Abstract
Hypoxia, a state of low oxygen, is a common feature of solid tumors and is associated with disease progression as well as resistance to radiotherapy and certain chemotherapeutic drugs. Hypoxic regions in tumors, therefore, represent attractive targets for cancer therapy. To date, five distinct classes of bioreactive prodrugs have been developed to target hypoxic cells in solid tumors. These hypoxia-activated prodrugs, including nitro compounds, N-oxides, quinones, and metal complexes, generally share a common mechanism of activation whereby they are reduced by intracellular oxidoreductases in an oxygen-sensitive manner to form cytotoxins. Several examples including PR-104, TH-302, and EO9 are currently undergoing phase II and phase III clinical evaluation. In this review, we discuss the nature of tumor hypoxia as a therapeutic target, focusing on the development of bioreductive prodrugs. We also describe the current knowledge of how each prodrug class is activated and detail the clinical progress of leading examples.
Keywords: Bioreductive, prodrug, tumor hypoxia, clinical trial, oxidoreductase
Hypoxia plays a central role in cancer progression. Indeed, tumor hypoxia can promote resistance to apoptosis[1],[2], encourage hypermutation by inhibiting DNA repair[3], alter cell metabolism to favor cell growth[4],[5], up-regulate angiogenesis[6], enhance local invasiveness[7], drive metastatic spread[8], and provide a sanctuary for cancer stem cells[9]. This plethora of effects on tumor biology is orchestrated in large part by the oxygen labile transcriptional regulator, hypoxia-inducible factor 1[10].
Tumor hypoxia also plays an important role in resistance to radiotherapy and chemotherapy[11]. Molecular oxygen is a potent radiosensitizer, as it facilitates oxidation of free radicals in DNA generated during tissue irradiation. Accordingly, hypoxic cells in tumors are directly and significantly resistant to radiotherapy[12],[13]. In addition, hypoxic cells can exhibit considerable resistance to chemotherapy via several mechanisms. For example, hypoxic cells—often quiescent because of the lack of oxygen and nutrients—can escape the actions of chemotherapeutic drugs with anti-proliferative properties, such as anti-metabolites[14]. These S-phase–specific agents are incorporated into the DNA of dividing cells, a process necessary for their activity. Resistance may also be due to the inherent limitations of delivering chemotherapy to distal tumor regions; hypoxic cells reside in a pharmacological sanctuary[15],[16].
With the increasing global incidence of cancer, efficient and specific strategies for cancer treatment are urgently required. The hypoxic microenvironment of solid tumors has attracted significant attention as a target for the development of a novel therapeutics for cancer treatment. Bioreductive prodrugs can be designed for selective activation under low oxygen conditions typical of many solid tumors. These hypoxia-activated prodrugs can target and kill hypoxic cells, and their effect can extend to include sterilization of surrounding tumor cells when the activated metabolites are sufficiently stable to diffuse beyond their primary site of action. The majority of normal tissues are devoid of hypoxic region, although several tissues can exhibit regions of mild physiological hypoxia. Therefore, the severity of hypoxia observed in many solid tumors represents an attractive basis for tumor selectivity. In this article, we aim to highlight the various classes of bioreductive prodrugs and their metabolism by endogenous oxidoreductases. We also discuss the lessons learnt from past drug design and the future of this field.
Mechanisms of Bioreductive Prodrug Activation
Hypoxia-activated prodrugs are deactivated or masked cytotoxins that undergo biotransformation following reductive metabolism by endogenous human cellular oxidoreductases. This process is usually inhibited by molecular oxygen, thereby imparting specificity for the hypoxic tumor microenvironment. Oxygen inhibition involves direct competition for the single electron of the initial reduced drug species, and direct scavenging of this single electron by oxygen prevents net reduction of the prodrug. The superoxide radical byproduct of this process is readily detoxified by superoxide dismutase, ensuring bioreductive drugs exhibit minimal toxicity to normal tissues[11],[17].
This activation step is catalyzed by a variety of oxidoreductases and differs depending on the bioreductive drug class[18]. In the majority of cases, this reduction is inhibited in the presence of oxygen. Preclinical models have increased our understanding of the enzymes involved in bioreductive metabolism, but further studies are needed. In addition, few studies have examined, in the clinical setting, the roles these enzymes play.
One-electron and two-electron oxidoreductases typically catalyze oxygen-sensitive and oxygen-insensitive activation of bioreductive prodrugs, respectively. One-electron oxidoreductases generate prodrug free radical species that are readily back-oxidized resulting in a futile metabolic cycle[19]. This reversible step ensures prodrug activation is restricted to tissues experiencing limited oxygen availability. In contrast, two-electron reduction by certain oxidoreductases fails to generate an oxygen-sensitive radical intermediate. This metabolic process is therefore irreversible and may occur in tumors and normal tissues. In some cases, this can result in oxygen-independent prodrug activation.
Although a variety of one-electron or two-electron oxidoreductases are known to be involved in prodrug reduction, the frequency and amplitude of their expression in human tumors is poorly defined. To date, we have shown that the diflavin oxidoreductases, such as cytochrome P450 oxidoreductase (POR), activate bioreductive prodrugs in human cell cultures; however, the frequency of expression in human cancers appears to be low and generally does not overlap with biological markers of hypoxia such as carbonic anhydrase IX[20]. Several two-electron oxidoreductases such as DT-diaphorase (NQO1) and aldo-keto reductase 1C3 (AKR1C3) are expressed in certain tumor types, but NQO1 and AKR1C3 have also been found in normal tissues.
Progress in the Development of Hypoxia-Activated Prodrugs
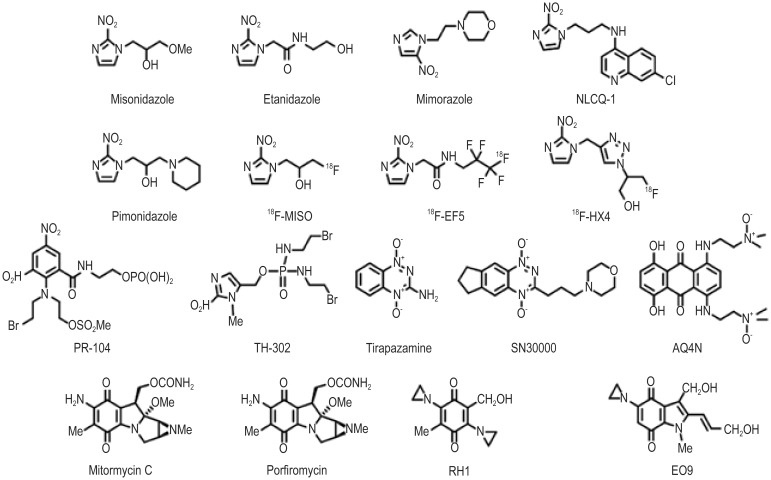
Five classes of bioreductive compounds that can undergo enzymatic reduction to active species have been developed. These can be divided into nitro(hetero)cyclic compounds, aromatic N-oxides, aliphatic N-oxides, quinones, and metal complexes (for example structures, as shown in Figure 1). Although no registered agents have been used in clinical therapy, several hypoxia-specific prodrugs are in various stages of clinical development.
Figure 1. Structure of the bioreductive compounds.
Nitro(hetero)cyclic compounds
Several nitroaromatic compounds have been evaluated in clinical trials, including the nitroimidazoles misonidazole, etanidazole, and nimorazole. These agents were primarily designed as radiosensitizers (i.e., oxygen mimetics), and derivatives have been developed for hypoxic cell imaging using immunohistochemistry [e.g., pimonidazole, EF5 [2-(2-Nitro-1H-imidazol-1-yl)-N-(2,2,3,3,3-pentafluoropropyl)acetamide] or positron emission tomography (PET; e.g., [18F]-floromisonidazole, [18F]-EF5, [18F]-flortanidazole). More recently, the transition in electron density resulting from reduction of a nitro group (NO2) to a hydroxylamine (NHOH) or amine (NH2) has been utilized to design the hypoxic cytotoxin PR-104.
PR-104 is a water-soluble phosphate ester pre-prodrug that undergoes rapid hydrolysis in vivo to the prodrug PR-104A[21]. PR-104A is a dinitrobenzamide mustard that can be reduced to para-hydroxylamine and para-amine metabolites—PR-104H and PR-104M, respectively—by various oxidoreductases. These cytotoxic metabolites give rise to DNA interstrand cross-links, which can kill tumor cells[22]. Flavoenzymes mediate the nitro reduction of PR-104A under anoxia in vitro, with the one-electron reductase POR appearing to account for the majority of activity in human tumor cell lines[20],[23]. In addition, the two-electron oxidoreductase AKR1C3 can also catalyze the reduction of PR-104A and has been identified as an oxygen-insensitive metabolic pathway[24]. The anti-cancer agent PR-104 is currently in phase II clinical trials.
Another promising clinical stage nitro compound is TH-302, a 2-nitroimidazole-based nitrogen mustard prodrug. Reduction by one-electron oxidoreductases in hypoxic cells leads to fragmentation of the 2-nitroimidazole trigger unit and release of bromo-isophosphoramide mustard[25]. The mustard moiety acts as a DNA cross-linking agent and appears particularly effective in cell lines deficient in homologous recombination DNA repair pathways[26]. TH-302 shows good selectivity for hypoxic cells, with reported aerobic-to-hypoxic cytotoxicity ratios in vitro of up to 550[26]. Efficacy in vitro was translated to efficacy in preclinical in vivo studies[27],[28] and enabled TH-302 to progress to clinical trial. The reported phase I trial demonstrated encouraging evidence of tumor response in a monotherapy setting[29]. TH-302 in combination with chemotherapy is currently being evaluated in several phase II and phase III clinical trials.
Aromatic N-oxides
The N-oxide tirapazamine (TPZ; SR4233) has been the most extensively evaluated compound in the clinic to date. TPZ was first reported in 1986 and was shown to exhibit up to 300-fold greater toxicity under anoxic conditions than aerobic conditions in vitro[30]. TPZ undergoes one-electron reduction to generate a TPZ radical; in the absence of oxygen, the TPZ radical undergoes spontaneous conversion to generate the benzotriazinyl radical, leading to DNA breaks and other complex lesions[31]–[33]. When oxygen is present, TPZ undergoes futile cycling back to the parent compound with the concomitant formation of superoxide. A number of enzymes catalyze the one-electron reduction of TPZ[34], with POR being the most extensively reported in the literature[35],[36]. In addition to the one-electron reduction of TPZ, two-electron reduction has also been reported by enzymes such as NQO1[34]. Two-electron reduction of TPZ is bioprotective to cells because it bypasses formation of the TPZ radical to generate the mono N-oxide, a relatively non-toxic metabolite[37].
Preclinical in vivo studies in which TPZ was combined with radiotherapy or cisplatin[30],[38],[39] showed great promise, and TPZ progressed to clinical trials in the early 1990s. TPZ has been evaluated in a number of phase II trials, with promising results reported in most trials[40]–[43]. However, phase II results have not been translated into increased efficacy over conventional treatment in phase III trials[44],[45]. The failure of TPZ in phase III trials may reflect the need to identify patient populations with high levels of tumor hypoxia and thus allow TPZ to be administered to patients most likely to benefit from the drug.
SN30000 is an analogue of TPZ that has undergone extensive optimization of its diffusion and metabolism characteristics[46]. This allows the prodrug to reach the hypoxic tumor cell compartment in higher concentrations than TPZ. Consequently, hypoxic radiation-resistant tumor cells are more effectively sterilized[46]. SN30000 is presently scheduled to enter phase I clinical trials.
Aliphatic N-oxides
The leading aliphatic N-oxide AQ4N (banoxantrone) is metabolized under hypoxia to AQ4, a high affinity DNA intercalator that inhibits topoisomerase II[47]. Unlike aromatic N-oxides, oxygen-sensitive reduction of AQ4N involves a two-electron step carried out by cytochrome P450 isozymes (CYP)[48]–[52] or inducible nitric oxide synthase (NOS2A)[53]. Selectivity for hypoxic conditions occurs because this enzymatic step is inhibited in the presence of oxygen. Preclinical studies combining AQ4N with radiation or chemotherapy in vivo demonstrated significant activity, enabling AQ4N to advance to clinical trials[54],[55]. Metabolism of AQ4N to AQ4 in tumor tissue has been demonstrated in clinical studies[56].
Quinones
The development of quinones as bioreductive drugs stems from an observation made in 1980 that the quinone mitomycin C (MMC) is preferentially activated in hypoxic tumor cells[57]. However, although MMC is preferentially activated in hypoxic cells, this effect is minor, prompting development of other quinone compounds that show greater selectivity towards hypoxic cells, including porfiromycin[58], RH1[59], and EO9 (apaziquone)[60]. Activation of quinones under hypoxia is carried out by one-electron reductases such as POR[61],[62]. The greatest selectivity towards hypoxic cells has been observed for the indolequinone EO9[63]–[65]. Hypoxic selectivity is lost in cells expressing the two-electron oxidoreductase NQO1[66],[67]. One of the drawbacks of EO9 is poor pharmacokinetic property. Thus, EO9 has been evaluated in a phase II trial in bladder cancer, where loco-regional administration of the drug is possible[68]. In addition, reported expression of NQO1 in a subset of bladder cancer patients ensures activation of EO9 in this setting[69]. EO9 is currently being evaluated against bladder cancer in phase III trials.
Metal complexes
Complexes of transition metals have the potential to be used as hypoxia-selective agents, but to date, none have been developed for clinical use. The first record of metal complexes as hypoxia-selective agents was in 1993, when a series of nitrogen mustard-cobalt complexes were developed[70]. The rationale behind this class of compound is that cytotoxicity depends on the electron density on the nitrogen mustard. Coordination of the nitrogen lone pair of electrons to Co(III) suppresses the alkylating reactivity. Under hypoxic conditions, one-electron reduction of Co(III) to Co(II) can occur and lead to an increase in mustard reactivity[70]. More recently, hypoxia-selective complexes of cobalt/chloromethylbenzindoline DNA minor groove alkylators[71] and copper/nitrogen mustards[72] have been reported.
Summary and Future Perspectives
Hypoxia, a common phenomenon of solid tumors, is a unique physiological feature of cancer that can be exploited with rational drug design. To this end, a number of bioreductive prodrugs have been designed in the last three decades. Although several hypoxia-selective prodrugs have progressed to clinical trials, none have yet been approved for clinical use. This partly reflects some of the many challenges and limitations that have come to light during the development of these agents. For example, TPZ, the most advanced clinical candidate, suffers from excessive metabolic consumption as it penetrates the extravascular space, a phenomenon that diminishes its apparently impressive selectivity for hypoxic cells from 50–300 fold in vitro to the more modest range of 3–5 fold in vivo[73],[74]. EO9 encountered similar issues of poor extravascular transport, compounded by an extraordinarily brief plasma half-life and oxygen-independent metabolism by NQO1[60]. Metabolic reduction by concerted two-electron oxidoreductases can unexpectedly corrupt the oxygen-inhibited activation as recently reported for PR-104[24]. Here, AKR1C3 bypasses the desired mechanism of one-electron reduction, rendering all AKR1C3-positive tissues potentially susceptible to PR-104 toxicity, including the gastrointestinal tract and bone marrow. An interesting exception is AQ4N, for which concerted two-electron reduction is inhibited by molecular oxygen, presumably via direct competition in the active site of the cytochrome P450 isozymes. Beyond these idiosyncratic examples of hypoxia prodrug design challenges lies a fundamental need to balance small molecule stability against reactivity in a reduction/oxidation equilibrium, such that toxicity is only “delivered” to tissues that oxygen is unable to reach. One example where this harmony has apparently been achieved is TH-302[26]. Indeed, early reports of the anti-tumor efficacy of TH-302 in clinical trials garner optimism[29],[75],[76]. Most recently, the departure from DNA damaging chemistries to a new generation of prodrugs that release kinase inhibitors under hypoxia may signal an exciting new direction in prodrug design[77]. The knowledge gained from preclinical and clinical studies, as well as an increased understanding of cancer biology, can assist in the rational development of novel drugs or drug analogues. This may lead to more efficient targeting of the hypoxic tumor environment in the future. It is likely that the future will see a move towards a more personalized medicine approach, with the identification of patients that may benefit most from hypoxia-selective drugs. Achieving this aim will involve the use of imaging agents to detect hypoxia in the clinic, thus enabling hypoxia-selective prodrugs to be directed towards the patients most likely to benefit most from treatment. In addition, developing a thorough understanding of the enzymology of bioreductive drugs is an important step in identifying tumor types that express high levels of the activating enzymes and enables targeting to these tumor types. Whether prodrug activation in a clinical setting depends on a limited number of key enzymes or whether it occurs from activation of a wide variety of enzymes remains to be seen.
Acknowledgments
This study was supported in part by grants from the Health Research Council of New Zealand (Programme Grant 11/1103), Key Project on Innovative Drug of Guangdong Province (No. 2011A080501013), and the Chinese Academy of Sciences.
References
- 1.Graeber TG, Osmanian C, Jacks T, et al. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 2.Kim CY, Tsai MH, Osmanian C, et al. Selection of human cervical epithelial cells that possess reduced apoptotic potential to low-oxygen conditions. Cancer Res. 1997;57:4200–4204. [PubMed] [Google Scholar]
- 3.Chan N, Koritzinsky M, Zhao H, et al. Chronic hypoxia decreases synthesis of homologous recombination proteins to offset chemoresistance and radioresistance. Cancer Res. 2008;68:605–614. doi: 10.1158/0008-5472.CAN-07-5472. [DOI] [PubMed] [Google Scholar]
- 4.Semenza GL. Regulation of cancer cell metabolism by hypoxia-inducible factor 1. Semin Cancer Biol. 2009;19:12–16. doi: 10.1016/j.semcancer.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Vaupel P. Metabolic microenvironment of tumor cells: a key factor in malignant progression. Exp Oncol. 2010;32:125–127. [PubMed] [Google Scholar]
- 6.Harris AL. Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 7.Vaupel P, Hoeckel M. Predictive power of the tumor oxygenation status. Adv Exp Med Biol. 1999;471:533–539. doi: 10.1007/978-1-4615-4717-4_63. [DOI] [PubMed] [Google Scholar]
- 8.Lunt SJ, Chaudary N, Hill RP. The tumor microenvironment and metastatic disease. Clin Exp Metastasis. 2009;26:19–34. doi: 10.1007/s10585-008-9182-2. [DOI] [PubMed] [Google Scholar]
- 9.Kim Y, Lin Q, Glazer PM, et al. Hypoxic tumor microenvironment and cancer cell differentiation. Curr Mol Med. 2009;9:425–434. doi: 10.2174/156652409788167113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denny WA. The role of hypoxia-activated prodrugs in cancer therapy. Lancet Oncol. 2000;1:25–29. doi: 10.1016/S1470-2045(00)00006-1. [DOI] [PubMed] [Google Scholar]
- 12.Nordsmark M, Overgaard M, Overgaard J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother Oncol. 1996;41:31–39. doi: 10.1016/s0167-8140(96)91811-3. [DOI] [PubMed] [Google Scholar]
- 13.Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–239. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- 14.Huxham LA, Kyle AH, Baker JHE, et al. Microregional effects of gemcitabine in HCT-116 xenografts. Cancer Res. 2004;63:6537–6541. doi: 10.1158/0008-5472.CAN-04-0986. [DOI] [PubMed] [Google Scholar]
- 15.Minchinton AI, Tannock IF. Drug penetration in solid tumors. Nat Rev Cancer. 2006;6:583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 16.Tredan O, Galmarini CM, Patel K, et al. Drug resistance and the solid tumour microenvironment. J Natl Cancer Inst. 2007;99:1441–1454. doi: 10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]
- 17.Denny WA. Hypoxia-activated anticancer drugs. Expert Opinion Ther Pat. 2005;15:635–646. [Google Scholar]
- 18.Chen Y, Hu L. Design of anticancer prodrugs for reductive activation. Med Res Rev. 2009;29:29–64. doi: 10.1002/med.20137. [DOI] [PubMed] [Google Scholar]
- 19.Stratford IJ, Workman P. Bioreductive drugs into the next millennium. Anticancer Drug Des. 1998;13:519–528. [PubMed] [Google Scholar]
- 20.Guise CP, Abbattista MR, Tipparaju SR, et al. Diflavin oxidoreductases activate the bioreductive prodrug PR-104A under hypoxia. Mol Pharmacol. 2012;81:31–40. doi: 10.1124/mol.111.073759. [DOI] [PubMed] [Google Scholar]
- 21.Patterson AV, Ferry DM, Edmunds SJ, et al. Mechanism of action and preclinical antitumor activity of the novel hypoxia-activated DNA crosslinking agent PR-104. Clin Cancer Res. 2007;13:3922–3932. doi: 10.1158/1078-0432.CCR-07-0478. [DOI] [PubMed] [Google Scholar]
- 22.Singleton RS, Guise CP, Ferry DM, et al. DNA crosslinks in human tumor cells exposed to the prodrug PR-104A: relationships to hypoxia, bioreductive metabolism and cytotoxicity. Cancer Res. 2009;69:3884–3891. doi: 10.1158/0008-5472.CAN-08-4023. [DOI] [PubMed] [Google Scholar]
- 23.Guise CP, Wang A, Thiel A, et al. Identification of human reductases that activate the dinitrobenzamide mustard prodrug PR-104A: a role for NADPH:cytochrome P450 oxidoreductase under hypoxia. Biochem Pharmacol. 2007;74:810–820. doi: 10.1016/j.bcp.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Guise CP, Abbattista M, Singleton RS, et al. The bioreductive prodrug PR-104A is activated under aerobic conditions by human aldo-keto reductase 1C3. Cancer Res. 2010;70:1573–1584. doi: 10.1158/0008-5472.CAN-09-3237. [DOI] [PubMed] [Google Scholar]
- 25.Duan JX, Jiao H, Kaizerman J, et al. Potent and highly selective hypoxia-activated achiral phosphoramidate mustards as anticancer drugs. J Med Chem. 2008;51:2412–2420. doi: 10.1021/jm701028q. [DOI] [PubMed] [Google Scholar]
- 26.Meng F, Evans JW, Bhupathi D, et al. Molecular and cellular pharmacology of the hypoxia-activated prodrug TH-302. Mol Cancer Ther. 2012;11:740–751. doi: 10.1158/1535-7163.MCT-11-0634. [DOI] [PubMed] [Google Scholar]
- 27.Sun JD, Liu Q, Wang J, et al. Selective tumor hypoxia targeting by hypoxia-activated prodrug TH-302 inhibits tumor growth in preclinical models of cancer. Clin Cancer Res. 2012;18:758–770. doi: 10.1158/1078-0432.CCR-11-1980. [DOI] [PubMed] [Google Scholar]
- 28.Liu Q, Sun JD, Wang J, et al. TH-302, a hypoxia-activated prodrug with broad in vivo preclinical combination therapy efficacy: optimization of dosing regimens and schedules. Cancer Chemother Pharmacol. 2012;69:1487–1498. doi: 10.1007/s00280-012-1852-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss GJ, Infante JR, Chiorean EG, et al. Phase 1 study of the safety, tolerability and pharmacokinetics of TH-302, a hypoxia-activated prodrug, in patients with advanced solid malignancies. Clin Cancer Res. 2011;17:2997–3004. doi: 10.1158/1078-0432.CCR-10-3425. [DOI] [PubMed] [Google Scholar]
- 30.Zeman EM, Brown JM, Lemmon MJ, et al. SR-4233: a new bioreductive agent with high selective toxicity for hypoxic mammalian cells. Int J Radiat Oncol Biol Phys. 1986;12:1239–1242. doi: 10.1016/0360-3016(86)90267-1. [DOI] [PubMed] [Google Scholar]
- 31.Shinde SS, Maroz A, Hay MP, et al. Characterization of radicals formed following enzymatic reduction of 3-substituted analogues of the hypoxia-selective cytotoxin 3-amino-1,2,4-benzotriazine 1,4-dioxide (tirapazamine) J Am Chem Soc. 2010;132:2591–2599. doi: 10.1021/ja908689f. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Biedermann KA, Brown JM. Repair of DNA and chromosome breaks in cells exposed to SR 4233 under hypoxia or to ionizing radiation. Cancer Res. 1992;52:4473–4477. [PubMed] [Google Scholar]
- 33.Daniels JS, Gates KS, Tronche C, et al. Direct evidence for bimodal DNA damage induced by tirapazamine. Chem Res Toxicol. 1998;11:1254–1257. doi: 10.1021/tx980184j. [DOI] [PubMed] [Google Scholar]
- 34.Patterson AV, Saunders MP, Chinje EC, et al. Enzymology of tirapazamine metabolism: a review. Anticancer Drug Des. 1998;13:541–573. [PubMed] [Google Scholar]
- 35.Patterson AV, Saunders MP, Chinje EC, et al. Overexpression of human NADPH:cytochrome c (P450) reductase confers enhanced sensitivity to both tirapazamine (SR 4233) and RSU 1069. Br J Cancer. 1997;76:1338–1347. doi: 10.1038/bjc.1997.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patterson AV, Barham HM, Chinje EC, et al. Importance of P450 reductase activity in determining sensitivity of breast tumour cells to the bioreductive drug, tirapazamine (SR 4233) Br J Cancer. 1995;72:1144–1150. doi: 10.1038/bjc.1995.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baker MA, Zeman EM, Hirst VK, et al. Metabolism of SR 4233 by Chinese hamster ovary cells: basis of selective hypoxic cytotoxicity. Cancer Res. 1988;48:5947–5952. [PubMed] [Google Scholar]
- 38.Siim BG, Menke DR, Dorie MJ, et al. Tirapazamine-induced cytotoxicity and DNA damage in transplanted tumors: relationship to tumor hypoxia. Cancer Res. 1997;57:2922–2928. [PubMed] [Google Scholar]
- 39.Brown JM, Lemmon MJ. Tumor hypoxia can be exploited to preferentially sensitize tumors to fractionated irradiation. Int J Radiat Oncol Biol Phys. 1991;20:457–461. doi: 10.1016/0360-3016(91)90057-b. [DOI] [PubMed] [Google Scholar]
- 40.Marcu L, Olver I. Tirapazamine: from bench to clinical trials. Curr Clin Pharmacol. 2006;1:71–79. doi: 10.2174/157488406775268192. [DOI] [PubMed] [Google Scholar]
- 41.Rischin D, Peters L, Fisher R, et al. Tirapazamine, cisplatin, and radiation versus fluorouracil, cisplatin, and radiation in patients with locally advanced head and neck cancer: a randomized phase II trial of the Trans-Tasman Radiation Oncology Group (TROG 98.02) J Clin Oncol. 2005;23:79–87. doi: 10.1200/JCO.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 42.Miller VA, Ng KK, Grant SC, et al. Phase II study of the combination of the novel bioreductive agent, tirapazamine, with cisplatin in patients with advanced non-small-cell lung cancer. Ann Oncol. 1997;8:1269–1271. doi: 10.1023/a:1008219125746. [DOI] [PubMed] [Google Scholar]
- 43.Treat J, Johnson E, Langer C, et al. Tirapazamine with cisplatin in patients with advanced non-small-cell lung cancer: a phase II study. J Clin Oncol. 1998;16:3524–3527. doi: 10.1200/JCO.1998.16.11.3524. [DOI] [PubMed] [Google Scholar]
- 44.Rischin D, Peters LJ, O'Sullivan B, et al. Tirapazamine, cisplatin, and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): a phase III trial of the Trans-Tasman Radiation Oncology Group. J Clin Oncol. 2010;28:2989–2995. doi: 10.1200/JCO.2009.27.4449. [DOI] [PubMed] [Google Scholar]
- 45.Williamson SK, Crowley JJ, Lara PN, Jr, et al. Phase III trial of paclitaxel plus carboplatin with or without tirapazamine in advanced non-small-cell lung cancer: Southwest Oncology Group Trial S0003. J Clin Oncol. 2005;23:9097–9104. doi: 10.1200/JCO.2005.01.3771. [DOI] [PubMed] [Google Scholar]
- 46.Hicks KO, Siim BG, Jaiswal JK, et al. Pharmacokinetic/pharmacodynamic modeling identifies SN30000 and SN29751 as tirapazamine analogues with improved tissue penetration and hypoxic cell killing in tumors. Clin Cancer Res. 2010;16:4946–4957. doi: 10.1158/1078-0432.CCR-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patterson LH, Craven MR, Fisher GR, et al. Aliphatic amine N-oxides of DNA binding agents as bioreductive drugs. Oncol Res. 1994;6:533–538. [PubMed] [Google Scholar]
- 48.Patterson LH, McKeown SR, Robson T, et al. Antitumour prodrug development using cytochrome P450 (CYP) mediated activation. Anticancer Drug Des. 1999;14:473–486. [PubMed] [Google Scholar]
- 49.Raleigh SM, Wanogho E, Burke MD, et al. Involvement of human cytochromes P450 (CYP) in the reductive metabolism of AQ4N, a hypoxia activated anthraquinone di-N-oxide prodrug. Int J Radiat Oncol Biol Phys. 1998;42:763–767. doi: 10.1016/s0360-3016(98)00308-3. [DOI] [PubMed] [Google Scholar]
- 50.Nishida CR, Lee M, Ortiz de Montellano PR. Efficient hypoxic activation of the anticancer agent AQ4N by CYP2S1 and CYP2W1. Mol Pharmacol. 2010;78:497–502. doi: 10.1124/mol.110.065045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patterson LH, Murray GI. Tumour cytochrome p450 and drug activation. Curr Pharm Des. 2002;8:1335–1347. doi: 10.2174/1381612023394502. [DOI] [PubMed] [Google Scholar]
- 52.Yakkundi A, McErlane V, Murray M, et al. Tumor-selective drug activation: a GDEPT approach utilizing cytochrome P450 1A1 and AQ4N. Cancer Gene Ther. 2006;13:598–605. doi: 10.1038/sj.cgt.7700933. [DOI] [PubMed] [Google Scholar]
- 53.Chinje EC, Cowen RL, Kong Z, et al. Elevated inducible NOS activity in human fibrosarcoma HT1080 tumor cells enhances AQ4N reductive metabolism in vitro. Proc AACR. 2004;45:564. [Google Scholar]
- 54.McKeown SR, Hejmadi MV, McIntyre IA, et al. AQ4N: an alkylaminoanthraquinone N-oxide showing bioreductive potential and positive interaction with radiation in vivo. Br J Cancer. 1995;72:76–81. doi: 10.1038/bjc.1995.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patterson LH, McKeown SR, Ruparelia K, et al. Enhancement of chemotherapy and radiotherapy of murine tumours by AQ4N, a bioreductively activated anti-tumour agent. Br J Cancer. 2000;82:1984–1990. doi: 10.1054/bjoc.2000.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Albertella MR, Loadman PM, Jones PH, et al. Hypoxia-selective targeting by the bioreductive prodrug AQ4N in patients with solid tumors: results of a phase I study. Clin Cancer Res. 2008;14:1096–1104. doi: 10.1158/1078-0432.CCR-07-4020. [DOI] [PubMed] [Google Scholar]
- 57.Kennedy KA, Rockwell S, Sartorelli AC. Preferential activation of mitomycin C to cytotoxic metabolites by hypoxic tumor cells. Cancer Res. 1980;40:2356–2360. [PubMed] [Google Scholar]
- 58.Haffty BG, Wilson LD, Son YH, et al. Concurrent chemo-radiotherapy with mitomycin C compared with porfiromycin in squamous cell cancer of the head and neck: final results of a randomized clinical trial. Int J Radiat Oncol Biol Phys. 2005;61:119–128. doi: 10.1016/j.ijrobp.2004.07.730. [DOI] [PubMed] [Google Scholar]
- 59.Ward TH, Coe N, Hargreaves R, et al. Toxicity, cellular uptake and DNA crosslinking studies on the novel bioreductive anticancer drug RH1. Br J Cancer. 2000;83:66. [Google Scholar]
- 60.Phillips RM, Hendriks HR, Peters GJ. EO9 (Apaziquone): from the clinic to the laboratory and back again. Br J Pharmacol. 2013;168:11–18. doi: 10.1111/j.1476-5381.2012.01996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cowen RL, Patterson AV, Telfer BA, et al. Viral delivery of P450 reductase recapitulates the ability of constitutive overexpression of reductase enzymes to potentiate the activity of Mitomycin C in human breast cancer xenografts. Mol Cancer Ther. 2003;2:901–909. [PubMed] [Google Scholar]
- 62.Saunders MP, Jaffar M, Patterson AV, et al. The relative importance of NADPH: cytochrome c (P450) reductase for determining the sensitivity of human tumour cells to the indolequinone EO9 and related analogues lacking functionality at the C-2 and C-3 positions. Biochem Pharmacol. 2000;59:993–996. doi: 10.1016/s0006-2952(99)00405-0. [DOI] [PubMed] [Google Scholar]
- 63.Plumb JA, Workman P. Unusually marked hypoxic sensitization to indoloquinone EO9 and mitomycin C in a human colon-tumour cell line that lacks DT-diaphorase activity. Int J Cancer. 1994;56:134–139. doi: 10.1002/ijc.2910560124. [DOI] [PubMed] [Google Scholar]
- 64.Plumb JA, Gerritsen M, Milroy R, et al. Relative importance of DT-diaphorase and hypoxia in the bioactivation of EO9 by human lung tumor cell lines. Int J Radiat Oncol Biol Phys. 1994;29:295–299. doi: 10.1016/0360-3016(94)90278-x. [DOI] [PubMed] [Google Scholar]
- 65.Robertson N, Haigh A, Adams GE, et al. Factors affecting sensitivity to EO9 in rodent and human tumour cells in vitro: DT-diaphorase activity and hypoxia. Eur J Cancer. 1994;30A:1013–1019. doi: 10.1016/0959-8049(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 66.Ross D, Siegel D. NAD(P)H:quinone oxidoreductase 1 (NQO1, DT-diaphorase), functions and pharmacogenetics. Methods Enzymol. 2004;382:115–144. doi: 10.1016/S0076-6879(04)82008-1. [DOI] [PubMed] [Google Scholar]
- 67.Danson S, Ward TH, Butler J, et al. DT-diaphorase: a target for new anticancer drugs. Cancer Treat Rev. 2004;30:437–449. doi: 10.1016/j.ctrv.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 68.Hendricksen K, Cornel EB, de Reijke TM, et al. Phase 2 study of adjuvant intravesical instillations of apaziquone for high risk nonmuscle invasive bladder cancer. J Urol. 2012;187:1195–1199. doi: 10.1016/j.juro.2011.11.101. [DOI] [PubMed] [Google Scholar]
- 69.Choudry GA, Stewart PA, Double JA, et al. A novel strategy for NQO1 (NAD(P)H:quinone oxidoreductase, EC 1.6.99.2) mediated therapy of bladder cancer based on the pharmacological properties of EO9. Br J Cancer. 2001;85:1137–1146. doi: 10.1054/bjoc.2001.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ware DC, Palmer BD, Wilson WR, et al. Hypoxia-selective antitumor agents. 7. Metal complexes of aliphatic mustards as a new class of hypoxia-selective cytotoxins. Synthesis and evaluation of cobalt(III) complexes of bidentate mustards. J Med Chem. 1993;36:1839–1846. doi: 10.1021/jm00065a006. [DOI] [PubMed] [Google Scholar]
- 71.Ahn GO, Botting KJ, Patterson AV, et al. Radiolytic and cellular reduction of a novel hypoxia-activated cobalt(III) prodrug of a chloromethylbenzindoline DNA minor groove alkylator. Biochem Pharmacol. 2006;71:1683–1694. doi: 10.1016/j.bcp.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 72.Parker LL, Lacy SM, Farrugia LJ, et al. A novel design strategy for stable metal complexes of nitrogen mustards as bioreductive prodrugs. J Med Chem. 2004;47:5683–5689. doi: 10.1021/jm049866w. [DOI] [PubMed] [Google Scholar]
- 73.Denny WA, Wilson WR. Tirapazamine: a bioreductive anticancer drug that exploits tumour hypoxia. Expert Opin Investig Drugs. 2000;9:2889–2901. doi: 10.1517/13543784.9.12.2889. [DOI] [PubMed] [Google Scholar]
- 74.Hicks KO, Pruijn FB, Secomb TW, et al. Use of three-dimensional tissue cultures to model extravascular transport and predict in vivo activity of hypoxia-targeted anticancer drugs. J Natl Cancer Inst. 2006;98:1118–1128. doi: 10.1093/jnci/djj306. [DOI] [PubMed] [Google Scholar]
- 75.Borad MJ, Reddy S, Uronis H, et al. Randomized phase II study of the efficacy and safety of gemcitabine + TH-302 (G+T) vs gemcitabine (G) alone in previously untreated patients with advanced pancreatic cancer. Cancer Res. 2012;72(Suppl 1):abstract LB-121. [Google Scholar]
- 76.Chawla SP, Ganjoo KN, Adkins D, et al. A phase 2 study of TH-302 in combination with doxorubicin in advanced soft tissue sarcoma. Connective Tissue Oncology Society (CTOS) Annual Meeting, Chicago, USA 26-29 October, 2011.
- 77.Smaill JB, Jaiswal JK, Abbattista MR, et al. Mechanism of action of the hypoxia-activated irreversible pan-HER inhibitor SN29966. Mol Cancer Ther. 2011;10:A247. [Google Scholar]