Abstract
Objective
To examine differences in behavioral symptoms and cognitive functioning between males and females with autism spectrum disorder (ASD).
Method
We analyzed data from 2,418 probands with autism (304 females, 2,114 males) included in the Simons Simplex Collection. Sex differences were evaluated across measures of autism symptoms, cognitive and motor functioning, adaptive behavior, and associated behavior problems. Measurement bias was examined using latent variable models of symptoms. Unadjusted and propensity-adjusted analyses were computed to ensure sex differences were not due to unbalanced sampling. Moderator and mediator analyses evaluated whether sex differences were modified by clinical characteristics or driven by cognitive ability.
Results
Females with ASD had greater social communication impairment, lower levels of restricted interests, lower cognitive ability, weaker adaptive skills, and greater externalizing problems relative to males. Symptom differences could not be accounted for by measurement differences, indicating that diagnostic instruments captured autism similarly in males and females. IQ reductions mediated greater social impairment and reduced adaptive behavior in females with ASD, but did not mediate reductions in restricted interests or increases in irritability.
Conclusions
A specific female ASD phenotype is emerging that cannot be accounted for by differential symptom measurement. The present data suggest that the relatively low proportion of high functioning females may reflect the effect of protective biological factors or may be due to under-identification. Additional carefully-accrued samples are needed to confirm the present pattern and to evaluate whether observed sex ratios in high functioning cases are reduced if female-specific indicators of restricted interests are included.
Keywords: autism spectrum disorder (ASD), behavior problems, cognitive, females, restricted interests
Being male is one of the most powerful and well-established risk factors for autism spectrum disorders (ASD). Sex ratio estimates have indicated 3–4 males per female across the diagnostic spectrum.1 Even more severe ratios (as high as 9:1) are observed in cognitively higher functioning cases.2, 3 Consequently, original descriptions, subsequent diagnostic criteria, and the vast majority of phenotypic data are from males with ASD. Yet, recent data suggest that females may be underidentified4 or, at minimum, show different behavioral, cognitive, neuroanatomical, and/or molecular expression patterns.5–10 Unfortunately, studies of sex differences in the ASD phenotype have often included modest female sample sizes, focused exclusively on adults, and/or focused on a limited number of domains (e.g., symptoms or cognition). Thus, no previous studies have been able to comprehensively evaluate whether major differences in core symptoms or overall cognitive ability may drive other sex differences in children with ASD.
Previous investigations of sex differences in autism symptoms have suggested decreased repetitive behavior in females, with no consistent differences in social communication symptoms. In one of the earliest and largest studies of sex differences in ASD, Lord et al.11 found that females with autism had lower levels of unusual visual interests. Contemporary studies have confirmed lower levels of repetitive sensory motor behavior (Cohen’s d across studies =.16–.42),4, 12 at least in older children with ASD where repetitive behaviors are often more apparent and impairing.13 It is not yet clear whether this reduction is broadly present across all sub-domains (e.g., repetitive sensory motor, need for sameness, and restricted interests). One recent study suggested that sex differences may be most pronounced for repetitive symptoms characteristic of higher functioning individuals (e.g., large store of factual information).4 Additionally, no previous studies have carefully evaluated whether sex differences reflect true differences in symptom expression or an artifact of differential symptom measurement in females with ASD. This is crucial given that diagnostic criteria were primarily based on male-centric descriptions. Furthermore, no previous studies have examined whether sex differences may reflect unbalanced sampling of demographic or clinical factors across male and female cases. For example, female ASD cases from families of lower socioeconomic strata may be differentially under-represented. Careful evaluation of measurement equivalence and sample comparability are key to ensuring observed sex differences reflect true differences.
Studies of sex differences in cognitive functioning have produced inconsistent findings. Several investigations have found worse verbal/language abilities in females11, 13 while others have found no significant differences.4, 14 Findings for full scale IQ, nonverbal IQ, and motor functioning have been variable and potentially dependent on age and sample characteristics.4, 13–15 Data on adaptive functioning and associated behavior problems have also been variable, with the largest studies suggesting decreased adaptive behavior, weaker social competence, and more emotional difficulties in older females with ASD.4, 11 A key next step will be estimating these effects simultaneously in a large sample. This will assist in determining whether previous discrepancies across studies are accounted for by statistical power or sampling variation as well as examining whether cognitive differences between males and females mediate other sex differences.
The primary aim of the present study was to evaluate sex differences in behavioral and cognitive characteristics of youth with ASD using a large sample from the Simons Simplex Collection (SSC). The SSC affords the advantage of simultaneously evaluating an array of phenotypic measures, including core ASD symptoms, cognitive and language measures, adaptive functions, and associated behavior problems. To address this overarching objective, the study first evaluated the possibility that any observed symptom differences between males and females may result from differential symptom measurement. We hypothesized that females and males would show highly similar autism symptom structure, implying equivalent measurement between sexes in the SSC. If established, measurement equivalence would facilitate investigation of mean differences between males and females. Sex differences were then evaluated across the full range of phenotypic measures, with careful correction for any unbalanced sampling between males and females that may distort sex differences. Based on previous literature, we anticipated lower repetitive behavior symptom levels, greater cognitive impairment (particularly for verbal IQ), weaker adaptive behavior, and higher levels of associated behavior problems in females relative to males with ASD. Finally, clinical characteristics were examined as potential moderators and overall cognitive ability was examined as a mediator of sex differences in other phenotypic domains.
Method
Sample
Data were obtained from the Simons Simplex Collection (SSC; version 14.1) and included 2,418 probands with complete demographic and diagnostic data, including 304 females and 2,114 males with ASD. Previous reports have described the SSC data collection process, as well as the extensive phenotypic data available.16 Informed consent was obtained at each data collection site included in the SSC. The procedures of the present study were reviewed and approved by the institutional review board of the Cleveland Clinic.
Measures
Demographics and clinical characteristics
Age and sex of the proband and their sibling (for quad families), race/ethnicity (coded white non-Hispanic, other race/ethnicity), family type (trios vs. quads), family structure (zero or one biological parent in the home vs. two biological parents in the home), number of siblings, highest parental education, family income, evidence of regression, presence of a phrase speech delay, proband height, proband body mass index, SSC diagnostic certainty rating, and Collaborative Programs of Excellence in Autism (CPEA) proband diagnosis (e.g., Autism, ASD, Asperger’s). The SSC includes probands with DSM-IV-TR spectrum diagnoses, including an “ASD” designation functionally similar to DSM-IV Pervasive Developmental Disorder Not Otherwise Specified. SSC families were included if the proband was age 4–18, had nonverbal mental age >18 months, was absent severe neurologic deficits or birth complications, met CPEA criteria for an autism spectrum disorder, and did not have a first-degree relative with an autism spectrum disorder.16
Core autism symptoms
Autism symptom data were obtained from the Autism Diagnostic Interview–Revised (ADI-R)17 total and domain scores (social, nonverbal communication, and restricted/repetitive behavior), Autism Diagnostic Observation Schedule (ADOS) calibrated severity score and scale scores (reciprocal social, communication, social affect, and restrictive/repetitive),18 Social Responsiveness Scale (SRS) total raw and subscale scores,19 and Repetitive Behavior Scale–Revised (RBS-R) total raw score.20 ADI-R repetitive behavior current item scores and RBS-R items were coded into sub-scales based on recent empirical work by Bishop et al.21 identifying a different factor structure than originally used for scoring these instruments. Specifically, ADI-R repetitive behavior item scores were summed to create insistence on sameness and repetitive sensory motor subscales. RBS-R items were summed to create stereotypy, restricted interests, self-injury, compulsive, and sameness sub-scales. For conceptual clarity, symptom measures were presented in clusters (global autism severity, social communication/interaction, and restricted/repetitive behavior).
Cognitive and motor
Cognitive data included full scale intelligence quotient (FSIQ), verbal IQ, and nonverbal IQ derived from multiple instruments.22–24 As a result, only overall, verbal, and non-verbal ability standard scores or ratio (deviation) scores were obtained. The absolute difference between verbal and nonverbal IQ scores (VIQ minus PIQ) was also examined based on previous literature indicating an increased rate of discrepancies in autism.25 Language measurements included standard scores from the Peabody Picture Vocabulary Test–Fourth edition (PPVT),26 scaled scores from the non-word repetition subtest of the Comprehensive Test of Phonological Processing (CTOPP),27 and the difference between language level defined by the ADOS module received and nonverbal mental age (coded 0=no discrepancy between ADOS module received and expected ADOS module, 1= one level lower than the expected ADOS module, and 2= two levels lower than the expected ADOS module). Motor functioning was assessed using the total number of pegs completed using the dominant (Pegs Dominant) and nondominant (Pegs Non-Dominant) hands in the Grooved Pegboard test.28 Caregiver reports of motor function were obtained using the fine motor, coordination during movement, general coordination, and composite scores from the Developmental Coordination Disorder Questionnaire (DCDQ).29
Adaptive behavior and associated behavior problems
Adaptive behavior was evaluated using the composite and subscale standard scores from the Vineland Adaptive Behavior Scale–Second Edition.30 Associated behavior problems were measured using the total, internalizing, and externalizing T-scores of the Child Behavior Checklist (CBCL)31 and the total and subscale raw scores from the Aberrant Behavior Checklist (ABC).32, 33
Data Analysis
Differences in sample demographic and clinical characteristics across females and males with ASD were estimated using independent samples t-tests or Chi-square statistics.
Measurement invariance
Detailed measurement invariance methods are provided in Supplement 1. Briefly, a series of increasingly restrictive factor models were estimated with the 3 ADI-R domain scores, 3 ADOS domain scores, SRS total raw score, and RBS-R total raw score as indicators (Table S1 provides indicator-factor correspondence). Comparisons of fit across models were used to determine whether males and females show similar factor structure (factor loadings, intercepts, and error variances).
Sex differences
To examine unadjusted sex differences across all phenotypic measures, independent samples t-tests were computed with each measure as a dependent variable. Unadjusted analyses do not account for possible demographic or clinical differences (unbalanced sampling) between females and males with ASD. To account for these potential differences, a logistic regression was computed predicting participant sex using all other demographic and clinical factors. The log probability of being male (linear propensity score) was saved for each case and used to adjust for demographic and clinical differences between females and males with ASD. Preliminary analyses also examined one-to-one matching based on the linear propensity score. However, all standardized covariate differences were less than 10% of the pooled standard deviation (Cohen’s d<.10) and the linear propensity score difference was also small (Cohen’s d=.14). The pattern of results was highly similar. Therefore, the following presentation focused only on covariate-adjustment.
Moderator analyses
To examine moderation, demographic and clinical characteristics and their interaction with proband sex were included as predictors in linear regression models. Dependent variables included phenotypic measures with the largest, statistically significant sex differences in each domain. A significant interaction between sex and other demographic or clinical characteristics would imply potential moderator effects.34
Mediational models
To examine whether sex differences in other phenotypic measures were mediated by reductions in full scale IQ, a series of regression analyses were computed following Baron and Kenny.35 Analogous structural equation models were also computed to simultaneously estimate standardized coefficients. It is important to note that all data were cross-sectional. Thus, mediation can only be described in the statistical sense and could not be logically tested following MacArthur framework guidelines.34, 36
Multiple comparison corrections
For all analyses, false discovery rate corrections were applied within each domain (global autism symptoms, social communication/interaction, restricted/repetitive behavior, cognitive, motor, adaptive behavior, and associated behavior problems) to maintain Type 1 error rate at .05.37, 38 Univariate statistics (t and F) were converted to Cohen’s d to represent the magnitude of effect sizes across all measures.39 Effect sizes conventions were very small (d<.10), small (d=.20), medium (.50), and large (d=.80).40 Latent variable models were estimated using MPlus v5.2. All other analyses used SPSS v20.
Results
Sample Characteristics
Table 1 presents demographic and clinical characteristics, separately for males and females with ASD. The SSC included 2,418 ASD-affected individuals (304 females, 2,114 males; age range=4–18) with complete demographic and clinical data. Bachelor’s and graduate degrees were common among parents and the socioeconomic status of families was higher than the general population (income ~$75,000). The majority of children lived with both biological parents. Consistent with the broad but relatively higher functioning composition of the SSC,16 phrase speech delay was reported in roughly a third of cases. Females and males with ASD were surprisingly well-matched across the full array of demographic and clinical variables (largest absolute standardized difference - Cohen’s d=.07). The propensity score representing combined differences on all covariates indicated that the level of overlap between females and males with ASD was quite high (Figure S1).
Table 1.
Simons Simplex Collection (SSC) Sample Characteristics.
| Females with ASD | Males with ASD | t/X2 | (p) | Cohen’s d | |
|---|---|---|---|---|---|
|
| |||||
| M (SD) | M (SD) | ||||
| n | 304 | 2114 | |||
| Age | 9.32 (3.67) | 9.01 (3.56) | 1.42 | (.157) | .06 |
| White non-Hispanic (n, %) | 231 (76.0%) | 1560 (73.8%) | 0.67 | (.415) | .03 |
| SSC family type (quads, n, %) | 248 (81.6%) | 1741 (82.4%) | 0.11 | (.740) | .01 |
| Living with both biological parents (n, %) | 280 (92.1%) | 1956 (92.5%) | 0.25 | (.884) | .02 |
| Number of siblings | 1.38 (0.39) | 1.42 (0.88) | −0.79 | (.432) | −.03 |
| Highest parent education | 5.90 (1.19) | 5.89 (1.22) | 0.23 | (.818) | .01 |
| Family income | 5.90 (2.19) | 5.88 (2.22) | 0.21 | (.831) | .01 |
| Regression (definite language loss, n, %) | 34 (11.2%) | 268 (12.7%) | 0.54 | (.462) | .03 |
| Phrase speech delay (>33 months, n, %) | 109 (35.9%) | 827 (39.1%) | 1.19 | (.275) | .04 |
| Height (z-score) | 0.26 (1.21) | 0.38 (1.14) | −1.71 | (.088) | −.07 |
| Body mass index (z-score) | 0.71 (1.23) | 0.66 (1.40) | 0.61 | (.545) | .02 |
| Diagnostic certainty | 13.00 (2.52) | 13.11 (2.38) | −0.74 | (.460) | −.03 |
| CPEA Diagnosis (n, %) | 0.26 | (.878) | .02 | ||
| Autism | 273 (89.8%) | 1912 (90.4%) | |||
| ASD | 24 (7.9%) | 162 (7.7%) | |||
| Asperger’s disorder | 7 (2.3%) | 40 (1.9%) | |||
Note: Collaborative Programs of Excellence in Autism (CPEA) diagnosis was computed by collapsing the ASD and Asperger’s categories. Highest parent education was ordinally coded from 1=no high school diploma to 7=graduate school degree. Family income was ordinally coded from 1=<$20,000 annually to 9=>$160,000 annually. Diagnostic certainty was ordinally coded from 1=high certainty youth did not have autism to 6=uncertain whether ASD, 10=high certainty of ASD, but not Autism to 15=high certainty youth had autism. The effective range for this sample was from 6 to 15. CPEA diagnostic coding scheme used the Autism Diagnostic Observation Schedule, Autism Diagnostic Interview–Revised, and clinician best estimate diagnosis. ASD=Autism Spectrum Disorder.
Measurement Equivalence
Table S2 presents fit statistics from increasingly restrictive measurement models. Fit was stable and in some cases improved across increasingly restrictive models, implying strict measurement invariance across sexes. The presence of strict measurement invariance for each assessment measure permits evaluation of mean differences in symptoms by demonstrating that observed differences in scores were not a function of differences in measurement between males and females included in the SSC. The DSM-5 factor model also estimated equivalent correlations between social communication/interaction and restricted/repetitive behavior factors in males (r=.63, 95% CI=.57–.69) and females (r=.71, 95% CI=.56–.86).
Autism Symptoms
Table 2 presents unadjusted means and standard deviations for autism symptoms in males and females with ASD. There were no significant differences in global autism severity metrics, although a non-significant trend was noted for higher SRS total raw scores in females. For specific symptom measures, females with ASD showed higher social and communication symptom levels on the ADOS and two of the four SRS social subscales. Conversely, females with ASD had significantly lower repetitive behavior symptom levels on the ADI-R repetitive domain score and the RBS-R restricted interests subscale. The latter effect was the largest absolute male-female difference across symptom measures, but was small in magnitude (Cohen’s d=−.13). Adjustment for the propensity score did not alter the pattern or magnitude of results.
Table 2.
Descriptive Statistics for Autism Symptom Measures in Females and Males With Autism Spectrum Disorder (ASD).
| Females with ASD n=304 | Males with ASD n=2114 | t | (p) | Cohen’s d | |||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | ||||
| Global Autism Severity | |||||||
| ADI-R total | 36.16 | (10.07) | 36.04 | (9.17) | −0.21 | (.836) | .01 |
| ADOS CSS | 7.45 | (1.76) | 7.43 | (1.67) | −0.17 | (.868) | .01 |
| SRS total raw | 100.69 | (26.49) | 97.77 | (26.79) | −1.78 | (.075) | .07 |
|
| |||||||
| Social Communication/Interaction | |||||||
| ADI-R social | 20.58 | (6.05) | 20.24 | (5.67) | −0.99 | (.323) | .04 |
| ADI-R non-verbal communication | 9.33 | (3.73) | 9.22 | (3.41) | −0.49 | (.627) | .02 |
| ADOS reciprocal social | 8.51 | (2.80) | 8.16 | (2.56) | −2.19 | (.028) | .09 |
| ADOS communication | 4.73 | (2.32) | 4.41 | (2.05) | −2.50 | (.013) | .10 |
| ADOS social affect | 11.55 | (4.24) | 11.01 | (3.96) | −2.18 | (.029) | .09 |
| SRS social cognition | 19.27 | (5.24) | 18.52 | (5.62) | −2.20 | (.028) | .09 |
| SRS social communication | 34.59 | (9.61) | 33.32 | (9.91) | −2.09 | (.036) | .09 |
| SRS social awareness | 12.80 | (3.65) | 12.53 | (3.64) | −1.20 | (.230) | .05 |
| SRS social motivation | 15.21 | (5.82) | 14.73 | (5.67) | −1.37 | (.171) | .06 |
|
| |||||||
| Restricted/Repetitive Behavior | |||||||
| ADI-R RRB total | 6.25 | (2.47) | 6.58 | (2.51) | 2.16 | (.031) | −.09 |
| ADI-R repetitive sensory motor | 3.86 | (2.64) | 3.99 | (2.66) | 0.81 | (.416) | .03 |
| ADI-R insistence on sameness | 5.72 | (3.00) | 5.84 | (3.08) | 0.64 | (.523) | .03 |
| ADOS restrictive and repetitive | 4.01 | (2.21) | 3.96 | (2.05) | −0.44 | (.661) | .02 |
| SRS autism mannerisms | 18.83 | (7.08) | 18.67 | (6.76) | −0.37 | (.708) | .02 |
| RBS-R total score | 26.86 | (16.93) | 27.10 | (17.29) | 0.23 | (.817) | −.01 |
| RBS-R stereotypy | 4.60 | (3.76) | 5.13 | (3.80) | 1.89 | (.059) | .08 |
| RBS-R compulsive | 5.16 | (4.42) | 4.88 | (4.55) | 1.05 | (.294) | .04 |
| RBS-R sameness | 5.99 | (4.79) | 5.82 | (4.88) | 0.61 | (.546) | .02 |
| RBS-R restricted interests | 2.13 | (1.81) | 2.49 | (1.82) | 3.19 | (.001) | −.13 |
Note: Descriptive and inferential statistics are unadjusted. Positive effect sizes imply higher symptom levels in females. Repetitive Behavior Scale–Revised (RBS-R) sub-scales were scored using unit weighting of items based on factor loadings from Bishop et al. (2012). Autism Diagnostic Interview–Revised (ADI-R) Restricted/repetitive behavior (RRB) total is based on the algorithm scores using ever or most abnormal coding. ASD=Autism Spectrum Disorder; ADOS=Autism Diagnostic Observation Schedule; CSS=ADOS Calibrated Severity Score; SRS=Social Responsiveness Scale.
Cognition and Motor
Table 3 presents unadjusted means and standard deviations for cognitive and motor measures in males and females with ASD. Females with ASD showed significantly lower overall, verbal, nonverbal cognitive scores as well as reduced language scores. Overall, the discrepancy between verbal and nonverbal IQ was less pronounced in females with ASD, with males more likely to show a discrepancy (≥8 points) in favor of nonverbal IQ (males 48.8% vs. females 40.5%) and females more likely to show a discrepancy in favor of verbal IQ (males 18.9% vs. females 23.6%; χ2(2)=9.04, p=.011; see Figure S2). Interestingly, at higher overall cognitive levels (full scale IQ≥80), females with ASD did not show a verbal/nonverbal discrepancy (females M=0.27, SD=14.26; males M=−3.72, SD=16.51; F(1,1388)=6.83, p=.009). Effect sizes for cognitive and language measures were small, but this is not surprising given the large score ranges. The average standard score difference for full scale IQ was approximately 8 points. Additionally, lower cognitive ability in females with ASD appears to be largely due to a lower proportion of females with IQ≥80 (9.6%) relative to IQ<80 (16.5%: χ2(1)=24.87, p<.001), and there were no sex differences in IQ when analyses were conducted separately in low (IQ<80) and high (IQ≥80) IQ groups (smallest p=.313). Females and males generally showed comparable motor function, with the only exception being females with ASD reported to have worse gross motor coordination (throwing or catching a ball, jumping, running, etc.), possibly due to rater biases in judging gross motor success in girls.
Table 3.
Descriptive Statistics for Measures of Cognitive, Motor, Adaptive, and Associated Behavior Problems in Females and Males With Autism Spectrum Disorder (ASD).
| Females with ASD n=304 | Males with ASD n=2114 | t/χ2 | (p) | Cohen’s d | |||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | ||||
| Cognitive | |||||||
| Full Scale IQ | 74.70 | (27.59) | 82.56 | (27.59) | 4.63 | (<.001) | .19 |
| Verbal IQ | 73.43 | (31.95) | 79.16 | (30.74) | 3.02 | (.003) | .12 |
| Non-Verbal IQ | 77.40 | (26.17) | 85.96 | (25.81) | 5.39 | (<.001) | .22 |
| Verbal IQ - Non-Verbal IQ | −3.97 | (16.34) | −6.79 | (17.04) | 2.72 | (.007) | .11 |
| PPVT | 80.02 | (29.50) | 86.03 | (28.72) | 3.34 | (.001) | .14 |
| CTOPP: Non-Word Repetition | 7.29 | (2.91) | 7.85 | (2.79) | 2.82 | (.005) | .13 |
| Language Deficit (N, %) | 11.03 | (.004) | .14 | ||||
| No Deficit | 189 | (62.2%) | 1494 | (71.0%) | |||
| Mild to Moderate Deficit | 86 | (28.3%) | 483 | (22.9%) | |||
| Severe Deficit | 29 | (9.5%) | 128 | (6.1%) | |||
|
| |||||||
| Motor | |||||||
| Pegboard: Dominant Hand | 9.23 | (3.39) | 8.99 | (3.48) | −0.99 | (.323) | −.05 |
| Pegboard: Non-Dominant Hand | 8.22 | (3.42) | 8.22 | (3.63) | 0.00 | (.999) | .00 |
| DCDQ Total | 37.16 | (12.90) | 38.54 | (12.28) | 1.70 | (.089) | .07 |
| DCDQ Fine Motor | 9.87 | (4.63) | 9.97 | (4.51) | 0.35 | (.726) | .02 |
| DCDQ Movement Coordination | 15.56 | (5.94) | 16.53 | (5.83) | 2.55 | (.011) | .11 |
| DCDQ General Coordination | 11.75 | (4.52) | 12.07 | (4.38) | 1.12 | (.261) | .05 |
|
| |||||||
| Adaptive | |||||||
| Vineland Composite | 70.64 | (11.68) | 73.58 | (11.99) | 4.01 | (<.001) | .16 |
| Vineland Social | 69.08 | (12.34) | 71.31 | (12.45) | 2.92 | (.004) | .12 |
| Vineland Communication | 74.30 | (13.71) | 77.59 | (14.58) | 3.71 | (<.001) | .15 |
| Vineland Daily Living Skills | 73.51 | (13.53) | 76.88 | (13.81) | 3.99 | (<.001) | .16 |
|
| |||||||
| Associated Behavior Problems | |||||||
| CBCL Total Problems | 63.69 | (8.08) | 62.22 | (9.15) | −2.66 | (.008) | .11 |
| CBCL Internalizing | 60.15 | (9.76) | 60.37 | (9.47) | 0.37 | (.715) | −.01 |
| CBCL Externalizing | 58.04 | (10.10) | 56.37 | (10.62) | −2.58 | (.010) | .10 |
| ABC Total | 48.55 | (25.84) | 46.12 | (25.61) | −1.55 | (.122) | .06 |
| ABC Irritability | 13.17 | (9.09) | 11.22 | (8.70) | −3.63 | (<.001) | .15 |
| ABC Lethargy | 11.03 | (7.75) | 9.58 | (7.02) | −3.32 | (.001) | .14 |
| ABC Stereotypy | 4.86 | (4.47) | 4.89 | (4.28) | 0.13 | (.898) | −.01 |
| ABC Hyperactivity | 15.72 | (10.04) | 16.78 | (10.53) | 1.65 | (.099) | −.07 |
| ABC Inappropriate Speech | 3.78 | (3.03) | 3.65 | (2.95) | −0.72 | (.471) | .03 |
| RBS-R Self-Injury | 2.40 | (3.13) | 2.04 | (2.81) | 2.01 | (.044) | .09 |
Note: Bold designated p>.05. Descriptive and inferential statistics are unadjusted. IQ, Peabody Picture Vocabulary Test (PPVT), and Vineland scores are given as Standard Scores (M=100, SD=15). CTOPP scores are given as scales scores (M=10, SD=3). Child Behavior Checklist (CBCL) scores are given as standard scores (T=50, SD=10). Developmental Coordination Disorder Questionnaire (DCDQ) and Aberrant Behavior Checklist (ABC) scores are given as raw scores. Positive effect sizes imply worse cognition, motor function, adaptive behavior, or associated behavior problems in females. Scaled scores were used from the Comprehensive Test of Phonological Processing (CTOPP): non-word repetition sub-test. For language deficit, Cohen’s d was computed by converting the linear by linear association to a standardized mean difference metric. Nine male ASD participants were missing data used to compute language deficit. ASD=Autism Spectrum Disorder. RBS-R=Repetitive Behavior Scale-Revised.
Adaptive and Associated Behavior Problems
Table 3 also presents unadjusted descriptive and inferential statistics for measures of adaptive behavior and associated behavior problems in females and males with ASD. Females with ASD had worse adaptive behavior in all areas. Females with ASD also had greater total and externalizing behavior problems, irritability, lethargy, and self-injurious behavior. Adjustment for the propensity score did not alter findings for any phenotypic measure.
False Discovery Rate Correction
Table 4 summarizes findings that survive false discovery rate correction within each domain. Significant increases in social communication/interaction symptoms in females were no longer significant after false discovery correction. However, 5 of the 9 tests were nominally significant (p<.05) and the pattern of p-values deviated substantially from expectation (see Figure S2 Q-Q plot), suggesting a small trend toward poorer social communication/interaction ability in females with ASD. RBS-R restricted interests survived false discovery rate correction within the repetitive behavior domain, implying that females with ASD are likely to show fewer circumscribed interests. All of the cognitive measures survived false discovery rate correction, as did the four nominally significant Vineland adaptive behavior measures (composite, social, communication, and daily living skills). All of the significant findings within the associated behavior problems domain survived false discovery rate correction except RBS-R self-injurious behavior.
Table 4.
Summary of Major Phenotypic Differences Surviving False Discovery Rate Correction Between Females and Males With Autism Spectrum Disorder.
| Domains | Females With ASD Show… |
|---|---|
|
| |
| Social Communication/Interaction | Greater Symptoms (trend on q-q plot) |
| Restricted Interests | Fewer Symptoms |
| Overall, Verbal, and Non-Verbal IQ | Lower Ability |
| Language Processing | Lower Ability |
| Social, Communication, and Daily Living Skills | Lower Adaptive Function |
| Total and Externalizing Behavior Problems | Greater Problems |
| Irritability and Lethargy | Greater Problems |
Moderators of Sex Differences
Sex differences in social communication symptoms, full scale IQ, gross motor coordination, and adaptive behavior were larger at older ages (smallest t(2379)=2.01, p=.045). Reductions of adaptive behavior in females with ASD were less pronounced in youth of other race/ethnicity (t(2383)=1.94, p=.053) and youth from higher income families (t(2383)=2.29, p=.022). Lower IQ levels in females with ASD were also less pronounced in higher income families (t(2379)=2.06, p=.040). Irritability levels decreased with age (t(2358)=− 6.90, p<.001), but this general trend was modified by substantially greater irritability in older females with ASD relative to older males with ASD (t(2358)=2.83, p=.005). For both sexes, a history of regression was associated with lower full scale IQ and adaptive behavior (t(2360)=3.60, p<.001). However, a history of regression did not moderate sex differences for any measure (all p>.05). Lower levels of restricted interests in females with ASD was not moderated by any demographic or clinical characteristic (all p>.05).
Mediation of Sex Differences
Figure 1 presents mediational model results. IQ was chosen as a mediator to determine whether reductions in IQ in females with ASD, resulting from a lower proportion of females with IQ≥80, was driving other phenotypic differences. Reductions in full scale IQ in females with ASD fully mediated higher social communication/interaction symptoms and lower adaptive function. Lower restricted interests in females with ASD was independent of reductions in IQ (standardized direct effect =−.065, standardized indirect effect < .001). Similarly, greater irritability in females with ASD was largely independent of reductions in IQ (standardized direct effect = .051, standardized indirect effect = .009). A similar pattern of findings was obtained when verbal or non-verbal IQ scores were included as mediators as well as when externalizing behavior replaced ABC-irritability as a downstream effect.
Figure 1.
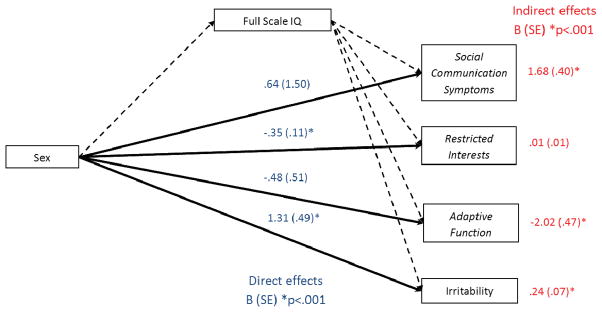
Full scale IQ as a mediator of greater social communication/interaction symptoms, lower restricted interests, poorer adaptive function, and increased irritability in females with autism spectrum disorder. Note: Lower IQ drove greater social communication/interaction symptoms and lower adaptive function. Lower restricted interests and greater irritability in females with autism spectrum disorder were largely independent of IQ. All coefficients are unstandardized with standard errors in parentheses. Social communication symptoms were measured using Social Responsiveness Scale: Social Communication subscale and restricted interests were measured using the Repetitive Behavior Scale–Revised: Restricted Interests subscale. Adaptive function was measured using the Vineland composite score and Irritability was measured using the Aberrant Behavior Checklist: Irritability subscale.
Discussion
The present study represents the largest and most comprehensive study of the cognitive and behavioral characteristics of females with ASD conducted to date. The findings clarify a growing literature on the female ASD phenotype, elucidating both the pattern and magnitude of sex differences after cautiously considering possible sampling and measurement biases. Consistent with several smaller-scale investigations, females with ASD had generally greater impairment than males, including more social communication/interaction symptoms,11, 13 lower cognitive and language abilities,11, 13 and poorer adaptive function,11, 13 and increased problem behavior by parent report4—particularly externalizing problems and irritability.
A lower proportion of females with IQ≥80 in the SSC is congruent with increasingly disparate sex ratios at the highest functioning end of the autism spectrum (~9:1 male to female).2, 3 This sex disparity raises the question of whether females are protected from ASD, requiring additional deleterious “hits” to express the ASD phenotype or whether high functioning ASD females are differentially underidentified; explanations that are not mutually exclusive. Recent data support the notion of etiologic protective factors in females, identifying requirement of a higher genetic liability for expression of ASD in females, particularly for the repetitive behavior domain.12 The idea of separate protective factors across different ASD symptom domains has also been identified by behavioral genetic studies,41 further supporting the potential for sex- and domain-specific protective factors.
The possibility of a higher liability threshold for expression of repetitive behavior in females with ASD is intriguing in light of the present finding of lower restricted interests, especially in higher functioning individuals. This observation may explain the very high sex ratios at the high end of the spectrum, where restricted interests are a key symptom for ASD identification. Less circumscribed interests may be a protective factor or may simply represent a less obvious phenotype during clinical evaluation. This also raises the question of whether high functioning females without restricted interests but with social communication/interaction difficulties and need for sameness will qualify for the newly posited Social Communication (Pragmatic) Disorder or whether a relaxation of proposed DSM-5 criteria for ASD should be implemented for females. Additional study of the specific DSM-5 symptom patterns in females will be needed before determining whether sex-specific algorithms are useful.
Aside from a higher liability threshold, it is also possible that high functioning females with ASD are differentially under-identified. Original descriptions of autism tended to be male-dominated42, 43, although not exclusively,44, 45 and even contemporary descriptions of high functioning ASD emphasize attributes related to traditionally- (albeit stereotypically) male interests.46, 47 At present, symptom exemplars specific to female presentations are not clearly emphasized in diagnostic instruments. The inclusion of behavior exemplars more characteristic of female presentations in commonly-used assessment tools may improve identification of high functioning females with ASD. Interestingly, the present results indicate that currently-applied symptom measurements are not evaluating ASD in a substantively different way, at least in the majority of ASD-affected females in the SSC (including 135 females with full scale IQ≥80). This observation further supports a role for differential liability thresholds in males and females, but does not completely rule out measurement as an additional explanation for under-identification, as particular items may still show minor variations in sensitivity to males and females.
The possibility of under-identification of females with ASD was further supported by moderator analyses. Larger sex differences at older ages, and modification of sex differences in IQ and adaptive behavior by race and income, may imply under-identification of females. The fact that sex differences were less prominent in younger cohorts may imply sampling bias in older cohorts of the SSC or may indicate that identification of females with ASD is improving as general knowledge of autism in the public and in primary care professionals is heightened. The need to reduce any disparity in identification of females, particularly at the higher end of the spectrum, highlights the importance of evaluating rater effects. Mothers are typically the primary source of information for subjective report and interview-based tools and they may report differently for female and male children, at least for “high functioning” symptoms. Less pronounced discrepancy of verbal and non-verbal IQ may also contribute to underidentification of females with ASD. Higher nonverbal ability, relative to verbal ability, is often considered a clinical clue to the presence of ASD. Whether this represents a true protective factor for females or simply contributes to under-identification merits further exploration.
The third major finding from this investigation was that lower levels of restricted interests and greater irritability/externalizing behavior could not be explained by a lower proportion of cognitively-able females in the SSC. Yet, it is not clear if these findings relate more directly to being female rather than to ASD etiology or if there is an interaction between background genetics of females and etiologic factors driving ASD. Regardless, the presence of higher irritability and externalizing behavior highlights that females with ASD should be monitored carefully for these symptoms. It is also crucial to note that the above described pattern of sex differences could not be completely explained by selection bias of diagnostic instruments. The two primary diagnostic instruments, ADI-R and ADOS, separately showed measurement equivalence across sexes and DSM-5 symptom domains had equivalent correlations in males and females. Additionally, results were not likely attributable to unbalanced sampling of females, as results remained consistent both in pattern and magnitude following propensity adjustment. However, unbalanced sampling in this context is distinct from under-identification of female cases with higher IQ. Under-identification of females with higher IQ may still be present, even when sampling is balanced on other clinical and demographic factors. Future large-scale studies that carefully attend to sample ascertainment and measurement bias are needed to confirm the present findings. This research should use multiple distinct instruments and item sets to further investigate ASD factor structure and its equivalence across males and females. Future investigations should also attend to age at ASD diagnosis as this is an important factor in symptom expression.
The primary limitations of the present investigation were evaluation of a large number of phenotypic measures clustered into conceptual domains, the lack of correlation between phenotypic sex-differences and genotypic measurements, and the inherent limitations of the SSC cohort. Specifically, some female ASD cases may have been missed due to the strict diagnostic requirements of the sample, including a requirement to at least meet autism spectrum criteria on the ADI-R and ADOS. This concern is somewhat diminished because of the high functioning nature of the SSC sample and inclusion of clinician best estimate diagnostic judgments. Future studies of sex differences in ASD should include individuals from single and multiple incidence families who do not meet full criteria for ASD but have other “mimic” conditions, including social (pragmatic) communication disorder. Examining the patterns of autism symptoms across a fuller range of syndromal and subsyndromal presentations, using both raw and sex-adjusted scores on quantitative trait and clinical diagnostic measures, is needed to better understand any ascertainment biases and to inform diagnostic judgments.
The extensive array of phenotypic measures coupled with a large sample sizes raises the question of whether observed sex-differences are simply Type 1 errors. To mitigate against this possibility, the present study applied false discovery corrections within each domain and focused on effect sizes in the interpretation of significant findings. Furthermore, application of moderator and mediator analyses permitted building a conceptual model of the pattern of findings, rather than focusing on each measure in isolation. Additional studies that delineate factors leading to sex differences and that link differences across levels of analysis (symptoms, cognition, brain structure and function, cellular, and molecular) are needed to develop a comprehensive understanding of sex differences in ASD. These studies should also attend to the magnitude of observed differences as small effects may or may not be clinically meaningful.
The present study provided a broad picture across a range of behavioral symptom and cognitive/motor domains. As such, it represents an important large-scale “first pass” at female-male differences within ASD, identifying lower restricted interests and higher irritability/externalizing behavior as phenotypic differences that could not be explained by measurement or sampling. Future studies that take a more focused look at behavioral exemplars and specific facets of cognitive processes (e.g. prosody or affect recognition) are needed to further refine our understanding of sex-differences in ASD. Finally, the SSC has recently provided access to large-scale genotype data. An important next step will be to correlate identified sex-differences in restricted interests and irritability/externalizing behavior with genetic variation. Several regions and genomic features show sex differences48, 49 across males and females and these may be candidates for risk or protective factors driving sex-differences in ASD.
Supplementary Material
Clinical Guidance.
Females with autism spectrum disorder (ASD) may show lower levels of restricted interests on currently used measures. Evaluators should be aware of this difference when conducting evaluations and consider looking for female-specific indicators of restricted interests.
Females with ASD may show lower cognitive ability and adaptive function but generally similar symptom levels to males with ASD. Clinicians should consider evaluating cognition and adaptive function when engaging in treatment planning as the symptom pattern may not provide an accurate picture of the functional capacity of females with ASD.
Clinicians should consider both raw and sex-adjusted scores for parent-reported quantitative symptom measures, particularly for social communication behavior, as females with ASD appear to be equally or more impaired than males with ASD on this domain.
Clinicians may consider Social (Pragmatic) Communication Disorder as an alternative to ASD for females who do not meet full DSM-5 restricted and repetitive behavior criteria for ASD.
Acknowledgments
This work was made possible by a pilot research grant from the Simons Foundation Autism Research Initiative (SFARI) and by funding to the Case Western Reserve University/Cleveland Clinic Clinical and Translational Science Award (CTSA) grant UL1 RR024989 provided by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (NIH).
The authors acknowledge the contributions of families participating in the Simons Simplex Collection.
Footnotes
Supplemental material cited in this article is available online.
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Simons Foundation or NIH.
T.W.F. and A.Y.H. designed the present study. T.W.F. obtained funding to support analyses. S.L.B. contributed to outcome measure selection. T.W.F. supervised data interpretation of the study. T.W.F. conducted data management and data analyses. All authors contributed to writing and revision. Dr. Frazier served as the statistical expert for this research.
Disclosure: Dr. Frazier has received federal funding or research support from, acted as a consultant to, received travel support from, and/or received a speaker’s honorarium from the Ingalls Foundation, Forest Laboratories, Ecoeos, IntegraGen, Shire Development, Bristol-Myers Squibb, NIH, and the Brain and Behavior Research Foundation. Dr. Hardan has received research funding from Forest Pharmaceuticals and Bristol Myers Squibb and is a consultant to IntegraGen. Drs. Georgiades and Bishop report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dr. Thomas W. Frazier, Center for Pediatric Behavioral Health and Center for Autism, Cleveland Clinic.
Dr. Stelios Georgiades, McMaster University and Offord Centre for Child Studies.
Dr. Somer L. Bishop, Center for Autism and the Developing Brain, Weill Cornell Medical College.
Dr. Antonio Y. Hardan, Stanford University.
References
- 1.Blumberg SJ, Bramlett MD, Kogan MD, Schieve LA, Jones JR, Lu MC. Changes in Prevalence of Parent-reported Autism Spectrum Disorder in School-aged US Children: 2007 to 2011–2012. Hyattsville, MD: National Center for Health Statistics; 2013. [PubMed] [Google Scholar]
- 2.Volkmar FR, Szatmari P, Sparrow SS. Sex differences in pervasive developmental disorders. J Autism Dev Disord. 1993 Dec;23(4):579–591. doi: 10.1007/BF01046103. [DOI] [PubMed] [Google Scholar]
- 3.Bryson SE, Clark BS, Smith IM. First report of a Canadian epidemiological study of autistic syndromes. J Child Psychol Psychiatry. 1988 Jul;29(4):433–445. doi: 10.1111/j.1469-7610.1988.tb00735.x. [DOI] [PubMed] [Google Scholar]
- 4.Mandy W, Chilvers R, Chowdhury U, Salter G, Seigal A, Skuse D. Sex differences in autism spectrum disorder: Evidence from a large sample of children and adolescents. J Autism Dev Disord. 2012;42(7):1304–1313. doi: 10.1007/s10803-011-1356-0. [DOI] [PubMed] [Google Scholar]
- 5.Lai MC, Lombardo MV, Suckling J, et al. Biological sex affects the neurobiology of autism. Brain. 2013 Sep;136(Pt 9):2799–2815. doi: 10.1093/brain/awt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beacher FD, Minati L, Baron-Cohen S, et al. Autism attenuates sex differences in brain structure: a combined voxel-based morphometry and diffusion tensor imaging study. AJNR Am J Neuroradiol. 2012 Jan;33(1):83–89. doi: 10.3174/ajnr.A2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai MC, Lombardo MV, Pasco G, et al. A behavioral comparison of male and female adults with high functioning autism spectrum conditions. PLoS One. 2011;6(6):e20835. doi: 10.1371/journal.pone.0020835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beacher FD, Radulescu E, Minati L, et al. Sex differences and autism: brain function during verbal fluency and mental rotation. PLoS One. 2012;7(6):e38355. doi: 10.1371/journal.pone.0038355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai MC, Lombardo MV, Ruigrok AN, et al. Cognition in males and females with autism: similarities and differences. PLoS One. 2012;7(10):e47198. doi: 10.1371/journal.pone.0047198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarz E, Guest PC, Rahmoune H, et al. Sex-specific serum biomarker patterns in adults with Asperger’s syndrome. Mol Psychiatry. 2010 Dec;16(12):1213–1220. doi: 10.1038/mp.2010.102. [DOI] [PubMed] [Google Scholar]
- 11.Lord C, Schopler E, Revicki D. Sex differences in autism. J Autism Dev Disord. 1982 Dec;12(4):317–330. doi: 10.1007/BF01538320. [DOI] [PubMed] [Google Scholar]
- 12.Szatmari P, Liu XQ, Goldberg J, et al. Sex differences in repetitive stereotyped behaviors in autism: implications for genetic liability. Am J Med Genet B Neuropsychiatr Genet. 2012 Jan;159B(1):5–12. doi: 10.1002/ajmg.b.31238. [DOI] [PubMed] [Google Scholar]
- 13.Carter AS, Black DO, Tewani S, Connolly CE, Kadlec MB, Tager-Flusberg H. Sex differences in toddlers with autism spectrum disorders. J Autism Dev Disord. 2007 Jan;37(1):86–97. doi: 10.1007/s10803-006-0331-7. [DOI] [PubMed] [Google Scholar]
- 14.Lemon JM, Gargaro B, Enticott PG, Rinehart NJ. Executive functioning in autism spectrum disorders: a gender comparison of response inhibition. J Autism Dev Disord. 2011 Mar;41(3):352–356. doi: 10.1007/s10803-010-1039-2. [DOI] [PubMed] [Google Scholar]
- 15.Lord C. Follow-up of two-year-olds referred for possible autism. J Child Psychol Psychiatry. 1995 Nov;36(8):1365–1382. doi: 10.1111/j.1469-7610.1995.tb01669.x. [DOI] [PubMed] [Google Scholar]
- 16.Fischbach GD, Lord C. The Simons Simplex Collection: a resource for identification of autism genetic risk factors. Neuron. 2010 Oct 21;68(2):192–195. doi: 10.1016/j.neuron.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview-Revised Manual. Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- 18.Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule: ADOS manual. Los Angeles, CA: Western Psychological Services; 2002. [Google Scholar]
- 19.Constantino JN, Gruber CP. Social Responsiveness Scale: Manual. Los Angeles, CA: Western Psychological Services; 2005. [Google Scholar]
- 20.Lam KS, Aman MG. The Repetitive Behavior Scale-Revised: independent validation in individuals with autism spectrum disorders. J Autism Dev Disord. 2007 May;37(5):855–866. doi: 10.1007/s10803-006-0213-z. [DOI] [PubMed] [Google Scholar]
- 21.Bishop SL, Hus V, Duncan A, et al. Subcategories of restricted and repetitive behaviors in children with autism spectrum disorders. J Autism Dev Disord. 2013 Jun;43(6):1287–1297. doi: 10.1007/s10803-012-1671-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elliott CD. Differential Ability Scales: Introductory and technical handbook. San Antonio, TX: The Psychological Corporation; 1990. [Google Scholar]
- 23.Wechsler D. Manual for the Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- 24.Wechsler D. The Wechsler Intelligence Scale for Children - Fourth Edition. London: Pearson; 2004. [Google Scholar]
- 25.Joseph RM, Tager-Flusberg H, Lord C. Cognitive profiles and social-communicative functioning in children with autism spectrum disorder. J Child Psychol Psychiatry. 2002 Sep;43(6):807–821. doi: 10.1111/1469-7610.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunn LM, Dunn DM. Peabody Picture Vocabulary Test. 4. San Antonio, Tx: NCS Pearson, Inc; 2007. [Google Scholar]
- 27.Wagner RK, Torgesen JK, Rashotte CA. Comprehensive test of phonological processing. Austin, TX: PRO-ED; 1999. [Google Scholar]
- 28.Lezak MD. Neuropsychological assessment. 3. New York, NY, USA: Oxford University Press; 1995. [Google Scholar]
- 29.Wilson BN, Crawford SG, Green D, Roberts GW, Aylott A, Kaplan BJ. Psychometric properties of the revised developmental coordination disorder questionnaire. Physical and Occupational Therapy in Pediatrics. 2009;20(2):184–204. doi: 10.1080/01942630902784761. [DOI] [PubMed] [Google Scholar]
- 30.Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales, Second Edition. San Antonio, TX: Pearson; 2005. [Google Scholar]
- 31.Achenbach TM, Rescorla LA. Manual for the ASEBA school-age forms and profiles. Burlington, VT: University of Vermont, Department of Psychiatry; 2001. [Google Scholar]
- 32.Aman MG, Singh NN, Stewart AW, Field CJ. Psychometric characteristics of the aberrant behavior checklist. Am J Ment Defic. 1985;89:492–502. [PubMed] [Google Scholar]
- 33.Aman MG, Singh NN, Stewart AW, Field CJ. The Aberrant Behavior Checklist: a behavior rating scale for the assessment of treatment effects. Am J Ment Defic. 1985;89:485–491. [PubMed] [Google Scholar]
- 34.Kraemer HC, Kiernan M, Essex M, Kupfer DJ. How and why criteria defining moderators and mediators differ between the Baron and Kenny and MacArthur approaches. Health Psychol. 2008 Mar;27(2 Suppl):S101–108. doi: 10.1037/0278-6133.27.2(Suppl.).S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 36.Kraemer HC, Lowe KK, Kupfer DJ. To Your Health: How to Understand What Research Tells Us About Risk. New York: Oxford University Press; 2005. [Google Scholar]
- 37.Benjamini Y, Hochberg T. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995;85:289–300. [Google Scholar]
- 38.Benjamini Y, Hochberg T. On the adaptive control of the false discovery rate in multiple testing with independent statistics. Journal of Educational and Behavioral Statistics. 2000;26:60–83. [Google Scholar]
- 39.Rosenthal R, Rosnow RL. Essentials of behavioral research: Methods and data analysis. 2. New York: McGraw-Hill, Inc; 1991. [Google Scholar]
- 40.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Erlbaum; 1987. [Google Scholar]
- 41.Ronald A, Happe F, Bolton P, et al. Genetic heterogeneity between the three components of the autism spectrum: A twin study. J Am Acad Child Adolesc Psychiatry. 2006;45(6):691–699. doi: 10.1097/01.chi.0000215325.13058.9d. [DOI] [PubMed] [Google Scholar]
- 42.Wing L. Asperger’s syndrome: a clinical account. Psychol Med. 1981;11(1):115–129. doi: 10.1017/s0033291700053332. [DOI] [PubMed] [Google Scholar]
- 43.Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- 44.Bartak L, Rutter M. Differences between mentally retarded and normally intelligent autistic children. J Autism Child Schizophr. 1976 Jun;6(2):109–120. doi: 10.1007/BF01538054. [DOI] [PubMed] [Google Scholar]
- 45.Kanner L. Follow-up study of eleven autistic children originally reported in 1943. J Autism Child Schizophr. 1971 Apr-Jun;1(2):119–145. doi: 10.1007/BF01537953. [DOI] [PubMed] [Google Scholar]
- 46.Buchen L. Scientists and autism: When geeks meet. Nature. 2011;479:25–27. doi: 10.1038/479025a. [DOI] [PubMed] [Google Scholar]
- 47.Lawson J, Baron-Cohen S, Wheelwright S. Empathising and systemising in adults with and without Asperger Syndrome. J Autism Dev Disord. 2004 Jun;34(3):301–310. doi: 10.1023/b:jadd.0000029552.42724.1b. [DOI] [PubMed] [Google Scholar]
- 48.Liu J, Morgan M, Hutchison K, Calhoun VD. A study of the influence of sex on genome wide methylation. PLoS One. 2010;5(4):e10028. doi: 10.1371/journal.pone.0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mittelstrass K, Ried JS, Yu Z, et al. Discovery of sexual dimorphisms in metabolic and genetic biomarkers. PLoS Genet. 2011 Aug;7(8):e1002215. doi: 10.1371/journal.pgen.1002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kenny DA, Kashy DA. Analysis of the multi-trait multi-method matrix by confirmatory factor analysis. Psychol Bull. 1992;112(1):165–172. [Google Scholar]
- 51.Cheung GW, Rensvold RB. Evaluating goodness-of-fit indexes for testing measurement invariance. Structural Equation Modeling: A Multidisciplinary Journal. 2002;9(2):233–255. [Google Scholar]
- 52.Vandenberg RJ, Lance CE. A review and synthesis of the measurement equivalence literature: Suggestions, practices, and recommendations for organizational research. Organizational Research Methods. 2000;3:4–70. [Google Scholar]
- 53.Chen FF. Sensitivity of goodness of fit indexes to lack of measurement invariance. Structural equation modeling. 2007;14(3):464–504. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


