Dear Editor,
Hepatic ischemia–reperfusion (I/R) injury is a significant medical emergency occurring upon trauma, hepatic surgery, and hemorrhagic shock which leads to acute liver failure, remote organ damage and may limit both liver transplantation and partial hepatectomy surgery. The release of inflammatory mediators is the consequence of ischemia/hypoxia and reperfusion-mediated hepatic injury. Upon severe I/R injury, endogenous danger signals activate the inflammasome complex recruiting caspase-1 cleaving pro-interleukin (IL)-1β into its mature form (Besnard et al., 2012). Upon the IL-1R/Toll-like receptor (TLR) activation and phosphorylation of IκB, NF-κB translocates into the nucleus, and initiates gene expression of IL-23, IL-6, IL-1β, and TGF-β (Llacuna et al., 2009). These mediators together with IL-1β favor the differentiation of IL-17A-producing (Th17) cells expressing IL-23 receptors allowing their expansion by IL-23 produced by innate immune cells (Bettelli et al., 2008). The inflammatory cytokines IL-1 and IL-17A are induced upon organ I/R injury, but their roles in hepatic I/R are elusive. We revisited the role of IL-1 and IL-17A using a model of hepatic I/R injury with 1 h partial ischemia (70%) followed by 6 h reperfusion resulting in hepatic damage.
First, we demonstrate upregulation of hepatic IL-1 and report that hepatic I/R injury, liver inflammation, and neutrophils infiltration are drastically attenuated in IL-1R1 deficient mice (Supplementary Figure S1). We reported before that IL-17A is upregulated in IL-1-dependent lung injury (Gasse et al., 2011), and show here that upon hepatic I/R injury, IL-17A expression is upregulated and IL-1R1 signaling dependent (Supplementary Figure S1F). Moreover, in the liver I/R model, liver damage along with neutrophils influx were significantly attenuated in the absence of IL-17RA signaling (Supplementary Figure S2), which is consistent with a previous report (Kono et al., 2011) and underscores the role of IL-17A in neutrophil recruitment and activation. Interestingly, IL-17RA deficiency or IL-17A antibody neutralization was associated with diminished IL-1β, KC and TGF-β expression suggesting that IL-17A is critical for the inflammatory response (Supplementary Figure S2F).
To confirm the role of IL-1 or IL-17A, we used a neutralization strategy. Using anakinra, IL-1ra, the soluble IL-1R antagonist, we found reduced centroacinar cell necrosis and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining with diminished neutrophil recruitment and serum alanine transaminase (ALT) activity. Furthermore, IL-17A antibody neutralization had a comparable protective effect on hepatic I/R injury and inflammation (Figure 1A). Therefore, we firmly established that either IL-1R1 or IL-17RA signaling are critically involved in hepatic I/R induced injury and inflammation. Further, IL-17A expression in the liver is reduced in IL-1ra treated mice, which is consistent with the findings in gene knock-out mice suggesting that IL-17A expression relying on IL-1R signaling (Figure 1A). We showed that the transcription of IL-6, IL-23p19, and transforming growth factor beta (TGF-β) which favor the production of IL-17A is induced upon I/R, and is IL-1R1-dependent (Supplementary Figure S1F). IL-23 plays a critical role in liver injury, neutrophil infiltration and IL-17A production was greatly attenuated in IL-23p19 knock-out mice (Supplementary Figure S2G), supporting an IL-1–IL-23–IL-17-axis as reported in experimental autoimmune encephalomyelitis and allergic asthma (Besnard et al., 2012).
Figure 1.
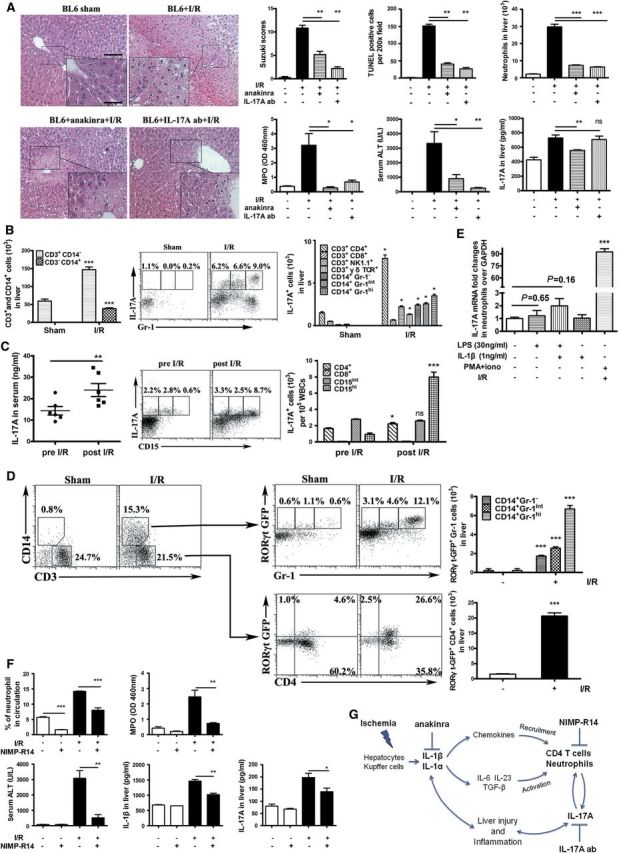
The role of neutrophil and the IL-1–IL-23–IL-17 axis in liver ischemia/reperfusion injury was assessed by performing hepatic I/R model on mice and acquired clinic specimen from patients who suffered liver I/R injury. (A) Representative H&E staining of liver from C57BL/6 and anakinra or IL-17A antibody treated mice (200×, 400×). IL-1R1 and IL-17A neutralization largely protect the liver from I/R injury, showed by Suzuki score, terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) positive cells counting, absolute number of liver infiltrated neutrophils analyzed by flow cytometry, liver MPO activity, and serum alanine transaminase (ALT) levels. IL-17A expression in liver homogenate (ELISA) was attenuated in anakinra treated mice subjected to I/R surgery. (B) Infiltrating mononuclear cells isolated from the mouse liver 6 h after I/R and controls were permeabilized, stained by specific antibodies and analyzed by FACS. Absolute number of CD3+ or CD14+ cells was described. IL-17A production was analyzed by gating on CD3−CD14+Gr-1hi neutrophils and CD3−CD14+Gr-1int macrophages. Absolute numbers of all IL-17A+ subpopulations in the liver were shown, CD4+ and Gr-1hi neutrophils presented as the main sources of IL-17A in mice liver. (C) Investigation on IL-17A in PBMC from human patients following partial hepatectomy. IL-17A serum level was measured by ELISA. CD15hi neutrophils were gated and examined for IL-17A expression. Total numbers of the different cell subpopulation expressing IL-17A are described suggesting that CD15hi neutrophil contribute to the IL-17A production in patients' PBMC. Results from flow cytometry are from one representative experiment of seven independent studies. (D) The Th17 lineage transcription factor expressed in neutrophils and CD4+ T cells. CD14+CD3− and CD14−CD3+ cells were gated, respectively. RORγt expressed in Gr-1hi neutrophils, as Gr-1int macrophages also have the potential to express RORγt. CD4+ T cells were selected in CD3+CD14− population, exhibited an increase of RORγt expression. (E) IL-17A mRNA expression was measured in neutrophils from naïve peritoneal which activated in vitro with LPS (30 ng/ml) ± IL-1β (1 ng/ml) for 12 h; neutrophils from the I/R challenged liver were restimulated with PMA/ionomycin only and mRNA was extracted as the same procedures; mRNA expression was analyzed as fold over GAPDH. (F) NIMP-R14 antibody was injected into C57BL/6 mice 12 h before I/R challenge to deplete neutrophils and peripheral blood neutrophils and liver MPO activity were measured. Neutrophil depletion results in reduced serum ALT levels, hepatic IL-1β and IL-17A at 6 h after reperfusion. (G) Proposed mechanisms of hepatic I/R injury and inflammation. I/R causes intestinal barrier injury allowing microbial leakage activating TLRs and release of danger-signals triggering the inflammasome complex resulting in IL-1α, IL-6, TGF-β, and IL-23 and chemokine expression leading to recruitment and activation of CD4+ T cells and neutrophils, which produce IL-17A, attract more neutrophils infiltrating the liver and augmenting hepatic I/R. The data represent mean ± SEM (n=6–8 mice per group from three independent experiments, *P< 0.05, **P<0.01, ***P<0.001).
IL-17A is required for neutrophil recruitment and angiogenesis, and plays a critical role in chronic inflammatory diseases such as rheumatoid arthritis, asthma, systemic lupus erythematosus, or allograft rejection (Bettelli et al., 2008). Previous reports showed that CD4+ T cells, NKT cells, γδ T cells and more recently innate lymphoid cells and Paneth cells produce IL-17A (Park et al., 2011). Furthermore, neutrophils may express IL-17A in inflamed synovium (Moran et al., 2011), and renal I/R (Cua and Tato, 2010). Since the source of IL-17A in liver I/R injury is uncertain, we isolated the cells from the liver of BL6 mice at 6 h after reperfusion and restimulated with phorbol 12-myristate 13-acetate (PMA) and ionomycin. Intracellular staining with IL-17A antibody and flow cytometry analysis revealed increased IL-17A+ liver infiltrating cells upon I/R when compared with sham control mice (Supplementary Figure S3A). To determine which cells express IL-17A, we gated on CD3−CD14+ and CD3+CD14− cells, respectively (Supplementary Figure S3B). We found that among T cell subsets, increased IL-17A expressing CD4+ T cells in the liver upon I/R when compared with sham operated controls, while CD8+, NK, and γδ T cells were unchanged (Figure 1B and Supplementary Figure 3C).
For CD3−CD14+ cells, we distinguished Gr-1+ intermediate (macrophages) and high cells (neutrophils) as described (Daley et al., 2008) and observed a drastic increase of IL-17A expressing cells upon I/R when compared with controls (Gr-1+ intermediate 0% versus 6.6% and Gr-1+ high 0.2% versus 9%), indicating that neutrophils, but also macrophages represent an important source of IL-17A (Figure 1B). Furthermore, we calculated the relative contribution to IL-17A production, from each population (Figure 1B). Therefore, CD4+ T cells, but also neutrophils and possibly macrophages, dendritic cells represent a major source of IL-17A producing infiltrating cells in the liver upon I/R in mice.
In order to explore the clinical relevance of the experimental data, IL-17A expression was investigated in patients with I/R. Informed consents for blood analysis were obtained prior to surgery and the study was approved by Institutional Ethics Committee of Nanjing Medical University. Peripheral blood mononuclear cells from seven patients suffering from early phase or localized hepatocellular carcinoma requiring partial hepatectomy were analyzed for IL-17A expression. IL-17A levels in serum are significantly higher at 6 h after reperfusion (Figure 1C), and IL-17A expression in blood cells is greatly upregulated (Supplementary Figure S3D). The percentage of IL-17A expressing CD4+ cells in peripheral blood mononuclear cells is slightly increased upon surgical ischemia, while CD8+ T cells were unchanged (Supplementary Figure S3E). Importantly, circulating CD15hi neutrophils expressing IL-17A were significantly increased (0.6% versus 8.7%) upon surgical intervention associated with I/R (Figure 1C) in peripheral blood. Therefore, neutrophils likely represent an important source of systemic IL-17A production in human patients (Figure 1C). Although NK cells greatly increased in the PBMC, NK, or NKT cells expressed low levels of IL-17A as shown by histograms and median fluorescence intensity (MFI) (Supplementary Figure S3F). Taken together, neutrophils and CD4+ T cells represent the major source of IL-17A in liver following experimental I/R in mice. In addition to a direct effect on hepatocytes and sinus endothelial cells, neutrophils may attract more inflammatory cells through IL-17A production upon hepatic I/R injury.
The morphological features of I/R injury consisting of massive microvacuolar degeneration of hepatocytes with apoptosis and necrosis in the centroacinar area with infiltration of neutrophils and mononuclear cells adhering to the sinusoidal endothelial lining in the liver (Figure 1A). Activation of the respiratory burst in neutrophils produces large quantity of superoxide and other effector molecules with cytotoxic properties which may have potent cytopathic effects (Llacuna et al., 2009). Recent reports suggest that neutrophils and other innate immune cells may express IL-17A (Ferretti et al., 2003). Since we show here that neutrophils are an important source of IL-17A, we asked whether they express the retinoic acid receptor-related orphan receptor-γt (RORγt) transcription factor. RORγt controls the lineage fate of IL-17 differentiation (Cua and Tato, 2010), but its expression in neutrophils is unknown. Therefore, we used RORγt-EGFP reporter mice to verify whether the hepatic infiltrating IL-17A+ neutrophils and CD4+ T cells express IL-17A relying on RORγt following hepatic I/R. Using flow cytometry analysis we gated CD14+ and CD3+ infiltrating neutrophils and CD4+ T cells expressing RORγt-EGFP (Figure 1D). During hepatic I/R, 12.1% Gr-1+ neutrophils exhibit RORγt-EGFP expression comprising 2.5% of the total infiltrating cells (Figure 1D), which is novel and unexpected. It is established that activated IL-17A+CD4+ T cells express RORγt (Bettelli et al., 2008). Here, upon I/R injury about 26.6% of all CD4+ T cells expressed RORγt, which is about 6% of the total infiltrating cells (Figure 1D), representing early innate IL-17 producing T cells. Furthermore, to ascertain that neutrophils may express IL-17A in vitro, we isolated neutrophils from I/R challenged mouse liver as well as thioglycollate elicited peritoneal lavage from naive mice, after that, Gr-1hiF4/80−CD3− cells were sorted by FACS and checked IL-17A expression upon combined IL-1β and/or LPS and/or PMA/ionomycin stimulation. Infiltrating activated neutrophils express IL-17A mRNA after restimulation with PMA/ionomycin (Figure 1E), but not naïve neutrophils stimulated with IL-1β and/or LPS. Recent reports indicated that neutrophils express IL-1R1 during inflammatory conditions (Mantovani et al., 2011), but do not respond to IL-1, suggesting an indirect activation through chemokines. Reduced neutrophil accumulation in IL-1R1 deficient mice is likely a result of the absence of the down-stream cytokines such as IL-6, IL-23, CXCL1/KC, and TGF-β (Mantovani et al., 2011). Those results together with the flow cytometry data are strong evidence that RORγt expressing neutrophil have the capacity to produce IL-17A.
Since neutrophils are recruited from the blood into liver, while T cell are a less prominent component of the hepatic infiltrate upon I/R (Figure 1B and C), we examined the role of neutrophils in hepatic I/R using a neutrophil depleting antibody, NIMP-R14 (Xiao et al., 2005). Neutrophils in peripheral circulation are significantly reduced 12 h after the injection of depleting antibody (Figure 1F). After I/R, NIMP-R14 injected mice have reduced neutrophils in blood and liver, and liver injury is dramatically reduced (Figure 1F). Importantly, IL-1β and IL-17A concentrations in liver homogenate are diminished following NIMP-R14 administration (Figure 1F). These results strongly suggest IL-17A producing neutrophils in peripheral blood or in liver may directly contribute to tissue injury and amplify the neutrophils recruitment into liver and augment further damage (Figure 1G).
In conclusion, our data emphasize a critical role of an IL-1–IL-23–IL-17 axis in the pathogenesis of I/R induced hepatic inflammation and damage. IL-17A released from RORγt+ neutrophils and CD4+ T cells is dependent on IL-1R1 signaling and downstream cytokines and mediators like IL-23p19. Hepatic I/R injury and neutrophil recruitment are attenuated in IL-1R1 deficient mice or by IL-1ra administration. Importantly, neutrophils depletion drastically reduced inflammation as well as IL-17A production. Therefore, neutrophils appear to amplify the response and locking the IL-1–IL-23–IL-17-axis or neutrophil depletion may prevent I/R injury, which may be of benefit to human patients following liver transplantation, hepatectomy or trauma.
[Supplementary material is available at Journal of Molecular Cell Biology online. This work was supported by ‘Agence Nationale pour la Recherche’ (ANR 2007 MIME-103-02), the ‘Fondation pour la Recherche Médicale’ (FRM allergy DAL 2007 0822007), the ‘Fond européen de développement regional’ (FEDER Asthme 1575-32168) and Le Studium Orleans, CNRS, Orléans, France and the National Natural Science Foundation (81225017, 91029721, 81072029 to B.S. and 81201528 to R.J.), National Basic Research Program of China (2012CB910800 to B.S.). We thank Prof. François Erard, Dieudonnée Togbe and Isabelle Maillet (CNRS UMR7377, Orleans) for advice and technical support.]
Supplementary Material
References
- Besnard A.G., Togbe D., Couillin I., et al. Inflammasome-IL-1-Th17 response in allergic lung inflammation. J. Mol. Cell Biol. 2012;4:3–10. doi: 10.1093/jmcb/mjr042. [DOI] [PubMed] [Google Scholar]
- Bettelli E., Korn T., Oukka M., et al. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cua D.J., Tato C.M. Innate IL-17-producing cells: the sentinels of the immune system. Nat. Rev. Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- Daley J.M., Thomay A.A., Connolly M.D., et al. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- Ferretti S., Bonneau O., Dubois G.R., et al. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J. Immunol. 2003;170:2106–2112. doi: 10.4049/jimmunol.170.4.2106. [DOI] [PubMed] [Google Scholar]
- Gasse P., Riteau N., Vacher R., et al. IL-1 and IL-23 mediate early IL-17A production in pulmonary inflammation leading to late fibrosis. PLoS One. 2011;6:e23185. doi: 10.1371/journal.pone.0023185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono H., Fujii H., Ogiku M., et al. Role of IL-17A in neutrophil recruitment and hepatic injury after warm ischemia-reperfusion mice. J. Immunol. 2011;187:4818–4825. doi: 10.4049/jimmunol.1100490. [DOI] [PubMed] [Google Scholar]
- Llacuna L., Mari M., Lluis J.M., et al. Reactive oxygen species mediate liver injury through parenchymal nuclear factor-kappaB inactivation in prolonged ischemia/reperfusion. Am. J. Pathol. 2009;174:1776–1785. doi: 10.2353/ajpath.2009.080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A., Cassatella M.A., Costantini C., et al. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- Moran E.M., Heydrich R., Ng C.T., et al. IL-17A expression is localised to both mononuclear and polymorphonuclear synovial cell infiltrates. PLoS One. 2011;6:e24048. doi: 10.1371/journal.pone.0024048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.W., Kim M., Brown K.M., et al. Paneth cell-derived interleukine-17A causes multiorgan dysfunction after hepatic ischemia and reperfusion injury. Hepatology. 2011;53:1662–75. doi: 10.1002/hep.24253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H., Heeringa P., Liu Z., et al. The role of neutrophils in the induction of glomerulonephritis by anti-myeloperoxidase antibodies. Am. J. Pathol. 2005;167:39–45. doi: 10.1016/S0002-9440(10)62951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


