Abstract
Objective To evaluate the effectiveness of electronic monitoring and feedback to improve adherence in children taking daily asthma controller medications. Method Five patients with asthma and considered nonadherent participated. Inhalers were electronically monitored with the MDILogIITM device, and feedback was given by medical staff. Using a nonconcurrent multiple-baseline design, patients and their parents received bimonthly feedback regarding medication use. Following treatment, feedback was withdrawn and effects of monitoring alone were observed. Results Three participants showed improvements in adherence following treatment, with more notable increases when baseline adherence was low. Improvements in the inhaler technique occurred for all patients. Some patients demonstrated improvements in lung functioning and functional severity. When feedback was withdrawn, adherence decreased for some participants, but technique improvements maintained. Conclusions Results support the use of objective monitoring devices for assessing pediatric asthma patients’ adherence and indicate that feedback from medical staff may improve and maintain medication adherence for some patients.
Keywords: adherence, electronic monitoring, intervention, pediatric asthma
Introduction
Treatment of asthma typically involves some combination of daily maintenance medications that serve as anti-inflammatory agents (e.g., inhaled corticosteroids) and medications that produce fast-acting relief for acute symptoms (e.g., beta-adrenergic agonists or bronchodilators; U.S. Department of Health and Human Services, 2007). Although medication management coupled with avoidance of environmental triggers is effective, nonadherence with treatment regimens remains a particularly problematic issue (McQuaid, Kopel, Klein, & Fritz, 2003). Rates of nonadherence to medication in pediatric asthma samples vary depending on the measurement methods and type of medication but range from 10% to 90% (Baum & Creer, 1986; Bender, Milgrom, & Rand, 1997; Lemanek, 1990; McQuaid et al., 2003).
Electronic monitoring has emerged as a standard method for assessing patients’ adherence to inhaled medication because it has been found to be more accurate than other measures, although due to the expensiveness and technological limitations of devices, it is not yet considered the “gold standard” (Quittner, Modi, Lemanek, Ievers-Landis, & Rapoff, 2008). Compared to self-report (e.g., asthma diary cards), electronic monitoring has shown that patients consistently over-report their use of asthma maintenance medications (e.g., Bender et al., 2000; Berg, Dunbar-Jacob, & Rohay, 1998; Milgrom et al., 1996). Riekert and Rand (2002) reviewed the advantages and disadvantages of electronic monitoring devices. In short, these devices are seen as objective and reliable ways to examine adherence and its relation to health outcomes. Riekert and Rand suggested that electronic monitoring devices are useful in behavioral interventions to promote adherence, though one major limitation is their high cost.
Despite the advantages of electronic monitoring, these devices are not routinely used as part of an intervention to improve medication adherence. In the few published pediatric asthma studies that used electronic monitoring as part of an intervention, the devices measured medication adherence rather than functioning as an actual intervention component (e.g., Berg, Rapoff, Snyder, & Belmont, 2007; Celano et al., 2010; da Costa, Rapoff, Lemanek, & Goldstein, 1997; Rohan et al., 2010). On the other hand, a study by Bartlett and colleagues (2002) conducted a nurse-administered in-home intervention with inner-city youth using electronic monitoring as part of a multifaceted intervention and demonstrated an increase in the percent of participants using their medications as prescribed over the course of the 4-week intervention. One limitation of this study was that the time and training requirements for four to five home visits by a nurse are unlikely to be feasible on a larger scale. In a recent study with a clinic-based intervention similar to our own, electronic monitoring and feedback about inhaler use improved adherence but did not result in clinically significant improvements in lung function (Burgess, Sly, & Devadason, 2010). It is also noteworthy that this study did not investigate the effect of feedback on patient's inhaler technique.
For many asthma interventions within a typical outpatient clinic setting, success depends on ease of implementation. If implementation requires extensive, time-consuming procedures to achieve patient adherence, it may prove too cumbersome for general practice. Although the complexity presented by some patients—medically or psychosocially—may require highly involved support plans, for the most part psychologist-guided behavioral interventions are most useful when medical staff can implement them easily. In line with this thinking, a few studies have included nurses or physicians in treatments designed to improve children's adherence with asthma medication (e.g., Bartlett et al., 2002; Peterson, 1992). Because medical providers focus on delivering “standard” medical care (e.g., assessment of lung function and provision of medication instructions), psychologists have been responsible for implementing behavioral interventions to improve adherence. Yet, by providing feedback regarding objective measures of patient adherence, medical team members have the potential to directly influence patient behavior with support from psychologists. Objective monitoring and feedback were found to be important components of Bartlett et al.'s (2002) intervention to improve adherence in pediatric patients with asthma.
The main goal of this study was to evaluate the effect of objective monitoring and feedback from medical staff regarding medication use and inhaler technique as an intervention to improve children's adherence to inhaled corticosteroids. The intervention was designed to occur within a busy medical clinic setting and require minimal effort from medical staff, and thus another main goal was to evaluate whether such a minimal intervention would be sufficient for behavior change. This study used a single-subject, multiple-baseline experimental design to assess the effects of the intervention on each individual (Barlow, Nock, & Hersen, 2009; Horner et al., 2005; Parsonson & Baer, 1978). It was hypothesized that implementation of objective monitoring and feedback would improve adherence with prescribed dosage of controller medication as well as inhaler technique for all patients and be better than monitoring alone. Additional hypotheses predicted that the intervention would produce improvements in measures of patient lung functioning and functional severity of asthma, and that patient's use of rescue (medication) inhalers would decrease or remain stable.
Methods
Participants
Five children (four female; 100% Caucasian) between the ages of 11 and 14 years (M = 12.4, SD = 1.52) diagnosed with persistent asthma for at least 6 months participated. Inclusion criteria were age between 8 and 17 years, living within 60-min travel time to clinic (for home visits), prescription of daily inhaled corticosteroid treatment and referred as “nonadherent” by the patients’ pediatric allergist. Patients were considered nonadherent when patients or their parents reported not taking medications as prescribed. All patients who were approached agreed to participate. Three participants were prescribed inhaled corticosteroids two puffs twice daily; one participant was prescribed two puffs once daily; and one participant was prescribed three puffs once daily. Families were from lower to middle socioeconomic status. All patient names reported in this article are pseudonyms.
Experimental Design
A nonconcurrent, multiple-baseline across participants design was used to examine the effects of the intervention on adherence (Barlow et al., 2009). A nonconcurrent approach was used because participants meeting all inclusion criteria were not numerous enough to enroll simultaneously (Harvey, May, & Kennedy, 2004). Following a baseline condition to establish adherence rates for each participant, the main treatment condition of monitoring plus feedback was introduced. To investigate possible effects of monitoring without feedback from staff, a modified withdrawal condition followed, resulting in the monitoring alone condition.
Measures
Patient Information
The parent(s) of each child completed a questionnaire regarding child and family demographic characteristics (e.g., race and income).
Medication Use
Prescribed regimens for each patient were obtained from physicians. Three of the five patients switched medications at the beginning of the study to ensure compatibility between medication dispenser and monitoring device. Medication changes occurred under physician supervision. Children's use of inhaled corticosteroids was assessed using an electronic recording device, the MDILogIITM (manufactured by Westmed, Inc., Englewood, CO, USA), which records aspects of each actuation from a metered-dose inhaler (i.e., date and time, inhalation timing, canister shaking, and multiple dispenses) with adequate reliability and objectivity (Apter, Tor, & Feldman, 2001). Adherence was calculated as the percentage of prescribed doses the child inhaled (0–100%). Although overuse of medication (>100%) was recorded, adherence rates were capped at 100%. Correct inhaler technique required both shaking the canister and an on-time inhalation and ranged from 0 to 100% of inhaled actuations, independent of adherence with prescribed number of doses.
Reliability Assessment
Prior to the study, doctoral students who were members of the research team conducted reliability testing with all MDILogIITM devices. Testing involved a series of 20 dispenses from each device. Each dispense simulated a different combination of inhaler use that could be performed by patients across three variables noted in the MDILogIITM data output: canister shaking (yes/no), number of actuations, and inhalation “timing” (yes/late/none). To avoid ingesting medication during testing, inhalation timing was simulated by blowing into an opening on the back of the device, as recommended by the device manufacturer. However, this procedure (which was not actual inhalation) may have resulted in lower agreement scores for inhalation timing. Each dispense sequence was recorded by hand and compared with usage data logged in and downloaded from the device. Percent agreement was calculated for each device by dividing the number of agreements by 20 (total dispenses) and multiplying by 100. Percent agreement of total actuations ranged from 95% to 100%; agreement for canister shaking ranged from 85% to 100%; and agreement scores for inhalation timing ranged from 55% to 80%. Each device was tested prior to use; following each replacement of medication canisters; and at the end of the study to confirm that the devices were operating and to help ensure reliability of outcome data with regard to shaking, actuation, and inhalation timing functions.
Pulmonary Function
Lung functioning was measured in the clinic by a pediatric allergy nurse at three points in the study through spirometry testing: enrollment, end of the monitoring plus feedback phase, and end of the monitoring alone phase. These tests measured large-airway volume (FEV1), the total volume of exhalation during the first 1-s interval, and small-airway volume (FEF25–75%). In general, an FEV1 increase of 10% or an FEF25–75% increase of 30% is a clinically significant improvement in lung function (Castile, 1998). Values are based on normative data for age, height, weight, race, and gender, with 100% considered to be normal (Miller, 1987).
Asthma Functional Severity Scale
The Asthma Functional Severity Scale (AFSS; Rosier et al., 1994) is a six-item parent-report measure of symptom activity and the impact of asthma on daily activities for school-aged children. The AFSS yields a total score, which can be categorized into four severity ranges (low: 0–4, mild: 5–8, moderate: 9–14, and severe: 15–24). This scale has demonstrated predictive validity with functional outcomes, including school absences due to wheezing, functional impairment, and physician visits for care (Rosier et al., 1994). An additional question, designed to assess patient's use of rescue inhaler medications (i.e., albuterol), was added. Specifically, parents reported the frequency per week that their children used a rescue inhaler or nebulizer over the past month.
Procedure
Patients referred by the pediatric allergist as nonadherent to their medication plans were approached during regularly scheduled clinic visits. All patients and families who were approached (n = 5) by research staff agreed to participate and no participants withdrew during the course of the intervention. All participants and their parent(s) provided informed assent and consent, respectively, at the time of enrollment in accordance with and as approved by the authors’ Institutional Review Board. For three patients, both parents participated in the study, and for the remaining two patients only mothers participated. When both parents participated, one response was obtained from the family for self-report measurement. Participants received monetary compensation for their time for completing each phase of the study.
Each participant in the study was provided with an electronic MDILogIITM device attached to an inhaler containing corticosteroid medication, according to their prescribed regimen, for the duration of their participation. At the direction of the medical staff, volumatic spacing devices were not used for any participants. Per standard clinic procedure, medical staff reviewed medication prescription as well as inhaler use and technique with each family at their initial enrollment visit. During the baseline condition, research staff visited families at their homes on a weekly basis to download data from the participant's MDILogIITM onto a laptop computer. Since these visits were part of the research protocol to gather baseline levels of adherence, no feedback was given at these visits. An exception occurred for one participant (Kathy). In the middle of her baseline phase, data from her device were collected at the clinic during an appointment unrelated to the study. Upon observing that she was using double her prescribed dose, this information was given to the medical staff, who in turn provided her with this feedback.
Baseline adherence levels were established for each participant using visual analysis (Kratochwill et al., 2010; Parsonson & Baer, 1978) of patient adherence rates. Baseline lengths were staggered from 2 to 8 weeks to help control for potential threats to internal validity (e.g., maturation) and to establish stability (level, trend, and variability) assessed at weekly data-collection intervals. Patients then were scheduled for a clinic visit to begin the monitoring plus feedback treatment condition.
During the monitoring plus feedback treatment condition, families returned to the clinic at 2-week intervals to meet with the pediatric allergist to review the strengths and weaknesses of patient inhaler use in terms of adherence with regimen (overuse/underuse, missed days) and technique (canister shaking, inhalation timing, and multiple dispenses), based on the allergist's review of the data from electronic monitoring. The technique was practiced if determined to be a problem area. A maximum of four feedback sessions was set to maintain the simplicity of the intervention. The duration of the feedback condition ranged from 27 to 50 days and was determined by participant adherence. Shorter phases (e.g., Kathy) occurred due to highly stable data (only 4 days not at 100% adherence), whereas the longest phase (e.g., Gail) was a result of the patient wanting to improve her adherence by reducing her corticosteroid inhaler overuse. Research staff continued to collect adherence data via home visits, conveyed to the allergist during patient-scheduled clinic visits. Following the treatment condition, the feedback component was withdrawn, allowing for an evaluation of monitoring in the absence of feedback from medical staff. This condition lasted 30 days, the approximate span of a medication canister using a four-puff daily dosing regimen. Due to a scheduling conflict, Tony was unable to return for a final clinic visit until 36 days had passed.
At the start of baseline, the end of the monitoring plus feedback condition, and the end of monitoring alone condition, each participant completed the following assessments: lung functioning (i.e., spirometry assessment from medical staff) and daily activity functioning (i.e., parent report on AFSS including rescue medication use).
Data Analysis
Results focused on three areas: (a) adherence to prescribed dose of inhaled corticosteroids, (b) accuracy of inhalation technique, and (c) lung functioning and parental report of functional severity.
Adherence was calculated as a percentage of the prescribed doses, truncated at 100% (e.g., Milgrom et al., 1996; Walders, Kopel, Koinis-Mitchell, & McQuaid, 2005). However, to visually preserve actual patterns of over- or underuse, we graphed adherence data as deviating above or below 100%. That is, a participant who took five puffs in a day when he or she was prescribed four puffs was graphed at 125% for the day, but adherence was calculated as 100% (visually preserving the pattern of overuse). The inhalation technique was calculated as a percentage of technique components performed correctly (i.e., canister shaking prior to use, on-time inhalation) for each prescribed dose of inhaled corticosteroids.
Following the Design and Evidence Standards for single-case designs (Kratochwill et al., 2010), results for adherence and technique first were examined for each participant via visual inspection. Evaluation of treatment effect focused on changes in level, trend, and variability across conditions (Horner et al., 2005; Parsonson & Baer, 1978). Next, three effect-size estimates were created to evaluate the effect of the intervention for each participant at each phase change: (a) monitoring plus feedback compared to baseline, (b) monitoring alone compared to monitoring plus feedback, and (c) monitoring alone compared to baseline. The standard mean difference (SMD) was used as a measure of effect size, as recommended by Durlak (2009) and Olive and Smith (2005). The SMD is calculated by subtracting the mean of the baseline condition from the mean of the intervention condition and then dividing by the standard deviation of the baseline data (Olive & Smith, 2005). SMDs can be interpreted as .2 indicating a small effect, .5 indicating a medium effect, and .8 indicating a large effect (Cohen, 1988).
Results
Adherence data for all participants are presented in Figure 1 and technique data for all participants are presented in Figure 2. Although participants were not enrolled concurrently, these data are presented contiguously to allow comparison of treatment effects across participants (Harvey et al., 2004).
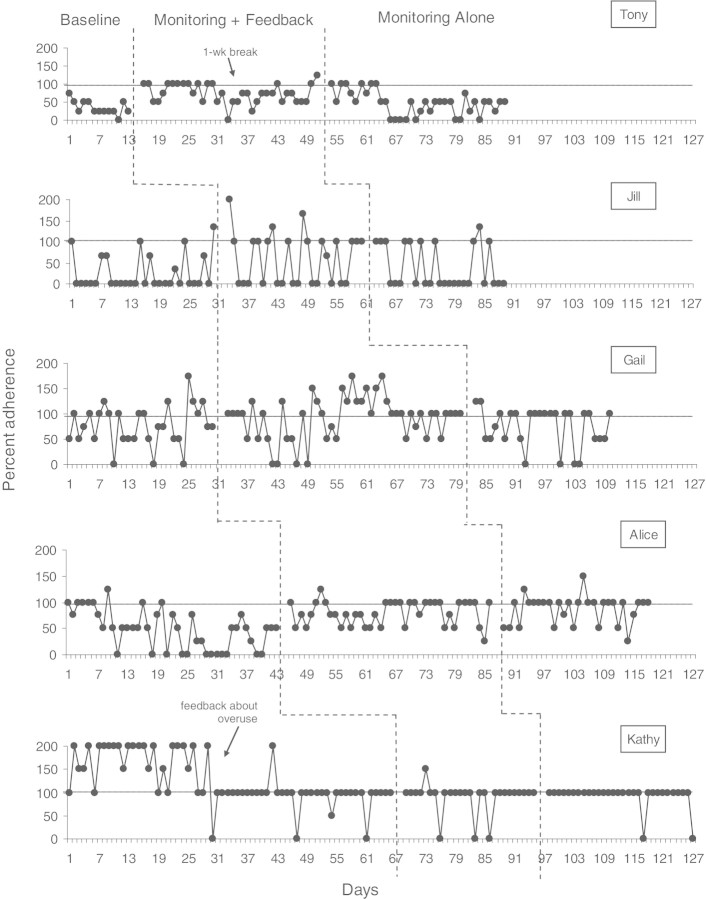
Figure 1.
Percent adherence for each participant across all three conditions.
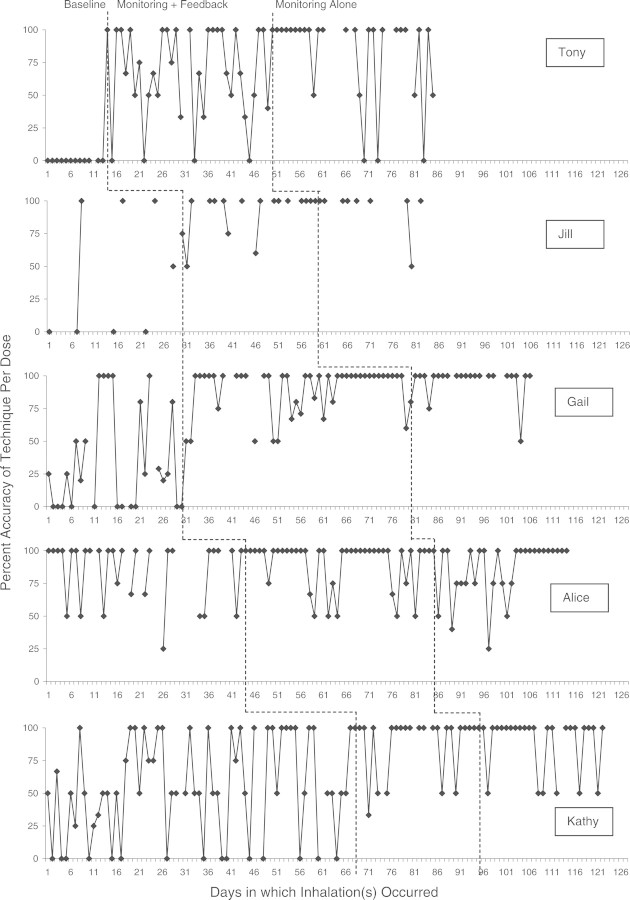
Figure 2.
Percent accuracy of technique for each participant across all three conditions. Note. There were no technique accuracy data available for days when participants did not use their medication, resulting in some missing data.
Medication Adherence
As illustrated in Figure 1, there were noticeable patterns of medication overuse for two patients during the study (Gail and Kathy). Medical staff informed patients of medication overuse after discovery—during baseline in the clinic for Kathy and during the third clinic visit of the monitoring plus feedback phase for Gail—to provide optimal clinical care. These patients generally showed improvement following physician feedback regarding inappropriate medication use. During the baseline condition, the average percent adherence for each participant was as follows: Tony: 34.6%, Jill: 23.3%, Gail: 70.8%, Alice: 47.1%, and Kathy: 94.7%.
Visual analysis suggested treatment effects from baseline to the monitoring plus feedback condition for Tony, Jill, and Alice. Gail's data showed a less convincing effect, and Kathy's data showed negligible effect once her initial overuse was removed.
When calculating effect sizes without regard to visual inspection, four of five participants demonstrated improvement from baseline to the monitoring plus feedback condition when using average percent adherence: Tony: 72.9% (SMD = 1.99), Jill: 52.4% (SMD = .76), Gail: 79.7% (SMD = .27), and Alice: 78.7% (SMD = .87). Kathy demonstrated a slight reduction in adherence, though had the greatest percent adherence of all participants throughout the study (88.9%; SMD = −.27). Effect sizes were large for Tony and Alice, medium for Jill, and small for Gail and Kathy (the average effect size across participants was .41). More noticeable improvements occurred for the patients whose baseline medication use was low and a small decrease was noted for Kathy, who had very high adherence during baseline.
When feedback regarding medication use was withdrawn (i.e., monitoring alone condition), patients’ medication use was variable across participants, with Tony at 46.5%, Jill at 37.0%, Gail at 72.3%, Alice at 85.8%, and Kathy at 93.3%. Changes from the monitoring plus feedback to the monitoring alone condition, whether improvement or decline in adherence, were small for all participants except Tony (SMD ranged from −.31 to .32). Tony's data showed a large decrease in adherence (SMD = −1.03).
Compared to the baseline condition, four of five participants demonstrated improvements during the maintenance, or monitoring alone, condition (SMD = .05 for Gail, .36 for Jill, .62 for Tony, and 1.07 for Alice). Kathy demonstrated a slight decrease compared to baseline (SMD = −.06).
Inhaler Technique
Percent accuracy of technique per inhalation for all participants is presented in Figure 2 (note that there were no technique accuracy data available for days when participants did not use their medication). Mean accuracy for each participant during the baseline condition was 0% (Tony), 47.2% (Jill), 43.4% (Gail), 54.0% (Kathy), and 84.9% (Alice).
Visual analysis of inhaler technique showed improvement for all five participants from baseline to the monitoring plus feedback condition. These improvements maintained when feedback was withdrawn. Effect-size estimates for this phase change also showed improvement during the monitoring plus feedback condition: Tony: 67.8% (SMD = 1.62), Jill: 92.3% (SMD = .95), Gail: 90.3% (SMD = 1.43), Kathy: 88.9% (SMD = .93), and Alice: 90.4% (SMD = .23). Effect sizes for these improvements were large, with the exception of Alice, whose high accuracy during baseline minimized appreciable intervention improvements. Maintenance during the monitoring alone condition was generally stable, with Tony showing a slight improvement: 82.1% (Tony), 95.0% (Jill), 94.4% (Gail), 89.3% (Kathy), and 86.3% (Alice). Changes from the monitoring plus feedback to the monitoring alone condition were small for all participants (SMD ranged from −.23 to .42).
Lung Functioning and Functional Severity
Participants’ baseline large airway lung functioning ranged from 77% to 125%. Two out of five participants (Tony and Kathy) showed clinically significant improvement (i.e., >10%) in large airway functioning from baseline to posttreatment and follow-up. The other three participants showed slight improvements or maintenance across time. One participant (Gail) demonstrated above normal functioning throughout the study.
Tony and Kathy showed clinical gains in small airway function from baseline to follow-up (24% and 26%, respectively). Three patients (Tony, Kathy, and Alice) showed some improvement in small airway function from baseline to the end of treatment (i.e., monitoring plus feedback), with improvements of 18%, 16%, and 12%, respectively. Alice's values fall back into the abnormal range at follow-up. Although changes in small airway function between 16% and 18% may be viewed as small, such changes could likely alter physician's management if associated with improvement in patient symptoms. The increases in small airway function for Jill were minimal and may not indicate a treatment effect for lung functioning. Gail had excellent lung-function readings throughout the study, suggesting that additional improvements other than changes in her clinical asthma control would not be expected.
Parent-reported functional severity on the AFSS ranged from 4 to 16 at baseline, with one patient in the low category (Kathy), two in the mild category (Gail and Alice), one in the moderate category (Jill), and one in the severe category (Tony). All five participants showed a decrease in parent-reported functional severity from baseline to posttreatment, which generally continued to decline or was maintained through follow-up. At follow-up, three patients were categorized as low severity (Kathy, Gail, and Alice) and two as mild (Jill and Tony). In addition, three participants’ parents reported a decrease in albuterol rescue medication use across the study (Gail, Alice, and Kathy; reductions ranged from 2 puffs to 14 puffs). One patient (Tony) did not use rescue medication during the study and another (Jill) increased albuterol use from 1–2 times to 2–3 times per week at follow-up. These results suggest that lung functioning and functional severity improvements were not due to increased use of rescue medications.
Discussion
The main goal of the study was to evaluate the effect of objective monitoring and feedback from medical staff regarding medication use and inhaler technique as an intervention to improve children's adherence to inhaled corticosteroids. Overall, as hypothesized, medication adherence improved (a) for four of five participants (Tony, Jill, Gail, and Alice) when using the standard mean difference as an effect size estimate, and (b) for Tony, Jill, and Alice (and possibly Gail) when only using visual analysis. Once feedback was withdrawn, treatment effects for adherence became more variable and/or declined for three participants (Tony, Jill, and Gail), suggesting the importance of regular feedback from medical staff as a critical component in improving adherence rates. This variability could also be a function of fewer clinic visits (and thus physician contact) in the monitoring alone condition, as compared to the monitoring plus feedback condition. The importance of ongoing objective monitoring and feedback was demonstrated for patients who were nonadherent in the sense of underusing medications as well as overusing medications. Despite physician review of dosing at enrollment for the study, two patients demonstrated a pattern of overuse that may not have been identified without the monitoring.
In general practice, some patterns of medication overuse may be medication dose dumping (i.e., intentionally dispensing medication without inhaling it). However, the MDILogIITM devices tracked multiple dispenses (e.g., multiple actuations with no inhalations within at least 7s), allowing us to separate these noninhaled, often clustered actuations from prescribed dispenses. This pattern may have occurred during baseline for Jill. Her data indicated no inhaler use until just prior to weekly home visits to collect data from the device, with 4 days where multiple, noninhaled dispenses of 25, 4, 3, and 3 occurred. The total number of actuations during baseline for Jill (both inhaled and “dumped”) approximate the actuations that would have occurred through correct inhaler use. When the monitoring plus feedback phase was introduced (and the nature of monitoring shared with Jill), this pattern of dose dumping ended. Alice's data showed 7 days with multiple dispenses during baseline (clusters of 1–12) and only 1 day with multiple dispenses (two actuations) following the first-phase change and 2 days in the monitoring alone phase.
All participants showed substantial improvements in inhaler technique following introduction of the treatment condition, regardless of their overall adherence rates. These improvements were maintained after feedback was discontinued, suggesting that once the skill was learned, feedback was no longer necessary. Although we did not measure the amount of practice during the monitoring plus feedback condition, the pediatric allergist provided specific technique feedback and practice during this condition. Our results are consistent with other studies showing that comprehensive instructions with repeated follow-up or monitoring and feedback produce improvements in the technique (Kamps, van Ewijk, Roorda, & Brand, 2000; Walia et al., 2006). Further, the maintenance of these gains through the monitoring alone phase suggest that the more “active” coaching and practice intervention may lead to lasting behavior change, consistent with research suggesting that behavior change requires more than education (Burkhart, Rayens, Oakley, Abshire, & Zhang, 2007). Clinically, our results suggest that electronic monitoring with feedback (including individual instruction and practice at visits) can be used to improve the inhaler technique. Although our 30-day follow-up period was rather short, these initial findings suggest that once technique is improved, further monitoring and feedback may be provided less frequently unless there is a change in the child's prescription. However, additional investigation of the maintenance of these skills is needed to strengthen this argument.
In addition to improvements in adherence and technique, participants showed improvements in lung function and functional impairment, as well as decreased use of rescue medication (per family report) over the course of the intervention. Interestingly, Tony demonstrated the largest treatment effect for adherence and technique, and also demonstrated significant improvement in lung functioning as well as a reduction in functional severity. These results suggest a possible relation between improved adherence and technique and reduced treatment costs (e.g., reduction in rescue medication use). Since this study relied on parent-report of rescue medication use, it would be important to more objectively evaluate rescue medication use in future studies to specifically determine the potential benefits (e.g., health outcome and financial savings) of providing families with costly electronic monitoring devices such as the MDILogIITM.
Less clear from our results is the direct relation between improvement in adherence, technique, and lung functioning. Although three participants demonstrated clinical gains in lung functioning during the study, these improvements may have occurred even without the intervention. Tony's data seemed the most consistent, with improvements in lung function, adherence, and technique, but other participants’ outcomes were mixed. Kathy, who showed clinical improvements in lung function at the end of each study condition, had smaller improvements in technique and a slight decrease in adherence. The relatively modest improvement may have been due to the fact that these were not new patients but had been receiving asthma treatment for at least 6 months.
Overall, the results of this study support the utility of electronic monitoring devices in providing patient care information to physicians and medical staff in order to provide feedback to patients and their families about adherence. Although we did not collect information regarding the amount of clinic time devoted to reviewing electronic monitoring data with families, medical staff required only brief training in interpreting the data outputs. Our research staff required training to attach monitoring devices to inhalers and to download and interpret data from them on a weekly basis, but these activities would be simplified in general clinic use, where monitoring devices can collect data for up to 30 days’ worth of daily inhaler use. Compared with Bartlett et al. (2002), investigating a nurse-administered in-home intervention, this study demonstrated the feasibility of conducting a simple intervention using electronic monitoring and feedback from medical staff within a clinic setting, which may be more practical for settings where there is not adequate staff time to devote to home visits. Our results also reflect findings reported by Burgess et al. (2010), which indicated that feedback about adherence can improve children's use of preventive asthma medicines, while having a less marked immediate impact on lung functioning.
Our work also lends support to future research that more closely examines the specific mechanisms that may play a role in patients’ behavior change. For example, our results suggest that positive feedback from staff, or the absence of negative feedback from staff, may have a favorable effect on adherence for some children where a more comprehensive, multi-faceted intervention (e.g., diaries, self-efficacy encouragement, tangible incentives, and family problem solving) is not warranted (Bartlett et al., 2002). In addition, our intervention may have involved a negative reinforcement contingency to increase adherence in some patients. Specifically, patients may have been motivated to use their inhalers correctly in order to avoid a clinic visit where data clearly show nonadherence and incorrect technique. The results are consistent with other studies that illustrate how performance-based feedback using negative reinforcement contingencies can drive efficient and powerful interventions (DiGennaro, Martens, & McIntyre, 2005; Noell et al., 2000). Psychologists can provide guidance to physicians and their medical staff by identifying the minimal level of intervention complexity most likely to result in improvements to adherence.
Despite clinically significant treatment effects, there were several limitations to this study. For example, device cost, associated software and hardware costs, technical expertise required to manage the devices, frequent patient contact to change medication canisters (approximately once monthly), coordination of data collection between home locations and clinics, and the medication-specific nature of the device (i.e., not applicable for all available asthma medications; Riekert & Rand, 2002) limit the widespread use of devices for all clinic patients. Another limitation stems from the possible unreliability of the device's tracking of late inhalations. We included late inhalation in our adherence calculations, as poor technique can reduce the actual amount of medication ingested. However, because the reliability of the MDILogIITM devices to detect inhalation timing was variable, the impact of late inhalations on actual adherence should be interpreted with caution. In any case, it is unclear whether the recommended test to simulate inhalation is the best method for testing such reliability. Though we observed some short-term improvements in adherence, not all patients demonstrated lasting effects, and a more intense intervention may be necessary in some cases. Another limitation of this study was that three participants switched medications at the beginning of the study to use one consistent with the MDILogIITM devices. Although this did not appear to impact adherence (as rates were lower during baseline), it may have impacted lung functioning and functional severity results. Although no systematic steps were included to ensure that patients did not use other, nonprescribed asthma medications during the study (e.g., from a relative or a spare inhaler), such use was not reported by participants during the physician feedback about patient adherence and inhaler technique. The use of rescue medication decreased for most participants, but this result was based on retrospective parent report of children old enough to manage their own behavior, making the finding potentially unreliable. Finally, replication of these results with a broader range of patients, both in characteristics and number, would help extend the generalizability of these initial findings.
In conclusion, this study demonstrated that providing pediatric patients with asthma and their families with feedback about inhaler use based on objective measures resulted in improvements in both medication adherence and inhaler technique, though maintenance of adherence improvements was variable. Although there are costs involved in obtaining the monitoring devices for patients, the benefits of integrating such feedback into standard clinical care may outweigh initial expenses and actually reduce cost and lower use of other medications (i.e., rescue) in the long-term. Future investigations should directly explore the cost/benefit ratio of electronic monitoring. These studies should focus on keeping the intervention simple and possibly transferring the responsibility of feedback from medical staff to parents and families. As suggested by Riekert and Rand (2002), this strategy could assist families in appropriately transferring responsibility of asthma care from parents to adolescents. Future studies might also compare the practical aspects of simple feedback (i.e., the intervention in this study) against more demanding interventions (e.g., contingency management approaches) to evaluate relative efficacy and long-term maintenance. Advances in web-based communication technology and mobile technology could also be leveraged by physicians to provide feedback remotely, further increasing the feasibility and efficiency of the intervention. Lastly, it seems that the critical question for future research is how to maintain treatment effects across time. For example, is it necessary to maintain electronic monitoring and feedback throughout the child's pediatric care, and if so, at what frequency? Do treatment effects diminish as the feedback possibly loses saliency across time? These are empirical questions to address to improve future care.
Conflicts of interest: None declared.
References
- Apter A J, Tor M, Feldman H I. Testing the reliability of old and new features of a new electronic monitor for metered dose inhalers. Annals of Allergy, Asthma, & Immunology. 2001;86:421–424. doi: 10.1016/S1081-1206(10)62488-X. [DOI] [PubMed] [Google Scholar]
- Barlow D H, Nock M K, Hersen M. Single-case experimental designs: Strategies for studying behavior change. 2009. (3rd ed.). Boston: Allyn & Bacon. [Google Scholar]
- Bartlett S J, Lukk P, Butz A, Lampros-Klein F, Rand C S. Enhancing medication adherence among inner-city children with asthma: Results from pilot studies. Journal of Asthma. 2002;39(1):47–54. doi: 10.1081/jas-120000806. [DOI] [PubMed] [Google Scholar]
- Baum D B, Creer T L. Medication adherence in children with asthma. Journal of Asthma. 1986;23(2):49–59. doi: 10.3109/02770908609077475. [DOI] [PubMed] [Google Scholar]
- Bender B, Milgrom H, Rand C. Non-adherence in asthmatic patients: Is there a solution to the problem? Annals of Allergy, Asthma, & Immunology. 1997;79:177–186. doi: 10.1016/S1081-1206(10)63001-3. [DOI] [PubMed] [Google Scholar]
- Bender B, Wamboldt F S, O'Connor S L, Rand C, Stanley S, Milgrom H, Wamboldt M Z. Measurement of children's asthma medication adherence by self-report, mother report, canister weight, and Doser CT. Annals of Allergy, Asthma, & Immunology. 2000;85:416–421. doi: 10.1016/s1081-1206(10)62557-4. [DOI] [PubMed] [Google Scholar]
- Berg C J, Rapoff M A, Snyder C R, Belmont J M. The relationship of children's hope to pediatric asthma treatment adherence. The Journal of Positive Psychology. 2007;2:176–184. [Google Scholar]
- Berg J, Dunbar-Jacob J, Rohay J M. Adherence with inhaled medications: The relationship between diary and electronic monitor. Annals of Behavioral Medicine. 1998;20:36–38. doi: 10.1007/BF02893807. [DOI] [PubMed] [Google Scholar]
- Burgess S W, Sly P D, Devadason S G. Providing feedback on adherence increases use of preventive medication by asthmatic children. Journal of Asthma. 2010;47:198–201. doi: 10.3109/02770900903483840. [DOI] [PubMed] [Google Scholar]
- Burkhart P V, Rayens M K, Oakley M G, Abshire D A, Zhang M. Testing an intervention to promote children's adherence to asthma self management. Journal of Nursing Scholarship. 2007;39(2):133–140. doi: 10.1111/j.1547-5069.2007.00158.x. [DOI] [PubMed] [Google Scholar]
- Castile R G. Pulmonary function testing in children. In: Chernick V, Boat T F, Kendig E L, editors. Kendig's disorders of the respiratory tract in children. 6th. Philadelphia: W. B. Saunders Company; 1998. pp. 196–212. [Google Scholar]
- Celano M P, Linzer J F, Demi A, Bakeman R, Smith C O, Croft S, Kobrynski L J. Treatment adherence among low-income, African American children with persistent asthma. Journal of Asthma. 2010;47:317–322. doi: 10.3109/02770900903580850. [DOI] [PubMed] [Google Scholar]
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Erlbaum.
- da Costa I G, Rapoff M A, Lemanek K, Goldstein G L. Improving adherence to medication regimens for children with asthma and its effect on clinical outcome. Journal of Applied Behavior Analysis. 1997;30:687–691. doi: 10.1901/jaba.1997.30-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGennaro F D, Martens B K, McIntyre L L. Increasing treatment integrity through negative reinforcement: Effects on teacher and student behavior. School Psychology Review. 2005;34:220–231. [Google Scholar]
- Durlak J. How to select, calculate, and interpret effect sizes. Journal of Pediatric Psychology. 2009;34:917–928. doi: 10.1093/jpepsy/jsp004. [DOI] [PubMed] [Google Scholar]
- Harvey M T, May M E, Kennedy C H. Nonconcurrent multiple baseline designs and the evaluation of educational systems. Journal of Behavioral Education. 2004;13:267–276. [Google Scholar]
- Horner R H, Carr E G, Halle J, McGee G, Odom S, Wolery M. The use of single-subject research to identify evidence-based practice in special education. Exceptional Children. 2005;71:165–179. [Google Scholar]
- Kamps A, van Ewijk B, Roorda R, Brand P. Poor inhalation technique, even after inhalation instructions, in children with asthma. Pediatric Pulmonology. 2000;29:39–42. doi: 10.1002/(sici)1099-0496(200001)29:1<39::aid-ppul7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Kratochwill T R, Hitchcock J, Horner R H, Levin J R, Odom S L, Rindskopf D M, Shadish W R. Single-case designs technical documentation. 2010. Retrieved from What Works Clearinghouse website: http://ies.ed.gov/ncee/wwc/pdf/wwc_scd.pdf. [Google Scholar]
- Lemanek K. Adherence issues in the medical management of asthma. Journal of Pediatric Psychology. 1990;15:437–458. doi: 10.1093/jpepsy/15.4.437. [DOI] [PubMed] [Google Scholar]
- McQuaid E L, Kopel S J, Klein R B, Fritz G K. Medication adherence in pediatric asthma: Reasoning, responsibility, and behavior. Journal of Pediatric Psychology. 2003;28(5):323–333. doi: 10.1093/jpepsy/jsg022. [DOI] [PubMed] [Google Scholar]
- Milgrom H, Bender B, Ackerson L, Bowry P, Smith B, Rand C. Non-adherence and treatment failure in children with asthma. Journal of Allergy and Clinical Immunology. 1996;98:1051–1057. doi: 10.1016/s0091-6749(96)80190-4. [DOI] [PubMed] [Google Scholar]
- Miller A. Spirometry and maximum expiratory flow-volume curves. In: Miller A, editor. Pulmonary function tests: A guide for the student and house officer. New York: Harcourt Brace Jovanovich; 1987. pp. 15–32. [Google Scholar]
- Noell G H, Witt J C, LaFleur L H, Mortenson B P, Ranier D D, LeVelle J. Increasing intervention implementation in general education following consultation: A comparison of two follow-up strategies. Journal of Applied Behavior Analysis. 2000;33:271–284. doi: 10.1901/jaba.2000.33-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive M L, Smith B W. Effect size calculations and single subject designs. Educational Psychology. 2005;25:313–324. [Google Scholar]
- Parsonson B, Baer D. The analysis and presentation of graphic data. In: Kratochwill T, editor. Single-subject research: Strategies for evaluating change. New York: Academic Press; 1978. pp. 105–165. [Google Scholar]
- Peterson S. Ensuring compliance in children. European Respiratory Journal. 1992;5:143–145. [PubMed] [Google Scholar]
- Quittner A L, Modi A C, Lemanek K L, Ievers-Landis C E, Rapoff M A. Evidence-based assessment of adherence to medical treatments in pediatric psychology. Journal of Pediatric Psychology. 2008;33:916–936. doi: 10.1093/jpepsy/jsm064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riekert K A, Rand C S. Electronic monitoring of medication adherence: When is high-tech best? Journal of Clinical Psychology in Medical Settings. 2002;9(1):25–34. [Google Scholar]
- Rohan J, Drotar D, McNally K, Schluchter M, Reikert K, Vavrek P, Schmidt A, Redline S, Kercsmar C. Adherence to pediatric asthma treatment in economically disadvantaged African-American children and adolescents: An application of growth curve analysis. Journal of Pediatric Psychology. 2010;35:394–404. doi: 10.1093/jpepsy/jsp074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosier M J, Bishop J, Nolan T, Robertson C F, Carlin J B, Phelan P D. Measurement of functional severity of asthma in children. American Journal of Respiratory Critical Care Medicine. 1994;149:1434–1441. doi: 10.1164/ajrccm.149.6.8004295. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program. Expert panel report 3 (EPR3): Guidelines for the diagnosis and management of asthma. 2007. (NIH Publication No. 08-4051). Retrieved from http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf. [Google Scholar]
- Walders N, Kopel S J, Koinis-Mitchell D, McQuaid E L. Patterns of quick-relief and long-term controller medication use in pediatric asthma. The Journal of Pediatrics. 2005;146:177–182. doi: 10.1016/j.jpeds.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Walia M, Paul L, Satyavani A, Lodha R, Kalaivani M, Kabra SK. Assessment of inhalation technique and determinants of incorrect performance among children with asthma. Pediatric Pulmonology. 2006;41:1082–1087. doi: 10.1002/ppul.20498. [DOI] [PubMed] [Google Scholar]