Abstract
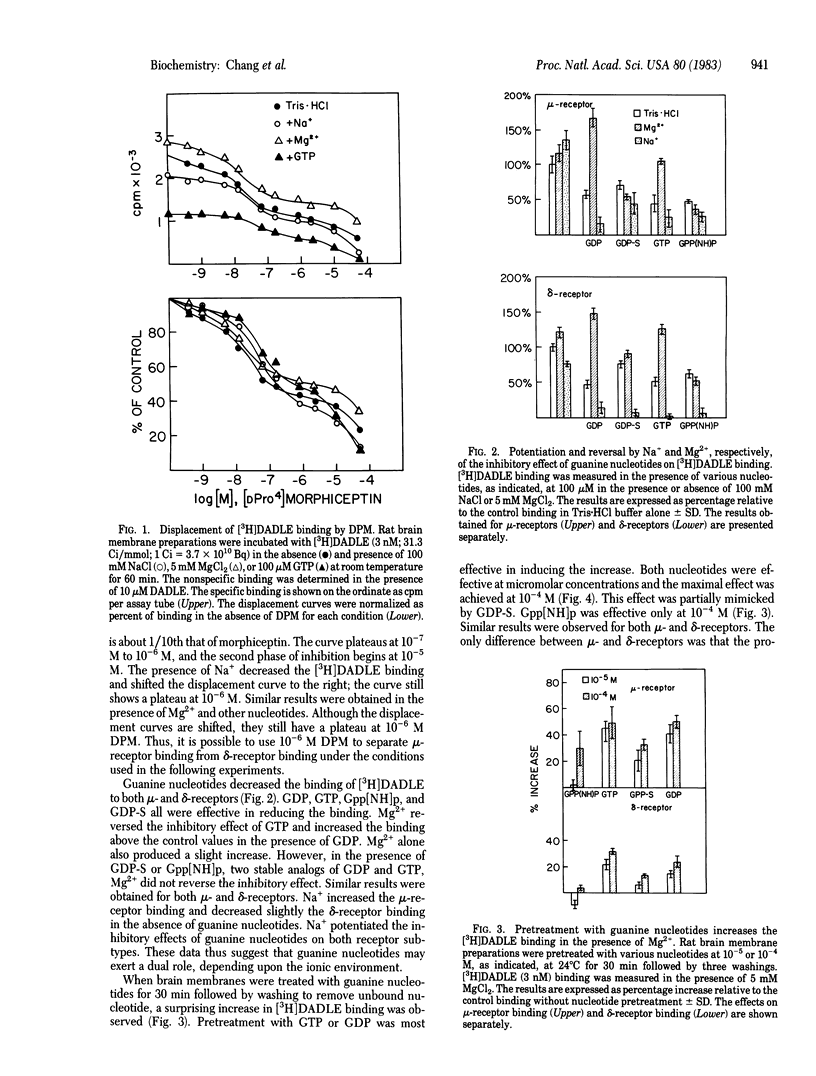
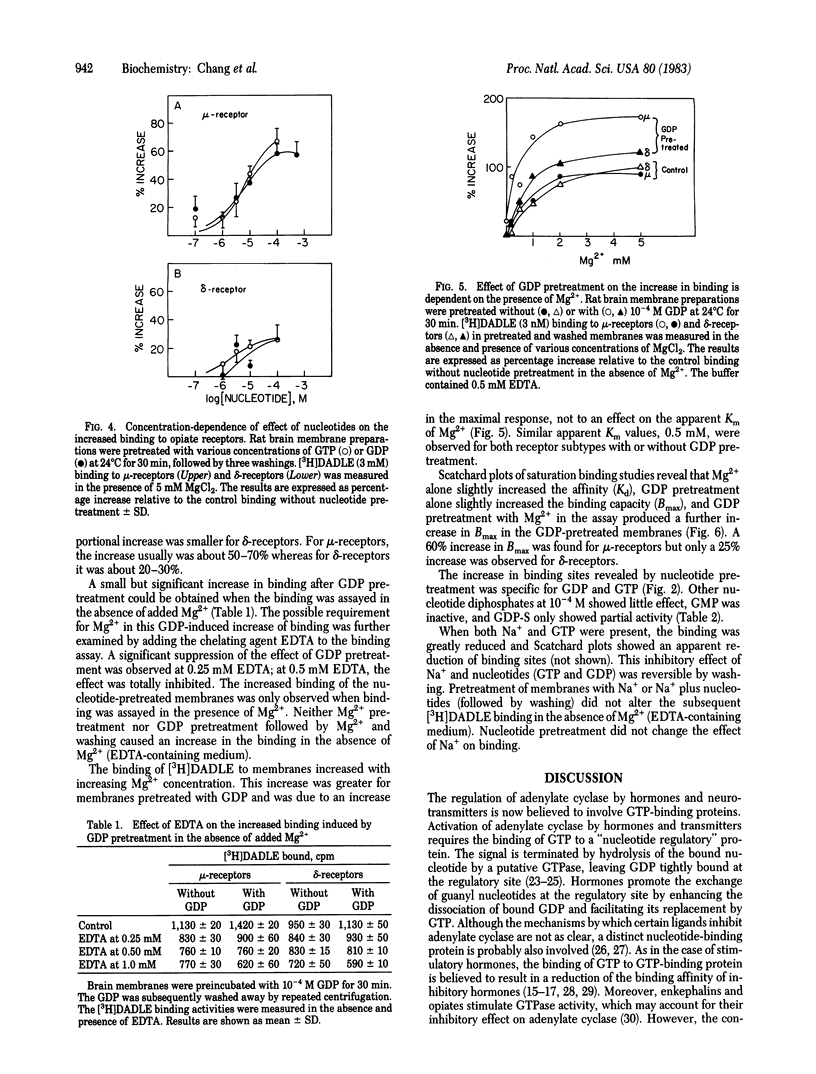
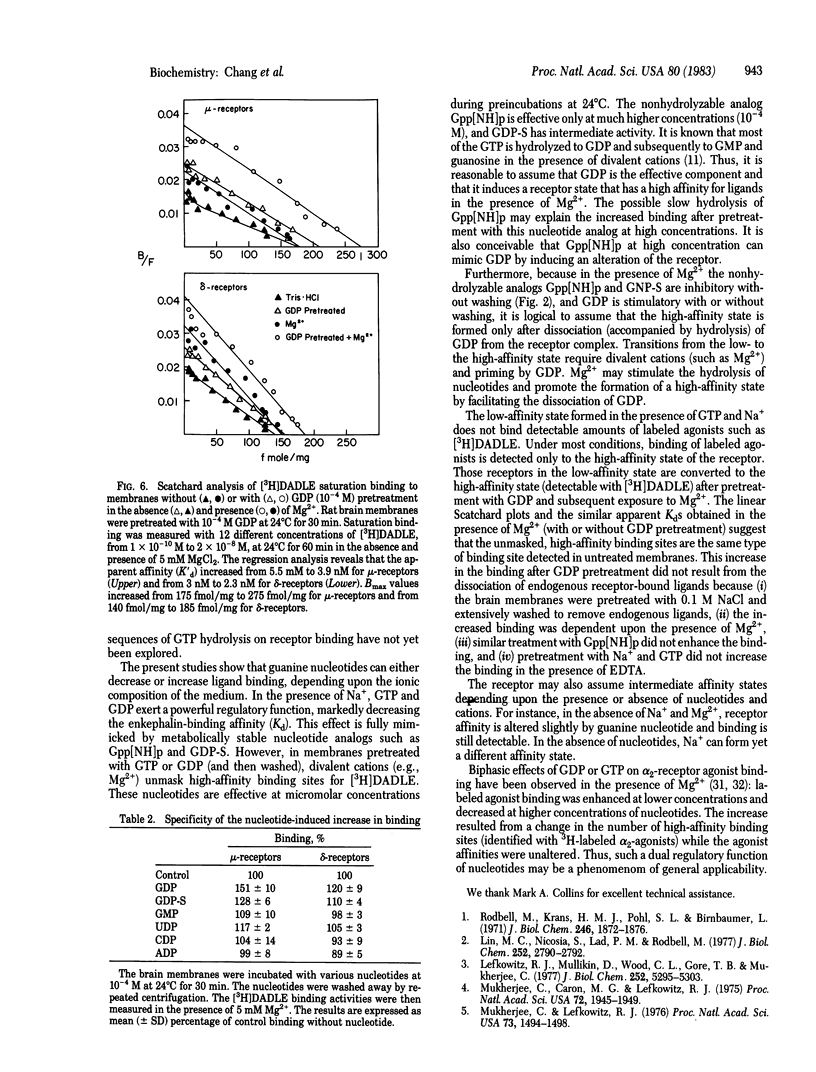
The regulation of mu- and delta-opiate receptors by guanine nucleotides and cations was studied by examining the binding of [3H][DAla2, DLeu5]enkephalin to rat brain membranes. The binding to mu-opiate receptors could be suppressed by 1 microM [DPro4]morphiceptin, a highly specific mu-agonist, thus permitting separate assessment of delta-opiate receptor binding. GTP, GDP, and the nonhydrolyzable analogs 5'-guanylyl imidodiphosphate (Gpp[NH]p) and guanosine 5'-O-(2-thiodiphosphate) (GDP-S) effectively decreased the binding to both receptor types. This inhibitory effect was potentiated by Na+. The inhibitory effect of GTP and GDP, but not of the nonhydrolyzable analogs, was reversed by Mg2+. Pretreatment of membranes with GDP or GTP increased substantially the subsequently measured high-affinity binding, and this effect required the presence of Mg2+ in the binding assay. Similar pretreatment with GDP-S resulted in only a partial increase of binding compared to GTP or GDP, and Gpp[NH]p was relatively ineffective. Similar results were observed for both mu- and delta-receptors, although the effects on mu-receptors were quantitatively more profound. These data suggest that guanine nucleotides play a dual function in regulating opiate receptor binding in a manner dependent upon the presence of Na+ or Mg2+.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blume A. J., Foster C. J. Neuroblastoma adenylate cyclase. Role of 2-chloroadenosine, prostaglandin E1, and guanine nucleotides in regulation of activity. J Biol Chem. 1976 Jun 10;251(11):3399–3404. [PubMed] [Google Scholar]
- Blume A. J. Interaction of ligands with the opiate receptors of brain membranes: regulation by ions and nucleotides. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1713–1717. doi: 10.1073/pnas.75.4.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume A. J. Opiate binding to membrane preparations of neuroblastoma x glioma hybrid cells NG108-15: effects of ions and nucleotides. Life Sci. 1978 May 22;22(20):1843–1852. doi: 10.1016/0024-3205(78)90602-1. [DOI] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Catecholamine-stimulated GTPase activity in turkey erythrocyte membranes. Biochim Biophys Acta. 1976 Dec 8;452(2):538–551. doi: 10.1016/0005-2744(76)90206-0. [DOI] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Mechanism of adenylate cyclase activation by cholera toxin: inhibition of GTP hydrolysis at the regulatory site. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3307–3311. doi: 10.1073/pnas.74.8.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Mechanism of adenylate cyclase activation through the beta-adrenergic receptor: catecholamine-induced displacement of bound GDP by GTP. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4155–4159. doi: 10.1073/pnas.75.9.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. J., Cuatrecasas P. Heterogeneity and properties of opiate receptors. Fed Proc. 1981 Nov;40(13):2729–2734. [PubMed] [Google Scholar]
- Chang K. J., Cuatrecasas P. Multiple opiate receptors. Enkephalins and morphine bind to receptors of different specificity. J Biol Chem. 1979 Apr 25;254(8):2610–2618. [PubMed] [Google Scholar]
- Chang K. J., Hazum E., Cuatrecasas P. Novel opiate binding sites selective for benzomorphan drugs. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4141–4145. doi: 10.1073/pnas.78.7.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. J., Lillian A., Hazum E., Cuatrecasas P., Chang J. K. Morphiceptin (NH4-tyr-pro-phe-pro-COHN2): a potent and specific agonist for morphine (mu) receptors. Science. 1981 Apr 3;212(4490):75–77. doi: 10.1126/science.6259732. [DOI] [PubMed] [Google Scholar]
- Childers S. R., Snyder S. H. Differential regulation by guanine nucleotides or opiate agonist and antagonist receptor interactions. J Neurochem. 1980 Mar;34(3):583–593. doi: 10.1111/j.1471-4159.1980.tb11184.x. [DOI] [PubMed] [Google Scholar]
- Childers S. R., Snyder S. H. Guanine nucleotides differentiate agonist and antagonist interactions with opiate receptors. Life Sci. 1978 Aug 21;23(7):759–761. doi: 10.1016/0024-3205(78)90077-2. [DOI] [PubMed] [Google Scholar]
- Creese I., Pasternak G. W., Pert C. B., Snyder S. H. Discrimination by temperature of opiate agonist and antagonist receptor binding. Life Sci. 1975 Jun 15;16(12):1837–1842. doi: 10.1016/0024-3205(75)90287-8. [DOI] [PubMed] [Google Scholar]
- Jakobs K. H. Inhibition of adenylate cyclase by hormones and neurotransmitters. Mol Cell Endocrinol. 1979 Dec;16(3):147–156. doi: 10.1016/0303-7207(79)90023-6. [DOI] [PubMed] [Google Scholar]
- Jakobs K. H., Lasch P., Minuth M., Aktories K., Schultz G. Uncoupling of alpha-adrenoceptor-mediated inhibition of human platelet adenylate cyclase by N-ethylmaleimide. J Biol Chem. 1982 Mar 25;257(6):2829–2833. [PubMed] [Google Scholar]
- Kahn D. J., Mitrius J. C., U'Prichard D. C. Alpha 2-adrenergic receptors in neuroblastoma x glioma hybrid cells. Characterization with agonist and antagonist radioligands and relationship to adenylate cyclase. Mol Pharmacol. 1982 Jan;21(1):17–26. [PubMed] [Google Scholar]
- Koski G., Klee W. A. Opiates inhibit adenylate cyclase by stimulating GTP hydrolysis. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4185–4189. doi: 10.1073/pnas.78.7.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lefkowitz R. J., Mullikin D., Wood C. L., Gore T. B., Mukherjee C. Regulation of prostaglandin receptors by prostaglandins and guanine nucleotides in frog erythrocytes. J Biol Chem. 1977 Aug 10;252(15):5295–5303. [PubMed] [Google Scholar]
- Lefkowitz R. J., Williams L. T. Catecholamine binding to the beta-adrenergic receptor. Proc Natl Acad Sci U S A. 1977 Feb;74(2):515–519. doi: 10.1073/pnas.74.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitzki A., Sevilla N., Steer M. L. The regulatory control of beta-receptor dependent adenylate cyclase. J Supramol Struct. 1976;4(3):405–418. doi: 10.1002/jss.400040311. [DOI] [PubMed] [Google Scholar]
- Limbird L. E., Speck J. L., Smith S. K. Sodium ion modulates agonist and antagonist interactions with the human platelet alpha 2-adrenergic receptor in membrane and solubilized preparations. Mol Pharmacol. 1982 May;21(3):609–617. [PubMed] [Google Scholar]
- Lin M. C., Nicosia S., Lad P. M., Rodbell M. Effects of GTP on binding of (3H) glucagon to receptors in rat hepatic plasma membranes. J Biol Chem. 1977 Apr 25;252(8):2790–2792. [PubMed] [Google Scholar]
- Lucas M., Bockaert J. Use of (-)-[3H]dihydroalprenolol to study beta adrenergic receptor-adenylate cyclase coupling in C6 glioma cells: role of 5'-guanylylimidodiphosphate. Mol Pharmacol. 1977 Mar;13(2):314–329. [PubMed] [Google Scholar]
- Mukherjee C., Caron M. G., Lefkowitz R. J. Catecholamine-induced subsensitivity of adenylate cyclase associated with loss of beta-adrenergic receptor binding sites. Proc Natl Acad Sci U S A. 1975 May;72(5):1945–1949. doi: 10.1073/pnas.72.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee C., Lefkowitz R. J. Desensitization of beta-adrenergic receptors by beta-adrenergic agonists in a cell-free system: resensitization by guanosine 5'-(beta, gamma-imino)triphosphate and other purine nucleotides. Proc Natl Acad Sci U S A. 1976 May;73(5):1494–1498. doi: 10.1073/pnas.73.5.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodbell M., Krans H. M., Pohl S. L., Birnbaumer L. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. IV. Effects of guanylnucleotides on binding of 125I-glucagon. J Biol Chem. 1971 Mar 25;246(6):1872–1876. [PubMed] [Google Scholar]
- Rodbell M., Lin M. C., Salomon Y., Londos C., Harwood J. P., Martin B. R., Rendell M., Berman M. Role of adenine and guanine nucleotides in the activity and response of adenylate cyclase systems to hormones: evidence for multisite transition states. Adv Cyclic Nucleotide Res. 1975;5:3–29. [PubMed] [Google Scholar]
- Smith S. K., Limbird L. E. Evidence that human platelet alpha-adrenergic receptors coupled to inhibition of adenylate cyclase are not associated with the subunit of adenylate cyclase ADP-ribosylated by cholera toxin. J Biol Chem. 1982 Sep 10;257(17):10471–10478. [PubMed] [Google Scholar]
- U'Prichard D. C., Snyder S. H. Guanyl nucleotide influences on 3H-ligand binding to alpha-noradrenergic receptors in calf brain membranes. J Biol Chem. 1978 May 25;253(10):3444–3452. [PubMed] [Google Scholar]
- U'Prichard D. C., Snyder S. H. Interactions of divalent cations and guanine nucleotides at alpha 2-noradrenergic receptor binding sites in bovine brain mechanisms. J Neurochem. 1980 Feb;34(2):385–394. doi: 10.1111/j.1471-4159.1980.tb06608.x. [DOI] [PubMed] [Google Scholar]
- Zahniser N. R., Molinoff P. B. Effect of guanine nucleotides on striatal dopamine receptors. Nature. 1978 Oct 5;275(5679):453–455. doi: 10.1038/275453a0. [DOI] [PubMed] [Google Scholar]


