Abstract
The accumulation of microbial biofilms on ships' hulls negatively affects ships' performance and efficiency while also moderating the establishment of even more detrimental hard-fouling communities. However, there is little quantitative information on how the accumulation rate of microbial biofilms is impacted by the balance of the rates of cell settlement, in situ production (ie growth), dispersal to surrounding waters and mortality induced by grazers. These rates were quantified on test panels coated with copper-based antifouling or polymer-based fouling-release coatings by using phospholipids as molecular proxies for microbial biomass. The results confirmed the accepted modes of efficacy of these two types of coatings. In a more extensive set of experiments with only the fouling-release coatings, it was found that seasonally averaged cellular production rates were 1.5 ± 0.5 times greater than settlement and the dispersal rates were 2.7 ± 0.8 greater than grazing. The results of this study quantitatively describe the dynamic balance of processes leading to microbial biofilm accumulation on coatings designed for ships' hulls.
Keywords: marine microbial biofilms, ship's hull biofouling, microfouling, intact polar lipids, microbial phospholipids, sediment microelectrode
Introduction
Microbial biofilms (ie slimes) on ships' hulls attenuate the efficiency and performance of every surface vessel. By effectively doubling the roughness of ships' hulls, microbial biofilms cause hydrodynamic drag that can result in powering penalties of 7 – 21% and as much as a10 % increase in overall operational fuel consumption (Schultz 2007, Schultz et al. 2011). In addition, microbial biofilms play a key role in the recruitment of larger fouling organisms, such as algae (Joint et al. 2002, Callow & Callow 2005, Mieszkin et al. 2012, Dobretsov et al. 2013, Mieszkin et al. 2013) and tubeworms (Huang et al. 2007, Chung et al. 2010, Hadfield 2011, Huang et al. 2012, Salta et al. 2013), which further reduce efficiency, limit top-end speeds, and necessitate costly and environmentally hazardous hull cleaning (Schultz 2007, Schultz, et al. 2011). Microbial biofouling costs the world's navies, merchant shipping corporations, and private boat-owners billions of dollars each year through the combination of lost fuel efficiency and increased maintenance (Swain 2011). Furthermore, by contributing to increased fuel consumption, ship's hull microbial biofilms directly result in increased emissions of green-house gasses and other combustion-derived pollutants (Swain 2011).
Humans have sought novel ship's hull coatings to mitigate biofouling for millennia (WHOI 1952). Present-day coatings may be broadly categorized as either anti-fouling or fouling-release (Lejars et al. 2012). The former category is characterized by coatings that either contain or produce bioactive chemical agents, which deter the settlement of biofouling organisms and/or inhibit their growth. In contrast, fouling-release coatings are biologically benign and are designed to reduce the adhesion of fouling organisms. In theory, biofouling organisms should be released from the fouling-release coating by application of hydrodynamic forces (eg forward vessel motion). Although Casse and Swain (2006) showed that hydrodynamic forces might only remove some components of biofilm communities from a fouling-release coating, it appears that specific formulations may be more effective than others (Dobretsov & Thomason 2011). In practice, fouling-release coatings are unsuitable for vessels, such as surface warships, which are in port for extended periods; unchecked fouling may ultimately accumulate to the point that the fouling-release potential of a coating can no longer be realized. A velocity of 11 m s-1 (21.4 knots) led to the release of only 30% of barnacles from a silicone-based fouling-release coating (Larsson et al. 2010), and thus continuous hull grooming has been proposed as a solution for ships with fouling-release coating that spend extended periods in port (Tribou & Swain 2009). While the goal of antifouling and fouling-release coatings is the same, to reduce the accumulation rate of marine organisms on ships' hulls, the mode of efficacy is quite different. This had been evidenced by recent observations that different coatings yield different microbial biofilm communities (Casse & Swain 2006, Molino et al. 2009a, Molino et al. 2009b, Dobretsov & Thomason 2011, Zargiel et al. 2011).
Over the last few years, investigators applying state-of-the-art methods targeting microbial proteins, RNA, DNA and metabolites have made rapid progress on improving the understanding of the environmental mechanisms, community interactions, and microbial physiology behind marine microbial biofouling (eg, Thiyagarajan et al. 2009, Chen et al. 2011, Huang et al. 2012). Significant strides have also been made in understanding the complex chemical ecology of biofilm microbes (Thiyagarajan 2010, Hadfield 2011); based on this research, numerous formulations for coatings to prevent ship's hull microbial biofouling have been proposed (Holm 2012, Dobretsov et al. 2013, Qian et al. 2013). Despite the ever-increasing mechanistic understanding of marine microbial biofilm communities, predicting the success of coatings from first principles has remained elusive. The current gold standard for assessing the promise of new coatings during the early stages of coating development is simply to expose coatings to laboratory cultures or natural microbial populations and then visually score the extent and type of fouling. This approach cuts directly to the question of coating performance, but it does not provide much information about how the coating affects fundamental microbial processes; new approaches are needed that provide a more-detailed view of hull coating performance under in situ conditions.
Fundamentally, the accumulation rate of microbes that develop into a biofilm on a solid surface must be equal to the balance of the sources and losses of microbes at the surface; it is proposed that the primary source processes are cell settlement on the surface and cell production (ie growth) within the microbial biofilm, while the primary loss processes are cell dispersal from the surface and mortality of biofilm microbes by grazers. Little quantitative information is available on how anti-fouling and fouling-release coatings impact the rates of these fundamental processes. In this study, phospholipid-based molecular methods have been developed for determining the accumulation rates and growth rates of biofilm microbes on test panels of ship's hull coatings. These methods were validated using classical radioisotope-based kinetics and state-of-the-art electrochemistry. The results with anti-fouling and fouling-release coatings agreed with the currently accepted modes of efficacy. The results from two extensive experiments with the fouling-release coating underscore the importance of microbial cellular production, dispersal and grazing mortality processes that are relatively poorly understood compared to settlement and accumulation, which tend to be the focus of studies of microbial biofilms on coatings for ships' hulls. By providing the ability to quantify all of the fundamental processes that contribute to biofilm accumulation, the present approach could be used as an exploratory tool during coating development to improve performance of existing types of coatings and aid the discovery of novel coatings.
Materials and methods
All experiments described were conducted with coastal seawater obtained off Woods Hole Massachusetts (41.5°N 70.7°W). Experimental surfaces composed of test coupons (3 cm × 7 cm) or panels (10 cm × 25 cm), which were coated with: Interspeed 640® (International Paint) a copper-based, ablative, antifouling paint; Intersleek 425® (International Paint) a silicone-based fouling-release paint; or Intersleek 900® (International Paint) a fluoropolymer-based fouling-release paint. The coatings are manufactured by International Paint (http://www.international-marine.com). All experimental surfaces were conditioned by soaking for at least one month in distilled water and gently drying them with laboratory paper wipes prior to their first use. Triplicate experimental surfaces (ie three independent coupons or panels) were harvested and analyzed for each experimental condition at each time-point. In general, time-points were taken about half way through the incubation time period, three quarters of the way through the incubation time period, and at the end of the incubation time period. Data are presented as mean ± standard deviation. All reported statements of similarity or differences between data are based on Student's t-tests (significance threshold, p = 0.05).
Design of ‘in situ’ experiments
Three separate time-course experiments with whole seawater under in situ conditions (hereafter ‘in situ’ experiments; Table 1) were conducted to examine the accumulation and production rates of microbial phospholipids. In 2007, a time-course experiment was conducted from January to March (designated ‘winter’) with Intersleek 425 and Interspeed 640 coupons. The coupons were glued to a 60 cm × 70 cm PVC plate using food-grade silicone cement. The plate was deployed on a piling in Woods Hole at a depth of approximately 1 m below mean low tide. Three time points were collected over the course of the experiment. At each time-point (21, 45 and 54 days), the plate was retrieved by research divers using SCUBA, triplicate coupons were quickly pried off the plate for immediate sampling and the plate was redeployed. Similar experiments were conducted in April of 2008 (‘spring’) and July of 2007 (‘summer’) with only Intersleek 425 coupons.
Table 1.
Summary of conditions for each experiment in the study. The figures displaying the results for each of these experiments are referred to in the far right column.
| Experiment | Season | Surface | Format | Period | Analyses | Figure |
|---|---|---|---|---|---|---|
| Microelectrode | Winter 2008 | Glass | Coupon | 12 days | 1 | 2 |
| Isotope dilution | Summer 2011 | Intersleek 900 | Panel | 10 days | 1, 2 | 3 |
| In situ #1 | Winter 2007 | Intersleek 425 & Interspeed 640 | Coupon | 54 days | 2, 3, 4, 5 | 4, 5, 6 |
| In situ #2 | Spring 2008 | Intersleek 425 | Coupon | 23 days | 2, 3, 4 | 4 |
| In situ #3 | Summer 2007 | Intersleek 425 | Coupon | 10 days | 2, 3, 4 | 4 |
| Process exclusion #1 | Spring 2010 | Intersleek 900 | Panel | 25 days | 2, 3, 4 | 7 |
| Process exclusion #2 | Summer 2011 | Intersleek 900 | Panel | 17 days | 2, 3, 4 | 7 |
1Phosphate microelectrode;
2Dissolved phosphate;
3Phospholipid production;
4Phospholipid accumulation;
5Fatty acid analysis
Design of ‘process-exclusion’ experiments
Two separate time-course experiments designed to estimate the accumulation, production, dispersal and grazing rates of microbial phospholipids were undertaken in April of 2010 (‘spring’) and July of 2011 (‘summer’) using panels coated with Intersleek 900. Microbial biofilms were allowed to form on these panels by incubating them in glass aquaria (≈ 10 L) with continuously flowing (≈ 100 L d-1) unfiltered Woods Hole seawater for seven days. The test panels were then switched to separate aquaria that were flushed with Woods Hole seawater that was pre-filtered through a 0.2 μm, 1 μm or 20 μm pore-size hydrophilic polyethersulfone membrane. These membrane pore-sizes were chosen to exclude various processes that contribute to microbial biofilm accumulation, and these experiments are hereafter referred to as ‘process-exclusion’ experiments (Table 1). Since the aquaria were flushed relatively rapidly (≈ 10 d-1) such that any microbe that had dispersed from the microbial biofilm (ie became planktonic) would be swept out of the experiment at a rate that was much faster than its in situ growth (estimated approximately 0.3 d-1 (Ducklow 2000)) in order to preclude the establishment of resident planktonic communities with the experiment.
Phospholipid accumulation rates
At time-points during the aforementioned in situ and process-exclusion experiments, microbial biofilms were removed from the test coupons and panels by first gently scraping with a sterile razor blade and then rinsing with sterile seawater; a cursory inspection by epifluorescence microscopy confirmed that this procedure removed > 90% of the microbial biofilm by area. The microbial biofilms were then transferred to glass centrifuge tubes, and the amount of biofilm and seawater was determined gravimetrically. Next, methanol and dichloromethane were added such that the final mixture of methanol:dichloromethane:seawater was 2:1:0.8 by volume. The mixtures were then spiked with the internal recovery standard dinitrophenyl phosphatidlyethanolamine (DNP-PE). The succeeding steps in the lipid extraction generally follow the Bligh and Dyer (1959) method and were conducted as described (Popendorf et al. 2013). These total lipid extracts were stored under argon gas at -80 °C until analysis.
Microbial phospholipids, phosphatidylglycerol (PG), phosphatidlyethanolamine (PE), and phosphatidylcholine (PC), contained in the total lipid extracts were analyzed by high performance liquid chromatography/mass spectrometry (HPLC-MS) with an Agilent 1200 HPLC and Thermo Scientific TSQ Vantage triple quadrupole MS with a heated electrospray ionization (ESI) interface. HPLC and MS instrument conditions and details of quantification were described previously by Popendorf et al (2013). Briefly, 20 μL aliquots of the lipid extracts were injected and analyzed. Positively charged molecular ions of the phospholipids were fragmented in the MS to produce diagnostic product ions (PC) and neutral fragments (PG and PE) that were readily quantified. Chromatographic peaks of phospholipids were above background levels (signal: noise > 500:1) and were baseline-resolved. The recovery of phospholipids in the microbial biofilm extracts was then normalized to DNP-PE internal standard concentrations; recoveries were generally 95 - 100%. Lipid concentrations in the extracts were converted to areal mole inventories (pmol cm-2). Accumulation rates were determined by conducting a linear regression of phospholipid concentrations over the sampling time-points using SigmaPlot software; the standard error of the regression is applied as the error of the accumulation rate. The composition of the fatty acids within each phospholipid class was determined as described (Van Mooy & Fredricks 2010).
Phospholipid production rates
Production rates of microbial phospholipids were determined using the radiotracer 33P-phosphate, based on the method described by Van Mooy et al. (2008). At time-points during the aforementioned experiments, test panels were transferred to custom-made, small-volume, polycarbonate incubators (approximately 300 mL) filled with seawater taken directly from the experimental aquaria. The seawater in the incubators was then spiked with approximately 3.7 MBq of 33P-phosphate (approximately 130 pmol L-1 final concentration). Samples of the seawater in the incubators were taken for phosphate analysis using a standard colorimetric methods (Strickland and Parsons 1972), and for determining the exact quantity of 33P by liquid scintillation counting; these data together were used to calculate the specific 33P radioactivity of phosphate in the overlying seawater. The test panels were then incubated at in situ temperature and light conditions for 24 hours. Next, the microbial biofilms were samples and extracted as described above. An aliquot of the lipid extract was taken to determine the 33P radioactivity of total phospholipids by scintillation counting. Using the specific 33P radioactivity of phosphate in the incubations, total phospholipid production rates were determined. These rates were normalized to the area of the coupon or panel, and expressed in units of pmol cm-2 d-1.
The phospholipids PG, PE and PC in total lipid extracts for the 33P experiments were isolated from one another by using the same HPLC conditions are used for phospholipid quantification. Baseline separation of the PG, PE and PC on the HPLC were occasionally checked by injecting phospholipid standards and monitoring their elution from the HPLC using a Sedere evaporative light scattering detector (ELSD). The 33P radioactivity of the fractions containing either PG, PE or PC was then determined by liquid scintillation counting, and the production rates of these individual classes of phospholipids were calculated.
Estimation of phospholipids gained via microbial settlement and loss via dispersal and grazing mortality in the process-exclusion experiments
The accumulation of microbial biomass, and thereby phospholipids, in natural microbial biofilms is dictated by a balance of natural processes that act as sources and losses of cells to microbial biofilms:
| (Equation 1) |
For the purposes of this study, it is assumed that the primary sources were in situ cell production (ie growth) and settlement, and that the primary losses were grazing mortality and dispersal (Figure 1):
Figure 1.
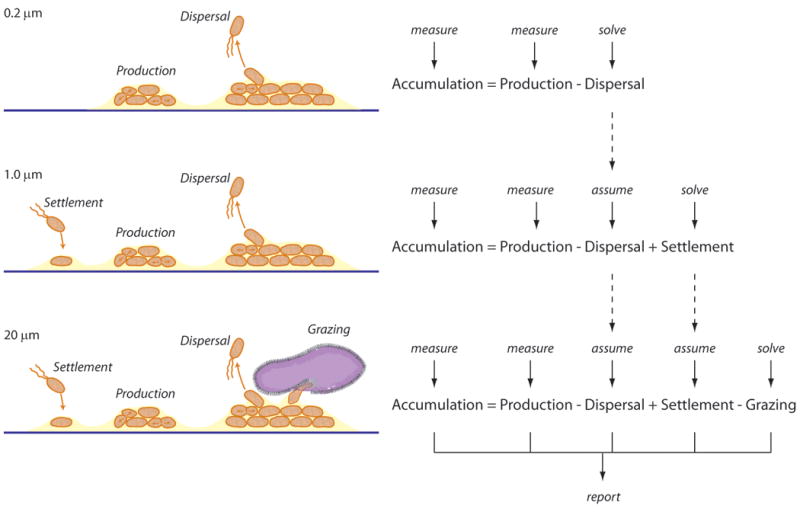
Schematic illustrating the design of the process-exclusion experiments. Microbial biofilms on test panels developed for ships' hulls in Woods Hole seawater were tranferred to aquaria that were flushed with seawater that had been filtered, thereby excluding the organisms responsible for key microbial processes. Only the accumulation and production rates of phospholipids were directly measured; rates of dispersal, settlement, and grazing were determined by difference.
| (Equation 2) |
The process-exclusion experiments (Fuhrman and Azam 1982, Kirchman and Ducklow 1993, Ducklow 2000; and references therein) conducted with seawater filtered through either 0.2 μm, 1 μm or 20 μm pore-size membranes (described above), were intended to exclude different functional classes of microbes from the experiments and thereby exclude different sets of key microbial biofilm processes. The microbial phospholipid accumulation and production rates are surrogates of microbial accumulation and production; measuring these rates in experiments where different processes are excluded enables differentiation and estimation of rates of microbial losses via grazing and dispersal.
In the experiments with seawater filtered through 0.2 μm membranes, all organisms were excluded, thereby excluding the processes of settlement and grazing. It was then assumed that only microbial processes at play in these 0.2 μm experiments were accumulation, production and dispersal; the difference in the measured rates of the accumulation and production yielded an estimate of the rate of phospholipid loss via dispersal (Figure 1). In the 1 μm incubations, only grazing was excluded while accumulation, production, dispersal and settlement were at play. It was then assumed that the rate of dispersal was the same in the 0.2 μm and 1 μm incubations, and settlement rate was calculated by difference (Figure 1). In the 20 μm treatment all processes were at play, the rate of settlement was assumed to be the same as the 1 μm and 20 μm incubations, and grazing was calculated by difference (Figure 1).
Interstitial phosphate concentrations
The exchange of phosphate between overlying seawater and interstitial microbial biofilm water was observed directly by using a phosphate selective microelectrode. The chemistry for phosphate detection follows the protocols of Ungerer et al. (1993) and Kinoshita et al. (1995) but with the following modifications. The microelectrode sensor was designed around a 25μm carbon fiber, inserted through and bonded into a 30μm tip pipette (Smith et al. 1999, Porterfield et al. 2001). The protruding fiber was broken back and pipettes with a recess of approximately 10 μm were chosen. The enzymes, xanthine oxidase (0.0025-0.005 Units μL-1) and nucleoside phosphorylase (50 Units μL-1) were loaded sequentially. A reservoir pipette, loaded with the enzymes and gluteraldehyde (5% by volume in phosphate buffered saline) was prepared and used in the manner described for manufacturing potentiometric electrodes (Smith, et al. 1999). The sensing microelectrode was advanced into the reservoir and withdrawn such that a droplet of mix adhered to and entered the tip and cavity. This was dried at room temperature and the second enzyme layer deposited. Sensors could be stored overnight at 4°C. Inorganic phosphate, in the company of inosine in the medium, was detected through hydrogen peroxide generated by the two enzyme process and reacted on the carbon surface held at 600mV. Headstage and amplifiers, as well as positional control of the sensor (BRC, Woods Hole), were as described for current generated during enzymatic glucose detection by Jung et al. (2001) and reviewed by Smith et al. (2007). In contrast to these two papers the data presented here are in picoamps representing the localized bulk activity of the analyte and not as a flux where the microelectrode sensor is used in a translational mode. Observations were made on an inverted Zeiss compound microscope, housed in a Faraday box as described by Smith et al. (1999, 2007). Microelectrode movement, data collection and applied voltages were controlled through IonView Software (BRC, Woods Hole). All chemicals and enzymes were purchased from Sigma, USA.
In January of 2008 (‘winter’), a glass coupon (ie slide) was incubated in Woods Hole seawater for ten days to establish a natural marine microbial biofilm (Table 1). This was then incubated for another two days in an aquarium containing natural seawater spiked with inosine at a final concentration of 8 mmol L-1, which acts as a co-substrate for the reaction described by Ungerer et al. (1993) and Kinoshita et al. (1995). The coupon, which had become coated with a microbial biofilm of approximately 300 to 500 μm in thickness, was transferred to the experimental platform equipped with a custom-made, temperature-controlled, stirred reservoir that held the coupon in place under approximately 100 mL of seawater, and allowed the incubation to proceed while being directly observed via the Zeiss microscope. A number of experiments were conducted where the phosphate microelectrode was inserted into the microbial biofilm (approximately 300 μm into the surface of the microbial biofilm and approximately 10 to 200 μm from the surface of the glass coupon) by using a stepper-motor micromanipulator. The process was observed by microscopy and repeated at numerous locations across the microbial biofilm surface. In locations where the microelectrode pierced the microbial biofilm such that a seal between the walls of the microelectrode and the microbial biofilm was observed, the overlying water was flooded with phosphate buffer to a final concentration of 800 μmol L-1 over the course of several seconds and the electrochemically generated current was recorded using the IonView Software.
The concentration of phosphate in interstitial waters was estimated using the 33P isotope-dilution method laid out by Forsdyke (1971) and advanced by Moriarty et al. (1985) and Freeman and Lock (1995) for studies of marine microbial biofilms. This method was applied in August of 2011 (‘summer’) in a third process-exclusion incubation of the same design as that conducted in April of 2010 and July of 2011 (Table 1). Replicate sets of triplicate panels from each of the three prefiltration conditions were amended with a phosphate solution to yield concentrations that were either 100 nmol L-1 or 500 nmol L-1 above ambient concentrations. A set of triplicate panels that received an amendment of only the distilled water used to make the phosphate solutions served as non-amended (ie ambient phosphate only) controls. Microbial phospholipid production rates were then determined using 33P-phosphate incubations as described above.
Results
Validation of the use of the 33P-phosphate tracer
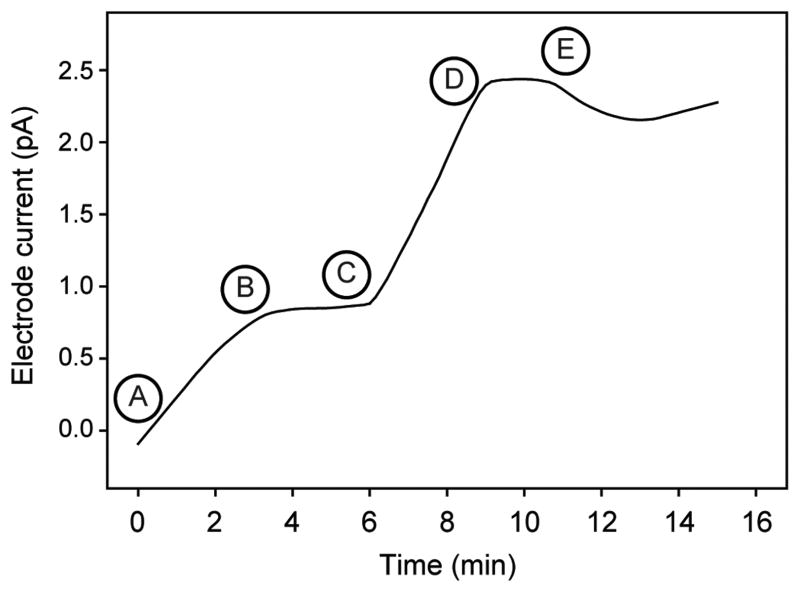
The exchange of phosphate between the interstitial waters of natural marine microbial biofilms and overlying waters using a phosphate selective microelectrode was assessed. In a typical experiment (Figure 2), the electrode current, which is directly proportional to inorganic phosphate activity, stabilized shortly after being inserted deep into the microbial biofilm. The overlying waters were then flooded with phosphate, and immediately a response by the microelectrode deep within the microbial biofilm was observed. The electrode current then stabilized again after only a few minutes, suggesting equilibrium inorganic phosphate activity had been reached within the microbial biofilm. Upon withdrawing the electrode, the current tended to drift slightly but returned to equilibrium levels showing that the activities of inorganic phosphate within the microbial biofilm and the overlying waters were similar.
Figure 2.
A typical profile of the current recorded by the phosphate selective microelectrode. A) The microelectrode was inserted into a densely populated area of the biofilm as confirmed by microscopy. B) The microelectrode equilibrated with interstitial phosphate concentrations within the biofilm. C) Phosphate was added to the seawater overlying the biofilm. D) The microelectrode current responded to the transport of the phosphate from the overlying seawater into the biofilm. E) The microelectrode was withdrawn from the biofilm and after equilibration the current reflected the phosphate in the overlying seawater. Experimental conditions summarized in Table 1.
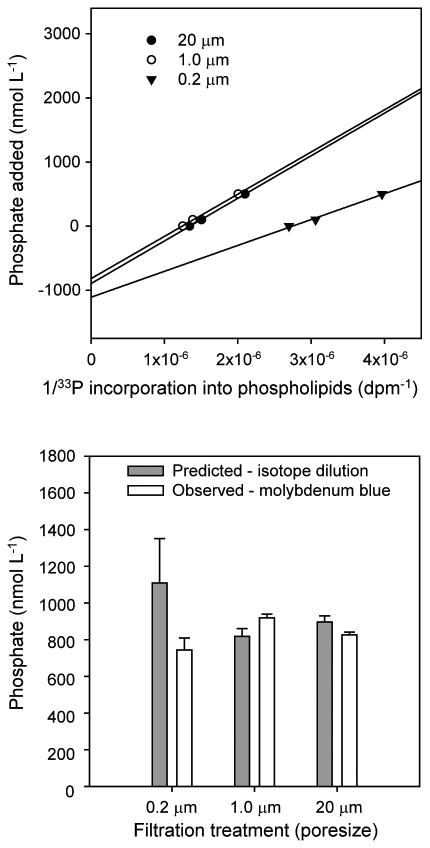
The concentrations of phosphate in interstitial waters were further examined using a 33P-phosphate isotope dilution approach. Linear regressions of the inverse of 33P incorporation into lipids versus phosphate amendments concentration yielded straight lines (all R2 > 0.95) (Figure 3). The y-intercepts of these linear regressions yield estimates of ambient phosphate concentrations experienced by the microbial biofilm microbes engaged in phospholipid biosynthesis. This approach indicated that interstitial phosphate concentrations were between 800 and 1,200 nmol L-1. Also, phosphate data from colorimetric analyses were statistically indistinguishable (p>0.05) from those estimated by isotope dilution (Figure 3), and with published data from the area (Borkman and Turner 1993a). This result shows that microbes experienced phosphate concentrations that were almost the same as the overlying water, and validated the extrapolation of phosphate concentrations values from overlying waters to interstitial waters, which is essential to calculate phospholipid synthesis rates.
Figure 3.
Determinations of interstitial phosphate concentrations in process-exclusion experiments. Top panel: Forsdyke (1970) plots showing predicted interstitial phosphate concentrations (y-intercept) based on 33P-phosphate isotope dilution experiments; symbols delineate the data from the experiments conducted with seawater filtered through various pore-size filters. Bottom panel: Comparison between the predicted interstitial phosphate concentrations, and the phosphate concentrations of the overlying seawater as determined by a colorimetric assay method. Experimental conditions summarized in Table 1.
Production and accumulation rates of total phospholipids in microbial biofilms under in situ conditions
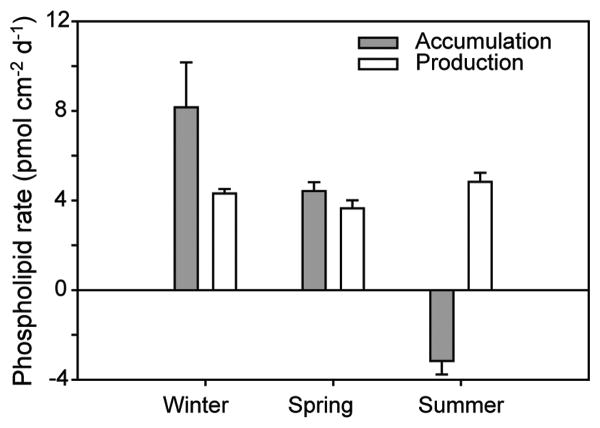
Total phospholipid accumulation and production rates were obtained on fouling-release (Intersleek 425) test panels in the in situ experiments conducted during winter, spring and summer (Figure 4). These relatively simple experiments showed that total phospholipid accumulation rates varied substantially between seasons, from about 8 to -4 pmol cm2 d-1. By contrast, total phospholipid production rates remained steady at about 4 pmol cm2 d-1. In the winter, total phospholipid accumulation rates exceeded total phospholipid production rates (p<0.01), indicating that microbial settlement contributed significantly to total phospholipid accumulation, and that losses of biomass were relatively minor. In the spring, the rates of total phospholipid accumulation and total phospholipid production were nearly equal (p>0.05), which showed that settlement rates were offset by losses (Equation 2). Losses were dominant over sources in the summer (p<0.01) as evidenced by an overall dissipation of microbial biofilm biomass (ie negative total phospholipid accumulation) despite steady total phospholipid production.
Figure 4.
Accumulation and production rates of total microbial biomass (based on total phospholipids) in the in situ experiments. Experimental conditions summarized in Table 1.
In a separate winter experiment, a more sophisticated approach was taken, where the accumulation and production rates of PG, PE and PC were independently measured on test coupons coated with either an anti-fouling paint (Interspeed 640) or fouling-release paint (Intersleek 425). First and foremost, it was found that accumulation rates of all three microbial phospholipids were about two orders of magnitude greater on the fouling-release paint versus the antifouling paint (Figure 5). The difference in production rates between the two paints appeared to be even greater, although the production rates in microbial biofilms on surfaces coated with the antifouling paint were nearly undetectable. The accumulation rates of PG and PE, which in North Atlantic waters are thought to be primarily from heterotrophic bacteria (Van Mooy et al. 2009, Van Mooy and Fredricks 2010, Popendorf et al. 2011) were nearly twice as great as production rate on the fouling-release surface (p<0.05); this imbalance is consistent with the measurements of total phospholipid accumulation and suggests substantial sources from settlement (Equation 2). By contrast, the accumulation and production rates of PC, which is primarily from eukaryotes (Van Mooy, et al. 2009, Van Mooy and Fredricks 2010, Popendorf, et al. 2011) were relatively equal on the fouling-release coating, indicating that eukaryote settlement was balanced by losses (Equation 2).
Figure 5.
Accumulation and production rates of bacterial biomass (based on PG and PE) and eukaryotic biomass (based on PC) in the in situ experiments. Top left: Results from test panels coated with fouling-release coating. Top right: Results from test panels coated with antifouling coating. Bottom left: Epifluorescence photomicrographs (approximate dimensions: 1.5 × 1.0 mm) showing the accumulated biofilms on test panels coated with fouling-release coating. Bottom right: similar images for an antifouling coating. In these images, biofilm components that are enriched in chlorophyll, such as phytoplankton, appear red. Experimental conditions summarized in Table 1.
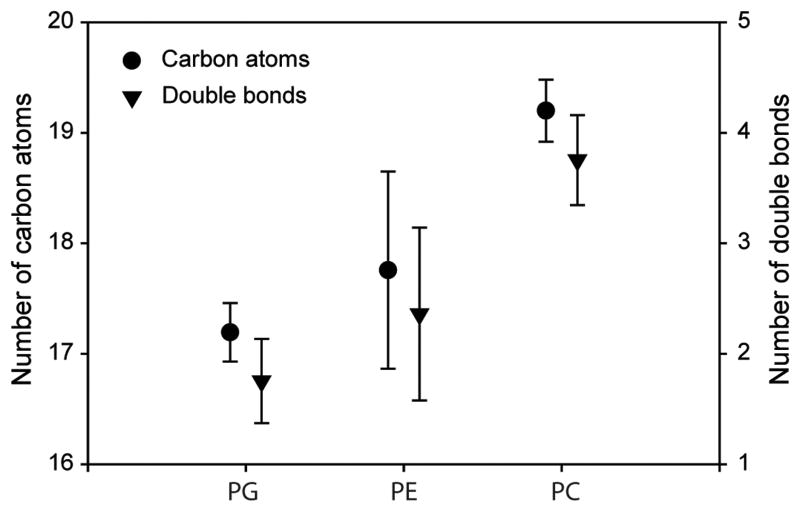
The distributions of fatty acids with PE and PG molecules were similar to each other, while that of PC was distinct (Figure 6). As will be discussed below, these data affirm the delineation of PG and PE as bacterial phospholipids and PC as a eukaryotic phospholipid.
Figure 6.
Characteristics of the fatty acid moieties bacterial phospholipids (PG and PE) and eukaryotic phospholipids (PC). These results are from the winter experiment comparing fouling-release and antifouling coatings (Figure 5). There were no discernable difference between the data collected for the two different types of surfaces, and thus they were averaged. Experimental conditions summarized in Table 1.
Relative contribution of microbial production, settlement, detachment and grazing to phospholipid accumulation
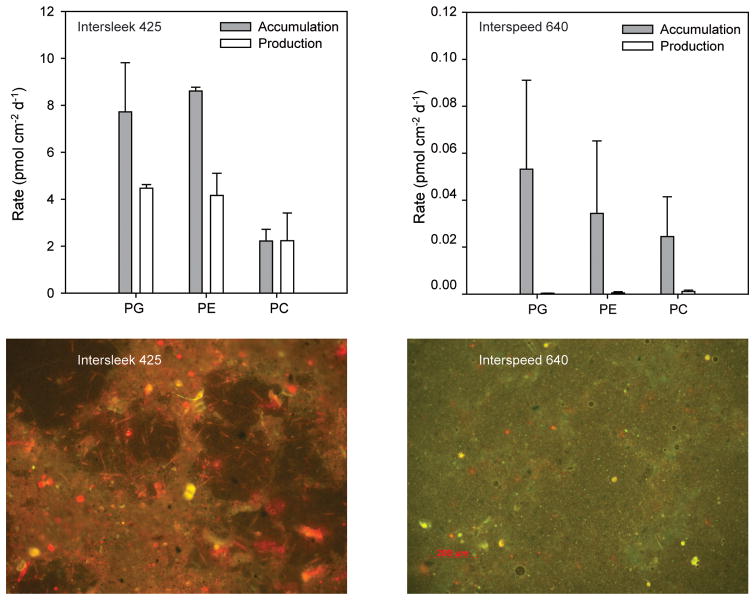
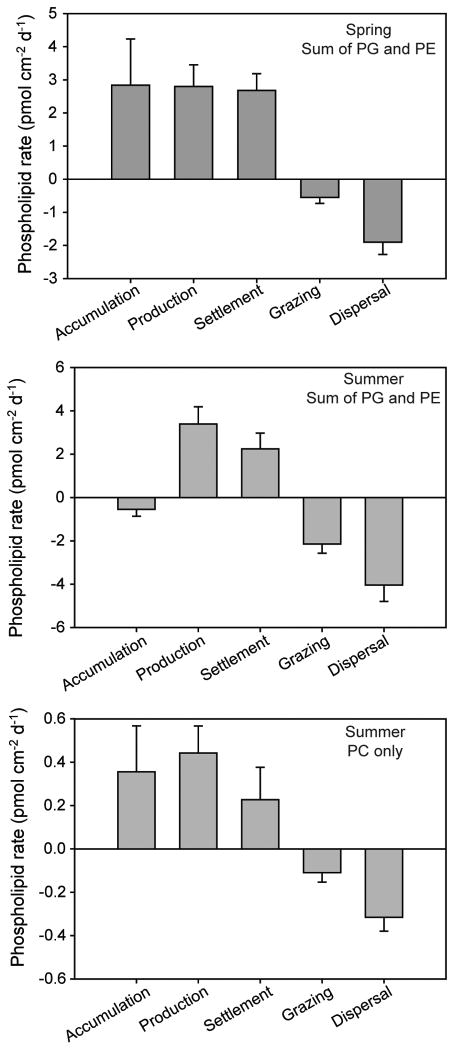
In the process-exclusion experiments conducted on Intersleek 900 test panels in the spring, the sum of the sources of bacterial phospholipids (PG and PE) exceeded the sum of the losses, resulting in net accumulation of bacterial phospholipids (Figure 7). The production and settlement rates of bacterial phospholipids were of similar magnitude to one another, whereas the losses of bacterial phospholipids from dispersal were about three times greater than grazing (Figure 7). This molecular-level quantification for PG and PE is similar to the total phospholipid data (Figure 4); spring time is a period of net bacterial biomass accumulation in biofilms in the waters off Woods Hole. Parallel analyses with PC were not possible during this time period because growth rates were below limits of quantification.
Figure 7.
Rates of key bacterial processes (based on PG and PE) and eukaryotic processes (based on PC) in the process-exclusion experiments. Top: Results showing bacterial processes in the spring experiment. Middle: Results showing bacterial processes in the summer experiment. Bottom: Results showing eukaryotic processes in the summer experiment. Experimental conditions summarized in Table 1.
Bacterial processes in the summer differed somewhat from those in the spring (Figure 7). The primary difference was that accumulation rate of bacterial phospholipids was effectively zero, indicating a nearly steady-state balance between sources and losses of bacterial biomass. Production and settlement rates in summer were similar in magnitude to spring. Grazing contributed a greater fraction of total losses rates in the summer, but remained less than dispersal rates. Again the molecular-level characterization of bacterial phospholipids was consistent with that from total phospholipids (Figure 4): summer is not a period of intense bacterial biomass accumulation.
There was a net accumulation of eukaryotic biomass during the summer experiment as indicated by the balance of PC sources and losses. Cell production was a larger source process than settlement, and dispersal was a greater loss process than grazing. Note that the absolute rates for PC were about an order of magnitude less than rates for PE and PG.
On average, across both groups of organisms during both seasons, growth rates were 1.5 ± 0.5 times greater than settlement and the dispersal rates were 2.7 ± 0.8 greater than grazing.
Discussion
Insights on the physical structure of marine microbial biofilms
The phosphate microelectrode and 33P-phosphate isotope dilution experiments (Figures 2 and 3) were designed to constrain interstitial phosphate concentrations and to determine whether diffusion limitation of 33P-phosphate into microbial biofilms grown in Woods Hole waters might significantly extend isotopic equilibration time, skewing the accuracy of the 33P-phospholipid synthesis rates. Three key results from these experiments together appear to preclude significant isotopic disequilibrium. First, the Forsdyke plots of 33P-phosphate incorporation into phospholipids yield nearly straight lines, which is a hallmark signal of isotopic equilibrium. Second, the isotope dilution experiments showed that the concentrations of phosphate in the interstitial waters were effectively equal to overlying waters; this might be a coincidence, but a more reasonable explanation is that there is rapid exchange between interstitial and overlying water. This rapid exchange is supported by the third key result: the phosphate microelectrode that was embedded in the microbial biofilm showed rapid changes in interstitial phosphate activities in response to perturbations of phosphate concentrations in overlying waters. Not only do these three results preclude significant isotopic disequilibrium and affirm the accuracy of the phospholipid synthesis rates, they suggest that there was relatively unfettered exchange of phosphate (and probably other solutes) between the interstitial waters of microbial biofilms and the overlying waters. Structural heterogeneity of the microbial biofilms on fouling-release test panels was evident in photomicrographs (Figure 5) and suggested the presence of interconnected voids that are thought to function as channels to facilitate seawater exchange (Costerton et al. 1995, Dobretsov 2010). Monds and O'Toole (2008) point out that explicit experimental evidence of this function in biofilms is scarce; the data presented in this study (Figures 2, 3 and 5) may provide a foundation for further studies of physical structure and solute transport of biofilms on ships' hulls.
Using phospholipids as proxies for microbial biomass in biofilms
This study hinges critically on the strength of phospholipid accumulation and production rates as molecular proxies for rates of microbial biomass accumulation and production. Most existing studies apply either microscopic techniques (Casse & Swain 2006, Molino et al. 2009a, Molino et al. 2009b, Dobretsov & Thomason 2011) or molecular methods that target microbial genes (Huggett et al. 2009, Chung et al. 2010) to access microbial biomass accumulation. To our knowledge phospholipids have not seen broad use in the study of biofouling on coatings for ship's hulls, and methodological biases should be considered when comparing this study to previous studies. This stated, applying phospholipid concentrations to estimate microbial biomass in microbial biofilms is a long-standing approach in microbial ecology; the seminal studies by White and colleagues that established phospholipids as quantitative proxies for microbial biomass were first developed for marine sediments, and continue to see broad application in marine and aquatic settings (White & Tucker 1969, White et al. 1979, Middelburg et al. 2000, Spivak et al. 2007). Thus, the interpretation of phospholipid accumulation as a signal of microbial biomass accumulation rests on a solid foundation from the literature.
By contrast, the application of phospholipid production as a proxy for microbial cell production in marine microbial biofilms has seen considerably less application. However, the 33P-phospholipid approach has been critically and thoroughly assessed in two studies (Moriarty, et al. 1985, Freeman & Lock 1995) and found to be as robust as other commonly accepted techniques for measuring microbial cell production, such as tracing 3H-thymidine incorporation into to DNA. The primary recommendations to emerge from these studies are to accurately determine interstitial phosphate concentrations and to be vigilant about the potential effects of 33P-phosphate disequilibrium, both of which were addressed in this study and examined in the previous section (Figures 2 and 3). Furthermore, 33P-phosphate was shown to be incorporated by a broader spectrum of marine microbes than other radiolabeled growth substrates (Longnecker et al. 2010). Therefore it is concluded that the phospholipid production rates presented here are probably also reasonably sound estimates of microbial biomass production.
While total phospholipid data are an established proxy for total microbial biomass, these types of data provide no information on the relative contributions of bacterial and eukaryotic cells. By examining PG, PE, and PC independently, an attempt was made to distinguish these two broad groups of microbes. Since PG and PE accumulation and production rates consistently exceed those for PC (Figures 5 and 7), the present data lead to the conclusion that bacterial biomass dominates over eukaryotic biomass.
Clearly, the specificities of PG and PE as biomarkers for marine bacteria and PC as a biomarker for marine micro-eukaryotes are critical for supporting the conclusion that bacterial biomass outweighs eukaryotic biomass in biofilms on ships' hulls. Fortunately, the recent application of HPLC/MS to the study of phospholipids in marine environments has allowed the microbial sources of phospholipids to be assessed (Van Mooy et al. 2006, Van Mooy et al. 2009, Van Mooy & Fredricks 2010, Popendorf et al. 2011). In a presentation of data from marine microbes in the South Pacific Ocean in conjunction with a review of existing literature, Van Mooy and Fredricks (2010) concluded that PG and PE in samples of marine microbes were derived primarily from heterotrophic bacteria and PC was derived primarily from microeukaryotes. This delineation was supported by a subsequent study that employed a suite of both cultivation-dependent and cultivation-independent methods in the North Atlantic Ocean, including stations in relatively close proximity to Woods Hole (Popendorf et al. 2011). The fatty acid composition of microbial biofilm PG, PE and PC (Figure 6) provides some additional confidence in extrapolating insights gained from the offshore environments on the microbial origins of PG, PE and PC to the microbial origins of these molecules in biofilms growing on antifouling/fouling release coatings. The delineation of PG and PE as bacterial phospholipids and PC as a eukaryotic phospholipid was supported by the distribution of the various fatty acids contained in the phospholipids (Figure 6). The distributions of fatty acids with PE and PG molecules were similar to each other, while that of PC was distinct. The shorter, saturated fatty acids within PG and PE are suggestive of bacterial sources, while longer, unsaturated fatty acids in PC are diagnostic of eukaryotic sources. The fatty acid compositions of PG, PE, and PC in the microbial biofilms reported here (Figure 6) is indistinguishable from the planktonic microbes reported by Van Mooy and Fredricks (2010).
In situ microbial biofilm accumulation and cell production
Total phospholipid data from the in situ experiments showed that microbial production rates were relatively constant across the winter, spring and summer time points; however, the accumulation rates decreased across these seasons (Figure 4). Although these data were collected in different years, they nonetheless support a model where settlement rates decrease and loss processes intensify as the seasons of the year progress, corresponding to more and more rapid recycling of microbial biofilm biomass. Using the averages of the total phospholipid synthesis rates (Figure 4) and total phospholipid concentrations (not shown), it is estimated that the steady-state turnover rate of the total microbial community within the biofilms increased from 0.02 d-1 in winter, to 0.03 d-1 in spring to 0.08 d-1 in summer. These trends in the biofilm microbial community parallel that of the planktonic community in nearby waters. The abundance of heterotrophic bacteria in waters near Woods Hole was shown to increase rapidly in the late spring and summer months (Borkman & Turner 1993b), which could have been a signal, in part, to the dispersal of bacteria from biofilms. In addition, the abundance of their grazers also increased in lockstep (Borkman & Turner 1993b), which introduces the possibility that intensification in grazing rates during the summer could lead to greater mortality for biofilm microbes as well, thereby decreasing accumulation rates. On the other hand, the eukaryotic phytoplankton community typically transitions from diatoms, which are known to contribute to microbial biofilms, to other classes of phytoplankton that might be less inclined toward settling (Turner et al. 2009); this could also lead to a decrease in accumulation rates.
The total phospholipid data provide the opportunity to elucidate fundamental differences between the life strategies of planktonic microbes and those within biofilms in the study area. Interestingly, the aforementioned turnover rates of microbial biofilm biomass are nearly an order of magnitude slower than what might be expected for the planktonic community in productive coastal waters (eg Sosik et al. 2003). This suggests that the biofilm life strategy conferred a greater average lifespan for biofilm microbes than for planktonic microbes. The mechanisms that might confer greater longevity to microbial biofilm microbes are myriad and complex, and these have been reviewed extensively (Jefferson 2004, Monds & O'Toole 2008, Moons et al. 2009, Dobretsov 2010, McDougald et al. 2011). Understanding the mechanisms that underpin the advantages of the biofilm life strategy on ship's hull microbial biofilms demands the quantitative elucidation of the balance of settlement, production, grazing and dispersal to microbial biofilm accumulation.
Balance of production, settlement, dispersal and grazing in process-exclusion experiments
The process-exclusion experiments on fouling-release (Intersleek 900) test panels provide a more detailed view of the processes that contribute to microbial biofilm accumulation than in the aforementioned in situ experiments. The obvious trade-off for this increased level of information is that by excluding different processes, these experiments may no longer accurately reflect in situ conditions. However, in general, results from the process-exclusion experiments reflect those from the in situ experiments, which would suggest that the process-exclusion experiments are not necessarily inaccurate. First, both the process-exclusion and in situ experiments capture the transition between strong positive accumulation in the winter and negligible or negative accumulation in the summer (Figures 4 and 7). Second, the absolute magnitude of the production rates in the process-exclusion and in situ experiments are of the same magnitude (Figures 4 and 6). These similarities, and more importantly the lack of major discrepancies, lead to the conclusion that the process-exclusion experiments probably provide a reasonable approximation of in situ processes.
The results quantitatively illustrate how settlement on ship's hull test panels conferred benefits to the marine microbes. Settlement rates were generally similar to production rates (Figure 7), indicating to a first approximation that microbes which settled on the test panels doubled once. Thus, on average the biofilm microbes experienced gross growth. Furthermore, in the spring settlement and production contributed to accumulation of biomass on test panels in this environment, while in the summer these processes contributed to significant rates of dispersal. This is consistent with a seasonal model where winter and spring conditions are conducive to a sessile existence focused on reproduction, while summer conditions are favorable for a planktonic life strategy that affords microbes the opportunity to proliferate geographically. The microbial biofilm life strategy clearly also offered marine microbes refuge from predation by grazers; regardless of the season or the class of microbe, dispersal rates exceeded grazing rates. This observation underscores how the microbial biofilm life strategy offered refuge for microbial biofilm microbes in Woods Hole. It has long been predicted that microbial biofilms function as refuges in marine environments and the data quantitatively affirm this model (Jefferson 2004).
One important caveat needs to be kept in mind when considering how the results of this study reflect overall mortality: it is possible that viral lysis could contribute to the observed dispersal rates. Dispersal rates are defined based on the disappearance of intact phospholipids; these molecules are thought to be very labile, and could quickly be degraded once the cell membrane of a microbe is compromised. Thus, if viral lysis rates were significant, then the methods employed in this study would be unable to distinguish this process from dispersal. Data are limited on the role of viruses in microbial biofilms, although one study suggested that marine microbial biofilm microbes may be fairly resistant to viral attack (Thomas et al. 2008). Clearly the role of viral lysis in biofilms growing on hull coatings deserves further investigation (Dobretsov 2010).
Relevance to the development of coatings for ship's hulls
The comparison of microbial processes on surfaces coated with an antifouling paint versus a fouling-release coating (Figure 5) reflected the expected modes of efficacy of these two classes of coatings. Fouling-release coatings such as Intersleek 425 and 900 are designed to create a surface that is difficult for marine organisms to adhere to strongly, resulting in them sloughing when hydrodynamic forces intensify. Thus, fouling-releasecoatings are designed to derive their efficacy from encouraging accelerated rates of microbial dispersal. However, even under dynamic conditions some components of microbial biofilm communities may still persist (Casse & Swain 2006). Clearly, in the hydrodynamically quiescent (ie pier-side) conditions under which the experiments were conducted, fouling-release coatings would not be expected to impact dispersal rates at all. Furthermore, these coatings do not appear to significantly deter settlement, nor would they inhibit production because they are non-toxic. Thus it is reasonable to expect that both microbial accumulation and production rates would proceed unchecked. By contrast, cooper-based, ablative antifouling coatings such as Interspeed 640 are highly toxic and their surfaces are continually renewed, characteristics that would decrease growth rates and increase dispersal rates, respectively. Thus, the existing understanding of how silicone/fluoropolymer fouling-release and cooper-based antifouling coatings function would lead us to predict that microbial accumulation and production rates would be much higher on the former coating than the latter coating (Equation 2). The observations reported here reflect these predictions and lend additional support for the phospholipid-based methods developed for this study.
Owing to a regulatory environment that is increasingly hostile to copper-based anti-fouling coatings on ships' hulls (Dafforn et al. 2011), the search for new anti-fouling coatings continues to accelerate (Dobretsov et al. 2013, Qian et al. 2013). The insights this study provides on the microbial processes that contribute to biofilm formation on two basic types of coatings could inform the development of a new coatings. Complementary molecular methods targeting microbial proteins, RNA, DNA or metabolites provide a detailed view into the physiological underpinnings of production, settlement, dispersal and grazing, but these methods do not readily yield quantitative estimates for the absolute rates of these processes. Future application of the methods employed in this study could provide useful feedback to product developers by distinguishing the effects of novel coating properties on the major processes contributing to the accumulation of microbial biofilms. For example, coatings designed to stimulate the rates at which natural communities of grazers check the accumulation of biofilms on coatings can now be more rigorously tested. Importantly, even if a coating ‘fails’ field trials, the methods applied in this study have the potential to reveal its positive attributes (ie attenuated rates of settlement and growth or accelerated rates of dispersal and grazing), which might otherwise be veiled under high rates of net accumulation.
Acknowledgments
We thank Doug Handy and Ed O'Brien for assistance with diving activities at WHOI pier, Eric Holm, Dean Webster, and David Christianson for coating our ship's hull test coupon and panels. We are also grateful for assistance with lipid analyses and illustrations by Kimberly Popendorf and Jack Cook, respectively. This work was supported by grants from the Office of Naval Research to B.A.S.V.M. (N000140610134, N000140810764, N000140910091, and N000141110181) and grants from the National Institutes of Health to P.J.S.S. (NCRR P41RR01395).
References
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Borkman DG, Turner JT. Plankton studies in Buzzards Bay, Massachusetts, USA. II. Nutrients, chlorophyll a, and phaeopigments, 1987 to 1990. Mar Ecol Prog Ser. 1993a;100(27-34) [Google Scholar]
- Borkman DG, Turner JT. Plankton studies in Buzzards Bay, Massachusetts, USA. I. Hydrography and bacterioplankton, 1987 to 1990. Mar Ecol Prog Ser. 1993b;100(17-26) [Google Scholar]
- Callow JA, Callow ME. Biofilms. Prog Mol Subcell Biol. 2005;42:141–169. doi: 10.1007/3-540-30016-3_6. [DOI] [PubMed] [Google Scholar]
- Casse F, Swain GW. The development of microfouling on four commercial antifouling coatings under static and dynamic immersion. International biodeterioration & biodegradation. 2006;57(3):179–185. [Google Scholar]
- Chen ZF, Matsumura K, Wang H, Arellano SM, Yan X, Alam I, Archer JAC, Bajic VB, Qian PY. Toward an Understanding of the Molecular Mechanisms of Barnacle Larval Settlement: A Comparative Transcriptomic Approach. PLos One. 2011;6(7):e22913. doi: 10.1371/journal.pone.0022913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HC, Lee OO, Huang YL, Mok SY, Kolter R, Qian PY. Bacterial community succession and chemical profiles of subtidal biofilms in relation to larval settlement of the polychaete Hydroides elegans. ISME J. 2010;4(6):817–828. doi: 10.1038/ismej.2009.157. [DOI] [PubMed] [Google Scholar]
- Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- Dafforn KA, Lewis JA, Johnston EL. Antifouling strategies: History and regulation, ecological impacts and mitigation. Marine Pollution Bulletin. 2011;62(3):453–465. doi: 10.1016/j.marpolbul.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Dobretsov S. Biofouling. Oxford: Wiley-Blackwell; 2010. Marine biofilms. [Google Scholar]
- Dobretsov S, Thomason JC. The development of marine biofilms on two commercial non-biocidal coatings: a comparison between silicone and fluoropolymer technologies. Biofouling. 2011;27(8):869–880. doi: 10.1080/08927014.2011.607233. [DOI] [PubMed] [Google Scholar]
- Dobretsov S, Abed RMM, Teplitski M. Mini-review: Inhibition of biofouling by marine microorganisms. Biofouling. 2013;29(4):423–441. doi: 10.1080/08927014.2013.776042. [DOI] [PubMed] [Google Scholar]
- Ducklow H. Microbial ecology of the ocean. New York: Wiley-Liss; 2000. Bacterial production and biomass in the oceans; pp. 85–120. [Google Scholar]
- Forsdyke DR. Application of the Isotope-Dilution Principle to the Analysis of Factors Affecting the Incorporation of [3H]Uridine and [3H]Cytidine into Cultured Lymphocytes: Evaluation of Pools in Serum and Culture Media. Biochem J. 1971;125:721–732. doi: 10.1042/bj1250721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman C, Lock MA. Isotope dilution analysis and rates of 32P incorporation into phospholipid as a measure of microbial growth rates in biofilms. Wat Res. 1995;29:789–792. [Google Scholar]
- Fuhrman JA, Azam F. Thymidine incorporation as a measure of heterotrophic bacterioplankton production in marine surface waters: evaluation and field results. Marine Biology. 1982;66:109–120. [Google Scholar]
- Hadfield MG. Biofilms and Marine Invertebrate Larvae: What Bacteria Produce That Larvae Use to Choose Settlement Sites. Annual Review of Marine Science. 2011;3(1):453–470. doi: 10.1146/annurev-marine-120709-142753. [DOI] [PubMed] [Google Scholar]
- Holm ER. Barnacles and Biofouling. Integrative and Comparative Biology. 2012;52(3):348–355. doi: 10.1093/icb/ics042. [DOI] [PubMed] [Google Scholar]
- Huang Y, Callahan S, Hadfield MG. Recruitment in the sea: bacterial genes required for inducing larval settlement in a polychaete worm. Sci Rep. 2012;2 doi: 10.1038/srep00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YL, Dobretsov S, Ki JS, Yang LH, Qian PY. Presence of Acyl-Homoserine Lactone in Subtidal Biofilm and the Implication in Larval Behavioral Response in the Polychaete Hydroides elegans. Microb Ecol. 2007 doi: 10.1007/s00248-007-9210-9. [DOI] [PubMed] [Google Scholar]
- Huggett MJ, Nedved BT, Hadfield MG. Effects of initial surface wettability on biofilm formation and subsequent settlement of Hydroides elegans. Biofouling. 2009;25(5):387–399. doi: 10.1080/08927010902823238. [DOI] [PubMed] [Google Scholar]
- Jefferson KK. What drives bacteria to produce a biofilm? Fems Microbiology Letters. 2004;236(2):163–173. doi: 10.1016/j.femsle.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Joint I, Tait K, Callow ME, Callow JA, Milton D, Williams P, Camara M. Cell-to-cell communication across the prokaryote-eukaryote boundary. Science. 2002;298:1207–1208. doi: 10.1126/science.1077075. [DOI] [PubMed] [Google Scholar]
- Jung SK, Trimarchi JT, Sanger RH, Smith PJS. Development and application of a self-referencing glucose microsensor for the measurement of glucose consumption by pancreatic ♌-cells. Anal Chem. 2001;73:3759–3767. doi: 10.1021/ac010247u. [DOI] [PubMed] [Google Scholar]
- Kinoshita H, Yoshida D, Miki K, Usui T, Ikeda T. An amperometric-enzymatic method for assays of inorganic phosphate and adenosine deaminase in serum based on the measurement of uric acid with a dialysis membrane-covered carbon electrode. Anal Chim Acta. 1995;303:301–307. [Google Scholar]
- Kirchman DL, Ducklow HW. Handbook of Methods in Microbial Ecology. Boca Raton: Lewis Publishers; 1993. Estimating conversion factors for the thymidine and leucine methods for measuring bacterial production. [Google Scholar]
- Larsson AI, Mattsson-Thorngren L, Granhag LM, Berglin M. Fouling-release of barnacles from a boat hull with comparison to laboratory data of attachment strength. Journal of Experimental Marine Biology and Ecology. 2010;392(1):107–114. [Google Scholar]
- Lejars M, Margaillan A, Bressy C. Fouling Release Coatings: A Nontoxic Alternative to Biocidal Antifouling Coatings. Chemical Reviews. 2012;112(8):4347–4390. doi: 10.1021/cr200350v. [DOI] [PubMed] [Google Scholar]
- Longnecker K, Lomas MW, Van Mooy BAS. Abundance and diversity of heterotrophic bacterial cells assimilating 33P-phosphate in the subtropical North Atlantic Ocean. Environ Microbiol. 2010;12:2773–2782. doi: 10.1111/j.1462-2920.2010.02247.x. [DOI] [PubMed] [Google Scholar]
- McDougald D, Rice SA, Barraud N, Steinberg PD, Kjelleberg S. Should we stay or should we go: mechanisms and ecological consequences for biofilm dispersal. Nature Reviews Microbiology. 2011;10:39–50. doi: 10.1038/nrmicro2695. [DOI] [PubMed] [Google Scholar]
- Middelburg JJ, Barranguet C, Boschker HTS, Herman PMJ, Moens T, Heip CHR. The fate of intertidal microphytobenthos carbon: an in situ 13C-labelling study. Limnol Oceanogr. 2000;45(6):1224–1234. [Google Scholar]
- Mieszkin S, Callow ME, Callow JA. Interactions between microbial biofilms and marine fouling algae: a mini review. Biofouling. 2013;29(9):1097–1113. doi: 10.1080/08927014.2013.828712. [DOI] [PubMed] [Google Scholar]
- Mieszkin S, Martin-Tanchereau P, Callow ME, Callow JA. Effect of bacterial biofilms formed on fouling-release coatings from natural seawater and Cobetia marina, on the adhesion of two marine algae. Biofouling. 2012;28(9):953–968. doi: 10.1080/08927014.2012.723696. [DOI] [PubMed] [Google Scholar]
- Molino PJ, Campbell E, Wetherbee R. Development of the initial diatom microfouling layer on antifouling and fouling-release surfaces in temperate and tropical Australia. Biofouling. 2009a;25(8):685–694. doi: 10.1080/08927010903089912. [DOI] [PubMed] [Google Scholar]
- Molino PJ, Childs S, Eason Hubbard MR, Carey JM, Burgman MA, Wetherbee R. Development of the primary bacterial microfouling layer on antifouling and fouling release coatings in temperate and tropical environments in Eastern Australia. Biofouling. 2009b;25(2):149–162. doi: 10.1080/08927010802592917. [DOI] [PubMed] [Google Scholar]
- Monds RD, O'Toole GA. The developmental model of microbial biofilms: ten years of a paradigm up for review. Trends in Microbiol. 2008;17:73–87. doi: 10.1016/j.tim.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Moons P, Michiels CW, Aertsen A. Bacterial interactions in biofilms. Critical Reviews in Microbiology. 2009;35(3):157–168. doi: 10.1080/10408410902809431. [DOI] [PubMed] [Google Scholar]
- Moriarty DJW, White DC, Wassenberg TJ. A Convenient Method for Measuring Rates of Phospholipid-Synthesis in Seawater and Sediments - Its Relevance to the Determination of Bacterial Productivity and the Disturbance Artifacts Introduced by Measurements. Journal of Microbiological Methods. 1985;3(5-6):321–330. [Google Scholar]
- Popendorf KJ, Lomas MW, Van Mooy BAS. Microbial sources of intact polar diacylglycerolipids in the western North Atlantic Ocean. Org Geochem. 2011;42:803–811. [Google Scholar]
- Popendorf KJ, Fredricks HF, Van Mooy BAS. Molecular-ion independent quantitation of intact polar diacylglycerolipids in marine plankton using triple quadrupole MS. Lipids. 2013;48:185–195. doi: 10.1007/s11745-012-3748-0. [DOI] [PubMed] [Google Scholar]
- Porterfield DM, Laskin JD, Jung SK, Malchow RP, Billack B, Smith PJS, Heck DE. Direct measurement of nitric oxide fluxes from macrophages using a novel self-referencing electrode. Am J Physiol. 2001;281:L904–L912. doi: 10.1152/ajplung.2001.281.4.L904. [DOI] [PubMed] [Google Scholar]
- Qian PY, Chen L, Xu Y. Mini-review: Molecular mechanisms of antifouling compounds. Biofouling. 2013;29(4):381–400. doi: 10.1080/08927014.2013.776546. [DOI] [PubMed] [Google Scholar]
- Salta M, Wharton JA, Blache Y, Stokes KR, Briand JF. Marine biofilms on artificial surfaces: structure and dynamics. Environmental Microbiology. 2013 doi: 10.1111/1462-2920.12186. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- Schultz MP. Effects of coating roughness and biofouling on ship resistance and powering. Biofouling. 2007;23:331–341. doi: 10.1080/08927010701461974. [DOI] [PubMed] [Google Scholar]
- Schultz MP, Bendick JA, Holm ER, Hertel WM. Economic impact of biofouling on a naval surface ship. Biofouling. 2011;27(1):87–98. doi: 10.1080/08927014.2010.542809. [DOI] [PubMed] [Google Scholar]
- Smith PJS, Sanger RS, Messerli MA. Methods and New Frontiers in Neuroscience. CRC Press; 2007. Principles, Development and Applications of Self-Referencing Electrochemical Microelectrodes to the Determination of Fluxes at Cell Membranes. [PubMed] [Google Scholar]
- Smith PJS, Hammar K, Porterfield DM, Sanger RH, Trimarchi JR. A self-referencing, non-invasive, ion selective electrode for single cell detection of trans-plasma membrane calcium flux. 1999;46:398–417. doi: 10.1002/(SICI)1097-0029(19990915)46:6<398::AID-JEMT8>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Sosik HM, Olson RJ, Neubert MG, Shalapyonok A, Solow AR. Growth rates of coastal phytoplankton from time-series measurements with a submersible flow cytometer. Limnol Oceanogr. 2003;48:1756–1765. [Google Scholar]
- Spivak AC, Canuel EA, Duffy J, Richardson J. Top-down and bottom-up controls on sediment organic matter composition in an experimental seagrass ecosystem. Limnology and Oceanography. 2007;52(6):2595–2607. [Google Scholar]
- Strickland JDH, Parsons TR. A practical handbook of seawater analysis. Bull Fish Res Bd Can 167 1972 [Google Scholar]
- Swain GW. The importance of ship hull coatings and maintenence as drivers for environmental sustainibility. Quat J Ship Hull Perfor. 2011;1:48–56. [Google Scholar]
- Thiyagarajan V. A review on the role of chemical cues in habitat selection by barnacles: New insights from larval proteomics. Journal of Experimental Marine Biology and Ecology. 2010;392(1-2):22–36. [Google Scholar]
- Thiyagarajan V, Wong T, Qian PY. 2D Gel-Based Proteome and Phosphoproteome Analysis During Larval Metamorphosis in Two Major Marine Biofouling Invertebrates. Journal of Proteome Research. 2009;8(6):2708–2719. doi: 10.1021/pr800976u. [DOI] [PubMed] [Google Scholar]
- Thomas T, Evans FF, Schleheck D, Mai-Prochnow A, Burke C, Penesyan A, Dalisay DS, Stelzer-Braid S, Saunders N, Johnson J, et al. Analysis of the Pseudoalteromonas tunicata Genome Reveals Properties of a Surface-Associated Life Style in the Marine Environment. PLos One. 2008;3(9):e3252. doi: 10.1371/journal.pone.0003252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribou M, Swain G. The use of proactive in-water grooming to improve the performance of ship hull antifouling coatings. Biofouling. 2009;26(1):47–56. doi: 10.1080/08927010903290973. [DOI] [PubMed] [Google Scholar]
- Turner JT, Borkman DG, Lincoln JA, Gauthier DA, Petitpas CM. Plankton studies in Buzzards Bay, Massachusetts, USA. VI. Phytoplankton and water quality, 1987 to 1998. Mar Ecol Prog Ser. 2009;376:103–122. [Google Scholar]
- Ungerer J, Oosthuizen HM, Bissbort SH. An enzymatic assay of inorganic phosphate in serum using nucleoside phosphorylase and xanthine oxidase. Clinica Chimica Acta. 1993;223:149–157. doi: 10.1016/0009-8981(93)90071-b. [DOI] [PubMed] [Google Scholar]
- Van Mooy BAS, Fredricks HF. Bacterial and eukaryotic intact polar lipids in the eastern subtropical South Pacific: water-column distribution, planktonic sources, and fatty acid composition. Geochimica et Cosmochimica Acta. 2010;74:6499–6516. [Google Scholar]
- Van Mooy BAS, Rocap G, Fredricks HF, Evans CT, Devol AH. Sulfolipids dramatically decrease phosphorus demand by picocyanobacteria in oligotrophic marine environments. Proc Natl Acad Sci. 2006;103:8607–8612. doi: 10.1073/pnas.0600540103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Mooy BAS, Moutin T, Duhamel S, Rimmelin P, Van Wambeke F. Phospholipid synthesis rates in the eastern subtropical South Pacific Ocean. Biogeosciences. 2008;5:133–139. [Google Scholar]
- Van Mooy BAS, Fredricks HF, Pedler BE, Dyhrman ST, Karl DM, Lomas MW, Mincer T, Moore LR, Moutin T, Rappé MS, et al. Phytoplankton in the ocean use non-phosphorus lipids in response to phosphorus scarcity. Nature. 2009;458:69–72. doi: 10.1038/nature07659. [DOI] [PubMed] [Google Scholar]
- White DC, Tucker AN. Phospholipid metabolism during bacterial growth. Journal of Lipid Research. 1969;10(2):220–233. [PubMed] [Google Scholar]
- White DC, Davis WM, Nickels JS, King JD, Bobbie RJ. Determination of the sedimentary microbial biomass by extractable lipid phosphate. Oecologia. 1979;40:51–62. doi: 10.1007/BF00388810. [DOI] [PubMed] [Google Scholar]
- WHOI. Woods Hole Oceanographic Institution. Annapolis, MD: United States Naval Institute; 1952. Marine fouling and its prevention. [Google Scholar]
- Zargiel KA, Coogan JS, Swain GW. Diatom community structure on commercially available ship hull coatings. Biofouling. 2011;27(9):955–965. doi: 10.1080/08927014.2011.618268. [DOI] [PubMed] [Google Scholar]