Abstract
OBJECTIVE
Peroxynitrite, a toxic nitrogen species, has been implicated in the development of ischemia/reperfusion injury. The aim of the present study was to investigate the effects of the potent peroxynitrite decomposition catalyst, FP15, on myocardial, endothelial, and pulmonary function in an experimental model of cardioplegic arrest and extracorporal circulation.
METHODS
Twelve anesthetized dogs underwent hypothermic cardiopulmonary bypass. After 60 min of hypothermic cardiac arrest, reperfusion was started and either saline vehicle (control, n = 6) or FP15 (n = 6) was administered. Left-ventricular preload-recruitable stroke work (PRSW) was measured by a combined pressure–volume conductance catheter at baseline and after 60 min of reperfusion. Left anterior descending (LAD) coronary (CBF) and pulmonary blood flow (PBF), endothelium-dependent vasodilatation to acetylcholine (ACh), and alveolo–arterial O2 gradient were determined.
RESULTS
The administration of FP15 led to a significantly better recovery of PRSW (given as percent of baseline: 93 ± 9 vs 62 ± 6%, p < 0.05). CBF was also significantly higher in the FP15 group (44 ± 6 vs 25 ± 4 ml min−1, p < 0.05). Injection of ACh resulted in a significantly higher increase in CBF (70 ± 6 vs 35 ± 5%, p < 0.05) in the FP15-treated animals. The alveolo–arterial O2 gradient was significantly lower after FP15 administration (83 ± 7 vs 49 ± 6 mmHg, p < 0.05). Catalytic peroxynitrite decomposition did not affect baseline cardiovascular and pulmonary functions.
CONCLUSIONS
Application of FP15 improves myocardial, endothelial, and pulmonary function after cardiopulmonary bypass with hypothermic cardiac arrest. The observed protective effects imply that catalytic peroxynitrite decomposition could be a novel therapeutic option in the treatment of ischemia/reperfusion injury.
Keywords: Cardiopulmonary bypass, Ischemia/reperfusion injury, Peroxynitrite, FP15, Endothelial function
INTRODUCTION
Ischemia/reperfusion injury following cardiac surgery is a common condition, which develops after cardiopulmonary bypass (CPB) operations with cardioplegic arrest. Moreover, temporary dysfunction of the heart can be observed frequently, presumably as a consequence of this phenomenon. Even if cardiac dysfunction is not always clinically remarkable, reduction of myocardial contractility may occur as described in a human study using pressure–volume relationships [1,2]. In addition, coronary endothelial and peripheral vascular dysfunction may further complicate the postoperative course [3].
There is evidence that pulmonary injury (ranging from sub-clinical functional changes to acute respiratory distress syndrome (ARDS)) occurs in the context of cardiac surgery as a consequence of extracorporal circulation-induced inflammatory reaction and reduced pulmonary perfusion [4]. Significantly increased alveolar–arterial oxygen-pressure difference and pulmonary shunt fraction, together with decreased functional residual capacity and carbon-monoxide-transfer factor, have frequently been observed in patients after CPB.
Extracorporal circulation is also known to induce systemic inflammatory reactions [5], with leucocyte activation and uncontrolled release of toxic oxidants and free radicals. A toxic nitrogen species, peroxynitrite, is formed by the reaction of the vascular mediator NO and the superoxide anion radical. Potential biological actions of peroxynitrite include: cardiac depression, lipid peroxidation, nitration of tyrosine residues on proteins, inducing DNA strand breaks and activation of the poly(adenosine diphosphate (ADP)-ribose) polymerase (PARP) pathway [6,7]. Increased nitrotyrosine staining (as a ‘footprint’ of peroxynitrite) has been reported in the myocardial tissue of experimental animals [8] and patients [9] undergoing cardiac surgery with CPB and cardioplegic arrest. These studies suggest the important role of reactive nitrogen species (and especially peroxynitrite) in the development of myocardial and endothelial dysfunction after CPB.
A previous study has shown that a peroxynitrite scavenger effectively reduced systemic inflammatory response in a rodent model of CPB [10]. Another work from our research group demonstrated improved cardiovascular functions after CPB by interrupting the peroxynitrite–PARP pathway via pharmacological PARP inhibition [11]. However, to date there are no data on the effects of pharmacological peroxynitrite decomposition on cardiovascular and pulmonary function after CPB. Peroxynitrite reacts very rapidly and efficiently with synthetic metalloporphyrins. One of them, FP15, an N-polyethyleneglycol (PEG)ylated-2-pyridyl iron porphyrin, has shown a superior performance as a peroxynitrite decomposition catalyst [12]. The aim of our study was to investigate the effect of FP15 on ischemia/reperfusion injury in a clinically relevant canine model of CPB with cardioplegic arrest [13–15].
MATERIALS AND METHODS
Animals, experimental groups
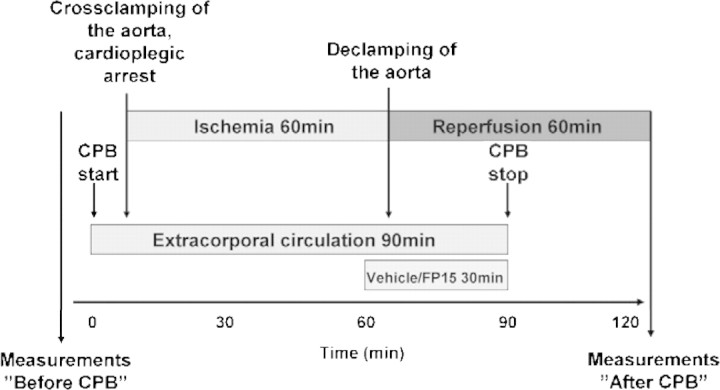
Twelve dogs (foxhounds) weighing 23–32 kg (26 ± 2 kg) were used in this experiment. All animals received humane care in compliance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH Publication No. 86-23, revised 1996). The experiments were approved by the Ethical Committee of the Land Baden-Württemberg for Animal Experimentation. Six animals received FP15 (FeCl tetrakis-2-(triethylene glycol monomethyl ether) pyridyl porphyrin) (0.3 mg kg−1 bodyweight (BW), a dose comparable to that used in previous studies [16–18]) as a short IV infusion starting 5 min before aortic declamping and continued during the first 25 min of reperfusion (Fig. 1). Six animals treated with vehicle served as controls.
Figure 1:
Timeline of the experiment. Schematic representation of the experiment timeline. CPB: cardiopulmonary bypass.
CPB
Surgical preparation and general management during the operation were performed as described previously [11,13–15]. After systemic anticoagulation with heparin (300 U kg−1), the left subclavian artery was cannulated for arterial perfusion. The venous cannula was placed in the right atrium. The extracorporal circuit consisted of a heat exchanger, a venous reservoir, a roller pump, and a membrane oxygenator primed with Ringer’s lactate solution (1000 ml) supplemented with heparin (150 U kg−1) and 20 ml sodium bicarbonate (8.4%). Hypothermic CPB was performed for 90 min at a lowest temperature of 28 °C. After initiation of CPB, the hearts were arrested with 1000-ml crystalloid cardioplegia (Custodiol®). After 60 min, the aortic cross-clamp was released, and the reperfusion phase was initiated. After weaning from CPB, heparin was antagonized by protamine, and the animals were monitored for 1 h (Fig. 1).
Cardiac- and pulmonary functional measurements were performed at baseline (before CPB) and 60 min after starting reperfusion (after treatment and CPB, Fig. 1). After completing the experiment, all animals were euthanized.
Hemodynamic measurements – cardiac function
Aortic flow was monitored with a perivascular ultrasonic probe attached to the ascending aorta. Arterial pressure was monitored with 6F Millar catheter inserted into the abdominal aorta via the femoral artery (Millar Instruments, Houston, TX, USA). Left-ventricular (LV) pressures and volumes were measured by combined 6F Millar pressure–volume conductance catheters with 6-mm spacing, which were inserted into the left ventricle via the apex. Cardiac output as the equivalent of aortic flow was monitored continuously. Stroke volume was calculated from the integrated flow signal and was used to calibrate the volume signal from the conductance catheter. Parallel conductance was estimated by rapid injection of 1 ml of hypertonic saline into the left atrium.
LV pressure–volume loops were constructed on-line. Vena-cava occlusions were performed to obtain a series of loops for calculation of the slope (Ees) of the LV end-systolic pressure–volume relationships (ESPVRs). Ees and preload-recruitable stroke work (PRSW) were calculated as load-independent indices of myocardial contractility.
Coronary blood flow (CBF) was measured by an ultrasonic probe placed on the left anterior descending coronary artery. Coronary endothelium-dependent vasodilatation was assessed after intracoronary administration of a single bolus of acetylcholine (ACh, 10−7 mol) and endothelium-independent vasodilatation after sodium nitroprusside (SNP, 10−4 mol) administration. The vasoresponse was expressed as percent change of baseline CBF.
Pulmonary function
In addition to routine blood-gas analysis, blood gases were determined before and after weaning from CPB at 60 min of reperfusion at room-air ventilation. Pulmonary function was characterized by the alveolar–arterial oxygen difference, which was calculated according to the standard formulas. In addition, the left-lower-lobar pulmonary artery was dissected, blood flow was measured by a 4-mm-diameter ultrasonic flow probe, and pulmonary vascular resistance was calculated. Pulmonary endothelium-dependent vasodilatation was assessed after intra-arterial administration of a single bolus of ACh (10−7 mol). The vasoresponse was expressed as percentage change of PBF.
Materials
All materials used were purchased from Sigma–Aldrich (Taufkirchen, Germany), unless specified otherwise. FP15 was dissolved in 5% glucose solution.
Statistical analysis
All values were expressed as mean ± standard error of the mean (SEM). A paired t-test was used to compare two means within a group (comparison of ‘before’ and ‘after’ values). Means between the groups were compared by an unpaired two-sided Student’s t-test (comparison of control and FP15 groups). A p value < 0.05 was considered statistically significant.
RESULTS
Hemodynamic parameters
Hemodynamic variables are shown in Table 1 . Baseline parameters did not differ between the groups and were within the physiological range. Heart rate (HR) did not change either in the control or in the FP15 group. After 60 min of cardioplegic arrest and 60 min of reperfusion, cardiac output (CO) decreased significantly (p < 0.05) in the control group, while it remained unchanged in the FP15 group. There was a significant decrease in mean arterial pressure values after CPB in both groups.
Table 1:
Hemodynamic variables
| Before CPB |
After CPB |
|||
|---|---|---|---|---|
| Control | FP15 | Control | FP15 | |
| HR (min−1) | 123 ± 10 | 115 ± 8 | 109 ± 16 | 119 ± 9 |
| MAP (mmHg) | 91 ± 8 | 86 ± 7 | 67 ± 9 | 68 ± 7# |
| CO (l min−1) | 2.56 ± 0.47 | 2.73 ± 0.38 | 1.98 ± 0.24* | 2.56 ± 0.30 |
Heart rate (HR), mean arterial pressure (MAP) and cardiac output (CO) values are shown before and after cardiopulmonary bypass (CPB) in both groups. All values are given as mean ± SEM.
#p < 0.05 versus before CPB.
* p < 0.05 versus control.
LV systolic and diastolic function
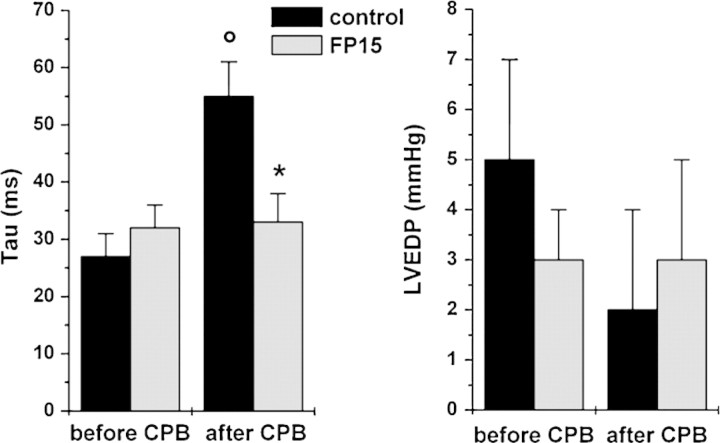
LV function – characterized by the load-independent, sensitive contractility indices Ees and PRSW (Fig. 2) – showed a significant decrease (p < 0.05) after extracorporal circulation and reperfusion in the control group, while it remained unchanged in the FP15-treated group. The myocardial relaxation constant (Tau) increased significantly (p < 0.05) in the control group at 60 min of reperfusion, but it remained at baseline level in the FP15 group (Fig. 3).
Figure 2:
Left-ventricular systolic function. The slope of the left-ventricular end-systolic pressure–volume relationship (Ees, left side) and preload-recruitable stroke work (PRSW, right side) before and after cardiopulmonary bypass (CPB) at 60 min of reperfusion. All values are given as mean ± SEM; o: p < 0.05 versus before CPB, *: p < 0.05 versus control.
Figure 3:
Left-ventricular diastolic function. Time constant of left-ventricular pressure decay (Tau, left side) and left-ventricular end-diastolic pressure (LVEDP, right side) before and after cardiopulmonary bypass (CPB) at 60 min of reperfusion. All values are given as mean ± SEM; o: p < 0.05 versus before CPB, *: p < 0.05 versus control.
CBF and vascular function
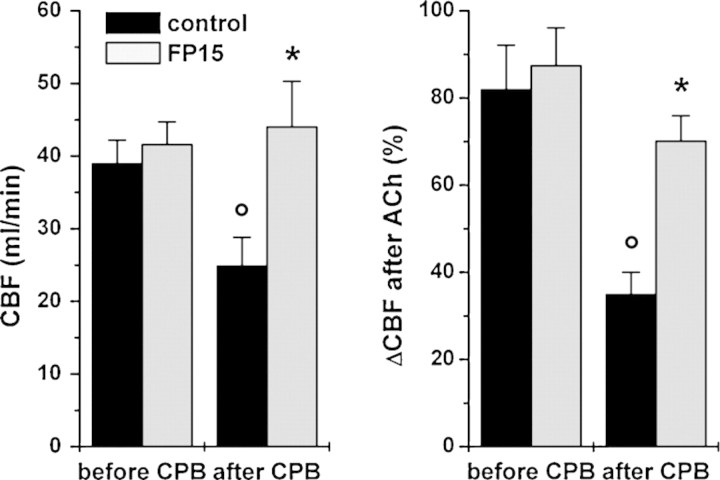
CBF was similar in both groups before cardioplegic arrest. After 60 min of reperfusion, the control group showed significantly decreased CBF, but, in the FP15 group, CBF remained unchanged after CPB (Fig. 4). Endothelium-dependent coronary vasodilatation after ACh was significantly reduced in control animals after 60 min of reperfusion in comparison to pre-CPB values, which was prevented by FP15 treatment (Fig. 4). Endothelium-independent vasodilatation – as reflected by the increase of CBF after SNP injection – did not differ either between groups or over time (67 ± 6% before CPB vs 58 ± 8% after CPB in controls and 71 ± 9% before CPB vs 66 ± 11% after CPB in the FP15 group).
Figure 4:
Coronary arterial function. Coronary blood flow (CBF, left side) and endothelium-dependent vasodilatation after acetylcholine (ACh, 10−7 mol, right side) expressed as percent change of CBF before and after cardiopulmonary bypass (CPB) at 60 min of reperfusion. All values are given as mean ± SEM; o: p < 0.05 versus before CPB, *: p < 0.05 versus control.
Pulmonary function
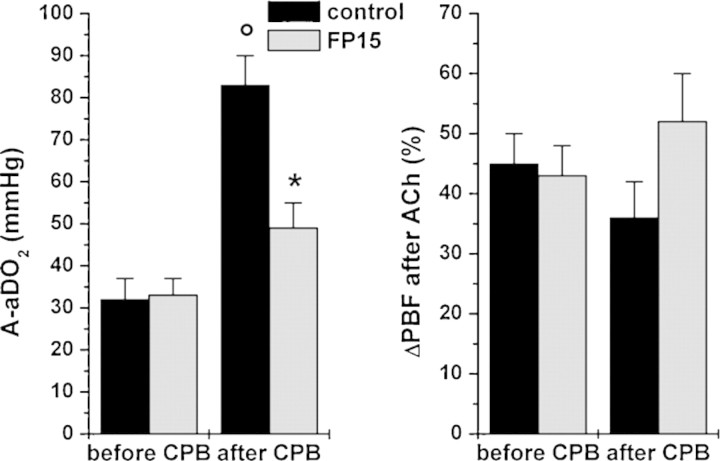
Alveolar–arterial oxygen difference at room-air ventilation was similar in both groups at baseline and increased significantly after CPB in the control group, indicating pulmonary dysfunction, while it remained unchanged in the FP15 group (Fig. 5). Pulmonary vasodilatory response to ACh did not differ either between groups or over time.
Figure 5:
Pulmonary function. Alveolar–arterial oxygen difference (A-aDO2) at room-air ventilation (left side), and endothelium-dependent pulmonary arterial vasodilatation after acetylcholine (ACh, 10−7 mol, right side) expressed as percent change of pulmonary blood flow (PBF) before and after cardiopulmonary bypass (CPB) at 60 min of reperfusion. All values are given as mean ± SEM; o: p < 0.05 versus before CPB, *: p < 0.05 versus control.
DISCUSSION
In this study, the benefits of application of the peroxynitrite decomposition catalyst FP15 during reperfusion were assessed in a canine model of crystalloid cardioplegia and extracorporal circulation. In accordance with the literature [1,3], hypothermic cardioplegic arrest and reperfusion resulted in a decline in LV, pulmonary, and endothelial function. To the best of our knowledge, this is the first study demonstrating that pharmacological peroxynitrite decomposition improves cardiovascular recovery and pulmonary function in terms of pulmonary endothelial function and oxygenation during early reperfusion in a clinically relevant animal model of CPB.
Myocardial and endothelial damage with temporary cardiac dysfunction is a well-described phenomenon in the context of cardiac surgery. Hearts undergoing coronary bypass surgery or other surgical procedures requiring CPB and elective cardioplegia experience episodes of global ischemia and reperfusion, which leads to endothelial injury as well as contractile dysfunction and morphological injury despite the use of cardioprotective cardioplegic solutions and other strategies for myocardial protection [3].
Due to leucocyte activation in the extracorporal circuit and during reperfusion after global myocardial ischemia, high levels of reactive oxygen radicals and other related oxidants are produced and are central mediators of reperfusion injury. A potent oxidant species, peroxynitrite (ONOO−) is formed by the reaction of superoxide anion radical (O2−) and the vascular mediator nitric oxide (NO). Due to its high diffusibility across lipid membranes in the protonated form, peroxynitrite can easily penetrate cells and tends to attack various biomolecules and cellular structures, thereby inactivating functionally important receptors and enzymes and causing various forms of DNA injury (strand breaks and base modifications). Peroxynitrite-induced DNA damage leads to the activation of the nuclear enzyme PARP, which initiates an energy-consuming metabolic cycle by transferring ADP-ribose units from nicotinamide adenine dinucleotide (NAD+) to nuclear proteins. This process results in rapid depletion of intracellular ATP pools, eventually leading to cellular dysfunction and death [19]. There are several studies in the literature showing the activation of the peroxynitrite–PARP pathway during extracorporal circulation [9,11], thereby offering the possibility of therapeutic intervention. Accordingly, inhibition of the pathway by scavenging peroxynitrite with quercetin has been reported to reduce the systemic inflammatory response after CPB in a rodent model [10]. In previous studies, our research group showed that pharmacological inhibition of PARP improves myocardial and endothelial dysfunction after CPB and also after heart transplantation [11,20]. Pharmacological catalytic decomposition of peroxynitrite with FP15 has been demonstrated to effectively eliminate peroxynitrite and prevent PARP activation both in vitro and in vivo [12,18], thereby improving cardiovascular function in various disease models.
For a sensitive and reliable assessment of LV myocardial contractility in the present study, we calculated the pressure–volume loop-derived, load-independent indices, Ees (slope of ESPVR) and PRSW. These parameters clearly demonstrated a significant decrease of contractile function of the left ventricle, which is consistent with previous studies [1]. Similar to the effects of PARP inhibition [11], pharmacological peroxynitrite decomposition completely prevented LV contractile dysfunction as indicated by the assessed load-independent contractility indices; moreover, diastolic dysfunction after CPB as characterized by the prolonged time constant of LV pressure decay (Tau) has been significantly improved (Figs. 2 and 3). The reported beneficial cardiac effects of FP15 on global cardiac ischemia/reperfusion are in correspondence with the results of Bianchi et al., showing reduced infarct size and improved mycardial function after FP15 treatment in a porcine coronary artery ligation model [17].
Although enhanced formation of reactive oxygen species (ROS) has been reported to occur in both cardiomyocytes and endothelial cells [21], leucocyte–endothelial cell interactions and increased release of ROS from leucocytes affect first and foremost mainly the endothelium, resulting in endothelial dysfunction. The damaged dysfunctional coronary endothelium is responsible for the impaired endothelial vasodilatory function of coronary arteries, which limits CBF (as demonstrated in the present experiments, Fig. 4) and triggers a range of problems including platelet and leucocyte adhesion and aggregation, leading to impaired cardiac performance. The present study is the first which demonstrates that catalytic peroxynitrite decomposition improves not only myocardial but also endothelial function after CPB. The observed endothelial effect is comparable to those with application of nitric-oxide precursors [22] or PARP inhibitors [11]. As pharmacological elimination of peroxynitrite restores adenosine triphosphate (ATP) levels, this may contribute to improved endothelial function.
As pulmonary dysfunction is common after cardiac surgery (overviewed in Ref. [4]), we assessed pulmonary function in terms of alveolar–arterial oxygen gradient as well as pulmonary vascular function. Corresponding to the literature, we observed deteriorated gas exchange and a tendency toward impaired pulmonary endothelial function after extracorporal circulation in the control group. Summarizing the recent literature, pulmonary dysfunction after extracorporal circulation is the result of multiple insults, which include general anesthesia, thoracotomy, and breach of the pleura, as well as blood contact with artificial material, hypothermia, pulmonary ischemia and lung ventilatory arrest [4,5]. Many of these factors may induce an inflammatory cascade with subsequent free-radical production. Naidu et al. [23] reported a reduction of lung injury (reduced accumulation of neutrophils and attenuated pro-inflammatory cytokine expression) after application of FP15 in a rat model of lung ischemia/reperfusion. Beneficial effects of WW85, another peroxynitrite decomposition catalyst, have also been shown on lung injury induced by intratracheal application of lipopolysaccharide and mechanical ventilation [24]. While previous studies focused on histological and biochemical changes, the present study demonstrates a functional improvement (preserved gas exchange) by pharmacological peroxynitrite decomposition after lung injury in a model of CPB.
Regarding the improved cardiac, endothelial, and pulmonary function, rapid pharmacological decomposition of peroxynitrite by FP15 in our model seems to be comparable to or better than the efficacy of blocking the pathway by PARP inhibition. By eliminating peroxynitrite, nitro-oxidative stress can be reduced and severe damage of DNA can be effectively prevented. In addition, using this concept we can avoid peroxynitrite-induced modifications of enzymes, receptors, and structural proteins and may help to restore the normal bioavailability of the crucial physiological mediator nitric oxide (as confirmed by the improved endothelium-dependent vasodilatation).
Based on the data presented in the current report, we propose that pharmacological decomposition of peroxynitrite may represent a potential therapy approach to reduce ischemia/reperfusion injury during cardiac surgery.
Funding
This work was supported by the Land Baden-Württemberg, by the German Research Foundation (SFB 414), and by a grant from the National Development Agency of Hungary (TÁMOP 4.2.2-08/1/KMR-2008-0004).
Conflict of interest: none declared.
ACKNOWLEDGMENTS
The expert technical assistance of Christiane Miesel-Gröschel, Karin Sonnenberg, and Lutz Hoffmann is gratefully acknowledged.
REFERENCES
- 1.Wallace A, Lam HW, Nose PS, Bellows W, Mangano DT. Changes in systolic and diastolic ventricular function with cold cardioplegic arrest in man. The Multicenter Study of Perioperative Ischemia (McSPI) Research Group. J Card Surg. 1994;9:497–502. doi: 10.1111/jocs.1994.9.3s.497. [DOI] [PubMed] [Google Scholar]
- 2.Lewis ME, Al Khalidi AH, Townend JN, Coote J, Bonser RS. The effects of hypothermia on human left ventricular contractile function during cardiac surgery. J Am Coll Cardiol. 2002;39:102–8. doi: 10.1016/s0735-1097(01)01694-1. [DOI] [PubMed] [Google Scholar]
- 3.Vinten-Johansen J, Sato H, Zhao ZQ. The role of nitric oxide and NO-donor agents in myocardial protection from surgical ischemia-reperfusion injury. Int J Cardiol. 1995;50:273–1. doi: 10.1016/0167-5273(95)02388-d. [DOI] [PubMed] [Google Scholar]
- 4.Ng CS, Wan S, Yim AP, Arifi AA. Pulmonary dysfunction after cardiac surgery. Chest. 2002;121:1269–7. doi: 10.1378/chest.121.4.1269. [DOI] [PubMed] [Google Scholar]
- 5.Wan S, LeClerc JL, Vincent JL. Inflammatory response to cardiopulmonary bypass: mechanisms involved and possible therapeutic strategies. Chest. 1997;112:676–92. doi: 10.1378/chest.112.3.676. [DOI] [PubMed] [Google Scholar]
- 6.Szabó C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–80. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 7.Jagtap P, Szabó C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005;4:421–40. doi: 10.1038/nrd1718. [DOI] [PubMed] [Google Scholar]
- 8.Fischer UM, Klass O, Stock U, Easo J, Geissler HJ, Fischer JH, Bloch W, Mehlhorn U. Cardioplegic arrest induces apoptosis signal-pathway in myocardial endothelial cells and cardiac myocytes. Eur J Cardiothorac Surg. 2003;23:984–90. doi: 10.1016/s1010-7940(03)00113-1. [DOI] [PubMed] [Google Scholar]
- 9.Mehlhorn U, Krahwinkel A, Geissler HJ, LaRosee K, Fischer UM, Klass O, Suedkamp M, Hekmat K, Tossios P, Bloch W. Nitrotyrosine and 8-isoprostane formation indicate free radical-mediated injury in hearts of patients subjected to cardioplegia. J Thorac Cardiovasc Surg. 2003;125:178–83. doi: 10.1067/mtc.2003.97. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi Y, Sawa Y, Nishimura M, Fukuyama N, Ichikawa H, Ohtake S, Nakazawa H, Matsuda H. Peroxynitrite, a product between nitric oxide and superoxide anion, plays a cytotoxic role in the development of post-bypass systemic inflammatory response. Eur J Cardiothorac Surg. 2004;26:276–80. doi: 10.1016/j.ejcts.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 11.Szabó G, Soós P, Mandera S, Heger U, Flechtenmacher C, Bährle S, Seres L, Cziráki A, Gries A, Zsengellér Z, Vahl CF, Hagl S, Szabó C. INO-1001 a novel poly(ADP-ribose) polymerase (PARP) inhibitor improves cardiac and pulmonary function after crystalloid cardioplegia and extracorporal circulation. Shock. 2004;21:426–32. doi: 10.1097/00024382-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Szabó C, Mabley JG, Moeller SM, Shimanovich R, Pacher P, Virag L, Soriano FG, Van Duzer JH, Williams W, Salzman AL, Groves JT. FP 15, a novel potent peroxynitrite decomposition catalyst: in vitro cytoprotective actions and protection against diabetes mellitus and diabetic cardiovascular complications. Mol Med. 2002;8:571–80. [PMC free article] [PubMed] [Google Scholar]
- 13.Szabó G, Radovits T, Veres G, Krieger N, Loganathan S, Sandner P, Karck M. Vardenafil protects against myocardial and endothelial injuries after cardiopulmonary bypass. Eur J Cardiothorac Surg. 2009;36:657–64. doi: 10.1016/j.ejcts.2009.03.065. [DOI] [PubMed] [Google Scholar]
- 14.Radovits T, Korkmaz S, Miesel-Gröschel C, Seidel B, Stasch JP, Merkely B, Karck M, Szabó G. Pre-conditioning with the soluble guanylate cyclase activator Cinaciguat reduces ischaemia-reperfusion injury after cardiopulmonary bypass. Eur J Cardiothorac Surg. 2011;39:248–55. doi: 10.1016/j.ejcts.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 15.Szabó G, Seres L, Soós P, Gorenflo M, Merkely B, Horkay F, Karck M, Radovits T. Tetrahydrobiopterin improves cardiac and pulmonary function after cardiopulmonary bypass. Eur J Cardiothorac Surg. 2011;40:695–700. doi: 10.1016/j.ejcts.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 16.Pacher P, Liaudet L, Bai P, Mabley JG, Kaminski PM, Virág L, Deb A, Szabó E, Ungvári Z, Wolin MS, Groves JT, Szabó C. Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction. Circulation. 2003;107:896–904. doi: 10.1161/01.cir.0000048192.52098.dd. [DOI] [PubMed] [Google Scholar]
- 17.Bianchi C, Wakiyama H, Faro R, Khan T, McCully JD, Levitsky S, Szabó C, Sellke FW. A novel peroxynitrite decomposer catalyst (FP-15) reduces myocardial infarct size in an in vivo peroxynitrite decomposer and acute ischemia-reperfusion in pigs. Ann Thorac Surg. 2002;74:1201–7. doi: 10.1016/s0003-4975(02)03953-x. [DOI] [PubMed] [Google Scholar]
- 18.Radovits T, Seres L, Gero D, Lin LN, Beller CJ, Chen SH, Zotkina J, Berger I, Groves JT, Szabó C, Szabó G. The peroxynitrite decomposition catalyst FP15 improves ageing-associated cardiac and vascular dysfunction. Mech Ageing Dev. 2007;128:173–81. doi: 10.1016/j.mad.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Virág L, Szabó C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- 20.Szabó G, Bährle S, Stumpf N, Sonnenberg K, Szabó EE, Pacher P, Csont T, Schulz R, Dengler TJ, Liaudet L, Jagtap PG, Southan GJ, Vahl CF, Hagl S, Szabó C. Poly(ADP-ribose) polymerase inhibition reduces reperfusion injury after heart transplantation. Circ Res. 2002;90:100–6. doi: 10.1161/hh0102.102657. [DOI] [PubMed] [Google Scholar]
- 21.Zweier JL, Talukder MA. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res. 2006;70:181–90. doi: 10.1016/j.cardiores.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 22.Soós P, Andrási T, Buhmann V, Kohl B, Vahl C, Hagl S, Szabó G. Myocardial protection after systemic application of l-arginine during reperfusion. J Cardiovasc Pharmacol. 2004;43:782–8. doi: 10.1097/00005344-200406000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Naidu BV, Fraga C, Salzman AL, Szabo C, Verrier ED, Mulligan MS. Critical role of reactive nitrogen species in lung ischemia-reperfusion injury. J Heart Lung Transplant. 2003;22:784–93. doi: 10.1016/s1053-2498(02)00556-9. [DOI] [PubMed] [Google Scholar]
- 24.Vaschetto R, Kuiper JW, Musters RJ, Eringa EC, Della Corte F, Murthy K, Groeneveld AB, Plötz FB. Renal hypoperfusion and impaired endothelium-dependent vasodilation in an animal model of VILI: the role of the peroxynitrite-PARP pathway. Crit Care. 2010;14:R45. doi: 10.1186/cc8932. [DOI] [PMC free article] [PubMed] [Google Scholar]