Abstract
Both platinum-based doublet chemotherapy (PBC) and epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) prolong the survival of patients with advanced non-small cell lung cancer (NSCLC). In early studies, most patients underwent PBC as first-line treatment, but not all patients could afford EGFR-TKIs as second-line treatment. To understand the impact of PBC and EGFR-TKIs on NSCLC prognosis, we evaluated the association between the receipt of both regimens and overall survival (OS). Using MEDLINE and EMBASE, we identified prospective, randomized, controlled phase III clinical trials in advanced NSCLC that met the inclusion criteria: in general population with advanced NSCLC, the percentage of patients treated with both PBC and EGFR-TKIs was available in the trial and OS was reported. After collecting data from the selected trials, we correlated the percentage of patients treated with both PBC and EGFR-TKIs with the reported OS, using a weighted analysis. Fifteen phase III clinical trials—involving 11,456 adult patients in 32 arms—were included in the analysis, including 6 trials in Asian populations and 9 in non-Asian (predominantly Caucasian) populations. The OS was positively correlated with the percentage of patients treated with both PBC and EGFR-TKIs (r = 0.797, P < 0.001). The correlation was obvious in the trials in Asian populations (r = 0.936, P < 0.001) but was not statistically significant in the trials in predominantly Caucasian populations (r = 0.116, P = 0.588). These results suggest that treatment with PBC and EGFR-TKIs may provide a survival benefit to patients with advanced NSCLC, highlighting the importance of having both modalities available for therapy.
Keywords: NSCLC, platinum-based doublet chemotherapy, correlation, EGFR-TKIs, overall survival
Lung cancer remains the leading cause of cancer mortality worldwide, with 1.4 million deaths per year. Non–small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancer cases. Upon initial diagnosis, approximately 25%–30% of NSCLC patients present with locally advanced disease, whereas 40%–50% present with metastatic disease[1],[2]. The third-generation platinum-based doublet chemotherapy (PBC) has long been considered the standard care for advanced NSCLC patients with a good performance status (PS)[3]. Although 70%–80% of patients experience clinical benefit after undergoing first-line chemotherapy, the overall survival (OS) remains disappointing, with a 5-year survival rate of less than 1%[4]. The OS was 8–10 months, and the 1-year survival rate was 30%–35%[5]. Different PBC combinations involving paclitaxel, gemcitabine, vinorelbine, and docetaxel have shown equal efficacies but distinct toxicity profiles in clinical trials[5],[6]. In addition to the medicine mentioned above, pemetrexed, another cytotoxic drug, was more effective in lung adenocarcinoma than in squamous cell carcinoma with mild toxicity, but the reported survival was still similar to the previously reported result (about 10 months)[7],[8].
In recent years, advances in targeted therapy have provided new treatment options. Small-molecule tyrosine kinase inhibitors (TKIs) of epidermal growth factor receptor (EGFR), such as gefitinib and erlotinib, have shown antitumor activity in patients with advanced NSCLC, especially those with EGFR mutation. A Japanese study compared survival before and after gefitinib treatment in patients with advanced NSCLC and showed that OS was significantly prolonged in patients after gefitinib treatment[9].
In most clinical trials about advanced NSCLC during the last decade, monotherapy with either EGFR-TKIs or chemotherapy was administered as a salvage regimen in post-study treatment, though to different extents. The reported OS varied in these trials. Notably, there was no significant difference in patient selection, and the trials were conducted within a relatively short time for an individual patient. Thus, the variance in survival time was likely due to differences in the proportion of patients who underwent post-study treatment[10]. Similarly, in a research involving patients with colorectal cancer, the percentage of patients who received fluorouracil-leucovorin, irinotecan, and oxaliplatin (first- or second-line and third-line) was positively correlated with the reported median survival[10],[11]. However, to our knowledge, no similar study has been conducted in NSCLC. Hence, our study was undertaken to determine the impact of both PBC and EGFR-TKIs on OS in phase III clinical trials of advanced NSCLC.
Materials and Methods
Literature search
To ensure all relevant studies (randomized controlled trials) on the topic were retrieved, we used a broad search strategy with key words related to lung cancer. Using the search terms “non–small cell lung cancer,” “lung adenocarcinoma,” or “lung squamous carcinoma,” we identified all related clinical trials of NSCLC published within the past 12 years (January 2001 to February 2012) from PubMed and EMBASE. All results were limited to phase III randomized controlled clinical trials published in English. We also searched the reference lists of articles and reviews.
Literature selection
Two reviewers screened all literature independently to verify compliance with the predetermined inclusion criteria. When there were disagreements between the two reviewers, a third reviewer was involved to facilitate consensus.
The inclusion criteria were as follows: (1) the study was a randomized controlled trial; (2) the patients enrolled were >18 years with pathologically proven advanced NSCLC, and the majority had a baseline Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–1 (PS = 2 in less than 20% of the patients); (3) the OS was reported, and the percentage of patients treated with both PBC and EGFR-TKIs anytime during the course of treatment was available in the papers; and (4) the patients enrolled were from the general population and not selected on the basis of molecular status (to guarantee homogeneity).
The following trials were excluded: (1) trials involving only patients over 70 years of age or patients previously exposed to other antitumor treatments for an indeterminate time, and (2) trials comparing the combination of chemotherapy and EGFR-TKIs with chemotherapy alone. However, trials comparing chemotherapy and the combination of chemotherapy and other targeted agents such as cetuximab, bevacizumab, vadimezan, and bexarotene were included.
Data collection and analysis
The following data were collected from each selected study: first authors, publication year, study regimens, number of patients, median age, tumor stage, percentage of Asian and Caucasian subjects, percentage of female subjects, tumor pathologic type (proportions of adenocarcinoma and squamous carcinoma), PS, percentage of patients treated with both PBC and EGFR-TKIs, OS, and median progression-free survival (PFS). OS was calculated from the time of first-line treatment, which meant that the patients in the trials should be chemo-naïve.
Statistical analysis
Correlation between the percentage of patients receiving both PBC and EGFR-TKIs and the OS was examined using linear regression analysis. For sensitivity analysis, a weighted regression was performed, with weight proportional to the trial's sample size. Trials were also divided on the basis of the primary population enrolled—Asian or non-Asian (primarily Caucasian)—by determining the country in which the study was conducted or the proportion of patients of different races. Correlation between the percentage of patients treated with both PBC and EGFR-TKIs and the OS among different ethnicities was determined using the same weighted regression analysis. The influence of patient or tumor characteristics such as sex, PS, ethnicity, tumor histologic subtypes, and tumor stage on OS, PFS, and response rate was also examined with student t test or chi-square test. P values <0.05 were considered statistically significant, and all reported P values were two-sided. All statistical analyses were performed with STATA SE 10.0 package (StataCorp, College Station, TX, USA).
Results
Characteristics of the selected studies
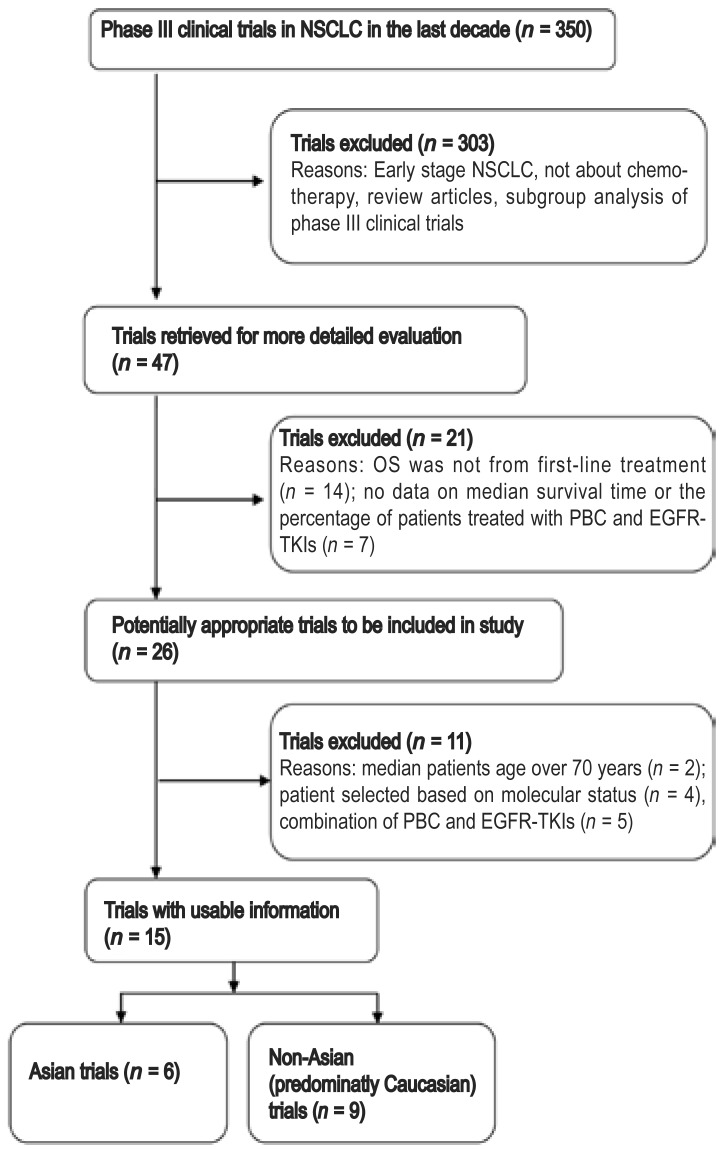
Our literature search yielded 350 trials, of which 303 were excluded and 47 were considered potentially relevant. The remaining 47 trials were retrieved for more detailed evaluation. Of these, 21 were excluded for the following reasons: 14 trials were second- or third-line treatments, and the OS was not calculated from first-line treatment; and 7 trials failed to provide survival time or information about the patients receiving TKIs. Upon further analysis of the remaining 26 trials, 11 were excluded: 2 focused on patients over 70 years of age, 4 (OPTIMAL, NEJ002, EURATAC, and WJTOG3405) on patients with EGFR mutations, and 5 on the combination of chemotherapy and TKIs. Thus, 15 trials, which involved 11,456 adult patients in 32 arms, met all of the inclusion criteria and were used in our study. The flowchart of trial selection is shown in Figure 1.
Figure 1. Flow chart showing the process of trial selection in patients with non–small cell lung cancer (NSCLC).
OS, overall survival; PBC, platinum-based doublet chemotherapy; TKIs, tyrosine kinase inhibitors.
For patients in the 15 selected trials, the median age was 61 years (range, 56–65 years), and the majority (range, 82%–100%) had a PS of 0–1. Six trials were performed in predominantly Asian populations, whereas 9 were performed in non-Asian (predominantly Caucasian) populations. The basic characteristics of the 15 trials are shown in Table 1.
Table 1. Characteristics of the trials included in the analysis.
| First author/year | Study regimens | No. of Pts | PS (%) |
Median age (years) | Stage (%) |
PFS (months) | Female (%) | ||
| 0-1 | ≥2 | IIIB | IV | ||||||
| Mok TS/2010 [23] | Gefitinib | 609 | 90 | 10 | 57 | 24.6 | 75.4 | 5.7 | 79.5 |
| TC | 608 | 89.3 | 10.7 | 57 | 23.8 | 76.2 | 5.8 | 79.1 | |
| Okamoto I/2010 [31] | TC | 281 | 100 | 0 | 63 | 24.2 | 75.8 | 4.8 | 23.5 |
| Carboplatin + S-1 | 282 | 100 | 0 | 64 | 24.1 | 75.9 | 4.1 | 23.0 | |
| Kubota K/2008 [32] | GN followed docetaxela | 196 | 100 | 0 | 64 | 17.0 | 83 | 5.5 | 27.0 |
| TC | 197 | 100 | 0 | 65 | 17.0 | 83 | 5.8 | 31.0 | |
| Ohe Y/2007 [33] | IP | 145 | 100 | 0 | 62 | 21.4 | 78.6 | 4.7 | 33.1 |
| TC | 145 | 100 | 0 | 63 | 19.3 | 80.7 | 4.5 | 31.7 | |
| GP | 146 | 100 | 0 | 61 | 20.5 | 79.5 | 4.0 | 30.8 | |
| NP | 145 | 100 | 0 | 61 | 17.9 | 82.1 | 4.1 | 30.3 | |
| Kubota K/2004 [34] | DP | 151 | 96 | 4 | 63 | 0 | 100 | - | 35.8 |
| Vindesine + cisplatin | 151 | 96.7 | 3.3 | 64 | 0 | 100 | - | 31.8 | |
| Han JY/2012 [24] | Gefitinib | 159 | 91.2 | 8.8 | 57 | 10.7 | 89.3 | 5.8 | 88.0 |
| GP | 150 | 90.7 | 9.3 | 56.5 | 9.3 | 90.7 | 6.4 | 89.3 | |
| Lara PN Jr/2011 [35] | TC + vadimezan | 649 | 99.7 | - | 62 | 8.2 | 91.8 | 5.5 | 37.9 |
| TC + placebo | 650 | 98.8 | - | 61 | 9.1 | 90.9 | 5.5 | 37.7 | |
| Reck M/2010 [36] | Placebo + GP | 347 | 100 | 0 | 59 | 23.0 | 77 | 6.1 | 36.0 |
| Bevacizumab7.5 + GP | 345 | 100 | 0 | 57 | 22.0 | 78 | 6.7 | 35.0 | |
| Bevacizumab15 + GP | 351 | 100 | 0 | 59 | 23.0 | 77 | 6.5 | 38.0 | |
| Lynch TJ/2010 [37] | TC + C225 | 338 | 98 | 2 | 64 | 12.0 | 88 | 4.4 | 43.0 |
| TC | 338 | 99 | 1 | 65 | 14.0 | 86 | 4.24 | 40.0 | |
| Pirker R/2009 [38] | NP + cetuximab | 557 | 83 | 17 | 59 | 6.0 | 94 | 4.8 | 31.0 |
| NP | 568 | 82 | 18 | 60 | 6.0 | 94 | 4.8 | 29.0 | |
| Tan EH/2009 [39] | NP | 194 | 62.1 | 37.9 | 59.4 | 19.5 | 80.5 | 4.9 | 26.8 |
| DP | 196 | 62.3 | 37.7 | 62.1 | 15.2 | 84.8 | 5.1 | 23.6 | |
| Scagliotti GV/2008 [8] | GP | 830 | 99.9 | NA | 61.1 | 24.3 | 75.7 | 5.1 | 29.9 |
| AP | 839 | 99.8 | NA | 61 | 23.8 | 76.2 | 4.8 | 29.8 | |
| Ramlau R/2008 [40] | Bexarotene + NP | 311 | 100 | 0 | 61 | 17.0 | 83.0 | 4.3 | 28.0 |
| NP | 312 | 100 | 0 | 61 | 19.0 | 81.0 | 5.0 | 28.0 | |
| Blumenschein GR Jr /2008 [41] | TC + bexarotene | 306 | 100 | 0 | 63 | 13.0 | 87.0 | 4.1 | 34.0 |
| TC | 306 | 100 | 0 | 63 | 13.0 | 87.0 | 4.9 | 34.0 | |
| Sandler A/2011 [42] | TC + bevacizumab | 417 | 100 | 0 | NA | 22.0 | 78.0 | 6.2 | 50.0 |
| TC | 433 | 100 | 0 | NA | 26.0 | 74.0 | 4.5 | 42.0 | |
Pts, patients; PS, performance status; PFS, median progression-free survival; TC, paclitaxel + carboplatin; GN, gemcitabine + vinorelbine; IP, irinotecan + cisplatin; GP, gemcitabine + cisplatin; NP, vinorelbine + cisplatin; DP, docetaxel + cisplatin; AP, pemetrexed + cisplatin; NA, not available. aThis arm was excluded, because the percentage of patients treated with both platinum-based doublet chemotherapy (PBC) and epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) was unknown.
Correlation between trial characteristics and treatment results
The impact of trial characteristics on OS, PFS, and response rate is summarized in Table 2. Trials primarily involving Asian populations showed longer OS than did trials in non-Asian populations (P < 0.001). Similarly, the response rate to first-line treatment in Asian populations was also better than that in non-Asian populations (P = 0.007). In study arms with a larger proportion of adenocarcinomas, the OS and PFS were longer and the response rate was higher than those in arms with a smaller proportion of adenocarcinomas (P < 0.001, P = 0.017, and P = 0.012, respectively). The proportion of stage IV cases enrolled had no impact on OS (P = 0.560), PFS (P = 0.760), or response rate (P = 0.950). Most of the trials included patients with a PS of 0–1, with only a small portion having a PS of 2. The small proportion of patients with PS = 2 had no impact on the OS (P = 0.170) but did affect the response rate (P = 0.014). The trials with higher proportion of female subjects showed longer PFS (P = 0.005), but the OS and response rates were similar.
Table 2. The influence of trial characteristics on treatment results.
| Characteristic of the trials | No. of arms | OSc (months) | P | PFSc,d (months) | P | Response ratec (%) | P |
| Ethnicity | |||||||
| Asian population | 13 | 15.1 ± 4.1 | <0.001 | 5.1 ± 0.9 | 0.832 | 34.7 ± 9.2 | 0.007 |
| Non-Asian population (Caucasian) | 19 | 10.8 ± 1.8 | 5.1 ± 0.8 | 26.6 ± 6.8 | |||
| Proportion of femalesa | |||||||
| < 34% | 16 | 11.5 ± 2.0 | 0.11 | 4.8 ± 0.5 | 0.005 | 29.7 ± 5.5 | 0.92 |
| ≥34% | 16 | 13.6 ± 4.5 | 5.5 ± 0.9 | 30.0 ± 11.3 | |||
| Proportion of adenocarcinomasa | |||||||
| < 68% | 16 | 10.4 ± 1.9 | <0.001 | 4.8 ± 0.5 | 0.017 | 26.1 ± 6.1 | 0.012 |
| ≥68% | 16 | 14.6 ± 3.8 | 5.4 ± 0.9 | 32.6 ± 9.5 | |||
| Proportion of patients with PS ≥2b | |||||||
| 0 | 17 | 11.9 ± 2.3 | 0.17 | 5.0 ± 0.9 | 0.56 | 26.9 ± 7.6 | 0.014 |
| > 0 | 11 | 13.9 ± 5.3 | 5.2 ± 0.7 | 35.5 ± 9.4 | |||
| Proportion of patients with stage IVa | |||||||
| < 83% | 16 | 12.9 ± 2.6 | 0.56 | 5.2 ± 0.9 | 0.74 | 30.0 ± 6.7 | 0.95 |
| ≥83% | 16 | 12.1 ± 4.6 | 5.1 ± 0.7 | 29.8 ± 10.8 |
OS, overall survival; PFS, progression-free survival; PS, performance status. a The characteristics of all study arms were divided into two groups according to the median and then were compared. b Most of the trials had enrolled patients with PS 0-1. Two trials did not provide the data of patients with PS ≥2. c All the values are presented as mean ± standard deviation. d In one of the trials (two arms), there were no PFS reported.
Correlation between the percentage of patients treated with PBC and EGFR-TKIs and the OS
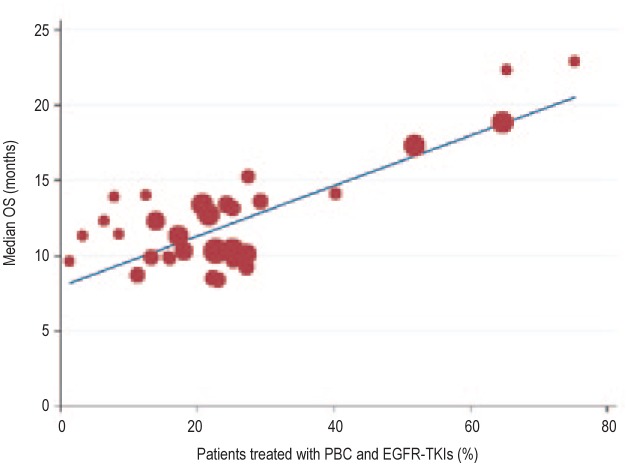
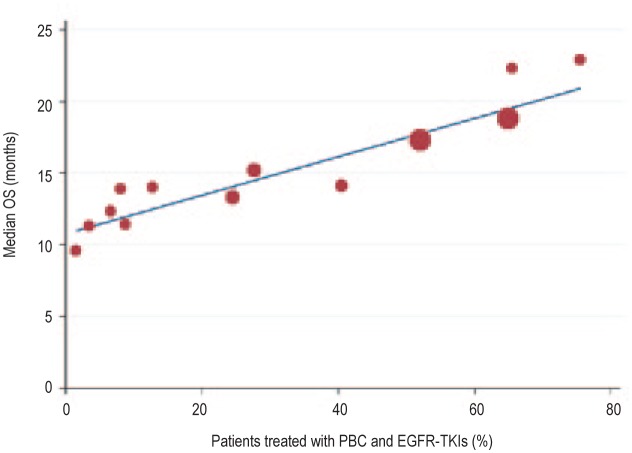
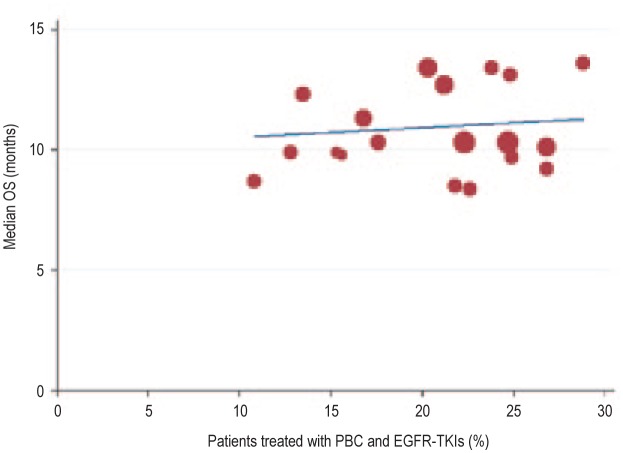
Plotting the data collected from the studies shown in Table 3, we found that the percentage of patients who underwent both PBC and EGFR-TKIs during the course of treatment was positively correlated with the reported OS (r = 0.797, P < 0.001; Figure 2). The trials with a higher proportion of patients treated with both PBC and EGFR-TKIs during the course of treatment showed longer OS. We further divided these trials by race group—Asian and non-Asian (predominantly Caucasian). We found that the correlation between the percentage of patients who underwent both PBC and EGFR-TKIs and the OS was strong in the 6 Asian trials (r = 0.936, P < 0.001; Figure 3) but not in the 9 non-Asian trials (r = 0.116, P = 0.588; Figure 4).
Table 3. Correlation between percentage of patients treated with both PBC and EGFR-TKIs during the course of the disease and the reported OS.
| First author/year | No. of Pts (%) | Response rate (%) | Reported OS (months) | Asian (%) | Caucasian (%) | Adenocarcinoma (%) | Squamous (%) | |
| Mok TS/2010 [23] | 609 | (64.5) | 43.0 | 18.8 | 99.7 | NR | 95.4 | 0 |
| 608 | (51.5) | 32.2 | 17.3 | 99.8 | NR | 97.2 | 0 | |
| Okamoto I/2010 [31] | 281 | (24.0) | 29.0 | 13.3 | 100 | 0 | 69.4 | 30.6 |
| 282 | (27.2) | 20.4 | 15.2 | 100 | 0 | 69.1 | 30.9 | |
| Kubota K/2008 [32] | 196 | NA | 25.0 | 13.6 | 100 | 0 | 66 | 23 |
| 197 | (40.0) | 37.0 | 14.1 | 100 | 0 | 76 | 15 | |
| Ohe Y/2007 [33] | 145 | (7.6) | 31.0 | 13.9 | 100 | 0 | 83.4 | 11 |
| 145 | (6.2) | 32.4 | 12.3 | 100 | 0 | 71.7 | 21.4 | |
| 146 | (12.3) | 30.1 | 14 | 100 | 0 | 74 | 19.9 | |
| 145 | (8.3) | 33.1 | 11.4 | 100 | 0 | 75.2 | 20 | |
| Kubota K/2004 [34] | 151 | (3.0) | 25 | 11.3 | 100 | 0 | 79.5 | 11.3 |
| 151 | (1.0) | 37 | 9.6 | 100 | 0 | 68.2 | 21.9 | |
| Han JY/2012 [24] | 159 | (65.0) | 55.4 | 22.3 | 100 | 0 | 100 | 0 |
| 150 | (75.0) | 46.0 | 22.9 | 100 | 0 | 100 | 0 | |
| Lara PN Jr/2011 [35] | 649 | (20.5) | 24.7 | 13.4 | 25 | 71.5 | 66.5 | 20.3 |
| 650 | (21.4) | 24.6 | 12.7 | 25.2 | 71.5 | 67.1 | 20.5 | |
| Reck M/2010 [36] | 347 | (25.0) | 21.6 | 13.1 | 8 | 92 | 82 | NR |
| 345 | (29.0) | 37.8 | 13.6 | 9 | 91 | 85 | NR | |
| 351 | (24.0) | 34.6 | 13.4 | 9 | 91 | 85 | NR | |
| Lynch TJ/2010 [37] | 338 | (25.1) | 25.7 | 9.69 | 2 | 88 | 51 | 20 |
| 338 | (22.8) | 17.2 | 8.38 | 3 | 89 | 54 | 19 | |
| Pirker R/2009 [38] | 557 | (17.0) | 36.0 | 11.3 | 11 | 84 | 46 | 34 |
| 568 | (27.0) | 29.0 | 10.1 | 10 | 85 | 49 | 33 | |
| Tan EH/2009 [39] | 194 | (15.5) | 31.2 | 9.9 | 0 | 100 | 41.6 | 34.2 |
| 196 | (15.8) | 29.6 | 9.8 | 0 | 100 | 39.3 | 33.5 | |
| Scagliotti GV/2008 [8] | 830 | (22.5) | 28.2 | 10.3 | 12.1 | 78.8 | 47.6 | 26.5 |
| 839 | (24.9) | 30.6 | 10.3 | 13.5 | 77.6 | 50.6 | 28.3 | |
| Ramlau R/2008 [40] | 311 | (11.0) | 16.7 | 8.7 | 1 | 89 | 39 | 38 |
| 312 | (13.0) | 24.4 | 9.9 | 1 | 92 | 41 | 36 | |
| Blumenschein GR Jr /2008 [41] | 306 | (22.0) | 19.3 | 8.5 | NR | 88 | 55 | 20 |
| 306 | (27.0) | 23.5 | 9.2 | NR | 89 | 50 | 21 | |
| Sandler A/2011 [42] | 417 | (13.7) | 35.0 | 12.3 | 25.0 | 91.0 | 88.0 | 20.3 |
| 433 | (17.8) | 15.0 | 10.3 | 25.2 | 90.0 | 88.0 | 20.5 | |
Pts, patients; PS, performance status; OS, overall survival; NA, not available. NR, not reported.
Figure 2. Linear regression curve showing positive correlation between the percentage of patients treated with both PBC and EGFR-TKIs during the course of treatment and the OS (r = 0.797, R2 = 0.636, P < 0.001) in all selected trials.
Mathematic equation of regression (based on a weighted model): OS (months) = 8.01 + 16.7 × (percentage of patients treated with both PBC and EGFR-TKIs).
Figure 3. Linear regression curve showing positive correlation between the percentage of patients treated with both PBC and EGFR-TKIs during the course of treatment and the OS (r = 0.936, R2 = 0.876, P < 0.001) in Asian trials.
Mathematic equation of regression (based on a weighted model): OS (months) =10.82 + 13.42 × (percentage of patients treated with both PBC and EGFR-TKIs).
Figure 4. Linear regression curve showing no obvious correlation correlation between the percentage of patients treated with both PBC and EGFR-TKIs during the course of treatment and the OS in non-Asian (predominantly Caucasian) trials (r = 0.116, R2 = 0.013, P = 0.588).
Mathematic equation of regression (based on a weighted model): OS (months) = 8.83 + 10.52 × (percentage of patients treated with both PBC and EGFR-TKIs).
In the non-Asian trials, the percentage of patients treated with both PBC and EGFR-TKIs was less than 30%. The correlation of that percentage with OS was not statistically significant, but the OS varied from 8.4 months to 13.6 months. OS did not differ significantly between study regimens—third-generation PBC and the combination of chemotherapy and targeted agents (P = 0.356).
Discussion
Our research demonstrated a strong correlation between the percentage of patients treated with both PBC and EGFR-TKIs and OS. This finding highlights the importance of making both PBC and EGFR-TKIs available to patients with advanced NSCLC during the course of treatment to achieve maximal survival benefit. In most trials included in our analysis, PBC was first-line treatment and EGFR-TKIs were second-line or third-line. EGFR-TKIs were taken as a salvage treatment. Here, we found that the specific salvage therapy patients underwent impacted the OS. Our findings suggest that patients who undergo first-line PBC need EGFR-TKIs as second-line or third-line treatment to maximize the survival benefit.
After EGFR-TKIs were approved for advanced NSCLC, the OS of advanced NSCLC patients increased[9]. In the BR.21[12] and TRUST[13] studies, erlotinib monotherapy had better efficacy and was safer in second-line or third-line treatment than placebo. Recently, the Tailor study indicated that patients with wild-type EGFR who underwent previous treatment did not obtain a PFS benefit from second-line erlotinib compared with docetaxel[14]. Furthermore, the impact of second-line treatment with EGFR-TKI on NSCLC patient survival was not yet known.
It is now apparent that EGFR mutation status is a determinant of survival benefit from TKI treatment. Currently, erlotinib is recommended as first-line treatment for NSCLC patients with EGFR mutation. Gefitinib is also a recommended option for Asian patients. Riely et al.[15] reported that the overall EGFR mutation rate in non-selected cases of NSCLC was 16.7%. EGFR mutations are also more prevalent in Asian patients compared with Caucasian patients (30.6% vs. 7.6%)[16]. Most of the trials in our study did not report the EGFR mutation rate, likely because this predictive factor was not yet confirmed when the trials were conducted. Nevertheless, these findings suggest that the more patients receiving EGFR-TKIs after chemotherapy in the trial, the higher the probability that patients with EGFR mutation benefit from treatment. Furthermore, the high prevalence of EGFR mutation in Asian populations may explain the strong correlation between the percentage of patients treated with both PBC and EGFR-TKIs and OS which was observed in our study. By contrast, the correlation was not as strong in Caucasian populations, in which EGFR mutation is less prevalent. Notably, the percentage of patients treated with both PBC and EGTR-TKIs was less than 30% in the predominantly Caucasian trials, which may also underlie the weak correlation.
The discrepancy of the chemotherapy effect in different races was reported in another meta-analysis, which compared the survival of patients with advanced NSCLC. In that study, the median OS with third-generation PBC in Asian and Caucasian trials was 11.3 and 9.5 months, respectively[17],[18]. In a historical comparison, the presence of EGFR mutation was reported to be an independent, favorable prognostic factor for advanced NSCLC patients. Without EGFR-TKI treatment, patients with EGFR mutation still showed longer survival than those with wild-type EGFR[9]. Comparing Figures 3 and 4, we found that at 0% of patients with both treatments, the median OS was 10.8 months in Asian trials and 8.8 months in non-Asian (predominantly Caucasian) trials. In fact, at the point of 0, the survival outcome was mainly an effect of chemotherapy. Our finding was consistent with the results of the meta-analysis.
We did not extrapolate the predicted OS if 100% of patients underwent both PBC and EGFR-TKIs, because this value was outside the range of data in our linear regression model. Instead, we used other available data to prove the regression model. We analyzed 6 clinical trials in which first-line EGFR-TKIs was compared with chemotherapy. Of these trials, 4 were not included in our correlation analysis because the patients were selected according to molecular status (positive for EGFR mutation)[19]–[22]. The other 2 trials—IPASS[23] and First-signal[24]—were included in our correlation analysis. Here, however, we only analyzed the subgroup of patients with EGFR mutation. These studies had significant overlap between EGFR-TKIs and PBC treatment regimens, and therefore, the percentage of patients treated with both PBC and EGFR-TKIs was high. Because all patients were positive for EGFR mutation, the prognosis should be better than the prognosis for the Asian population. Based on our mathematic equation of regression in Asian populations and the available data, we compared the calculated OS of these trials with the reported results (Table 4). Theoretically, the reported OS should be longer than the calculated OS, and we found that the result was consistent with our hypotheses.
Table 4. Baseline characteristics of the six trials comparing EGFR-TKIs with chemotherapy for patients with previously untreated NSCLC with mutated EGFR.
| Trial | First author/year | Regimens | No. of patients (%) | Reported OS (months) | Calculated OS (months) | |
| IPASS | Mok TS/2009[23] | Gefitinib 250 mg | 132 | (75) | 21.6 | 20.9 |
| TC | 129 | (64.3) | 21.9 | 19.4 | ||
| First SIGNAL | HAN JY/2012[24] | Gefitinib 250 mg | 26 | NR | 30.6 | NR |
| GP | 16 | NR | 26.5 | NR | ||
| NEJ 002 | Maemondo M/2011[22] | Gefitinib 250 mg | 114 | (67.5) | 30.5 | 19.8 |
| TC | 114 | (94.6) | 23.6 | 23.5 | ||
| WJTOG3405 | Mitsudomi T/2011[20] | Gefitinib 250 mg | 86 | (61) | 36 | 19.0 |
| DP | 86 | (91) | 39 | 23.5 | ||
| OPTIMAL | Zhou CC/2011[19] | Erlotinib 150 mg | 82 | (52) | 22.7 | 17.8 |
| GC | 72 | (71) | 28.9 | 20.3 | ||
| EURTAC | Rosell R/2012[21] | Erlotinib 150 mg | 77 | NR | 19.3 | NR |
| Standard PBC | 76 | (76) | 19.5a | 21.0 | ||
TC, paclitaxel + carboplatin; GP, gemcitabine + cisplatin; DP, docetaxel + cisplatin; GC, gemcitabine + carboplatin; OS, overall survival; NR, not reported. a The reported OS was not mature.
The patients who most benefited from EGFR-TKIs were those with EGFR mutation. EGFR mutation test is now recommended for NSCLC patients[25]. However, the recommendation has not been well applied in China. Indeed, only 7.8% of patients with advanced NSCLC were tested for EGFR mutation, according to a national survey of medical treatment status for NSCLC conducted in 12 Chinese cities[26]. This low rate was mainly due to inadequate samples and high cost for testing, as well as limited testing technology. Nevertheless, the survey indicated that EGFR-TKIs were frequently used for salvage treatment.
The results of our present study show that it is important to make both PBC and EGFR-TKIs available for advanced NSCLC patients. In addition, sequential treatment with chemotherapy and EGFR-TKIs, rather than concurrent treatment, provided a survival benefit for patients. This is consistent with results from several trials[27]–[29], which show that concurrent treatment was not superior to chemotherapy alone. One possible reason for the failure of concurrent treatment is that the patients were not screened and selected, as there was no available test with which to identify those likely to benefit from EGFR-TKIs at that time[30]. Notably, these concurrent treatment trials[25]–[27], such as TRIBUTE, INTACT 1 and INTACT 2, were conducted in non-Asian countries, where the EGFR mutation rate is low. Another reason might be an unidentified negative interaction when EGFR-TKIs are given concurrently with chemotherapy[27].
There was an inherent bias in our analysis. One might argue that patients who live longer have greater opportunity to be treated with both PBC and EGFR-TKIs. In fact, all the trials included in our study were conducted after the approval of TKIs, after which the survival of advanced NSCLC did improve[9]. In all trials in our study, the median PFS of patients who underwent first-line chemotherapy was 4–6 months[29]–[42]. Hence, most patients had the chance to undergo second- or third-line treatment after disease progression. Our findings suggest that EGFR-TKIs should be considered an option. Nevertheless, this study was not conducted as a formal meta-analysis, as only reported data, and not individual patient data, were used for analysis. Hence, the study should be taken as hypothesis generating. However, we provide evidence in support of our hypothesis that the percentage of patients with both PBC and EGFR-TKIs positively correlated with OS using other data from clinical trials.
In conclusion, we have found a strong relationship between the percentage of patients treated with sequential treatment with PBC and EGFR-TKIs and OS. The differences in the strength of the correlation in Asians and Caucasians might be caused by the distinct EGFR mutation rates in each population. Our results support the concept of making these two treatments available to NSCLC patients over the whole course of treatment to maximize the survival benefit.
References
- 1.Landis SH, Murray T, Bolden S, et al. Cancer statistics, 1999. CA Cancer J Clin. 1999;49:8–31. doi: 10.3322/canjclin.49.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Novello S, Le Chevalier T. Chemotherapy for non-small cell lung cancer. Part 1: early-stage disease. Oncology (Williston Park) 2003;17:357–364. [PubMed] [Google Scholar]
- 3.Group NM-AC Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol. 2008;26:4617–4625. doi: 10.1200/JCO.2008.17.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 5.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 6.Stinchcombe TE, Socinski MA. Current treatments for advanced stage non-small cell lung cancer. Proc Am Thorac Soc. 2009;6:233–241. doi: 10.1513/pats.200809-110LC. [DOI] [PubMed] [Google Scholar]
- 7.Gronberg BH, Bremnes RM, Flotten O, et al. Phase III study by the Norwegian lung cancer study group: pemetrexed plus carboplatin compared with gemcitabine plus carboplatin as first-line chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:3217–3224. doi: 10.1200/JCO.2008.20.9114. [DOI] [PubMed] [Google Scholar]
- 8.Scagliotti GV, Parikh P, Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naïve patients with advanced stage non-small cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 9.Takano T, Fukui T, Ohe Y, et al. EGFR mutations predict survival benefit from gefitinib in patients with advanced lung adenocarcinoma: a historical comparison of patients treated before and after gefitinib approval in Japan. J Clin Oncol. 2008;26:5589–5595. doi: 10.1200/JCO.2008.16.7254. [DOI] [PubMed] [Google Scholar]
- 10.Grothey A, Sargent D, Goldberg R, et al. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22:1209–1214. doi: 10.1200/JCO.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 11.Grothey A, Sargent D. Overall survival of patients with advanced colorectal cancer correlates with availability of fluorouracil, irinotecan, and oxaliplatin regardless of whether doublet or single-agent therapy is used first line. J Clin Oncol. 2005;23:9441–9442. doi: 10.1200/JCO.2005.04.4792. [DOI] [PubMed] [Google Scholar]
- 12.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 13.Laack E, Schneider C, Gutjahr T, et al. Association between different potential predictive markers from TRUST, a trial of erlotinib in non-small cell lung cancer (NSCLC) J Clin Oncol. 2007;18(Suppl):abstr 7651. [Google Scholar]
- 14.Garassino MC, Martelli O, Bettini A, et al. A phase III trial comparing erlotinib versus docetaxel as second-line treatment of NSCLC patients with wild-type EGFR. J Clin Oncol. 2012;30(Suppl):abstr LBA7501. [Google Scholar]
- 15.Riely GJ, Politi KA, Miller VA, et al. Update on epidermal growth factor receptor mutation in non-small cell lung cancer. Clin Cancer Res. 2006;12:7232–7241. doi: 10.1158/1078-0432.CCR-06-0658. [DOI] [PubMed] [Google Scholar]
- 16.Chan SK, Gullick WJ, Hill ME. Mutations of the epidermal growth factor receptor in non-small cell lung cancer: search and destroy. Eur J Cancer. 2006;42:17–23. doi: 10.1016/j.ejca.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 17.Soo RA, Loh M, Mok TS, et al. Ethnic differences in survival outcome in patients with advanced stage non-small cell lung cancer: results of a meta-analysis of randomized controlled trials. J Thorac Oncol. 2011;6:1030–1038. doi: 10.1097/JTO.0b013e3182199c03. [DOI] [PubMed] [Google Scholar]
- 18.Zhou W, Christiani DC. East meets west: ethnic differences in epidemiology and clinical behaviors of lung cancer between East Asians and Caucasians. Chin J Cancer. 2011;30:287–292. doi: 10.5732/cjc.011.10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou CC, Wu YL, Chen GY, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 20.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harboring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 21.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 22.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 23.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 24.Han JY, Park L, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30:1122–1128. doi: 10.1200/JCO.2011.36.8456. [DOI] [PubMed] [Google Scholar]
- 25.National Comprehensive Cancer Network-Asia Non-small cell lung cancer: treatment guidelines for patients. Version 1. < http://www.nccn-asia.org/cn/>. [Google Scholar]
- 26.Xue C, Hu ZH, Jiang W, et al. National survey of the medical treatment status for non-small-cell lung cancer in China. Lung Cancer. 2012;77:371–375. doi: 10.1016/j.lungcan.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Herbst RS, Prager D, Hermann R, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small cell lung cancer. J Clin Oncol. 2005;23:5892–5899. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 28.Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial-intact 1. J Clin Oncol. 2004;22:777–784. doi: 10.1200/JCO.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small cell lung cancer: a phase III trial-intact 2. J Clin Oncol. 2004;22:785–794. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 30.Baselga J. Combining the anti-EGFR agent gefitinib with chemotherapy in non-small cell lung cancer: how do we go from INTACT to impact? J Clin Oncol. 2004;22:759–761. doi: 10.1200/JCO.2004.12.903. [DOI] [PubMed] [Google Scholar]
- 31.Okamoto I, Yoshioka H, Morita S, et al. Phase III trial comparing oral S-1 plus carboplatin with paclitaxel plus carboplatin in chemotherapy-naïve patients with advanced non-small-cell lung cancer: results of a west Japan oncology group study. J Clin Oncol. 2010;28:5240–5246. doi: 10.1200/JCO.2010.31.0326. [DOI] [PubMed] [Google Scholar]
- 32.Kubota K, Kawahara M, Ogawara M, et al. Vinorelbine plus gemcitabine followed by docetaxel versus carboplatin plus paclitaxel in patients with advanced non-small-cell lung cancer: a randomised, open-label, phase III study. Lancet Oncol. 2008;9:1135–1142. doi: 10.1016/S1470-2045(08)70261-4. [DOI] [PubMed] [Google Scholar]
- 33.Ohe Y, Ohashi Y, Kubota K, et al. Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small cell lung cancer: Four-Arm Cooperative Study in Japan. Ann Oncol. 2007;18:317–323. doi: 10.1093/annonc/mdl377. [DOI] [PubMed] [Google Scholar]
- 34.Kubota K, Watanabe K, Kunitoh H, et al. Phase III randomized trial of docetaxel plus cisplatin versus vindesine plus cisplatin in patients with stage IV non-small cell lung cancer: The Japanese Taxotere Lung Cancer Study Group. J Clin Oncol. 2004;22:254–261. doi: 10.1200/JCO.2004.06.114. [DOI] [PubMed] [Google Scholar]
- 35.Lara PN, Jr, Douillard JY, Nakagawa K, et al. Randomized phase III placebo-controlled trial of carboplatin and paclitaxel with or without the vascular disrupting agent vadimezan (ASA404) in advanced non-small cell lung cancer. J Clin Oncol. 2011;29:2965–2971. doi: 10.1200/JCO.2011.35.0660. [DOI] [PubMed] [Google Scholar]
- 36.Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabinewith either placebo or bevacizumab as first-line therapy for nonsquamous non-small cell lung cancer: AVAiL. J Clin Oncol. 2009;27:1227–1234. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 37.Lynch TJ, Patel T, Dreisbach L, et al. Cetuximab and first-line taxane/carboplatin chemotherapy in advanced non-small cell lung cancer: results of the randomized multicenter phase III trial BMS099. J Clin Oncol. 2010;28:911–917. doi: 10.1200/JCO.2009.21.9618. [DOI] [PubMed] [Google Scholar]
- 38.Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009;373:1525–1531. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 39.Tan EH, Rolski J, Grodzki T, et al. Global Lung Oncology Branch trial 3 (GLOB3): final results of a randomised multinational phase III study alternating oral and I.V. vinorelbine plus cisplatin versus docetaxel plus cisplatin as first-line treatment of advanced non-small cell lung cancer. Ann Oncol. 2009;20:1249–1256. doi: 10.1093/annonc/mdn774. [DOI] [PubMed] [Google Scholar]
- 40.Ramlau R, Zatloukal P, Jassem J, et al. Randomized phase III trial comparing bexarotene (l1069-49)/cisplatin/vinorelbine with cisplatin/vinorelbine in chemotherapy-naive patients with advanced or metastatic non-small cell lung cancer: SPIRIT I. J Clin Oncol. 2008;26:1886–1892. doi: 10.1200/JCO.2007.12.2614. [DOI] [PubMed] [Google Scholar]
- 41.Blumenschein GR, Jr, Khuri FR, von Pawel J, et al. Phase III trial comparing carboplatin, paclitaxel, and bexarotene with carboplatin and paclitaxel in chemotherapy-naive patients with advanced or metastatic non-small cell lung cancer: SPIRIT II. J Clin Oncol. 2008;26:1879–1885. doi: 10.1200/JCO.2007.12.2689. [DOI] [PubMed] [Google Scholar]
- 42.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]