Abstract
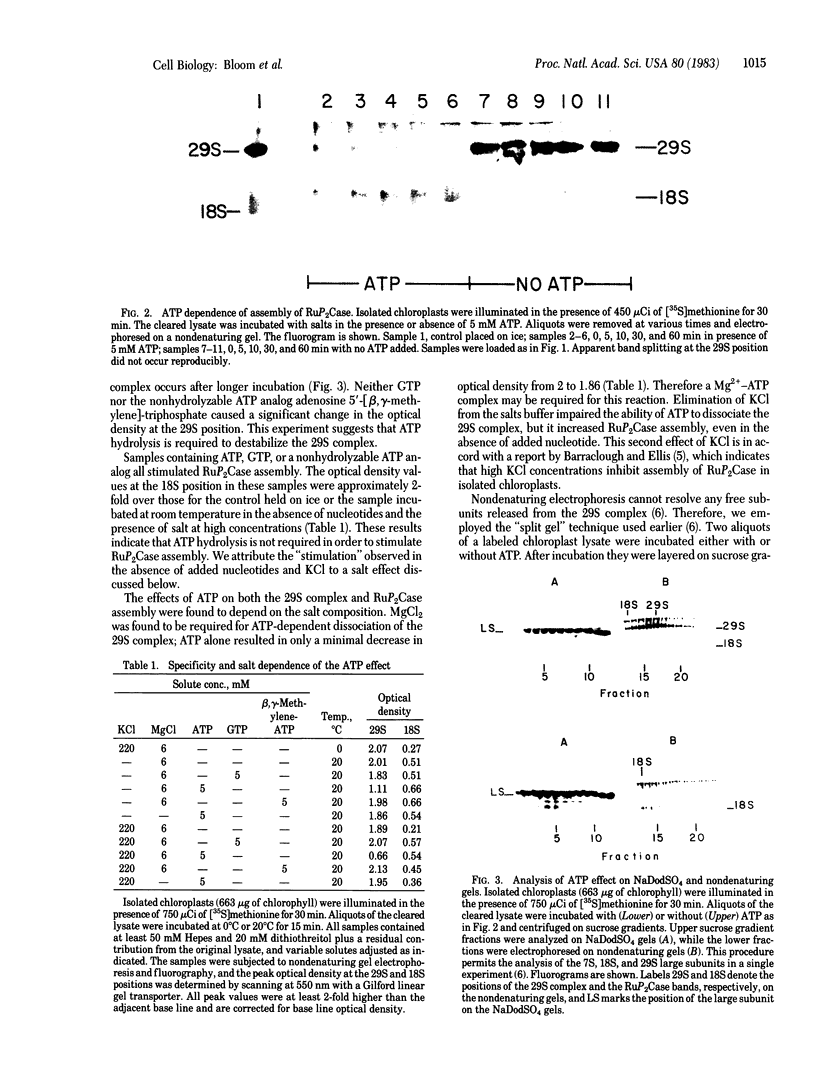
Ribulose-1,5-bisphosphate carboxylase [RuP2Case; 3-phospho-D-glycerate carboxy-lyase (dimerizing), EC 4.1.1.39] is composed of eight small subunits (Mr, 14,000) and eight large subunits (Mr, 55,000). Newly synthesized large subunits are associated with two complexes having sedimentation coefficients of 7 and 29 S. Assembly of RuP2Case occurs in isolated intact chloroplasts in the light but not in the dark. When extracts of chloroplasts are treated with ATP or GTP, RuP2Case assembly is accelerated while the 29S large subunit complex is maintained. In the presence of Mg2+, ATP brings about almost complete dissociation of the 29S complex, whereas GTP and a nonhydrolyzable analog of ATP are without effect. These results indicate the existence of a complex set of reactions involving nucleotides, Mg2+, and several putative intermediates in RuP2Case assembly. It is postulated that these reactions at least partly account for the light dependence of RuP2Case assembly. In particular, ATP and GTP promote the assembly of large subunits into RuP2Case.
Keywords: chloroplast, ATP, protein synthesis
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barraclough R., Ellis R. J. Protein synthesis in chloroplasts. IX. Assembly of newly-synthesized large subunits into ribulose bisphosphate carboxylase in isolated intact pea chloroplasts. Biochim Biophys Acta. 1980 Jun 27;608(1):19–31. doi: 10.1016/0005-2787(80)90129-x. [DOI] [PubMed] [Google Scholar]
- Blair G. E., Ellis R. J. Protein synthesis in chloroplasts. I. Light-driven synthesis of the large subunit of fraction I protein by isolated pea chloroplasts. Biochim Biophys Acta. 1973 Aug 24;319(2):223–234. doi: 10.1016/0005-2787(73)90013-0. [DOI] [PubMed] [Google Scholar]
- Bowes G., Ogren W. L., Hageman R. H. Phosphoglycolate production catalyzed by ribulose diphosphate carboxylase. Biochem Biophys Res Commun. 1971 Nov 5;45(3):716–722. doi: 10.1016/0006-291x(71)90475-x. [DOI] [PubMed] [Google Scholar]
- Chua N. H., Schmidt G. W. Post-translational transport into intact chloroplasts of a precursor to the small subunit of ribulose-1,5-bisphosphate carboxylase. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6110–6114. doi: 10.1073/pnas.75.12.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Fish L. E., Jagendorf A. T. High rates of protein synthesis by isolated chloroplasts. Plant Physiol. 1982 Oct;70(4):1107–1114. doi: 10.1104/pp.70.4.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Timasheff S. N. In vitro reconstitution of calf brain microtubules: effects of solution variables. Biochemistry. 1977 Apr 19;16(8):1754–1764. doi: 10.1021/bi00627a037. [DOI] [PubMed] [Google Scholar]
- Morgenthaler J. J., Price C. A. Photosynthetic activity of spinach chloroplasts after isopycnic centrifugation in gradients of silica. Plant Physiol. 1974 Oct;54(4):532–534. doi: 10.1104/pp.54.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich S. A., Estes J. E. Detection of conformational changes in actin by proteolytic digestion: evidence for a new monomeric species. J Mol Biol. 1976 Jul 15;104(4):777–792. doi: 10.1016/0022-2836(76)90181-9. [DOI] [PubMed] [Google Scholar]
- Roy H., Bloom M., Milos P., Monroe M. Studies on the assembly of large subunits of ribulose bisphosphate carboxylase in isolated pea chloroplasts. J Cell Biol. 1982 Jul;94(1):20–27. doi: 10.1083/jcb.94.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner A. Head to tail polymerization of actin. J Mol Biol. 1976 Nov;108(1):139–150. doi: 10.1016/s0022-2836(76)80100-3. [DOI] [PubMed] [Google Scholar]