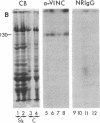
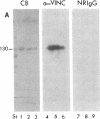
Abstract
We have found that vinculin is localized at the sarcolemma of skeletal muscle cells in a two-dimensional orthogonal lattice. Perpendicular to the longitudinal axis of the cell, bands of vinculin encircle the muscle cell and repeat along its length with a periodicity corresponding to the subjacent sarcomeres. Because of their appearance and probable function, we call the transverse elements of the lattice "costameres" (Latin costa, rib; Greek meros, part). Costameres have a substructure consisting of densely clustered patches of vinculin; the patches are segregated into two rows which flank the Z line and overlie the I band of the underlying sarcomere. It is likely that the costameres are physically coupled to the underlying myofibrils because: (i) the costameres broaden and narrow in concert with the underlying I band in stretched and contracted muscle, and (ii) adjacent but misaligned myofibrils are mirrored by corresponding discontinuities in the overlying costameres. We hypothesize that the sarcolemmal lattice, detected because vinculin is one of its molecular components, integrates the contractile apparatus with the sarcolemma during lengthening and shortening of the muscle cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERGMAN R. A. An experimental study of the non-fibrillar components in frog striated muscle. Bull Johns Hopkins Hosp. 1958 Dec;103(6):267–280. [PubMed] [Google Scholar]
- Burridge K., Feramisco J. R. Microinjection and localization of a 130K protein in living fibroblasts: a relationship to actin and fibronectin. Cell. 1980 Mar;19(3):587–595. doi: 10.1016/s0092-8674(80)80035-3. [DOI] [PubMed] [Google Scholar]
- Chiesi M., Ho M. M., Inesi G., Somlyo A. V., Somlyo A. P. Primary role of sarcoplasmic reticulum in phasic contractile activation of cardiac myocytes with shunted myolemma. J Cell Biol. 1981 Dec;91(3 Pt 1):728–742. doi: 10.1083/jcb.91.3.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig S. W., Lancashire C. L., Cooper J. A. Preparation of smooth muscle alpha-actinin. Methods Enzymol. 1982;85(Pt B):316–321. doi: 10.1016/0076-6879(82)85031-3. [DOI] [PubMed] [Google Scholar]
- Craig S. W., Pardo J. V. alpha-Actinin localization in the junctional complex of intestinal epithelial cells. J Cell Biol. 1979 Jan;80(1):203–210. doi: 10.1083/jcb.80.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feramisco J. R., Smart J. E., Burridge K., Helfman D. M., Thomas G. P. Co-existence of vinculin and a vinculin-like protein of higher molecular weight in smooth muscle. J Biol Chem. 1982 Sep 25;257(18):11024–11031. [PubMed] [Google Scholar]
- Garamvölgyi N. Inter-Z bridges in the flight muscle of the bee. J Ultrastruct Res. 1965 Dec;13(5):435–443. doi: 10.1016/s0022-5320(65)90006-7. [DOI] [PubMed] [Google Scholar]
- Geiger B. A 130K protein from chicken gizzard: its localization at the termini of microfilament bundles in cultured chicken cells. Cell. 1979 Sep;18(1):193–205. doi: 10.1016/0092-8674(79)90368-4. [DOI] [PubMed] [Google Scholar]
- Geiger B., Dutton A. H., Tokuyasu K. T., Singer S. J. Immunoelectron microscope studies of membrane-microfilament interactions: distributions of alpha-actinin, tropomyosin, and vinculin in intestinal epithelial brush border and chicken gizzard smooth muscle cells. J Cell Biol. 1981 Dec;91(3 Pt 1):614–628. doi: 10.1083/jcb.91.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B., Tokuyasu K. T., Dutton A. H., Singer S. J. Vinculin, an intracellular protein localized at specialized sites where microfilament bundles terminate at cell membranes. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4127–4131. doi: 10.1073/pnas.77.7.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockusch B. M., Isenberg G. Interaction of alpha-actinin and vinculin with actin: opposite effects on filament network formation. Proc Natl Acad Sci U S A. 1981 May;78(5):3005–3009. doi: 10.1073/pnas.78.5.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. T., Burgess D. R. SDS microslab linear gradient polyacrylamide gel electrophoresis. Anal Biochem. 1978 Jul 1;87(2):386–396. doi: 10.1016/0003-2697(78)90688-7. [DOI] [PubMed] [Google Scholar]
- Nunzi M. G., Franzini Armstrong C. The structure of smooth and striated portions of the adductor muscle of the valves in a scallop. J Ultrastruct Res. 1981 Aug;76(2):134–148. doi: 10.1016/s0022-5320(81)80012-3. [DOI] [PubMed] [Google Scholar]
- Page S. G. Structure and some contractile properties of fast and slow muscles of the chicken. J Physiol. 1969 Nov;205(1):131–145. doi: 10.1113/jphysiol.1969.sp008956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siliciano J. D., Craig S. W. Meta-vinculin--a vinculin-related protein with solubility properties of a membrane protein. Nature. 1982 Dec 9;300(5892):533–535. doi: 10.1038/300533a0. [DOI] [PubMed] [Google Scholar]
- Singer I. I. Association of fibronectin and vinculin with focal contacts and stress fibers in stationary hamster fibroblasts. J Cell Biol. 1982 Feb;92(2):398–408. doi: 10.1083/jcb.92.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer I. I., Paradiso P. R. A transmembrane relationship between fibronectin and vinculin (130 kd protein): serum modulation in normal and transformed hamster fibroblasts. Cell. 1981 May;24(2):481–492. doi: 10.1016/0092-8674(81)90339-1. [DOI] [PubMed] [Google Scholar]
- Spray T. L., Waugh R. A., Sommer J. R. Peripheral couplings in adult vertebrate skeletal muscle. Anatomical observations and functional implications. J Cell Biol. 1974 Jul;62(1):223–227. doi: 10.1083/jcb.62.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K. T., Dutton A. H., Geiger B., Singer S. J. Ultrastructure of chicken cardiac muscle as studied by double immunolabeling in electron microscopy. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7619–7623. doi: 10.1073/pnas.78.12.7619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins J. A., Lin S. High-affinity interaction of vinculin with actin filaments in vitro. Cell. 1982 Jan;28(1):83–90. doi: 10.1016/0092-8674(82)90377-4. [DOI] [PubMed] [Google Scholar]