Abstract
Anti-angiogenic therapy has shown promising but insufficient efficacy on gliomas. Recent studies suggest that vasculogenic mimicry (VM), or the formation of non-endothelial, tumor-cell-lined microvascular channels, occurs in aggressive tumors, including gliomas. There is also evidence of a physiological connection between the endothelial-lined vasculature and VM channels. Tumor cells, by virtue of their high plasticity, can form vessel-like structures themselves, which may function as blood supply networks. Our previous study on gliomas showed that microvessel density was comparably less in VM-positive tumors than in VM-negative tumors. Thus, VM may act as a complement to ensure tumor blood supply, especially in regions with less microvessel density. Patients with VM-positive gliomas survived a shorter period of time than did patients with VM-negative gliomas. Although the detailed molecular mechanisms for VM are not fully understood, glioma stem cells might play a key role, since they are involved in tumor tissue remodeling and contribute to neovascularization via transdifferentiation. In the future, successful treatment of gliomas should involve targeting both VM and angiogenesis. In this review, we summarize the progress and challenges of VM in gliomas.
Keywords: Glioma, vasculogenic mimicry, target therapy
Gliomas are the most common primary brain tumors in adults. In clinical studies, surgery followed by radiotherapy and chemotherapy with temozolomide prolonged the survival of patients with tumors showing methylation of the O-6-methylguanine DNA methyltransferase (MGMT) promoter[1]–[3]. Nevertheless, the median survival time of patients with glioblastoma (GBM), an aggressive glioma, is still less than two years[1]–[3]. Histologically, GBMs are highly angiogenic and characterized by microvascular proliferation[4]. However, the role of microvessel density (MVD) as a histopathologic factor that influences prognosis was controversial in a retrospective series of malignant gliomas[5]. Likewise, the clinical benefit of anti-angiogenic therapy for glioma patients remains unsatisfactory[6],[7]. To optimize anti-angiogenic therapy and improve patient outcomes, a better understanding of glioma vascularization is needed. A key question that must be addressed is whether tumors can acquire blood supply through other mechanisms and thereby escape conventional anti-angiogenesis therapy, which targets the endothelium.
Vascularization is crucial for the growth and metastasis of tumors[8],[9]. For years, endothelium-dependent vessels were considered the exclusive means of supplying blood to tumors. In 1999, Maniotis et al.[10] first reported a new vascular type: vasculogenic mimicry (VM). VM describes the ability of aggressive tumor cells to form extracellular matrix (ECM)–rich, vasculogenic-like networks to complement the endothelial-cell-dependent vasculature[11]–[16]. We previously reported the presence of these non-endothelium-dependent vessels in gliomas[17]. In a subsequent study, EI Hallani et al.[18] found evidence of a physiological connection between the endothelial-lined vasculature and VM channels. In further studies, we observed that patients with VM-positive gliomas survived a shorter period of time than those with VM-negative gliomas[19], similar to the results reported for other aggressive tumors[20]–[23]. VM is conspicuously different from angiogenesis and vasculogenesis. One study has indicated that VM may represent an important survival mechanism contributing to the failure of current anti-angiogenic therapy, which aims to completely deprive tumors of their blood supply[24]. Although the biologic features of tumor cells that exhibit VM remain unknown, understanding the molecular mechanisms that regulate VM is an important first step towards developing new vasculogenic therapies for glioma.
Current Understanding of the Vascularization Process in Tumors
Angiogenesis and vasculogenesis are widely accepted processes of tumor vascularization, particularly for endothelium-dependent vessels. In both processes, tumor vascular endothelial cells develop from host cells located in normal tissues around the tumor or from endothelial progenitor cells. During the transition from endothelium-dependent vessels to mimicked vessels, mosaic vessels occur as a transitional type between endothelium-dependent vessels and VM channels, wherein both the host endothelium and tumor cells participate in tumor vascularization[25]. VM is completely different from angiogenesis and vasculogenesis, in another word, the blood supply to tumors is proposed to involve three types: tumor-cell-lined vessels, mosaic vessels, and endothelium-dependent vessels[26]. A study suggest that VM channels—the tumor-cell-lined vessels—could be the main source of blood supply in the early stage of tumor growth[11]. Endothelium-dependent vessels could then replace VM channels via a transitional step as mosaic vessels to become the dominant blood supply pattern at the late stage of tumor growth[25].
Cancer stem cells (CSCs), or tumor-initiating cells, were identified as a unique subpopulation with stem cell features in many types of cancer. Current CSC studies provide novel insight into tumor angiogenesis and its interplay with the tumor microenvironment. CSCs have been shown to promote tumor angiogenesis by secreting vascular endothelial growth factor (VEGF) and via their potential for transdifferentiation into endothelial cells. Rebetz et al.[27] showed that CD133+ cells could originate from either tumor blood vessels or gliomas. In another study, He et al.[28] found that blood vessels near CD133+ or nestin+ niches as well as some CD31+ vessels co-expressed CD133 or nestin. Dong et al.[29] reported that glioma stem cells (GSCs) were involved in tumor tissue remodeling in a xenograft model. Similarly, Ricci-Vitiani et al.[30] and Wang et al.[31] found that a subpopulation of cells within glioma can give rise to endothelial cells. In summary, the angiogenesis capacity of GSCs has been demonstrated; however, the detailed relationship of GSCs and the phenomenon VM in glioma is still unclear.
Biological Characteristics and Clinical Significance of VM in Gliomas
VM channels are negative for CD34 (and other endothelial blood vessel markers, such as CD31) and positive for periodic acid-schiff (PAS). VM can be identified histologically based on three elements: the plasticity of malignant tumor cells, the remodeling of the ECM, and the connection of VM channels to the host microcirculation system[32],[33]. VM has been observed in many human tumors, including melanoma[10],[34], clear cell renal cell carcinoma[35], breast cancer[36], ovarian carcinoma[37], primary gallbladder carcinoma[38], malignant esophageal stromal carcinoma[39], mesothelial sarcoma and alveolar rhabdomyosarcoma[20], hepatocellular carcinoma[40]–[43], prostatic carcinoma[44], bladder carcinoma[45], osteosarcoma[46], and pheochromocytoma[47]. We[17] and others[18] reported the presence of VM in glioblastoma. We used CD34 staining to identify the endothelium in glioma tissue sections and PAS staining to determine the basement membrane of tumor blood vessels. Real tumor vessels stained positive for CD34 on their luminal surface and for PAS in their walls. However, in a subset of gliomas, we observed PAS-positive tubular structures that contained red blood cells but were lined by CD34-negative cells in the luminal surface, i.e., VM. VM was also found in human glioma cell line xenografts[38].
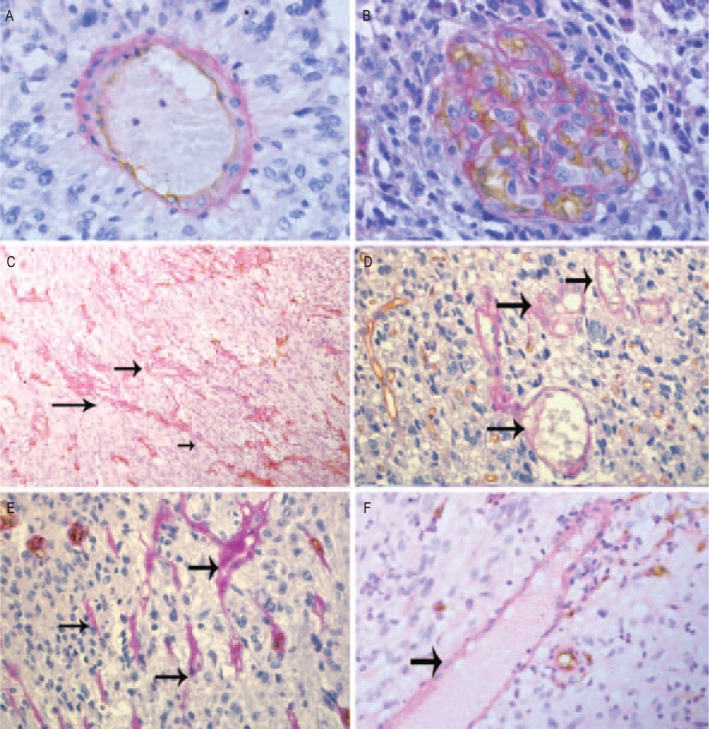
As described by Folberg et al.[32], seven morphologic patterns of VM channels were identified in uveal melanoma: straight channels, arrangements of parallel straight channels, straight channels that cross-link, arcs (incompletely closed loops), arcs with branching, closed loops, and networks (networks were defined arbitrarily as at least three back-to-back, closed, PAS-positive loops). In our study, we found that VM channels can be sorted into two distinct types: tumor cell-dependent (wherein CD34-negative tumor cells imitate the functions of the endothelium; Figure 1) and ECM-dependent (which involves only PAS-positive ECM without tumor cells; Figure 1)[17]. Neither type of tubing is dependent on the endothelium, but both contribute to microcirculation in gliomas.
Figure 1. Vasculogenic mimicry (VM) in human gliomas.
These representative images show gliomas dual stained for CD34 and periodic acid-schiff (PAS) using immunohistochemistry. A, endothelial cells are detected with anti-CD34 (dark brown), and vascular basement membrane is detected with PAS (purple magenta) in normal tubular blood vessels. B, microvascular proliferation of glioblastoma. C–F, typical VM channels (denoted by black arrows). The channels are located in a viable area of the tumor, far from necrosis. C and E, seven morphologic patterns of PAS-positive channels. D and F, VM channels containing red blood cells positive for PAS but negative for CD34: large cross section (D) and longitudinal section (F). Magnification: A, B, D, E, and F, ×400; C, ×100. (Cited from Yue et al.[17]. The authors have got the permission to reprint this image.)
Maniotis' discovery of VM originally spawned controversy. However, different approaches have since confirmed that VM channels provide a mechanism of perfusion and a dissemination route within the tumor that functions either independently of or simultaneously with angiogenesis. Studies have demonstrated the functional role of VM channels in tumor circulation using several methods, including microinjection[12],[13],[29], Doppler ultrasonography[14], magnetic resonance imaging (MRI)[15], laser scanning confocal angiography[16],[33], and injection of absorbite particles[18] (Figure 2). In our study, we found that the distribution of VM channels in gliomas looked patchy and abundant by the CD34-PAS double staining[17]. Furthermore, VM channels were always located in regions where endothelium-dependent vessels were not found, and no necrosis or surrounding inflammatory cells were observed nearby. We investigated the potential association between VM and MVD in 48 glioblastomas and found that the MVD was comparably less in VM-positive tumors than in VM-negative tumors[19]. This evidence supports that VM channels may be a complementary system to ensure tumor blood supply, especially in regions with less MVD.
Figure 2. Connection between the endothelial-lined vasculature and VM channels.

A, this longitudinal section dual stained for CD34 and periodic acid-schiff (PAS) shows a blood vessel with distinctive CD34+ (dark brown) and CD34− but PAS+ portions (purple magenta) (magnification: ×400). (Cited from El Hallani et al.[18]. The authors have got the permission to reprint this image.) B, absorbite particles, injected into the jugular vein, could be seen at human glioma stem cells, derived intracranial tumor vessels of various sizes. The inner lumens of these tumor vessels were irregular and discontinuous, and absorbite particles aggregated with red blood cells (HE staining, magnification: ×400). (Cited from Dong et al.[29]. The authors have got the permission to reprint this image.)
For cancer patients, VM is associated with poor prognosis, as the unique structure of VM channels facilitates tumor cell metastasis. Tumor cells, which line the inner surface of VM channels, are directly exposed to blood flow, allowing them to leak out, migrate through the blood stream, and metastasize to other regions. Furthermore, tumor cells that line the VM channel are highly malignant, are poorly differentiated, and have high plasticity. These cells can degrade adjacent connective tissue and penetrate the basement membrane of blood vessels by secreting proteins that mediate tumor invasion and metastasis. This phenomenon has been confirmed in liver cancer[40]–[43], breast cancer[36], gastrointestinal stromal tumors[36], and glioblastoma[18]. We performed a retrospective analysis on 101 glioma patients[19]. Tumor samples were co-stained for CD34 and PAS. Then, the dual stained samples were stained for Ki-67, cyclooxygenase-2 (COX-2), and matrix metalloproteinase-9 (MMP-9). VM was detected in 13 of 101 samples and was more frequent in high-grade gliomas than in low-grade gliomas. VM channels were also associated with expression of COX-2 and MMP-9. Patients with VM-positive tumors survived a shorter period of time than did patients with VM-negative tumors.
Targeting Vasculogenic Mimicry for Glioma Therapy
In 1971, Folkman[9] reported that tumors require a blood supply for survival, growth, and metastasis, and argued, for the first time, that anti-angiogenic therapy would have significant efficacy on tumors. Anti-angiogenic therapy aims at inhibiting endothelial cell proliferation and/or migration to hypoxic tumor regions, thereby diminishing the supply of oxygen and nutrition to tumor cells and inducing cell death. However, this approach has shown a promising but incomplete efficacy. Traditional anti-angiogenesis drugs such as angiostatin and endostatin, which target normal endothelial cells, have little effect on VM channels, which lack normal endothelial cells[48]. How, then, can the supply of oxygen and nutrition to tumor cells be blocked effectively and completely? One solution is to focus on both endothelium-dependent vessels and non-endothelium-dependent vessels.
Recently, several genes such as matrix metalloproteinase-2 (MMP-2), membrane type-1 matrix metalloproteinase (MT1-MMP), vascular endothelial growth factor (VEGF), COX-2, and vascular endothelial cadherin (VE-cadherin) have been implicated in VM channel formation in human tumors, making them potential targets for therapy with anti-sense oligonucleotides or monoclonal antibodies. Indeed, agents targeting these genes have specific anti-VM effects. Thalidomide influences VM channel formation in melanoma by inhibiting MMP-2 and VEGF expression[49]. Genistein suppresses VM by inhibiting vascular endothelial (VE)–cadherin expression[50]. Celecoxib, a COX-2 inhibitor, may block vascular channel formation, but addition of prostaglandin E2 (PGE2) abrogates these inhibitory effects[51]. Doxycycline inhibits MMP-2 expression[52]. Chemically modified tetracycline-3 inhibits the expression of VE-cadherin, MMP-2, and MT1-MMP[53]. Other strategies to inhibit VM in preclinical studies include suppressing tyrosine kinase activity, knocking out the erythropoietin-producing hepatocellula A2 (EphA2) gene[54]–[56], down-regulating VE-cadherin, targeting human MMPs and the Laminin-5γ2 chain[57], and inhibiting the phosphatidylinositol 3-kinase (PI3K) pathway[58]. Furthermore, GSCs are suggested to be critical for VM formation, which will have significant implications for the design of novel anti-tumor therapies.
Advances and Challenges
Abnormal, dysfunctional tumor vasculature and GSCs are believed to be major obstacles for effective glioma treatment. VM may represent an important tumor survival mechanism and may contribute to the failure of current anti-angiogenic therapy, which aims to completely deprive tumors of their blood supply[59]. Targeting VM along with endothelium-dependent vessels may thus block the supply of oxygen and nutrition to tumor cells effectively and completely. Furthermore, the unique structure of VM channels directly exposes tumor cells, which line the channels' inner surface, to blood vessels, thereby facilitating metastasis. VM is frequently seen in the regions between the tumor and surrounding normal tissues and is associated with poor prognosis. Therefore, therapies targeting VM channels have the potential to destroy the niche that maintains GSCs, block the passage through which tumor cells metastasize, and reduce cancer recurrence[60].
Nevertheless, tumor vascularization is a complex process that involves concomitant activity of several distinct pathways that may vary according to the patient, tumor type, tumor grade, and therapeutic effect. Successful treatment of gliomas should involve targeting one or more stages in the VM signaling cascade. Three factors affect VM channel formation: the plasticity of VM channel–associated tumor cells, the remodeling of extracellular matrix, and the connection of VM channels with the host microcirculation[61]. Thus, anti-VM therapy should focus on inhibiting tumor cell plasticity as well as remodeling the ECM and tumor microenvironment by blocking the biochemical and molecular pathways underlying VM. However, further studies on the mechanisms of VM are needed to determine the potential for future translational studies and clinical applications for glioma treatment.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Hegi ME, Diserens AC, Godard S, et al. Clinical trial substantiates the predictive value of O-6-methylguanine-DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide. Clin Cancer Res. 2004;10:1871–1874. doi: 10.1158/1078-0432.ccr-03-0384. [DOI] [PubMed] [Google Scholar]
- 3.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 4.Louis DN, Ohgaki H, Wiestler OD, et al. WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Preusser M, Heinzl H, Gelpi E, et al. Histopathologic assessment of hot-spot microvessel density and vascular patterns in glioblastoma: poor observer agreement limits clinical utility as prognostic factors—a translational research project of the European Organization for Research and Treatment of Cancer Brain Tumor Group. Cancer. 2006;107:162–170. doi: 10.1002/cncr.21973. [DOI] [PubMed] [Google Scholar]
- 6.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 7.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Folkman J. Clinical applications of research on angiogenesis. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. New Engl J Med. 1995;333:1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- 9.Folkman J. Tumour angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 10.Maniotis AJ, Folberg R, Hess A, et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155:739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang S, Guo H, Zhang D, et al. Microcirculation patterns in different stages of melanoma growth. Oncol Rep. 2006;15:15–20. [PubMed] [Google Scholar]
- 12.Clarijs R, Otte-Höller I, Ruiter DJ, et al. Presence of a fluid-conducting meshwork in xenografted cutaneous and primary human uveal melanoma. Invest Ophthalmol Vis Sci. 2002;43:912–918. [PubMed] [Google Scholar]
- 13.Shirakawa K, Kobayashi H, Heike Y, et al. Hemodynamics in vasculogenic mimicry and angiogenesis of inflammatory breast cancer xenograft. Cancer Res. 2002;62:560–566. [PubMed] [Google Scholar]
- 14.Shirakawa K, Kobayashi H, Sobajima J, et al. Inflammatory breast cancer: vasculogenic mimicry and its hemodynamics of an inflammatory breast cancer xenograft model. Breast Cancer Res. 2003;5:136–139. doi: 10.1186/bcr585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi H, Shirakawa K, Kawamoto S, et al. Rapid accumulation and internalization of radiolabeled herceptin in an inflammatory breast cancer xenograft with vasculogenic mimicry predicted by the contrast-enhanced dynamic MRI with the macromolecular contrast agent G6-(1B4M-Gd)(256) Cancer Res. 2002;62:860–866. [PubMed] [Google Scholar]
- 16.Ruf W, Seftor EA, Petrovan RJ, et al. Differential role of tissue factor pathway inhibitors 1 and 2 in melanoma vasculogenic mimicry. Cancer Res. 2003;63:5381–5389. [PubMed] [Google Scholar]
- 17.Yue WY, Chen ZP. Does vasculogenic mimicry exist in astrocy-toma? J Histochem Cytochem. 2005;53:997–1002. doi: 10.1369/jhc.4A6521.2005. [DOI] [PubMed] [Google Scholar]
- 18.El Hallani S, Boisselier B, Peglion F, et al. A new alternative mechanism in glioblastoma vascularization: tubular vasculogenic mimicry. Brain. 2010;133:973–982. doi: 10.1093/brain/awq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu XM, Zhang QP, Mu YG, et al. Clinical significance of vasculogenic mimicry in human gliomas. J Neurooncol. 2011;105:173–179. doi: 10.1007/s11060-011-0578-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun B, Zhang S, Zhao X, et al. Vasculogenic mimicry is associated with poor survival in patients with mesothelial sarcomas and alveolar rhabdomyosarcomas. Int J Oncol. 2004;25:1609–1614. [PubMed] [Google Scholar]
- 21.Zhao H, Gu XM. Study on vasculogenic mimicry in malignant esophageal stromal tumors. World J Gastroenterol. 2008;14:2430–2433. doi: 10.3748/wjg.14.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baeten CI, Hillen F, Pauwels P, et al. Prognostic role of vascu-logenic mimicry in colorectal cancer. Dis Colon Rectum. 2009;52:2028–2035. doi: 10.1007/DCR.0b013e3181beb4ff. [DOI] [PubMed] [Google Scholar]
- 23.Vartanian AA, Stepanova EV, Gutorov SL, et al. Prognostic significance of periodic acid-Schiff-positive patterns in clear cell renal cell carcinoma. Can J Urol. 2009;16:4726–4732. [PubMed] [Google Scholar]
- 24.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Huang J, Yao WY, et al. The origins of vacularization in tumors. Frontiers in Bioscience. 2012;17:2559–2565. doi: 10.2741/4071. [DOI] [PubMed] [Google Scholar]
- 26.Zhang S, Guo H, Zhang D, et al. Microcirculation patterns in different stages of melanoma growth. Oncol Rep. 2006;15:15–20. [PubMed] [Google Scholar]
- 27.Rebetz J, Tian D, Persson A, et al. Glial progenitor-like phenotype in low-grade glioma and enhanced CD133-expression and neuronal lineage differentiation potential in high-grade glioma. PLoS One. 2008;3:e1936. doi: 10.1371/journal.pone.0001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He H, Niu CS, Li NW. Correlation between glioblastoma stem-like cells and tumor vascularization. Oncol Rep. 2012;27:45–50. doi: 10.3892/or.2011.1484. [DOI] [PubMed] [Google Scholar]
- 29.Dong J, Zhang Q, Huang Q, et al. Glioma stem cells involved in tumor tissue remodeling in a xenograft model. J Neurosurg. 2010;113:249–260. doi: 10.3171/2010.2.JNS09335. [DOI] [PubMed] [Google Scholar]
- 30.Ricci-Vitiani L, Pallini R, Biffoni M, et al. Tumor vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824–828. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 31.Wang R, Chadalavada K, Wilshire J, et al. Glioblastoma stem-like cells give rise to tumor endothelium. Nature. 2010;468:829–833. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 32.Folberg R, Hendrix MJ, Maniotis AJ. Vasculogenic mimicry and tumor angiogenesis. Am J Pathol. 2000;156:361–381. doi: 10.1016/S0002-9440(10)64739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frenkel S, Barzel I, Levy J, et al. Demonstrating circulation in vasculogenic mimicry patterns of uveal melanoma by confocal indocyanine green angiography. Eye (Lond) 2008;22:948–952. doi: 10.1038/sj.eye.6702783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warso MA, Maniotis AJ, Chen X, et al. Prognostic significance of periodic acid-Schiff-positive patterns in primary cutaneous melanoma. Clin Cancer Res. 2001;7:473–477. [PubMed] [Google Scholar]
- 35.Vartanian AA, Stepanova EV, Gutorov SL, et al. Prognostic significance of periodic acid-Schiff-positive patterns in clear cell renal cell carcinoma. Can J Urol. 2009;16:4726–4732. [PubMed] [Google Scholar]
- 36.Shirakawa K, Wakasugi H, Heike Y, et al. Vasculogenic mimicry and pseudo-comedo formation in breast cancer. Int J Cancer. 2002;99:821–828. doi: 10.1002/ijc.10423. [DOI] [PubMed] [Google Scholar]
- 37.Sood AK, Fletcher MS, Zahn CM, et al. The clinical significance of tumor celllined vasculature in ovarian carcinoma: implications for antivasculogenic therapy. Cancer Biol Ther. 2002;1:661–664. doi: 10.4161/cbt.316. [DOI] [PubMed] [Google Scholar]
- 38.Fan YZ, Sun W, Zhang WZ, et al. Vasculogenic mimicry in human primary gallbladder carcinoma and clinical significance thereof. Zhonghua Yi Xue Za Zhi. 2007;87:145–149. [in Chinese] [PubMed] [Google Scholar]
- 39.Zhao H, Gu XM. Study on vasculogenic mimicry in malignant esophageal stromal tumors. World J Gastroenterol. 2008;14:2430–2433. doi: 10.3748/wjg.14.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao XL, Du J, Zhang SW, et al. A study on vasculogenic mimicry in hepatocellular carcinoma. Zhonghua Gan Zang Bing Za Zhi. 2006;14:41–44. [in Chinese] [PubMed] [Google Scholar]
- 41.Sun B, Zhang S, Zhang D, et al. Vasculogenic mimicry is asso-ciated with high tumor grade, invasion and metastasis, and short survival in patients with hepatocellular carcinoma. Oncol Rep. 2006;16:693–698. [PubMed] [Google Scholar]
- 42.Zhao J, Huang JS, Yang AJ, et al. Three-dimensional cell culture and histology of vasculogenic mimicry in hepatocellular carcinoma. Ai Zheng. 2007;26:123–126. [in Chinese] [PubMed] [Google Scholar]
- 43.Guzman G, Cotler SJ, Lin AY, et al. A pilot study of vasculogenic mimicry immunohistochemical expression in hepatocellular carcinoma. Arch Pathol Lab Med. 2007;131:1776–1781. doi: 10.5858/2007-131-1776-apsovm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma N, Seftor RE, Seftor EA, et al. Prostatic tumor cell plasticity involves cooperative interactions of distinct phenotypic subpopulations: role in vasculogenic mimicry. Prostate. 2002;50:189–201. doi: 10.1002/pros.10048. [DOI] [PubMed] [Google Scholar]
- 45.Fujimoto A, Onodera H, Mori A, et al. Tumour plasticity and extravascular circulation in ECV304 human bladder carcinoma cells. Anticancer Res. 2006;26:59–69. [PubMed] [Google Scholar]
- 46.Cai XS, Jia YW, Mei J, et al. Tumor blood vessels formation in osteosarcoma: vasculogenesis mimicry. Chin Med J (Engl) 2004;117:94–98. [PubMed] [Google Scholar]
- 47.Favier J, Plouin PF, Corvol P, et al. Angiogenesis and vascular architecture in pheochromocytomas: distinctive traits in malignant tumors. Am J Pathol. 2002;161:1235–1246. doi: 10.1016/S0002-9440(10)64400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hillen F, Griffioen AW. Tumour vascularization: sprouting angio-genesis and beyond. Cancer Metastasis Rev. 2007;26:489–502. doi: 10.1007/s10555-007-9094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang S, Li M, Gu Y, et al. Thalidomide influences growth and vasculogenic mimicry channel formation in melanoma. J Exp Clin Cancer Res. 2008;27:60. doi: 10.1186/1756-9966-27-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cong R, Sun Q, Yang L, et al. Effect of Genistein on vasculogenic mimicry formation by human uveal melanoma cells. J Exp Clin Cancer Res. 2009;28:124. doi: 10.1186/1756-9966-28-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Basu GD, Liang WS, Stephan DA, et al. A novel role for cyclooxygenase-2 in regulating vascular channel formation by human breast cancer cells. Breast Cancer Res. 2006;8:R69. doi: 10.1186/bcr1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moir LM, Ng HY, Poniris MH, et al. Doxycycline inhibits matrix metalloproteinase-2 secretion from TSC2-null mouse embryonic fibroblasts and lymphangioleiomyomatosis cells. Br J Pharmacol. 2011;164:83–92. doi: 10.1111/j.1476-5381.2011.01344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lokeshwar BL, Selzer MG, Zhu BQ, et al. Inhibition of cell proliferation, invasion, tumor growth and metastasis by an oral non-antimicrobial tetracycline analog (COL-3) in a metastatic prostate cancer model. Int J Cancer. 2002;98:297–309. doi: 10.1002/ijc.10168. [DOI] [PubMed] [Google Scholar]
- 54.Hendrix MJ, Seftor EA, Meltzer PS, et al. Expression and functional significance of VE-cadherin in aggressive human melanoma cells: role in vasculogenic mimicry. Proc Natl Acad Sci USA. 2001;98:8018–8023. doi: 10.1073/pnas.131209798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hess AR, Seftor EA, Gardner LM, et al. Molecular regulation of tumor cell vasculogenic mimicry by tyrosine phosphorylation: role of epithelial cell kinase (Eck/EphA2) Cancer Res. 2001;61:3250–3255. [PubMed] [Google Scholar]
- 56.Hess AR, Seftor EA, Gruman LM, et al. VE-cadherin regulates EphA2 in aggressive melanoma cells through a novel signaling pathway: implications for vasculogenic mimicry. Cancer Biol Ther. 2006;5:228–233. doi: 10.4161/cbt.5.2.2510. [DOI] [PubMed] [Google Scholar]
- 57.Hendrix MJ, Hess AR. EphA2 as a promoter of melanoma tumorigenicity. Cancer Biol Ther. 2009;8:279–288. doi: 10.4161/cbt.8.3.7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hess AR, Margaryan NV, Seftor EA, et al. Deciphering the signaling events that promote melanoma tumor cell vasculogenic mimicry and their link to embryonic vasculogenesis: role of the Eph receptors. Dev Dyn. 2007;236:3283–3296. doi: 10.1002/dvdy.21190. [DOI] [PubMed] [Google Scholar]
- 59.Ruzinova MB, Benezra R. Id proteins in development, cell cycle and cancer. Trends Cell Biol. 2003;13:410–418. doi: 10.1016/s0962-8924(03)00147-8. [DOI] [PubMed] [Google Scholar]
- 60.Yao XH, Ping YF, Bian XW. Contribution of cancer stem cells to tumor vasculogenic mimicry. Protein Cell. 2011;2:266–272. doi: 10.1007/s13238-011-1041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fujimoto A, Onodera H, Mori A, et al. Tumour plasticity and extravas-cular circulation in ECV304 human bladder carcinoma cells. Anticancer Res. 2006;26:59–69. [PubMed] [Google Scholar]