Abstract
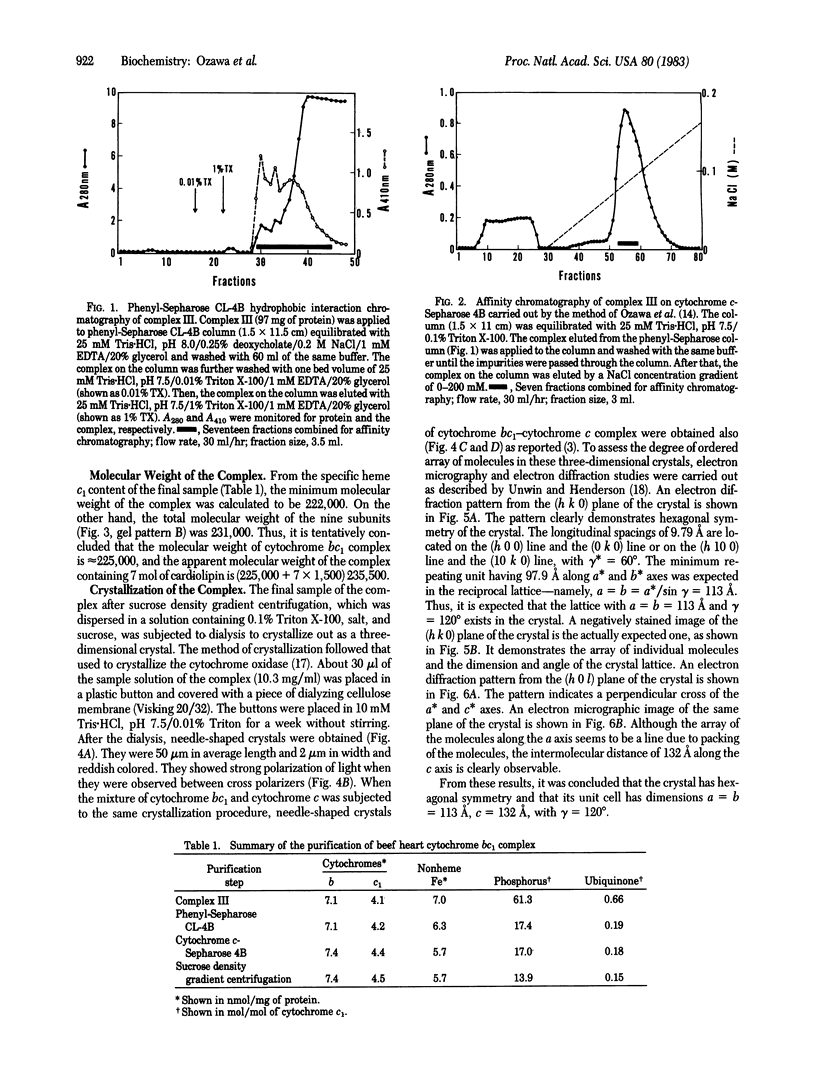
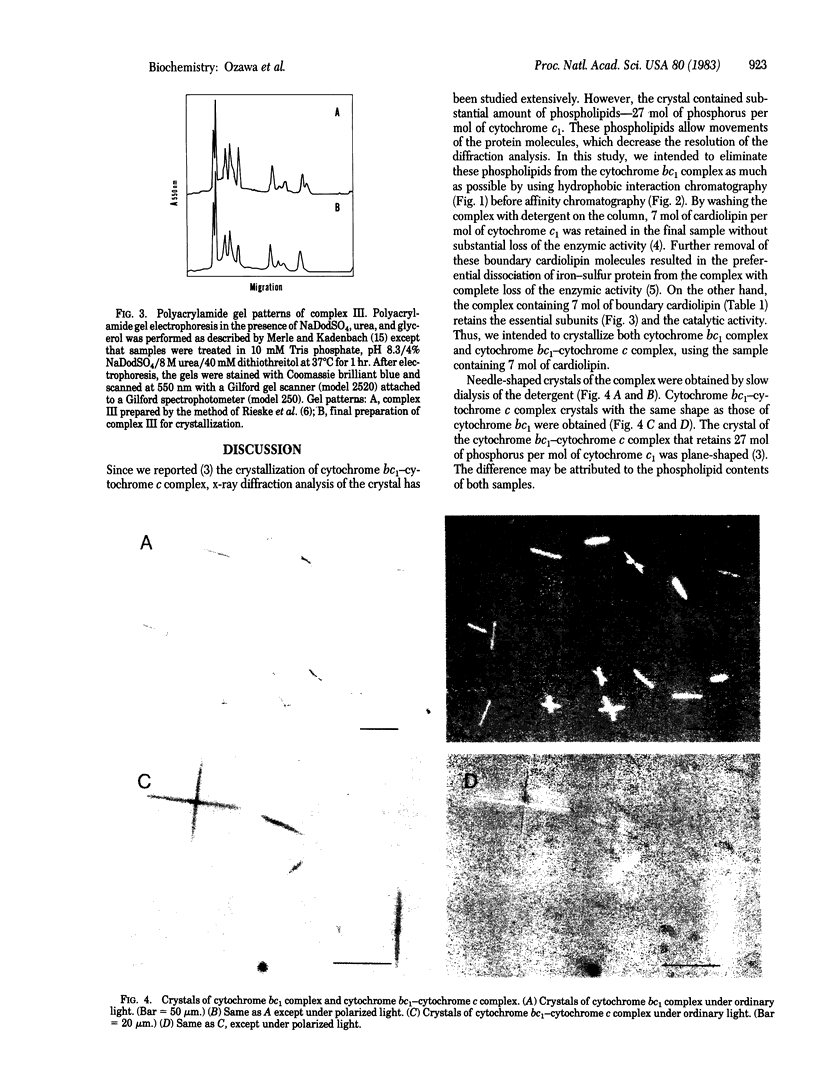
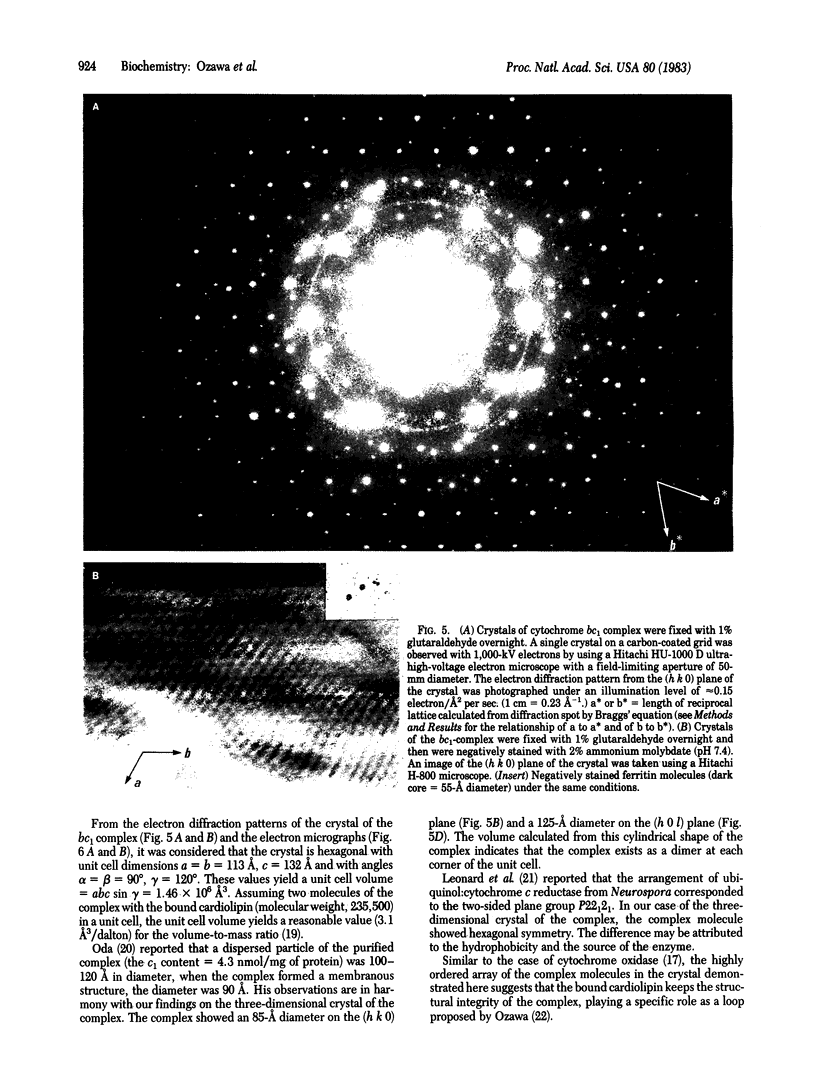
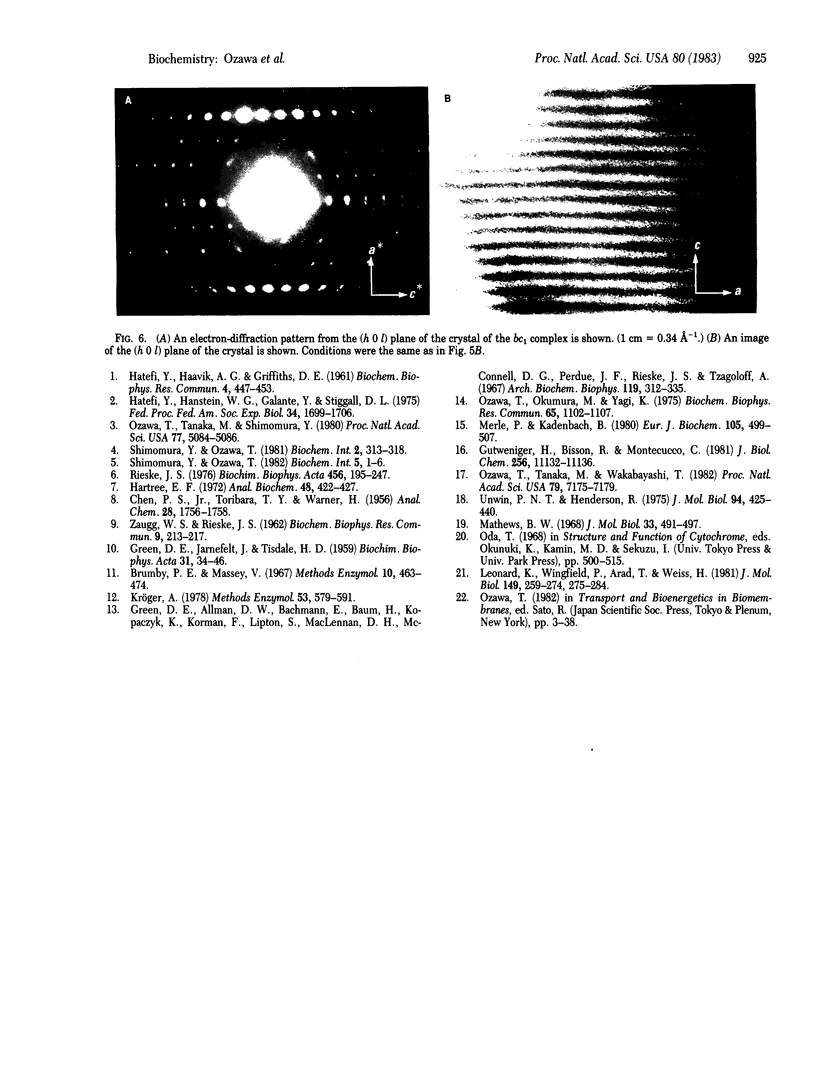
Complex III (cytochrome bc1 particle; ubiquinol:ferricytochrome c oxidoreductase, EC 1.10.2.2) was purified from beef heart mitochondria by a combination of hydrophobic interaction and affinity chromatography. By washing the complex with detergent on the hydrophobic interaction column, phospholipids were effectively depleted; 7 mol of cardiolipin per mol of cytochrome c1 was retained in the final sample. NaDodSO4 gel electrophoresis showed nine polypeptide subunits in the sample. The molecular weight of the complex was estimated to be approximately equal to 225,000 from the specific heme c1 content and the subunit composition. The purified complex was crystallized by slow removal of the detergent in which the complex was dispersed. Electron micrographs and electron diffraction patterns showed that the crystal is hexagonal with unit cell dimensions a = b = 113 A, c = 132 A, and with angles alpha = beta = 90 degrees, gamma = 120 degrees. The role of bound cardiolipin in the structural integrity of the complex was discussed.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GREEN D. E., JARNEFELT J., TISDALE H. D. Studies on the elecron transport system. XIV. The isolation and properties of soluble cytochrome c1. Biochim Biophys Acta. 1959 Jan;31(1):34–46. doi: 10.1016/0006-3002(59)90436-6. [DOI] [PubMed] [Google Scholar]
- Green D. E., Allmann D. W., Bachmann E., Baum H., Kopaczyk K., Korman E. F., Lipton S., MacLennan D. H., McConnell D. G., Perdue J. F. Formation of membranes by repeating units. Arch Biochem Biophys. 1967 Mar;119(1):312–335. doi: 10.1016/0003-9861(67)90461-4. [DOI] [PubMed] [Google Scholar]
- Gutweniger H., Bisson R., Montecucco C. Membrane topology of beef-heart ubiquinone-cytochrome c reductase (complex III). J Biol Chem. 1981 Nov 10;256(21):11132–11136. [PubMed] [Google Scholar]
- HATEFI Y., HAAVIK A. G., GRIFFITHS D. E. Reconstitution of the electron transport system II. Reconstitution of DPNH-cvtochrome c reductase. succinic-cytochrome c reductase and DPNH, succinic-cytochrome c reductase. Biochem Biophys Res Commun. 1961 Apr 28;4:447–453. doi: 10.1016/0006-291x(61)90306-0. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Hatefi Y., Hanstein W. G., Galante Y., Stiggall D. L. Mitochondrial ATP-Pi exchange complex and the site of uncoupling of oxidative phosphorylation. Fed Proc. 1975 Jul;34(8):1699–1706. [PubMed] [Google Scholar]
- Leonard K., Wingfield P., Arad T., Weiss H. Three-dimensional structure of ubiquinol:cytochrome c reductase from Neurospora mitochondria determined by electron microscopy of membrane crystals. J Mol Biol. 1981 Jun 25;149(2):259–274. doi: 10.1016/0022-2836(81)90301-6. [DOI] [PubMed] [Google Scholar]
- Matthews B. W. Solvent content of protein crystals. J Mol Biol. 1968 Apr 28;33(2):491–497. doi: 10.1016/0022-2836(68)90205-2. [DOI] [PubMed] [Google Scholar]
- Merle P., Kadenbach B. The subunit composition of mammalian cytochrome c oxidase. Eur J Biochem. 1980 Apr;105(3):499–507. doi: 10.1111/j.1432-1033.1980.tb04525.x. [DOI] [PubMed] [Google Scholar]
- Ozawa T., Okumura M., Yagi K. Purification of cytochrome oxidase by using Sepharose-bound cytochrome c. Biochem Biophys Res Commun. 1975 Aug 4;65(3):1102–1107. doi: 10.1016/s0006-291x(75)80499-2. [DOI] [PubMed] [Google Scholar]
- Ozawa T., Tanaka M., Shimomura Y. Crystallization of the middle part of the mitochondrial electron transfer chain: cytochrome bc1-cytochrome c complex. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5084–5086. doi: 10.1073/pnas.77.9.5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa T., Tanaka M., Wakabayashi T. Crystallization of mitochondrial cytochrome oxidase. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7175–7179. doi: 10.1073/pnas.79.23.7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieske J. S. Composition, structure, and function of complex III of the respiratory chain. Biochim Biophys Acta. 1976 Sep 27;456(2):195–247. doi: 10.1016/0304-4173(76)90012-4. [DOI] [PubMed] [Google Scholar]
- Unwin P. N., Henderson R. Molecular structure determination by electron microscopy of unstained crystalline specimens. J Mol Biol. 1975 May 25;94(3):425–440. doi: 10.1016/0022-2836(75)90212-0. [DOI] [PubMed] [Google Scholar]
- ZAUGG W. S., RIESKE J. S. The quantitative estimation of cytochrome b in sub-mitochondrial particles from beef heart. Biochem Biophys Res Commun. 1962 Oct 17;9:213–217. doi: 10.1016/0006-291x(62)90060-8. [DOI] [PubMed] [Google Scholar]










