Abstract
The present studies determined whether clinically relevant phosphodiesterase 5 (PDE5) inhibitors interacted with clinically relevant chemotherapies to kill gastrointestinal/genitourinary cancer cells. In bladder cancer cells, regardless of H-RAS mutational status, at clinically achievable doses, PDE5 inhibitors interacted in a greater than additive fashion with doxorubicin/mitomycin C/gemcitabine/cisplatin/paclitaxel to cause cell death. In pancreatic tumor cells expressing mutant active K-RAS, PDE5 inhibitors interacted in a greater than additive fashion with doxorubicin/gemcitabine/paclitaxel to cause cell death. The most potent PDE5 inhibitor was sildenafil. Knock down of PDE5 expression recapitulated the combination effects of PDE5 inhibitor drugs with chemotherapy drugs. Expression of cellular FLICE-like inhibitory protein-short did not significantly inhibit chemotherapy lethality but did significantly reduce enhanced killing in combination with sildenafil. Overexpression of B-cell lymphoma–extra large suppressed individual and combination drug toxicities. Knock down of CD95 or Fas-associated death domain protein suppressed drug combination toxicity. Combination toxicity was also abolished by necrostatin or receptor interacting protein 1 knock down. Treatment with PDE5 inhibitors and chemotherapy drugs promoted autophagy, which was maximal at ∼24 hour posttreatment, and 3-methyl adenine or knock down of Beclin1 suppressed drug combination lethality by ∼50%. PDE5 inhibitors enhanced and prolonged the induction of DNA damage as judged by Comet assays and γhistone 2AX (γH2AX) and checkpoint kinase 2 (CHK2) phosphorylation. Knock down of ataxia telangiectasia mutated suppressed γH2AX and CHK2 phosphorylation and enhanced drug combination lethality. Collectively our data demonstrate that the combination of PDE5 inhibitors with standard of care chemotherapy agents for gastrointestinal/genitourinary cancers represents a novel modality.
Introduction
The majority of bladder cancers are defined as transitional cell carcinomas; carcinomas of the epithelial cells that are the inner lining of the bladder, including squamous cell and adenocarcinoma. In the United States there are ∼73,000 cases of bladder cancer diagnosed every year with ∼15,000 deaths, with the majority of patients presenting with superficial bladder tumors (stage 0 or stage 1) (Hudson and Herr, 1995; American Cancer Society, 2013, http://www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-figures-2013). Surprisingly, even in patients with disseminated disease >T1, there is the possibility of long-term complete response with multimodal therapies (Torti and Lum, 1984; Catalona et al., 1987; Thrasher and Crawford, 1993).
In patients with superficial disease, the morphologic differentiation status of the tumor cells also determines their response to the most widely used therapy against bladder cancer, the immunotherapy Bacillus Calmette-Guerin (BCG). Of patients who present with poorly differentiated superficial carcinoma cells and who have a complete initial response to BCG, ∼20% show disease progression at 5 years. However in individuals who do not initially fully respond to BCG there is a >90% likelihood of disease progression and metastasis (Lamm et al., 1991; Lacombe et al., 1996; Stein et al., 1998; Quek et al., 2005). Several modalities are routinely used to treat bladder cancer patients, including BCG; urethral surgery and cystectomy; intravesicle chemotherapy with mitomycin C, gemcitabine (Gemzar; Eli Lilly, Indianapolis, IN), and doxorubicin (DOX); and systemic chemo-/radiotherapy with Gemzar (Witjes et al., 1998; Malmstrom et al., 1999; Singal et al., 2000; Rivera, 2003; Shen et al., 2008; Grivas et al., 2013). DOX usefulness has been limited because of myelosuppression and cardiotoxicity. Clearly novel approaches to use DOX, which will reduce normal tissue toxicity, would be helpful. Collectively, these chemotherapeutic approaches have been shown to provide at least 80–90% of patients with an enhanced 5-year disease-free survival.
The erection dysfunction drugs sildenafil (Viagra; Pfizer, Groton, CT), vardenafil (Levitra; Bayer, Leverkusen, Germany), and tadalafil (Cialis; Eli Lilly) inhibit phosphodiesterase 5 (PDE5), the predominant phosphodiesterase enzyme in the corpus cavernosum that is essential for the regulation of vascular smooth muscle contraction through elevation of cGMP levels (Bender and Beavo, 2006). PDE5 inhibitors also protect the heart against ischemia/reperfusion injury and DOX-induced cardiomyopathy. The cardioprotective effect of PDE5 inhibitors is attributed to suppressing apoptosis and necrosis. Downstream of reduced PDE5 function, the effects of elevated cGMP levels include enhanced expression of nitric oxide synthase (NOS) enzymes, particularly endothelial NOS and inducible NOS and activation of protein kinase C isoforms and protein kinase G (Ockaili et al., 2002; Salloum et al., 2003, 2006, 2008; Das et al., 2004, 2005, 2008, 2009; Fisher et al., 2005). Thus increased NOS levels lead to increased nitric oxide (NO) production.
PDE5 expression is also increased in multiple human carcinoma cell types, including breast, colon, bladder, and lung cancers (Bender and Beavo, 2006; Eggen et al., 2012; Karami-Tehrani et al., 2012; Zhang et al., 2012). At high nonphysiologic levels sildenafil and vardenafil suppress tumor cell growth and induce caspase-dependent apoptosis in B-CLL cells (Sarfati et al., 2003). The PDE5 inhibitors sildenafil and vardenafil are also multidrug resistance transporter inhibitors, suggesting they may be useful in the treatment of central nervous system localized diseases where drug penetration across the blood-brain barrier is an issue (Chen et al., 2012). More recently we showed in prostate cancer cells and flank tumors that high concentrations of PDE5 inhibitors (∼10 μM) enhance DOX lethality and protect the heart from DOX toxicity (Das et al., 2010). Mitochondrial reactive oxygen species (ROS) is one key component of antitumor activity of DOX in tumor cells. In prostate cancer cells, ROS /reactive nitrogen species (RNS) levels in DOX- and sildenafil-treated cells were greater than those in either PDE5 inhibitor alone or DOX alone.
The present studies were designed to determine whether PDE5 inhibitors interacted with standard of care cancer chemotherapeutic agents to kill gastrointestinal/genitourinary tumor cells in vitro. It is known that NO donors such as exisulind inhibit bladder carcinogenesis and that PDE5 inhibitors increase NO levels (Piazza et al., 2001). Our data show that PDE5 inhibitors interact in an on-target fashion with DOX, mitomycin C, and Gemzar to kill bladder cancer cells and do so through increased death receptor signaling mediated by caspase 8 and increased autophagy mediated by receptor interacting protein 1 (RIP-1).
Materials and Methods
Phospho-/total antibodies were purchased from Cell Signaling Technology (Danvers, MA) and Santa Cruz Biotechnology (Santa Cruz, CA). All drugs were purchased from Selleckchem (Houston, TX). Commercially available validated short hairpin RNA molecules to knock down RNA/protein levels were from Qiagen (Valencia, CA). Antibody reagents, other kinase inhibitors, caspase inhibitors, cell culture reagents, and noncommercial recombinant adenoviruses have been previously described (Park et al., 2008; Zhang et al., 2008; Bareford et al., 2011; Cruickshanks et al., 2012). Cell death assays were performed using both trypan blue exclusion and the Millipore Scepter system with 60-μm tips (Billerica, MA).
Cell Culture and In Vitro Exposure of Cells to Drugs
All established cancer lines were cultured at 37°C [5% (v/v CO2)] in vitro using RPMI supplemented with 5% (v/v) fetal calf serum and 10% (v/v) nonessential amino acids. For short-term cell killing assays and immunoblotting, cells were plated at a density of 3 × 103/cm2 and 24 hour after plating were treated with various drugs as indicated. In vitro small molecule inhibitor treatments were from a 100-mM stock solution of each drug, and the maximal concentration of vehicle (dimethylsulfoxide) in media was 0.02% (v/v). Cells were not cultured in reduced serum media during any study.
Cell Treatments, SDS-PAGE, and Western Blot Analysis
Cells were treated with various drug concentrations, as indicated in the figure legends. SDS-PAGE and immunoblotting was performed as described in Park et al. (2008), Zhang et al. (2008), Bareford et al. (2011), and Cruickshanks et al. (2012).
Recombinant Adenoviral Vectors: Infection In Vitro
We generated and purchased previously noted recombinant adenoviruses as per Park et al. (2008), Zhang et al. (2008), Bareford et al. (2011), and Cruickshanks et al. (2012). Cells were infected with these adenoviruses at an approximate multiplicity of infection as indicated in the figures (usually 50 multiplicity of infection). Cells were incubated for 24 hour to ensure adequate expression of transduced gene products prior to drug exposures.
Assessment of ROS Generation
Cancer cells were plated in 96-well plates. Cells were preincubated with dihydro-dichlorofluorescein (5 mM for 30 minutes). Fluorescence measurements were obtained 0–30 minutes after drug addition with a Vector 3 plate reader. Data are presented corrected for basal fluorescence of vehicle-treated cells at each time point and expressed as a fold increase in ROS levels.
Detection of Cell Death by Trypan Blue, Hoechst, Terminal Deoxynucleotidyl Transferase UTP Nick End Labeling, and Flow Cytometric Assays
Cells were harvested by trypsinization with Trypsin/EDTA for ∼10 minutes at 37°C. Cell death assays were performed as described in Park et al. (2008), Bareford et al. (2011), and Cruickshanks et al. (2012).
Assessment of Autophagy
Cells were transfected with a plasmid to express a green fluorescent protein (GFP) tagged form of LC3 (ATG8). For analysis of cells transfected with the GFP-LC3 construct, the GFP-LC3–positive vesicularized cells were examined under the ×40 objective of a Zeiss Axiovert fluorescent microscope (Carl Zeiss AG, Jena, Germany) (Park et al., 2008; Bareford et al., 2011; Cruickshanks et al., 2012).
Plasmid Transfection
Plasmids.
Cells were plated as described above, and 24 hours after plating they were transfected. Plasmids (0.5 μg) expressing a specific mRNA or appropriate vector control plasmid DNA were diluted in 50 μl serum- and antibiotic-free medium (1 portion for each sample). Concurrently, 2 μl Lipofectamine 2000 (Invitrogen, Carlsbad, CA), was diluted into 50 μl of serum-free and antibiotic-free medium. Diluted DNA was added to the diluted Lipofectamine 2000 for each sample and incubated at room temperature for 30 minutes. This mixture was added to each well/dish of cells containing 200 μl serum- and antibiotic-free medium for a total volume of 300 μl, and the cells were incubated for 4 hours at 37°C. An equal volume of 2× medium was then added to each well. Cells were incubated for 48 hours and then treated with drugs. To assess transfection efficiency of plasmids we used a plasmid to express GFP and defined the percentage of cells being infected as the percentage of GFP+ cells. For all cell lines, the infection efficiency was >70%.
siRNA.
Cells were plated in 60-mm dishes from a fresh culture growing in log phase as described above, and 24 hours after plating they were transfected. Prior to transfection, the medium was aspirated and 1 ml serum-free medium was added to each plate. For transfection, 10 nM of the annealed siRNA, the positive sense control doubled-stranded siRNA targeting GAPDH or the negative control (a “scrambled” sequence with no significant homology to any known gene sequences from mouse, rat, or human cell lines) were used (predominantly Qiagen; occasional alternate siRNA molecules were purchased from Ambion, Inc., Austin, TX). Ten nanomoles siRNA (scrambled or experimental) was diluted in serum-free media. Four microliters Hiperfect (Qiagen) was added to this mixture, and the solution was mixed by pipetting up and down several times. This solution was incubated at room temperature for 10 minutes and then added drop-wise to each dish. The medium in each dish was swirled gently to mix and then incubated at 37°C for 2 hours. One milliliter of 10% (v/v) serum-containing medium was added to each plate, and cells were incubated at 37°C for 24–48 hours before replating (50 × 103 cells each) onto 12-well plates. Cells were allowed to attach overnight and then treated with drugs (0–48 hours). Trypan blue exclusion assays and SDS PAGE/immunoblotting analyses were then performed at the indicated time points.
Data Analysis
Comparison of the effects of various treatments was performed using one-way analysis of variance and a two-tailed Student’s t test. Synergy was measured by the method of Chou and Talalay (1984): combination index values of less than 1.00 were considered synergistic. Differences with a P value of <0.05 were considered statistically significant. Experiments shown are the means of multiple individual points from multiple experiments (± S.E.M.).
Results
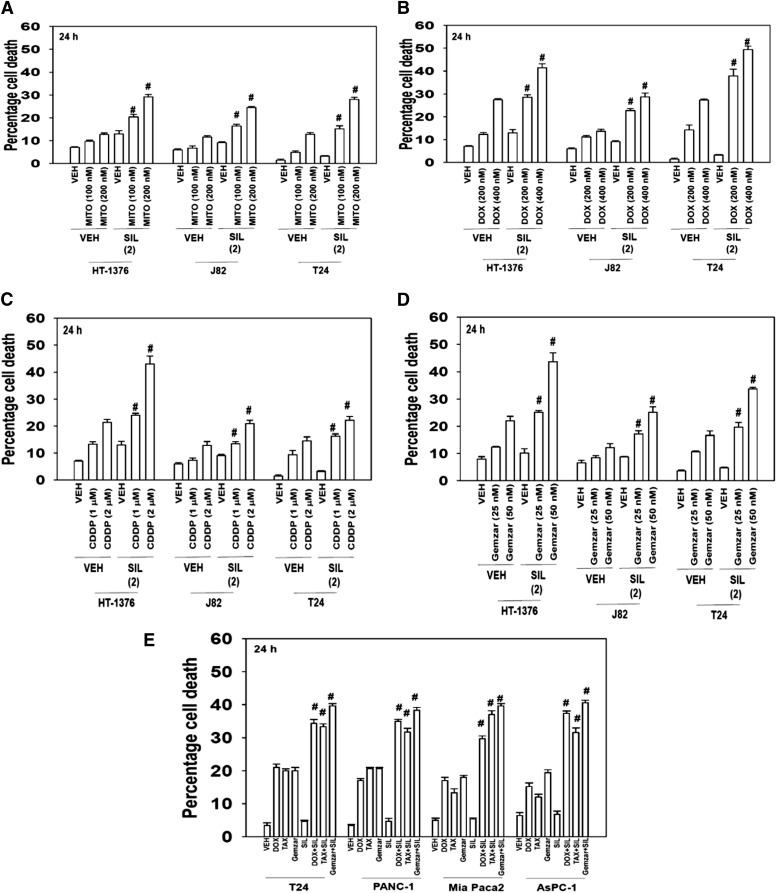
Initial studies examined whether there was a lethal interaction between Food and Drug Administration-approved PDE5 inhibitors such as sildenafil and standard of care chemotherapeutic agents for bladder cancer including mitomycin C, doxorubicin, cisplatin, and gemcitabine. Sildenafil enhanced the lethality of mitomycin C, doxorubicin, cisplatin, and gemcitabine in bladder cancer cell lines in short-term survival assays (Fig. 1, A–D; P < 0.05). The toxic interaction of PDE5 inhibitors with chemotherapeutic agents was not just restricted to bladder cancer cells, as in pancreatic cancer cells, sildenafil also enhanced the lethality of doxorubicin, paclitaxel, and gemcitabine (Fig. 1E; (P < 0.05).
Fig. 1.
The PDE5 inhibitor sildenafil interact with established cytotoxic chemotherapy agents to kill multiple bladder cancer cell lines. (A) Bladder cancer cells (HT-1376; J82; T24) were treated with mitomycin C (MITO 100–200 nM) and/or sildenafil (SIL, 2.0 μM). Cells were isolated after 24 hours, and viability was determined by trypan blue exclusion (n = 3, ± S.E.M.). #P < 0.05 greater than corresponding value in vehicle (VEH) control. (B) Bladder cancer cells (HT-1376; J82; T24) were treated with DOX (200–400 nM) and/or SIL (2.0 μM). Cells were isolated after 24 hours, and viability was determined by trypan blue exclusion (n = 3, ± S.E.M.). #P < 0.05 greater than corresponding value in vehicle control. (C) Bladder cancer cells (HT-1376; J82; T24) were treated with cisplatin [cisplatinum (CDDP); 1000–2000 nM] and/or SIL (2.0 μM). Cells were isolated after 24 hours, and viability was determined by trypan blue exclusion (n = 3, ± S.E.M.). #P < 0.05 greater than corresponding value in vehicle control. (D) Bladder cancer cells (HT-1376; J82; T24) were treated with Gemzar (25–50 nM) and/or SIL (2.0 μM). Cells were isolated after 24 hours, and viability was determined by trypan blue exclusion (n = 3, ± S.E.M.). #P < 0.05 greater than corresponding value in vehicle control. (E) Bladder and pancreatic cancer cells (T24, PANC-1, Mia Paca2, AsPC-1) were treated with Gemzar (25 nM) and/or paclitaxel (TAX, 10 nM) and/or SIL (2.0 μM). Cells were isolated after 24 hours, and viability was determined by trypan blue exclusion (n = 3, ± S.E.M.). #P < 0.05 greater than corresponding value in vehicle control.
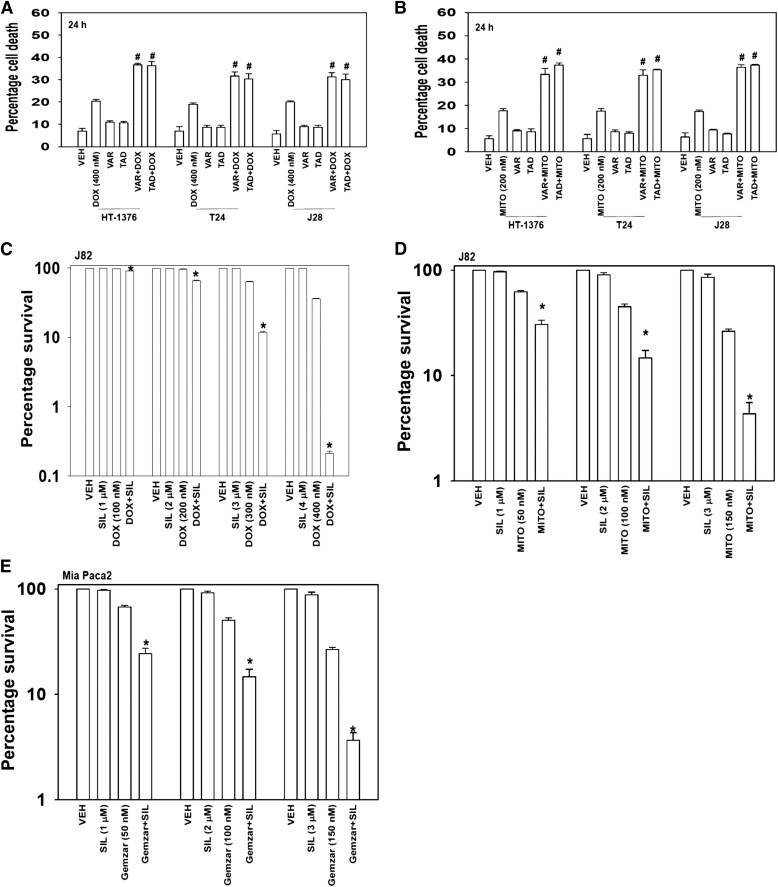
Sildenafil is not the only Food and Drug Administration–approved PDE5 inhibitor, with the chemically related vardenafil and chemically dissimilar tadalafil also being approved for use. Parallel combinatorial killing data to that using sildenafil were obtained using the PDE5 inhibitors vardenafil and tadalafil (Fig. 2, A and B; P < 0.05). In long-term colony formation assays, sildenafil enhanced the lethality of doxorubicin, mitomycin C, and gemcitabine in an apparently greater than additive fashion (Fig. 2, C–E; P < 0.05). As measured by the method of Chou and Talalay (1984), the range of combination index values for each of these panels were Fig. 2C, 0.36–0.19; Fig. 2D, 0.58–0.43; Fig. 2E, 0.65–0.55. As the measured combination indexes were less than 1.00, our data tend to argue that we were observing a synergy of drug interaction in terms of cell killing.
Fig. 2.
PDE5 inhibitors enhance doxorubicin or mitomycin C toxicity. (A) Bladder cancer cells (HT-1376; J82; T24) were treated with DOX (400 nM) and/or vardenafil (VAR, 0.5 μM) and/or tadalafil (TAD, 2 μM). Cells were isolated after 24 hours, and viability was determined by trypan blue exclusion (n = 3, ± S.E.M.). #P < 0.05 greater than corresponding value in vehicle control. (B) Bladder cancer cells (HT-1376; J82; T24) were treated with mitomycin C (MITO, 200 nM) and/or VAR (0.5 μM) and/or TAD (2 μM). Cells were isolated after 24 hours, and was viability determined by trypan blue exclusion (n = 3, ± S.E.M.). #P < 0.05 greater than corresponding value in vehicle control. (C) J82 cells were plated as single cells in sextuplicate (250–500 cells per well). Twelve hours after plating cells were treated with vehicle, sildenafil (SIL, 1–4 μM), DOX (100–400 nM), or the combination in a fixed dose ratio. Cells were treated with drugs for 24 hours followed by culture in drug-free media for ∼10 days. Colonies were fixed, stained, and counted (n = 3, ± S.E.M.). *P < 0.05 less than DOX alone value. (D) J82 cells were plated as single cells in sextuplicate (250–500 cells per well). Twelve hours after plating cells were treated with vehicle, SIL (1–3 μM), MITO (50–150 nM), or the combination in a fixed dose ratio. Cells were treated with drugs for 24 hours followed by culture in drug-free media for ∼10 days. Colonies were fixed, stained, and counted (n = 3, ± S.E.M.). *P < 0.05 less than MITO alone value. (E) Mia Paca 2 cells were plated as single cells in sextuplicate (250–500 cells per well). Twelve hours after plating cells were treated with vehicle, SIL (1–3 μM), Gemzar (50–150 nM), or the combination in a fixed dose ratio. Cells were treated with drugs for 24 hours followed by culture in drug-free media for ∼10 days. Colonies were fixed, stained, and counted (n = 3, ± S.E.M.). *P < 0.05 less than Gemzar alone value.
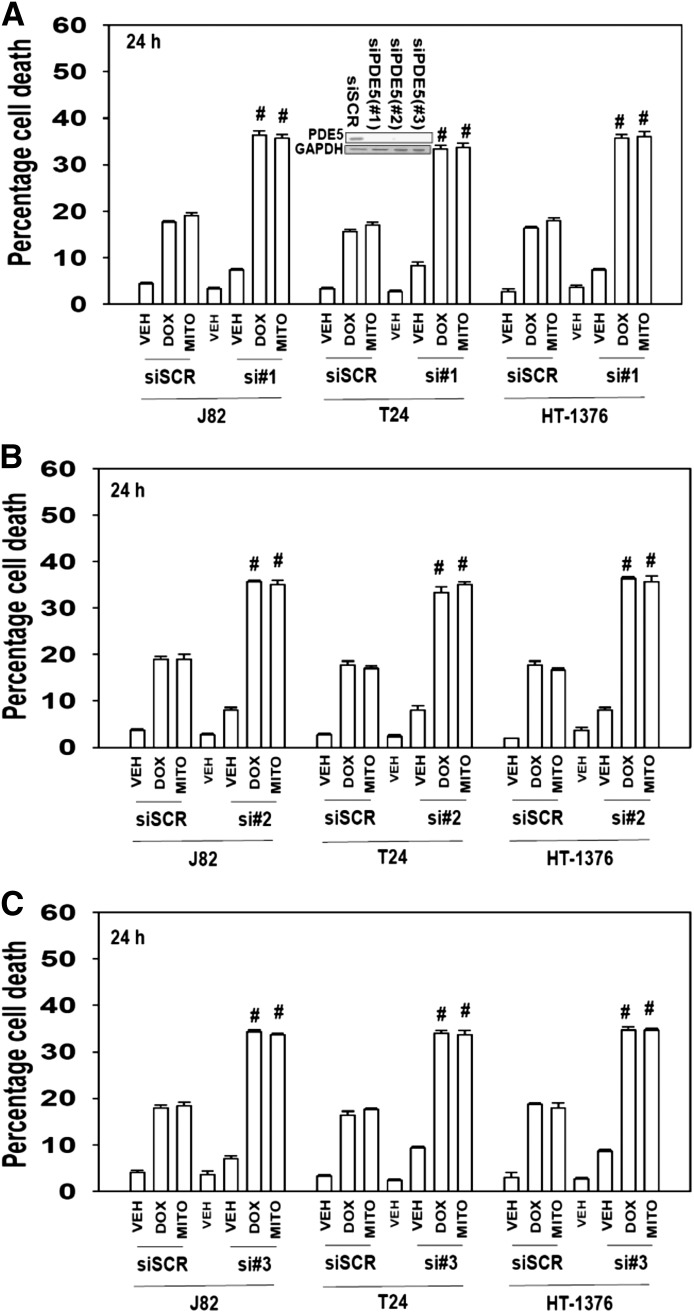
The PDE5 inhibitors we have used can also have inhibitory effects on other PDE enzymes. Thus we wished to determine whether inhibition of PDE5 itself was playing any role in the observed combinatorial killing effect. Knock down of PDE5 using three different siRNA molecules enhanced chemotherapy toxicity in vitro (Fig. 3, A–C; P < 0.05). Thus, regardless of H-/K-RAS mutational status or side-effects on other PDE isoforms, the drug combination of PDE5 inhibitor plus chemotherapy agent resulted in greater than additive killing effects in tumor cells.
Fig. 3.
Knock down of PDE5 expression enhances doxorubicin or mitomycin C lethality. Bladder cancer cells (HT-1376; J82; T24) were transfected with scrambled siRNA (siSCR) or one of three different siRNA molecules to knock down expression of PDE5 (si#1, si#2, si#3; A–C). Thirty-six hours after transfection cells were treated with vehicle, mitomycin C (MITO, 100 nM), DOX (200 nM) as indicated. Cells were isolated after 24 hours, and viability was determined by trypan blue exclusion (n = 3, ± S.E.M.). #P < 0.05 greater than corresponding value in siSCR control.
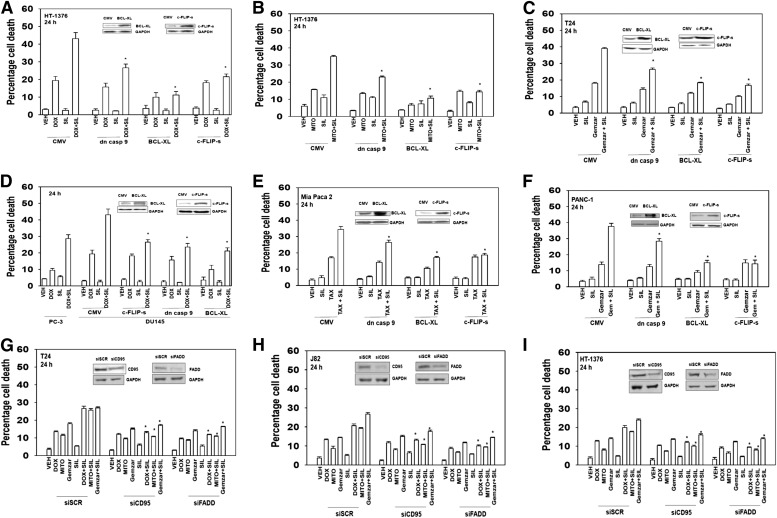
We next attempted to determine the mechanisms by which the drug combination killed cells, i.e., the mitochondrial/caspase 9 intrinsic pathway, the caspase 8 extrinsic pathway, autophagy, or necrosis. Expression of dominant negative caspase 9 reduced overall killing but did not prevent sildenafil enhancing chemotherapy toxicity (Fig. 4, A–C). However, overexpression of either the mitochondrial protective protein B-cell lymphoma–extra large (BCL-XL) or the caspase 8 inhibitor cellular FLICE-like inhibitory protein short (c-FLIP-s) prevented sildenafil enhancing chemotherapy toxicity. Similar data were obtained in another GU cell type: prostate cancer cells (Fig. 4D). Comparable data to that in bladder and prostate tumor cells were obtained when sildenafil was combined with paclitaxel and gemcitabine in GI pancreatic cancer cells (Fig. 4, E and F). The data obtained expressing the caspase 8 inhibitor c-FLIP-s or the mitochondrial protective protein BCL-XL suggests that both death receptor and aberrant mitochondrial signaling are part of the killing process. In agreement with the protective effect of the caspase 8 inhibitor c-FLIP-s, knock down of death receptor expression (CD95) or the linker protein Fas-associated death domain protein expression suppressed the toxicity enhancing activity of sildenafil (Fig. 4, G–I). The combination of sildenafil with chemotherapies increased plasma membrane surface levels of the death receptor CD95, demonstrating that the sildenafil and chemotherapy drug combinations induced receptor activation (Fig. 5).
Fig. 4.
The toxic interaction between PDE5 inhibitors and chemotherapy is blocked by overexpression of BCL-XL or c-FLIP-s. Bladder cancer cells (HT-1376; J82; T24) were infected with empty vector adenovirus (CMV) or three other viruses to express dominant negative caspase 9 (dn casp 9); BCL-XL; c-FLIP-s. (A–C) Thirty-six hours after infection cells were treated with vehicle, sildenafil (SIL, 2 μM), mitomycin C (MITO, 100 nM), DOX (200 nM), Gemzar (50 nM) as indicated. Cells were isolated after 24 hours, and viability was determined by trypan blue exclusion (n = 3, ± S.E.M.). *P < 0.05 less than corresponding value in CMV control. (D) Prostate cancer cells (DU145) were infected with CMV or three other viruses to express dn casp 9, BCL-XL, c-FLIP-s. Thirty-six hours after infection cells were treated with vehicle, SIL (2 μM), DOX (200 nM), as indicated. PC-3 prostate cancer cells that lack phosphatase and tensin homolog and have constitutive AKT activity were used as an internal control. Cells were isolated after 24 hours, and viability was determined by trypan blue exclusion (n = 3, ± S.E.M.). *P < 0.05 less than corresponding value in CMV control. (E and F) Pancreatic cancer cells (PANC-1, Mia Paca2) were infected with CMV or three other viruses to express dn casp 9: BCL-XL, c-FLIP-s treated with vehicle, SIL (2 μM), and/or gemcitabine (Gemzar, 50 nM) and/or paclitaxel (TAX, 10 nM) as indicated. Cells were isolated after 24 hours, and viability was determined by trypan blue exclusion (n = 3, ± S.E.M.). (G–I) Bladder cancer cells (HT-1376; J82; T24) were transfected with scrambled siRNA (siSCR) or siRNA molecules to knock down expression of CD95 or Fas-associated death domain protein (FADD) (siCD95, siFADD). Thirty-six hours after transfection cells were treated with vehicle, SIL (2 μM), MITO (100 nM), DOX (200 nM), Gemzar (50 nM) as indicated. Cells were isolated after 24 hours, and viability was determined by trypan blue exclusion (n = 3, ± S.E.M.). *P < 0.05 less than corresponding value in siSCR control.
Fig. 5.
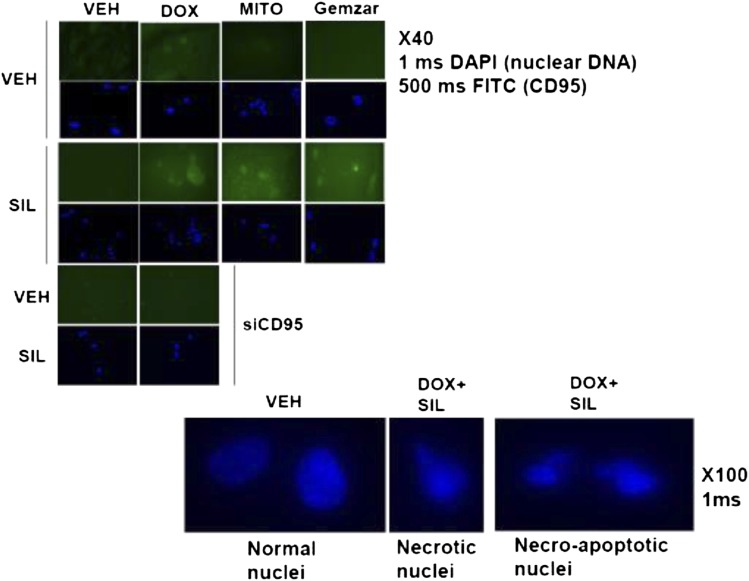
The toxic interaction between PDE5 inhibitors and chemotherapy is associated with increased plasma membrane levels. Bladder T24 cells were grown in chamber slides. Twenty-four hours after plating cells were treated with vehicle, sildenafil (SIL, 2 μM), mitomycin C (MITO, 100 nM), DOX (200 nM), Gemzar (50 nM) as indicated. Cells were fixed 6 hours after exposure, and the cell surface levels of CD95 under each condition were determined by immunohistochemistry. DAPI, 4′,6-diamidino-2-phenylindole; FITC, fluorescein isothiocyanate.
On the basis of the known actions of the individual drugs, treatment of cells with chemotherapy and/or sildenafil is expected to increase the levels of ROS and RNS. Incubation of cells with the nitric oxide synthase inhibitor N(G)-nitro-l-arginine (l-NAME) or the ROS quenching agent N-acetyl cysteine (NAC) significantly reduced the increase in CD95 plasma membrane localization by 67 ± 8 and 79 ± 9%, respectively (P < 0.05).
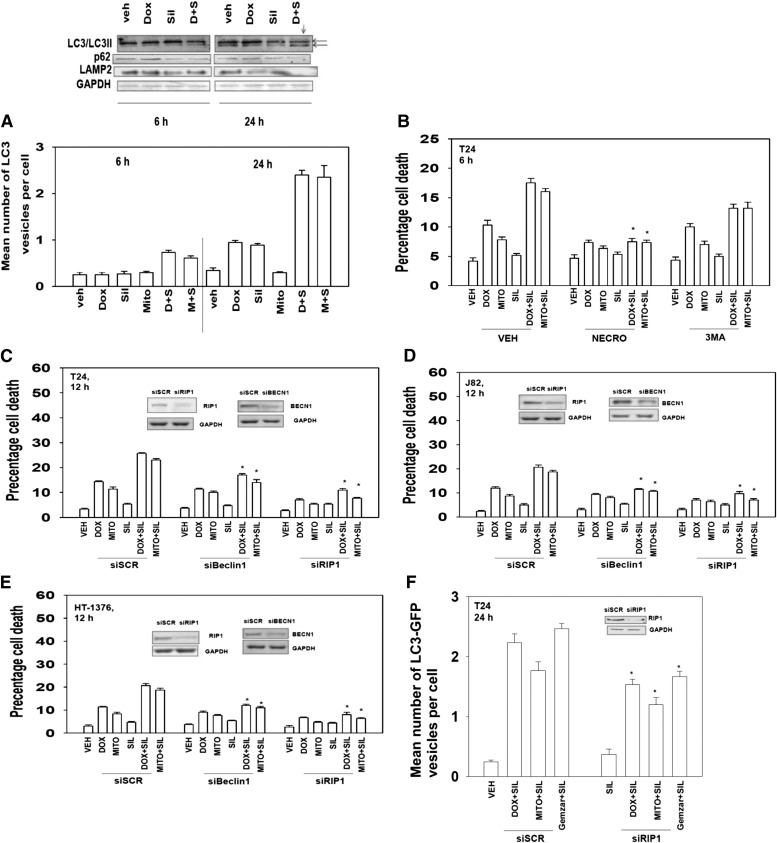
Prior studies by our laboratory have shown that activation of the death receptor CD95 can increase the vesicularization of an expressed LC3-GFP fusion protein and elevate LC3II processing, both suggestive of autophagosome formation (autophagy). In the present studies, combined exposure of cells to sildenafil and chemotherapy resulted in enhanced LC3-GFP vesicularization that correlated with enhanced processed LC3II levels and reduced levels of p62 and LAMP2, all indicative of autophagy flux, i.e., formation of vesicles followed by their fusion with acidic endosomes and finally protein digestion (Fig. 6A; 24-hour time point, observe lane below arrow). Treatment of cells with the small molecule inhibitor of the class III phosphatidyl inositol 3-kinase vps34 (3-methyl adenine), an enzyme essential for vesicle formation, suppressed the lethality of sildenafil and chemotherapy at the 6-hour time point, whereas the small molecule inhibitor of RIP-1 (necrostatin; inhibition of necrotic cell killing) abolished killing by all agents at this time (Fig. 6B). Knock down of the autophagy regulatory protein Beclin1 suppressed the induction of LC3-GFP vesicles and reduced killing by the drug combinations (Fig. 6, C–E, data not shown). Knock down of RIP-1, in a manner similar to necrostatin, abolished killing by all agents at this time (Fig. 6, C–E). In general agreement with these findings, knock down of RIP-1 partially, although significantly, reduced the levels of autophagy caused by the drug combination treatments (Fig. 6F). This suggests that downstream of CD95, RIP-1 may stimulate cell death through autophagy-dependent and -independent pathways.
Fig. 6.
Sildenafil and chemotherapy-induced lethality is mediated through RIP-1 and increased autophagy. (A) (Bottom) T24 cells were transfected with a plasmid to express LC3-GFP. Twenty-four hours after transfection cells were treated with vehicle, sildenafil (SIL, 2 μM), mitomycin C (MITO, 100 nM), DOX (200 nM), Gemzar (50 nM) as indicated. Cells were examined 6 and 24 hours after treatment using a fluorescent microscope, and the mean number of LC3-GFP+ vesicles was determined in >40 cells (n = 3, ± S.E.M.). (Top) T24 cells were treated with vehicle, SIL (2 μM), DOX (200 nM) as indicated. Cells were isolated 6 and 24 hours after treatment, and immunoblotting was performed to examine the phosphorylation/expression of the indicated proteins (n = 3). (B) T24 cells were treated with vehicle, SIL (2 μM), MITO (100 nM), DOX (200 nM) as indicated. In parallel, cells were treated with vehicle, necrostatin (1.0 μM) or 3-methyl adenine (3MA, 10 mM). Cells were isolated after 6 hours, and viability was determined by trypan blue exclusion (n = 3, ± S.E.M.). *P < 0.05 less than corresponding value in vehicle control. (C–E) Bladder cancer cells (HT-1376; J82; T24) were transfected with scrambled siRNA (siSCR) or siRNA molecules to knock down expression of RIP-1 or Beclin1 (siRIP-1, siBeclin1). Thirty-six hours after transfection, cells were treated with vehicle, SIL (2 μM), MITO (100 nM), or DOX (200 nM) as indicated. Cells were isolated after 12 hours, and viability was determined by trypan blue exclusion (n = 3, ± S.E.M.). *P < 0.05 less than corresponding value in siSCR control. (F) T24 cells were transfected to express LC3-GFP and with siSCR or siRNA molecules to knock down expression of RIP-1 (siRIP-1). Thirty-six hours after transfection cells were treated with vehicle, SIL (2 μM), MITO (100 nM), or DOX (200 nM) as indicated. Cells were examined 24 hours after treatment using a fluorescent microscope, and the mean number of LC3-GFP+ vesicles was determined in >40 cells (n = 3, ± S.E.M.). GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
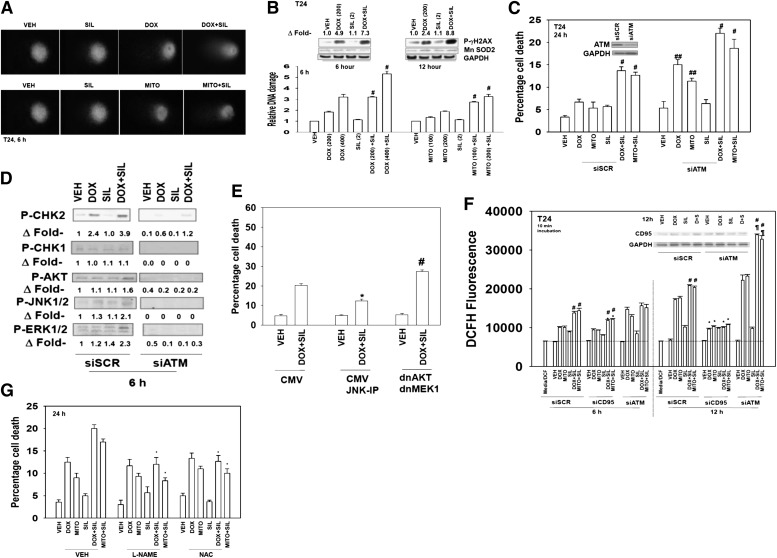
Standard of care chemotherapy agents such as doxorubicin are known to cause DNA damage as part of their killing mechanism. Sildenafil rapidly and significantly enhanced the amount of DNA damage caused by doxorubicin or mitomycin C as judged in Comet assays (Fig. 7, A, images, and B, quantified tail moments). The drug combination significantly increased the phosphorylation of γhistone 2AX (γH2AX) 6 hours after exposure (Fig. 7B, upper blots). Of greater interest was that at the 12-hour time point where doxorubicin-induced γH2AX phosphorylation was declining whereas that of the drug combination was increasing. The phosphorylation of γH2AX is thought to be regulated via the ataxia telangiectasia mutated (ATM) protein. As previously published by us and others, knock down of ATM reduced drug-induced γH2AX phosphorylation (data not shown; Golding et al., 2007; Booth et al., 2013). Knock down of ATM increased the toxicity of doxorubicin and of mitomycin C (Fig. 7C). Knock down of ATM further enhanced the toxicity of sildenafil combined with doxorubicin or mitomycin C. Knock down of ATM suppressed doxorubicin-induced phosphorylation of checkpoint kinase 2 (CHK2) within 6 hours (Fig. 7D). The drug combination modestly activated the c-Jun NH2-terminal kinase (JNK1/2), extracellular signal-regulated kinase (ERK) 1/2, and thymoma viral proto-oncogene (AKT) signal transduction pathways within 6 hours; inhibition of JNK1/2 signaling suppressed killing and inhibition of ERK kinase (MEK) 1 and AKT enhanced killing (Fig. 7E).
Fig. 7.
Sildenafil increases and prolongs chemotherapy-induced DNA damage; knock down of ATM enhances drug combination toxicity. (A) T24 cells were grown in soft agar and treated as indicated with vehicle, sildenafil (SIL, 2 μM), mitomycin C (MITO, 100 nM), DOX (200 nM) as indicated for 6 hours. Cells were subjected to electrophoresis and stained. Images are a representative (n = 4). (B) Graph: the tail moments of cells imaged in Awere measured and plotted (n = 4, ± S.E.M.). #P < 0.05 greater than corresponding value in vehicle-treated cells. Blot: T24 cells were treated with vehicle, SIL (2 μM), DOX (200 nM) as indicated. Cells were isolated 6 and 12 hours after exposure, and the phosphorylation of γH2AX was determined, with the fold increase in phosphorylation shown (n = 3, ± S.E.M.). (C) T24 cells were transfected with scrambled siRNA (siSCR) or siRNA molecules to knock down expression of ATM (siATM). Thirty-six hours after transfection cells were treated with vehicle, SIL (2 μM), MITO (100 nM), or DOX (200 nM) as indicated. Cells were isolated after 24 hours, and viability was determined by trypan blue exclusion (n = 3, ± S.E.M.). #P < 0.05 greater than DOX/MITO only treated cells; ##P < 0.05 greater than corresponding value in siSCR control. (D and E) Blot: T24 cells were transfected with siSCR or siRNA molecules to knock down expression of ATM (siATM). Thirty-six hours after transfection cells were treated with vehicle, SIL (2 μM), and/or DOX (200 nM) as indicated. Cells were isolated after 6 hours, and the expression and the phosphorylation of the indicated proteins was determined (n = 3). Graph: T24 cells were transfected with an empty vector plasmid (CMV) or plasmids to express dominant negative MEK1 and dominant negative AKT. Twenty-four hours after transfection, cells were as indicated treated with the JNK inhibitory peptide (JNK-IP, 10 μM) and then treated with vehicle or SIL (2 μM) and DOX (200 nM) as indicated. Cells were isolated after 24 hours, and viability was determined by trypan blue exclusion (n = 3, ± S.E.M.). #P < 0.05 greater than SIL+DOX in CMV cells; *P < 0.05 less than corresponding value in CMV. (F) T24 cells were plated in 96-well plates in phenol red free media. T24 cells were transfected with siSCR or siRNA molecules to knock down expression of ATM (siATM) or CD95 (siCD95). Thirty-six hours after transfection cells were treated with vehicle, SIL (2 μM), MITO (100 nM), or DOX (200 nM) as indicated. Cells were incubated with dihydro-dichlorofluorescein (5 mM for 10 minutes). Fluorescence measurements were obtained 6 and 12 hours after drug addition with a Vector 3 plate reader. Data are presented including basal fluorescence of vehicle-treated cells at each time point (n = 3, ± S.E.M.). #P < 0.05 greater than cells treated with only DOX/MITOs; ¶P < 0.05 greater than corresponding value in siSCR control; *P < 0.05 less than sildenafil/doxorubicin alone. (G) Bladder cancer cells (HT-1376; J82; T24) were treated with vehicle or l-NAME (10 mM) or NAC (10 mM) followed 30 minutes later by vehicle, SIL (2 μM), MITO (100 nM), DOX (200 nM), Gemzar (50 nM) as indicated. Cells were isolated after 24 hours, and viability was determined by trypan blue exclusion (n = 3, ± S.E.M.).
PDE5 inhibitors increase the levels of cGMP and of nitric oxide (NO) in cells. NO regulates many cellular processes, including the protection of normal tissues from noxious stimuli; however, high levels of NO combined with high levels of ROS in tumor cells can result in the generation of the toxic molecule preoxy-nitrate (ONOO−). Thus we determined whether the generation of ROS/NO was involved in the toxic interaction between doxorubicin and sildenafil. Doxorubicin/mitomycin C and sildenafil interacted to increase the levels of ROS, as judged using dischlorofluorescein (Fig. 7F). Knock down of CD95 suppressed the production of ROS and knock down of ATM increased ROS production. Cells were incubated in the presence of the nitric oxide synthase inhibitor l-NAME. l-NAME or the ROS quenching agent NAC abolished the toxic interaction between doxorubicin and sildenafil (Fig. 7G).
Discussion
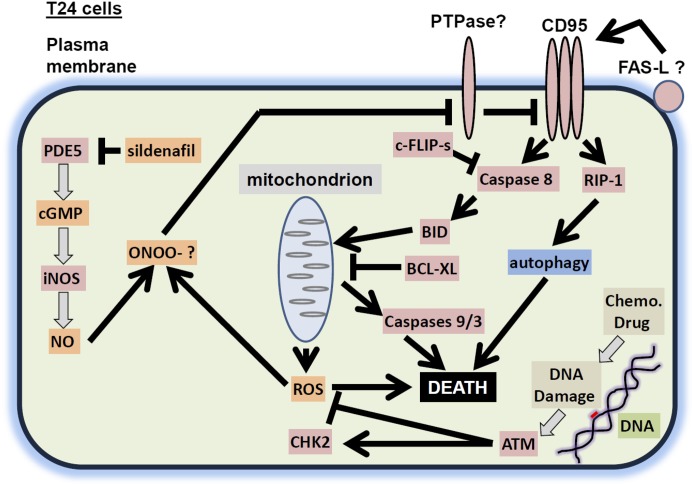
The present studies were performed to determine whether clinically relevant PDE5 inhibitors interacted with standard of care chemotherapy agents to kill bladder and pancreatic cancer cells. PDE5 inhibitors, regardless of whether the bladder cancer cell expressed a mutant active H-RAS protein, interacted with standard of care chemotherapy agents in a greater than additive fashion to kill bladder tumor cells. Similar findings were made in pancreatic cancer cells that express a mutant active K-RAS protein. The interaction between PDE5 inhibitors and standard of care chemotherapy agents was dependent on activation of the extrinsic pathway/death receptors. However, although inhibition of caspase 8, through c-FLIP-s, or mitochondrial protection using BCL-XL abolished the drug interaction, inhibition of caspase 9 only partially reduced overall killing. This implied another signal was emanating from the death receptor to cause killing and to enhance mitochondrial dysfunction. Thus, downstream of CD95, we discovered that knock down of RIP-1 also was found to profoundly suppress the induction of both autophagy and tumor cell killing by the drug combination. Molecular inhibition of autophagy was partially protective. Hence the ability of PDE5 inhibitors to facilitate cytotoxic chemotherapy killing, killing that acts through autophagy and mitochondrial dysfunction, utilizes both the caspase 8 and RIP-1 pathways downstream of death receptors in bladder cancer (T24) cells (Fig. 8).
Fig. 8.
A schematic of how PDE5 inhibitors and DNA damaging drugs interact to kill T24 cells. PDE5 inhibitors such as sildenafil inhibit PDE5, which leads to increased cGMP levels, increased iNOS expression, and elevated levels of NO. DNA damaging drugs stimulate the production of ROS that is counteracted by ATM. ROS and NO generate peroxynitrite, which inhibits the protein tyrosine phosphatase that inhibits CD95. CD95 becomes activated, stimulating (1) the extrinsic apoptosis pathway and (2) through RIP-1, an autophagy pathway that also leads to cell killing. BID, BH3 interacting-domain death agonist.
Prior studies have shown that activation of CD95 can stimulate the induction of LC3-GFP vesicle formation and processing to LC3II (Zhang et al., 2008). Treatment of cells with sildenafil and doxorubicin increased LC3-GFP vesicle numbers and LC3II processing as well as lowering the levels of p62/LAMP2, all indicative of autophagic flux. Knock down of Beclin1 reduced cell killing to a lesser extent than knock down of RIP-1, which strongly suppressed death. The small molecule inhibitor of RIP-1, necrostatin, blocks the death process known as “necroptosis,” a nonapoptotic cell death pathway. Inhibition of RIP-1/necroptosis has been shown by others to not inhibit FAS-L–induced apoptosis nor does it alter the classic appearance of apoptotic morphology (Liedtke et al., 2011). In our hands we observed necrotic nuclei and nuclei that had a mixed morphology of necrosis and apoptosis. Because both caspase 8, RIP-1, and elevated autophagy are downstream of death receptors, our data tend to suggest that RIP-1/autophagy signaling complements caspase 8 signaling in our specific drug combination in bladder cancer cells (Fig. 8). These findings are in contrast to those that argue autophagy and apoptosis can be antagonistic, e.g., Amir et al. (2013). Clearly, our data in the present manuscript using agents that cause a DNA damage response are different from those using other non-DNA damaging drugs. Thus, the mechanisms by which sildenafil can stimulate this form of killing will need further investigation.
One initial concern over our studies was that PDE5 inhibitors were enhancing the toxicity of multiple chemotherapies, implying that we may be observing an off-target phenomenon with respect to PDE5 inhibitor action. However, knock down of PDE5 using three different siRNAs argued that loss of PDE5 function did enhance chemotherapy toxicity. In addition, use of l-NAME and NAC argued that drug combination killing required the generation of reactive nitrogen/oxygen species, most probably downstream of elevated cGMP concentrations. Loss of ATM function is known to increase basal levels of ROS (Rosato et al., 2010). In our system, knock down of ATM did not appreciably increase basal levels of ROS production but did facilitate the induction of ROS after exposure to chemotherapy or chemotherapy plus sildenafil. To our surprise, knock down of CD95 suppressed the overall induction of ROS by all agents, except sildenafil, and l-NAME and NAC both suppressed CD95 activation as judged by plasma membrane localization. These findings argue that CD95 by mechanisms unknown regulates ROS production shortly after drug combination exposure. Additional studies outside the scope of the present manuscript will be required to further understand the biology by which nitric oxide/ROS/cGMP signaling and the CD95 pathway interact.
A frequent mode of cell killing by “traditional” cytotoxic chemotherapies involves the damaging of DNA and the subsequent inability of cells to fully repair this DNA damage. With doxorubicin, an agent that causes single- and double-stranded DNA breaks, we used two read-outs for DNA damage, the Comet assay and γH2AX phosphorylation. By both measures, drug treatment caused DNA damage. The amount of damage caused by the chemotherapeutic drug was enhanced and prolonged by sildenafil. Sildenafil itself did not cause any DNA damage, and chemically this drug would not be expected to damage DNA. It is known that protein nitration can alter the formation of DNA repair complexes as well as regulate the phosphatases that regulate protein complex formation, and our data argue that sildenafil treatment results in greater levels of DNA damage that are not repaired over time (Sturla et al., 2005; Jones et al., 2009). In general agreement with this hypothesis we note that the amount of cell killing observed in colony formation assays is much greater than that observed in short-term death assays at 24 hours, indicating that prolonged DNA damage is resulting in an inability of cells to form colonies. Further studies beyond the scope of the present manuscript will be required to define how changes in protein repair complex formation are altered by sildenafil coexposure.
The ATM protein controls multiple aspects of cell biology after DNA damage, including cell cycle arrest, activation of signaling pathways, activation of apoptosis pathways, and DNA repair protein complex formation (and as previously described, ROS production) (Golding et al., 2007; Booth et al., 2013). We noted that not only was γH2AX phosphorylation increased by the drug combination of sildenafil and doxorubicin compared with DNA damaging agent alone, but so too was phosphorylation of the ATM downstream target CHK2 (Matsuoka et al., 2000; Duong et al., 2013). In general it is believed that beyond a certain threshold, DNA damage-induced activation of ATM/CHK2 in a cell switches from an arrest/survival outcome to a programmed cell death outcome (Scafoglio et al., 2013). CHK2 can signal cell cycle arrest through CDC25C/CDC2, suppress CDC25A expression that leads to elevated ERK1/2 activity and cytoprotection, and shift the apoptotic rheostat toward cell survival through modulation of reactive oxygen species levels and the transcriptional regulation of pro- and antiapoptotic genes (Roos and Kaina, 2006; Antoni et al., 2007; Lavin and Kozlov, 2007; Stolz et al., 2011). Of note is that the PP2C family protein phosphatase Wip-1 is induced by CHK2, which then acts to dephosphorylate and inactivate ATM and CHK2, i.e., a feedback loop (Lu et al., 2005). In the presence of elevated ROS and RNS levels, however, any elevation in Wip-1 activity will be somewhat negated because of covalent modification of the phosphatase by elevated ROS/RNS levels (Fang et al., 2004; Sturla et al., 2005). This will permit CHK2 activity to be prolonged and proapoptotic, as we observed in our time course. Further studies into the biology of Wip-1 in our system will require additional investigation beyond our present studies.
Our prior studies in prostate cancer cells demonstrated, regardless of phosphatase and tensin homolog expression, that sildenafil and doxorubicin interacted to suppress tumor growth in vivo. The present studies focused in greater detail on in vitro mechanisms, and animal studies in bladder and pancreatic tumor cells are a planned future direction. It is intriguing to wonder, however, whether, based on the known vascular response to sildenafil exposure, i.e., vessel dilation, we may also cause an increased penetration of our chemotherapy agents into a tumor itself, which will also result in a greater level of tumor cell killing. Additional in vivo studies measuring both antitumor effects and chemotherapy agent concentrations in the tumor using gas chromatography–mass spectrometry will be required to prove or refute this hypothesis.
In conclusion, our findings strongly argue that at physiologically achievable concentrations in the 1–3 μM range, obtainable after 50–200 mg doses of sildenafil, clinically relevant PDE5 inhibitors enhance the lethality of multiple well established chemotherapy agents (Milligan et al., 2002; Kanjanawart et al., 2011). The drug interaction was dependent in part on expression of death receptors, i.e., CD95, and was observed in multiple tumor cell types. At present, based on our prior studies in prostate cancer cells, a phase I trial is open at Virginia Commonwealth University Massey Cancer Center combining doxorubicin with sildenafil with an assessment end-point of measuring cardiotoxicity (Das et al., 2010; NCT01375699). In this study sildenafil is given only proximal to the period of doxorubicin infusion (DOX being infused every 21 days). This transient level of dosing would reduce the likelihood of negative sequelae caused by prolonged exposure to PDE5 inhibitors. Hence, the present studies further argue for the addition of PDE5 inhibitors to multiple existing treatment regimens in bladder and pancreatic cancer patients. Further laboratory based and clinical studies will be required to understand more fully the mechanisms of drug interaction and the clinical utility of this therapeutic approach.
Abbreviations
- ATM
ataxia telangiectasia mutated
- BCG
Bacillus Calmette-Guerin
- BCL-XL
B-cell lymphoma–extra large
- c-FLIP-s
cellular FLICE-like inhibitory protein short
- CHK2
checkpoint kinase 2
- DOX
doxorubicin
- Gemzar
gemcitabine
- GFP
green fluorescent protein
- γH2AX
γhistone 2AX
- l-NAME
N(G)-nitro-l-arginine
- NAC
N-acetyl cysteine
- NO
nitric oxide
- NOS
nitric oxide synthase
- PDE
phosphodiesterase
- RIP-1
receptor interacting protein 1
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- si
small interfering
Authorship Contributions
Participated in research design: Durrant, Das, Kukreja, Grant, Poklepovic, Dent.
Conducted experiments: Booth, Roberts, Cruickshanks, Conley.
Contributed new reagents or analytic tools: Fisher.
Performed data analysis: Dent.
Wrote or contributed to the writing of the manuscript: Dent.
Footnotes
This work was funded by Public Health Service grants from the National Institutes of Health National Cancer Institute [Grants R01-CA141704 and R01-CA150214]; the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant R01-DK52825]; and the Department of Defense [W81XWH-10-1-0009].
P.D. is the holder of the Universal, Inc., Professorship in Signal Transduction Research. The authors have no conflicts of interest to report.
References
- Amir M, Zhao E, Fontana L, Rosenberg H, Tanaka K, Gao G, Czaja MJ. (2013) Inhibition of hepatocyte autophagy increases tumor necrosis factor-dependent liver injury by promoting caspase-8 activation. Cell Death Differ 20:878–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni L, Sodha N, Collins I, Garrett MD. (2007) CHK2 kinase: cancer susceptibility and cancer therapy - two sides of the same coin? Nat Rev Cancer 7:925–936 [DOI] [PubMed] [Google Scholar]
- Bareford MD, Park MA, Yacoub A, Hamed HA, Tang Y, Cruickshanks N, Eulitt P, Hubbard N, Tye G, Burow ME, et al. (2011) Sorafenib enhances pemetrexed cytotoxicity through an autophagy-dependent mechanism in cancer cells. Cancer Res 71:4955–4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender AT, Beavo JA. (2006) Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev 58:488–520 [DOI] [PubMed] [Google Scholar]
- Booth L, Cruickshanks N, Ridder T, Dai Y, Grant S, Dent P. (2013) PARP and CHK inhibitors interact to cause DNA damage and cell death in mammary carcinoma cells. Cancer Biol Ther 14:458–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalona WJ, Hudson MA, Gillen DP, Andriole GL, Ratliff TL. (1987) Risks and benefits of repeated courses of intravesical bacillus Calmette-Guerin therapy for superficial bladder cancer. J Urol 137:220–224 [DOI] [PubMed] [Google Scholar]
- Chen JJ, Sun YL, Tiwari AK, Xiao ZJ, Sodani K, Yang DH, Vispute SG, Jiang WQ, Chen SD, Chen ZS. (2012) PDE5 inhibitors, sildenafil and vardenafil, reverse multidrug resistance by inhibiting the efflux function of multidrug resistance protein 7 (ATP-binding Cassette C10) transporter. Cancer Sci 103:1531–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TC, Talalay P. (1984) Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22:27–55 [DOI] [PubMed] [Google Scholar]
- Cruickshanks N, Tang Y, Booth L, Hamed H, Grant S, Dent P. (2012) Lapatinib and obatoclax kill breast cancer cells through reactive oxygen species-dependent endoplasmic reticulum stress. Mol Pharmacol 82:1217–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Salloum FN, Xi L, Rao YJ, Kukreja RC. (2009) ERK phosphorylation mediates sildenafil-induced myocardial protection against ischemia-reperfusion injury in mice. Am J Physiol Heart Circ Physiol 296:H1236–H1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Xi L, Kukreja RC. (2008) Protein kinase G-dependent cardioprotective mechanism of phosphodiesterase-5 inhibition involves phosphorylation of ERK and GSK3beta. J Biol Chem 283:29572–29585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Durrant D, Mitchell C, Mayton E, Hoke NN, Salloum FN, Park MA, Qureshi I, Lee R, Dent P, et al. (2010) Sildenafil increases chemotherapeutic efficacy of doxorubicin in prostate cancer and ameliorates cardiac dysfunction. Proc Natl Acad Sci USA 107:18202–18207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Ockaili R, Salloum F, Kukreja RC. (2004) Protein kinase C plays an essential role in sildenafil-induced cardioprotection in rabbits. Am J Physiol Heart Circ Physiol 286:H1455–H1460 [DOI] [PubMed] [Google Scholar]
- Das A, Xi L, Kukreja RC. (2005) Phosphodiesterase-5 inhibitor sildenafil preconditions adult cardiac myocytes against necrosis and apoptosis. Essential role of nitric oxide signaling. J Biol Chem 280:12944–12955 [DOI] [PubMed] [Google Scholar]
- Duong HQ, Hong YB, Kim JS, Lee HS, Yi YW, Kim YJ, Wang A, Zhao W, Cho CH, Seong YS, et al. (2013) Inhibition of checkpoint kinase 2 (CHK2) enhances sensitivity of pancreatic adenocarcinoma cells to gemcitabine. J Cell Mol Med 17:1261–1270 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggen T, Sager G, Berg T, Nergaard B, Moe BT, Ørbo A. (2012) Increased gene expression of the ABCC5 transporter without distinct changes in the expression of PDE5 in human cervical cancer cells during growth. Anticancer Res 32:3055–3061 [PubMed] [Google Scholar]
- Fang Y, Han SI, Mitchell C, Gupta S, Studer E, Grant S, Hylemon PB, Dent P. (2004) Bile acids induce mitochondrial ROS, which promote activation of receptor tyrosine kinases and signaling pathways in rat hepatocytes. Hepatology 40:961–971 [DOI] [PubMed] [Google Scholar]
- Fisher PW, Salloum F, Das A, Hyder H, Kukreja RC. (2005) Phosphodiesterase-5 inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation 111:1601–1610 [DOI] [PubMed] [Google Scholar]
- Golding SE, Rosenberg E, Neill S, Dent P, Povirk LF, Valerie K. (2007) Extracellular signal-related kinase positively regulates ataxia telangiectasia mutated, homologous recombination repair, and the DNA damage response. Cancer Res 67:1046–1053 [DOI] [PubMed] [Google Scholar]
- Grivas PD, Hussain M, Hafez K, Daignault-Newton S, Wood D, Lee CT, Weizer A, Montie JE, Hollenbeck B, Montgomery JS, et al. (2013) A phase II trial of neoadjuvant nab-paclitaxel, carboplatin, and gemcitabine (ACaG) in patients with locally advanced carcinoma of the bladder. Urology 82: 111–117 [DOI] [PubMed] [Google Scholar]
- Hudson MA, Herr HW. (1995) Carcinoma in situ of the bladder. J Urol 153:564–572 [DOI] [PubMed] [Google Scholar]
- Jones LE, Jr, Ying L, Hofseth AB, Jelezcova E, Sobol RW, Ambs S, Harris CC, Espey MG, Hofseth LJ, Wyatt MD. (2009) Differential effects of reactive nitrogen species on DNA base excision repair initiated by the alkyladenine DNA glycosylase. Carcinogenesis 30:2123–2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanjanawart S, Gaysonsiri D, Tangsucharit P, Vannaprasaht S, Phunikhom K, Kaewkamson T, Wattanachai N, Tassaneeyakul W. (2011) Comparative bioavailability of two sildenafil tablet formulations after single-dose administration in healthy Thai male volunteers. Int J Clin Pharmacol Ther 49:525–530 [DOI] [PubMed] [Google Scholar]
- Karami-Tehrani F, Moeinifard M, Aghaei M, Atri M. (2012) Evaluation of PDE5 and PDE9 expression in benign and malignant breast tumors. Arch Med Res 43:470–475 [DOI] [PubMed] [Google Scholar]
- Lacombe L, Dalbagni G, Zhang ZF, Cordon-Cardo C, Fair WR, Herr HW, Reuter VE. (1996) Overexpression of p53 protein in a high-risk population of patients with superficial bladder cancer before and after bacillus Calmette-Guérin therapy: correlation to clinical outcome. J Clin Oncol 14:2646–2652 [DOI] [PubMed] [Google Scholar]
- Lamm DL, Blumenstein BA, Crawford ED, Montie JE, Scardino P, Grossman HB, Stanisic TH, Smith JA, Jr, Sullivan J, Sarosdy MF, et al. (1991) A randomized trial of intravesical doxorubicin and immunotherapy with bacille Calmette-Guérin for transitional-cell carcinoma of the bladder. N Engl J Med 325:1205–1209 [DOI] [PubMed] [Google Scholar]
- Lavin MF, Kozlov S. (2007) ATM activation and DNA damage response. Cell Cycle 6:931–942 [DOI] [PubMed] [Google Scholar]
- Liedtke C, Bangen JM, Freimuth J, Beraza N, Lambertz D, Cubero FJ, Hatting M, Karlmark KR, Streetz KL, Krombach GA, et al. (2011) Loss of caspase-8 protects mice against inflammation-related hepatocarcinogenesis but induces non-apoptotic liver injury. Gastroenterology 141:2176–2187 [DOI] [PubMed] [Google Scholar]
- Lu X, Nannenga B, Donehower LA. (2005) PPM1D dephosphorylates Chk1 and p53 and abrogates cell cycle checkpoints. Genes Dev 19:1162–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmström PU, Wijkström H, Lundholm C, Wester K, Busch C, Norlén BJ, Swedish-Norwegian Bladder Cancer Study Group (1999) 5-year followup of a randomized prospective study comparing mitomycin C and bacillus Calmette-Guerin in patients with superficial bladder carcinoma. J Urol 161:1124–1127 [PubMed] [Google Scholar]
- Matsuoka S, Rotman G, Ogawa A, Shiloh Y, Tamai K, Elledge SJ. (2000) Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc Natl Acad Sci USA 97:10389–10394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan PA, Marshall SF, Karlsson MO. (2002) A population pharmacokinetic analysis of sildenafil citrate in patients with erectile dysfunction. Br J Clin Pharmacol 53 (Suppl 1):45S–52S [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockaili R, Salloum F, Hawkins J, Kukreja RC. (2002) Sildenafil (Viagra) induces powerful cardioprotective effect via opening of mitochondrial K(ATP) channels in rabbits. Am J Physiol Heart Circ Physiol 283:H1263–H1269 [DOI] [PubMed] [Google Scholar]
- Park MA, Zhang G, Martin AP, Hamed H, Mitchell C, Hylemon PB, Graf M, Rahmani M, Ryan K, Liu X, et al. (2008) Vorinostat and sorafenib increase ER stress, autophagy and apoptosis via ceramide-dependent CD95 and PERK activation. Cancer Biol Ther 7:1648–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza GA, Thompson WJ, Pamukcu R, Alila HW, Whitehead CM, Liu L, Fetter JR, Gresh WE, Jr, Klein-Szanto AJ, Farnell DR, et al. (2001) Exisulind, a novel proapoptotic drug, inhibits rat urinary bladder tumorigenesis. Cancer Res 61:3961–3968 [PubMed] [Google Scholar]
- Quek ML, Stein JP, Nichols PW, Cai J, Miranda G, Groshen S, Daneshmand S, Skinner EC, Skinner DG. (2005) Prognostic significance of lymphovascular invasion of bladder cancer treated with radical cystectomy. J Urol 174:103–106 [DOI] [PubMed] [Google Scholar]
- Rivera E. (2003) Liposomal anthracyclines in metastatic breast cancer: Clinical update. Oncologist 8 (Suppl 2):3–9 [DOI] [PubMed] [Google Scholar]
- Roos WP, Kaina B. (2006) DNA damage-induced cell death by apoptosis. Trends Mol Med 12:440–450 [DOI] [PubMed] [Google Scholar]
- Rosato RR, Kolla SS, Hock SK, Almenara JA, Patel A, Amin S, Atadja P, Fisher PB, Dent P, Grant S. (2010) Histone deacetylase inhibitors activate NF-kappaB in human leukemia cells through an ATM/NEMO-related pathway. J Biol Chem 285:10064–10077 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Salloum F, Yin C, Xi L, Kukreja RC. (2003) Sildenafil induces delayed preconditioning through inducible nitric oxide synthase-dependent pathway in mouse heart. Circ Res 92:595–597 [DOI] [PubMed] [Google Scholar]
- Salloum FN, Abbate A, Das A, Houser JE, Mudrick CA, Qureshi IZ, Hoke NN, Roy SK, Brown WR, Prabhakar S, et al. (2008) Sildenafil (Viagra) attenuates ischemic cardiomyopathy and improves left ventricular function in mice. Am J Physiol Heart Circ Physiol 294:H1398–H1406 [DOI] [PubMed] [Google Scholar]
- Salloum FN, Ockaili RA, Wittkamp M, Marwaha VR, Kukreja RC. (2006) Vardenafil: a novel type 5 phosphodiesterase inhibitor reduces myocardial infarct size following ischemia/reperfusion injury via opening of mitochondrial K(ATP) channels in rabbits. J Mol Cell Cardiol 40:405–411 [DOI] [PubMed] [Google Scholar]
- Sarfati M, Mateo V, Baudet S, Rubio M, Fernandez C, Davi F, Binet JL, Delic J, Merle-Beral H. (2003) Sildenafil and vardenafil, types 5 and 6 phosphodiesterase inhibitors, induce caspase-dependent apoptosis of B-chronic lymphocytic leukemia cells. Blood 101:265–269 [DOI] [PubMed] [Google Scholar]
- Scafoglio C, Smolka M, Zhou H, Perissi V, Rosenfeld MG. (2013) The co-repressor SMRT delays DNA damage-induced caspase activation by repressing pro-apoptotic genes and modulating the dynamics of checkpoint kinase 2 activation. PLoS One 8:e59986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen F, Chu S, Bence AK, Bailey B, Xue X, Erickson PA, Montrose MH, Beck WT, Erickson LC. (2008) Quantitation of doxorubicin uptake, efflux, and modulation of multidrug resistance (MDR) in MDR human cancer cells. J Pharmacol Exp Ther 324:95–102 [DOI] [PubMed] [Google Scholar]
- Singal PK, Li T, Kumar D, Danelisen I, Iliskovic N. (2000) Adriamycin-induced heart failure: mechanism and modulation. Mol Cell Biochem 207:77–86 [DOI] [PubMed] [Google Scholar]
- Stein JP, Grossfeld GD, Ginsberg DA, Esrig D, Freeman JA, Figueroa AJ, Skinner DG, Cote RJ. (1998) Prognostic markers in bladder cancer: a contemporary review of the literature. J Urol 160:645–659 [DOI] [PubMed] [Google Scholar]
- Stolz A, Ertych N, Bastians H. (2011) Tumor suppressor CHK2: regulator of DNA damage response and mediator of chromosomal stability. Clin Cancer Res 17:401–405 [DOI] [PubMed] [Google Scholar]
- Sturla LM, Amorino G, Alexander MS, Mikkelsen RB, Valerie K, Schmidt-Ullrichr RK. (2005) Requirement of Tyr-992 and Tyr-1173 in phosphorylation of the epidermal growth factor receptor by ionizing radiation and modulation by SHP2. J Biol Chem 280:14597–14604 [DOI] [PubMed] [Google Scholar]
- Thrasher JB, Crawford ED. (1993) Current management of invasive and metastatic transitional cell carcinoma of the bladder. J Urol 149:957–972 [DOI] [PubMed] [Google Scholar]
- Torti FM, Lum BL. (1984) The biology and treatment of superficial bladder cancer. J Clin Oncol 2:505–531 [DOI] [PubMed] [Google Scholar]
- Witjes JA, Caris CT, Mungan NA, Debruyne FM, Witjes WP. (1998) Results of a randomized phase III trial of sequential intravesical therapy with mitomycin C and bacillus Calmette-Guerin versus mitomycin C alone in patients with superficial bladder cancer. J Urol 160:1668–1671, discussion 1671–1672 [PubMed] [Google Scholar]
- Zhang G, Park MA, Mitchell C, Hamed H, Rahmani M, Martin AP, Curiel DT, Yacoub A, Graf M, Lee R, et al. (2008) Vorinostat and sorafenib synergistically kill tumor cells via FLIP suppression and CD95 activation. Clin Cancer Res 14:5385–5399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yan G, Ji J, Wu J, Sun X, Shen J, Jiang H, Wang H. (2012) PDE5 inhibitor promotes melanin synthesis through the PKG pathway in B16 melanoma cells. J Cell Biochem 113:2738–2743 [DOI] [PubMed] [Google Scholar]