Abstract
The neurotrophin receptor p75NTR has been implicated in mediating neuronal apoptosis after injury to the CNS. Despite its frequent induction in pathologic states, there is limited understanding of the mechanisms that regulate p75NTR expression after injury. Here, we show that after focal cerebral ischemia in vivo or oxygen–glucose deprivation in organotypic hippocampal slices or neurons, p75NTR is rapidly induced. A concomitant induction of proNGF, a ligand for p75NTR, is also observed. Induction of this ligand/receptor system is pathologically relevant, as a decrease in apoptosis, after oxygen–glucose deprivation, is observed in hippocampal neurons or slices after delivery of function-blocking antibodies to p75NTR or proNGF and in p75NTR and ngf haploinsufficient slices. Furthermore, a significant decrease in infarct volume was noted in p75NTR−/− mice compared with the wild type. We also investigated the regulatory mechanisms that lead to post-ischemic induction of p75NTR. We demonstrate that induction of p75NTR after ischemic injury is independent of transcription but requires active translation. Basal levels of p75NTR in neurons are maintained in part by the expression of microRNA miR-592, and an inverse correlation is seen between miR-592 and p75NTR levels in the adult brain. After cerebral ischemia, miR-592 levels fall, with a corresponding increase in p75NTR levels. Importantly, overexpression of miR-592 in neurons decreases the level of ischemic injury-induced p75NTR and attenuates activation of pro-apoptotic signaling and cell death. These results identify miR-592 as a key regulator of p75NTR expression and point to a potential therapeutic candidate to limit neuronal apoptosis after ischemic injury.
Keywords: ischemia, microRNA, neurotrophin, NGF, p75NTR, stroke
Introduction
The receptor p75NTR is a member of the tumor necrosis factor (TNF) receptor superfamily. p75NTR binds to mature and precursor neurotrophins (proneurotrophins) and regulates numerous functions, including neuronal differentiation, neurite outgrowth, and cell death (Lee et al., 2001; Roux and Barker, 2002). p75NTR is expressed at high levels during development and in the perinatal period, time frames that coincide with programmed cell death and axonal pruning in the developing nervous system (Singh et al., 2008; Yang et al., 2009). p75NTR expression gradually decreases to reach its lowest levels in adulthood but is upregulated after injury or with disease, such as Alzheimer's disease, corticospinal axotomy, seizures, and hypo-osmolar stress (Fahnestock et al., 2001; Harrington et al., 2004; Volosin et al., 2006; Ramos et al., 2007). Reexpression of p75NTR after injury correlates with cell death, frequently mediated by proNGF, highlighting the pathologic role of this ligand/receptor system (Fahnestock et al., 2001; Harrington et al., 2004; Volosin et al., 2006). Previous studies have noted the incidental induction of p75NTR in neurons after brain ischemia; however, its specific role in ischemic injury and the identity of the p75NTR ligand mediating potential effects are unknown (Park et al., 2000; Jover et al., 2002).
Despite numerous models documenting that injury-induced p75NTR expression correlates with neuronal apoptosis, little is known about the regulatory mechanisms that restrict p75NTR expression in adulthood, or which lead to rapid upregulation after CNS injury. The p75NTR promoter resembles a housekeeping gene, with high CG content and multiple Sp1-binding sites, and recent studies have documented transcriptional induction of p75NTR after hypo-osmolar stress, using a Sp1-dependent mechanism (Carroll et al., 1995; Huber and Chao, 1995; Ramos et al., 2007). Although it is not known whether p75NTR is regulated post-transcriptionally, a prior report has shown that serial truncation of the 3′-untranslated region (UTR) of p75NTR mRNA leads to increased expression of p75NTR, implicating the regulation of p75NTR at its 3′UTR (Krygier and Djakiew, 2001). Regulation at the 3′UTR of p75NTR could be modulated by noncoding RNAs, such as microRNAs (miRNAs), that control protein translation by binding to the complementary seed sequences in the UTRs of their target mRNAs (Bartel, 2004). Since miRNAs are predicted to target ∼30% of all human genes and ischemic injury induces marked changes in the cerebral microRNAome, we considered whether miRNAs participate in the regulation of p75NTR after ischemia (Lewis et al., 2005; Jeyaseelan et al., 2008; Tan et al., 2011).
Here, we used experimental models of cerebral ischemia in vitro and in vivo to explore the regulation and consequences of ischemic induction of p75NTR. We observe that p75NTR and its ligand proNGF are rapidly induced in the postischemic neocortex and that the resulting activation of the proNGF-p75NTR pathway leads to cell death. We also identify a previously unrecognized post-transcriptional mechanism of p75NTR regulation through a microRNA, thus suggesting an approach to limit the postischemic induction of p75NTR and its pro-apoptotic actions.
Materials and Methods
Middle cerebral artery occlusion.
All procedures were performed in accordance with institutional and federal guidelines for the treatment of animals. Focal cerebral ischemia was induced using the intraluminal filament model of middle cerebral artery occlusion (MCAO), as described previously (Abe et al., 2010; Jackman et al., 2011; Hochrainer et al., 2012). All experiments were performed in male C57BL/6 mice (6–8 weeks) or p75NTR−/− mice, which were purchased from the Jackson Laboratory. Briefly, mice were anesthetized with a mixture of isoflurane (1.5–2%), oxygen, and nitrogen, and rectal temperature was monitored and maintained at ∼37°C. A heat-blunted 6-0 nylon suture was inserted into the right external carotid artery and advanced along the internal carotid artery until it obstructed the middle cerebral artery (MCA). The right common carotid artery was simultaneously ligated. Cerebral blood flow was monitored in the center of the ischemic territory using transcranial laser Doppler flowmetry (Periflux System 5010; Perimed) during the surgery, and only mice with >85% flow reduction were included in this study. The filament remained in place for 35 min and was then retracted, inducing reperfusion for transient, or left in position for permanent MCAO. For sham MCAO, mice were anesthetized, and vessels were visualized and cleared of connective tissue, but no further manipulations were made. Mice were allowed to recover. Infarct volume (in cubic millimeters) was quantified 72 h after transient MCAO in cresyl violet-stained coronal brain sections obtained throughout the infarcted territory, as described previously (Jackman et al., 2011). Infarct volume data were corrected for swelling. In other studies, mice were killed after 8 h of permanent MCAO or sham surgery, and two adjacent 1 mm slices of brain tissue were isolated within the MCA territory (approximately +1.2 to −0.8 mm bregma) using a brain matrix. Slices were subsequently dissected into ipsilateral (damaged) and contralateral cortices.
Organotypic hippocampal slice cultures.
Organotypic slice cultures from hippocampus were prepared according to the technique described previously (Stoppini et al., 1991). In brief, C57BL/6 mice at postnatal day 6 (P6) were decapitated, the brain was removed, and hippocampi were isolated and cut into transverse slices of 500 μm thickness on a McIlwain tissue chopper (Vibratome). The slices were then cultured on membrane culture inserts (Millicell CM; pore size, 0.4 μm; Millipore) in six-well culture plates with 1 ml slice culture media consisting of 50% minimum essential medium (MEM; with 25 mm HEPES modification), 25% Eagle's basal medium, 25% heat-inactivated horse serum, 2 mm glutamine, and 0.65% glucose (Sigma-Aldrich), pH 7.2, per well. All reagents were purchased from (Invitrogen) unless specified. Cultures were maintained for 14 d at 37°C in a humidified atmosphere with 5% CO2, and media were replaced every third day.
Primary hippocampal cultures.
Briefly, hippocampi were dissected from embryonic day 18 (E18) Sprague Dawley rat embryos, cells were dissociated with 0.05% trypsin, and the cell suspension was plated and maintained in culture for 5–7 d in vitro (DIV5–7) on poly-d-lysine-coated dishes in Neurobasal medium with B27 supplement penicillin (100 U/ml), streptomycin (0.1 mg/ml), and 1 mm l-glutamine. Mitotic inhibitor 5-fluoro-2-deoxyuridine (Sigma-Aldrich) was added to the medium (10 μm) at DIV2, and the medium was half-changed at DIV4. Amaxa nucleofection with control or microRNA coding constructs was performed with the Amaxa Rat Neuron Nucleofector kit (Lonza). Briefly, 3–5 million E18 rat primary neurons were electroporated on DIV0 with 3 μg of indicated plasmid (described below) using the predefined program O-003 of the Nucleofector II (Lonza), according to the manufacturer's instructions. Nucleofected neurons were plated in medium containing MEM supplemented with 45% glucose (Sigma-Aldrich), 1% sodium pyruvate, penicillin–streptomycin (0.1 mg/ml), and 0.05 mm l-glutamine. The medium was completely changed 2 h after transfection with Neurobasal medium consisting of B27 supplement penicillin (100 U/ml), penicillin–streptomycin (0.1 mg/ml), and 1 mm l-glutamine; maintained in culture until DIV5; subjected to experimental analysis; and harvested for protein, RNA, or TUNEL assay. All reagents were obtained from Invitrogen unless mentioned otherwise.
For function-blocking studies, anti-p75NTR serum REX (Weskamp and Reichardt, 1991) or AB1554 [Millipore (Gehler et al., 2004)] and nonimmune rabbit IgG (Millipore) were used at a dilution of 1:100. A well characterized function-blocking antibody against the pro-domain of proNGF (Beattie et al., 2002; Harrington et al., 2004) was used to block proNGF activity at a concentration of 25 μg/ml in hippocampal slices.
Oxygen glucose deprivation.
Slices were first rinsed twice with warm oxygen glucose deprivation (OGD) medium (in mm: 125 NaCl, 5 KCl, 1.2 Na2PO4, 26 NaHCO3, 1.8 CaCl2, 0.9 MgCl2, and 10 HEPES, pH 7.4) and incubated in the same buffer for 10 min. The slices were then transferred to an OGD chamber (Billups-Rothenberg) and flushed with anoxic gas (95% N2 and 5% CO2) at 21 liters/min for 8 min. The chamber was then sealed and placed in an incubator maintained at 37°C for 30 min. Controls were rinsed with OGD buffer supplemented with 10 mm glucose and incubated under normoxic conditions. Primary hippocampal cultures were similarly subjected to OGD with incubation time prolonged to 1 h. At the end of OGD, slices and primary neurons were washed in normal culture medium and incubated under normal culture conditions until the time of harvest. All reagents were obtained from Sigma-Aldrich unless mentioned otherwise.
Western blot.
Tissue was lysed using radioimmunoprecipitation buffer (50 mm Tris HCl, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1% NP-40, 0.5% deoxycholate, and 0.1% SDS, pH 8.0) supplemented with protease inhibitor mixture (Sigma-Aldrich). After a 15 min centrifugation step at 4°C, the protein concentration of the supernatant was determined using a protein assay kit (Bio-Rad) as per the manufacturer's instructions. Proteins were separated by SDS-PAGE and transferred to a polyvinylidene fluoride membrane (Millipore). For blotting, membranes were blocked in 5% skim milk and 0.05% Tween 20 (Bio-Rad) in Tris-buffered saline (TBS) for 1 h at room temperature. Membranes were then incubated with the respective primary antibody for 1 h at room temperature or overnight at 4°C, and then with the secondary antibody for 1 h at room temperature. Three washes were performed with 0.05% Tween 20 in PBS after both incubations. Primary antibodies were diluted in 5% skim milk or 4% bovine serum albumin and 0.05% Tween 20 in TBS as follows: rabbit anti-proNGF (Harrington et al., 2004), rabbit anti-p75NTR (9992, antisera against the p75NTR intracellular domain), rabbit anti-p75NTR (R&D Systems, against p75NTR ectodomain), rabbit anti-furin (Santa Cruz Biotechnology), rabbit anti-NGF (Santa Cruz Biotechnology), rabbit anti-cleaved caspase-3 (Cell Signaling), mouse anti-β-actin (Sigma-Aldrich), rabbit anti-ERK (Santa Cruz Biotechnology), and mouse anti-tubulin (Sigma-Aldrich).
Immunohistochemistry and immunofluorescence.
Ten-micrometer cryostat sections of PFA-fixed brain tissue were rehydrated in PBS and incubated with a blocking solution consisting of 5% serum of the host of secondary antibody/0.1% Triton X-100 in PBS. The sections were then incubated with the primary antibody against proNGF (pro-domain specific, 1:100; Harrington et al., 2004) overnight at 4°C. After incubation with biotinylated secondary antibodies, immunoreactivity was detected using the VIP Peroxidase Substrate kit as per the manufacturer's instructions (Vector Laboratories).
For hippocampal slices and primary neurons, TUNEL assay was performed with the DeadEnd Fluorometric TUNEL system (Promega) as per the manufacturer's instructions. For hippocampal slices, the reaction mix was incubated overnight at 4°C to allow for better penetration.
Image acquisition for primary neurons and tissue sections was done with Axio Observer.Z1 (Carl Zeiss). For hippocampal slices, all images were obtained under identical settings with a 20 × objective on an LSM 700 laser-scanning confocal microscope (Carl Zeiss). Quantification for staining was done with ImageJ software.
RT-PCR.
Total RNA was isolated from primary hippocampal neurons or brain tissue in Trizol (Invitrogen). Reverse transcription reactions were performed using the Superscript First Strand cDNA synthesis system (Invitrogen) according to manufacturer's protocol. cDNA was amplified for 40 cycles using Taqman gene expression assays (Applied Biosystems) for p75NTR and 18S. All reactions were done in triplicate and normalized to the housekeeping gene 18S. For microRNA analysis, small RNAs were extracted using the RNA aqueous kit (Ambion/Invitrogen) as per the manufacturer's instructions. cDNA was synthesized using microRNA-specific primers (Taqman Microrna Assays) as per the manufacturer's protocol. Amplification and data acquisition were done with an ABI 7900 HT Fast RT-PCR system (Applied Biosystems) with microRNA-specific primers (Applied Biosystems). Analysis of the data was done with ABI 7700 Prism SDS software. For quantification of microRNA levels, all reactions were done in triplicate and normalized to endogenous small RNA U6.
Precursor miRNA and antagomiR vectors.
Vectors (pEZX-MR03) expressing the precursor miR-592 under a CMV promoter or a control vector were obtained from Genecopiea. Lentiviral plasmids (pEZX-MR03 and pEZX-AM03) expressing miR-592 and antagomiR-592, respectively, with their control vectors were purchased from Genecopiea.
Lentiviral generation and transduction.
Lentivirus was packaged by cotransfection of shRNA vectors with second-generation packaging plasmids pMD2.G and pCMV-dR8.9 using endofectin (Genecopiea) into HEK293-FT (ATCC) cells. The medium was changed 24 h later, and the 48 h supernatant was filtered through a 0.45 μm filter and pelleted by centrifugation with PEG-IT (System Biosciences). Viral pellets were resuspended in Neurobasal medium. Primary hippocampal neurons were infected with lentiviruses on DIV0, and the medium was changed the following day. Neurons were harvested for analysis on DIV5–7.
Target gene 3′UTR luciferase assays.
The 3′UTR of p75NTR from the rat hippocampus was amplified using the following primers: forward, 5′ GCGCGGCCGCACTCACACAGGACTGGGAGCCCCT 3′; and reverse, 5′ GCCTCGAGTTGCTATTTAATGGTCATCATG 3′. The ∼2000 bp PCR fragment containing the 3′UTR of p75NTR was ligated into the dual luciferase reporter vector psiCheck2 (Promega). The p75NTR 3′UTR was preceded by a renilla luciferase reporter gene, and the vector also contained a separate cassette of the Firefly luciferase gene serving as an endogenous transfection control. The control vector contained only the two luciferase genes. 293T cells (2 × 105) were plated in a 24-well plate and transfected 24 h later with the 3′UTR target or control plasmid (10 ng/well), with 10- to 50-fold excess, of pre-miRNA-592 or a control Scramble pre-miRNA plasmid (Genecopiea), using Lipofectamine 2000 (Invitrogen). Twenty-four hours after transfection, cells were lysed, and luciferase activities were measured with a Synergy 4 Luminometer (Biotek) using the Dual-Luciferase Assay kit (Promega) according to the manufacturer's directions. Mutations in the 3′UTR were introduced using the Quikchange II mutagenesis kit (Agilent) using the following primers: mutation (M1) 1 forward, 5′GCAAAAGAGCAGACTGGTGTGAGAAGCAAAGAAAAAGCAGATGCTGG 3′; reverse, 5′ CCAGCATCTGCTTTTTCTTTGCTTCTCACACCAGTCTGCTCTTTTGC 3′; mutation (M2) 2 forward, 5′ AAAAGAGCAGACTGGTGTGAGACCGAAGGAAAAAGCAGATGCTGGCCCTG 3′; reverse, 5′ CAGGGCCAGCATCTGCTTTTTCTTCGGTCTCACACCAGTCTGCTCTTTT 3′.
Statistics.
Calculations were performed in Excel (Microsoft) and graphed using Prism version 5.0 (GraphPad Software). Data in the text and figures are expressed as mean ± SEM. Two group comparisons were evaluated by the Student's t test and multiple comparisons by one- or two-way ANOVA, as indicated. The p values <0.05 were considered statistically significant.
Results
p75NTR is increased after brain ischemia
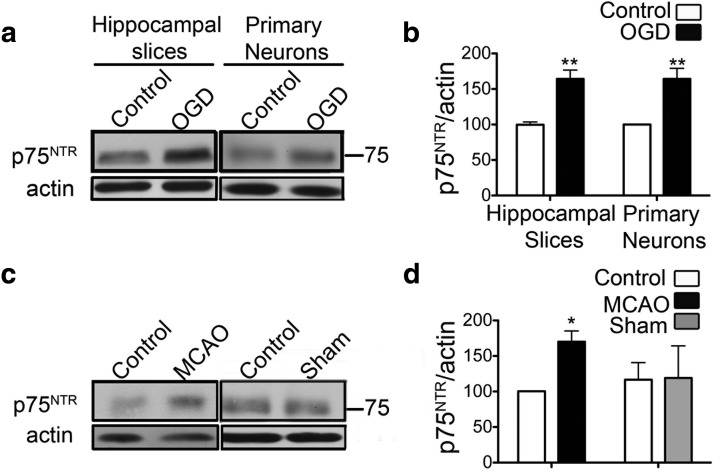
To determine whether ischemia elicits changes in the levels of p75NTR, we used oxygen and glucose deprivation in organotypic hippocampal slice cultures, a well characterized in vitro model of ischemia (Noraberg et al., 2005). Hippocampal slices were subjected to OGD for 30 min, followed by exposure to normal oxygen and glucose conditions for 8 h. At this time, analysis of hippocampal lysates demonstrated elevated levels of p75NTR when compared with control slices (Fig. 1a,b). A similar result was observed in primary hippocampal neurons subjected to 60 min of OGD (Fig. 1a,b).
Figure 1.
p75NTR is increased after ischemia. a, Organotypic hippocampal slice cultures and primary hippocampal neurons subjected to OGD show increased levels of p75NTR at 8 h after OGD. b, Quantification of p75NTR protein normalized to β-actin from a (n = 3). c, Increased levels of p75NTR are similarly observed in the cortex of mice subjected to permanent MCAO. d, Quantification of p75NTR protein normalized to β-actin from c (n = 3). All statistical analyses were done by the Student's t test. *p < 0.05; **p < 0.01. Error bars indicate SEM.
To determine whether the increase in p75NTR also occurred after cerebral ischemia in vivo, we examined p75NTR levels in the brains of male C57BL/6 mice subjected to permanent MCAO or sham surgery. As the cortex is more susceptible than the hippocampus to MCAO injury, cortical samples from the ischemic hemisphere and the contralateral control hemisphere were analyzed. Immunoblots demonstrate elevated levels of p75NTR in the ischemic hemisphere at 8 h after occlusion, whereas sham surgery did not affect the levels of p75NTR (Fig. 1c,d).
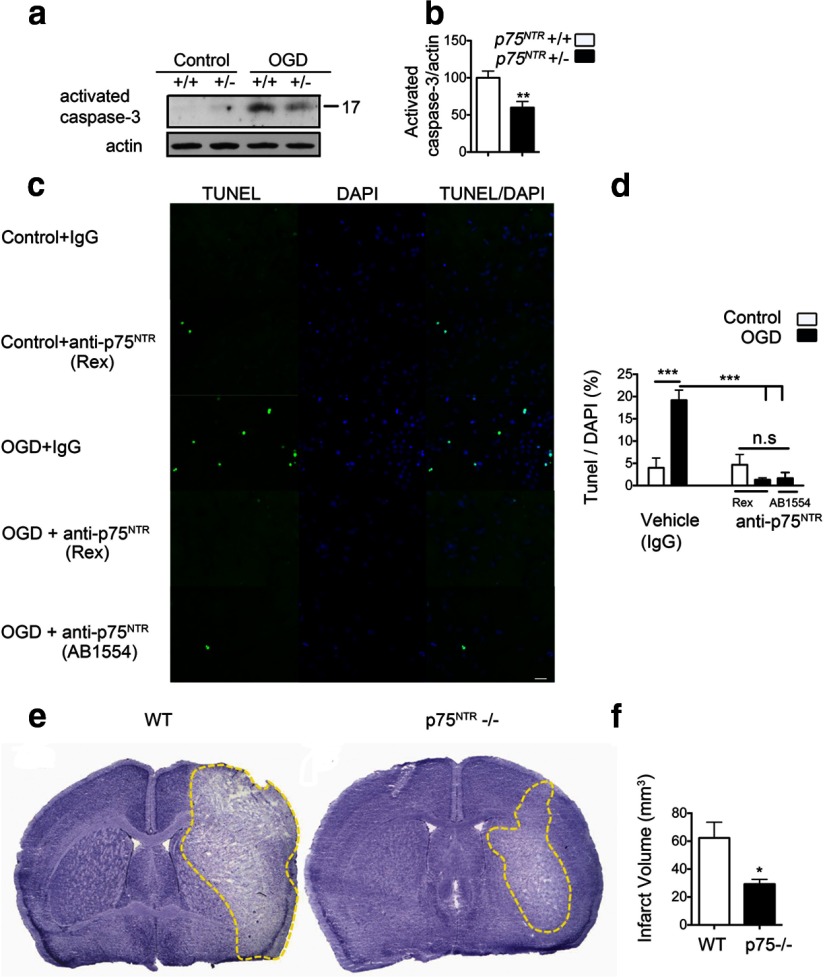
It has been shown previously that p75NTR-mediated signaling leads to activation of caspase-3, contributing to cell death (Wang et al., 2001). To determine whether the increase in p75NTR after ischemic injury is pathologically relevant, we subjected organotypic hippocampal slices from p75NTR haploinsufficient mice or wild-type littermates to OGD. OGD-induced activation of caspase-3 was significantly decreased in p75NTR+/− slices compared with wild-type slices (Fig. 2a,b). Furthermore, delivery of function-blocking antibodies directed against the extracellular domain led to a complete rescue of OGD-induced apoptosis in primary neurons (Fig. 2c,d). To conclusively evaluate the role of p75NTR in ischemia, we performed transient middle cerebral artery occlusion in wild-type and p75NTR−/− adult mice. Mice were euthanized at 72 h after ischemia, and coronal sections of the brains were stained with cresyl violet for determination of infarct volume (Jackman et al., 2011). Infarct volume was significantly decreased in the p75NTR−/− mice (29.2 ± 7.5 mm3) compared with the age-matched wild-type controls (62.3 ± 11.25 mm3) (Fig. 2e,f). Together, these results highlight the deleterious effects of ischemia-induced p75NTR in the neuronal damage that ensues.
Figure 2.
Blockade of p75NTR rescues ischemic cell death. a, Organotypic hippocampal slices from p75NTR haploinsufficient mice and wild-type littermates were subjected to 30 min of OGD and harvested at 8–12 h after OGD. OGD-induced activated caspase-3 was significantly decreased in hippocampal slices from p75NTR haploinsufficient mice. b, Quantification of activated caspase-3 after OGD. Values are shown relative to activated caspase-3 in the wild type (n = 3; Student's t test). c, Apoptotic cell death is significantly reduced in the presence of p75NTR function-blocking antibodies. Primary hippocampal neurons were treated with anti-p75NTR antibody (REX) or AB1554 (Millipore). Control cells were treated with rabbit IgG (Millipore). Antibodies were added 2 h before OGD, and TUNEL assay was performed 24 h after OGD. Representative images show a decrease in OGD-induced apoptosis on blockade of p75NTR. Scale bar, 20 μm. d, Quantification of TUNEL-positive cells represented as a percentage of DAPI cells in the field. A total of three fields were imaged from each coverslip (n = 4). ANOVA analysis was performed. e, Representative cresyl violet-stained coronal brain sections from wild-type and p75NTR−/− mice 72 h after MCAO. Note the smaller infarct (unstained white area) in p75NTR−/− mice. f, Quantification of the infarct volume 72 h after MCAO in wild-type (n = 7) and p75NTR−/− (n = 5) mice shows a significant infarct reduction in p75NTR−/− mice. *p < 0.05; **p < 0.01; ***p < 0.001, Student's t test.
ProNGF is increased after brain ischemia
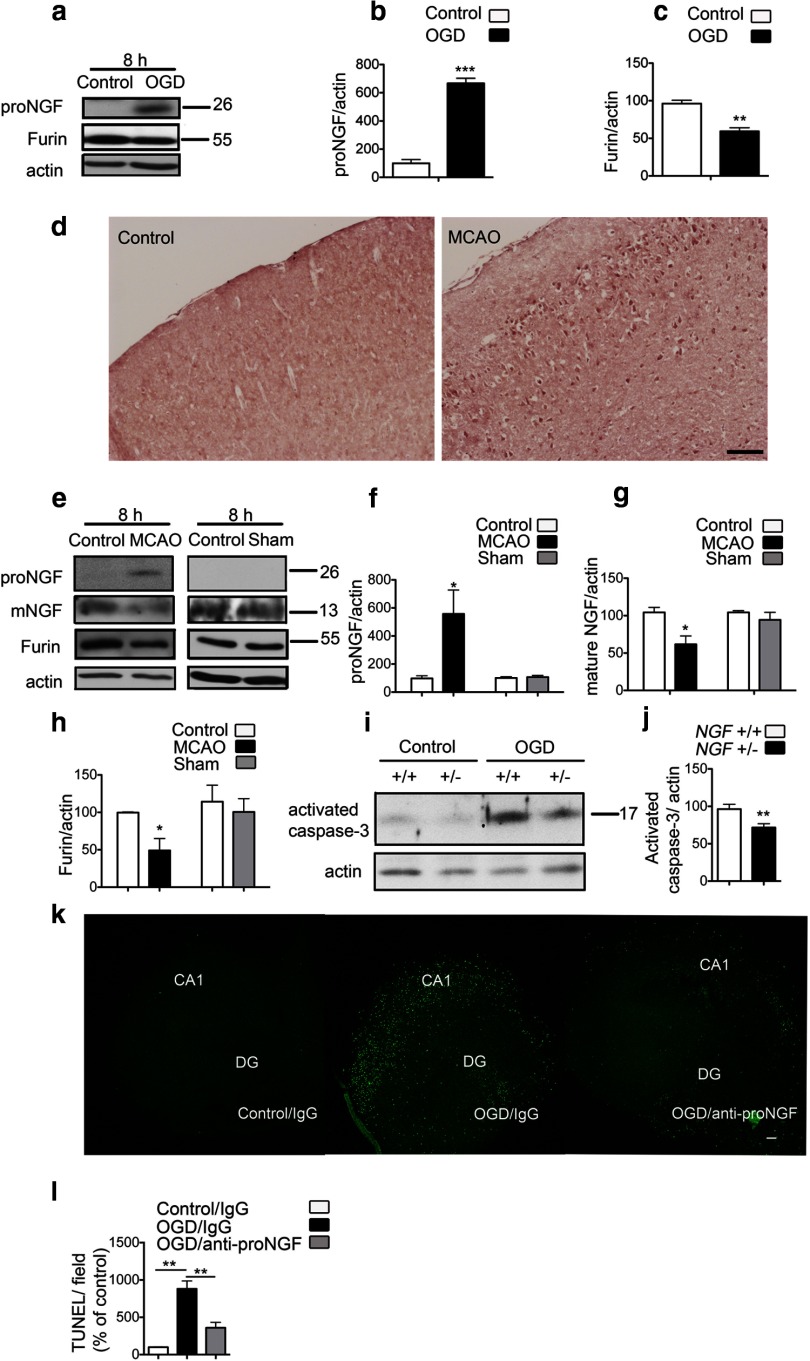
As the apoptotic actions of p75NTR are mediated by its interaction with its ligand proNGF, we investigated whether a coordinate induction of proNGF occurs after ischemia. An antibody specific for the pro-domain of proNGF was used to detect proNGF of ∼28 kDa (Beattie et al., 2002; Harrington et al., 2004). Elevated levels of proNGF were observed in organotypic hippocampal slices subjected to OGD when compared with control slices (Fig. 3a,b). Immunoblots probed with an antibody specific for the mature NGF domain did not detect endogenous mature NGF in control slices or after OGD (data not shown). To provide insight into how ischemia might selectively increase proNGF, we analyzed the levels of furin, an intracellular enzyme known to cleave proNGF to mature NGF during intracellular trafficking. In slices subjected to OGD, we observed a significant reduction in the levels of furin (Fig. 3a,c), suggesting that impaired cleavage of proNGF to mature NGF could contribute to the overall increase in proNGF after OGD.
Figure 3.
ProNGF is increased after ischemia. a, ProNGF protein expression is increased with a concurrent decrease in furin in organotypic hippocampal slices subjected to OGD. b, Quantification of proNGF levels from a (n = 3; Student's t test). c, Quantification of furin levels from a (n = 3; Student's t test). d, Immunohistochemistry using an anti-pro-domain antibody shows induction of proNGF protein in the ischemic cortex 8 h after MCAO, compared with the contralateral control hemisphere. Images are representative of data from three different animals. Scale bar, 100 μm. e, Immunoblots from the ischemic cortex show elevation of proNGF after MCAO. Mature NGF is decreased in the ischemic cortex. A concomitant decrease in furin is also observed in the ischemic hemisphere. Sham surgeries do not affect the levels of proNGF, NGF, or furin expression. f, Quantification of proNGF levels from e (n = 4). Student's t test was used to compare between the MCAO or sham groups with the contralateral control hemisphere. g, Quantification of mature NGF from e (n = 3; Student's t test). h, Quantification of furin levels from e (n = 4). i, OGD-induced activation of caspase-3 is decreased in slices from ngf +/− mice compared with slices from wild-type littermates. j, Quantification of activated caspase-3 from i. Values are shown relative to activated caspase-3 in the wild type 8–12 h after OGD (n = 3; Student's t test). k, Anti-proNGF treatment in organotypic hippocampal slices rescues OGD-induced apoptotic cell death. The function-blocking antibody specific for the pro-domain of proNGF or nonimmune rabbit IgG was added to slices 2 h before OGD, and TUNEL assay was performed at 24 h after OGD. Representative images of slices are shown. DG, Dentate gyrus. Scale bar, 100 μm. I, Quantification of TUNEL positivity per field shows significant reduction in apoptosis on treatment with the anti-proNGF antibody. Three to five slices in each group were analyzed from four independent experiments (n = 4; ANOVA). *p < 0.05; **p < 0.01; ***p < 0.001.
Immunohistochemistry and Western blot analysis of cortical lysates from the ischemic hemisphere after MCAO confirmed the elevation of proNGF in vivo (Fig. 3d–f). ProNGF was not detectable in the nonischemic control hemisphere or after sham surgery (Fig. 3d,e). In addition, mature NGF was significantly decreased in the adult cortex after cerebral ischemia (Fig. 3e,g). Consistent with the results obtained in hippocampal slices, we observed a concomitant decrease in the levels of furin in the ischemic cortex, suggesting that postischemic elevation of proNGF may be caused by decreased processing of the precursor to mature NGF (Fig. 3e,h). To establish whether the increase in proNGF after ischemic injury activated cell-death pathways, we subjected organotypic hippocampal slice cultures from ngf haploinsufficient mice or wild-type littermates to OGD. We observed that OGD-induced activation of caspase-3 was significantly diminished in the slices from ngf+/− mice, highlighting the pro-apoptotic role of ischemia-induced proNGF (Fig. 3i,j). Furthermore, we used a well characterized function-blocking antibody to the pro-domain of proNGF (Beattie et al., 2002; Harrington et al., 2004), or nonimmune rabbit IgG, to examine the effects of increased proNGF after OGD. Slices were maintained in the presence of the antibody for 24 h after OGD. Inclusion of anti-proNGF, but not nonimmune IgG, resulted in a significant decrease in OGD-induced apoptosis as assessed by TUNEL analysis (Fig. 3k,l).
Increased expression of p75NTR protein after OGD occurs in a post-transcriptional, translation-dependent mechanism
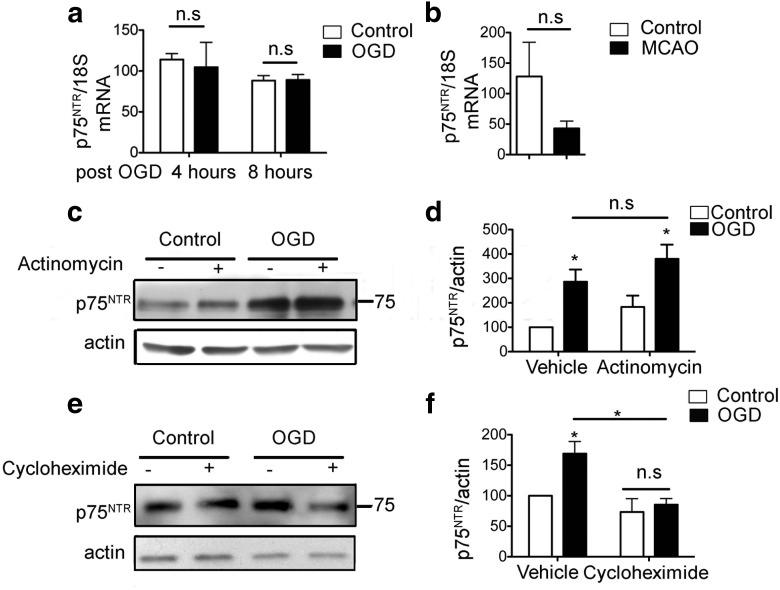
To determine whether the increase after p75NTR protein in ischemia is accompanied by changes in mRNA levels consistent with a transcriptional mechanism, we performed quantitative PCR (qPCR) for p75NTR mRNA in primary hippocampal neurons subjected to OGD. Although p75NTR protein was robustly induced 8 h after OGD in neurons, there was no concomitant elevation in the mRNA at 4 or 8 h after OGD (Fig. 4a). Similarly, the increase in p75NTR protein in the ischemic cortex after MCAO was not associated with an increase in p75NTR mRNA (Fig. 4b). To determine whether the changes in p75NTR protein were the result of a post-transcriptional mechanism, we treated primary hippocampal neurons with the transcriptional inhibitor actinomycin D. Treatment with actinomycin D (0.1 μg/ml) did not decrease the levels of OGD-induced p75NTR (Fig. 4c,d). However, similar treatment with the translation inhibitor cycloheximide (10 μm) completely eliminated the OGD-induced increase in p75NTR (Fig. 4e,f). Together, these results indicate that the increase in p75NTR protein after ischemic injury does not require active transcription but is dependent on active translation.
Figure 4.
Increased expression of p75NTR protein after OGD occurs in a post-transcriptional, translation-dependent mechanism. a, Primary hippocampal neurons show no change in p75NTR mRNA at 4 and 8 h after OGD (n = 3; Student's t test). b, Quantitative real-time PCR for p75NTR mRNA from cortex of animals subjected to MCAO does not show an increase compared with the contralateral control hemisphere. Graphs depict p75NTR mRNA normalized to endogenous control 18S RNA (n = 4; Student's t test). c, OGD in the presence of the transcriptional inhibitor actinomycin D does not decrease induction of p75NTR. d, Densitometry of c (n = 3; ANOVA). e, Application of the translation inhibitor cycloheximide decreases the induction of p75NTR protein after OGD. f, Densitometry of e (n = 3; ANOVA, *p < 0.05). Error bars indicate SEM. n.s., Not significant.
Endogenous miR-592 regulates the levels of p75NTR
As the increase in p75NTR after ischemic injury seems to be a translation-dependent process, we sought to establish whether the regulation of p75NTR involves a microRNA-mediated mechanism. The rodent and human p75NTR transcripts have a large 3′UTR of ∼2000 bp. Prior studies have identified a strong correlation between the length of the 3′UTR of transcripts of several mRNAs and the probability of its regulation by miRNAs (Cheng et al., 2009). Furthermore, cerebral ischemia is known to alter the miRNA profile in a spatiotemporal manner (Jeyaseelan et al., 2008; Tan et al., 2011). Thus, we considered whether a reduction in the levels of specific miRNAs after ischemia could contribute to the misregulation of their target mRNAs, specifically p75NTR. We performed an unbiased approach to identify miRNAs that are altered by ischemia, using a microRNA microarray analysis in organotypic hippocampal slices, 4 and 8 h after OGD (data not shown). miRNAs that were decreased by at least 50% at both 4 and 8 h after ischemia were further analyzed through algorithms (miRanda) to identify candidate miRNAs that were predicted to interact with the 3′UTR of p75NTR.
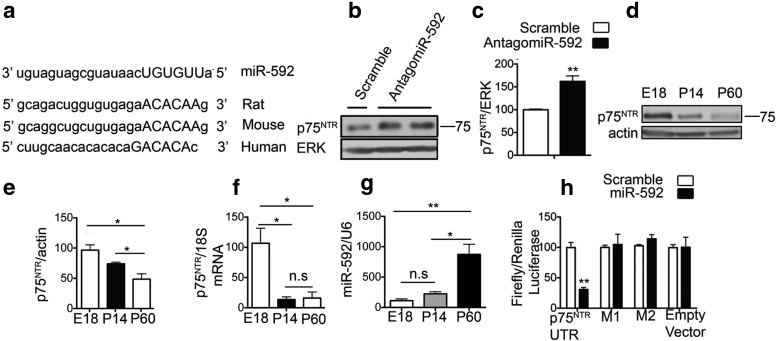
miR-592 emerged as a candidate, as it was decreased by >50% after OGD in hippocampal slices at both time points examined and was further predicted to target the 3′UTR of p75NTR at a sequence conserved in mouse, rat, and humans (Fig. 5a). To assess the role of endogenous miR-592 in the regulation of p75NTR protein, we transduced primary hippocampal neurons with lentiviruses producing antagomiR-592 to block the activity of the endogenous miR-592 or a control scrambled antagomiR. Transduction with antagomiR-592 led to a significant increase in the level of p75NTR protein in hippocampal neurons, indicating that endogenous miR-592 participates in controlling the levels of p75NTR protein (Fig. 5b,c).
Figure 5.
Endogenous miR-592 regulates p75NTR levels. a, The 3′UTR of p75NTR has a conserved site for miR-592. Putative binding sites for miR-592 on the p75NTR 3′UTR are shown, with complementary sites in capitals. b, Primary neurons were infected with lentiviral vectors expressing the antagomiR-592 or scramble and analyzed for p75NTR levels after 4 d in vitro. Blocking the activity of endogenous miR-592 increases the levels of neuronal p75NTR. c, Quantification of p75NTR levels from b (n = 4; Student's t test). d, A temporal decrease in p75NTR protein levels is seen in the mouse cortex. e, Quantification of p75NTR levels from d (n = 3; ANOVA). f, Cortical p75NTR mRNA decreases in the first 2 weeks postnatally. Subsequent decline in p75NTR protein is not accompanied by a decrease in p75NTR mRNA (n = 3; ANOVA). g, Quantitative real-time PCR shows that miR-592 levels inversely correlate with p75NTR protein levels, with a robust increase in miR-592 seen in the adult mouse (P60) cortex. microRNA levels are normalized to endogenous small RNA control U6 (n = 3; ANOVA). h, miR-592 or a scramble control was cotransfected with plasmids containing psicheck-2 empty vector or psiCheck-2-p75NTR-3′UTR. A decrease in luciferase activity indicative of binding of miR-592 to the p75NTR UTR is seen. Mutations (M1, M2) of the miR-592-binding sites on the p75NTR 3′UTR abolish the effects of miR-592 on luciferase levels. miR-592 does not affect the luciferase levels in the empty vector lacking the p75NTR 3′UTR (n = 3). Student's t test was used to compare between scramble and miR-592 transfections. *p < 0.05; **p < 0.01. n.s., Not significant.
Interestingly, the levels of miR-592 inversely correlated with the levels of p75NTR in the developing and early postnatal mouse brain. p75NTR is most highly expressed in the early postnatal hippocampus (Yang et al., 2009) and has been shown to be critical for the pruning of axons in the developing nervous system (Singh et al., 2008). Immunoblots for p75NTR in the cortex show that the levels of p75NTR are highest in the perinatal days (Fig. 5d,e). At 2 weeks after birth, p75NTR protein levels decline, and this is associated with a simultaneous drop in the levels of p75NTR mRNA as assessed by qPCR (Fig. 5f). Therefore, p75NTR levels in this time frame are likely to be regulated in part by changes in transcriptional activity. Interestingly, in the perinatal period, the levels of miR-592 are low (Fig. 5g). The continued decline in p75NTR protein from P14 to its basal levels in the adult (P60) is not accompanied by a further drop in the levels of p75NTR mRNA (Fig. 5f). Rather, a robust induction of miR-592 was observed in adulthood (Fig. 5g), which could contribute to the low levels of expression of p75NTR in the adult. Together, these observations suggest that miR-592 contributes to the maintenance of p75NTR at low baseline levels in the adult noninjured brain.
To confirm that miR-592 regulates p75NTR protein levels by directly targeting the 3′UTR of p75NTR, the 3′UTR was amplified from rat brain and inserted into a dual luciferase plasmid. Coexpression of the p75NTR 3′UTR with miR-592 but not the control miRNA in heterologous cells led to significantly decreased luciferase activity 24 h after coexpression (Fig. 5h), indicating that miR-592 binds to the 3′UTR of p75NTR. This interaction was also confirmed to be specific at the predicted binding sites, as mutations at these sites on the p75NTR 3′UTR abolished the effect of miR-592 on luciferase levels. miR-592 did not affect the luciferase levels of a control empty vector lacking the p75NTR 3′UTR (Fig. 5h).
miR-592 regulates the induction of p75NTR in neurons after ischemic injury and contributes to cell death
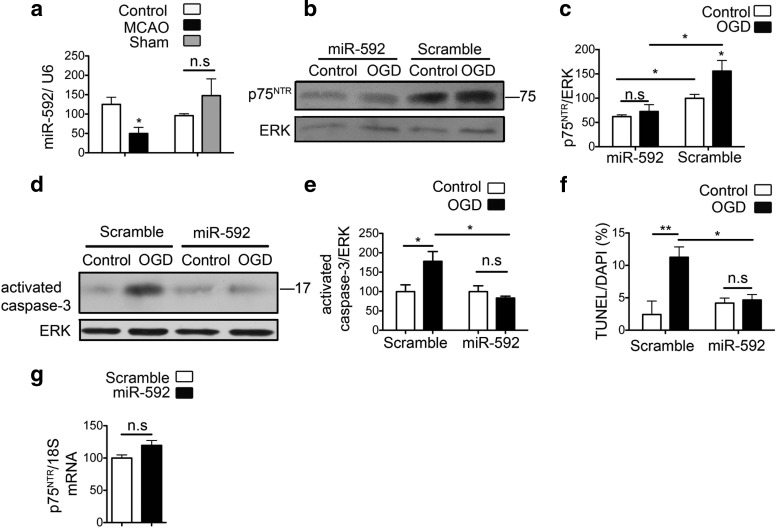
Quantitative PCR confirmed a significant reduction in the levels of miR-592 coinciding with the induction of p75NTR after MCAO, but not sham surgery (Fig. 6a). To determine whether overexpression of miR-592 in primary neuronal cultures can attenuate the increase in OGD-induced p75NTR, we transfected primary hippocampal neurons with precursor miRNA-592. Transfection with miR-592 but not the control scramble miRNA significantly decreased the levels of basal p75NTR. Furthermore, overexpression of miR-592 attenuated the OGD-induced increase in p75NTR protein (Fig. 6b,c). In addition, overexpression of miR-592 levels also significantly attenuated the OGD-induced activation of caspase-3 in primary hippocampal neurons (Fig. 6d,e). Quantification of apoptotic cells 24 h after OGD showed a significant protective effect by miR-592 in primary hippocampal neurons (Fig. 6f).
Figure 6.
MiR-592 regulates induction of p75NTR after ischemic injury in neurons and contributes to apoptosis. a, Quantitative real-time PCR shows decreased levels of miR-592 in the ischemic cortex of mice subjected to MCAO compared with the contralateral hemisphere. Sham surgeries do not affect the levels of miR-592. MicroRNA levels are normalized to endogenous small RNA U6 (n = 4). Student's t test was used to compare the miRNA levels between MCAO or sham with their respective contralateral control hemispheres. b, Primary hippocampal neurons were transfected with precursor miR-592 or a control vector and subjected to 60 min of OGD. Transfection with miR-592 decreases the levels of p75NTR under control conditions and also mitigates the OGD-induced increase in p75NTR levels. c, Quantification of p75NTR from b (n = 4; ANOVA). d, Activation of capsase-3 is observed at 8 h after OGD in primary hippocampal neurons. Overexpression of miR-592, but not the control scramble microRNA, significantly attenuates the OGD-induced activation of capsase-3. e, Quantification of activated caspase-3 from d (n = 5; ANOVA). f, Quantification of apoptotic cells after transduction with SCR or miR-592 in primary hippocampal neurons. TUNEL assay quantification shows decreased apoptosis in the presence of miR-592. An average of six fields were analyzed per coverslip, and values are shown as a percentage of TUNEL-positive cells to DAPI (n = 3; ANOVA). g, Transfection of precursor miR-592 in primary neurons does not affect the levels of p75NTR mRNA. p75NTR mRNA levels are normalized to endogenous 18S RNA (n = 4; Student's t test). *p < 0.05; **p < 0.001. n.s., Not significant.
MicroRNAs can alter protein expression by binding to the 3′UTR of their target mRNA to regulate translation either by cleavage and degradation of the target mRNA, or by repression of translation (Bartel, 2004). To distinguish between these actions, we transfected primary hippocampal neurons with miR-592, which led to a significant decrease in p75NTR protein without a concomitant decrease in the levels of p75NTR mRNA (Fig. 6g). This observation suggests that miR-592 likely acts by inhibiting p75NTR translation, possibly at its initiation or postinitiation stage. In summary, these findings suggest that deregulation of miR-592 after injury leads to enhanced translation of p75NTR.
Discussion
In the current study, we identify that miR-592 maintains basal levels of p75NTR in neurons and that after ischemic injury, miR-592 levels decline, likely releasing p75NTR mRNA from translational repression, thus contributing to the postischemic induction of p75NTR. We also demonstrate that modulation of the levels of miR-592 in neurons attenuates induction of p75NTR after injury and the apoptotic cell death that ensues.
Although p75NTR is rapidly induced in several injury models, few mechanisms have been identified that regulate its gene expression. A previous study has identified that O-GlcNAcylation of the Sp1 transcription factor regulates p75NTR transcription in hypo-osmolar injury (Ramos et al., 2007; Kommaddi et al., 2011). The pro-inflammatory cytokines IL-1b and TNF-α have also been shown to contribute to p75NTR upregulation after seizures (Choi and Friedman, 2009). In these two models of injury, the increase in p75NTR protein was accompanied by an increase in p75NTR mRNA, indicating transcriptional regulation of the gene. However, other studies analyzing p75NTR levels in tumor models have reported that altered p75NTR protein levels are associated with a change in polysomal loading without affecting mRNA levels, implicating a translation-based mechanism (Provenzani et al., 2006). Our studies show that ischemic injury induces p75NTR without affecting its transcription, as evidenced by the lack of change in p75NTR mRNA after ischemia. Furthermore, blockade of transcription fails to decrease injury-induced p75NTR elevation. On the contrary, we show that active translation is necessary for ischemia-induced elevation of p75NTR.
The 3′UTR of an mRNA is a key regulator of translation, through modulation of mRNA localization, poly-adenylation, and stability, which occur in part through interaction with RNA-binding proteins and/or microRNAs (Barrett et al., 2012). A prior report has shown that serial truncation of the 3′UTR of p75NTR leads to significantly higher levels of p75NTR protein, thus implicating regulation of p75NTR by its 3′UTR (Krygier and Djakiew, 2001). The relatively large size of the 3′UTR of p75NTR (∼2 KB) suggests that it may be regulated at the UTR by numerous interacting factors including miRNAs. Our study is the first to report the regulation of p75NTR by a microRNA. However, as p75NTR is induced in multiple models of neuronal injury and prior studies have demonstrated that numerous microRNAs are dynamically regulated after CNS injury, it is possible that additional microRNAs could also participate in the regulation of p75NTR (Bhalala et al., 2013).
miRNAs are spatiotemporally regulated in the nervous system during development (Kapsimali et al., 2007). p75NTR is also differentially expressed during development, with the highest levels being in the embryonic and early postnatal periods, consistently decreasing over time in the adult brain. Interestingly, our observations show that, correspondingly, miR-592 is low in the perinatal days when the peak expression of p75NTR is seen. miR-592 is expressed severalfold higher in the adult brain, correlating with the low presence of p75NTR. A previous study has identified region-specific differences in the levels of miR-592 in the adult rodent brain (Shu et al., 2013). However, further study will be required to determine whether these levels inversely correlate to the highly restricted regional localization of p75NTR in adulthood. In addition, miR-592 has been previously reported to be differentially expressed in several tumors, and it is plausible that it responds similarly to tumor hypoxia, thereby modulating the effects of p75NTR in tumors (Sarver et al., 2009; Wang et al., 2012a, b).
The altered levels of miR-592 have important consequences in the nervous system through modulation of p75NTR expression. miR-592 is located in the intron of mGluR8, a metabotropic glutamate receptor widely expressed in the brain (Niswender and Conn, 2010). Intronic miRNAs can be transcribed either as part of their host genes or independently under the control of their own promoter (Monteys et al., 2010). Alternatively, the levels of miR-592 may be regulated at a post-transcriptional stage wherein the miRNA processing or mature miRNA stability is altered during injury.
The concomitant postischemic induction of the ligand proNGF is conducive to the pro-apoptotic actions of p75NTR. ProNGF is selectively increased after ischemic injury, with a corresponding decrease in mature NGF. Whereas a transcriptional response of the ngf gene cannot be ruled out, altered processing of proNGF may also contribute to the selective elevation of proNGF. Indeed, after seizures, a decline in the activity of MMP-7, an enzyme known to regulate the extracellular cleavage of proNGF, has been shown to contribute to the increase in proNGF (Le and Friedman, 2012). The intracellular processing enzyme furin has been similarly shown to regulate the balance between proNGF and mature NGF (Seidah et al., 1996). We report here that the elevation of proNGF in ischemic injury likely results from a drastic decrease in the levels of soluble furin. Previous studies have reported an increase in the mRNA for furin after ischemia, which is possibly driven by positive feedback because of the decreased postischemic activity of furin (Yokota et al., 2001).
Although several models of CNS injury have implicated the pathophysiologic role of proNGF and p75NTR in mediating neuronal apoptosis, this report is the first to document the effect of this pro-apoptotic complex in a model of a major form of death and disability in humans, ischemic stroke. This is significant particularly because measures to control delayed damage through apoptosis may be critical in salvaging the ischemic penumbra (Moskowitz et al., 2010). As proNGF is also known to bind p75NTR to affect neuronal morphology, it is conceivable that the effects of their accumulation extend beyond cell death to alter neuronal morphology and synaptic plasticity in the adult brain (Deinhardt et al., 2011).
Our findings elicit further interest in the post-transcriptional regulation of p75NTR. Future studies directed at elucidating the biogenesis of miR-592 in development and disease will be crucial to our understanding of p75NTR regulation. As our observations have identified the detrimental role of miR-592 in ischemia through p75NTR, modulation of miR-592 is a putative therapeutic strategy to limit ischemic damage.
Footnotes
This work was supported by NIH Grants NS30687 (B.L.H.) and NS34179 and NS73666 (C.I.), by German Research Foundation Grant Un34/25 (K.U.), and by the Australian NHMRC C. J. Martin Overseas Biomedical Fellowship to K.A.J. We thank Louis Reichardt for generously providing the anti-p75NTR (REX) antibody.
The authors declare no competing financial interests.
References
- Abe T, Shimamura M, Jackman K, Kurinami H, Anrather J, Zhou P, Iadecola C. Key role of CD36 in Toll-like receptor 2 signaling in cerebral ischemia. Stroke. 2010;41:898–904. doi: 10.1161/STROKEAHA.109.572552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LW, Fletcher S, Wilton SD. Regulation of eukaryotic gene expression by the untranslated gene regions and other non-coding elements. Cell Mol Life Sci. 2012;69:3613–3634. doi: 10.1007/s00018-012-0990-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Beattie MS, Harrington AW, Lee R, Kim JY, Boyce SL, Longo FM, Bresnahan JC, Hempstead BL, Yoon SO. ProNGF induces p75-mediated death of oligodendrocytes following spinal cord injury. Neuron. 2002;36:375–386. doi: 10.1016/S0896-6273(02)01005-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalala OG, Srikanth M, Kessler JA. The emerging roles of microRNAs in CNS injuries. Nat Rev Neurol. 2013;9:328–339. doi: 10.1038/nrneurol.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll SL, Schweitzer JB, Holtzman DM, Miller ML, Sclar GM, Milbrandt J. Elements in the 5′ flanking sequences of the mouse low-affinity NGF receptor gene direct appropriate CNS, but not PNS, expression in transgenic mice. J Neurosci. 1995;15:3342–3356. doi: 10.1523/JNEUROSCI.15-05-03342.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Bhardwaj N, Gerstein M. The relationship between the evolution of microRNA targets and the length of their UTRs. BMC Genomics. 2009;10:431. doi: 10.1186/1471-2164-10-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Friedman WJ. Inflammatory cytokines IL-1beta and TNF-alpha regulate p75NTR expression in CNS neurons and astrocytes by distinct cell-type-specific signalling mechanisms. ASN Neuro. 2009;1.pii:e00010. doi: 10.1042/AN20090009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deinhardt K, Kim T, Spellman DS, Mains RE, Eipper BA, Neubert TA, Chao MV, Hempstead BL. Neuronal growth cone retraction relies on proneurotrophin receptor signaling through Rac. Sci Signal. 2011;4:ra82. doi: 10.1126/scisignal.2002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahnestock M, Michalski B, Xu B, Coughlin MD. The precursor pro-nerve growth factor is the predominant form of nerve growth factor in brain and is increased in Alzheimer's disease. Mol Cell Neurosci. 2001;18:210–220. doi: 10.1006/mcne.2001.1016. [DOI] [PubMed] [Google Scholar]
- Gehler S, Gallo G, Veien E, Letourneau PC. p75 neurotrophin receptor signaling regulates growth cone filopodial dynamics through modulating RhoA activity. J Neurosci. 2004;24:4363–4372. doi: 10.1523/JNEUROSCI.0404-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington AW, Leiner B, Blechschmitt C, Arevalo JC, Lee R, Mörl K, Meyer M, Hempstead BL, Yoon SO, Giehl KM. Secreted proNGF is a pathophysiological death-inducing ligand after adult CNS injury. Proc Natl Acad Sci U S A. 2004;101:6226–6230. doi: 10.1073/pnas.0305755101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochrainer K, Jackman K, Anrather J, Iadecola C. Reperfusion rather than ischemia drives the formation of ubiquitin aggregates after middle cerebral artery occlusion. Stroke. 2012;43:2229–2235. doi: 10.1161/STROKEAHA.112.650416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber LJ, Chao MV. Mesenchymal and neuronal cell expression of the p75 neurotrophin receptor gene occur by different mechanisms. Dev Biol. 1995;167:227–238. doi: 10.1006/dbio.1995.1019. [DOI] [PubMed] [Google Scholar]
- Jackman K, Kunz A, Iadecola C. Modeling focal cerebral ischemia in vivo. Methods Mol Biol. 2011;793:195–209. doi: 10.1007/978-1-61779-328-8_13. [DOI] [PubMed] [Google Scholar]
- Jeyaseelan K, Lim KY, Armugam A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke. 2008;39:959–966. doi: 10.1161/STROKEAHA.107.500736. [DOI] [PubMed] [Google Scholar]
- Jover T, Tanaka H, Calderone A, Oguro K, Bennett MV, Etgen AM, Zukin RS. Estrogen protects against global ischemia-induced neuronal death and prevents activation of apoptotic signaling cascades in the hippocampal CA1. J Neurosci. 2002;22:2115–2124. doi: 10.1523/JNEUROSCI.22-06-02115.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapsimali M, Kloosterman WP, de Bruijn E, Rosa F, Plasterk RH, Wilson SW. MicroRNAs show a wide diversity of expression profiles in the developing and mature central nervous system. Genome Biol. 2007;8:R173. doi: 10.1186/gb-2007-8-8-r173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kommaddi RP, Dickson KM, Barker PA. Stress-induced expression of the p75 neurotrophin receptor is regulated by O-GlcNAcylation of the Sp1 transcription factor. J Neurochem. 2011;116:396–405. doi: 10.1111/j.1471-4159.2010.07120.x. [DOI] [PubMed] [Google Scholar]
- Krygier S, Djakiew D. Molecular characterization of the loss of p75(NTR) expression in human prostate tumor cells. Mol Carcinog. 2001;31:46–55. doi: 10.1002/mc.1038. [DOI] [PubMed] [Google Scholar]
- Le AP, Friedman WJ. Matrix metalloproteinase-7 regulates cleavage of pro-nerve growth factor and is neuroprotective following kainic acid-induced seizures. J Neurosci. 2012;32:703–712. doi: 10.1523/JNEUROSCI.4128-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Monteys AM, Spengler RM, Wan J, Tecedor L, Lennox KA, Xing Y, Davidson BL. Structure and activity of putative intronic miRNA promoters. RNA. 2010;16:495–505. doi: 10.1261/rna.1731910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noraberg J, Poulsen FR, Blaabjerg M, Kristensen BW, Bonde C, Montero M, Meyer M, Gramsbergen JB, Zimmer J. Organotypic hippocampal slice cultures for studies of brain damage, neuroprotection and neurorepair. Curr Drug Targets CNS Neurol Disord. 2005;4:435–452. doi: 10.2174/1568007054546108. [DOI] [PubMed] [Google Scholar]
- Park JA, Lee JY, Sato TA, Koh JY. Co-induction of p75NTR and p75NTR-associated death executor in neurons after zinc exposure in cortical culture or transient ischemia in the rat. J Neurosci. 2000;20:9096–9103. doi: 10.1523/JNEUROSCI.20-24-09096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzani A, Fronza R, Loreni F, Pascale A, Amadio M, Quattrone A. Global alterations in mRNA polysomal recruitment in a cell model of colorectal cancer progression to metastasis. Carcinogenesis. 2006;27:1323–1333. doi: 10.1093/carcin/bgi377. [DOI] [PubMed] [Google Scholar]
- Ramos A, Ho WC, Forte S, Dickson K, Boutilier J, Favell K, Barker PA. Hypo-osmolar stress induces p75NTR expression by activating Sp1-dependent transcription. J Neurosci. 2007;27:1498–1506. doi: 10.1523/JNEUROSCI.4806-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol. 2002;67:203–233. doi: 10.1016/S0301-0082(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Sarver AL, French AJ, Borralho PM, Thayanithy V, Oberg AL, Silverstein KA, Morlan BW, Riska SM, Boardman LA, Cunningham JM, Subramanian S, Wang L, Smyrk TC, Rodrigues CM, Thibodeau SN, Steer CJ. Human colon cancer profiles show differential microRNA expression depending on mismatch repair status and are characteristic of undifferentiated proliferative states. BMC Cancer. 2009;9:401. doi: 10.1186/1471-2407-9-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidah NG, Benjannet S, Pareek S, Savaria D, Hamelin J, Goulet B, Laliberte J, Lazure C, Chrétien M, Murphy RA. Cellular processing of the nerve growth factor precursor by the mammalian pro-protein convertases. Biochem J. 1996;314:951–960. doi: 10.1042/bj3140951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu SY, Qing D, Wang B, Zeng QY, Chen YC, Jin Y, Zeng CC, Bao R. Comparison of microRNA expression in hippocampus and the marginal division (MrD) of the neostriatum in rats. J Biomed Sci. 2013;20:9. doi: 10.1186/1423-0127-20-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KK, Park KJ, Hong EJ, Kramer BM, Greenberg ME, Kaplan DR, Miller FD. Developmental axon pruning mediated by BDNF-p75NTR-dependent axon degeneration. Nat Neurosci. 2008;11:649–658. doi: 10.1038/nn.2114. [DOI] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-M. [DOI] [PubMed] [Google Scholar]
- Tan JR, Koo YX, Kaur P, Liu F, Armugam A, Wong PT, Jeyaseelan K. microRNAs in stroke pathogenesis. Curr Mol Med. 2011;11:76–92. doi: 10.2174/156652411794859232. [DOI] [PubMed] [Google Scholar]
- Volosin M, Song W, Almeida RD, Kaplan DR, Hempstead BL, Friedman WJ. Interaction of survival and death signaling in basal forebrain neurons: roles of neurotrophins and proneurotrophins. J Neurosci. 2006;26:7756–7766. doi: 10.1523/JNEUROSCI.1560-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Zhao LJ, Tan YX, Ren H, Qi ZT. MiR-138 induces cell cycle arrest by targeting cyclin D3 in hepatocellular carcinoma. Carcinogenesis. 2012a;33:1113–1120. doi: 10.1093/carcin/bgs113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Zhao LJ, Tan YX, Ren H, Qi ZT. Identification of deregulated miRNAs and their targets in hepatitis B virus-associated hepatocellular carcinoma. World J Gastroenterol. 2012b;18:5442–5453. doi: 10.3748/wjg.v18.i38.5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Bauer JH, Li Y, Shao Z, Zetoune FS, Cattaneo E, Vincenz C. Characterization of a p75(NTR) apoptotic signaling pathway using a novel cellular model. J Biol Chem. 2001;276:33812–33820. doi: 10.1074/jbc.M010548200. [DOI] [PubMed] [Google Scholar]
- Weskamp G, Reichardt LF. Evidence that biological activity of NGF is mediated through a novel subclass of high affinity receptors. Neuron. 1991;6:649–663. doi: 10.1016/0896-6273(91)90067-A. [DOI] [PubMed] [Google Scholar]
- Yang J, Siao CJ, Nagappan G, Marinic T, Jing D, McGrath K, Chen ZY, Mark W, Tessarollo L, Lee FS, Lu B, Hempstead BL. Neuronal release of proBDNF. Nat Neurosci. 2009;12:113–115. doi: 10.1038/nn.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota N, Uchijima M, Nishizawa S, Namba H, Koide Y. Identification of differentially expressed genes in rat hippocampus after transient global cerebral ischemia using subtractive cDNA cloning based on polymerase chain reaction. Stroke. 2001;32:168–174. doi: 10.1161/01.STR.32.1.168. [DOI] [PubMed] [Google Scholar]