Abstract
O6-methylguanine DNA methyltransferase (MGMT) can remove DNA alkylation adducts, thereby repairing damaged DNA and contributing to the drug resistance of gliomas to alkylating agents. In addition, glioma stem-like cells (GSCs) have been demonstrated to be involved in the recurrence and treatment resistance of gliomas. In this study, we aimed to investigate MGMT expression and regulatory mechanisms in GSCs and the association of MGMT with temozolomide (TMZ) sensitivity. GSCs were enriched from one MGMT-positive cell line (SF-767) and 7 MGMT-negative cell lines (U251, SKMG-4, SKMG-1, SF295, U87, MGR1, and MGR2) through serum-free clone culture. GSCs from the U251G, SKMG-4G, SF295G, and SKMG-1G cell lines became MGMT-positive, but those from the U87G, MGR1G, and MGR2G cell lines remained MGMT-negative. However, all the GSCs and their parental glioma cell lines were positive for nuclear factor-κB (NF-κB). In addition, GSCs were more resistant to TMZ than their parental glioma cell lines (P < 0.05). However, there was no significant difference in the 50% inhibition concentration (IC50) of TMZ between MGMT-positive and MGMT-negative GSCs (P > 0.05). When we treated the MGMT-positive GSCs with TMZ plus MG-132 (an NF-κB inhibitor), the antitumor activity was significantly enhanced compared to that of GSCs treated with TMZ alone (P < 0.05). Furthermore, we found that MGMT expression decreased through the down-regulation of NF-κB expression by MG-132. Our results show that MG-132 may inhibit NF-κB expression and further decrease MGMT expression, resulting in a synergistic effect on MGMT-positive GSCs. These results indicate that enhanced MGMT expression contributes to TMZ resistance in MGMT-positive GSCs.
Keywords: Glioma stem cell, MGMT, temozolomide, drug resistance, NF-κB
Gliomas are the most common primary brain tumors in adults. Despite the progress made in the molecular aspects of malignant gliomas, the prognosis of brain tumors continues to be dismal. Glioblastoma multiform (GBM) is a highly aggressive brain tumor with a median patient survival of 14.6 months[1]; moreover, it is generally incurable and responds poorly to chemotherapy. Chemotherapy has been used for decades to treat GBM, and the oral methylator temozolomide (TMZ) is currently the most widely used chemotherapeutic agent[2]. TMZ achieves cytotoxicity mostly by methylating the O6 position of guanine. Because the O6-methylguanine DNA methyltransferase (MGMT) can reverse alkylation at the O6 position of guanine and neutralize the cytotoxic effects of alkylating agents[3], MGMT expression in brain tumors represents a key mechanism of resistance toward alkylating agent therapy[4].
In addition, several groups have confirmed the existence of glioma stem-like cells (GSCs) with self-renewal capability, as measured by serial neurosphere assays and the proliferation of tumor cells by ex vivo intracranial limiting dilution assays[5]–[7]. Many studies have demonstrated that GSCs promote therapeutic resistance and that they are likely to be responsible for the relapse of GBM[8]–[10]. Amit et al.[11] revealed that a large proportion of glioma tumor cells display high constitutive activity of nuclear factor-κB (NF-κB). Such tumors usually show increased resistance to chemotherapy[12]. NF-κB is a family of dimeric transcription factors. DNA damage induced by alkylating agents results in a marked increase in NF-κB activity. Therefore, it was hypothesized that the role of NF-κB in anti-apoptotic mechanisms in human tumors contributes to the high incidence of chemoresistance to alkylating agents. However, there are limited data on the relationship between MGMT, NF-κB expression and TMZ resistance in GSCs.
Materials and Methods
Culture and identification of glioma stem-like cells
The human glioma cell lines SF767, U251, SKMG-4, SKMG-1, SF295, U87, MGR1, and MGR2 were cultured in Dulbecco's modified Eagle's medium (DMEM, Invitrogen-Gibco, China) supplemented with 10% fetal bovine serum (FBS, Invitrogen-Hyclone, China) in a humidified atmosphere containing 5% CO2 at 37°C. GSCs were enriched through serum-free clone formation with serum-free DMEM/F12 supplemented with 2% B27, 20 ng/mL epidermal growth factor (EGF), and 10 ng/mL basic fibroblast growth factor (bFGF)[13]. Molecular markers of GSCs [CD133, Nestin, Sox-2, glial fibrillary acidic protein (GFAP), and beta-tubulin-1 (TUJ-1)] were detected by immunofluorescent staining.
Detect MGMT and NF-κB expression by reverse transcription-polymerase chain reaction (RT-PCR)
The total RNA of glioma cells was extracted using the TRIzol reagent (Invitrogen, USA) according to the manufacturer's protocol. Reverse transcription was performed with 2 µg of total RNA from each sample in a total volume of 20 µL using the RevertAid™ First Strand cDNA Synthesis Kit (Fermentas, USA). GAPDH was used as a loading control. The sequences of the forward and reverse primers for each gene are listed in Table 1. Each polymerase chain reaction (PCR) mixture contained 1 µL of cDNA, 10 µL of Green GoTaq DNA polymerase (Promega, USA), 0.5 µL of forward primer (10 µmol/L), and 0.5 µL of reverse primer (10 µmol/L) in a final volume of 20 µL. PCR was carried out as follows: 94°C for 2 min, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 60°C for 30 s, and elongation at 72°C for 30 s, and then 72°C for another 2 min and a final hold at 4°C. The PCR products were separated on 2% agarose gel, visualized by ethidium bromide staining, and photographed with a Gel Doc XR (Bio-Rad Laboratories, USA).
Table 1. Sequences of primers used in polymerase chain reaction.
| Gene | Oligonucleotide | Sequence (5′-3′) | Size (bp) |
| MGMT | Forward | GTTATGAATGTAGGAGCCCTTATG | 239 |
| Reverse | TGACAACGGGAATGAAGTAATG | ||
| NF-κB | Forward | TGTGGGTTTCCTGTGCTAATG | 208 |
| Reverse | GAGACCAGCCTTTCTCCGTA | ||
| GAPDH | Forward | CGCTCTCTGCTCCTCCTGTTC | 108 |
| Reverse | ATCCGTTGACTCCGACCTTCAC |
MGMT, O6-methylguanine DNA methyltransferase; NF-κB, nuclear factor-κB.
Detect MGMT promoter methylation by methyaltion-specific PCR (MSP)
Genomic DNA was extracted from each glioma cell line using a DNA extraction kit (Tiangen, China). The isolated DNA (1.8 µg) was bisulfite-treated using the EpiTect Bisulfite Kit (QIAGEN, Germany) according to the manufacturer's protocol. The modified DNA was then amplified using primers specific for either the methylated or the unmethylated MGMT promoter sequences, as listed in Table 2. Each PCR mixture contained 2 µL of DNA, 25 µL of EpiTect MSP Master Mix (QIAGEN, Germany), 1 µL of forward primer (10 µmol/L), and 1 µL of reverse primer (10 µmol/L) in a final volume of 50 µL. PCR was carried out as follows: 94°C for 2 min, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 56°C for 30 s, and elongation at 72°C for 30 s, and then another 2 min at 72°C and a final hold at 4°C. The PCR products were separated on a 2% agarose gel, visualized by ethidium bromide staining, and photographed with a Gel Doc XR (Bio-Rad Laboratories, USA).
Table 2. Sequences of primers used in methylation-specific polymerase chain reaction.
| Gene | Oligonucleotide | Sequence (5′-3′) | Size (bp) |
| Methylated MGMT | Forward | TTTCGACGTTCGTAGGTTTTCGC | 81 |
| Reverse | GCACTCTTCCGAAAACGAAACG | ||
| Unmethylated MGMT | Forward | TTTGTGTTTTGATGTTTGTAGGTTTTTGT | 93 |
| Reverse | AACTCCACACTCTTCCAAAAACAAAACA |
Detect MGMT and NF-κB expression by Western blotting
The cells were washed using ice-cold PBS and lysed on ice using the radioimmunoprecipitation assay (RIPA) buffer (Beyotime, China). After the samples had been sonicated and centrifuged, the protein concentration of the supernatant was measured using the bicinchoninic acid (BCA) assay kit (Beyotime, China) according to the manufacturer's protocol. For each sample, equal amounts of total protein were mixed with 5× sample buffer [0.5 mol/L Tris-HCl pH 6.8, 10% (w/v) sodium dodecyl sulfate, 50% glycerol, 0.025% dithiothreitol, and 0.05% (w/v) bromophenol blue]. The samples were heated at 95°C for 5 min, separated on a 10% sodium dodecyl sulfate-polyacrylamide gel, and then trans-blotted onto a polyvinylidene difluoride (PVDF) membrane (Millipore, USA). The membrane was blocked in Tris-buffered saline (TBS) containing 0.1% Tween-20 and 5% skim milk at 37°C for 1.5 h and then incubated in primary antibodies against MGMT at 1:125 (Invitrogen, 357000, USA), NF-κB p65 at 1:50 (Biovision, P4336, USA) and β-actin at 1:1,000 (Beyotime, AA128, China) at 25°C for 2 h, followed by exposure to the horseradish peroxidase-conjugated secondary antibody (1:1,000) for 1 h. Proteins were visualized using the ECL system (Millipore, USA), and bands were exposed to Biomax MR film (Kodak, Japan).
Cytotoxicity assay
The cytotoxic effect of TMZ (Sigma-Aldrich, USA) on glioma cells was detected by WST-8 assay. Cells of each line were divided into four groups and remained untreated or treated with 500 µmol/L TMZ for 72 h, 1 µmol/L MG-132 (NF-ΚB inhibitor, Biovision, 1703-5, USA) for 72 h, or MG-132 for 24 h followed by TMZ for 48 h. After incubation, the cells from each group were subjected to the WST-8 assay. Cells were plated at a density of 5,000 cells/well in 96-well plates and allowed to attach overnight. Various concentrations of TMZ were then added, and the cells were cultured for 72 h. Four hours prior to harvest, 10 µL/well of the Cell Counting Kit-8 reagent (CCK-8, Dojindo, Japan) was added, and the cells were incubated for 4 h at 37°C. The increasing number of viable cells resulted in the overall increased activity of dehydrogenases in the samples, with an ensuing increase in formazan dye formation. The quantity of formazan dye was determined by measuring the absorbance of the dye solution at a wavelength of 490 nm. The cell survival rate was calculated by comparing the absorbance values of the treated samples to those of the untreated controls within each group. The concentration of TMZ required to inhibit cell growth by 50% (IC50) was calculated from survival curves using the Bliss Method[3]. The growth inhibition rates of treated cells were calculated by comparing with untreated cells. The growth inhibition rate of untreated cells was considered to be 0%.
Statistical analysis
Statistical analysis was performed using SPSS 16.0 software. For all tests, the level of statistical significance was P < 0.05. All data are expressed as mean ± standard deviation (SD). Unless otherwise specified, Student′s t test (two tailed) was used.
Results
Characteristics of GSCs
Eight GSC lines were successfully established by serum-free clone formation and began floating and propagating when cultured in serum-free medium containing EGF/bFGF/B27 (NSC medium) (Figure 1A). GSCs adhered to poly-lysine-coated plates when cultured in 10% FBS/DMEM/F12 medium (Figure 1B). Glioma spheres generated from all GSC lines showed high immunoreactivity for neural stem cell markers CD133, Nestin, and Sox-2 but low immunoreactivity for markers of differentiated lineages, such as GFAP and TUJ-1 (Figure 1C-G).
Figure 1. Characteristics of glioma stem-like cells (GSCs).
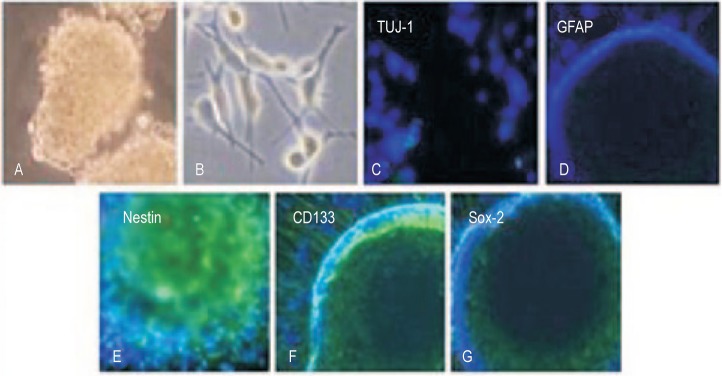
A, GSCs grow as neurosphere-like gliomaspheres in stem cell medium (original magnification × 20). B, GSCs adhered to poly-lysine-coated plates when cultured in 10% FBS/DMEM/F12 medium (original magnification × 100). C, the expression of neuronal class III beta-tubulin-1 (TUJ-1), a neuronal marker on the GSC sphere (original magnification × 200). D, the expression of glial fibrillary acidic protein (GFAP), an astrocytic marker in the GSC sphere (original magnification × 200). E-G, immunostaining of tumor cells for neural stem cell markers, Nestin (E), CD133 (F), and Sox-2 (G), in the GSC sphere (original magnification × 200).
MGMT and NF-κB expression in the GSC lines
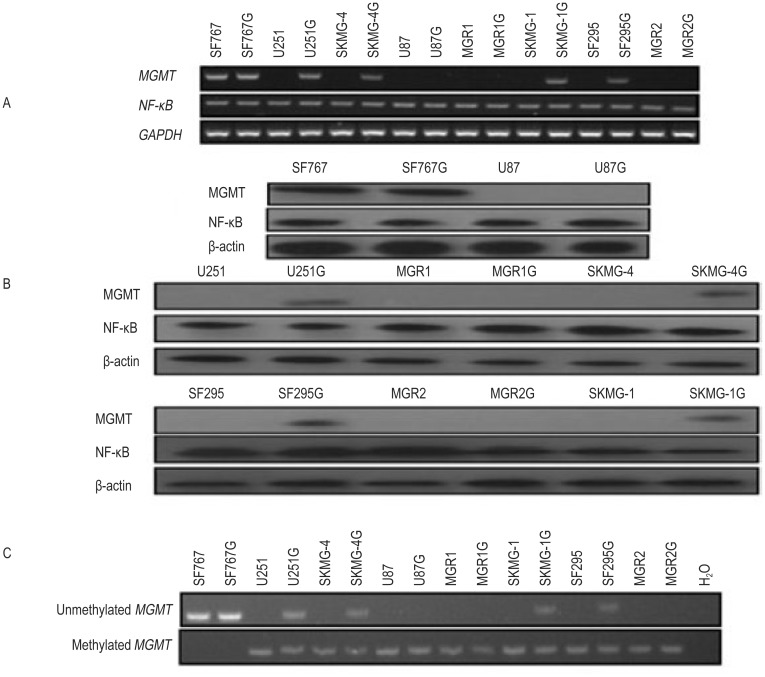
Both RT-PCR and Western blotting data showed that the glioma cell lines U251, SKMG-4, SF295, SKMG-1, U87, MGR1, and MGR2 were MGMT-negative and that SF767 was MGMT-positive. However, the GSC lines U251G, SKMG-4G, SF295G, SKMG-1G, and SF767G were MGMT-positive, whereas U87G, MGR1G, and MGR2G remained MGMT-negative. However, all the glioma cell lines and the GSC lines displayed positive NF-κB expression (Figure 2A, B).
Figure 2. O6-methylguanine DNA methyltransferase (MGMT) and nuclear factor-κB (NF-κB) expression in the GSC lines and the parental glioma cell lines.
A, reverse transcription-polymerase chain reaction (RT-PCR) shows that the glioma cell lines U251, SKMG-4, SF295, SKMG-1, U87, MGR1, and MGR2 are MGMT-negative, whereas the SF767 cell line is MGMT-positive. However, the GSC lines U251G, SKMG-4G, SF295G, SKMG-1G, and SF767G become MGMT-positive, whereas the U87G, MGR1G, and MGR2G lines remain MGMT-negative. Meanwhile, all GSC lines and their parental glioma cell lines displayed high NF-κB expression. B, the Western blotting data are consistent with the RT-PCR results. C, methylation-specific polymerase chain reaction (MSP) shows MGMT promoter methylation in all GSC lines and their parental glioma cell lines except for the SF767 and SF767G lines. In addition, unmethylated MGMT promoters exist in the glioma cell line SF767 and the GSC lines SF767G, U251G, SKMG-4G, SKMG-1G, and SF295G; H2O was used as the blank control.
MSP data showed MGMT promoter methylation in all the glioma cell lines and the GSC lines except for SF767 and SF767G. Unmethylated MGMT promoters were detected in the glioma cell line SF767 and in the GSC lines SF767G, U251G, SKMG-4G, SKMG-1G, and SF295G but were not detected in the U87G, MGR1G and MGR2G cell lines (Figure 2C). Our data indicate that some MGMT-negative glioma cell lines may convert to MGMT-positive GSCs through serum-free clone culture.
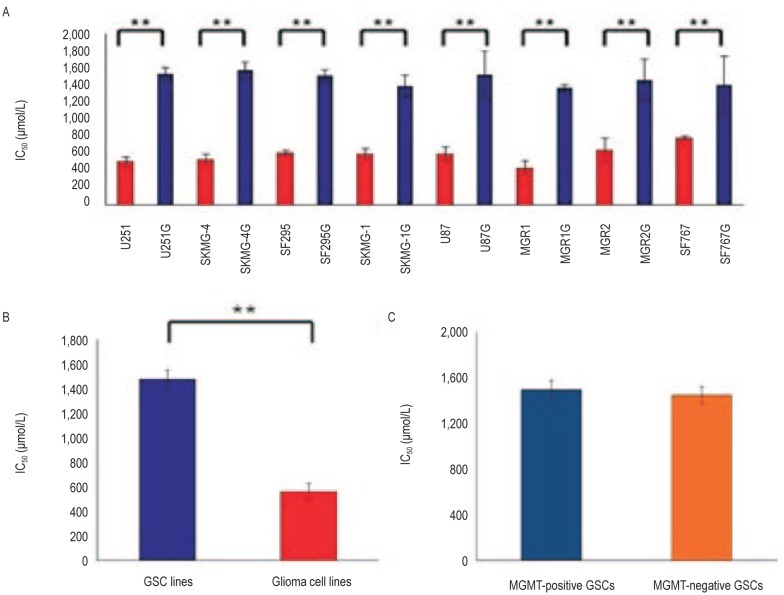
To evaluate the TMZ sensitivity in the glioma cells, the 8 GSC lines and the corresponding parental glioma cell lines were treated with various concentrations of TMZ and then assayed for cell viability using the WST-8 assay. The IC50 values of TMZ were (1,478.28 ± 84.19) µmol/L for GSCs and (626.06 ± 156.99) µmol/L for matched glioma cell lines (P < 0.05) (Figure 3A, B), indicating that GSCs are more resistant to TMZ treatment compared to the parental glioma cell lines. However, there was no significant difference in the IC50 values of TMZ between MGMT-positive and MGMT-negative GSC lines [(1,494.43 ± 88.43) µmol/L vs. (1,446.00 ± 80.35) µmol/L, P > 0.05] (Figure 3C).
Figure 3. The relationship between MGMT expression and resistance to temozolomide (TMZ ) in GSCs.
A, the 50% inhibition concentrations (IC50) of TMZ for the GSC lines are significantly higher than those for the parental glioma cell lines (**P < 0.05). B, the mean IC50 of TMZ for all GSC lines is significantly higher than that for all parental glioma cell lines (**P < 0.05). C, the IC50 of TMZ for MGMT-positive and MGMT-negative GSC lines are similar (P > 0.05).
Down-regulation of MGMT expression was associated with the inhibition of NF-κB in MGMT-positive GSC lines
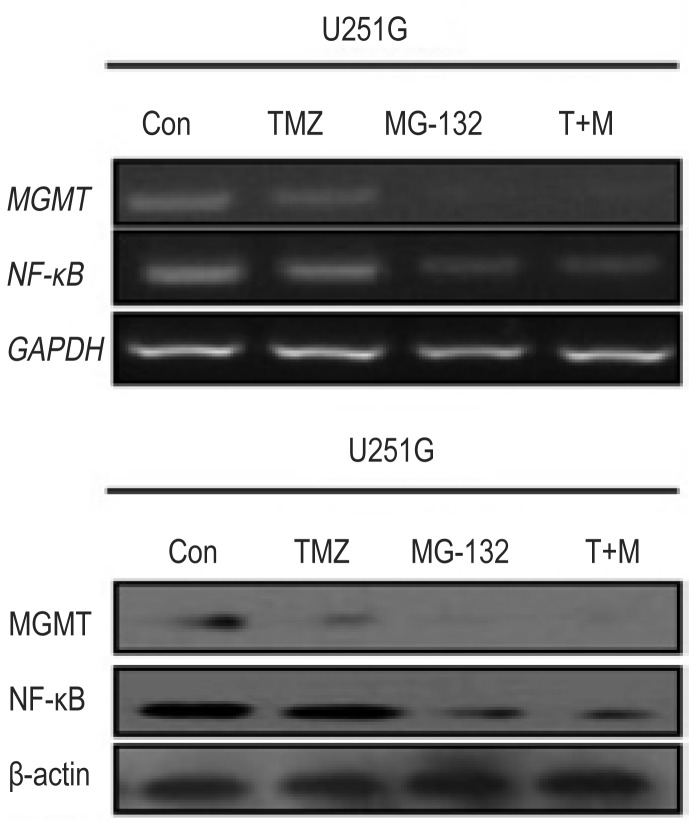
When MGMT-positive GSC lines were treated with TMZ, MGMT expression level slightly decreased, TMZ treatment did not affect the expression level of NF-κB, as determined by RT-PCR and Western blotting. However, both NF-κB and MGMT expression levels were significantly down-regulated when MGMT-positive GSC lines were treated with 1 µmol/L MG-132 alone or in combination with 500 µmol/L TMZ (Figure 4), indicating that the down-regulation of MGMT expression was related to NF-κB expression in MGMT-positive GSC lines.
Figure 4. Inhibiting NF-κB expression results in the down-regulation of MGMT expression in the GSC line U251G.
RT-PCR (upper panel) and Western blotting (lower panel) data show that TMZ treatment slightly down-regulated MGMT expression but did not affect NF-κB expression. MG-132 alone or in combination with TMZ down-regulated both NF-κB and MGMT expression. T+M, TMZ plus MG-132.
TMZ has a synergistic effect with NF-κB inhibitor on MGMT-positive GSCs
Previous studies have shown that MGMT contains two putative binding sites for NF-κB that play an important role in MGMT regulation[14]. We hypothesized that TMZ might have a synergistic effect with an NF-κB inhibitor (MG-132) on MGMT-positive GSCs. To test this hypothesis, the WST-8 assay was used to examine the drug sensitivity of MGMT-positive GSC lines and the parental glioma cell lines. The results revealed that MG-132 alone had mild inhibitory activity on the glioma cells. However, when the cells were co-incubated with TMZ and MG-132, the cytotoxic effect on MGMT-positive GSCs significantly increased, revealing a synergy between MG-132 and TMZ. In addition, TMZ plus MG-132 only showed an additive effect on the parental glioma cell lines and the MGMT-negative GSC line U87G (Table 3).
Table 3. Cytotoxic effects of temozolomide (TMZ) and MG-132 alone or in combination on MGMT-positive GSC lines and the parental glioma cell lines.
| Cell line | Growth inhibition rate (%) |
||
| TMZ | MG-132 | TMZ plus MG-132 | |
| U251 | 48.04 ± 1.00 | 15.49 ± 2.12 | 60.65 ± 2.49 |
| U251G | 17.48 ± 2.24 | 19.12 ± 4.27 | 62.99 ± 1.07 |
| SKMG-4 | 45.06 ± 2.46 | 16.52 ± 4.73 | 57.28 ± 8.35 |
| SKMG-4G | 20.44 ± 2.95 | 20.69 ± 3.84 | 60.87 ± 4.87 |
| SKMG-1 | 42.39 ± 1.60 | 15.41 ± 1.63 | 54.12 ± 2.07 |
| SKMG-1G | 19.33 ± 4.07 | 16.69 ± 1.79 | 57.02 ± 1.53 |
| SF295 | 43.72 ± 2.62 | 16.30 ± 4.49 | 55.43 ± 4.35 |
| SF295G | 19.18 ± 0.68 | 19.08 ± 3.18 | 57.37 ± 3.20 |
| U87 | 45.43 ± 2.97 | 21.08 ± 1.72 | 62.38 ± 3.11 |
| U87G | 21.16 ± 1.26 | 24.45 ± 2.12 | 33.94 ± 1.50 |
TMZ, temozolomide; GSC, glioma stem-like cell. Data are presented as mean ± standard deviation (SD) of 3 repeated experiments.
Discussion
GSCs are more efficient in repairing damaged DNA and more rapidly recover from DNA damage than non-stem glioma cells[15]. Therefore, effective treatments for GBM should target GSCs, which are resistant to current chemotherapy. MGMT is an important enzyme that can repair O6-alkylguanine adducts on DNA and plays a significant role in the resistance to alkylating agents in GBM[16],[17]. Our results show that GSCs obtained from MGMT-negative glioma cell lines may become MGMT-positive, which may partially explain why some patients with MGMT-negative gliomas initially respond well but eventually relapse with TMZ therapy. Similar results have been reported by Liu et al.[18], who postulated that MGMT expression was up-regulated in CD133-positive GSCs compared to CD133-negative cells.
Current available data derived from studies on glioma cell lines and human tumor samples suggest that resistance to TMZ is closely related to MGMT-mediated DNA repair in gliomas[19]. Our present study showed that GSCs were significantly more resistant to TMZ compared with their parental cell lines (P < 0.05), which is consistent with the data reported by Eramo et al.[20]. However, we failed to find any significant difference in the IC50 values of TMZ between MGMT-positive and MGMT-negative GSC lines (P >0.05), indicating that the resistance to TMZ in MGMT-negative GSCs was not fully related to MGMT expression. These results are consistent with previous findings that MGMT expression alone does not always predict the response to TMZ[21] and that MGMT status is only associated with TMZ sensitivity under differentiated conditions and not in stem-like cells under neurosphere conditions[22]. Actually, genes other than MGMT might generate resistance to alkylating agents in the GSCs. For example, raising the expression of the drug transporters, including ABC (ATP-binding cassette) drug transporters, that pump out chemotherapeutic agents is another potential mechanism, as ABC expression may be enriched for cancer stem cells[23]. In addition, multidrug resistance gene 1 (MDR1) has been found to be expressed in the majority of brain tumors, including glioblastomas. The effectiveness of the P-glycoprotein (P-gp) efflux pump may be influenced by other non-target substances inhibiting the pump transporter or by genetic variants having a functional impact on MDR1 transcription, leading to an altered ability of this P-gp to recognize target substrates[24]. Furthermore, DNA repair systems may also play a significant role in repairing the cross-linking and oxidative damage caused by alkylating agents. It has been shown that excision repair cross-complementing rodent repair deficiency gene 1 (ERCC1) and ERCC2, two critical factors in the nucleotide excision repair (NER) pathway, are associated with resistance to alkylating agents[25],[26]. In addition, a number of signaling pathways (e.g., RTK-Akt, Notch, BMPs, TGF-β, Hedgehog-Gli, Wnt-β-catenin, STAT3, GSK3-β) that are associated with GSC maintenance have been reported and could be related to drug resistance[27]. Therefore, high MGMT expression will result in resistance to O6-alkylating drugs, whereas low MGMT expression does not necessarily cause drug sensitivity. Nevertheless, since most GBMs are MGMT-positive[28], targeting MGMT with specific inhibitors could be an important strategy in GBM therapy [29].
A large proportion of tumor cells display high constitutive NF-κB activity[30]. NF-κB was activated in response to alkylating agents, and high NF-κB activation was related with chemoresistance[14]. Two putative NF-κB-binding sites in the MGMT promoter region can regulate MGMT expression, resulting in chemoresistance to alkylating agents[14]. To date, several therapeutic inhibitors that block the activation of NF-κB are under development to enhance the drug sensitivity of tumor cells. We have found that the inhibition of MGMT expression by an NF-κB inhibitor can increase TMZ sensitivity in MGMT-positive GSCs. Therefore, NF-κB inhibitors could be of great clinical value for sensitizing MGMT-positive cells to alkylating treatment and overcoming treatment-induced chemoresistance.
In conclusion, our findings provide evidence that MGMT is expressed in a subset of GSCs. Compared to the parental glioma cell lines, the GSC lines were more resistant to TMZ. However, there were no significant differences in TMZ resistance between MGMT-positive and MGMT-negative GSCs, suggesting that other mechanisms are also involved in TMZ resistance. Finally, treatment with an NF-κB inhibitor and TMZ showed a synergistic cytotoxic effect on MGMT-positive GSCs, as the down-regulation of NF-κB expression resulted in lower MGMT expression. These results suggest that enhanced MGMT expression contributes, at least in part, to TMZ resistance in MGMT-positive GSCs.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 30772551), the Science & Technology Program of Guangdong Province (No. 2011B031800178), National High-technology Research and Development Program of China (No. 2012AA02A508), and Specialized Research Fund for the Doctoral Program of Higher Education (No. 20110171110076).
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Hau P, Koch D, Hundsberger T, et al. Safety and feasibility of long-term temozolomide treatment in patients with high-grade glioma. Neurology. 2007;68:688–690. doi: 10.1212/01.wnl.0000255937.27012.ee. [DOI] [PubMed] [Google Scholar]
- 3.Pegg AE. Repair of O(6)-alkylguanine by alkyltransferases. Mutat Res. 2000;462:83–100. doi: 10.1016/s1383-5742(00)00017-x. [DOI] [PubMed] [Google Scholar]
- 4.Kaina B, Christmann M, Naumann S, et al. MGMT: key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Repair (Amst) 2007;6:1079–1099. doi: 10.1016/j.dnarep.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 6.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumor initiating cell. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 7.Dalerba P, Dylla SJ, Park IK, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bao S, Wu Q, Mclendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 9.Johannessen TC, Wang J, Staftnesmo KO, et al. Highly infiltrative brain tumours show reduced chemosensitivity associated with a stem cell-like phenotype. Neuropathol Appl Neurobiol. 2009;35:380–393. doi: 10.1111/j.1365-2990.2008.01008.x. [DOI] [PubMed] [Google Scholar]
- 10.Liu G, Yuan X, Zeng Z, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amit S, Ben-Neriah Y. NF-κB activation in cancer: a challenge for ubiquitination- and proteasome-based therapeutic approach. Semin Cancer Biol. 2003;13:15–28. doi: 10.1016/s1044-579x(02)00096-2. [DOI] [PubMed] [Google Scholar]
- 12.Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-κB. J Clin Invest. 2001;107:241–246. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Q, Zhang QB, Dong J, et al. Glioma stem cells are more aggressive in recurrent tumors with malignant progression than in the primary tumor, and both can be maintained long-term in vitro. BMC Cancer. 2008;8:304. doi: 10.1186/1471-2407-8-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavon I, Fuchs D, Zrihan D, et al. Novel mechanism where by nuclear factor kappa B mediates DNA damage repair through regulation of O(6)-methylguanine-DNA-methyltransferase. Cancer Res. 2007;67:8952–8959. doi: 10.1158/0008-5472.CAN-06-3820. [DOI] [PubMed] [Google Scholar]
- 15.Dey M, Ulasov IV, Lesniak MS. Virotherapy against malignant glioma stem cells. Cancer Lett. 2010;289:1–10. doi: 10.1016/j.canlet.2009.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esteller M, Garcia-Foncillas J, Andion E, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343:1350–1354. doi: 10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- 17.Kaina B, Ochs K, Grosch S, et al. BER, MGMT, and MMR in defense against alkylation-induced genotoxicity and apoptosis. Prog Nucl Acid Res Mol Biol. 2001;68:41–54. doi: 10.1016/s0079-6603(01)68088-7. [DOI] [PubMed] [Google Scholar]
- 18.Liu G, Yuan X, Zeng Z, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bredel M, Zentner J. Brain-tumour drug resistance: the bare essentials. Lancet Oncol. 2002;3:397–406. doi: 10.1016/s1470-2045(02)00786-6. [DOI] [PubMed] [Google Scholar]
- 20.Eramo A, Ricci-Vitiani L, Zeuner A, et al. Chemotherapy resistance of glioblastoma stem cells. Cell Death Differ. 2006;13:1238–1241. doi: 10.1038/sj.cdd.4401872. [DOI] [PubMed] [Google Scholar]
- 21.Blough MD, Westgate MR, Beauchamp D, et al. Sensitivity to temozolomide in brain tumor initiating cells. Neuro Oncol. 2010;12:756–760. doi: 10.1093/neuonc/noq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villalva C, Cortes U, Wager M, et al. O6-methylguanine-methyl-transferase (MGMT) promoter methylation status in glioma stem-like cells is correlated to temozolomide sensitivity under differentiation-promoting conditions. Int J Mol Sci. 2012;13:6983–6994. doi: 10.3390/ijms13066983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schatton T, Murphy GF, Frank NY, et al. Identification of cells initiating human melanomas. Nature. 2008;451:345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaich M, Kestel L, Pfirrmann M, et al. A MDR1 (ABCB1) gene single nucleotide polymorphism predicts outcome of temozolomide treatment in glioblastoma patients. Ann Oncol. 2009;20:175–181. doi: 10.1093/annonc/mdn548. [DOI] [PubMed] [Google Scholar]
- 25.Chen HY, Shao CJ, Shi HL, et al. Single nucleotide polymorphisms and expression of ERCC1 and ERCC2 vis-a-vis chemotherapy drug cytotoxicity in human glioma. J Neurooncol. 2007;82:257–262. doi: 10.1007/s11060-006-9290-2. [DOI] [PubMed] [Google Scholar]
- 26.Chen ZP, Malapetsa A, Monks A, et al. Nucleotide excision repair protein levels vis-à-vis anticancer drug resistance in 60 human tumor cell lines. Chin J Cancer. 2002;21:233–239. [PubMed] [Google Scholar]
- 27.Cheng L, Bao S, Rich JN. Potential therapeutic implications of cancer stem cells in glioblastoma. Biochem Pharmacol. 2010;80:654–665. doi: 10.1016/j.bcp.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Margison GP, Povey AC, Kaina B, et al. Variability and regulation of O6-alkylguanine-DNA alkyltransferase. Carcinogenesis. 2003;24:625–635. doi: 10.1093/carcin/bgg005. [DOI] [PubMed] [Google Scholar]
- 29.Javanmard S, Loktionova NA, Fang Q, et al. Inactivation of O(6)-alkylguanine-DNA alkyltransferase by folate esters of O(6)-benzyl-20-deoxygua-nosine and of O(6)-[4-(hydroxymethyl)benzyl] guanine. J Med Chem. 2007;50:5193–5201. doi: 10.1021/jm0705859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amit S, Ben-Neriah Y. NF-κB activation in cancer: a challenge for ubiquitination- and proteasome-based therapeutic approach. Semin Cancer Biol. 2003;13:15–28. doi: 10.1016/s1044-579x(02)00096-2. [DOI] [PubMed] [Google Scholar]