Abstract
Background
Poor adherence to inhaled corticosteroids (ICS) is a critical risk factor contributing to asthma morbidity among low-income minority adolescents.
Objective
This trial tested whether peer support group meetings and peer asthma messages delivered via mp3 players improved adherence to ICS.
Methods
Low-income African American and/or Hispanic adolescents, ages 11–16, with persistent asthma, and poor (≤ 48%) adherence to prescription ICS during the 3-week run-in were randomized to intervention or attention control groups (ATG) for the 10-week treatment. During treatment, the intervention arm participated in weekly coping peer group support sessions and received mp3 peer-recorded asthma messages promoting adherence. The ATG participated in weekly meetings with a research assistant and received an equivalent number of mp3 doctor-recorded asthma messages. Adherence was measured using self-report and the DoserCT, (Meditrac, Inc.), an electronic dose counter. The primary outcome was the difference in adherence at 10 weeks between the two arms.
Results
Thirty-four subjects were randomized to each arm. At 10 weeks, no statistical difference in objectively measured adherence could be detected between the two arms adjusting for baseline adherence (P = 0.929). Adherence declined in both groups over the course of the active treatment period. Participants’ in both study arms self-reported adherence was significantly higher than their objectively measured adherence at week 10 (P < 0.0001).
Conclusion
Improving medication adherence in longitudinal studies is challenging. Peer support and mp3-delivered peer asthma messages may not be of sufficient dose to improve outcomes.
Keywords: Childhood asthma, medication adherence, health status disparities, outcome assessment (health care), asthma knowledge, inner-city, adolescents
Asthma is the most common chronic illness of childhood in the United States, affecting 9.3% of children.1 Asthma prevalence in the pediatric population continues to rise, with greatest risk seen in minority racial/ethnic groups.2 African American (non-Hispanic) and Puerto Rican Hispanic children are disproportionately affected, compared to Caucasian children, with asthma prevalence of 14.6%, 18.4%, and 8.2% for each group, respectively.1 African American and Hispanic children experience higher rates of emergency department visits, hospitalizations, and deaths due to asthma than Caucasianchildren.3 In particular, African American children are over twice as likely to be hospitalized for asthma, and four times as likely to die from asthma, as Caucasian children.3 Age is a powerful predictor of negative outcomes. Across all three racial/ethnic groups, asthma death rates are approximately twice as high in 11–17 year old adolescents as 0–10 year old children.4
Lack of adherence to inhaled corticosteroid medications (ICS) is among the most significant risk factors associated with poor asthma outcomes.5–12 Although ICS reduce the frequency of asthma symptoms and severity of asthma attacks,13–15 adherence to this class of medications is dismally low and the benefits of ICS are often not received. In a city-wide Chicago cohort, only 11.9% of children with moderate or severe persistent asthma self-reported use of a controller medication.16 A large-scale community-wide intervention in Chicago was able to increase adequate ICS use in 5–9 year olds, but not in 10–17 year olds.17 Objective measurement of medication adherence—especially among African American and Hispanic youth—is rare.18–20 Interventions to improve adherence have not been rigorously tested in this population.7
A difficulty in the design of interventions for adolescents is that they must fit easily into adolescents existing lifestyles and peer group.21–25 Coping peers have been shown to be a valuable source of information, companionship, mutual understanding, and support in helping adolescents to face and manage their asthma.26–27 Technology-based interventions directed at adolescent adherence have begun to emerge and offer distinct advantages. Media consumption among adolescents overall is high, with two groups of youth standing out for exploding levels of media use: (1) those in the early teen years (11–14 year olds); and (2) African Americans and Hispanics.28 iPod/mp3 player ownership has increased from 18% to 76% among all 8–18 year olds between 2004 and 2009.28 Guided by social cognitive theory,21 the investigators sought to leverage this existing use of technology, specifically the iPod/mp3 player, and the coping peer model to deliver a culturally sensitive intervention aimed at improving medication adherence in minority adolescents with asthma.28
This randomized controlled trial assessed the efficacy of a coping peer support plus mp3 technology-assisted behavioral intervention, relative to an attention control, in improving adherence among non-adherent African American and Hispanic adolescents with persistent asthma.
Methods
Eligibility Criteria
Eligibility criteria specified that participants would be: 11 to 16 years of age and self-identified as African American or Hispanic, diagnosed with persistent asthma, and possessing an active prescription for a daily ICS for asthma. Persistent asthma was defined as asthma symptoms (e.g. cough, wheeze, shortness of breath, chest tightness) more than two days per week or nighttime awakenings more than twice a month; or being on a prescribed daily ICS for asthma.29 The latter requirement was met when the adolescent had, within the last 12 months: 1) an outpatient visit to Rush University Medical Center with asthma listed as a diagnosis code for that visit, and 2) a prescription for ICS. To verify that the ICS prescription was active, the participant had to either bring in the medication with his or her name on the pharmacy label, or have the pharmacy verify availability of an active prescription. Exclusion criteria included: a caregiver or child unable to speak English, the presence of co-morbidities that could interfere with study participation, or ≥ 48% adherence over 2 weeks during the run-in period. Participants with ≥ 48% adherence were excluded as the aim of the study was to target children with poor adherence (i.e. who could benefit most from this behavioral intervention). Observational studies that electronically monitored adherence to daily ICS in diverse samples of adolescents report rates between 40–50% as being typical. 18–19, 30–31 The most common dosing schedule for ICS administered via metered dose inhaler is two puffs twice daily (for a total of 56 puffs over a 2-week period). Thus, the investigators set the cutpoint for adherence eligibility as taking ≤ 26 puffs per 2-week period, or ≤ 48% adherence.
Recruitment and Study Follow-up
Patients were recruited between May 24, 2011 and February 28, 2012 from three primary care practices at Rush University Medical Center in Chicago, Illinois. All three practices have been recognized as level 3 (highest recognition awarded by the National Committee for Quality Assurance) Patient-Centered Medical Homes. The majority of pediatric patients in these practices receive public insurance through Medicaid or the State Children’s Health Insurance Program (SCHIP). The study protocol was approved by the Rush University Medical Center Institutional Review Board. After obtaining Request for Access to Personal Health Information for Reviews Preparatory to Research, eligible patients were identified by an electronic medical records search. Written consent and assent were obtained from eligible patients as well as their parent or guardian.
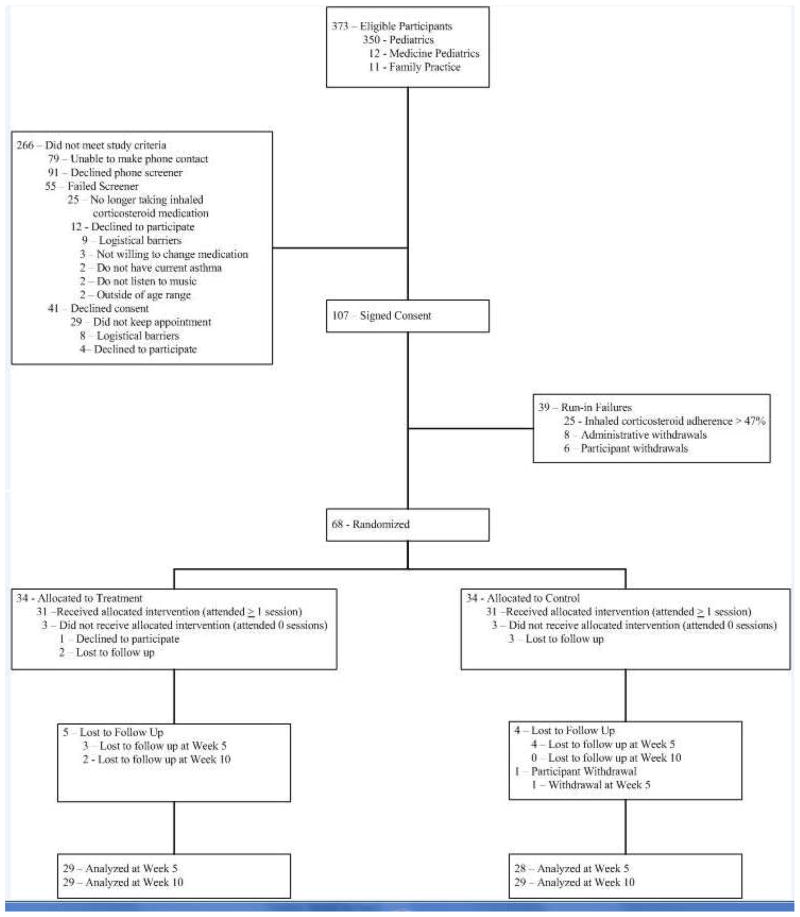
Figure 1 illustrates the flow of participants from recruitment through final data collection. Figure 2 shows the design of the study. Upon providing written consent, participants entered a 3-week run-in phase in which medication adherence eligibility was determined. Each participant was provided an electronic medication monitor (Doser CT, MediTrack, Inc., South Easton, MA) that was placed on their ICS. Participants and their caregivers were fully informed that the electronic monitor would record the number of times that they actuated their ICS on a daily basis, but were kept blind to the specific purpose of the monitoring.
Figure 1. CONSORT Diagram.
The CONSORT flow diagram illustrates the flow of participants from eligibility to completion of treatment.
Figure 2. Study Timeline.
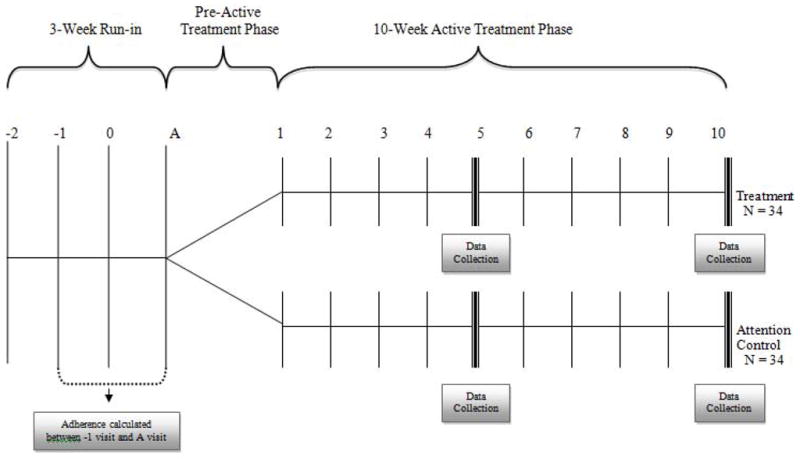
The study timeline illustrates the run-in, pre-active treatment, and active treatment phases. Data collection occurred at weeks 5 and 10 of the active treatment phase.
At the end of the 3-week run-in phase, eligible participants entered a pre-active treatment phase in which they attended weekly individual sessions until a cohort of 8 to 12 participants could be formed. Once a cohort of 8–12 participants was formed, the group was randomized (blocked group randomization, using a computer-generated allocation schedule) to receive either the active intervention or the attention control. A total of 7 cohorts were enrolled, resulting in a total of 68 participants. Participants were followed for 10 weeks after being randomized.
Study Interventions
During the run-in phase, all participants received: 1) medical supervision (provision of spacers and peak flow meters, as well as education on proper use of each);32 2) an iPod shuffle 2GB (4th Generation) (Apple, Cupertino, CA) locked at 70% volume to protect participant’s hearing33–34 and set in shuffle mode to ensure all contents had an equal chance of being played; and 3) clean and/or radio-edited mp3 music tracks.
During each of the 10 weeks of the active treatment phase, participants received music tracks and were to attend scheduled weekly sessions. Those in the active intervention group attended coping peer group sessions led by social workers during weeks 1 through 4 and 6 through 9. The session leaders were trained to use a motivational interviewing approach and to follow a topic guide designed specifically for this study. 35–38 During the sessions, participants discussed barriers to taking daily ICS and strategies to overcome them. The social workers received equivalent training in using motivational interviewing as a behavior change therapy, and asthma education39 prior to leading the coping peer groups. At the conclusion of each group session, participants developed and recorded 2 to 4 messages gleaned from the discussions at that visit and focused on encouraging each other to take their daily ICS. These messages were then produced with background music chosen by the participants and placed as selections on their iPods to be shuffled at random between the music tracks. In contrast, those in the attention control group met individually with a research assistant during weeks 1 through 4, and 6 through 9. The research assistant did not engage in conversation with the participants to promote adherence. At each of these sessions, the attention control group adolescents received the same number of iPod messages as their active intervention group counterparts, with content promoting adherence to daily controller medications. However, their messages were developed and recorded by an asthma doctor rather than by peers. These messages were placed on participants’ iPods to be shuffled at random between the music tracks.
Outcome Measures
Outcomes data were collected at baseline and at 5 and 10 weeks post-randomization (during the active treatment phase) by research assistants blinded to the participants’ group assignment. The primary study outcome was ICS adherence measured using the electronic medication monitor to ICS. Daily adherence was calculated as the following over a given 24-hour period: percentage of prescribed puffs = (puffs actuated/prescribed puffs) * 100%.6 Daily adherence was truncated at 100% of the prescribed dose because true adherence to ICS may be distorted by intentional overuse (medication dumping).40 To use a standard electronic monitoring system to measure adherence in all study participants, the investigators switched participants on different ICS formulations to a best approximation dose of Flovent HFA 110 mcg Inhalation Aerosol (provided by GlaxoSmithKline) for the duration of the study. If a participant declined the switch, he or she was allowed to continue to participate in the study as long as the Doser CT tracking cap fit onto his or her current ICS medication. Adherence was monitored continuously during study follow-up. The primary study outcome was measured using the average daily adherence over the previous 14 days. This measure was determined at baseline (using data obtained during the run-in phase) and post-randomization at 5 weeks and at 10 weeks.
Assessments at baseline included: demographics, asthma history, media use, asthma control, and depression. Asthma history included questions about currently prescribed controller and quick-relief medications for asthma as well as tobacco smoke exposure. Asthma control was assessed using questions that addressed domains of risk and impairment from the NHLBI EPR3 guidelines, specifically: recall of previous 2–4 weeks of daytime symptoms, nighttime awakenings, interference with normal activities, use of short-acting bronchodilator medication for symptom control; and number of asthma exacerbations requiring oral systemic steroid use in the prior year.29 The Children’s Depression Inventory 241 was used to evaluate levels of depression.
The following were assessed at baseline, as well as at weeks 5 and 10 of the active treatment period: 1) asthma knowledge;42 2) ICS knowledge;40 3) ICS self-efficacy;43 4) social support;44 5) asthma social support;44 and 6) asthma exacerbations.29 In addition, at the week 5 and week 10 visits only, participants were asked to self-report their ICS medication use over the past 14 days. Asthma knowledge was measured by the ZAP Asthma Knowledge Instrument. This 39-item questionnaire was adapted from the ZAP Caregiver Asthma Knowledge Survey Instrument.42 Asthma exacerbations included self-reported: missed school days; oral prednisone bursts; unscheduled urgent visits to the doctor’s office; emergency room visits; hospitalizations; intensive care unit admissions; and intubations.
Statistical Methods
This study employed a two-arm design in which participants were randomized to either the intervention arm or attention control arm. Whenever a cohort of 8–12 individuals met all eligibility criteria, the study biostatistician effected the randomization process. A 1:1 ratio block randomization scheme was used. Group membership was maintained and reinforced within the active intervention arm; however, the attention control was delivered individually. Thus, clustering was a potential confounder within the active intervention arm, but not within the control arm. Linear mixed effects modeling was used to assess the influence on outcomes of the clustering within the active intervention arm. This approach allows analysis of within- and between-cluster variance as well as estimation of the intraclass correlation.45 If the resulting model estimates are non-significant (P > 0.05), the effect of clustering can be assumed negligible and cluster-adjusted analyses not required.
Baseline demographic and assessment variables were compared between treatment groups to assess balance. The t-test or Wilcoxon rank sum test was used, depending on the appropriateness of the normality assumption, to compare continuous variables. Discrete variables were compared using the chi-square test or fisher exact test, depending on the pertinent sample size. Cluster-adjusted versions of these tests were used when analyses indicated a significant (at the 0.05 level) effect due to clustering within the intervention arm.
The primary outcome measure was adherence to ICS, computed as the average daily adherence rate over the previous 14-days. For each participant, this measure was taken at baseline and weeks 5 and 10 post-randomization. The primary analysis compared mean adherence at 10-weeks post-randomization between the two treatment groups. To assess the influence of missing data on this comparison, the analysis was also performed with missing outcomes values replaced with the maximal observed value (the so-called ‘best case’ scenario) and then with the missing outcomes replaced by the minimal observed value (the so-called ‘worst case’ scenario). To adjust for potentially meaningful pre-specified covariates (treatment group, visit attendance, asthma control, depression, music listening, and asthma severity), multilevel modeling was used.46 For the model, objectively measured 10-week adherence was the dependent variable, the covariates mentioned above were included as fixed-effects, and a random intercept was employed. All statistical analyses were performed using SAS v.9.2 software.
Results
Enrollment and Follow-up
See Figure 1. A total of 373 potentially eligible patients were identified from the electronic medical record. Two hundred and sixty-six participants did not meet study criteria. Of the 107 participants who completed informed consent and assent procedures, 39 failed the run-in, and 68 were randomized (34 to the treatment, and 34 to the attention control). Three participants randomized to the active intervention group did not attend any study visits due to: (n=1) declined to participate and (n=2) becoming lost to follow up. Three participants randomized to the attention control group did not attend any study visits, having become lost to follow up (n=3). Fifty-six percent (19/34) and 62% (21/34) of the treatment and attention control group participants, respectively, attended > 6 study visits. To be included in the data analysis, participants had to have either week 5 or week 10 data available with respect to adherence or asthma knowledge. A total of 57 participants, 29 (85%) and 28 (82%) in the treatment and attention control groups respectively, completed the 5 week follow-up visit. A total of 58 participants, 29 (85%) in each study arm, completed the 10 week follow up visit.
Baseline Characteristics
Baseline characteristics of the 68 randomized participants are presented in Table I. There were no differences between arms on any baseline characteristics except listening to music ≥ 1 hour/day where the control group listened more (97.1%) than the treated (76.5%) (P = 0.027). The sample is remarkable for a high rate of uncontrolled asthma (80.9%) and poor adherence to ICS (26.8%), despite all being prescribed ICS by their physician. There is also a high rate of participation in the Patient Centered Medical Home (75% of all participants) within this study cohort.
Table I.
Baseline Characteristics of 68 Randomized Participants
| Total (n=68) | Treatment (n=34) | Control (n=34) | P value | |
|---|---|---|---|---|
| Age, Mean (Min, Max) | 13.4 (11, 16) | 13.3 (11, 16) | 13.6 (11, 16) | 0.530 |
| Gender, n (%) | ||||
| Male | 32 (47.1) | 17 (50) | 16 (47.1) | 0.808 |
| Female | 36 (52.9) | 17 (50) | 18 (52.9) | |
| Ethnicity: Hispanic/Latino, n (%) | 9 (13.3) | 7 (20.6) | 2 (5.9) | 0.149 |
| Race, n (%) | ||||
| Black/African American | 58 (85.2) | 26 (83.9) | 32 (94.1) | 0.083 |
| Mixed African American* | 1 (1.5) | 1 (3.2) | 0 (0) | |
| Other | 9 (13.3) | 7 (20.6) | 2 (5.9) | |
| Child insurance status, n (%) | ||||
| Public | 54 (79.4) | 28 (82.4) | 26 (76.5) | 0.765 |
| Private | 14 (20.6) | 6 (17.6) | 8 (23.5) | |
| Receive free or reduced school lunch, 3 n (%) | 53 (80.3) | 27 (81.8) | 26 (78.8) | 0.757 |
| Patient Centered Medical Home Participant, n (%) | 51 (75.0) | 24 (70.6) | 27 (79.4) | 0.576 |
| ≥ 1 hour daily music listening, n (%) | 59 (86.8) | 26 (76.5) | 33 (97.1) | 0.027 |
| Smoking behavior reported by adolescent, n (%) | ||||
| Current smoker | 2 (2.9) | 2 (5.9) | 0 (0) | 0.492 |
| Exposed to second hand smoke at home | 5 (7.4) | 3 (8.8) | 2 (5.9) | >0.999 |
| Asthma controller medications, n (%) | ||||
| ICS monotherapy | 53 (77.9) | 25 (73.5) | 28 (82.4) | 0.559 |
| ICS and long-acting bronchodilator combination therapy‡ | 15 (22.1) | 9 (26.5) | 6 (17.7) | |
| Uncontrolled Asthma, n (%) | 55 (80.9) | 29 (85.3) | 26 (76.5) | 0.539 |
| Asthma exacerbation in the last 12 months | ||||
| ≥ 2 Requiring oral systemic corticosteroids, n (%) | 19 (27.9) | 9 (26.5) | 10 (29.4) | 0.787 |
| ≥ 1 Requiring emergency room visit or hospitalization, n (%) | 34 (50.8) | 19 (57.6) | 15 (44.1) | 0.332 |
| Children’s Depression Inventory 2, n (%) | ||||
| Very elevated | 3 (4.4) | 2 (5.9) | 1 (2.9) | 0.323 |
| Elevated | 5 (7.4) | 4 (11.8) | 1 (2.9) | |
| High average | 4 (5.9) | 3 (8.8) | 1 (2.9) | |
| Average or lower | 56 (82.4) | 25 (73.5) | 31 (91.2) | |
| ICS Knowledge Questionnaire, median (Q1, Q3) | 24.0 (22.0, 26.5) | 25.0 (22.0, 28.0) | 24.0 (21.0, 26.0) | 0.374 |
| ICS Self-Efficacy Questionnaire, median (Q1, Q3) | 51.0 (44.5, 59.0) | 50.5 (44.0, 58.0) | 52.0 (45.0, 59.0) | 0.370 |
| Social Support Questionnaire, median (Q1, Q3) | 33.0 (30.0, 35.0) | 33.0 (30.0, 35.0) | 33.0 (30.0, 35.0) | 0.792 |
| Asthma Social Support Questionnaire median (Q1, Q3) | 27.0 (24.0, 29.0) | 26.5 (22.0, 29.0) | 27.0 (24.0, 31.0) | 0.512 |
Mixed African American: Self-identified as a combination of African American and another race.
Criteria for Free and Reduced School Lunch in Chicago Public Schools July 1, 2011 – June 30, 2012.56
Inhaled corticosteroid and long-acting bronchodilator: Two participants who were taking inhaled corticosteroid and long acting bronchodilator combination therapy were also taking inhaled corticosteroid monotherapy.
Primary Outcomes
The mixed effects modeling indicated no significant effects of the clustering within the active intervention arm; thus, cluster adjusted statistical analysis methods were not used. Adherence to ICS and Asthma Knowledge at 5 weeks and 10 weeks are presented in Table II. Study participants in both arms did not demonstrate clinically or statistically significant differences in objectively measured adherence at weeks 5 and 10. Adherence in both groups was well below the clinically significant target of ≥70% throughout the study 9,40 With respect to the missing data analysis, the analysis under the ‘best case’ scenario produced similar results, indicating that the inability to achieve significance was not due to missing data. Additionally, adherence in both groups declined over time during study follow up. The multilevel modeling that allowed for group comparisons while adjusting for meaningful covariates produced similar results of no significant difference between groups in adherence at weeks 5 or 10.
Table II. Primary Outcomes.
This table depicts objectively measured adherence and asthma knowledge at baseline, week 5 and week 10 of the active treatment phase. There was 8% missing data at weeks 5 and 10.
| Adherence to ICS % Adherence | ZAP Asthma Knowledge Instrument % Items Correct | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Evaluation | Treatment | Control | P value | Treatment | Control | P value | ||||
| N | Median (Q1, Q3) | N | Median (Q1, Q3) | N | Median (Q1, Q3) | N | Median (Q1, Q3) | |||
| Baseline | 34 | 27.4 (14.3, 35.0) | 34 | 25.9 (14.0, 37.5) | 0.670 | 34 | 67.9 (59.0, 76.9) | 34 | 70.5 (61.5, 76.9) | 0.825 |
| 5 Weeks | 24 | 18.8 (5.4, 24.2) | 22 | 16.1 (7.14, 19.6) | 0.534 | 29 | 74.4 (59.0, 84.6) | 28 | 73.1 (56.4, 79.4) | 0.655 |
| 10 Weeks | 24 | 7.1 (0.9, 21.4) | 25 | 14.3 (5.4, 21.4) | 0.929 | 29 | 71.8 (59.0, 82.1) | 29 | 69.2 (61.5, 74.4) | 0.487 |
General asthma knowledge remained largely unchanged between baseline and weeks 5 and 10 of active follow up treatment, both within and between the treatment and attention control groups. Compared to baseline, at 10 weeks the treatment group percent items correct improved by 2.56% and the attention control group improved by 0.0% (P = 0.407 for the between group comparison).
Secondary Outcomes
Both active intervention (treatment) and attention control group participants’ self-reported adherence was significantly (P < 0.0001) higher than their objectively measured adherence at weeks 5 and 10 of the active treatment period (See Table III). For the treatment group, at week 5, the median objectively measured adherence was 16.1% and the median self-reported adherence was 50%. For the attention control group, at week 5, the median objectively measured adherence was 16.1% and the median self-reported adherence was 63.4%. For the treatment group, at week 10, the median objectively measured adherence was 6.3% and the median self-reported adherence was 50.0%. For the attention control group, at week 10, the median objectively measured adherence was 14.3% and the median self-reported adherence was 61.6%.
Table III. Objectively Measured and Self-Reported Adherence to Inhaled Corticosteroids.
This table shows differences in objectively measured versus self-reported adherence at week 5 and week 10 of the active treatment phase for treatment and attention control groups. Only participants whose self-reported data was available for the same two weeks as their objectively measured data were included.
| Week 5 | Week 10 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Group | Objectively Measured | Self-Reported | P value | Objectively Measured | Self-Reported | P value | ||||
| N | Median (Q1, Q3) | N | Median (Q1, Q3) | N | Median (Q1, Q3) | N | Median (Q1, Q3) | |||
| Treatment | 19 | 16.1 (3.6, 23.2) | 23 | 50.0 (30.4, 82.1) | 0.0007 | 18 | 6.3 (1.8, 14.3) | 21 | 50.0 (35.7, 78.6) | <.0001 |
| Control | 17 | 16.1 (14.3, 19.6) | 20 | 63.4 (49.1, 79.5) | <.0001 | 21 | 14.3 (5.4, 21.4) | 22 | 61.6 (48.2, 82.1) | <.0001 |
| Total | 36 | 16.1 (7.1, 22.3) | 43 | 57.1 (41.1, 82.1) | <.0001 | 39 | 7.1 (1.8, 21.4) | 43 | 57.1 (39.3, 78.6) | <.0001 |
Discussion
The active intervention under investigation combined weekly face-to-face coping peer support and coping peer asthma messages delivered between favorite music tracks on an mp3 player during the course of participants’ daily lives. Neither this intervention nor an attention control consisting of doctor asthma messages delivered via mp3 player demonstrated improvement in objectively measured adherence to ICS or asthma knowledge in our study population of low-income minority adolescents with persistent asthma. Adherence decreased and asthma knowledge did not change throughout study follow up in both groups.
There are several possible explanations for this lack of demonstrated efficacy. The original recruitment goal for this study was 90 participants, with 45 in each group, to achieve 80% power to detect at least a 25% difference in adherence rates between the two study groups while allowing for attrition and intraclass correlation within the treatment group. Recruitment was to be accomplished via school-based health centers, a strategy that proved to be unproductive. After eventually modifying the recruitment strategy to focus on use of the electronic medical record and Patient Centered Medical Home Project at Rush University Medical Center, remaining time and resources allowed for the enrollment of only 68 participants. The investigators note, however, that the study’s lower than expected attrition rate and intraclass correlation results in this sample size still meeting the 80% power criterion.
Although the clinically significant target adherence goal of ≥ 70% would be ideal, 9 the investigators consider it to be unrealistic for this minority adolescent population. The study targeted minority adolescents with low adherence (≤ 48%) because the investigators thought that the children with the most need could derive the greatest benefit from this intervention. A cutpoint of ≤ 48% adherence was chosen as: 1) observational only studies monitoring adherence to ICS in diverse samples of adolescents publish rates between 40–50%;18–19, 30–31 2) the typical dosing schedule for ICS delivered via metered dose inhaler is two puffs twice daily (for a total of 56 puffs over a 2-week period); and 3) the investigators sought to set the cutpoint just under 50% (which amounts to 26 out of 56 puffs per 2 weeks, or ≤ 48%).
While no validated instrument was used to measure asthma control, asthma control assessment followed the NHLBI EPR3 asthma guidelines and addressed both impairment and risk domains.29 Asthma exacerbation data was collected at baseline, 5 weeks, and 10 weeks, but impairment data were only collected at baseline. The investigators plan to measure outcomes during the active treatment phase, and use validated instruments to assess asthma control, in future studies.
A larger “dose” of the intervention may be needed. Adolescents may need two to three, rather than one, face-to-face coping peer support sessions per week to demonstrate improvement in adherence to ICSs. Within our study, much of the coping peer support sessions were devoted to providing group support to participants coping with stressful concerns for individual and familial health and well-being (e.g. grief for the recent loss of a friend or family member due to asthma or neighborhood violence). It was difficult to focus participants on discussions of barriers to adherence and strategies to overcome them given other compelling distractions. Asthma exacerbations are a systems problem; this study targeted only one piece of that system.
In addition, technological limitations did not allow effective tracking of their mp3 use. In particular, it was not possible to track which selections (e.g. songs, coping peer asthma messages, or doctor messages) were skipped, played ≤ 12 seconds in total duration, or listened to entirely. Thus, we do not know to what extent the active intervention was actually delivered.
The investigators’ conceptual model, based on social cognitive theory, led to an intervention aimed at increasing social support for and self-efficacy toward medication taking behavior. This model may need to be re-evaluated. If low-income minority adolescents with asthma have urgent competing needs, or are experiencing invulnerability47 and denial48 characteristic of this period of development, it may be ineffectual to attempt to increase social support and self- efficacy to promote a behavior that they are not at all interested in fostering.21,49
Participants were not provided with feedback on medication taking behavior. Study participants were kept blind to their electronically measured adherence to ICS throughout the 10-week active treatment period. In three studies successfully demonstrating improved adherence to ICS, participants were provided feedback and made accountable for their medication taking behavior.50–52 Studies objectively measuring adherence to ICS generally show a decline in adherence over time.50–51 Without the provision of feedback, participants’ may have been unaware that their adherence was poor.
An important finding from this research is the large discrepancy between objectively measured and self-reported adherence. This study confirms findings from other pediatric studies, that patient self-report exceeds objectively measured adherence to ICS10,53 In a study by Milgrom and colleagues, median electronic metered-dose inhaler monitor use was 58.4%, and median diary reported use was 95.4%.10 The Childhood Asthma Management Program ancillary clinical trial measured adherence in 140 children randomized to receive ICS or placebo using both self-report (daily diary cards) and objectively measured adherence (number of doses remaining in study inhalers). Self-reported adherence generally surpassed objectively measured adherence (93.6% vs. 60.8%, P < 0.0001).53 National asthma guidelines recommend physician assessment of patient adherence before stepping up therapy in patients not adequately controlled on their current controller medication regimen.29 While health care providers often believe they are able to ascertain their patients’ level of medication adherence, discordance between patient reports and objectively measured adherence should raise concerns.
The investigators have learned important lessons from this study in planning for future behavioral interventions aimed at increasing adherence to ICS in low income minority adolescents with persistent asthma. First, technology is attractive to inner-city teens and could potentially be a powerful way to intervene. Second, more immediate positive feedback could increase the potency of the messages. Collaborating with computer scientists, electrical engineers, and media arts experts to develop a mobile phone technology platform with mobile applications, ICS medication sensors, advanced analytics and feedback, would allow for continuous real time monitoring of asthma medication taking behavior and instant feedback. However, a randomized controlled trial of adolescents and adults with poorly controlled asthma did not demonstrate benefits of mobile phone supported self-monitoring and immediate feedback for asthma.54 Perhaps immediate positive feedback combined with back-up reinforcers would be more effective for changing medication taking behavior in this target population.55
Despite receiving asthma care at a hospital highly ranked for primary care, participation in a level 3 Patient-Centered Medical Home, and all having been prescribed the gold standard treatment for persistent asthma, 81% of our population had uncontrolled asthma, poor adherence to ICS, and a high rate of emergency room visits (51%). Poor adherence declined further throughout the study. Optimal medical care, coping peer support, and technology assisted intervention delivery were not sufficient to change behavior in this population.
Highlights.
What is already known about this topic?
Lack of adherence to inhaled corticosteroids is a significant risk factor for poor asthma outcomes among low-income African American and Hispanic adolescents with persistent asthma.
What does this article add to our knowledge?
Face-to-face coping peer support and mp3-delivered peer asthma messages do not appear to influence adherence to inhaled corticosteroids among inner-city minority adolescents with persistent asthma.
How does this study impact current management guidelines?
Asthma guidelines recommend monitoring patient adherence to his/her pharmacotherapeutic regimen at each visit. Reliance on self-report of adherence to inhaled corticosteroid medications among urban minority adolescents may give clinicians inadequate information to adjust their treatment regimens.
Acknowledgments
Funding: Supported by National Heart Lung and Blood Institute grants K23 HL092292 and R21 HL098812. Support in the form of study drug was provided by a grant from GlaxoSmithKline (FLV114794).
We thank the parents and children who participated in this study. We also thank the entire staff of The Rush Pediatric Primary Care Center, Rush Lifetime Medical Associates, and Rush University Family Physicians. We are grateful to Beth Volin, MD; Regina McClenton BSN, RN; Greda Erazo; Clarence Parks, MD; Denean Roberson NCMA; Steven K. Rothschild, MD, and Blanca Gonzalez, MOA for their assistance. We thank all of the study staff including: Tamara Olinger, MS; Samantha Fenno, PhD, MASW; Jessie Beebe, MASW; Abbie Daigle, MASW; Shelly Sital, MPH; Charon Smith, MPH; Jessica Ruggiero, BA; Scott Resnick, BS; Nicole Woodrick, BA; and Merlyn Abraham, BS.
Abbreviations
- ICS
Inhaled corticosteroids
- ATG
Attention control group
- mp3
Music file (MPEG Layer 3)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moorman JE, Zahran H, Truman B, Molla M. Current Asthma Prevalence – United States, 2006–2008. Centers for Disease Control & Prevention MMWR. 2011;60(Suppl):84–6. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Vital Signs: Asthma Prevalence, Disease Characteristics, and Self-Management Education — United States, 2001–2009. MMWR. 2011;60:547–52. [PubMed] [Google Scholar]
- 3.Moorman JE, Rudd RA, Johnson CA, King M, Minor P, Bailey C, et al. National Surveillance for Asthma – United States, 1980 – 2004. Centers for Disease Control & Prevention. Surveillance Summaries, October 19, 2007. MMWR. 2007;56(SS-8) [PubMed] [Google Scholar]
- 4.Akinbami LJ, Schoendorf KC. Trends in childhood asthma: prevalence, health care utilization, and mortality. Pediatrics. 2002;110:315–22. doi: 10.1542/peds.110.2.315. [DOI] [PubMed] [Google Scholar]
- 5.Williams LK, Pladevall M, Xi H, Peterson EL, Joseph C, Lafata JE, et al. Relationship between adherence to inhaled steroids and poor outcomes among adults with asthma. J Allergy Clin Immunol. 2004;114:1288–93. doi: 10.1016/j.jaci.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 6.Krishnan JA, Riekert KA, McCoy JV, Stewart DY, Schmidt S, Chanmugam A, et al. Corticosteroid use after hospital discharge among high-risk adults with asthma. Am J Respir Crit Care Med. 2004;170:1281–5. doi: 10.1164/rccm.200403-409OC. Epub 2004 Sep 16. [DOI] [PubMed] [Google Scholar]
- 7.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database of Syst Rev. 2008;(2):CD000011. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 8.Birkhead G, Attaway NJ, Strunk RC, Townsend MC, Teutsch S. Investigation of a cluster of deaths of adolescents from asthma: evidence implicating inadequate treatment and poor patient adherence with medications. J Allergy Clin Immunol. 1989;84:484–91. doi: 10.1016/0091-6749(89)90361-8. [DOI] [PubMed] [Google Scholar]
- 9.Williams LK, Peterson E, Wells K, Ahmedani B, Kumar R, Burchard E, et al. Quantifying the proportion of severe asthma exacerbations attributable to inhaled corticosteroid nonadherence. J Allergy Clin Immunol. 2011;128:1185–91. doi: 10.1016/j.jaci.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milgrom H, Bender B, Ackerson L, Bowry P, Smith B, Rand C. Noncompliance and treatment failure in children with asthma. J Allergy Clin Immunol. 1996;98:1051–7. doi: 10.1016/s0091-6749(96)80190-4. [DOI] [PubMed] [Google Scholar]
- 11.Wade S. Psychosocial components of asthma management in children. Dis Manage Health Outcomes. 2000;8:17–27. [Google Scholar]
- 12.McNabb W, Willson-Pessano S, Jacobs A. Critical self-management competencies for children with asthma. J Pediatr Psychol. 1986;11:103–17. doi: 10.1093/jpepsy/11.1.103. [DOI] [PubMed] [Google Scholar]
- 13.Agertoft L, Pedersen S. Effects of long-term treatment with an inhaled corticosteroid on growth and pulmonary function in asthmatic children. Res Med. 1994;88:373–81. doi: 10.1016/0954-6111(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 14.Barnes PJ, Pedersen S. Efficacy and safety of inhaled corticosteroids in asthma. Am Rev Respir Dis. 1993;148:S1–S26. doi: 10.1164/ajrccm/148.4_Pt_2.S1. [DOI] [PubMed] [Google Scholar]
- 15.Nelson H, Busse W, Kerwin E, Church N, Emmett A, Rickard K, et al. Fluticasone propionate/salmeterol combination provides more effective asthma control than low-dose inhaled corticosteroid plus montelukast. J Allergy Clin Immunol. 2000;106:1088–95. doi: 10.1067/mai.2000.110920. [DOI] [PubMed] [Google Scholar]
- 16.Weiss K, Shannon J, Sadowski L, Sharp L, Curtis L, Lyttle C, et al. The burden of asthma in the Chicago community fifteen years after the availability of national asthma guidelines: the design and initial results from the CHIRAH study. Contemp Clin Trials. 2009;30:246–55. doi: 10.1016/j.cct.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis SQ, Krishnan JA, Lee K, Persky V, Naureckas ET. Effect of a community-wide asthma intervention on appropriate use of inhaled corticosteroids. J Urban Health. 2011;88:S144–S155. doi: 10.1007/s11524-010-9476-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McQuaid EL, Kopel SJ, Klein RB, Fritz GK. Medication adherence in pediatric asthma: reasoning, responsibility, and behavior. J Pediatr Psychol. 2003;28:323–33. doi: 10.1093/jpepsy/jsg022. [DOI] [PubMed] [Google Scholar]
- 19.Naimi D, Freedman T, Ginsburg K, Bogen D, Rand C, Apter A. Adolescents and asthma: why bother with our meds? J Allergy Clin Immunol. 2009;123:1335–41. doi: 10.1016/j.jaci.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin MA, Olson D, Mosnaim GS, Ortega DA, Rothschild SK. Recruitment, asthma characteristics, and medication behaviors in midwest Puerto Rican youth: data from Project CURA. Ann Allergy Asthma Immunol. 2012;109:121–7. doi: 10.1016/j.anai.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bandura A. Self-efficacy: toward a unifying theory of behavior change. Psychol Rev. 1977;84:191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 22.Lemanek K. Adherence issues in the medical management of asthma. J Pediatr Psychol. 1990;15:437–58. doi: 10.1093/jpepsy/15.4.437. [DOI] [PubMed] [Google Scholar]
- 23.Ochieng BMN. Adolescent health promotion: the value of being a peer leader in a health education/promotion peer education programme. Health Educ J. 2003;62:61–72. [Google Scholar]
- 24.van Es SM, Nagelkerke AF, Colland VT, Scholten RJ, Bouter LM. An intervention programme using the ASE-model aimed at enhancing adherence in adolescents with asthma. Patient Educ Couns. 2001;44:193–203. doi: 10.1016/s0738-3991(00)00195-6. [DOI] [PubMed] [Google Scholar]
- 25.Williams PG, Holmbeck GN, Greenley RN. Adolescent health psychology. J Consult Clin Psychol. 2002;70:828–42. [PubMed] [Google Scholar]
- 26.Shah S, Peat JK, Mazurski EJ, Wang H, Sindhusake D, Bruce C, et al. Effect of peer led programme for asthma education in adolescents: cluster randomized controlled trial. BMJ. 2001 Mar 10;322(7286):583–5. doi: 10.1136/bmj.322.7286.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wysocki T, Hough BS, Ward KM, Green LB. Diabetes mellitus in the transition to adulthood: adjustment, self-care, and health status. J Dev Behav Pediatr. 1992;13:194–201. [PubMed] [Google Scholar]
- 28.Rideout V, Foehr U, Roberts D. Generation m2, media in the lives of 8 to 18 year-olds. A Kaiser Family Foundation Study. 2010:1–36. Available from: http://www.kff.org/entmedia/8010.cfm.
- 29.National Asthma Education and Prevention Program. Full Report. Bethedsa, MD: National Institutes of Health; 2007. Expert panel report 3: guidelines for the diagnosis and management of asthma; pp. 1–440. [Google Scholar]
- 30.Bender B, Zhang L. Negative affect, medication adherence, and asthma control in children. J Allergy Clin Immunol Pract. 2008;122:490–5. doi: 10.1016/j.jaci.2008.05.041. [DOI] [PubMed] [Google Scholar]
- 31.Walders N, Kopel SJ, Koinis-Mitchell D, McQuaid EL. Patterns of quick-relief and long-term controller medication use in pediatric asthma. J Pediatr. 2005;146:177–82. doi: 10.1016/j.jpeds.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 32.American College of Chest Physicians Patient Education Guide: using your MDI with a spacer [Internet] American College of Chest Physicians; 2006. Available from: http://www.chestnet.org/downloads/patients/guides/inhaledDevices/patientEducation8.pdf. [Google Scholar]
- 33.Researchers recommend safe listening levels for Apple iPod [Internet] 2006 Oct 17; [cited 2009 Jan 19]. Available from: http://www.physorg.com/news80304823.html.
- 34.Portnuff CDF, Fligor BJ. Output levels of portable digital music players [Internet] 2006 Oct 19; [cited 2009 Jan 19]. Available from: http://www.hearingconservation.org/docs/virtualPressRoom/portnuff.htm.
- 35.Naar-King S, Suarez M. Motivational interviewing with adolescents and young adults. New York: The Guilford Press; 2011. [Google Scholar]
- 36.Miller W, Rollnick S. Motivational interviewing: preparing people for change. 2. New York: The Guilford Press; 2002. [Google Scholar]
- 37.Mason P, Butler C. Health behavior change: a guide for practitioners. 2. Edinburgh: Elsevier Limited; 2010. [Google Scholar]
- 38.Weinstein AG, Laurenceau J, Vok J. The relationship between self-reported non-adherence (NA) to anti-inflammatory therapy and asthma management behaviors/attitudes related to NA in adult patients. J Allergy Clin Immunol. 2011;127:AB149. [Google Scholar]
- 39.Mosnaim GS, Li H, Damitz M, Sharp LK, Li Z, Talati A, et al. Evaluation of the Fight Asthma Now (FAN) program to improve asthma knowledge in urban youth and teenagers. Ann Allergy Asthma Immunol. 2011;107:310–6. doi: 10.1016/j.anai.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 40.Apter AJ, Reisine ST, Affleck G, Barrows E, ZuWallack RL. Adherence with twice-daily dosing of inhaled steroids: socioeconomic and health-belief differences. Am J Respir Crit Care Med. 1998;157:1810–7. doi: 10.1164/ajrccm.157.6.9712007. [DOI] [PubMed] [Google Scholar]
- 41.Kovacs M. Rating scales to assess depression in school-aged children. Acta Paedopsychiatr. 1981;46:305–15. [PubMed] [Google Scholar]
- 42.ZAP Asthma Project. Caregiver asthma knowledge survey instrument, Form 9 [Internet] Atlanta, GA: [revised 1997 Oct 3; cited 2007 Jun 8]. Available from: http://www.sph.emory.edu/zapasthma/pdf/a9.pdf. [Google Scholar]
- 43.Apter AJ, Boston RC, George M, Norfleet AL, Tenhave T, Coyne JC, et al. Modifiable barriers to adherence to inhaled steroids among adults with asthma: it’s not just black and white. J Allergy Clin Immunol. 2003;111:1219–26. doi: 10.1067/mai.2003.1479. [DOI] [PubMed] [Google Scholar]
- 44.Wight R, Botticello A, Aneshensel C. Socioeconomic context, social support, and adolescent mental health: a multilevel investigation. J Youth Adolesc. 2006;35:115–126. [Google Scholar]
- 45.Singer J. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. J Educ Behav Stat. 1998;24:323–55. [Google Scholar]
- 46.Donner A, Klar N. Design and analysis of cluster randomization trials in health research. United Kingdom: John Wiley & Sons, Ltd; 2000. Analysis quantitative outcome; pp. 111–27. [Google Scholar]
- 47.Friedman IM, Litt IF. Adolescents’ compliance with therapeutic regimens: Psychological and social aspects and intervention. J Adolesc Health Care. 1987;8:52–67. doi: 10.1016/0197-0070(87)90246-4. [DOI] [PubMed] [Google Scholar]
- 48.Logan D, Zelikovsky N, Labay L, Spergel J. The Illness Management Survey: identifying adolescents’ perceptions of barriers to adherence. J Pediatr Psychol. 2003;28:383–92. doi: 10.1093/jpepsy/jsg028. [DOI] [PubMed] [Google Scholar]
- 49.Bandura A. Social foundations of thought and action: a social cognitive theory. Englewood Cliffs, N.J: Prentice Hall; 1956. [Google Scholar]
- 50.Onyirimba F, Apter A, Reisine S, Litt M, McCusker C, Connors M, et al. Direct clinician-to-patient feedback discussion of inhaled steroid use: its effect on adherence. Ann Allergy Asthma Immunol. 2003;90:411–5. doi: 10.1016/S1081-1206(10)61825-X. [DOI] [PubMed] [Google Scholar]
- 51.Burgess S, Sly P, Devadason S. Providing feedback on adherence increases use of preventive medication by asthmatic children. J Asthma. 2010;47:198–201. doi: 10.3109/02770900903483840. [DOI] [PubMed] [Google Scholar]
- 52.Otsuki M. Adherence feedback to improve asthma outcomes among inner-city children. Pediatrics. 2009;124:1513. doi: 10.1542/peds.2008-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krishnan J, Bender B, Wamboldt F, Szefler S, Adkinson NF, Zeiger R, et al. Adherence to inhaled corticosteroids: an ancillary study of the Childhood Asthma Management Program clinical trial. J Allergy Clin Immunol. 2012;129:112–8. doi: 10.1016/j.jaci.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ryan D, Price D, Musgrave SD, Malhotra S, Lee AJ, Ayansina D, et al. Clinical and cost effectiveness of mobile phone supported self monitoring of asthma: Multicentre randomised controlled trial. BMJ. 2012;344:e1756. doi: 10.1136/bmj.e1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miltenberger R. Behavior modification: principles and procedures. 4. Australia: Wadsworth Cengage Learning; 2008. [Google Scholar]
- 56.Criteria for free and reduced school lunch in Chicago Public Schools [Internet] 2012 Dec 12; Available from: http://www.gpo.gov/fdsys/pkg/FR-2012-03-23/pdf/2012-7036.pdf3.