Abstract
Coral-excavating sponges are the most important bioeroders on Caribbean reefs and increase in abundance throughout the region. This increase is commonly attributed to a concomitant increase in food availability due to eutrophication and pollution. We therefore investigated the uptake of organic matter by the two coral-excavating sponges Siphonodictyon sp. and Cliona delitrix and tested whether they are capable of consuming dissolved organic carbon (DOC) as part of their diet. A device for simultaneous sampling of water inhaled and exhaled by the sponges was used to directly measure the removal of DOC and bacteria in situ. During a single passage through their filtration system 14% and 13% respectively of the total organic carbon (TOC) in the inhaled water was removed by the sponges. 82% (Siphonodictyon sp.; mean±SD; 13±17 μmol L−1) and 76% (C. delitrix; 10±12 μmol L−1) of the carbon removed was taken up in form of DOC, whereas the remainder was taken up in the form of particulate organic carbon (POC; bacteria and phytoplankton) despite high bacteria retention efficiency (72±15% and 87±10%). Siphonodictyon sp. and C. delitrix removed DOC at a rate of 461±773 and 354±562 μmol C h−1 respectively. Bacteria removal was 1.8±0.9×1010 and 1.7±0.6×1010 cells h−1, which equals a carbon uptake of 46.0±21.2 and 42.5±14.0 μmol C h−1 respectively. Therefore, DOC represents 83 and 81% of the TOC taken up by Siphonodictyon sp. and C. delitrix per hour. These findings suggest that similar to various reef sponges coral-excavating sponges also mainly rely on DOC to meet their carbon demand. We hypothesize that excavating sponges may also benefit from an increasing production of more labile algal-derived DOC (as compared to coral-derived DOC) on reefs as a result of the ongoing coral-algal phase shift.
Introduction
Coral-excavating sponges are usually the most abundant and destructive bioeroders on coral reefs and strong competitors for space [1], [2]. They account for 60 to >90% of total macroborer activity [3], [4] and can remove up to 30 kg CaCO3 m−2 year−1 [5], which is in the same range as coral reef calcification rates ([6] and references therein). Coral-excavating sponges thus influence the balance between reef accretion (calcification and cementation) and erosion (physical, chemical and bioerosion), whereby positive net accretion is crucial to maintain carbonate reef structures [7]. Coral reefs are increasingly subjected to anthropogenic disturbances that negatively impact the growth of calcifying organisms while favoring (bioeroding) suspension feeders [8]–[10]. This is of particular importance in the face of climate change, where rising seawater temperatures [11], [12] and ocean acidification [11], [13] are expected to further reduce calcification rates of these organisms. In turn, the same processes are expected to promote bioerosion or at least affect it to a lesser extent [14]–[17], thus further reducing the ability of reef communities to form and maintain three dimensional reef frameworks. Over the past three decades the abundance of excavating sponges has increased considerably, mostly tentatively linked to increased food availability (e.g., bacterioplankton and phytoplankton) in response to eutrophication and land-based pollution [8]–[10], [18]. Similar to non-excavating sponges, coral-excavating sponges are commonly assumed to be efficient suspension feeders [19], i.e. feeding on particulate food sources. Yet, apart from the contribution of photosynthetically-fixed carbon from symbiotic zooxanthellae to the nutrition of some coral-excavating sponges [16], [20], [21] little is known about their dietary composition and food uptake rates.
Traditionally, sponges were considered to be suspension feeders that efficiently remove bacterio-, phyto- [22]–[26] and even zooplankton [27] from water they actively pump through their filtration systems. However, already in 1974, Reiswig [28] hypothesized that sponges may also retain dissolved organic carbon (DOC), which was later confirmed for several sponges, ranging from tropical [29]–[31] to temperate sponge species [32]. These tropical coral reef sponges can take up >90% of the total organic carbon (TOC) as DOC, indicating that they foremost rely on DOC to meet their carbon demand [29], [30]. Since DOC also accounts for >90% of the TOC pool on coral reefs (e.g., [29]), the ability to utilize this food source may aid certain sponges to thrive under oligotrophic conditions, whereas most other heterotrophic reef organisms are unable to capitalize on this resource [31]. Therefore, the question arises if, and to what extent, coral-excavating sponges also rely on dissolved organic substances in their daily diet.
The dissolved organic matter (DOM) uptake of non-excavating sponges is estimated to be in the same order of magnitude as the gross primary production rates of entire coral reef ecosystems [31]. Moreover, they are at the base of a pathway that transfers the DOM into particulate detritus that is subsequently ingested by reef fauna. This sponge loop retains the energy and nutrients within the different reef communities and most likely affects the stable states of these communities. Coral-excavating sponges are not yet considered to participate in the sponge loop, of which the ability to feed on DOM is one of the prerequisites.
The often suggested importance of food availability to explain the current increase of coral-excavating sponges requires experimental proof, in particular to address (1) whether coral-excavating sponges, similar to non-excavating sponges, are capable of DOC uptake and, if confirmed, (2) to what extent it completes their total daily diet. To answer these questions we determined the uptake of DOC and bacteria by the common Caribbean coral-excavating sponges Siphonodictyon sp. (Berquist, 1965) and Cliona delitrix (Pang, 1973) in situ and estimated the respective contribution of DOC and POC (bacteria and phytoplankton) to their TOC uptake.
Materials and Methods
Ethics statement
Research on Curaçao was performed under the annual research permit (unnumbered) issued by the Curaçaoan Ministry of Health, Environment and Nature (GMN) to the CARMABI foundation. Research conducted on Bonaire was performed under research permit No. 2012004073 issued by the Bonaire National Marine Park (BNMP) authority.
Study area and sampling procedure
The study was conducted in May 2013 on the Southern Caribbean Islands of Curaçao and Bonaire (ESM table S1). Siphonodictyon sp. was sampled on the fore reef slope along the leeward coast of Curaçao at 19±1 m water depth (mean±SD) at stations Playa Jeremy (12° 33′ N, 69° 15′ W; n = 5) and Daaibooi (12° 21′ N, 69° 08′ W; n = 3). Both sites are characterized by narrow bays harboring a wide and sandy reef terrace (160–190 m) that leads to a fairly steep (>45°) fore reef slope off-shore [33]. Sampling of Cliona delitrix took place on the fore reef slope at 13±1 m water depth at station Playa Lechi (12° 16′ N, 68° 28′ W; n = 10) in front of Kralendijk, Bonaire. Here, the sandy reef terrace is narrow (approx. 65 m) and used as an anchorage zone for dive- and small fishing boats. The features of the reef slope are comparable to those of the two sites on Curaçao [33]. In situ water sampling was conducted on SCUBA. The simple and inexpensive point sampler (SIP) system [34], the so-called VacuSIP system designed by G. Yahel was slightly modified (see Fig. 1; for detailed description see http://web.uvic.ca/~yahel/GYWS/Other/VacuSIP%20usage%20and%20makeup.pdf) and used for the in situ measurement of the difference in DOC concentration and bacterial abundance between a pair of inhaled and exhaled water samples mediated by a sponge. This difference provided a measure of the net retention (or production) of a waterborne compound by the animal [35]. The sampling system used here consisted of two separate VacuSIP samplers attached to a stand which allowed simultaneous sampling of water inhaled and exhaled by the sponge (Fig. 1). Each sampler consisted of PEEK (polyetheretherketone) tubing (1/16”×25 μm, UpChurch Scientific) with a syringe needle connected to a male luer connector (IDEX Health and Science, P-655 1/4-28) at its distal end (outlet). Samplers were attached to a flexible arm so that the proximal end (inlet) of one sampler could be positioned in the osculum (excurrent aperture; Ex) and another one (In) outside of the osculum at a distance of approximately 20 cm from the inhalant surface (to ensure sampling of ambient water without contamination from substances emitted from the surface of the sponge). After positioning the VacuSIP, it was left untouched for at least 3 min to minimize possible disturbance effects that could have occurred during the installation of the device. Evacuated vials (Vacuette, 9 mL, no additive, Greiner Bio-One GmbH) were used to collect bacterial abundance samples and pre-combusted (4 h at 450°C) Epa vials (40 mL) were used to collect samples for DOC concentration. Vials were connected to the samplers by piercing their septa with the syringe needle. The pressure difference between the external water and the neutral (Epa vials) or evacuated (vacuettes) vials ensured that water flowed into the container during sampling. For DOC sampling, an inline stainless steel filter holder (13 mm, Swinney, Pall) with a pre-combusted (4 h at 450°C) GF/F filter (Whatmann, 0.7 μm) was added.
Figure 1. VacuSIP system for in situ sampling of DOC and bacteria.

VacuSIP system consisting of two separate samplers (In and Ex) attached to a stand to simultaneously take water samples of the ambient water (IN) and the water exhaled by the sponge (EX). Blue arrows indicate water pumped through the sponge.
To avoid contamination of the sampled water with ambient water, the VacuSIP water sampling rate was kept lower than the pumping – excurrent jet – rate of the sponge [35]. Therefore, the excurrent jet rate of each sponge was determined prior to sampling using the dye-front speed (DFS) technique [29]. A cut-open 15 mL Falcon tube (length 95 mm; diameter: 14 mm) was aligned with the osculum (diameter: 4–15 mm, Table 1) of the sponge (without touching it). A dye was released between osculum and tube and its movement with the excurrent jet through the tube was video-taped (three to five times). The resulting water transport speed (cm s−1) was multiplied with the cross-section area of tube (cm2) to yield the excurrent jet rate (mL min−1). During the In and Ex sampling the time to fill the containers was recorded to calculate the rate at which water was sampled. Mean sampling rate (±SD) for Siphonodictyon sp. and C. delitrix was 2.9±1.2 and 1.9±0.3 mL min−1 (ESM Table S1), respectively, which was two orders of magnitude less than the sponges' excurrent jet rate (table 1).
Table 1. Oscule diameter, water transport speed and excurrent jet rate for Siphonodictyon sp. and Cliona delitrix.
| Species | ID | Oscule diameter (cm) | Water transport speed (cm s−1) | Excurrent jet rate (mL min−1) |
| Siphonodictyon sp. | S1 | 0.7 | 5.0 | 466.1 |
| S2 | 0.7 | 2.9 | 263.8 | |
| S3 | 0.9 | 6.9 | 637.3 | |
| S4 | 1.1 | 7.6 | 706.3 | |
| S5 | 0.7 | 4.7 | 431.0 | |
| S6 | 0.5 | 4.7 | 431.0 | |
| S7 | 0.4 | 4.6 | 420.3 | |
| S8 | 0.5 | 7.9 | 727.4 | |
| Average (±SD) | 0.7±0.2 | 5.5±1.8 | 510.4±162.6 | |
| Cliona delitrix | C1 | 1.5 | 4.4 | 404.1 |
| C2 | 1.4 | 6.3 | 577.3 | |
| C3 | 1.4 | 4.6 | 427.9 | |
| C4 | 1.2 | 4.7 | 431.0 | |
| C5 | 1.0 | 8.8 | 808.2 | |
| C6 | 1.4 | 3.9 | 359.2 | |
| C7 | 1.0 | 3.9 | 363.7 | |
| C8 | 1.0 | 4.0 | 371.8 | |
| C9 | 1.5 | 4.8 | 440.8 | |
| C10 | 1.1 | 4.4 | 404.1 | |
| Average (±SD) | 1.1±0.2 | 5.0±1.5 | 458.8±137.7 | |
Prior to sampling, VacuSIP samplers were cleaned by flushing the sampler consecutively with 30 mL HCl (5%; except stainless steel filter holders to avoid corrosion), 30 mL MQ, and 30 mL Decon 90 (Decon Laboratories Limited; 5%). After in situ VacuSIP installment system samplers were flushed with 30 mL ambient seawater prior to sampling.
Processing of samples
Water samples were processed within 1h after sampling. Samples for DOC concentration (20 mL) were acidified with 6–7 drops of concentrated HCl (38%) to remove inorganic carbon and stored in the dark at 4°C until analysis. DOC concentrations were measured using the high-temperature catalytic oxidation (HTCO) technique in a total organic C analyzer (TOC-VCPN; Shimadzu). The instrument was calibrated with a standard addition curve of Potassium Phthalate (0; 25; 50; 100; 200 µmol C L−1). Consensus Reference Materials (CRM) provided by Hansell and Chen of the University of Miami (Batch 12; 2012; 41–44 µmol C L−1) were used as positive controls for our measurements. Concentrations measured for the batch gave average values (±SD) of 45±2 µmol C L−1. Average analytical variation of the instrument was <3% (5–7 injections per sample).
Samples for bacterial abundance (9 mL) were fixed in 4% paraformaldehyde (PFA) and filtered over a 0.2 μm polycarbonate filter (Millipore, 25 mm), supported by a 0.45 μm HA filter (Millipore, 25 mm). The filters were air-dried and stored in Eppendorf tubes at −20°C. Prior to bacterial cell counts, filters were mounted on a microscopy slide in a DAPI-mix. Bacterial numbers were counted using an epifluorescence microscope (Zeiss Axioplan; 1000×). Per slide 10 grids (36×36 μm, divided into 10 rows and columns) were counted or up to a minimum of 200 bacteria.
Data analysis
Differences in DOC concentration and bacterial abundance between In and Ex water samples were tested using the Wilcoxon Signed Rank test. To convert bacterial numbers to a corresponding amount of carbon biomass, a conversion factor for coastal bacteria of 30 fg per bacterial cell was used [36]. Net uptake (or release) rates are traditionally reported per unit of animal mass or volume. Yet, coral-excavating sponges such as Siphonodictyon sp. and C. delitrix live inside the substrate, which makes the quantification of such units difficult. Therefore, we followed the recommendation of Yahel et al. [35] and standardized fluxes to the excurrent jet rate. Uptake rates were calculated as the difference in concentration of an In-Ex pair (Δ concentrationIn-Ex) multiplied with the respective excurrent jet rate:
 |
The TOC pool is comprised of DOC and particulate organic carbon (POC). In tropical reef waters POC consists mainly of phytoplankton and bacterioplankton. However, phytoplankton concentrations were not directly measured. Generally, the contribution of phytoplankton carbon to the total carbon pool in tropical waters is low and roughly equal [37]–[39] or lower than bacterioplankton carbon (BC) [40], [41]. To quantify the contribution of DOC and POC to TOC we followed the formula suggested by de Goeij et al. [30]:
Results
Ambient DOC concentrations and net sponge DOC removal
Ambient DOC concentrations (mean±SD, derived from inhaled water) on Curaçao were 110±18 μmol L−1 and 95±5 μmol L−1 on Bonaire. POC concentrations were 4±1 μmol L−1 on both islands, so that ambient TOC concentrations were 114±18 and 99±13 μmol C L−1, for Curaçao and Bonaire, respectively. Both sponge species significantly removed amounts of DOC from the seawater pumped through their aquiferous system (Fig. 2A). DOC concentrations in the exhalant water were reduced by 13±17 μmol C L−1 for Siphonodictyon sp. (Wilcoxon Signed Rank: Z = −2.521, n = 8, p = 0.012) and 10±12 μmol C L−1 for C. delitrix (Wilcoxon Signed Rank: Z = −2.803, n = 10, p = 0.005), respectively, compared to the inhalant water. The majority of the TOC removed by the two coral-excavating sponges –82% (Siphonodictyon sp.) and 76% (C. delitrix) – consisted of DOC. The amount of DOC removed by both coral-excavating sponge species increased linearly with increasing ambient DOC concentrations (Siphonodictyon sp.: R2 = 0.88, p = 0.004; C. delitrix: R2 = 0.84, p = 0.002) encountered during the experiments (Siphonodictyon sp.: 98–151 μmol C L−1; C. delitrix: 80–124 μmol C L−1) (Fig. 3A). This indicates that no threshold or saturation effect occurred for the aforementioned ranges of ambient DOC concentrations.
Figure 2. Average DOC (A) and bacterial abundance (B) in the inhaled (black) and exhaled (grey) water of Siphonodictyon sp. and C. delitrix.

Error bars indicate SE. P values (Wilcoxon Signed Rank) indicate significance level of the difference in the concentrations between the inhaled and exhaled samples in n pairs of InEx samples.
Figure 3. Removal of DOC (A) and bacterial cells (B) by Siphonodictyon sp. (black) and C. delitrix (grey) plotted against ambient (inhaled) concentrations.
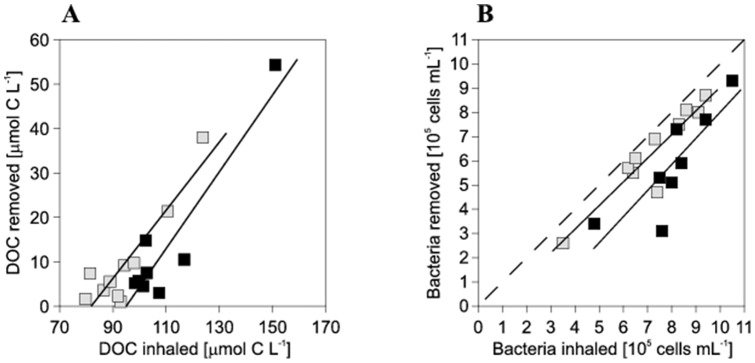
Both species responded linearly to elevated DOC (R2 = 0.88; p = 0.004 and R2 = 0.84; p = 0.002) and bacterial concentrations (R2 = 0.72; p = 0.045 and R2 = 0.87; p = 0.001) within the full concentration range encountered. Dashed line represents 100% bacterial removal.
Ambient bacterial abundance and net sponge bacterial removal
Ambient bacterial abundance (mean±SD, derived from inhaled water) on Curaçao (8.0±1.6×105 cells mL−1) and Bonaire (7.3±1.8×105 cells mL−1) corresponded to 2.0±0.4 and 1.8±0.4 μmol C L−1, respectively. Both, Siphonodictyon sp. and C. delitrix significantly reduced ambient bacterial concentrations by 5.89±2.11×105 (Wilcoxon Signed Rank: Z = −2.521, n = 8, p = 0.012) and 6.36±1.84×105 cells mL−1 (Wilcoxon Signed Rank: Z = −2.803, n = 10, p = 0.005), respectively (Fig. 2B). Bacteria removal efficiency was 72±15% and 87±10%, but despite these high efficiencies, bacterial removal accounted for only 9% (Siphonodictyon sp.) and 12% (C. delitrix) of the total TOC removal. Similar to the uptake of DOC, the number of bacteria cells removed by excavating sponges from the surrounding water increased linearly with increased cell abundance in the water column (Siphonodictyon sp.: R2 = 0.72, p = 0.0045; C. delitrix: R2 = 0.87, p = 0.001; Fig. 3B). Across the range of ambient bacterial concentrations encountered (Siphonodictyon sp.: 4.8–10.5×105 cells mL−1; C. delitrix: 3.5–9.4×105 cells mL−1) no indication of a threshold or saturation concentration occurred.
Sponge DOC and bacterial uptake rates
Water transport speed of Siphonodictyon sp. and C. delitrix were comparable at 5.4±1.8 and 5.0±1.5 cm s−1, respectively (table 1). And despite of 1.8 times larger mean oscule diameter for C. delitrix (table 1), mean excurrent jet rates were comparable as well (Siphonodictyon sp.: 510.4.5±162.6 mL min−1; C. delitrix: 458.8±137.7 mL min−1). Mean DOC uptake rate of Siphonodictyon sp. was 461±773 μmol C h−1 and, therefore, 1.3 times higher than that of C. delitrix (354±562 μmol C h−1) (table 2).
Table 2. Mean DOC and bacteria uptake rates (±SD) of Siphonodictyon sp. and Cliona delitrix standardized to excurrent jet rate.
| species | DOC (μmol C h−1) | Bacteria (1010 cells h−1) | Bacteria (μmol C h−1) |
| Siphonodictyon sp. | 461±773 | 1.8±0.9 | 46.0±21.2 |
| C. delitrix | 354±562 | 1.7±0.6 | 42.5±14.0 |
Mean bacteria uptake rate of Siphonodictyon sp. and C. delitrix were 1.8±0.9×1010 and 1.7±0.6×1010 cells h−1, respectively (table 2). These bacterial removal rates correspond to a BC uptake of 46.0±21.2 and 42.5±14.0 μmol C h−1. Therefore, DOC represents 83 and 81% of the TOC taken up by Siphonodictyon sp. and C. delitrix per hour.
Discussion
The coral-excavating sponges Siphonodictyon sp. and C. delitrix are both lacking photosynthetic symbionts ([42], pers. comm. C.H.L. Schönberg) and can therefore be considered as classic heterotrophs that depend on the uptake of organic matter as carbon and energy source. This study demonstrates that both species mainly rely on DOC uptake to meet their carbon demand. Despite high bacterial retention efficiencies, these sponges can be typified as DOM-feeders, retaining 83% and 81% of the TOC taken up in the form of DOC. This contribution of DOC in their daily diet is in the same range, and only slightly lower, than that reported for non-excavating sponges, such as the reef sponges Theonella swinhoei (Gray, 1868), Halisarca caerulea (Vacelet and Donadey, 1987), Mycale microsigmatosa (Arndt, 1927) and Merlia normani (Kirkpatrick, 1908) [29], [30]. Our results further suggest that, similar to bacteria (Fig. 3B) or phytoplankton (e.g., [29], [34]), sponges can efficiently take up DOC across a wide range of ambient DOC concentrations (Fig. 3A). This indicates that these sponges are well adapted to utilize DOC as food source [34], [43]. DOC uptake by sponges has been confirmed in an increasing number of species belonging to various orders of Demospongiae [29]–[32] and one order of Hexactinellida [44] (ESM table S2).
Sponge DOC and bacterial uptake rates
Since uptake rates were standardized to the excurrent jet rate and not to biomass or volume, results are primarily discussed in comparison to T. swinhoei in Yahel et al. [29], where necessary parameters are available. Largely similar retention efficiencies (DOC: 11–12%; bacteria: 72–87%) and water transport speeds (table 1) resulted in comparable DOC and bacterial uptake rates in Siphonodictyon sp. and C. delitrix (table 2). Yet, the DOC uptake rates were approximately three times higher than reported for T. swinhoei (DOC: 138 μmol C h−1). Similarly, bacterial uptake rates were twice as high for the excavating sponge species as for T. swinhoei (bacteria: 1.0×1010 cells h−1). This difference in uptake rates can be explained by a lower volume of water passing through T. swinhoei as indicated by a 2 times lower excurrent jet rate (230 mL min−1). Environmental factors (e.g. sediment in the water column) and mechanical stimuli are reported to reduce and/or arrest the pumping activity of sponges [45], [46]. Since the excurrent jet rate was only measured prior to the sampling it cannot be excluded that it varied during the sampling, which could explain the overall high variability in bacterial and DOC uptake rates in both excavating sponge species tested. Assuming an average daily pumping activity of 12 h [24] yields a TOC uptake of 6.6 and 5.2 mmol C d−1 for Siphonodictyon sp. and C. delitrix, respectively. It should be noted that the here presented uptake rates are given per excurrent jet and that both species are multi-oscular sponges and have therefore multiple excurrent jets. C. delitrix can grow up to a size of 1 m across with >30 oscules per specimen (B. Mueller pers. obs.). At Playa Lechi, our study site on Bonaire, the abundance of C. delitrix was with 0.03 individuals m−2 relatively low (Y. Mulders pers. obs.). However, densities of up to 0.23 and 0.54 individuals m−2 were reported for Grand Cayman and San Andrés, Columbia, respectively [10], [47]. When occurring in such high densities, C. delitrix is likely to have a significant effect on benthic carbon cycling by ingesting POC and especially DOC from the ambient water. Abundance data for Siphonodictyon sp. are rare, but with approximately 0.23 individuals m−2 on the south-western coast of Curaçao this species is quite common (B. Mueller and F.C. Van Duyl pers. obs.). However, specimens are comparable small at 48 cm2. Siphonodictyon coralliphagum (Rützler, 1971) is reported to grow up to a size of 600 cm2 [48] and individuals of >0.5 m across can be regularly encountered on Cozumel, Mexican Caribbean (B. Mueller pers. obs.). Therefore, also Siphonodictyon sp. might have a significant effect on benthic carbon cycling, when occurring in high densities and large sizes.
Potential effect of a coral-algal phase shift on coral-excavating sponges
The ability of sponges to take up and assimilate DOC [32], [49] has been proposed to be crucial to maintain biodiversity and high productivity on tropical coral reefs [32]. In the so-called “sponge loop”, analogously to the microbial loop, sponges make energy and nutrients stored in the dissolved organic matter (DOM) pool available to the benthic food web via DOM assimilation and subsequent detritus production by the sponges. Our study now shows that excavating sponges most likely also participate in the sponge loop, although it remains unclear to what extent these sponges produce detritus and what the nutritional value of this detritus is to other reef fauna. However, it is very clear that there is a current increase in the abundance of coral-excavating sponges throughout the Caribbean (e.g., [9], [42]). This increase is commonly attributed to a combination of an increase in the availability of new substrate due to coral declines [9], [50] and an increase in food availability as a result of eutrophication and pollution [8]–[10]. Regarding the latter, being suspension feeders, coral-excavating sponges were considered to benefit from elevated concentration of particulate resources, such as phytoplankton and bacteria (e.g., [8], [9], [47]). However, here we could show that coral-excavating sponges mainly rely on DOC to meet their carbon demand. Thus, an increase in DOC production, or quality, on coral reefs is likely to be beneficial for them. Shifts in the benthic reef community have caused major changes in the production and cycling of organic matter on reefs [51], [52]. Due to anthropogenic disturbances benthic algae are increasing at the expense of scleractinian corals on most coral reefs throughout the Caribbean region (e.g., [53]–[55]). Both, scleractinian corals and benthic algae release a substantial amount of their photosynthetically fixed carbon as organic matter in the surrounding water [56]–[58]. However, benthic algae are reported to release more DOM than corals (e.g., [52], [56], [59]) and algal-derived DOM appears to be of a higher quality [52], [60]. Sponges, including excavating species, could therefore benefit in two ways from an increase in DOM production and quality due to the shift in benthic communities: (1) directly via uptake of DOM and (2) indirectly by feeding on the heterotrophic planktonic microbial community, which is fueled by the DOM release of benthic algae. However, the competition between algae and (coral-excavating) sponges is controversial. A general negative correlation between the abundance of benthic algae and phototrophic excavating sponges was observed in the Mediterranean and on the Great Barrier Reef [61], [62]. Furthermore, competition for space between benthic algae and the phototrophic coral-excavating sponge Cliona tenuis (Zea and Weil, 2003) has been reported in the Caribbean [2], [63]. Despite possible DOM consumption by this sponge, the beneficial effects of the availability of algal-DOM might be reduced or even eliminated by the effects of sunlight shading by benthic algae, reducing the photosynthetic performance of the sponge [61], [62]. Interestingly, C. tenuis was reported to advance over turf algae [2], [64], which are known to release high amounts of DOC (e.g., [57], [59]) and do not shade the sponge. Moreover, a coexistence of sponges and benthic algae jointly dominating the benthic community was found on several Caribbean reefs and is suggested to become more frequent with increasing reef degradation [65], [66].
Here we could show that the coral-excavating sponges Siphonodictyon sp. and C. delitrix are capable of consuming DOC and mainly rely on DOC to meet their organic carbon demand. This suggests that coral-excavating sponges are likely to benefit from an increase in DOC production and quality as a result of the ongoing coral-algal phase shift.
Supporting Information
Sampling dates, locations, depth as well as sampling rates for Siphonodictyon sp. and Cliona delitrix.
(PDF)
Confirmed DOC feeding by sponges.
(PDF)
Acknowledgments
We thank the staff of Carmabi for their hospitality and logistic support during the field work on Curaçao. We further thank R. de León, F. Simal and P. Bertuol of STINAPA and R. Peachy of the CIEE research station for logistical support on Bonaire. We are grateful to S. Gonzalez and R. Doggen for their contribution to the DOC and bacterial analysis. The advice of G. Yahel on the construction of the VacuSIP is highly appreciated.
Funding Statement
The research leading to these results has received funding from the European Union Seventh Framework Programme (P7/2007-2013) under grant agreement no 244161 (Future of Reefs in a Changing Environment) and the Innovational Research Incentives Scheme of the Netherlands Organization for Scientific Research (NWO-VENI; 863.10.009; pers. grant to JMdG). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hein JF, Risk MJ (1975) Bioerosion of coral heads: inner patch reefs, Florida reef tract. Bull Mar Sci 25: 133–138. [Google Scholar]
- 2. Gonzalez-Rivero M, Yakob L, Mumby PJ (2011) The role of sponge competition on coral reef alternative steady states. Ecological Modelling 222: 1847–1853. [Google Scholar]
- 3. Risk MJ, Sammarco PW, Edinger EN (1995) Bioerosion in Acropora across the continental shelf of the Great Barrier Reef. Coral Reefs 14: 79–86. [Google Scholar]
- 4. Mallela J, Perry CT (2007) Calcium carbonate budget for two coral reefs affected by different terrestrial runoff regimes, Rio Bueno, Jamaica. Coral Reefs 26: 129–145. [Google Scholar]
- 5.Calcinai B, Azzini F, Bavestrello G, Gaggero L, Cerrano C (2007) Excavating rates and boring pattern of Cliona albimarginata (Porifera: Clionaidae) in different substrata. In: Custódio MR, Hajdu E, Lôbo-Hajdu G, Muricy G (eds) Porifera research: biodiversity, innovation and sustainability. Proc 7th Int Sponge Symp: 255–263.
- 6. Andersson AJ, Gledhill D (2013) Ocean acidification and coral reefs: Effects on breakdown, dissolution, and net ecosystem calcification. Annu Rev Mar Sci 5: 321–48. [DOI] [PubMed] [Google Scholar]
- 7.Glynn PW (1997) Bioerosion and coral reef growth: a dynamic balance. In: Birkeland C editor. Life and Death of Coral Reefs. New York: Chapman and Hall. 68–95.
- 8. Holmes KE (2000) Effects of eutrophication on bioeroding sponge communities with the description of new West Indian sponges, Cliona spp. (Porifera: Hadromerida: Clionidae). Invert Biol 119: 125–138. [Google Scholar]
- 9. Ward-Paige CA, Risk MJ, Sherwood OA, Jaap WC (2005) Clionid sponge surveys on the Florida Reef Tract suggest land-based nutrient inputs. Mar Pollut Bull 51: 570–570. [DOI] [PubMed] [Google Scholar]
- 10. Chaves-Fonnegra A, Zea S, Gómez ML (2007) Abundance of the excavating sponge Cliona delitrix in relation to sewage discharge at San Andrés Island, SW Caribbean, Colombia. Bol Investig Mar Costeras 36: 63–78. [Google Scholar]
- 11. Hoegh-Guldberg O, Mumby PJ, Steneck RS, Greenfield P, Gomez E, et al. (2007) Coral reefs under rapid climate change and ocean acidification. Science 318: 1737–1742. [DOI] [PubMed] [Google Scholar]
- 12. Cantin NE, Cohen AL, Karnauskas KB, Tarrant AM, McCorkle DC (2010) Ocean warming slows coral growth in the central Red Sea. Science 329: 322–325. [DOI] [PubMed] [Google Scholar]
- 13. Pandolfi JM, Connolly SR, Marshall DJ, Cohen AL (2011) Projecting coral reef futures under global warming and ocean acidification. Science 333: 418. [DOI] [PubMed] [Google Scholar]
- 14. Duckworth AR, Petersen BJ (2012) Effects of seawater temperature and pH on the boring rates of the sponge Cliona celata in scallop shells. Mar Biol 160: 27–35. [Google Scholar]
- 15. Fang JKH, Mello-Athayde MA, Schönberg CHL, Kline DI, Hoegh-Guldberg O, et al. (2013) Sponge biomass and bioerosion rates increase under ocean warming and acidification. Glob Change Biol 19: 3581–3591. [DOI] [PubMed] [Google Scholar]
- 16. Wisshak M, Schönberg CHL, Form A, Freiwald A (2012) Ocean acidification accelerates reef bioerosion. PLoS ONE 7(9): e45124 10.1371/journal.pone.0045124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wisshak M, Schönberg CHL, Form A, Freiwald A (2013) Effects of ocean acidification and global warming on reef bioerosion – lessons from a clionaid sponge. Aquat Biol 19: 11–127. [Google Scholar]
- 18.Schönberg CHL (2008) A history of sponge erosion: from past myths and hypotheses to recent approaches. In Wisshak M, Tapanila L (eds) Current developments in bioerosion. Springer-Verlag Berlin: 165–202.
- 19. Schönberg CHL, Wisshak M (2012) The perks of being endolithic. Aquat Biol 17: 1–5. [Google Scholar]
- 20. Hill MS (1996) Symbiotic zooxanthellae enhance boring and growth rates of the tropical sponge Anthosigmella varians forma varians. Mar Biol 125: 649–654. [Google Scholar]
- 21. Weisz JB, Massaro AJ, Ramsby BD, Hill MS (2010) Zooxanthellar symbionts shape host sponge trophic status through translocation of carbon. Biol Bull 219: 189–97. [DOI] [PubMed] [Google Scholar]
- 22. Reiswig HM (1971) Particle feeding in natural populations of three marine demosponges. Biol Bull 141: 568–591. [Google Scholar]
- 23. Reiswig HM (1975) Bacteria as food for temperate-water marine sponges. Can J Zool 533: 582–589. [Google Scholar]
- 24. Pile AJ, Patterson MR, Savarese M, Chernykh VI, Fialkov VA (1997) Trophic effects of sponge feeding within Lake Baikal's littoral zone. 2. Sponge abundance, diet, feeding efficiency, and carbon flux. Limnol Oceanogr 42: 178–184. [Google Scholar]
- 25. Ribes M, Coma R, Gili JM (1999) Natural diet and grazing rate of the temperate sponge Dysidea avara (Demospongiae, Dendroceratida) throughout an annual cycle. Mar Ecol Prog Ser 176: 179–190. [Google Scholar]
- 26. Perea-Blázquez A, Davy SK, Bell JJ (2012) Estimates of particulate organic carbon flowing from the pelagic environment to the benthos through sponge assemblages. PlosOne 7(1): e29569 10.1371/journal.pone.0029569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vacelet J, Boury-Esnault N (1995) Carnivorous sponges. Nature 373: 333–335. [Google Scholar]
- 28. Reiswig HM (1974) Water transport, respiration and energetics of three tropical marine sponges. J Exp Mar Biol Ecol 14: 231–249. [Google Scholar]
- 29. Yahel G, Sharp JH, Marie D, Haese C, Genin A (2003) In situ feeding and element removal in the symbiont-bearing sponge Theonella swinhoei: bulk DOC is the major source for carbon. Limnol Oceanogr 48: 141–149. [Google Scholar]
- 30. De Goeij JM, Van den Berg H, Van Oostveen MM, Epping EHG, Van Van Duyl FC (2008) Major bulk dissolved organic carbon (DOC) removal by encrusting coral reef cavity sponges. Mar Ecol Prog Ser 357: 139–151. [Google Scholar]
- 31. De Goeij JM, Van Oevelen D, Vermeij MJA, Osinga R, Middelburg JJ, et al. (2013) Surviving in a marine desert: the sponge loop retains resources within coral reefs. Science 342: 108–110. [DOI] [PubMed] [Google Scholar]
- 32. Ribes M, Jimenez E, Yahel G, Lopez-Sendino P, Diez B, et al (2012) Functional convergence of microbes associated with temperate marine sponges. Environ Microbiol 14: 1224–1239. [DOI] [PubMed] [Google Scholar]
- 33.Van Duyl FC (1985) Atlas of the living reefs of Curaçao and Bonaire (Netherlands Antilles). Studies of the flora and fauna of Surinam and the Netherlands Antilles Vol 117, Utrecht.
- 34. Yahel G, Whitney F, Reiswig HM, Eerkes-Medrano DI, Leys SP (2007) In situ feeding and metabolism of glass sponges (Hexactinellida, Porifera) studied in a deep temperate fjord with a remotely operated submersible. Limnol Oceanogr 52: 428–440. [Google Scholar]
- 35. Yahel G, Marie D, Genin A (2005) InEx – a direct in situ method to measure filtration rates, nutrition, and metabolism of active suspension feeders. Limnol Oceanogr Methods 3: 46–58. [Google Scholar]
- 36. Fukuda R, Ogawa H, Nagata T, Koike I (1998) Direct determination of carbon and nitrogen contents of natural bacterial assemblages in marine environments. Appl Environ Microbiol 64: 3352–3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ayukai T (1995) Retention of phytoplankton and planktonic microbes on coral reefs within the Great Barrier Reef, Australia. Coral Reefs 14: 141–147. [Google Scholar]
- 38. Yahel G, Post AF, Fabricius K, Marie D, Vaulot D, et al. (1998) Phytoplankton distribution and grazing near coral reefs. Limnol Oceanogr 43: 551–563. [Google Scholar]
- 39. Van Duyl FC, Gast GJ, Steinhoff W, Kloff S, Veldhuis MJW, et al. (2002) Factors influencing the short-term variation in phytoplankton composition and biomass in coral reef waters. Coral Reefs 21: 293–306. [Google Scholar]
- 40. Richter C, Wunsch M, Rasheed M, Kötter I, Badran MI (2001) Endoscopic exploration of Red Sea coral reefs reveals dense populations of cavity-dwelling sponges. Nature 413: 726–730. [DOI] [PubMed] [Google Scholar]
- 41.Kötter I (2003) Feeding ecology of coral reef sponges. PhD thesis, Universität Bremen.
- 42. Rützler K (2002) Impact of crustose clionid sponges in Caribbean coral reefs. Acta Geol Hisp 37: 61–72. [Google Scholar]
- 43. Coma R, Ribes M, Gili JM, Hughes RN (2001) The ultimate opportunists: consumers of seston. 44. Mar Ecol Prog Ser 219: 305–308. [Google Scholar]
- 44. Van Duyl FC, Hegeman J, Hoogstraten A, Maier C (2008) Dissolved carbon fixation by sponge-microbe consortia of deep water coral mounds in the northeastern Atlantic Ocean. Mar Ecol Prog Ser 358: 137–150. [Google Scholar]
- 45. Gerrodette T, Flechsig AO (1979) Sediment-induced reduction in the pumping rate of the tropical sponge Verongia lacunosa . Mar Biol 55: 103–110. [Google Scholar]
- 46. Tompkins-Mac Donald GJ, Leys SP (2008) Glass sponges arrest pumping in response to 48. sediment: implications for physiology of the hexactinellid conduction system. Mar Biol 154: 973–984. [Google Scholar]
- 47. Rose CS, Risk MJ (1985) Increase in Cliona delitrix infestation of Montastraea cavernosa heads on an organically polluted portion of the Grand Cayman. P.S.Z.N.I: Mar Ecol 6: 345–363. [Google Scholar]
- 48. Rützler K (1971) Bredin-Archbold-Smithsonian biological survey of Dominica: burrowing sponges, genus Siphonodictyon Bergquist, from the Caribbean. Smithson Contrib Zool 77: 37. [Google Scholar]
- 49. De Goeij JM, Moodley L, Houtekamer M, Carballeira NM, Van Duyl FC (2008) Tracing 13C-enriched dissolved and particulate carbon in Halisarca caerulea, a coral reef sponge with associated bacteria: evidence for DOM-feeding. Limnol Oceanogr 53: 1376–1386. [Google Scholar]
- 50. Maliao RJ, Turingan RG, Lin J (2008) Phase-shift in coral reef communities in the Florida Keys National Marine Sanctuary (FKNMS), USA. Mar Biol 154: 841–853. [Google Scholar]
- 51. Wild C, Haas AF, Naumann MS, Mayr C, el-Zidbah M (2008) Phase shifts in coral reefs – comparative investigation of corals and benthic algae as ecosystem engineers. Proc 11th Int Coral Reef Symp Ft. Lauderdale, 1319–1323. [Google Scholar]
- 52. Haas AF, Nelson CE, Rohwer F, Wegley-Kelly L, Quistad SD, et al. (2013) Influence of coral and algal exudates on microbially mediated reef metabolism. PeerJ 1: e90152; DOI 565 10.7717/peerj.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hughes TP (1994) Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 265(5178): 1547. [DOI] [PubMed] [Google Scholar]
- 54. McCook LJ, Jompa J, Diaz-Pulido G (2001) Competition between corals and algae on coral reefs: a review of evidence and mechanisms. Coral Reefs 19: 400–417. [Google Scholar]
- 55. Kennedy EV, Perry CT, Halloran PR, Iglesias-Prieto R, Schönberg CHL, et al. (2013) Avoiding coral reef functional collapse requires local and global action. Curr Biol 23: 912–918. [DOI] [PubMed] [Google Scholar]
- 56. Haas AF, Jantzen C, Naumann MS, Iglesias-Prieto R, Wild C (2010a) Organic matter release by the dominant primary producers in a Caribbean reef lagoon: implication for in situ O2 availability. Mar Ecol Prog Ser 409: 27–39. [Google Scholar]
- 57. Haas AF, Naumann MS, Struck U, Mayr C, el-Zidah M, et al. (2010b) Organic matter release by coral reef associated benthic algae in the Northern Red Sea. J Exp Mar Biol Ecol 389: 53–60. [Google Scholar]
- 58. Naumann MS, Haas AF, Struck U, Mayr C, el-Zibdah M, et al. (2010) Organic matter release by dominant scleractinian corals of the Northern Red Sea. Coral Reefs 29: 649–659. [Google Scholar]
- 59. Haas AF, Nelson CE, Wegley Kelly L, Carlson CA, Rohwer F, et al. (2011) Effects of coral reef benthic primary producers on dissolved organic carbon and microbial activity. PLoS ONE 6 11: 10.1371/journal.pone.0027973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nelson CE, Goldberg SJ, Wegley Kelly L, Haas AF, Smith JE, et al. (2013) Coral and macroalgal exudates vary in neutral sugar composition and differentially enrich reef bacterioplankton lineages. ISME J 7: 962–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cebrian E, Uriz MJ (2006) Grazing on fleshy seaweeds by sea urchins facilitates sponge Cliona viridis growth. Mar Ecol Prog Ser 323: 83–89. [Google Scholar]
- 62. Cebrian E (2010) Grazing on coral reefs facilitates growth of the excavating sponge Cliona orientalis (Clionaidae, Hadromerida). Mar Ecol 31: 533–538. [Google Scholar]
- 63. González-Rivero M, Ferrari R, Schönberg CHL, Mumby PJ (2012) Impacts of macroalgal competition and parrotfish predation on the growth of a common bioeroding sponge. Mar Ecol Prog Ser 444: 133–142. [Google Scholar]
- 64. López-Victoria M, Zea S, Weil E (2006) Competition for space between encrusting excavating Caribbean sponges and other coral reef organisms. Mar Ecol Prog Ser 312: 113–121. [Google Scholar]
- 65. Colvard NB, Edmunds PJ (2010) Decadal-scale changes in abundance of non-scleractinian invertebrates on a Caribbean coral reef. J Exp Mar Biol Ecol 397: 153–160. [Google Scholar]
- 66. Bell JJ, Davy SK, Jones T, Taylor MW, Webster NS (2013) Could some coral reefs become sponge reefs as our climate changes? Glob Change Biol 19: 2613–2624. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sampling dates, locations, depth as well as sampling rates for Siphonodictyon sp. and Cliona delitrix.
(PDF)
Confirmed DOC feeding by sponges.
(PDF)


