Abstract
Purpose
In this study, we aimed to investigate the prevalence of year-round respiratory viral infection in children with lower respiratory tract infection (LRTI) and the relationship between respiratory viral infection and allergen sensitization in exacerbating asthma.
Methods
We investigated the sources for acute LRTIs in children admitted to our hospital from May 2010 to April 2011. The 6 most common respiratory viruses were isolated from nasopharyngeal aspirate using multiplex reverse transcription-polymerase chain reaction in 309 children; respiratory syncytial virus (RSV), adenovirus (AV), parainfluenza virus (PIV), influenza virus (IFV), human metapneumovirus (hMPV), rhinovirus (RV). Atopic sensitization was defined if more than 1 serum specific Immunoglobulin E level measured using UniCAP (Pharmacia) was over 0.35 IU/mL.
Results
RSV was the most common pathogen of bronchiolitis in hospitalized children through the year. RV or IFV infection was more prevalent in asthma exacerbations compared to other LRTIs. AV and hMPV were more likely to cause pneumonia. RV and IFV were associated with asthma exacerbations in children with atopic sensitization, but not in nonatopic children.
Conclusion
RV and IFV are associated with hospitalization for asthma exacerbation in children with atopic sensitization.
Keywords: Asthma, Allergens, Child, Orthomyxoviridae, Rhinovirus
Introduction
Acute lower respiratory tract infections (LRTIs) such as pneumonia, bronchitis, bronchiolitis, and asthma are mostly caused by respiratory viruses in children. Respiratory syncytial virus (RSV), rhinovirus (RV), adenovirus (AV), human metapneumovirus (hMPV), influenza virus (IFV), and parainfluenza virus (PIV) are most common pathogens. Each virus usually follows seasonal patterns of activity and is mostly like to cause different LRTIs depending on the host age, virulence, and infected airway1,2).
Asthma is the most common chronic disease of childhood and the leading cause of childhood morbidity as measured by school absences, emergency department visits and hospitalizations3). Although no single specific cause of asthma has been found, a number of predisposing factors have been identified as target for ongoing clinical investigation.
Atopy is present in majority of children with asthma over the age of 3 and allergen-specific sensitization is one of the most important risk factors in the development of asthma. Acute viral respiratory infections can trigger wheezing and exacerbations of asthma in children4). In established asthma, viral infections are responsible for as many as 80% to 85% of exacerbations in children. The most commonly occurring viral triggers of asthma in children are RV and influenza, while in early childhood, RSV and PIV are more prevalent5-7).
Many previous studies have shown effects of respiratory viral infections on allergic sensitization suggesting a potential relationship. Respiratory viral infections prevented induction of tolerance to allergens or enhanced allergic sensitization in animal models8,9). Respiratory viral infections in animals previously sensitized to aeroallergens result in prolonged increases in inflammation and airway responsiveness, indicating that critical interactions between immune responses to allergen sensitization and the overall responses to infection may lead to more severe disease10).
The synergy between allergen sensitization with high allergen exposure and respiratory viral infection in exacerbating asthma has been reported in several studies11,12). Although the link between sensitization and RV infection has been suggested in children with wheezing, few studies have focused on the interaction between specific viral infections and allergen sensitization in exacerbating asthma in children13). Enhancing our understanding of the relationship between specific respiratory virus infections, allergen sensitization, and asthma in children is likely to produce new strategies for the prevention and treatment of asthma in childhood. Therefore, we investigated the prevalence of respiratory viral infections in lower respiratory tract diseases of children and the interaction between respiratory viral infections and allergen sensitization in precipitating acute asthma in children resulting in hospitalization.
Materials and methods
1. Patients and materials
The study was carried out in the Department of Pediatrics, Kangbuk Samsung Medical Center, Seoul, Korea. The study population consisted of 309 children who were hospitalized for acute LRTIs (pneumonia, bronchitis, bronchiolitis or asthma) between May 2010 and April 2011 and in whom detection of a specific sole respiratory virus by multiplex reverse transcription-polymerase chain reaction (RT-PCR) was possible. The children were diagnosed on the basis of clinical and radiologic findings14-17): pneumonia required rales on auscultation or demonstration of an infiltrate by chest x-ray; bronchiolitis was characterized by a cough, tachypnea, retraction and expiratory wheezes, often accompanied by rales; acute bronchitis required a cough and pulmonary signs such as wheezing, rhonchi and prolonged expiration and no evidence of consolidation; asthma required acute expiratory wheezing which recurred >2 times and was characterized by cough, breathlessness and nocturnal symptoms/awakenings. Four physicians and two radiologists determined the clinical diagnose on the basis of symptoms and signs as well as radiologic findings.
Family history and past medical history of allergic diseases were recorded. Blood eosinophil count was determined by a flow cytometry method (Beckman Coulter, Pasadena, CA, USA) according to the manufacturer's instructions. Serum total IgE and specific IgE for cat, dog, cockroach, alternaria, Dermatophagoides pteronyssinus, Dermatophagoides farina, weed mix, tree mix, milk, egg, soybean and peanut were measured using a fluoro-enzyme immunoassay (ImmunoCAP 250, Phadia, Uppsala, Sweden). Atopic sensitization was defined by a specific IgE level ≥0.35 IU/mL for more than one allergen.
This study was approved by the Institutional Review Board of Kangbuk Samsung Medical Center and written consent was obtained from parents.
2. Nasopharyngeal sample collection and virus analysis
On admission day, a nasopharyngeal aspirate sample was obtained through a nostril by inserting a disposable catheter connected to a mucus extractor to a depth of 5 to 7 cm and retracting it slowly while applying gentle suction with an electric suction device. All specimens were obtained without inserting any solution into the nostrils. Disposable plastic gloves were used to prevent contamination.
Viral RNA in nasopharyngeal aspirates was extracted using a QIAamp Viral RNA Mini kit (Qiagen, Hilden, Germany) in accordance with the manufacturer's instructions. cDNA was synthesized using a cDNA synthesis kit (Fermentas, Burlington, ON, Canada) according to manufacturer's instructions. Multiplex RT-PCR assays were utilized to detect 6 viruses; AV, hMPV, RSV, RV A, PIV 1/2/3, and IFV A and B.
3. Statistics
Statistical analyses were performed using STATA ver. 11.0 (Stata Corp LP., College Station, TX, USA). Serum total IgE values and blood total eosinophil counts were logarithmically transformed prior to analysis due to the skewed distribution. Differences among 4 groups were compared using one-way analysis of variance followed by the Bonferroni post hoc test for multiple comparisons. The significance of differences in categorical variables between groups was analyed using chi-square analysis. Logistic regression was used to determine the association between asthma and RV or IFV infection. Adjusted odds ratios (aORs) and 95% confidence intervals (CIs) were derived following adjustments for age and sex. Data were expressed as mean±standard deviation and significance was defined as a P value of less than 0.05.
Result
1. Patient characteristics
Patient characteristics including the distribution based on viral infections are shown in Table 1. The study population consisted of 309 children. Of the study population, 169 (54.7%) were male. The median age among children was 26.0 months (interquartile range, 16 to 39 months); 215 children (69.6%) were <3 years of age, 69 children (22.3%) were between 3-6 years of age, and 25 children (8.1%) were >6 years of age. The prevalence of viruses detected by nasopharyngeal aspiration was as follows: RSV was detected in 108 children (34.9%), hMPV in 70 (22.6%), RV in 45 (14.6%), AV in 38 (12.3%), IFV in 29 (9.4%) and PIV in 19 (6.1%).
Table 1.
Demographic characteristics of study population and detected viruses (n=309)
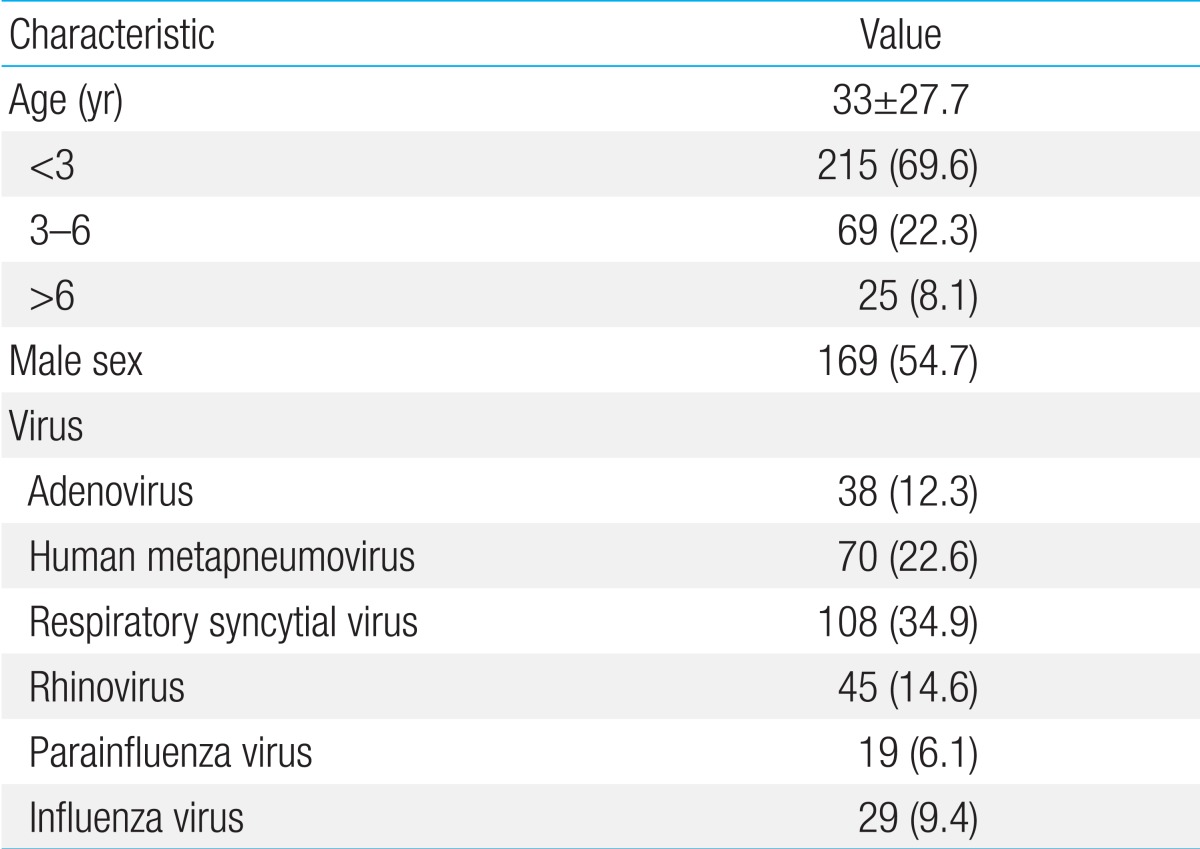
Values are presented as mean±standard deviation or number (%).
The age distributions of infants and children associated with each virus differed and the data are presented in Table 2. LRTIs caused by RSV were predominant among younger infants (mean age, 23 months) compared with LRTIs associated with AV, RV or IFV (mean age, 41 months, 38 months, 62 months, respectively). IFV was more frequently detected in older children (mean age, 62 months) compared to other viruses (P<0.05). There was no difference in the virus groups based on gender.
Table 2.
Blood total eosinophil counts and serum total immunoglobulin E levels according to respiratory viruses
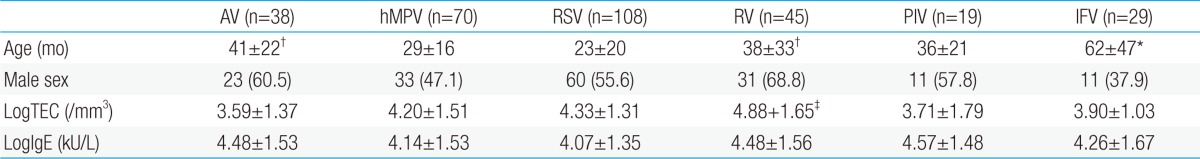
Values are presented as mean±standard deviation or number (%).
AV, adenovirus; hMPV, human metapneumovirus; RSV, respiratory syncytial virus; RV, rhinovirus; PIV, parainfluenza virus; IFV, influenza virus; logTEC, logarhythmic transformation of total eosinophil counts; logIgE, logarhythmic transformation of serum total IgE.
*P<0.05 vs. other viruses. †P<0.05 vs. RSV. ‡P<0.05 vs. AV.
LRTIs caused by RV were associated with high levels of blood eosinophils, compared with LRTIs associated with AV (P<0.05). No significant differences in serum total IgE levels were observed among individual virus groups.
2. Prevalence of detected viruses in each lower respiratory tract infection groups
The clinical diagnoses of the 309 patients with LRTIs associated with specific respiratory viruses are summarized in Table 3. The mean age of children with asthma and bronchitis (60 months, 58 months, respectively) was higher than that of children with pneumonia or bronchiolitis (33 months, 16 months, respectively, P<0.05). Asthma and bronchiolitis were more predominant in males compared to pneumonia (P<0.05). RSV was more likely to cause bronchiolitis (P<0.05) while AV was more likely to cause pneumonia compare to other LRTIs (P<0.05). hMPV was a more frequent cause of pneumonia compared to asthma (P<0.05). RV or IFV infection was more prevalent among asthma exacerbations compared to bronchiolitis or pneumonia (P<0.05) and IFV was also a more common pathogen of bronchitis than bronchiolitis (P<0.05).
Table 3.
Prevalence of virus infection in lower respiratory tract diseases

Values are presented as mean±standard deviation or number (%).
*P<0.05 vs. the others. †P<0.05 vs. pneumonia, bronchiolitis. ‡P<0.05 vs. pneumonia. §P<0.05 vs. asthma. ∥P<0.05 vs. bronchiolitis.
3. Atopic characteristics in each LRTI group
All atopy-related variables investigated in this study were associated with asthma except for family history of allergic diseases (Table 4). The atopic sensitization rate was 64.3% in asthma which was higher compared to other LRTIs. The frequency of allergic rhinitis and atopic dermatitis was also higher in asthma compared to other LRTIs (P<0.05). Blood total eosinophil count significantly increased in patients with asthma compared to bronchitis or bronchiolitis and serum total IgE levels were also augmented in asthma compared to pneumonia or bronchitis (P<0.05). While children with asthma had a relatively high rate of family history of allergic diseases, this association was not statistically significant.
Table 4.
Clinical characteristics of each lower respiratory tract infection group

Values are presented as number (%) or mean±standard deviation.
lgTEC, logarithmic transformation of total eosinophil count; lgIgE, logarithmic transformation of serum total IgE.
*P<0.05 vs. bronchiolitis. †P<0.05 vs. the others. ‡P<0.05 vs. pneumonia, bronchitis. §P<0.05 vs. bronchitis, bronchiolitis.
4. Association between asthma exacerbation and infection by RV and IFV according to atopic sensitization
Since the prevalence of RV and IFV infection was increased in asthmatic patients, we investigated the relative risk of asthma exacerbation by RV or IFV compare to other viruses. The adjusted odds ratio of RV and IFV with respect to asthma was 3.9 (95% CI, 1.4 to 10.5) and 4.0 (95% CI, 1.2 to 13.8), respectively following adjustments to age and sex. Following subcategorization of asthma into atopic asthma and nonatopic asthma, the association between RV and IFV infection and asthma exacerbation remained in atopic asthma (RV: aOR, 8.3; 95%CI, 1.5 to 43.3; and IFV: aOR, 11.9; 95%CI, 1.5 to 90.4, respectively), while RV and IFV had no associations with asthma in the non-atopic asthma group (RV: aOR, 2.4; 95% CI, 0.5 to 10.7; and IFV: aOR, 0.9; 95% CI, 0.1 to 12.3, respectively). This suggests that RV and IFV are important pathogens contributing to asthma exacerbation in children with atopic sensitization (Table 5).
Table 5.
Association between rhinovirus and influenza virus infection and asthma exacerbation according to atopic sensitization
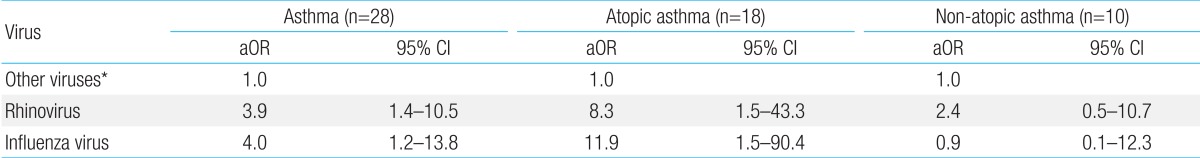
aOR, adjusted odd ratio to age and sex; CI, confidence interval.
*Adenovirus, human metapneumovirus, respiratory syncytial virus, parainfluenza virus.
Discussion
In this study, we assessed clinical diagnoses and atopic characteristics according to causal respiratory viruses identified by multiplex RT-PCR in children who were hospitalized for acute LRTIs. In addition, we evaluated the interaction between respiratory viral infections and allergen sensitization in precipitating acute asthma.
The clinical diagnoses of patients infected with different viruses were relatively distinct. Compared with other viruses, AV was associated with pneumonia, and RSV was frequently observed in patients with acute bronchiolitis. RV or IFV was more likely to be associated with asthma exacerbation, and this result was consistent with observation in previous study of Korea18). Although previous studies have shown that hMPV is more likely to result in acute bronchiolitis rather than pneumonia19,20), we found that hMPV is frequently associated with viral pneumonia. Our finding is in agreement with previous reports21,22). This observation of differences in the various studies may reflect differences in diagnostic definitions, study populations and design, or regions.
In this study, LRTIs caused by RV were associated with high levels of blood eosinophils compare to other viruses. Rakes et al.23) showed that RV infection in wheezing children is associated with nasal eosinophilia and elevated nasal eosinophil cationic protein (ECP). Kato at al.24) reported that serum concentrations of ECP were significantly elevated in children with RV-induced wheezing. These results collectively suggest that RV may be associated with eosinophilic inflammation.
In the present study, we show that hospital admission with acute asthma was strongly associated with a combination of sensitization and infections with RV or IFV compared to other viruses. However, this association was not presented in children without atopic sensitization. This implies atopic sensitization is a very important risk factor for RV or IFV-induced acute exacerbation of asthma.
RV has been shown to play an important role in acute asthma exacerbation as well as in the development of asthma25,26). In a study using inoculation of RV to mild asthmatics, airway hyperresponsiveness was augmented in conjunction with increased ECP in sputum5). Moreover, in a recent study, allergic sensitization appeared to be a risk factor for more severe RV induced illness in school age children with asthma27). While both allergen-sensitized and nonsensitized children had similar rates of viral infections, those who were sensitized experienced a more severe viral respiratory illness and asthma symptoms compared to nonsensitized children. Furthermore, nonsensitized children were more likely to experience asymptomatic infections. Collectively, our observations in keeping with previous reports indicate that a combination of infections with RV and sensitization is closely associated with exacerbation of asthma. The association between RV and atopic sensitization may be explained by the fact that allergic individuals with low interferon-γ level experience greater susceptibility to RV28), and atopic sensitization may promote the expression of the major RV receptor, intercellular adhesion molecule 129).
Several studies have shown the interaction between influenza and allergen sensitization30) as well as increased influenza infection rates in children with asthma31). In this study, we demonstrated an association between asthma exacerbation and IFV infection in atopic children and the present study is supported by our previous study showing the prevalence of H1N1 virus-induced severe LRTI is higher in children with atopic sensitization32).
Although previous reports18,21,22,27) have shown clinical manifestations of respiratory viruses in hospitalized children with LRTIs and association between allergen sensitization and viral infections with respect to the severity of respiratory symptom, this study includes certain unique features that build on existing knowledge. Specifically, the viral etiology and allergen sensitization in children who were hospitalized for LRTIs were confirmed, and the associations between asthma exacerbation and respiratory viruses were analyzed according to the existence of allergen sensitization. We have demonstrated that allergen sensitization may be involved in RV- or IFV-induced acute exacerbation of asthma in children. These findings suggest that potential interactions between allergen sensitization and RV or IFV may play an important role in asthma exacerbation.
Limitations of present study include the modest sample size, the selection of the study population, and the short duration, 1 year, of the study. This study was performed in a single medical center, and subjects were limited to children with LRTIs severe enough to require hospitalization. However, we confirmed viral etiology using PCR and disease diagnosis was made by 4 pediatricians and 2 radiologists using the same diagnostic criteria in the same hospital, which can reduce sample bias. Furthermore, we performed ImmunoCAP test to investigate the presence of allergic sensitization. Another limitation is we cannot show the cause-effect relationship between RV or IFV infection and allergic sensitization in exacerbation of asthma, since this is a cross-sectional study, not a cohort study. Large scale cohort study is needed to investigate the causal relationship between viral infection and atopic sensitization in children with asthma.
In conclusion, infections with RV or IFV may represent a significant risk factor for acute exacerbation of asthma in children who are sensitized to allergens. Influenza vaccination may contribute in preventing asthma exacerbation in those children who are sensitized. Since there are currently no effective antiviral treatments for these respiratory viruses, early detection of sensitized children as well as an improved understanding of the interactions between viral infection and asthma exacerbation is required. Designing novel treatments or preventive strategies for virus-induced exacerbations of asthma in sensitized individuals could provide a valuable therapeutic avenue in addressing these issues.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Ruuskanen O, Lahti E, Jennings LC, Murdoch DR. Viral pneumonia. Lancet. 2011;377:1264–1275. doi: 10.1016/S0140-6736(10)61459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosis S, Esposito S, Niesters HG, Zuccotti GV, Marseglia G, Lanari M, et al. Role of respiratory pathogens in infants hospitalized for a first episode of wheezing and their impact on recurrences. Clin Microbiol Infect. 2008;14:677–684. doi: 10.1111/j.1469-0691.2008.02016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masoli M, Fabian D, Holt S, Beasley R Global Initiative for Asthma (GINA) Program. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 4.Cypcar D, Stark J, Lemanske RF., Jr The impact of respiratory infections on asthma. Pediatr Clin North Am. 1992;39:1259–1276. doi: 10.1016/s0031-3955(16)38444-9. [DOI] [PubMed] [Google Scholar]
- 5.Grünberg K, Smits HH, Timmers MC, de Klerk EP, Dolhain RJ, Dick EC, et al. Experimental rhinovirus 16 infection. Effects on cell differentials and soluble markers in sputum in asthmatic subjects. Am J Respir Crit Care Med. 1997;156(2 Pt 1):609–616. doi: 10.1164/ajrccm.156.2.9610079. [DOI] [PubMed] [Google Scholar]
- 6.Murray CS, Simpson A, Custovic A. Allergens, viruses, and asthma exacerbations. Proc Am Thorac Soc. 2004;1:99–104. doi: 10.1513/pats.2306027. [DOI] [PubMed] [Google Scholar]
- 7.Tan WC. Viruses in asthma exacerbations. Curr Opin Pulm Med. 2005;11:21–26. doi: 10.1097/01.mcp.0000146781.11092.0d. [DOI] [PubMed] [Google Scholar]
- 8.Tsitoura DC, Kim S, Dabbagh K, Berry G, Lewis DB, Umetsu DT. Respiratory infection with influenza A virus interferes with the induction of tolerance to aeroallergens. J Immunol. 2000;165:3484–3491. doi: 10.4049/jimmunol.165.6.3484. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki S, Suzuki Y, Yamamoto N, Matsumoto Y, Shirai A, Okubo T. Influenza A virus infection increases IgE production and airway responsiveness in aerosolized antigen-exposed mice. J Allergy Clin Immunol. 1998;102:732–740. doi: 10.1016/S0091-6749(98)70012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarze J, Gelfand EW. Respiratory viral infections as promoters of allergic sensitization and asthma in animal models. Eur Respir J. 2002;19:341–349. doi: 10.1183/09031936.02.00254302. [DOI] [PubMed] [Google Scholar]
- 11.Murray CS, Poletti G, Kebadze T, Morris J, Woodcock A, Johnston SL, et al. Study of modifiable risk factors for asthma exacerbations: virus infection and allergen exposure increase the risk of asthma hospital admissions in children. Thorax. 2006;61:376–382. doi: 10.1136/thx.2005.042523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green RM, Custovic A, Sanderson G, Hunter J, Johnston SL, Woodcock A. Synergism between allergens and viruses and risk of hospital admission with asthma: case-control study. BMJ. 2002;324:763. doi: 10.1136/bmj.324.7340.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jartti T, Kuusipalo H, Vuorinen T, Soderlund-Venermo M, Allander T, Waris M, et al. Allergic sensitization is associated with rhinovirus-, but not other virus-, induced wheezing in children. Pediatr Allergy Immunol. 2010;21:1008–1014. doi: 10.1111/j.1399-3038.2010.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denny FW, Clyde WA., Jr Acute lower respiratory tract infections in nonhospitalized children. J Pediatr. 1986;108(5 Pt 1):635–646. doi: 10.1016/s0022-3476(86)81034-4. [DOI] [PubMed] [Google Scholar]
- 15.Knutson D, Braun C. Diagnosis and management of acute bronchitis. Am Fam Physician. 2002;65:2039–2044. [PubMed] [Google Scholar]
- 16.Pedersen SE, Hurd SS, Lemanske RF, Jr, Becker A, Zar HJ, Sly PD, et al. Global strategy for the diagnosis and management of asthma in children 5 years and younger. Pediatr Pulmonol. 2011;46:1–17. doi: 10.1002/ppul.21321. [DOI] [PubMed] [Google Scholar]
- 17.Jartti T, Lehtinen P, Vuorinen T, Osterback R, van den Hoogen B, Osterhaus AD, et al. Respiratory picornaviruses and respiratory syncytial virus as causative agents of acute expiratory wheezing in children. Emerg Infect Dis. 2004;10:1095–1101. doi: 10.3201/eid1006.030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim JS, Woo SI, Kwon HI, Baek YH, Choi YK, Hahn YS. Clinical characteristics of acute lower respiratory tract infections due to 13 respiratory viruses detected by multiplex PCR in children. Korean J Pediatr. 2010;53:373–379. [Google Scholar]
- 19.Caracciolo S, Minini C, Colombrita D, Rossi D, Miglietti N, Vettore E, et al. Human metapneumovirus infection in young children hospitalized with acute respiratory tract disease: virologic and clinical features. Pediatr Infect Dis J. 2008;27:406–412. doi: 10.1097/INF.0b013e318162a164. [DOI] [PubMed] [Google Scholar]
- 20.Williams JV, Harris PA, Tollefson SJ, Halburnt-Rush LL, Pingsterhaus JM, Edwards KM, et al. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350:443–450. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi EH, Lee HJ, Kim SJ, Eun BW, Kim NH, Lee JA, et al. The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000-2005. Clin Infect Dis. 2006;43:585–592. doi: 10.1086/506350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HY, Kim KM, Kim SH, Son SK, Park HJ. Clinical manifestations of respiratory viruses in hospitalized children with acute viral lower respiratory tract infections from 2010 to 2011 in Busan and Gyeongsangnam-do, Korea. Pediatr Allergy Respir Dis. 2012;22:265–272. [Google Scholar]
- 23.Rakes GP, Arruda E, Ingram JM, Hoover GE, Zambrano JC, Hayden FG, et al. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am J Respir Crit Care Med. 1999;159:785–790. doi: 10.1164/ajrccm.159.3.9801052. [DOI] [PubMed] [Google Scholar]
- 24.Kato M, Tsukagoshi H, Yoshizumi M, Saitoh M, Kozawa K, Yamada Y, et al. Different cytokine profile and eosinophil activation are involved in rhinovirus- and RS virus-induced acute exacerbation of childhood wheezing. Pediatr Allergy Immunol. 2011;22(1 Pt 2):e87–e94. doi: 10.1111/j.1399-3038.2010.01026.x. [DOI] [PubMed] [Google Scholar]
- 25.Cheuk DK, Tang IW, Chan KH, Woo PC, Peiris MJ, Chiu SS. Rhinovirus infection in hospitalized children in Hong Kong: a prospective study. Pediatr Infect Dis J. 2007;26:995–1000. doi: 10.1097/INF.0b013e3181586b63. [DOI] [PubMed] [Google Scholar]
- 26.Kusel MM, de Klerk NH, Kebadze T, Vohma V, Holt PG, Johnston SL, et al. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119:1105–1110. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olenec JP, Kim WK, Lee WM, Vang F, Pappas TE, Salazar LE, et al. Weekly monitoring of children with asthma for infections and illness during common cold seasons. J Allergy Clin Immunol. 2010;125:1001–1006.e1. doi: 10.1016/j.jaci.2010.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly JT, Busse WW. Host immune responses to rhinovirus: mechanisms in asthma. J Allergy Clin Immunol. 2008;122:671–682. doi: 10.1016/j.jaci.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bianco A, Sethi SK, Allen JT, Knight RA, Spiteri MA. Th2 cytokines exert a dominant influence on epithelial cell expression of the major group human rhinovirus receptor, ICAM-1. Eur Respir J. 1998;12:619–626. doi: 10.1183/09031936.98.12030619. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto N, Suzuki S, Shirai A, Suzuki M, Nakazawa M, Nagashima Y, et al. Dendritic cells are associated with augmentation of antigen sensitization by influenza A virus infection in mice. Eur J Immunol. 2000;30:316–326. doi: 10.1002/1521-4141(200001)30:1<316::AID-IMMU316>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 31.Kloepfer KM, Olenec JP, Lee WM, Liu G, Vrtis RF, Roberg KA, et al. Increased H1N1 infection rate in children with asthma. Am J Respir Crit Care Med. 2012;185:1275–1279. doi: 10.1164/rccm.201109-1635OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim YJ, Ryu SL, Jung SH, Shim JW, Kim DS, Jung HL, et al. Increased prevalence of H1N1-induced severe lower respiratory tract diseases in children with atopic sensitization. Allergy Asthma Immunol Res. 2012;4:277–283. doi: 10.4168/aair.2012.4.5.277. [DOI] [PMC free article] [PubMed] [Google Scholar]


