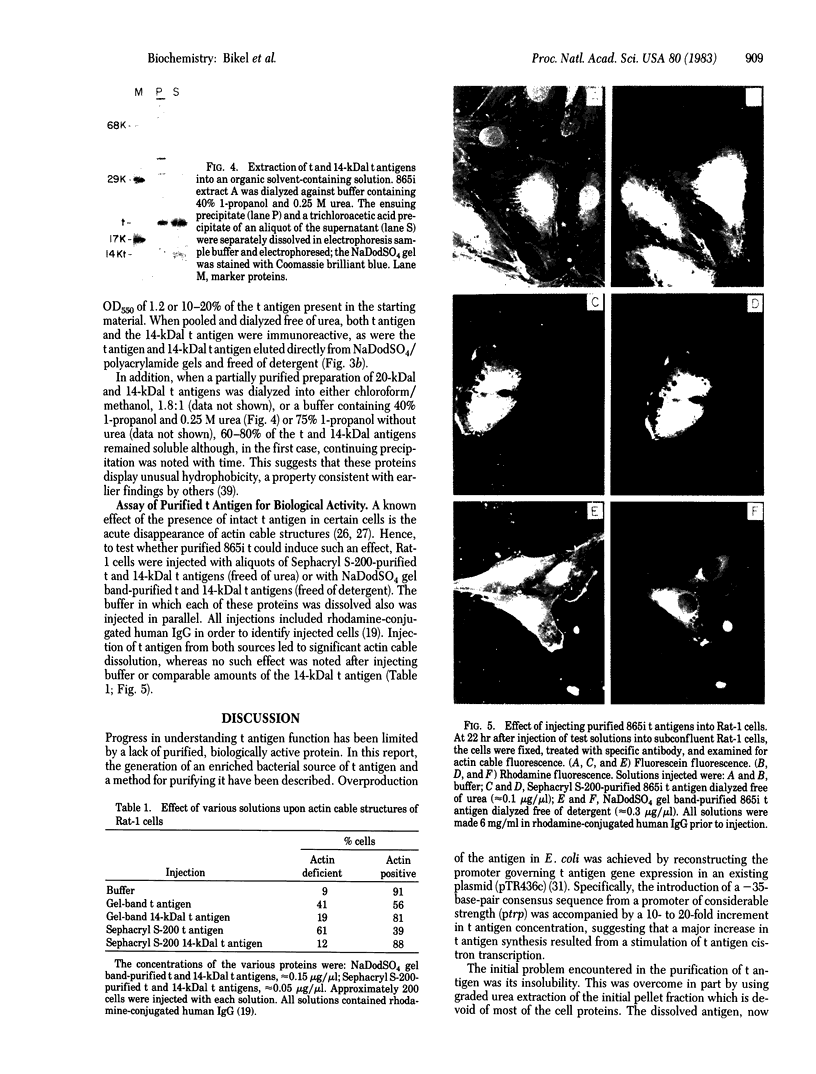
The simian virus 40 small tumor antigen (t antigen) gene has been cloned downstream from a hybrid Escherichia coli trp-lac promoter and a suitable ribosome binding site. A bacterial clone (865i) transformed by such a plasmid (pTR865) expresses this gene and, under optimal conditions, can produce greater than or equal to 5% of its total protein as t antigen. Soluble extracts of such a clone were relatively depleted in t antigen, which was found in the initial pellet fraction. The protein was recovered from this fraction in a significantly purified form by extraction with urea-containing buffer. After gel filtration of such t antigen-enriched solutions, highly purified protein was obtained. When either this fraction (freed of urea) or NaDodSO4 gel-purified 865i t antigen (rendered free of detergent) was injected into untransformed rat cells, dissolution of intracellular actin cable networks was observed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N. Human platelet-derived growth factor (PDGF): purification of PDGF-I and PDGF-II and separation of their reduced subunits. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7314–7317. doi: 10.1073/pnas.78.12.7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouck N., Beales N., Shenk T., Berg P., di Mayorca G. New region of the simian virus 40 genome required for efficient viral transformation. Proc Natl Acad Sci U S A. 1978 May;75(5):2473–2477. doi: 10.1073/pnas.75.5.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Butel J. S. Role of simian virus 40 gene A function in maintenance of transformation. J Virol. 1975 Mar;15(3):619–635. doi: 10.1128/jvi.15.3.619-635.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. DNA infectivity and the induction of host DNA synthesis with temperature-sensitive mutants of simian virus 40. J Virol. 1975 Jan;15(1):145–150. doi: 10.1128/jvi.15.1.145-150.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derom C., Gheysen D., Fiers W. High-level synthesis in Escherichia coli of the SV40 small-t antigen under control of the bacteriophage lambda pL promoter. Gene. 1982 Jan;17(1):45–54. doi: 10.1016/0378-1119(82)90099-3. [DOI] [PubMed] [Google Scholar]
- Feunteun J., Kress M., Gardes M., Monier R. Viable deletion mutants in the simian virus 40 early region. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4455–4459. doi: 10.1073/pnas.75.9.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluck M. M., Benjamin T. L. Comparisons of two early gene functions essential for transformation in polyoma virus and SV-40. Virology. 1979 Jul 15;96(1):205–228. doi: 10.1016/0042-6822(79)90185-5. [DOI] [PubMed] [Google Scholar]
- Fresno M., McVay-Boudreau L., Cantor H. Antigen-specific T lymphocyte clones. III. Papain splits purified T suppressor molecules into two functional domains. J Exp Med. 1982 Apr 1;155(4):981–993. doi: 10.1084/jem.155.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann T., Doolittle R. F., Walter G. Amino acid sequence homology between polyoma and SV40 tumour antigens deduced from nucleotide sequences. Nature. 1978 Jul 20;274(5668):291–293. doi: 10.1038/274291a0. [DOI] [PubMed] [Google Scholar]
- Frisque R. J., Rifkin D. B., Topp W. C. Requirement for the large T and small T proteins of SV40 in the maintenance of the transformed state. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):325–331. doi: 10.1101/sqb.1980.044.01.037. [DOI] [PubMed] [Google Scholar]
- Graessmann A., Graessmann M., Tjian R., Topp W. C. Simian virus 40 small-t protein is required for loss of actin cable networks in rat cells. J Virol. 1980 Mar;33(3):1182–1191. doi: 10.1128/jvi.33.3.1182-1191.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscott J. B., Defendi V. Simian virus 40 gene A regulation of cellular DNA synthesis. II. In nonpermissive cells. J Virol. 1981 Feb;37(2):802–812. doi: 10.1128/jvi.37.2.802-812.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Setlow V. P., Chepelinsky A. B., Seif R., Lewis A. M., Jr, Scher C. D., Stiles C. D., Avila J. Roles of the T antigens in transformation by SV40. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):311–324. doi: 10.1101/sqb.1980.044.01.036. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Setlow V. P., Edwards C. A., Vembu D. The roles of the simian virus 40 tumor antigens in transformation of Chinese hamster lung cells. Cell. 1979 Jul;17(3):635–643. doi: 10.1016/0092-8674(79)90271-x. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. Simian virus 40 gene A function and maintenance of transformation. J Virol. 1975 Mar;15(3):636–644. doi: 10.1128/jvi.15.3.636-644.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prives C., Gilboa E., Revel M., Winocour E. Cell-free translation of simian virus 40 early messenger RNA coding for viral T-antigen. Proc Natl Acad Sci U S A. 1977 Feb;74(2):457–461. doi: 10.1073/pnas.74.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassoulzadegan M., Perbal B., Cuzin F. Growth control in simian virus 40-transformed rat cells: temperature-independent expression of the transformed phenotype in tsA transformants derived by agar selection. J Virol. 1978 Oct;28(1):1–5. doi: 10.1128/jvi.28.1.1-5.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T. M., Bikel I., Yocum R. R., Livingston D. M., Ptashne M. Synthesis of simian virus 40 t antigen in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5596–5600. doi: 10.1073/pnas.76.11.5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H., Figge J., Bladon M. T., Chen L. B., Ellman M., Bikel I., Farrell M., Livingston D. M. Role of small t antigen in the acute transforming activity of SV40. Cell. 1982 Sep;30(2):469–480. doi: 10.1016/0092-8674(82)90244-6. [DOI] [PubMed] [Google Scholar]
- Rundell K., Major E. O., Lampert M. Association of cellular 56,000- and 32,000-molecular-weight protein with BK virus and polyoma virus t-antigens. J Virol. 1981 Mar;37(3):1090–1093. doi: 10.1128/jvi.37.3.1090-1093.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundell K. Presence in growth-arrested cells of cellular proteins that interact with simian virus 40 small-t antigen. J Virol. 1982 Jun;42(3):1135–1137. doi: 10.1128/jvi.42.3.1135-1137.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif R., Martin R. G. Growth state of the cell early after infection with simian virus 40 determines whether the maintenance of transformation will be A-gene dependent or independent. J Virol. 1979 Aug;31(2):350–359. doi: 10.1128/jvi.31.2.350-359.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif R., Martin R. G. Simian virus 40 small t antigen is not required for the maintenance of transformation but may act as a promoter (cocarcinogen) during establishment of transformation in resting rat cells. J Virol. 1979 Dec;32(3):979–988. doi: 10.1128/jvi.32.3.979-988.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk T. E., Carbon J., Berg P. Construction and analysis of viable deletion mutants of simian virus 40. J Virol. 1976 May;18(2):664–671. doi: 10.1128/jvi.18.2.664-671.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyamala M., Atcheson C. L., Kasamatsu H. Stimulation of host centriolar antigen in TC7 cells by simian virus 40: requirement for RNA and protein syntheses and an intact simian virus 40 small-t gene function. J Virol. 1982 Aug;43(2):721–729. doi: 10.1128/jvi.43.2.721-729.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh M. J., Topp W. C., Hanich R., Sambrook J. F. Mutants of SV40 with an altered small t protein are reduced in their ability to transform cells. Cell. 1978 May;14(1):79–88. doi: 10.1016/0092-8674(78)90303-3. [DOI] [PubMed] [Google Scholar]
- Spangler G. J., Griffin J. D., Rubin H., Livingston D. M. Identification and initial characterization of a new low-molecular-weight virus-encoded T antigen in a line of simian virus 40-transformed cells. J Virol. 1980 Nov;36(2):488–498. doi: 10.1128/jvi.36.2.488-498.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg B. M., Pollack R. Anchorage independence: analysis of factors affecting the growth and colony formation of wild-type and dl 54/59 mutant SV40-transformed lines. Virology. 1979 Dec;99(2):302–311. doi: 10.1016/0042-6822(79)90009-6. [DOI] [PubMed] [Google Scholar]
- Sugano S., Yamaguchi N., Shimojo H. Small t protein of simian virus 40 is required for dense focus formation in a rat cell line. J Virol. 1982 Mar;41(3):1073–1075. doi: 10.1128/jvi.41.3.1073-1075.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Function of simian virus 40 gene A in transforming infection. J Virol. 1975 Mar;15(3):613–618. doi: 10.1128/jvi.15.3.613-618.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Spillman T., Schuetz F. R. Purification and characterization of the SV40 F-gene protein. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):159–164. doi: 10.1101/sqb.1980.044.01.018. [DOI] [PubMed] [Google Scholar]
- Thummel C. S., Burgess T. L., Tjian R. Properties of simian virus 40 small t antigen overproduced in bacteria. J Virol. 1981 Feb;37(2):683–697. doi: 10.1128/jvi.37.2.683-697.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R., Fey G., Graessmann A. Biological activity of purified simian virus 40 T antigen proteins. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1279–1283. doi: 10.1073/pnas.75.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Green H., Swift M. R. Susceptibility of human diploid fibroblast strains to transformation by SV40 virus. Science. 1966 Sep 9;153(3741):1252–1254. doi: 10.1126/science.153.3741.1252. [DOI] [PubMed] [Google Scholar]
- Yang Y. C., Hearing P., Rundell K. Cellular proteins associated with simian virus 40 early gene products in newly infected cells. J Virol. 1979 Oct;32(1):147–154. doi: 10.1128/jvi.32.1.147-154.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip Y. K., Barrowclough B. S., Urban C., Vilcek J. Purification of two subspecies of human gamma (immune) interferon. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1820–1824. doi: 10.1073/pnas.79.6.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]