Abstract
High expression of fibrinogen and platelets are often observed in non–small cell lung cancer (NSCLC) patients with local regional or distant metastasis. However, the role of these factors remains unclear. The aims of this study were to evaluate the prognostic significance of plasma fibrinogen concentration and platelet count, as well as to determine the overall survival of NSCLC patients with brain metastases. A total of 275 NSCLC patients with brain metastasis were enrolled into this study. Univariate analysis showed that high plasma fibrinogen concentration was associated with age≥65 years (P = 0.011), smoking status (P = 0.009), intracranial symptoms (P = 0.022), clinical T category (P = 0.010), clinical N category (P = 0.003), increased partial thromboplastin time (P < 0.001), and platelet count (P < 0.001). Patients with low plasma fibrinogen concentration demonstrated longer overall survival compared with those with high plasma fibrinogen concentration (median, 17.3 months versus 11.1 months; P≤0.001). A similar result was observed for platelet counts (median, 16.3 months versus 11.4 months; P = 0.004). Multivariate analysis showed that both plasma fibrinogen concentration and platelet count were independent prognostic factors for NSCLC with brain metastases (R2 = 1.698, P < 0.001 and R2 = 1.699, P < 0.001, respectively). Our results suggest that high plasma fibrinogen concentration and platelet count indicate poor prognosis for NSCLC patients with brain metastases. Thus, these two biomarkers might be independent prognostic predictors for this subgroup of NSCLC patients.
Keywords: Plasma fibrinogen concentration, platelet counts, non–small cell lung cancer, brain metastasis, survival
Lung cancer, particularly non–small cell lung cancer (NSCLC), is the leading cause of cancer death worldwide[1]. Clinically, it appears that NSCLC can metastasize to specific target organs, such as the brain, bone, liver, and adrenal glands. Brain metastases affect approximately 20%–40% of NSCLC patients during their lifetime. The overall survival (OS) of patients with brain metastases is generally poor, ranging from 3 to 6 months[2]. To date, numerous tumor markers and prognostic indicators, such as carcino-embryonic antigen (CEA), neuron-specific enolase (NSE), and cytokeratin 19 fragments (CYFRA 21-1)[3],[4], have been described for predicting the survival of NSCLC patients with brain metastases. However, clinical studies have produced conflicting results, and reliable markers have limited availability.
Fibrinogen, the most abundant plasma coagulation factor, is synthesized by hepatocytes. The formation of platelet-fibrin-tumor cell aggregates may cause adhesion to endothelial cells and confer metastatic potential[5]. In clinical studies, fibrinogen and platelet count (PC) were reported to have prognostic significance in several cancers, including lung cancer[6]–[9]. However, the significance of plasma fibrinogen concentration (PFC) and PC in clinical settings and in the prognosis of NSCLC with brain metastases has not been elucidated.
We hypothesized that the formation of platelet-fibrin-tumor cell aggregates is crucial to developing brain metastases of NSCLC. Therefore, we evaluated the coagulation indices and PC in 275 NSCLC patients with brain metastases. The relationships between PFC, PC, clinicopathologic features, and prognosis were assessed to determine whether PFC and PC are prognostic factors for NSCLC patients with brain metastases.
Patients and Methods
Patient selection
A total of 275 patients with primary NSCLC and brain metastases who underwent treatment at Sun Yat-sen University Cancer Center between January 2000 and May 2011 were eligible for inclusion in the study. These cases fulfilled the following criteria: (1) newly diagnosed with NSCLC and brain metastases without previous treatment; (2) histologically or cytologically confirmed primary NSCLC and no other types of tumor in history; (3) brain metastases detected by cranial computed tomography (CT), cranial magnetic resonance imaging (MRI), or both; (4) coagulation indices and routine blood tested before treatment; (5) no history of coagulation disorders; and (6) complete profiles of clinical characteristics, and minimum 6 months of follow-up.
Clinical data were obtained from hospital records after treatment. Final confirming for vital status was conducted in November 2011.
Blood coagulation factors and platelet count tests
Coagulation function was assessed using plasma from a 4-mL blood sample to which 3.2% sodium citrate was added. Pretreatment PFC was determined using the Clauss method, with thrombin as the reagent (normal range, 2.0 to 4.0 g/L). In addition, PC and serum concentrations of CEA, CYFRA 21-1, NSE, cancer antigen 125 (CA125), cancer antigen 19-9 (CA19-9), and cancer antigen 153 (CA153) were also tested before treatment.
Statistical analysis
Statistical analysis was performed using SPSS software (standard version 16.0, SPSS, Chicago, IL). The relationships between coagulation indices and clinicopathologic features were analyzed by two independent sample t tests and chi-square test. Pearson's correlation coefficient analysis was used to analyze the correlation of PFC with PC and activated partial thromboplastin time (APTT). OS, defined as the time from diagnosis of brain metastases to death, was assessed using the Kaplan-Meier method and compared with the log-rank test. Multivariate survival analysis was performed using the Cox regression model for all of the variables that were significant in the univariate analysis. A two-sided probability less than 0.05 was considered statistically significant.
Results
Patient characteristics
The clinicalpathologic characteristics for all patients are presented in Table 1. There were 92 females and 183 males, with a median age of 56 years (range, 23–80 years)
Table 1. The relationship between blood coagulation and clinicopathologic features of non-small cell lung cancer (NSCLC) patients with brain metastases.
| Variable | No. of patients | PFC (g/L) | P | PC (×109/L) | P |
| Sex | 0.167 | 0.804 | |||
| Male | 183 | 4.0 ± 1.6 | 271.8 ± 107.1 | ||
| Female | 92 | 3.7 ± 1.3 | 274.9 ± 82. 9 | ||
| Age (years) | 0.011 | 0.470 | |||
| ≤65 | 215 | 3.8 ± 1.4 | 270.5 ± 91.8 | ||
| >65 | 60 | 4.3 ± 1.6 | 281.1 ± 124.0 | ||
| Smoking status | 0.009 | 0.503 | |||
| Never | 136 | 3.7 ± 1.3 | 268.8 ± 86.3 | ||
| Ever | 139 | 4.1 ± 1.6 | 276.8 ± 111.1 | ||
| Histology | 0.118 | 0.189 | |||
| AC | 246 | 3.9 ± 1.4 | 275.6 ± 101.7 | ||
| NAC | 29 | 4.3 ± 1.7 | 249.8 ± 76.6 | ||
| Number of BM | 0.592 | 0.628 | |||
| <3 | 166 | 3.9 ± 1.5 | 270.5 ± 102. 9 | ||
| ≥3 | 109 | 4.0 ± 1.5 | 276.4 ± 94.6 | ||
| Size of BM in diameter | 0.380 | 0.036 | |||
| <3 cm | 230 | 4.0 ± 1.5 | 267.3 ± 91.9 | ||
| ≥3 cm | 45 | 3.7 ± 1.5 | 301.3 ± 129.4 | ||
| Extracraninal lesions | 0.374 | 0.559 | |||
| No | 161 | 3.9 ± 1.4 | 275.8 ± 100.2 | ||
| Yes | 114 | 4.0 ± 1.6 | 268.7 ± 98.9 | ||
| Intracranial symptoms | 0.022 | 0.980 | |||
| Yes | 95 | 3.6 ± 1.2 | 272.7 ± 97.0 | ||
| No | 180 | 4.1 ± 1.6 | 273.0 ± 104. 7 | ||
| T category | 0.010 | 0.528 | |||
| T0 | 8 | 2.9 ± 1.1 | 247.0 ± 87.6 | ||
| T1 | 48 | 3.4 ± 1.1 | 271.7 ± 126.2 | ||
| T2 | 106 | 4.0 ± 1.5 | 265.2 ± 92.2 | ||
| T3 | 25 | 4.4 ± 1.4 | 299.8 ± 107.4 | ||
| T4 | 88 | 4.0 ± 1.6 | 277.4 ± 90.6 | ||
| N category | 0.003 | 0.297 | |||
| N0 | 80 | 3.5 ± 1.3 | 263.0 ± 100.2 | ||
| N1-3 | 195 | 4.1 ± 1.5 | 276.9 ± 99.2 |
Relationship between PFC, PC, and clinicopathologic features in NSCLC patients with brain metastases
We assessed several coagulation indices in our patient cohort. PFC was increased (higher than 4 g/L) in 41.1% (113/275) of patients. Median PFC was 3.92 g/L (range, 0.68–9.8 g/L) in pretreated patients. PFC was not associated with gender, number of brain metastases, size of brain metastases, extracranial lesions, or histologic subtype. However, a significant association between PFC and age was observed. Elder patients (≥65 years) had significantly higher level of PFCs than younger patients (4.3 g/L vs. 3.8 g/L, P = 0.011; Table 1). The association between PFC and the smoking status was evident (P = 0. 009). Patients with intracranial symptoms showed a slightly lower level of PFC compared to those without intracranial symptoms (P = 0.022). Moreover, T category (P = 0.010) and N category (P = 0.003) were associated with PFC (Figure 1). Patients with positive lymph nodes (N1+N2+N3) had a significantly higher PFC than those with negative lymph node (N0) (4.083 ± 1.503 vs. 3.511 ± 1.327, P = 0.003; Figure 1). PC was increased (> 300 × 109/L) in 27.3% (75/275) of patients. Size of brain metastasis was the only clinicopathologic feature associated with PC (P = 0.036, Table 1).
Figure 1. Relationship between pretreatment plasma fibrinogen concentration (PFC) and clinical T and N categories in 275 non–small cell lung cancer (NSCLC) patients with brain metastases.
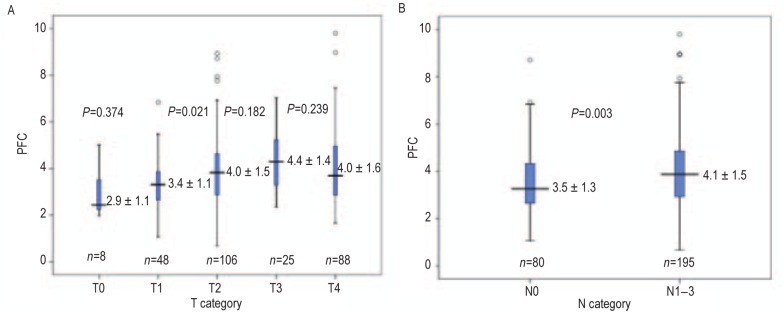
A, relationship between pretreatment PFC and clinical T category. B, relationship between pretreatment PFC and clinical N category.
Relationship between PFC, PC, and APTT
We observed a linear correlation between PFC and PC in NSCLC patients with brain metastases (R2 = 0.146, P < 0.001). We found a similar correlation between PFC and APTT (R2 = 0.117, P < 0.001), as shown in Figure 2. The correlation between PFC and other coagulation indices was not significant.
Figure 2. Linear correlations exist between PFC and platelet count (PC) or activated partial thromboplastin time (APTT) in 275 NSCLC patients with brain metastases.
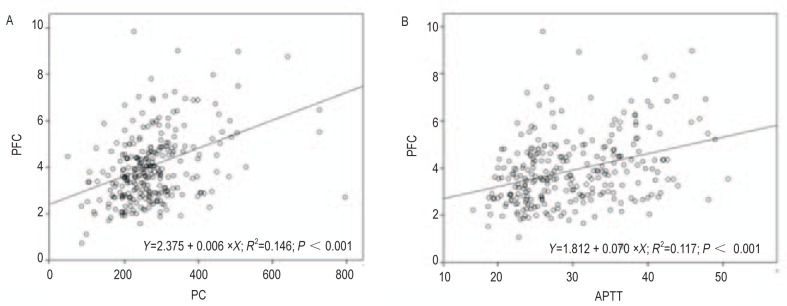
A, correlation between PFC and PC in 275 NSCLC patients with brain metastases. B, correlation between PFC and APTT in 275 NSCLC patients with brain metastases.
Coagulation factors and overall survival
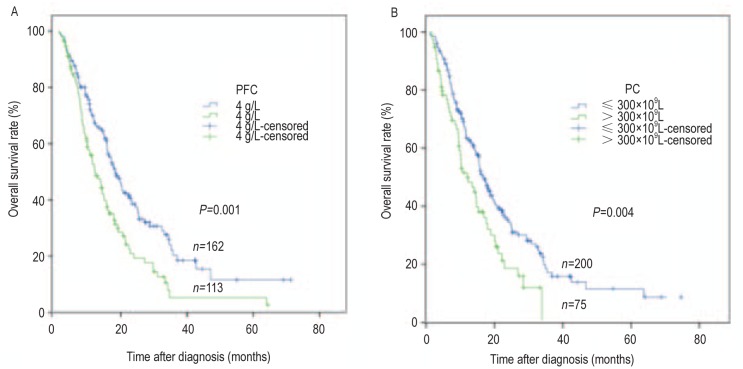
All 275 patients were rigorously followed up, with a median follow-up time of 20.7 months. Patients with normal PFC demonstrated longer OS compared with those with increased PFC (median, 17.3 months vs. 11.1 months, P < 0.001; Figure 3, Table 2). Patients with normal PC also demonstrated longer OS compared with those with lifted PC (median, 16.3 months vs. 11.4 months, P = 0.004; Figure 3, Table 2). Other coagulation indices—APTT, prothrombin time (PT), D-dimerization (D-D), and fibrinogen degradation product (FDP)—were also significantly associated with OS, whereas thromboplastin time (TT) was not (Table 2).
Figure 3. Kaplan-Meier overall survival curves for NSCLC patients with brain metastases.
A, overall survival curves according to pretreatment PFC. B, overall survival curves according to pretreatment PC.
Table 2. Kaplan-Meier survival analysis (log-rank test) according to the level of coagulation factors in NSCLC patients with brain metastases.
| Variable | No. of patients | Median overall survival (months) | 95% CI (months) | P |
| In total | 275 | 14.9 | 13.1–16.7 | |
| PFC | <0.001 | |||
| ≤4.0 g/L | 162 | 17.3 | 14.9–24.7 | |
| >4.0 g/L | 113 | 11.1 | 10.5–15.1 | |
| APTT | 0.001 | |||
| ≤34 s | 192 | 16.9 | 13.9–20.0 | |
| >34 s | 83 | 10.9 | 8.4–13.4 | |
| PT | 0.046 | |||
| ≤13.5 s | 243 | 15.4 | 13.4–17.3 | |
| >13.5 s | 32 | 10.4 | 4.3–16.6 | |
| TT | 0.280 | |||
| ≤21s | 247 | 15.0 | 13.0–16.9 | |
| >21s | 18 | 11.1 | 0.0–26.5 | |
| PC | 0.004 | |||
| ≤300×109/L | 200 | 16.3 | 14.1–18.5 | |
| >300×109/L | 75 | 11.4 | 7.2–15.5 | |
| D-D | 0.006 | |||
| ≤1.5 µg/mL | 54 | 24.4 | 12. 4–36.4 | |
| >1.5 µg/mL | 25 | 11.1 | 8.2–14.0 | |
| FDP | 0.001 | |||
| ≤5.0 µg/mL | 57 | 24.4 | 12.5–36.3 | |
| >5.0 µg/mL | 21 | 11.1 | 8.4–13.8 |
PFC, plasma fibrinogen concentration; PC, platelet count; PT, prothrombin time; APTT, activated partial thromboplastin time; TT, thromboplastin time; D-D, D-dimerization; FDP, fibrinogen degradation product.
Tumor biomarkers and overall survival
Patients with normal CA 19-9 level had longer OS compared with those with increased levels (median, 16.5 months vs. 9.7 months, P = 0.004; Table 3). Furthermore, patients with normal CEA level had non-significantly but potential longer OS compared with those with increased levels (median, 16.3 months vs. 14.8 months; Table 3). However, non-significant differences were observed between other lung cancer-related tumor markers and OS (Table 3).
Table 3. Kaplan-Meier survival analysis (log-rank test) according to tumor biomarkers in NSCLC patients with brain metastases.
| Variable | No. of patients | Median overall survival (months) | 95% CI (months) | P |
| In total | 275 | 14. 9 | 13.1–16.7 | |
| CEA | 0.246 | |||
| ≤5 g/L | 83 | 16.3 | 11.2–21.3 | |
| >5 g/L | 192 | 14.8 | 13.2–16.5 | |
| CYFR 21-1 | 0.083 | |||
| ≤3.5 g/L | 25 | 24.2 | 9.5–39.0 | |
| >3.5 g/L | 53 | 16.2 | 13.6–18.9 | |
| NSE | 0.050 | |||
| ≤15.2 g/L | 76 | 17.2 | 13.9–20.7 | |
| >15.2 g/L | 49 | 11.1 | 4.4–17.9 | |
| CA125 | 0.141 | |||
| ≤35 g/L | 35 | 18.7 | 13.7–23.7 | |
| >35 g/L | 65 | 14.9 | 11.5–18.3 | |
| CA153 | 0.257 | |||
| ≤25 g/L | 41 | 16.6 | 11.9–21.4 | |
| >25 g/L | 51 | 14.9 | 9.1–20.7 | |
| CA19-9 | 0.004 | |||
| ≤35 g/L | 84 | 16.5 | 13.0–20.0 | |
| >35 g/L | 32 | 9.7 | 7.5–11.8 |
CEA, carcino-embryonic antigen; CYFR21-1, cytokeratin 19 fragments; NSE, neuron-specific enolase; CA125, cancer antigen 125; CA153, cancer antigen 153; CA19-9, cancer antigen 19-9.
Clinicopathologic features and overall survival
Univariate analysis showed that the following variables significantly associated with OS: age, smoking status, number of brain metastases, size of brain metastasis, clinical T category, clinical N category, and treatment modality (Table 4).
Table 4. Univariate survival analyses (log-rank test) according to clinicopathologic features in NSCLC patients with brain metastases.
| Variable | No. of patients | Median overall survival (months) | 95% CI (months) | P |
| Total | 275 | 14.9 | 13.1 ± 16.7 | |
| Sex | 0.200 | |||
| Male | 183 | 14.9 | 12.7 ± 17.1 | |
| Female | 92 | 14.9 | 10.7 ± 19.1 | |
| Age (years) | 0.026 | |||
| ≤65 | 215 | 16.2 | 14.3 ± 18.1 | |
| >65 | 60 | 10.2 | 7.2 ± 13.1 | |
| Smoking status | 0.001 | |||
| Never | 136 | 16.9 | 13.1 ± 20.8 | |
| Ever | 139 | 13.4 | 10.0 ± 16.8 | |
| Histology | 0.327 | |||
| AC | 246 | 14.9 | 12.9 ± 16.9 | |
| NAC | 29 | 17.3 | 9.0 ± 25.7 | |
| Number of BM | 0.031 | |||
| <3 | 166 | 17.3 | 14.1 ± 20.4 | |
| ≥3 | 109 | 13.4 | 11.0 ± 15.7 | |
| Size of BM in diameter | 0.022 | |||
| <3 cm | 230 | 15.7 | 14.1 ± 17.4 | |
| ≥3 cm | 45 | 10.0 | 6.7 ± 13.4 | |
| Extracraninal lesions | 0.221 | |||
| No | 161 | 15.4 | 13.0 ± 17.7 | |
| Yes | 114 | 13.9 | 11.9 ± 15.8 | |
| Intracranial symptoms | 0.118 | |||
| Yes | 95 | 13.7 | 9.2 ± 18.2 | |
| No | 180 | 15.3 | 13.3 ± 17.5 | |
| T category | 0.024 | |||
| T0 | 8 | 33.6 | NA | |
| T1 | 48 | 16.5 | 12.1 ± 21.0 | |
| T2 | 106 | 15.2 | 12.3 ± 18.2 | |
| T3 | 25 | 11.4 | 9.4 ± 13.4 | |
| T4 | 88 | 13.7 | 10.9 ± 16.6 | |
| N category | <0.001 | |||
| N0 | 80 | 24.1 | 14.7 ± 33.4 | |
| N1-3 | 195 | 13.3 | 11.2 ± 15.4 | |
| Treatment modality | <0.001 | |||
| Symptomatic treatmenta | 57 | 9.0 | 5.4 ± 12.7 | |
| Systemic treatmentb | 218 | 16.5 | 14.3 ± 18.8 |
aSymptomatic treatment, given to reduce intracranial pressure: mannitol 125 mg twice per day via intravenous drip; bSystemic treatment, systemic chemotherapy plus local treatment. AC, adenocarcinoma; NAC, non-adenocarcinoma; BM, brain metastases; NA, not available.
Multivariate analyses
After multivariate analyses, the remaining independent prognostic factors for OS were smoking status, size of brain metastasis, clinical N category, PFC, PC, and treatment modality (Table 5).
Table 5. Multivariate survival analyses for overall survival according to the Cox regression model.
| Variable | RR | 95% CI | P |
| PFC | 1.5 | 1.1-2.1 | 0.008 |
| PC | 1.4 | 1.0-2.0 | 0.042 |
| Smoking status | 1.5 | 1.1-2.0 | 0.007 |
| Size of BM | 1.6 | 1.1-2.3 | 0.027 |
| N category | 1.8 | 1.3-2.5 | 0.001 |
| Treatment modality | 0.5 | 0.3-0.7 | <0.001 |
PFC, plasma fibrinogen concentration; PC, platelet count; RR, relative risk; CI, confidence interval; BM, brain metastasis.
Discussion
Our study has supported our hypothesis regarding the formation of platelet-fibrin-tumor cell aggregates in developing brain metastases of NSCLC. In our results, PFC and PC were increased in 41.1% (113 of 275) and 27.3% (75 of 275) of patients, respectively. These findings demonstrated that high coagulation state is a common phenomenon in advanced NSCLC[9]. Similar results have been observed in other human cancers, such as oral[10], ovarian[11], liver[12], and gastric cancers[13], such that high PFC is closely associated with increased tumor invasion, distant metastases, and poor prognosis for solid tumors. These results suggest that up-regulation of plasma fibrinogen may provide a selective advantage in tumor invasion and regional lymph node metastases in NSCLC.
Fibrinogen is a large glycoprotein synthesized and secreted by hepatic cells[14]. Platelet alpha granules are also rich in fibrinogen, which gets released into the blood upon activation by tumor cells[15]. Fibrinogen is recognized by multiple integrin and non-integrin receptors found on tumor cells, stromal cells, and inflammatory cells. The cellular interactions of fibrinogen mediated by specific receptors may control cell proliferation, cell migration, apoptosis, and expression of inflammatory mediators[16]. Fibrinogen may also play an important role in tumor metastasis, though the mechanism remains unclear. Current research suggests two hypotheses. One proposes that platelet adherence to tumor cells in peripheral blood may prolong the survival of malignant cells by protecting them from immune surveillance, turbulence, and sheer stress, and by enabling them to more easily adhere to the vessel wall[17]. The other proposes that fibrinogen is involved in tumor cell migration[18],[19]. Palumbo et al.[20] confirmed that the lack of plasma fibrinogen from rat models can reduce lymph node and blood metastases in rat. In our study, we found a linear correlation between PFC and PC in NSCLC patients with brain metastases, which supports the above hypotheses. In addition, tumor cells in the metastatic process require a large amount of coagulation factors, such as APTT and TT, which is also observed in our study.
Coagulation indices have prognostic significance in NSCLC. Pavey et al.[7] and Maeda et al.[21] found that plasma fibrinogen was associated with decreased survival and strongly with stage in 166 patients who underwent surgical resection of NSCLC. To the best of our knowledge, no study has focused on association between coagulation indices and NSCLC with brain metastases.
Distant metastases result poor outcome for NSCLC. Brain metastases is commonly observed in 20%–40% of NSCLC patients during the disease progression[22],[23]. Clinical prognostic biomarkers are largely unknown for this disease. Lee et al.[24] demonstrated that the pretreatment serum CEA level was significantly associated with brain metastases in advanced NSCLC. However, in our study, patients with normal CEA level had potential longer survival than those with lifted CEA. Only the level of CA 19-9 was significantly associated with OS. Furthermore, lifted PFC and PC were unfavorable prognostic factors on OS either in univariate or multivariate analyses for NSCLC patients with brain metastases. These results suggest that PFC and PC may be more effective prognostic predictors than tumor biomarkers in NSCLC patients with brain metastases.
For NSCLC, smoking cigarettes is not only the most established risk factor but also a prognostic factor[25]. Tuut et al.[26] suggested a primary role for increased synthesis of fibrinogen in producing the hyperfibrinogenemia associated with smoking status. In this study, there was a strong association between smoking status and PFC. NSCLC patients with brain metastases who have never smoked have longer OS than those have smoked.
Brain metastases are often presented with cerebral edema, which can endanger a patient's life. Applying adrenal cortical hormone was initial treatment to control intracranial hypertension of patients with cerebral edema and increased the median survival time to 2–3 months[27]. In our study, NSCLC patients with brain metastases and increased PFC were rarely observed with intracraninal symptoms compared to patients with normal PFC. However, the mechanism for this is unclear.
In summary, our results show that PFC and PC may serve as novel markers for predicting the prognosis of NSCLC with brain metastases. Moreover, anticoagulation therapy may improve the survival of patients with this disease. Further studies are required to validate our results.
Acknowledgments
This research was supported by grants from Ministry of Science and Technology Projects of China (No. 2012AA021502) and Provincial Science and Technology Projects of Guangdong (No. 2012B031800295).
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Kim SY, Kim JS, Park HS, et al. Screening of brain metastases with limited magnetic resonance imaging (MRI): clinical implications of using limited brain MRI during initial staging for non-small cell lung cancer patients. J Korean Med Sci. 2005;20:121–126. doi: 10.3346/jkms.2005.20.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cedres S, Nunez I, Longo M, et al. Serum tumor markers CEA, CYFRA21-1, and CA-125 are associated with worse prognosis in advanced non-small-cell lung cancer (NSCLC) Clin Lung Cancer. 2011;12:172–179. doi: 10.1016/j.cllc.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Barlesi F, Gimenez C, Torre JP, et al. Prognostic value of combination of Cyfra 21-1, CEA and NSE in patients with advanced non-small cell lung cancer. Respir Med. 2004;98:357–362. doi: 10.1016/j.rmed.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Biggerstaff JP, Seth N, Amirkhosravi A, et al. Soluble fibrin augments platelet/tumor cell adherence in vitro and in vivo, and enhances experimental metastases. Clin Exp Metastases. 1999;17:723–730. doi: 10.1023/a:1006763827882. [DOI] [PubMed] [Google Scholar]
- 6.Buccheri G, Ferrigno D, Ginardi C, et al. Haemostatic abnormalities in lung cancer: prognostic implications. Eur J Cancer. 1997;33:50–55. doi: 10.1016/s0959-8049(96)00310-3. [DOI] [PubMed] [Google Scholar]
- 7.Pavey SJ, Hawson GA, Marsh NA. Impact of the fibrinolytic enzyme system on prognosis and survival associated with non-small cell lung carcinoma. Blood Coagul Fibrinolysis. 2001;12:51–58. doi: 10.1097/00001721-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Seitz R, Rappe N, Kraus M, et al. Activation of coagulation and fibrinolysis in patients with lung cancer: relation to tumour stage and prognosis. Blood Coagul Fibrinolysis. 1993;4:249–254. doi: 10.1097/00001721-199304000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Wojtukiewicz MZ, Zacharski LR, Moritz TE, et al. Prognostic significance of blood coagulation tests in carcinoma of the lung and colon. Blood Coagul Fibrinolysis. 1992;3:429–437. [PubMed] [Google Scholar]
- 10.Ghosh M, Aroor AR, Raghavan MR. Clinical utility of serum fibrinogen degradation products (FDP) in the diagnostic and prognostic evaluation of oral cancer. Ann Dent. 1990;49:11–12, 45. [PubMed] [Google Scholar]
- 11.Sawaguchi K, Hojo T, Nozaki S, et al. The clinical significance of thrombin-antithrombin III complex (TAT) and fibrinogen and fibrin degradation products (FDP) levels in ovarian cancer. Nihon Sanka Fujinka Gakkai Zasshi. 1992;44:73–78. [PubMed] [Google Scholar]
- 12.Halota W, Lapniewska E, Bulik F. The gamma globulin-fibrinogen index in the differential diagnosis of decompensated cirrhosis and primary liver cancer. Wiad Lek. 1990;43:118–121. [PubMed] [Google Scholar]
- 13.Lee JH, Ryu KW, Kim S, et al. Preoperative plasma fibrinogen levels in gastric cancer patients correlate with extent of tumor. Hepatogastroenterology. 2004;51:1860–1863. [PubMed] [Google Scholar]
- 14.Duga S, Asselta R, Santagostino E, et al. Missense mutations in the human beta fibrinogen gene cause congenital afibrinogenemia by impairing fibrinogen secretion. Blood. 2000;95:1336–1341. [PubMed] [Google Scholar]
- 15.Handagama PJ, Shuman MA, Bainton DF. In vivo defibrination results in markedly decreased amounts of fibrinogen in rat megakaryocytes and platelets. Am J Pathol. 1990;137:1393–1399. [PMC free article] [PubMed] [Google Scholar]
- 16.Jones JM, Mcgonigle NC, Mcanespie M, et al. Plasma fibrinogen and serum C-reactive protein are associated with non-small cell lung cancer. Lung Cancer. 2006;53:97–101. doi: 10.1016/j.lungcan.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Palumbo JS, Kombrinck KW, Drew AF, et al. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood. 2000;96:3302–3309. [PubMed] [Google Scholar]
- 18.Yamazaki D, Kurisu S, Takenawa T. Regulation of cancer cell motility through actin reorganization. Cancer Sci. 2005;96:379–386. doi: 10.1111/j.1349-7006.2005.00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sahai E. Mechanisms of cancer cell invasion. Curr Opin Genet Dev. 2005;15:87–96. doi: 10.1016/j.gde.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Palumbo JS, Potter JM, Kaplan LS, et al. Spontaneous hemato-genous and lymphatic metastases, but not primary tumor growth or angiogenesis, is diminished in fibrinogen-deficient mice. Cancer Res. 2 [PubMed] [Google Scholar]
- 21.Maeda R, Yoshida J, Ishii G, et al. The prognostic impact of cigarette smoking on patients with non-small cell lung cancer. J Thorac Oncol. 2011;6:735–742. doi: 10.1097/JTO.0b013e318208e963. [DOI] [PubMed] [Google Scholar]
- 22.Schouten LJ, Rutten J, Huveneers HA, et al. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94:2698–2705. doi: 10.1002/cncr.10541. [DOI] [PubMed] [Google Scholar]
- 23.Han F, Xia YF, Lu LX, et al. Analysis of prognostic factors for 63 patients with brain metastases from lung cancer after radiochemotherapy. Ai Zheng. 2002;21:1141–1144. [in Chinese] [PubMed] [Google Scholar]
- 24.Lee DS, Kim YS, Jung SL, et al. The relevance of serum carcinoembryonic antigen as an indicator of brain metastases detection in advanced non-small cell lung cancer. Tumour Biol. 2012 doi: 10.1007/s13277-012-0344-0. [DOI] [PubMed] [Google Scholar]
- 25.Maeda R, Yoshida J, Ishii G, et al. The prognostic impact of cigarette smoking on patients with non-small cell lung cancer. J Thorac Oncol. 2011;6:735–742. doi: 10.1097/JTO.0b013e318208e963. [DOI] [PubMed] [Google Scholar]
- 26.Tuut M, Hense HW. Smoking, other risk factors and fibrinogen levels. evidence of effect modification. Ann Epidemiol. 2001;11:232–238. doi: 10.1016/s1047-2797(00)00226-x. [DOI] [PubMed] [Google Scholar]
- 27.Ebert BL, Niemierko E, Shaffer K, et al. Use of temozolomide with other cytotoxic chemotherapy in the treatment of patients with recurrent brain metastases from lung cancer. Oncologist. 2003;8:69–75. doi: 10.1634/theoncologist.8-1-69. [DOI] [PubMed] [Google Scholar]