Abstract
BACKGROUND
Hypertension impairs left ventricular (LV) diastolic and systolic function, which might be aggravated by inflammation or neurohumoral activation. We hypothesized that LV diastolic dysfunction is more common in patients with renovascular hypertension (RVHT) compared with essential hypertension (EHT).
METHODS
Hypertensive patients who underwent both renal imaging to exclude RVHT and cardiac echocardiography within a 3-year period were identified retrospectively. Patients with significant renovascular disease were included in the RVHT group (n = 75); those without significant renovascular disease were included in the EHT group (n = 69). Cardiac function and structure were compared.
RESULTS
Baseline renal function was preserved (serum creatinine ≤ 2mg/dl) in EHT patients and impaired (serum creatinine > 2mg/dl) in only 9 RVHT patients. RVHT patients had higher systolic blood pressure, E/e’ ratio, and greater prevalence of concentric hypertrophy but lower estimated glomerular-filtration-rate (eGFR) compared with EHT patients. Increased prevalence of LV diastolic dysfunction remained statistically significant in patients with RVHT after multivariable adjustment for age, sex, blood pressure, eGFR, diabetes, smoking, and statin use, with a relative risk (95% CI) for abnormal E/e’ of 1.70 (95% confidence interval = 1.05–2.90; P = 0.03) compared with EHT. RVHT patients with severe renal dysfunction showed greater impairments in cardiac systolic and diastolic function compared with those in EHT patients or preserved renal function RVHT patients.
CONCLUSIONS
Among hypertensive patients undergoing echocardiography, cardiac structure and diastolic function are impaired in RVHT patients compared with EHT patients and remain different after adjustment for multiple significant covariables. When associated with significant renal dysfunction, RVHT aggravates LV hypertrophy and both systolic and diastolic dysfunction. Hence, identification of RVHT and renal dysfunction warrants development of targeted management strategies.
Keywords: blood pressure, diastolic function, hypertension, left ventricular hypertrophy, renovascular hypertension.
Hypertension is one of the strongest predictors of cardiovascular disease and premature death.1–3 In the United States, of approximately 75 million adults with hypertension,4,5 approximately 5% have renovascular hypertension (RVHT)6–10 due to occlusive disease of the main renal arteries.11 RVHT usually results from renal artery stenosis (RAS) secondary to atherosclerotic renovascular disease, which is strongly associated with cardiovascular disease.12 Heart failure is not uncommon in patients with RVHT, and RVHT identifies a very high-risk cohort with decreased survival.13In particular, left ventricular (LV) diastolic dysfunction is more frequently observed than systolic dysfunction in patients with RVHT.13–15 Wright et al. reported that the majority of patients with RAS have LV hypertrophy (LVH) and diastolic dysfunction,14 both of which progress with increasing LV mass index (LVMI) and cardiac dilatation in a subgroup of patients.16 Notably, most of the studies involved RVHT and control patients with significant renal dysfunction and serum creatinine (SCr) >2mg/dl.13–16
Hemodynamic overload leading to LVH may partly account for LV diastolic dysfunction in patients with essential hypertension (EHT).4,5 In addition to hypertensive injury, patients with RVHT show elevated levels of various neurohumoral and growth regulatory factors. Activation and release of proinflammatory cytokines from stenotic kidneys, which might magnify cardiac remodeling and thereby diastolic dysfunction in patients with RVHT compared with patients with EHT facing similar elevation of blood pressure, have been identified in both experimental models17 and human subjects with RVHT.18 However, whether LV diastolic dysfunction is common in RVHT patients with and without marked renal functional abnormalities remains unclear. Therefore, this study tested the hypothesis that LV diastolic function is more impaired in patients with RVHT than in patients with EHT.
METHODS
Patient selection and data collection
The study was approved by the Mayo Foundation Institutional Review Board. A retrospective study cohort was selected from hypertensive patients who were seen at Mayo Clinic, Rochester, Minnesota, between 1 January 2004 and 31 August 2012, and had undergone imaging to exclude RAS. Patients were included in the study only if they had signed informed consent to allow use of their data for research purposes and had available cardiac echocardiography data collected within a 3-year period. The inclusion criteria included being aged >50 and <75 years and, for RAS, standardized criteria analogous to enrollment in Cardiovascular Outcomes for Renal Atherosclerotic Lesions (CORAL) study to identify presence of atherosclerotic RAS (NCT00081731).19 Details are provided in the Supplementary Material. Overall, 69 patients with evidence of RAS were included in the study in the RVHT group, and 75 unmatched hypertensive patients with no evidence of RAS were included in the study in the control (EHT) group. In EHT patients, either computed tomography or magnetic resonance angiography excluded RAS, whereas in all RVHT patients subsequent renal artery angiography confirmed RAS.
Clinical parameters
All of the clinical and anthropometric variables were recorded at the time of the echocardiogram. Retrospective chart reviews of all the identified patients were done. Clinical variables, including medication use, past medical history, and mortality data, were abstracted from the electronic medical records. Follow-up was censored at (i) the last observed clinical visit at Mayo Clinic; (ii) the end of the study period; or (iii) death. Follow-up in RVHT patients included blood pressure outcomes of revascularization. To assess the association of RAS and cardiac dysfunction, we compared the prevalence of LVH and LV systolic and diastolic dysfunction among patients with RVHT and EHT.
Echocardiographic parameters
The echocardiographic data used in this study were recorded by staff cardiologists with advanced training in echocardiography, and echocardiograms were performed by experienced sonographers according to American Society of Echocardiography guidelines. LV systolic function was assessed from LV ejection fraction (LVEF), LV stroke volume index (cardiac output/heart rate per body surface area), and LV cardiac index (cardiac output per body surface area). For the purpose of the study, cardiac structural changes were determined by evaluation of LVMI, presence or absence of LVH, left atrial volume index, and characterization of cardiac geometry. LVMI and relative wall thickness were used to classify cardiac geometry as normal, concentric remodeling, eccentric hypertrophy, and concentric hypertrophy. Diastolic function was assessed by M-mode and tissue Doppler echocardiography. M-mode parameters analyzed were peak early diastolic velocity (E), peak atrial velocity (A), E/A ratio, and isovolumic relaxation time. The tissue Doppler echocardiography parameters analyzed were peak early diastolic velocity (e’) and E/e’ ratio. Diastolic function was graded as normal, mild (grades I and Ia), moderate (grade II), or severe (grade III) diastolic dysfunction.20–22
Statistical analysis
Statistical analyses were performed using JMP version 8.0 (SAS Institute, Cary, NC). Results were expressed as mean ± SD for normally distributed data, and median (range) for non-normally distributed data. Baseline differences among the groups were determined by t tests or χ2 tests, as appropriate. By multivariable analysis, echocardiographic parameters were adjusted for age, sex, coronary artery disease, coronary artery bypass grafting, and other baseline variables where the observed difference between the groups was statistically significant. P < 0.05 was considered statistically significant. Survival analysis used Kaplan–Meier (log-rank) followed by multivariable Cox regression analysis, and revascularization outcomes in RVHT used t tests. Person-years of follow-up were calculated from the date of the echocardiography to the date of death or censoring (last clinic visit or 31 August 2012, whichever came first).
Detailed methods are provided in the Supplementary Methods.
RESULTS
Demographic information of the patients is provided in Table 1. RVHT patients were slightly older than EHT patients, and their baseline systolic and mean blood pressures were significantly higher. Body mass index, sex, and race distribution were similar between the 2 groups. Active smoking was more common among EHT patients, whereas diabetes mellitus requiring oral hypoglycemic agents and/or insulin was more prevalent among RVHT patients. The prevalence of coronary artery disease was high in both groups, and their likelihoods of undergoing surgical coronary artery revascularization were similar. RVHT patients used a higher number of antihypertensive drugs and were more commonly treated with statins. Renal function was slightly lower in RVHT patients, and plasma renin activity was higher, whereas protein excretion was similar to that of EHT patients. One RVHT patient was treated medically, whereas all others subsequently underwent renal artery revascularization.
Table 1.
Clinical characteristics of patients with essential (EHT) or renovascular (RVHT) hypertension
| Characteristic | EHT (n = 75) | RVHT (n = 69) | P value |
|---|---|---|---|
| Age, y | 65 (50–75) | 69 (52–75) | 0.003 |
| Sex, male/female | 41/34 | 32/37 | 0.32 |
| Body mass index, kg/m2 | 29±6 | 30±6 | 0.19 |
| Race, white/other/unknown | 72/1/2 | 63/1/5 | 0.30 |
| Duration of follow-up, y | 8.8 (2–9.1) | 8.8 (1.1–9.1) | 0.35 |
| Systolic blood pressure, mm Hg | 129±23 | 147±23 | <0.0001 |
| Diastolic blood pressure, mm Hg | 72±14 | 76±13 | 0.08 |
| Mean arterial pressure, mm Hg | 91±16 | 100±14 | 0.0007 |
| Heart rate, bpm | 66±12 | 68±13 | 0.50 |
| Total cholesterol, mg/dl | 169 (27–313) | 162 (88–382) | 0.79 |
| Hemoglobin, g/dl | 14±2 | 13±1 | 0.13 |
| Comorbidities | |||
| Diabetes mellitus | 15 (20%) | 27 (39%) | 0.01 |
| Coronary artery disease | 36 (48%) | 38 (55%) | 0.40 |
| Family history of coronary artery disease | 42 (57%) | 40 (59%) | 0.88 |
| Smoking status | 0.003 | ||
| Current smoker | 18 (24%) | 3 (4%) | |
| Former smoker | 35 (47%) | 45 (66%) | |
| Nonsmoker | 21 (28%) | 20 (29%) | |
| Recent myocardial infarction/stroke | 6 (8%) | 5 (7%) | 0.86 |
| Coronary artery bypass grafting | 11 (15%) | 18 (26%) | 0.09 |
| Atrial fibrillation | 15 (20%) | 12 (17%) | 0.69 |
| Sleep apnea | 17 (23%) | 19 (27%) | 0.50 |
| Cardiovascular medications | |||
| Antihypertensive | 3 (0–6) | 3 (0–7) | 0.03 |
| Diuretics | 48 (64%) | 50 (72%) | 0.28 |
| Calcium channel blockers | 21 (28%) | 29 (42%) | 0.08 |
| Beta-blockers | 57 (76%) | 53 (77%) | 0.91 |
| Angiotensin-converting enzyme inhibitors | 26 (35%) | 30 (43%) | 0.28 |
| Angiotensin receptor blockers | 14 (19%) | 20 (30%) | 0.14 |
| Alpha-blockers | 8 (11%) | 6 (9%) | 0.69 |
| Statins | 37 (50%) | 47 (68%) | 0.03 |
| Hormone replacement therapy | 5 (7%) | 3 (4%) | 0.54 |
| Renal function | |||
| Serum creatinine, mg/dl | 1 (0.5–1.9) | 1.2 (0.6–5.1) | <0.0001 |
| eGFR-MDRD, ml/min/1.73/m2 | 72 (34–143) | 49 (11–101) | <0.0001 |
| Proteinuria, mg/24h | 128 (23–1584) | 152 (27–1583) | 0.48 |
| Systemic plasma renin activity, ng/ml/ha | 0.6 (0.6–9.9) | 1 (0.6–22) | 0.03 |
Data are presented as median (range), number (%), or mean ± SD, as appropriate.
Abbreviation: eGFR-MDRD, estimated glomerular filtration rate–modification of diet in renal disease.
aAvailable for 18 (24%) and 27 (39%), of EHT and RVHT patients, respectively.
Cardiac structure and function
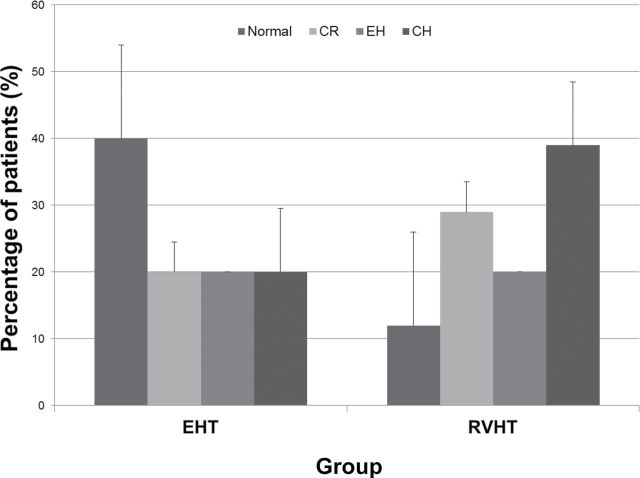
Cardiovascular assessments are reported in Table 2. There were significant differences in the prevalence of cardiac structural changes between the 2 groups. In the RVHT group, the E/e’ ratio was higher, fewer patients had normal cardiac geometry compared with the EHT group, and concentric hypertrophy and concentric remodeling were more prevalent (Figure 1). Moderate to severe impairment of LV diastolic function was prevalent among RVHT patients. Echocardiographic parameters were subsequently adjusted for sex, coronary artery disease, and demographic covariables that were significantly different between the groups (age, baseline blood pressure, diabetes mellitus, smoking status, number of antihypertensive medications, use of statins, and baseline renal function). The differences in cardiac structure and function between the 2 groups remained statistically significant after adjustment for these covariables. In addition, LV end diastolic diameter decreased, left atrial volume increased, and LV cardiac index became significantly lower in RVHT after multivariable analysis.
Table 2.
Echocardiographic assessments* of patients with essential (EHT) or renovascular (RVHT) hypertension
| Assessment | EHT (n = 75) | RVHT (n = 69) | Unadjusted P value | Adjusted∞ P value |
|---|---|---|---|---|
| Left ventricular end diastolic diameter, mm | 50 (37–72) | 49 (36–65) | 0.73 | <0.0001 |
| Left ventricular mass index, g/m2 | 100 (55–215) | 114 (57–237) | 0.03 | <0.0001 |
| Left atrial volume/BSA, ml/m2 | 35 (16–163) | 36 (15–97) | 0.28 | 0.004 |
| Medial annulus e’, m/sec | 0.06 (0.03–0.11) | 0.05 (0.02–0.11) | 0.02 | 0.05 |
| E/e’ ratio | 13±5 | 16±7 | 0.003 | <0.0001 |
| Left ventricular geometry | 0.0008 | 0.001 | ||
| Normal | 30 (40%) | 8 (11%) | ||
| Concentric remodeling | 15 (20%) | 20 (29%) | ||
| Eccentric LVH | 15 (20%) | 14 (20%) | ||
| Concentric LVH | 15 (20%) | 27 (39%) | ||
| Ejection fraction, % | 64 (20–77) | 65 (13–79) | 0.54 | <0.0001 |
| Cardiac index, L/min/m2 | 3.1 (2.2–8.1) | 3 (1.6–4.5) | 0.26 | 0.04 |
| Diastolic function | 0.01 | 0.001 | ||
| Normal | 19 (25%) | 7 (10%) | ||
| Mild (grade I and Ia) | 17 (23%) | 21 (30%) | ||
| Moderate (grade II) | 13 (17%) | 21 (30%) | ||
| Severe (grade III) | 5 (7%) | 11 (16%) |
Echocardiography performed 5 (0.03–33) and 2 (0.03–35) [median (range)] months before renal imaging in all EHT and RVHT patients, respectively. Data are presented as median (range), number (%), or mean ± SD, as appropriate.
Abbreviations: BSA, body surface area; e’, medial mitral annulus peak diastolic velocity; E, peak mitral inflow velocity; LVH, left ventricular hypertrophy.
aAdjusted for age, sex, baseline blood pressure, baseline hemoglobin levels, baseline renal function (serum creatinine and estimated glomerular filtration rate), use of statins, coronary artery disease, diabetes, smoking status, and number of antihypertensive medications.
Figure 1.
Distribution of cardiac geometry patterns in essential hypertension (EHT) and renovascular hypertension (RVHT) patients, presented as percentage of patients (%) with SE. P = 0.0008. Abbreviations: CH, concentric hypertrophy; CR, concentric remodeling; EH, eccentric hypertrophy.
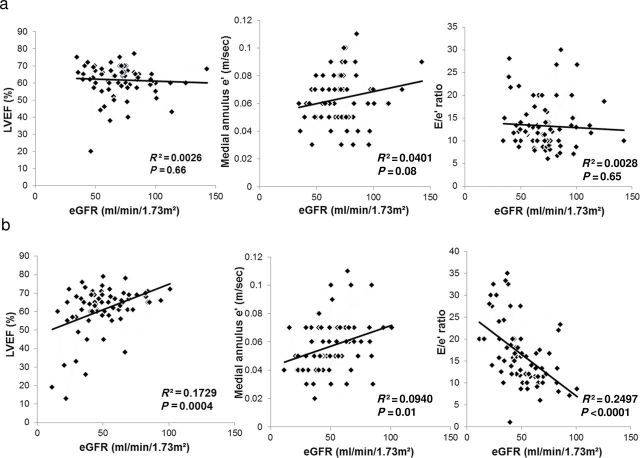
Correlation of baseline renal function with cardiac remodeling
In EHT patients, eGFR did not correlate with either systolic (LVEF) or diastolic functional parameters (medial annulus-e’, E/e’ ratio) (Figure 2a). In contrast, among RVHT patients, eGFR strongly correlated with systolic (directly with LVEF) and diastolic parameters (directly with medial annulus e’, and inversely with E/e’ ratio) (Figure 2b).
Figure 2.
Scatter plot of bivariable fit of left ventricular ejection fraction (LVEF), medial annulus e’, or E/e’ ratio by renal function (estimated glomerular filtration rate (eGFR)). (a) In essential hypertension (EHT). (b) In renovascular hypertension (RVHT).
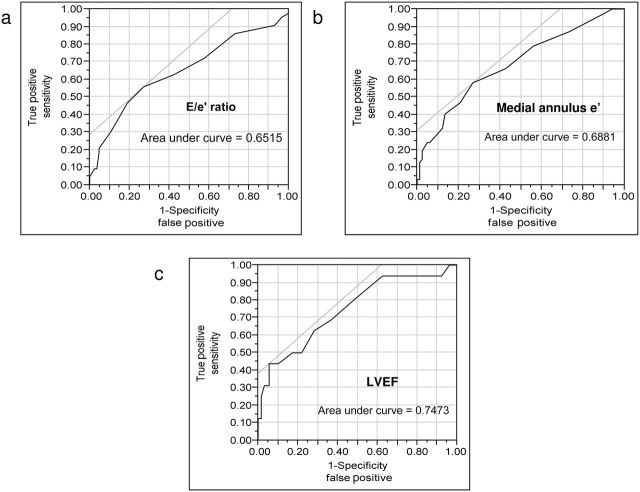
In receiver operating characteristics curves of SCr for E/e’ ratio (Figure 3a), medial annulus e’ (Figure 3b), and LVEF (Figure 3c), area under curves were 65%, 69%, and 75%, respectively. SCr provided improved sensitivity and specificity for predicting abnormal E/e’ ratio, medial annulus e’, and LVEF at a cutoff of 1.2mg/dl, 1.2mg/dl, and 1.8mg/dl, respectively. Patients with RVHT were more likely to have abnormal E/e’ ratio compared with EHT patients, (relative risk (RR) = 1.70; 95% confidence interval (CI) = 1.05–2.90; P = 0.03) but not abnormal LVEF (RR = 0.96; 95% CI = 0.85–1.1; P = 0.48).
Figure 3.
Receiver operating characteristics (ROC) curves of serum creatinine (SCr). (a) For E/e’ ratio. (b) For medial annulus e’. (c) For left ventricular ejection fraction (LVEF). For ROC curves of SCr and E/e’ ratio, values <8 were considered normal, E/e’ >15 was considered abnormal, and E/A >1.5 was used in borderline cases. For ROC analysis of SCr and medial annulus e’ or LVEF, values <0.06 m/s or <50%, respectively, were considered abnormal. Threshold cutoff of SCr of 1.2mg/dl, 1.2mg/dl, and 1.8mg/dl predicted abnormal E/e’ ratio, abnormal medial annulus e’, and abnormal LVEF, respectively. Further details are available in the Supplementary Materials.
Outcomes and predictors of mortality
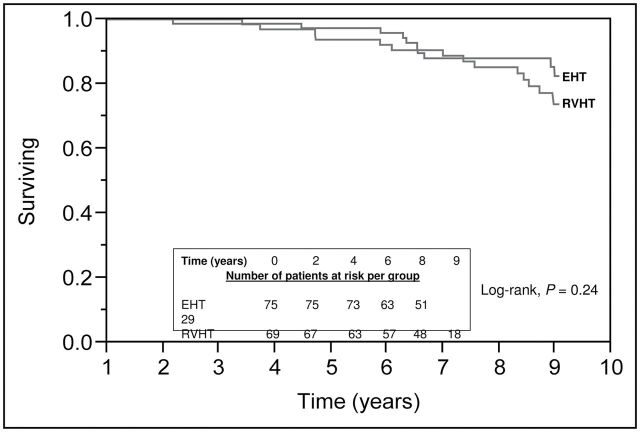
In RVHT patients, 6–12 months after revascularization, a fall in systolic, diastolic, and mean arterial blood pressures was noted (Table 3), but no change in renal function was noted. Indeed, 7% of RVHT patients developed end-stage renal disease and started chronic maintenance dialysis, compared with 1% of EHT patients (P = 0.08), although survival was similar in both groups (P = 0.24) (Figure 4). None of the baseline risk factors predicted mortality in univariable analysis (Table 3).
Table 3.
Age- and sex-adjusted univariable analysis of baseline risk factors for mortality in 144 hypertensive patients during 1,123.8 person-years (essential hypertension = 595.3 and renovascular hypertension = 528.5)
| Risk factor | Cases | HR (95%CI) | P value |
|---|---|---|---|
| Baseline blood pressure | — | 0.9 (0.9–1.0) | 0.90 |
| Coronary artery disease | 74 | 1.1 (0.8–1.5) | 0.88 |
| Diabetes mellitus | 42 | 0.8 (0.5–1.9) | 0.65 |
| Active smoking | 21 | 1.3 (0.8–2.0) | 0.70 |
| Baseline renal function (eGFR) | — | 0.9 (0.98–1.00) | 0.23 |
| Left ventricular ejection fraction | — | 0.99 (0.97–1.0) | 0.57 |
| Recent cardiovascular/ cerebrovascular event | 11 | 1.1 (0.6–2.0) | 0.88 |
| Statins | 84 | 0.9 (0.6–1.3) | 0.84 |
| Presence of hemodynamically significant RAS | 69 | 1.2 (0.9–1.7) | 0.62 |
| RAS + severe renal dysfunctiona | 9 | 1.2 (0.6–2.4) | 0.83 |
Abbreviations: CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; RAS, renal artery stenosis.
aIncludes renovascular hypertension patients with serum creatinine >2mg/dl.
Figure 4.
Survival plot of patients in essential hypertension (EHT) and renovascular hypertension (RVHT) groups. Numbers of events were 10 and 14 in the EHT and RVHT groups, respectively.
Subgroup analysis: RVHT with and without significant renal dysfunction
To examine the independent impact of renal dysfunction, the RVHT group was subdivided in a pilot fashion into 2 groups based on SCr level of ≤2mg/dl (RVHT-I, n = 60) or >2mg/dl (RVHT-II, n = 9). Systolic and mean blood pressures were higher in RVHT-I, whereas median age and prevalence of diabetes mellitus were higher in RVHT-II patients. Abnormal cardiac geometry, elevated LVMI, LV end-diastolic echo dimensions (LVEDD), and E/e’ ratio, and decreased LVEF in the RVHT-II group compared with the EHT and RVHT-I groups remained statistically significant after adjusting for age, sex, baseline blood pressure, eGFR, proteinuria, smoking status, diabetes mellitus, coronary artery disease, number of antihypertensive medications, and statin use. Of the patients in the RVHT-II group, 33% died during the course of follow-up, compared with 18% in the RVHT-I group and 13% in the EHT group (P = 0.28).
Please see details in the Supplementary Results.
DISCUSSION
The major finding of this study is that cardiac structure and function (particularly diastolic function) are worse in RVHT patients compared with EHT patients referred for echocardiography, even after adjustment for differences in the degree of hypertension and renal function. Furthermore, despite no further elevation of blood pressures, patients with RVHT accompanied by significant renal dysfunction (SCr >2mg/dl) show marked cardiac structural (concentric and eccentric hypertrophy) and functional (both LV systolic and diastolic) abnormalities. This study therefore underscores RVHT as a significant risk factor for cardiovascular disease. Single blood pressure measurements were obtained at the time of renal imaging, and although these readings may not necessarily reflect the patients’ blood pressure patterns over the preceding years, these findings may support the notion that elements beyond hypertension alone increase cardiovascular risk in RVHT.
Our results extend previous reports of more prevalent LV diastolic dysfunction in patients with RVHT.13–15 However, most previous studies included a smaller number of patients and control subjects with renal dysfunction14,16 and/or patients with RVHT undergoing renal artery revascularization.13,23 Our study included a larger number of patients, most of who had SCr levels <2mg/dl. Nevertheless, we found significant concentric cardiac hypertrophy or remodeling in patients with RVHT compared with EHT, possibly related to their higher systolic and mean blood pressures, whereas cardiac systolic function was relatively preserved. Although their number was small, we observed that compared with the EHT and RVHT-I groups, patients with substantial renal dysfunction in the RVHT-II group had significantly higher LVMI, LVEDD, and E/e’ ratio, suggesting involvement of factors other than pressure overload in cardiac remodeling. Receiver operating characteristics analysis revealed that renal insufficiency modestly increased the sensitivity of predicting abnormalities associated with LV diastolic and systolic dysfunction.
Among the RVHT patients, cardiac impairments ranging from abnormal cardiac geometry, elevated E/e’ ratio, and impaired LV diastolic function persisted after adjusting for multiple covariables, including age, sex, baseline blood pressure, diabetes mellitus, smoking status, use of statins, and baseline renal function. In particular, RVHT conferred elevated relative risk for abnormal E/e’. The mechanisms by which RVHT impairs cardiac function could be multifactorial. First and foremost, changes in cardiac structure and function are attributable to the elevated arterial pressure, which was higher in patients with RVHT than with EHT. Yet, in addition to hemodynamic overload, patients with RVHT show elevated levels of neurohumoral and growth regulatory factors.18 Plasma renin activity was also higher in RVHT patients compared with EHT patients, implicating amplified activation of the systemic renin-angiotensin-aldosterone system modulating target organ injury. However, PRA was measured under uncontrolled and variable conditions and was unavailable for some of the patients. Moreover, we have previously identified magnified systemic inflammation24 and release of proinflammatory markers from stenotic kidneys18 of patients with RVHT compared with matched patients with EHT. Inflammation can promote myocardial fibrosis and consequently dysfunction25 and has been linked to abnormal LV geometry and function in uremic, hypertensive, and elderly subjects.26 Although none of the study participants were on dialysis at the time of entry into the study, factors related to uremia may also aggravate vascular remodeling.27,28 Furthermore, in experimental renovascular disease, renal function modulates remote myocardial microvascular integrity, independent of hypertension.17 These observations underscore functionally important cardiorenal cross-talk, possibly mediated by renal injury signals, which may induce cardiac remodeling and impair its function beyond the hemodynamic effects of hypertension.
The prevalence of coronary artery disease was similar in patients with RVHT and EHT, as was the need for surgical coronary artery revascularization, possibly because of the relatively small number of patients included. The relationship between RVHT, cardiac geometry, diastolic function, and survival has been previously evaluated after renal artery revascularization. A decrease in LVMI after renal artery revascularization does not necessarily improve diastolic function,23 although it might confer a benefit in patients with cardiac symptoms29 and is independent of the change in blood pressure.30 RVHT is prevalent in patients with combined congestive heart failure and chronic kidney disease, but its severity does not correlate with LVEF, LVMI, or the extent of myocardial fibrosis.31 In addition to age, recent cardiovascular or cerebrovascular events, baseline eGFR, and presence of RAS with severe renal dysfunction were independent predictors of mortality. Cherr et al. demonstrated increased dialysis-free survival among patients showing improvement in eGFR after renal artery revascularization.32 Clearly, the relationship between kidney and cardiac dysfunction is complex and warrants further studies.
Patterns of LVH and cardiac geometry have been studied in hypertensive patients with33 and without chronic kidney disease,34 in black patients with chronic kidney disease,35,36 and in patients with diabetes and chronic kidney disease.37 Concentric37,38 and eccentric35,36 hypertrophy patterns characterize cardiac geometry in patients with moderate to severe renal insufficiency. Cardiac geometry patterns of concentric and eccentric hypertrophy reflect architectural reorganization of cardiac myocytes in response to pressure and volume overload states, respectively.39,40 Differences in prevalence of concentric hypertrophy between EHT and RVHT could be proportional to the degree of pressure overload. Although limited by small sample size, in our study eccentric hypertrophy was a more prevalent form of cardiac geometrical pattern than concentric hypertrophy in RVHT-II patients, which may suggest volume overload in these patients compared with EHT and RVHT-I. Furthermore, eccentric hypertrophy is reportedly more commonly associated with systolic dysfunction and concentric hypertrophy with diastolic dysfunction,41,42 which may account for the higher prevalence of eccentric hypertrophy in RVHT-II with decreased LVEF.
Being a retrospective study, our study is prone to several limitations, including selection bias inherent to a cohort identified from an institutional procedural database. Our cohort was largely white, limiting implications in a more diverse population. Including only those patients who had an echocardiogram also induced selection bias, which may account for the high prevalence of coronary artery disease among our patients. Our study also relied on standard but stringent radiological parameters to identify patients with hemodynamically significant RAS. Furthermore, the subsequent fall in arterial blood pressure (despite unaltered renal function) after revascularization supports the renovascular etiology of hypertension in the RVHT group. All the patients in the EHT group underwent either computed tomography or magnetic resonance angiography to rule out RAS, and in all RVHT patients initial imaging (renal computed tomography/magnetic resonance angiography or Doppler ultrasound) was subsequently confirmed by renal artery angiography. None of the EHT patients had SCr >2mg/dl, and a small sample size in the RVHT-II group limits elucidation of the association between cardiac dysfunction and severe renal dysfunction in RVHT, which requires further studies. Duration of hypertension was unknown, and the study groups were dissimilar in baseline blood pressure, renal function, and several comorbidities. Nevertheless, we observed significant differences in cardiac structure and function even after multivariable adjustment for all statistically significant covariables. The term “diastolic heart failure” has been replaced by “heart failure with preserved ejection fraction” because LV diastolic dysfunction is also seen in patients with reduced ejection fraction, as in our study. Therefore, for identification of our study cohort, we relied on echocardiographic evidence of LV diastolic dysfunction rather than a diagnosis code from the medical records. Nevertheless, we cannot exclude the possible contribution of differential ambient blood pressure levels during echocardiography to the differences in cardiac function between the groups.
Our study highlights the difference in pattern of LV geometry among essential hypertensive and renovascular hypertensive patients with relatively preserved renal function who were referred for echocardiography. Compared with patients with EHT, concentric cardiac hypertrophy/remodeling and diastolic dysfunction are more prevalent in patients with RVHT, suggesting that its deleterious effects on cardiac function start at an early stage. A decline in systolic function is exacerbated when marked renal dysfunction ensues and is responsible for the overall direct correlation between eGFR and LVEF. In addition to pressure overload, these associations might be speculatively attributable to increased circulating proinflammatory or uremia-related factors, which might magnify cardiac remodeling and aggravate dysfunction. These findings may be of value in identifying individuals with RVHT at risk for alterations in cardiac geometry and function and may support serial echocardiographic evaluations of these patients and monitoring the efficacy of treatment in reducing the incidence of cardiac dysfunction. Elucidating the independent impact of RVHT and renal failure on cardiac function may thus help direct preventive and management strategies in this group of patients.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENT
This work was partly supported by grants from the National Institues of Health (HL77131, DK76308, AG031750, HL092954, and UL1TR000135).
REFERENCES
- 1. Collins R, Peto R, MacMahon S, Hebert P, Fiebach NH, Eberlein KA, Godwin J, Qizilbash N, Taylor JO, Hennekens CH. Blood pressure, stroke, and coronary heart disease. Part 2, short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet 1990; 335:827–838 [DOI] [PubMed] [Google Scholar]
- 2. MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, Abbott R, Godwin J, Dyer A, Stamler J. Blood pressure, stroke, and coronary heart disease. Part 1, prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet 1990; 335:765–774 [DOI] [PubMed] [Google Scholar]
- 3. Mancia G. Blood pressure reduction and cardiovascular outcomes: past, present, and future. Am J Cardiol 2007; 100:3J–9J [DOI] [PubMed] [Google Scholar]
- 4. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990; 322:1561–1566 [DOI] [PubMed] [Google Scholar]
- 5. Schillaci G, Verdecchia P, Porcellati C, Cuccurullo O, Cosco C, Perticone F. Continuous relation between left ventricular mass and cardiovascular risk in essential hypertension. Hypertension 2000; 35:580–586 [DOI] [PubMed] [Google Scholar]
- 6. Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr, White CJ, White J, White RA, Antman EM, Smith SC, Jr., Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients with Peripheral Arterial Disease)—summary of recommendations. J Vasc Interv Radiol 2006; 17:1383–1397; quiz 1398. [DOI] [PubMed] [Google Scholar]
- 7. Przewlocki T, Kablak-Ziembicka A, Tracz W, Kopec G, Rubis P, Pasowicz M, Musialek P, Kostkiewicz M, Kozanecki A, Stompor T, Sulowicz W, Sokolowski A. Prevalence and prediction of renal artery stenosis in patients with coronary and supraaortic artery atherosclerotic disease. Nephrol Dial Transplant 2008; 23:580–585 [DOI] [PubMed] [Google Scholar]
- 8. Wang Y, Ho DS, Chen WH, Wang YQ, Lam WF, Shen ZJ, Lu CZ, Chui M. Prevalence and predictors of renal artery stenosis in chinese patients with coronary artery disease. Intern Med J 2003; 33:280–285 [DOI] [PubMed] [Google Scholar]
- 9. Jean WJ, al-Bitar I, Zwicke DL, Port SC, Schmidt DH, Bajwa TK. High incidence of renal artery stenosis in patients with coronary artery disease. Cathet Cardiovasc Diagn 1994; 32:8–10 [DOI] [PubMed] [Google Scholar]
- 10. Przewlocki T, Kablak-Ziembicka A, Tracz W, Kozanecki A, Kopec G, Rubis P, Kostkiewicz M, Roslawiecka A, Rzeznik D, Stompor T. Renal artery stenosis in patients with coronary artery disease. Kardiol Pol 2008; 66:856–862; discussion 863–854. [PubMed] [Google Scholar]
- 11. Textor SC. Current approaches to renovascular hypertension. Med Clin North Am 2009; 93:717–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kalra PA, Guo H, Kausz AT, Gilbertson DT, Liu J, Chen SC, Ishani A, Collins AJ, Foley RN. Atherosclerotic renovascular disease in united states patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int 2005; 68:293–301 [DOI] [PubMed] [Google Scholar]
- 13. Kane GC, Xu N, Mistrik E, Roubicek T, Stanson AW, Garovic VD. Renal artery revascularization improves heart failure control in patients with atherosclerotic renal artery stenosis. Nephrol Dial Transplant 2010; 25:813–820 [DOI] [PubMed] [Google Scholar]
- 14. Wright JR, Shurrab AE, Cooper A, Kalra PR, Foley RN, Kalra PA. Left ventricular morphology and function in patients with atherosclerotic renovascular disease. J Am Soc Nephrol 2005; 16:2746–2753 [DOI] [PubMed] [Google Scholar]
- 15. Ghanami RJ, Rana H, Craven TE, Hoyle J, Edwards MS, Hansen KJ. Diastolic function predicts survival after renal revascularization. J Vasc Surg 2011; 54:1720–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wright JR, Shurrab AE, Cooper A, Kalra PR, Foley RN, Kalra PA. Progression of cardiac dysfunction in patients with atherosclerotic renovascular disease. QJM 2009; 102:695–704 [DOI] [PubMed] [Google Scholar]
- 17. Urbieta-Caceres VH, Zhu XY, Jordan KL, Tang H, Textor K, Lerman A, Lerman LO. Selective improvement in renal function preserved remote myocardial microvascular integrity and architecture in experimental renovascular disease. Atherosclerosis 2012; 221:350–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eirin A, Zhu XY, Krier JD, Tang H, Jordan KL, Grande JP, Lerman A, Textor SC, Lerman LO. Adipose tissue-derived mesenchymal stem cells improve revascularization outcomes to restore renal function in swine atherosclerotic renal artery stenosis. Stem Cells 2012; 30:1030–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murphy TP, Cooper CJ, Dworkin LD, Henrich WL, Rundback JH, Matsumoto AH, Jamerson KA, D’Agostino RB. The cardiovascular outcomes with renal atherosclerotic lesions (coral) study: rationale and methods. J Vasc Interv Radiol 2005; 16:1295–1300 [DOI] [PubMed] [Google Scholar]
- 20. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 2009; 10:165–193 [DOI] [PubMed] [Google Scholar]
- 21. Moller JE, Pellikka PA, Hillis GS, Oh JK. Prognostic importance of diastolic function and filling pressure in patients with acute myocardial infarction Circulation 2006; 114:438–444 [DOI] [PubMed] [Google Scholar]
- 22. Gilman G, Nelson TA, Hansen WH, Khandheria BK, Ommen SR. Diastolic function: a sonographer’s approach to the essential echocardiographic measurements of left ventricular diastolic function. J Am Soc Echocardiogr 2007; 20:199–209 [DOI] [PubMed] [Google Scholar]
- 23. Corriere MA, Hoyle JR, Craven TE, D’Agostino RB, Jr, Edwards MS, Moore PS, Hansen KJ. Changes in left ventricular structure and function following renal artery revascularization. Ann Vasc Surg 2010; 24:80–84 [DOI] [PubMed] [Google Scholar]
- 24. Eirin A, Gloviczki ML, Tang H, Rule AD, Woollard JR, Lerman A, Textor SC, Lerman LO. Chronic renovascular hypertension is associated with elevated levels of neutrophil gelatinase-associated lipocalin. Nephrol Dial Transplant 2012; 27:4153–4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhu XY, Daghini E, Rodriguez-Porcel M, Chade AR, Napoli C, Lerman A, Lerman LO. Redox-sensitive myocardial remodeling and dysfunction in swine diet-induced experimental hypercholesterolemia. Atherosclerosis 2007; 193:62–69 [DOI] [PubMed] [Google Scholar]
- 26. Masiha S, Sundstrom J, Lind L. Inflammatory markers are associated with left ventricular hypertrophy and diastolic dysfunction in a population-based sample of elderly men and women. J Hum Hypertens 2013; 27:13–17 [DOI] [PubMed] [Google Scholar]
- 27. Talib A, Nakagawa N, Saito E, Matsuki M, Kobayashi M, Akasaka K, Hirayama T, Ishida H, Sato N, Hasebe N. The balance of fetuin-a and osteoprotegerin is independently associated with diastolic dysfunction in hemodialysis patients. Hypertens Res 2012; 35:426–433 [DOI] [PubMed] [Google Scholar]
- 28. Zhang X, Eirin A, Lerman A, Lerman LO. Osteopontin: an emerging therapeutic target in uremic vascular disease. Cardiovas Res 2013; 98:332–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kawarada O, Yokoi Y, Morioka N, Shiotani S, Higashimori A. Cardiac benefits of renal artery stenting. EuroIntervention 2010; 6:485–491 [DOI] [PubMed] [Google Scholar]
- 30. Rzeznik D, Przewlocki T, Kablak-Ziembicka A, Kozanecki A, Roslawiecka A, Lach J, Tracz W, Podolec P. Effect of renal artery revascularization on left ventricular hypertrophy, diastolic function, blood pressure, and the one-year outcome. J Vasc Surg 2011; 53:692–697 [DOI] [PubMed] [Google Scholar]
- 31. Emans ME, van der Putten K, Velthuis BK, de Vries JJ, Cramer MJ, America YG, Hillege HL, Meiss L, Doevendans PA, Braam B, Gaillard CA. Atherosclerotic renal artery stenosis is prevalent in cardiorenal patients but not associated with left ventricular function and myocardial fibrosis as assessed by cardiac magnetic resonance imaging. BMC Cardiovasc Disord 2012; 12:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cherr GS, Hansen KJ, Craven TE, Edwards MS, Ligush J, Jr, Levy PJ, Freedman BI, Dean RH. Surgical management of atherosclerotic renovascular disease. J Vasc Surg 2002; 35:236–245 [DOI] [PubMed] [Google Scholar]
- 33. Nardi E, Palermo A, Mule G, Cusimano P, Cottone S, Cerasola G. Left ventricular hypertrophy and geometry in hypertensive patients with chronic kidney disease. J Hypertens 2009; 27:633–641 [DOI] [PubMed] [Google Scholar]
- 34. Cuspidi C, Sala C, Negri F, Mancia G, Morganti A. Prevalence of left-ventricular hypertrophy in hypertension: an updated review of echocardiographic studies. J Hum Hypertens 2012; 26:343–349 [DOI] [PubMed] [Google Scholar]
- 35. Ulasi II, Arodiwe EB, Ijoma CK. Left ventricular hypertrophy in african black patients with chronic renal failure at first evaluation. Ethn Dis 2006; 16:859–864 [PubMed] [Google Scholar]
- 36. Fox ER, Taylor J, Taylor H, Han H, Samdarshi T, Arnett D, Myerson M. Left ventricular geometric patterns in the Jackson cohort of the Atherosclerotic Risk in Communities (ARIC) study: clinical correlates and influences on systolic and diastolic dysfunction. Am Heart J 2007; 153:238–244 [DOI] [PubMed] [Google Scholar]
- 37. Bayauli MP, Lepira FB, Kayembe PK, M’Buyamba-Kabangu JR. Left ventricular hypertrophy and geometry in type 2 diabetes patients with chronic kidney disease. An echocardiographic study. Cardiovasc J Afr 2012; 23:73–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sambi RS, Gaur AK, Hotchandani R, Aggarwal KK, Kaur S, Gupta M, Jain S, Krishna CK, Chopra HK, Anand V, Srivastava S, Gupta R, Parashar SK. Patterns of left ventricular hypertrophy in chronic kidney disease: an echocardiographic evaluation. Indian Heart J Teach Ser 2011; 63:259–268 [PubMed] [Google Scholar]
- 39. Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest 1975; 56:56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chahal NS, Lim TK, Jain P, Chambers JC, Kooner JS, Senior R. New insights into the relationship of left ventricular geometry and left ventricular mass with cardiac function: a population study of hypertensive subjects. Eur Heart J 2010; 31:588–594 [DOI] [PubMed] [Google Scholar]
- 41. Devereux RB, Bella JN, Palmieri V, Oberman A, Kitzman DW, Hopkins PN, Rao DC, Morgan D, Paranicas M, Fishman D, Arnett DK. Left ventricular systolic dysfunction in a biracial sample of hypertensive adults: the Hypertension Genetic Epidemiology Network (HYPERGEN) study. Hypertension 2001; 38:417–423 [DOI] [PubMed] [Google Scholar]
- 42. Wachtell K, Rokkedal J, Bella JN, Aalto T, Dahlof B, Smith G, Roman MJ, Ibsen H, Aurigemma GP, Devereux RB. Effect of electrocardiographic left ventricular hypertrophy on left ventricular systolic function in systemic hypertension (the Life Study). Losartan intervention for endpoint. Am J Cardiol 2001; 87:54–60 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.