Abstract
The bile salt export pump (BSEP, ABCB11) is predominantly responsible for the efflux of bile salts, and disruption of BSEP function is often associated with altered hepatic homeostasis of bile acids and cholestatic liver injury. Accumulating evidence suggests that many drugs can cause cholestasis through interaction with hepatic transporters. To date, a relatively strong association between drug-induced cholestasis and attenuated BSEP activity has been proposed. However, whether repression of BSEP transcription would contribute to drug-induced cholestasis is largely unknown. In this study, we selected 30 drugs previously reported as BSEP inhibitors to evaluate their effects on BSEP expression, farnesoid X receptor (FXR) activation, and correlations to clinically reported liver toxicity. Our results indicate that of the 30 BSEP inhibitors, five exhibited potent repression of BSEP expression (≥60% repression), ten were moderate repressors (20–60% repression), whereas others had negligible effects (≤20% repression). Of importance, two drugs (troglitazone and benzbromarone), previously withdrawn from the market because of liver injury, are among the potent repressors. Further investigation of the five potent repressors revealed that transcriptional repression of BSEP by lopinavir and troglitazone may occur through their interaction with FXR, whereas others are via FXR-independent yet unidentified pathways. Our data suggest that in addition to functional inhibition, repression of BSEP expression may play an important role in drug-induced cholestatic liver toxicity. Thus, a combination of the two would reveal a more accurate prediction of drug-induced cholestasis than does either repression or inhibition alone.
Introduction
The primary function of the ATP-binding cassette transporter bile salt export pump (BSEP, ABCB11) is to facilitate enterohepatic circulation by expelling bile salts from hepatocytes to the bile (Childs et al., 1995). Bile salts are synthesized in the liver via the catabolism of cholesterol; however, the majority of bile salts is recycled from the small intestine where they assist in the absorption of dietary fat (Esteller, 2008). BSEP represents one of the rate-limiting mechanisms involved in the enterohepatic circulation (Reichen and Paumgartner, 1976). Disruption of BSEP function has been linked to severe forms of cholestasis, characterized by accumulation of bile salts in the liver, jaundice caused by hyperbilirubinemia, and intestinal malabsorption of dietary fat (Ogimura et al., 2011). Cholestasis can occur either through inherited gene mutation or acquired via environmental factor-induced impairment of bile flow (Bull et al., 1998; Maddrey, 2005). The bile salts accumulated in the liver are polar molecules and, at high levels, can cause inflammation, apoptosis, and lead to various liver diseases (Stieger, 2009).
Although a close correlation between hereditary defects in BSEP gene and the progressive familial intrahepatic cholestasis type 2 has been firmly established, hereditary forms of cholestasis are clinically rare. In contrast, many xenobiotics including clinical used drugs are frequently associated with acquired cholestasis, becoming an increasingly recognized cause of liver disease (Bjornsson and Olsson, 2005). However, the mechanism(s) underlying the involvement of BSEP in the development of drug-induced cholestasis remains unclear. Previous reports have focused primarily on the ability of drugs to inhibit BSEP function, without adequately considering the potential drug-induced perturbation of BSEP expression (Kostrubsky et al., 2003; Morgan et al., 2010). Endpoints for inhibition studies often measure direct efflux competition between bile salts and drugs using plasma-membrane vesicles overexpressing BSEP instead of whole viable cells (van Staden et al., 2012). In some other reports that used rodent or human primary hepatocyte cultures, which provide a physiologically more relevant in vitro hepatic environment, transporter inhibition was evaluated over a short period of time (10–60 minutes) after drug exposure (Kostrubsky et al., 2003; Swift et al., 2010). Thus, contribution of BSEP expression in drug-induced cholestasis was largely unexplored in these studies.
Functioning as the major determinant of bile acids secretion and bile formation, BSEP gene is tightly controlled at the transcriptional level by a number of liver enriched transcription factors. The nuclear receptor farnesoid X-receptor (FXR), a ligand-activated nuclear receptor, plays a pivotal role in the inductive expression of BSEP (Ananthanarayanan et al., 2001). Several bile acids, such as chenodeoxycholic acid (CDCA) and lithocholic acid, are endogenous ligands for FXR, and when accumulated in the liver, these bile acids bind to FXR and trigger the expression of the BSEP gene (Makishima et al., 1999). This feedback mechanism ensures the removal of excess bile salts from the hepatocytes. Notably, BSEP expression is partially retained in the liver of FXR−/− mice, suggesting the existence of additional regulators of BSEP expression (Kubitz et al., 2012). Recent evidence reveals that expression of BSEP is also regulated by the nuclear factor erythroid-derived 2-like 2 (NRF2) and the liver receptor homolog-1 (LRH-1). Knockdown of NRF2 or knockout of LRH-1 was associated with decreased expression of BSEP, whereas activation of NRF2 by oltipraz increased the mRNA expression of BSEP (Song et al., 2008; Weerachayaphorn et al., 2009). Clearly, BSEP expression can be influenced by both endogenous and exogenous chemicals through their interaction with a number of transcription factors. Therefore, it is reasonable to speculate that drug-induced perturbation of BSEP expression in human liver can contribute to the acquired cholestasis.
In this study, we use sandwich-cultured human primary hepatocytes to investigate the role of BSEP repression in drug-induced cholestatic liver injury. Expression of BSEP, FXR, NRF2, and LRH-1 was determined in human hepatocytes upon treatment with an array of clinically used drugs that were reported BSEP inhibitors. Luciferase activation assay for FXR was carried out in HepG2 cells. Correlation of BSEP repression, inhibition, and clinical reported cholestatic injury for these drugs was analyzed. Collectively, our results emphasize that in addition to inhibition, drug-induced BSEP repression is another critical factor that contributes to drug-induced cholestatic liver injury.
Materials and Methods
Chemicals.
Troglitazone, erythromycin estolate, cinnarizine, 17-alpha ethynyl estradiol, simvastatin, benzbromarone, and rifampicin were purchased from Sigma-Aldrich (St. Louis, MO). Bosentan was acquired from Waterstone Technology (Carmel, IN). Glyburide and telmisartan were purchased from AK Scientific Inc. (Union City, CA). Pioglitazone, cyclosporine-A, gefitinb, lapatinib, sorafenib, and imatinib were purchased from Selleck Chemicals (Houston, TX). Glimepiride, lopinavir, paclitaxel, pazopanib, indinavir sulfate, fusidic acid sodium salt, and ketoconazole were obtained from Axxora (Farmingdale, NY). Nefazodone, nicardipine hydrochloride, fenofibrate, nelfinavir, saquinavir, itraconazole, ritonavir, and CDCA were generously provided by Dr. James Polli. The Dual-Luciferase reagent was obtained through Promega (Madison, WI). Primers were synthesized by Integrated DNA Technologies (Coralville, IA). Matrigel was received from BD Biosciences (Bedford, MA). All other cell culture reagents were purchased from Invitrogen (Carlsbad, CA) or Sigma-Aldrich.
Plasmids.
The FXR expression plasmid and BSEP luciferase reporter vector were kindly provided by Dr. David Mangelsdorf (UT Southwestern Medical Center, Dallas, TX) and Dr. Ruitang Deng (University of Rhode Island School of Pharmacy, Princeton, RI), respectively, as previously described (Lu et al., 2000; Deng et al., 2006). The pRL-TK Renilla luciferase plasmid used to normalize firefly luciferase activities were from Promega.
Human Primary Hepatocytes.
Human liver tissues were obtained after surgical resection by qualified pathology staff with donor consent and prior approval from the Institutional Review Board at the University of Maryland, School of Medicine. Hepatocytes were isolated from human liver specimens as described previously (LeCluyse et al., 2005) or obtained from Celsis In Vitro Technologies (Baltimore, MD). Hepatocytes were seeded at 7.5 × 105 cells/well in 12-well collagen-coated plates and cultured in the sandwich format as described previously (Faucette et al., 2006). Forty-eight hours after seeding, hepatocytes were treated with 0.1% DMSO or specified compounds for 24 and 72 hours for detection of mRNA and protein expression, respectively. Culture medium was replaced on a daily basis.
Real-Time Polymerase Chain Reaction.
Total RNA was isolated from hepatocytes using the TRIzol reagent and reverse transcribed using a High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) following the manufacturer's instruction. Primer sequences for real-time polymerase chain reaction (PCR) are as follows: BSEP: 5′-ACATGCTTGCGAGGACCTTTA-3′ (forward), 5′-GGAGGTTCGTGCACCAGGTA-3′ (reverse); FXR: 5′-ATGGGAATGTTGGCTGAATG-3′ (forward), 5′-CCTGCATGACTTTGTTGTCG-3′ (reverse); and GAPDH: 5′-CCCATCACCATCTTCCAGGAG-3′ (forward), 5′-GTTGTCATGGATGACCTTGGC-3′ (reverse). Target gene mRNA expressions were normalized against that of GAPDH. PCR assays were performed in 96-well optical plates on an Applied Biosystems StepOnePlus Real-Time PCR System with SYBR Green PCR Master Mix. Fold induction values were calculated according to the equation: fold over control = 2ΔΔCt, where ΔCt represents the differences in cycle threshold numbers between the target gene and GAPDH, and ΔΔCt represents the relative change in these differences between control and treatment groups.
Western Blotting.
Homogenate proteins (40 µg) from hepatocytes were resolved on SDS-polyacrylamide gels and transferred onto polyvinylidenefluoride membranes. Subsequently, membranes were incubated with specific antibodies against BSEP (Kamiya, Seattle, WA) or beta-actin (Santa Cruz Biotechnology, Santa Cruz, CA) followed with horseradish peroxidase-labeled IgG antibodies. Membranes were developed with West Femto chemiluminescent substrate (Thermo, Rockford, IL).
Transfection in HepG2 Cells.
HepG2 cells cultured in 24-well plates at 1 × 105 cells/well were transfected with the XtremeGene 9 DNA transfection reagent (Roche Applied Science, Indianapolis, IN) 24 hour after seeding. The transfection mixes contained the BSEP luciferase reporter plasmid, FXR expression plasmid, and the pRL-TK construct as internal control. Sixteen hours after transfection, cells were treated with vehicle control (DMSO 0.1%) or CDCA (50 µM) in the presence and absence of selected compounds for 24 hours before harvesting. Cell lysates were assayed for firefly activities and normalized against the activities of Renilla luciferase using the Dual-Luciferase Kit (Promega). Data are represented as mean ± S.D. of three individual transfections.
MTT Assay.
Human primary hepatocytes seeded 7.5 × 104 cells/well in 96-well plates were cultured for 48 hours before treatment with BSEP inhibitors at previously indicated concentrations. A typical 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay was carried out as described previously (Wang et al., 2013). Cell viability was expressed as percent of vehicle control (0.1% DMSO).
Data Analysis.
Results from real-time PCR and reporter gene assays are expressed as mean ± S.D. of triplicate determinations unless otherwise indicated. Statistical analyses were made where appropriate using one-way analysis of variance and χ2 tests.
Results and Discussion
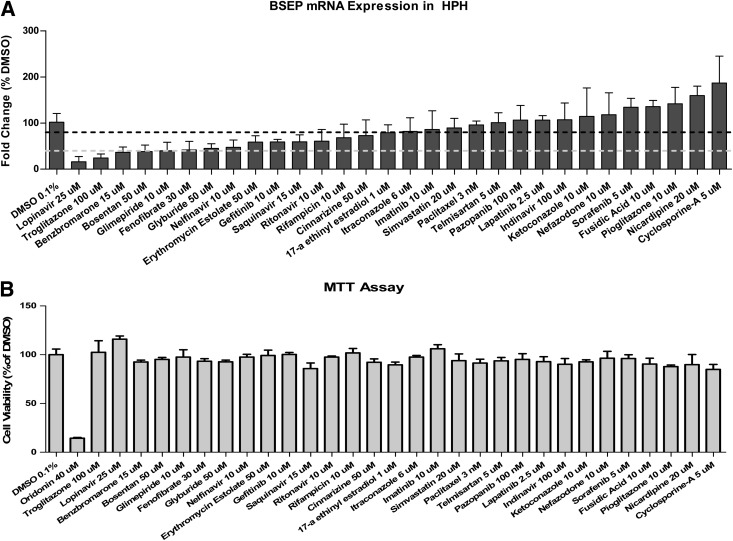
Preclinical prediction of drug-induced liver injury (DILI), the leading cause of attrition in drug development, has proven to be challenging. Oftentimes endogenous and/or exogenous chemicals were accumulated in the hepatocytes and led to inflammation, cholestasis, apoptosis, or necrosis during DILI (Dawson et al., 2012; Yamazaki et al., 2013). Currently, mounting evidence revealed that inhibition of BSEP function is correlated with a large number of drug-induced cholestatic liver liabilities (Ogimura et al., 2011). Many BSEP inhibitors have been identified. Nevertheless, whether transcriptional repression of BSEP expression could contribute to such correlation remains unclear. Utilizing membrane vesicles from transfected Sf9 insect cells, Morgan et al. (2010) evaluated more than 200 compounds including Food and Drug Administration-approved drugs and suggested a relatively strong association between the pharmacological interference with BSEP function and human liver toxicity. In the current study, 30 drugs with a BSEP IC50 value of 25 µM or less from Morgan’s report (Morgan et al., 2010) were analyzed for the BSEP mRNA expression in human primary hepatocyte sandwich cultures obtained from multiple liver donors. As shown in Fig. 1A, these known BSEP inhibitors could be further classified into three groups based on their effects on BSEP expression in human hepatocytes, which include five drugs as potent repressors of BSEP expression (≥60% repression) and ten as moderate repressors with decreased BSEP expression at 20–60% of vehicle control, whereas the rest of the drugs exhibited negligible repression (≤20% repression) to moderate induction. Importantly, all drugs were tested at a concentration selected from literature and were not associated with clear cytotoxicity in cultured human primary hepatocytes (Fig. 1B), suggesting the selective repression of BSEP was not a nonspecific cytotoxic reaction.
Fig. 1.
Repression of BSEP gene expression upon treatment with reported BSEP inhibitors in human primary hepatocytes. BSEP mRNA expression was measured using real-time PCR in hepatocytes from at least three donors upon treatment with BSEP inhibitors as described in Materials and Methods (A). Dotted lines indicate 20% (dark) and 60% (light) BSEP repression compared with vehicle control (0.1% DMSO). A standard MTT assay as described in Materials and Methods was performed in human primary hepatocytes to monitor cytotoxicities associated with BSEP inhibitors at selected concentrations (B). All the data are expressed as mean ± S.D. (n = 3).
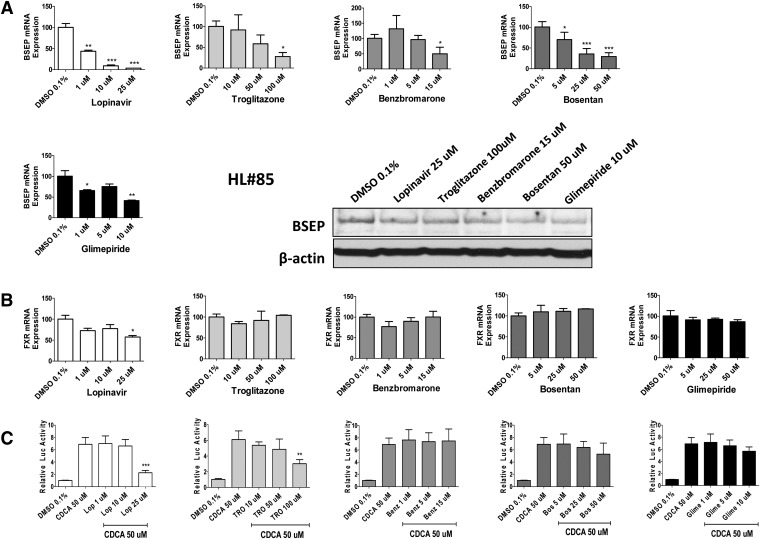
Unlike immediate functional inhibition, alteration of BSEP transcription is regulated by a number of transcription factors and can only be reliably measured in physiologically relevant viable cells or in vivo. Among others, FXR has been recognized as one of the key nuclear receptors that mediates drug-induced as well as basal expression of BSEP (Ananthanarayanan et al., 2001). Of the five potent repressors of BSEP, real-time PCR was used to measure the concentration-dependent mRNA expression of BSEP and FXR. As expected, all five drugs exhibited concentration-dependent repression of BSEP mRNA (Fig. 2A). Additional Western blotting analysis was carried out to examine the expression of BSEP protein upon treatment with each of the five repressors at a single concentration. In agreement with observations at the mRNA level, the identified potent repressors also suppressed BSEP expression at the protein level. On the other hand, expression of FXR was largely unchanged under the same panel of drug treatment (Fig. 2B). It is noteworthy to point out that although the expression level of some nuclear receptors correlates well with their target gene expression, prototypical agonists or antagonists often do not affect the expression of their target receptors (Aranda and Pascual, 2001; Li et al., 2012). To further evaluate whether the potent repressors of BSEP can inhibit the activity of FXR, cell-based luciferase assays by cotransfecting FXR expression and BSEP luciferase plasmids were carried out in HepG2 cells. As demonstrated in Fig. 2C, at higher concentrations, both lopinavir and troglitazone significantly reduced the FXR activity that was preinduced by CDCA, a known endogenous activator of FXR. Intriguingly, a previous report revealed that in Huh7 cells, although troglitazone (10 µM) moderately induces the basal expression of BSEP, it dose dependently represses CDCA-induced BSEP mRNA in a FXR-dependent manner (Kaimal et al., 2009). In contrast, benzbromarone, bosentan, or glimepiride exhibited only negligible effects on FXR activity (Fig. 2C). It appears that some of these potent repressors may suppress BSEP expression via FXR-independent mechanisms. Notably, the expression of NRF2 and LRH-1, two transcription factors that have recently been linked with BSEP expression (Song et al., 2008; Weerachayaphorn et al., 2009), was not significantly affected by the same panel of potent repressors in human hepatocytes (Supplemental Fig. 1). Further activity assays are warranted for elucidating the role of NRF2 and LRH-1 in drug-induced BSEP repression.
Fig. 2.
Concentration-dependent repression of BSEP and FXR. In human primary hepatocytes, five potent repressors of BSEP (≥60% BSEP repression, lopinavir, troglitazone, benzbromarone, bosentan, and glimepiride) were investigated in a concentration-dependent manner to determine their effects on BSEP (A) and FXR (B) mRNA expression as described in Materials and Methods. Repression of the BSEP protein was demonstrated in hepatocytes from liver donor #85 (HL#85). A cell-based luciferase assay was performed in HepG2 cells by cotransfection of the FXR expression and BSEP reporter constructs as detailed in Materials and Methods (C). The known FXR activator CDCA (50 μM) was used as a positive control. Influence of drugs on FXR activity was determined by comparison between CDCA alone and CDCA + drugs. All the data are expressed as a mean ± S.D. (n = 3). ***P < 0.001; **P < 0.01; *P < 0.05.
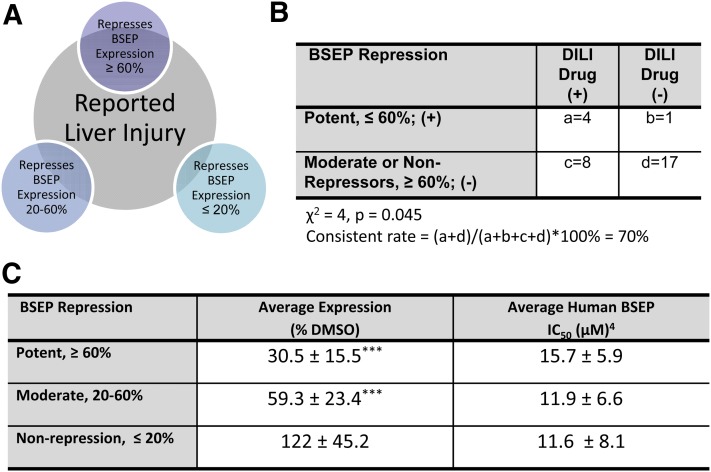
Correlation of the drug-induced BSEP expression profile with clinically reported DILI further reveals that among the five potent repressors, troglitazone and benzbromarone were withdrawn from the market because of severe liver toxicity, and the clinical use of bosentan, an endothelin receptor antagonist, requires monthly monitoring of liver function partly because of its well-known role in intracellular accumulation of cytotoxic bile salts (Fattinger et al., 2001) (Supplemental Table 1). Although it is difficult to quantify these clinically observed DILI, our findings imply that among the 30 selected BSEP inhibitors, the five potent repressors are generally associated with more severe clinical DILI (Fig. 3B) (Chen et al., 2011). Interestingly, the average BSEP IC50 values among the three groups of drugs classified based on BSEP repression were not significantly different (Fig. 3C), further supporting the contribution of BSEP expression in DILI. Meanwhile, we do recognize that a number of compounds as known inhibitors of BSEP exhibited moderate induction of BSEP mRNA in the current study. Although it is extremely challenging to dissect the net effects of inhibition versus induction on the same target molecule, moderate induction of BSEP is unlikely to be a risk factor that enhances BSEP inhibition-mediated liver injury.
Fig. 3.
Correlation between BSEP repression and clinically reported DILI. A, Number of DILI drugs in each group of BSEP repressors. B, The correlation between potent BSEP repression and DILI was analyzed by χ2 test. DILI was determined using criteria set forward in Chen et al., (2011). C, Average BSEP expression and reported IC50 for potent (5), moderate (10), and nonrepressors (15). One-way analysis of variance with post-hoc test Tukey analysis was performed. ***P < 0.001.
In conclusion, the present study in human primary hepatocytes identifies that a number of known BSEP inhibitors are also potent repressors of this gene. Dual inhibitors and repressors of BSEP are often associated with severe clinically reported DILI. Although further systematic and intensive investigations with a larger number of drugs are clearly needed, our current findings indicate that in addition to the well-studied BSEP inhibition, altered expression of this gene should be taken into consideration in predicting drug-induced liver toxicities.
Supplementary Material
Acknowledgments
The authors thank Drs. Ruitang Deng and David Mangelsdorf for providing BSEP luciferase and FXRα1 expression vectors and the University of Maryland Medical Center and Celsis In Vitro Technologies (Baltimore) for providing the human liver samples or hepatocytes used in this study.
Abbreviations
- BSEP
bile salt export pump
- CDCA
chenodeoxycholic acid
- DILI
drug-induced liver injury
- DMSO
dimethyl sulfoxide
- FXR
farnesoid x receptor
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- LRH-1
liver receptor homolog-1
- NRF2
nuclear factor (erythroid 2)-related factor 2
- PCR
polymerase chain reaction
Authorship Contributions
Participated in research design: Garzel, Zhang, Huang, Wang.
Conducted experiments: Garzel, Yang, Wang.
Performed data analysis: Garzel, Polli, Wang.
Wrote or contributed to the writing of the manuscript: Garzel, Yang, Zhang, Huang, Polli, Wang.
Footnotes
This work was supported by the US Food and Drug Administration [Award U01FD004320] and the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK061652]. The manuscript reflects the views of the authors and should not be construed to represent FDA’s views or policies.
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Ananthanarayanan M, Balasubramanian N, Makishima M, Mangelsdorf DJ, Suchy FJ. (2001) Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J Biol Chem 276:28857–28865 [DOI] [PubMed] [Google Scholar]
- Aranda A, Pascual A. (2001) Nuclear hormone receptors and gene expression. Physiol Rev 81:1269–1304 [DOI] [PubMed] [Google Scholar]
- Björnsson E, Olsson R. (2005) Outcome and prognostic markers in severe drug-induced liver disease. Hepatology 42:481–489 [DOI] [PubMed] [Google Scholar]
- Bull LN, van Eijk MJ, Pawlikowska L, DeYoung JA, Juijn JA, Liao M, Klomp LW, Lomri N, Berger R, Scharschmidt BF, et al. (1998) A gene encoding a P-type ATPase mutated in two forms of hereditary cholestasis. Nat Genet 18:219–224 [DOI] [PubMed] [Google Scholar]
- Chen M, Vijay V, Shi Q, Liu Z, Fang H, Tong W. (2011) FDA-approved drug labeling for the study of drug-induced liver injury. Drug Discov Today 16:697–703 [DOI] [PubMed] [Google Scholar]
- Childs S, Yeh RL, Georges E, Ling V. (1995) Identification of a sister gene to P-glycoprotein. Cancer Res 55:2029–2034 [PubMed] [Google Scholar]
- Dawson S, Stahl S, Paul N, Barber J, Kenna JG. (2012) In vitro inhibition of the bile salt export pump correlates with risk of cholestatic drug-induced liver injury in humans. Drug Metab Dispos 40:130–138 [DOI] [PubMed] [Google Scholar]
- Deng R, Yang D, Yang J, Yan B. (2006) Oxysterol 22(R)-hydroxycholesterol induces the expression of the bile salt export pump through nuclear receptor farsenoid X receptor but not liver X receptor. J Pharmacol Exp Ther 317:317–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteller A. (2008) Physiology of bile secretion. World J Gastroenterol 14:5641–5649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattinger K, Funk C, Pantze M, Weber C, Reichen J, Stieger B, Meier PJ. (2001) The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: a potential mechanism for hepatic adverse reactions. Clin Pharmacol Ther 69:223–231 [DOI] [PubMed] [Google Scholar]
- Faucette SR, Sueyoshi T, Smith CM, Negishi M, Lecluyse EL, Wang H. (2006) Differential regulation of hepatic CYP2B6 and CYP3A4 genes by constitutive androstane receptor but not pregnane X receptor. J Pharmacol Exp Ther 317:1200–1209 [DOI] [PubMed] [Google Scholar]
- Kaimal R, Song X, Yan B, King R, Deng R. (2009) Differential modulation of farnesoid X receptor signaling pathway by the thiazolidinediones. J Pharmacol Exp Ther 330:125–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostrubsky VE, Strom SC, Hanson J, Urda E, Rose K, Burliegh J, Zocharski P, Cai H, Sinclair JF, Sahi J. (2003) Evaluation of hepatotoxic potential of drugs by inhibition of bile-acid transport in cultured primary human hepatocytes and intact rats. Toxicol Sci 76:220–228 [DOI] [PubMed] [Google Scholar]
- Kubitz R, Dröge C, Stindt J, Weissenberger K, Häussinger D. (2012) The bile salt export pump (BSEP) in health and disease. Clin Res Hepatol Gastroenterol 36:536–553 [DOI] [PubMed] [Google Scholar]
- LeCluyse EL, Alexandre E, Hamilton GA, Viollon-Abadie C, Coon DJ, Jolley S, Richert L. (2005) Isolation and culture of primary human hepatocytes. Methods Mol Biol 290:207–229 [DOI] [PubMed] [Google Scholar]
- Li L, Sinz MW, Zimmermann K, Wang H. (2012) An insulin-like growth factor 1 receptor inhibitor induces CYP3A4 expression through a pregnane X receptor-independent, noncanonical constitutive androstane receptor-related mechanism. J Pharmacol Exp Ther 340:688–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, Mangelsdorf DJ. (2000) Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell 6:507–515 [DOI] [PubMed] [Google Scholar]
- Maddrey WC. (2005) Drug-induced hepatotoxicity: 2005. J Clin Gastroenterol 39(4, Suppl 2)S83–S89 [DOI] [PubMed] [Google Scholar]
- Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. (1999) Identification of a nuclear receptor for bile acids. Science 284:1362–1365 [DOI] [PubMed] [Google Scholar]
- Morgan RE, Trauner M, van Staden CJ, Lee PH, Ramachandran B, Eschenberg M, Afshari CA, Qualls CW, Jr, Lightfoot-Dunn R, Hamadeh HK. (2010) Interference with bile salt export pump function is a susceptibility factor for human liver injury in drug development. Toxicol Sci 118:485–500 [DOI] [PubMed] [Google Scholar]
- Ogimura E, Sekine S, Horie T. (2011) Bile salt export pump inhibitors are associated with bile acid-dependent drug-induced toxicity in sandwich-cultured hepatocytes. Biochem Biophys Res Commun 416:313–317 [DOI] [PubMed] [Google Scholar]
- Reichen J, Paumgartner G. (1976) Uptake of bile acids by perfused rat liver. Am J Physiol 231:734–742 [DOI] [PubMed] [Google Scholar]
- Song X, Kaimal R, Yan B, Deng R. (2008) Liver receptor homolog 1 transcriptionally regulates human bile salt export pump expression. J Lipid Res 49:973–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieger B. (2009) Recent insights into the function and regulation of the bile salt export pump (ABCB11). Curr Opin Lipidol 20:176–181 . [DOI] [PubMed] [Google Scholar]
- Swift B, Pfeifer ND, Brouwer KL. (2010) Sandwich-cultured hepatocytes: an in vitro model to evaluate hepatobiliary transporter-based drug interactions and hepatotoxicity. Drug Metab Rev 42:446–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Staden CJ, Morgan RE, Ramachandran B, Chen Y, Lee PH, Hamadeh HK.(2012) Membrane vesicle ABC transporter assays for drug safety assessment. Curr Protoc Toxicol 54:23.5.1–23.5.24 [DOI] [PubMed] [Google Scholar]
- Wang D, Li L, Yang H, Ferguson SS, Baer MR, Gartenhaus RB, Wang H. (2013) The constitutive androstane receptor is a novel therapeutic target facilitating cyclophosphamide-based treatment of hematopoietic malignancies. Blood 121:329–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerachayaphorn J, Cai SY, Soroka CJ, Boyer JL. (2009) Nuclear factor erythroid 2-related factor 2 is a positive regulator of human bile salt export pump expression. Hepatology 50:1588–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki M, Miyake M, Sato H, Masutomi N, Tsutsui N, Adam KP, Alexander DC, Lawton KA, Milburn MV, Ryals JA, et al. (2013) Perturbation of bile acid homeostasis is an early pathogenesis event of drug induced liver injury in rats. Toxicol Appl Pharmacol 268:79–89 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.