Abstract
Toll-like receptors (TLRs) play an essential role in initiating intracellular type I interferon (IFN)-mediated innate immunity against viral infections. We examined whether human neuronal cells (primary human neurons, NT2-N and CHP-212 cells) express TLRs and mount type I IFN-mediated innate immunity against herpes simplex virus-1 (HSV-1) infection. Human neuronal cells expressed TLR family members 1–10 and IFN-α/β. The activation of TLR3 or TLR8 by double-stranded RNA (poly-I:C) or single-stranded RNA (ssRNA) induced IFN-α/β expression. In addition, HSV-1 infection of human neuronal cells induced IFN-α expression. Investigation of the mechanisms showed that poly-I:C or ssRNA treatment enhanced the expression of several IFN regulatory factors. Importantly, the activation of TLR3 or TLR8 by poly-I:C or ssRNA prior to HSV-1 infection reduced the susceptibility of the neuronal cells to infection. These observations indicate that human neuronal cells possess intracellular TLR-mediated innate immune protection against HSV-1 infection.
Keywords: human neuronal cells, Toll-like receptor, poly-I:C, ssRNA, herpes simplex virus-1, type I interferons
The innate immune system serves as the first line of defense against pathogens by producing and releasing important cytokines and chemokines following recognition of pathogen signals by a family of pattern recognition receptors (Janeway and Medzhitov, 2002). Recognition of pathogen-associated molecular structures by cells via Toll-like receptors (TLRs) represents a key event in mounting the innate immune defense against pathogen invasion (Takeda and Akira, 2005). In humans, 10 different TLRs are currently known, and their expression and function have been extensively characterized, mostly with macrophages and dendritic cell populations. TLR binding by ligands triggers the activation of antiviral pathways, including the induction of type I interferons (IFNs; Akira et al., 2001; Janeway and Medzhitov, 2002; Akira, 2003). Among the members of the TLR family, TLRs 3, 4, 7, 8, and 9 are known to induce interferon (IFN)-α/β responses through the activation of IFN regulatory factors (IRFs; Doyle et al., 2002; Yamamoto et al., 2003; Honda et al., 2004; Yang et al., 2005). During viral infections, cells can recognize viral RNAs through TLRs. For example, it is known that TLR3 recognizes virus-derived double-stranded RNA (dsRNA), whereas TLR8 senses virus-derived single-stranded RNA (ssRNA; Alexopoulou et al., 2001; Heil et al., 2004).
The innate immune cells, such as natural killer cells, neutrophils, macrophages, and dendritic cells, are responsible for recognition and clearance of invading pathogens, insofar as these cells possess a functional TLR system (Gordon, 2002; Medzhitov and Janeway, 2002; Stuart and Ezekowitz, 2005). However, little is known about whether cells in the central nervous system (CNS) contain the integrated and functional TLR system that is able to mount an efficient antiviral response. Several studies have shown that the CNS of experimental mice expresses TLRs (Boivin et al., 2002; Bottcher et al., 2003; Koedel et al., 2003). Broad and regulated expression of TLRs was also reported for human microglial cells (Bsibsi et al., 2002) and astrocytes (Farina et al., 2005), both of which have been reported to have the ability to initiate immune responses to various pathogens by producing cytokines and chemokines. In addition to microglia and astrocytes, neuronal cells express TLRs (Prehaud et al., 2005). It has also been shown that human neuronal cells express IFN-α/β and APO-BEC3G, a newly identified anti-HIV-1 cellular factor (Argyris et al., 2007; Wang et al., 2008). In the present study, we examined whether TLRs expressed by human neuronal cells are biologically functional and can mount IFN-α/β-mediated innate antiviral immunity against herpes simplex virus-1 (HSV-1) infection. We were particularly interested in the role of the activation of TLR3 and TLR8 in protecting neuronal cells from HSV-1 infection, because these TLRs have a critical role in the activation of intracellular innate antiviral immunity.
MATERIALS AND METHODS
Reagents and Antibodies
Goat antibody against HSV-1 early/late antigens were purchased from Chemicon International (Temecula, CA). Mouse anti-IFN-α and goat anti-IFN-β antibodies were purchased from Sigma-Aldrich (St. Louis, MO). Rhodamine (TRITC)-conjugated goat anti-mouse IgG1 and fluorescein (FITC)-conjugated donkey anti-goat IgG1 were purchased from Southern Biotechnology Associates (Birmingham, AL). ELISA kits for IFN-α and IFN-β were purchased from PBL Biomedical Laboratories (Piscataway, NJ) and Fujirebio Inc. (Tokyo, Japan), respectively.
Human Neuronal Cells
Primary human neurons were prepared from 16–18-week-old human fetal brain tissues, as described previously (Hu et al., 2002). Briefly, human fetal brain cortical tissue obtained under a protocol approved by the Human Subjects Research Committee of the University of Minnesota were dissociated, trypsinized, and resuspended in DMEM (Gibco, Grand Island, NY) containing 10% heat-inactivated FBS (Hyclone, Logan, UT) plus 100 U/ml penicillin and 100 µg/ml streptomycin (Gibco) and plated onto collagen-coated plates (106 cells/well in 12-well plates). On day 5, cell cultures were treated with uridine (33.6 µg/ml) and fluorodeoxyuridine (13.6 µg/ml), followed by replacement with DMEM with 10% FBS on day 6 and every 4 days thereafter. On day 12, the neuronal cell cultures consisting of about 90% neurons (stained by anti-NeuN or anti-MAP-2 antibodies; Hu et al., 2002) were used for the experiments.
Human neuronal cells (NT2-N) were derived from differentiated Ntera-2clD/1 (NT2) cells as described previously (Pleasure et al., 1992). Briefly, NT2 cells were plated at a density of 2.3 × 106 per T75 flask and fed twice weekly with DMEM containing high glucose and 10% FBS with 100 U/ml penicillin, 100 µg/ml streptomycin, and 10−5 mol/liter retinoic acid (RA; Sigma-Aldrich) for 5 weeks. The cells were then divided (1:4) and cultured for an additional 48 hr in identical medium without RA. Neuronal cells growing above a monolayer of nonneuronal cells were dislodged with trypsin and plated at a density of 0.5 × 106 cells per well in a 24-well plate. NT2-N cells have morphologic features similar to those of primary human neurons and have processes that differentiate into axons and dendrites (Pleasure et al., 1992; Munir et al., 1995; Pekosz et al., 1996). NT2-N cells also express cytoskeletal proteins, secretory markers, and surface markers, which are characteristic of neurons. They also express functional neuropeptides (Li et al., 2003) and functional N-methyl-D-aspartate (NMDA) and non-NMDA glutamate receptors (Rootwelt et al., 1998). Undifferentiated human NT2 cells grafted into mouse brain differentiated into neuronal and glial cells (Ferrari et al., 2000). The neuroblastoma cell line CHP-212 was purchased from American Type Tissue Culture (No. CRL-2273; ATTC, Manassas, VA) and cultured according to the protocol provided by ATCC.
HSV-1 Infection of NT2-N Cells
HSV-1 17syn+ strain, a gift from Dr. James R Lokensgard (Department of Medicine, University of Minnesota Medical School), was propagated and purified from rabbit skin fibroblasts as previously described (Lokensgard et al., 2002). NT2-N cells were incubated with HSV-1 at a multiplicity of infection (MOI) of 0.01 for 2 hr and then washed three times with plain DMEM to remove the input viruses. HSV-1 infection of NT2-N was analyzed by immunostaining with the specific antibody to HSV-1 early/late antigens or by real-time PCR using the specific primers 5′-TCTCCGTCCAGTCGT TTAT-3′ (sense) and 5′-ATCCGAACGCAGCCCCGC-3′ (antisense).
Poly-I:C, ssRNA, and IFN-α/β Treatment
Human neuronal cells were treated with or without poly-I:C (10 µg/ml) or ssRNA (10 µg/ml) for either 4 or 12 hr or were treated with different concentrations (0.1 µg/ml, 1 µg/ml, and 10 µg/ml) for 12 hr. Untreated cells were included as a negative control (N.C.). As for HSV-1 infection experiments, NT2-N cells were pretreated with or without poly-I:C (10 µg/ml), ssRNA (10 µg/ml), or IFN-α (20 U/ml) and IFN-β (20 U/ml) for 12 hr, then incubated with HSV-1 at an MOI of 0.01. After 2 hr of incubation, the cells were washed three times with plain DMEM and cultured with complete 10% DMEM.
Real-Time RT-PCR
Total cellular RNA was extracted from cells using Tri-Reagent (Molecular Research Center, Cincinnati, OH) as described elsewhere (Li et al., 2003). Total RNA (1 µg) was reverse transcribed to cDNA using the reagents from Promega (Madison, WI). The real-time RT-PCR for the quantification of TLRs 1–10; IFN-α; IFN-β; IFN regulatory factors (IRFs) 1, 3, 5, 7, and 9; and GAPDH mRNA was performed with the iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA) as described elsewhere (Li et al., 2003). The levels of GAPDH mRNA were used as an endogenous reference to normalize the quantities of target mRNA. The oligonucleo-tide primers used in this study are listed in Table I. The oligo-nucleotide primers were synthesized by Integrated DNA Technologies Inc. (Coralville, IA).
TABLE I.
Primer Sets for Real-Time RT-PCR
| Primer | Accession No. | Orientation | Sequences | Product size (bp) |
|---|---|---|---|---|
| GAPDH | NM_002046 | Sense | 5′-GGTGGTCTCCTCTGACTTCAACA-3′ | 127 |
| Antisense | 5′-GTTGCTGTAGCCAAATTCGTTGT-3′ | |||
| IFN-α | NM_002175 | Sense | 5′-TTTCTCCTGCCTGAAGGACAG-3′ | 373 |
| Antisense | 5′-GCTCATGATTTCTGCTCTGACA-3′ | |||
| IFN-β | NM_002176 | Sense | 5′-GCCGCATTGACCATCTATGAGA-3′ | 346 |
| Antisense | 5′-GAGATCTTCAGTTTCGGAGGTAAC-3′ | |||
| IRF1 | NM_002198 | Sense | 5′-TGAAGCTACAACAGATGAGG-3′ | 111 |
| Antisense | 5′-AGTAGGTACCCCTTCCCATC-3′ | |||
| IRF3 | NM_001571 | Sense | 5′-ACCAGCCGTGGACCAAGAG-3′ | 60 |
| Antisense | 5′-TACCAAGGCCCTGAGGCAC-3′ | |||
| IRF5 | NM_001098631 | Sense | 5′-AAGCCGATCCGGCCAA-3′ | 63 |
| Antisense | 5′-GGAAGTCCCGGCTCTTGTTAA-3′ | |||
| IRF7 | NM_001572 | Sense | 5′-TGGTCCTGGTGAAGCTGGAA-3′ | 134 |
| Antisense | 5′-GATGTCGTCATAGAGGCTGTTGG-3′ | |||
| IRF9 | NM_006084 | Sense | 5′-GCATCAGGCAGGGCACGCTGCACC-3′ | 135 |
| Antisense | 5′-GCCTGCATGTTTCCAGGGAATCCG-3′ | |||
| TLR1 | NM_003263 | Sense | 5′-GCCTATATGCAAAGAGTTTGGC-3′ | 134 |
| Antisense | 5′-CTCTCCTAAGACCAGCAAGACC-3′ | |||
| TLR2 | NM_003264 | Sense | 5′-GGCTTCTCTGTCTTGTGACC-3′ | 292 |
| Antisense | 5′-GGGCTTGAACCAGGAAGACG-3′ | |||
| TLR3 | NM_003265 | Sense | 5′-AGCCACCTGAAGTTGACTCAGG-3′ | 268 |
| Antisense | 5′-CAGTCAAATTCGTGCAGAAGGC-3′ | |||
| TLR4 | NM_138554 | Sense | 5′-CAGAGTTTCCTGCAATGGATCA-3′ | 85 |
| Antisense | 5′-GCTTATCTGAAGGTGTTGCACAT-3′ | |||
| TLR5 | NM_003268 | Sense | 5′-AGCCATCTGACTGCATTAAGG-3′ | 336 |
| Antisense | 5′-GACTTCCTCTTCATCACAACC-3′ | |||
| TLR6 | NM_006068 | Sense | 5′-ATTGAAAGCATTCGTGAAGAAG-3′ | 123 |
| Antisense | 5′-ACGGTGTACAAAGCTGTCTGTG-3′ | |||
| TLR7 | NM_016562 | Sense | 5′-AAAATGGTGTTTCCAATGTGG-3′ | 107 |
| Antisense | 5′-GGCAGAGTTTTAGGAAACCATC-3′ | |||
| TLR8 | NM_138636 | Sense | 5′-TTATGTGTTCCAGGAACTCAGAGAA-3′ | 83 |
| Antisense | 5′-TAATACCCAAGTTGATAGTCGATAAGTTTG-3′ | |||
| TLR9 | NM_017442 | Sense | 5′-TACCAACATCCTGATGCTAGACTC-3′ | 231 |
| Antisense | 5′-TAGGACAACAGCAGATACTCCAGG-3′ | |||
| TLR10 | NM_001017388 | Sense | 5′-GGCCAGAAACTGTGGTCAAT-3′ | 205 |
| Antisense | 5′-AAATGACTGCATCCAGGGAG-3′ | |||
| 18s RNA | NR_003286 | Sense | 5′-CATGGTGACCACGGGTGA-3′ | 79 |
| Antisense | 5′-TTCCTTGGATGTGGTAGCCG-3′ |
Immunofluorescence Assay
The neuronal cells were cultured on glass coverslips at a density of 0.2 × 106 cells/well in 24-well plates. For immunofluorescent evaluation of IFN-α/β, neuronal cells on glass coverslips were fixed with 4% paraformaldehyde-4% sucrose in PBS for 20 min at 48C and then permeated in cold 100% methanol for additional 10 min, followed by 0.2% Triton X-100 for another 10 min. Cells were blocked in blocking solution for 1 hr at room temperature. The coverslips were then incubated with mouse anti-IFN-α (1:50) and goat anti-IFN-β (1:50) antibodies in blocking solution at room temperature for 60 min and were subsequently incubated with the secondary antibodies as TRITC-conjugated goat anti-mouse IgG1 antibody (1:250), FITC-conjugated donkey anti-goat IgG1 antibody (1:100) for 1 hr. For immunofluorescent evaluation of HSV-1 infection, the coverslips were incubated with goat anti-HSV antibody (1:100) in blocking solution at room temperature for 1 hr and were subsequently incubated with FITC-conjugated donkey anti-goat IgG1 antibody (1:100) for 1 hr. The coverslips were washed six times with PBS, mounted in Vectorshield (Vector Laboratories), and viewed with a fluorescence microscope (Olympus).
ELISA
Total cell lysates from the cultured NT2-N cells with or without HSV-1 infection (24 hr) were prepared using a radio-immune precipitation assay (RIPA) buffer (Promega, Madison, WI). ELISA assays for IFN-α and IFN-β were performed according to the protocol provided by the manufacturer.
Statistical Analysis
Where appropriate, data are expressed as mean ± SD of triplicate cultures. For comparison of the mean of two groups (treated vs. untreated), statistical significance was assessed by Student’s t-test. If there were more than two groups, one-way repeated-measures of ANOVA was used. Statistical analyses were performed with GraphPad Instat statistical software. Statistical significance was defined as P < 0.05.
RESULTS
Expression of TLRs and Type I IFNs in Human Neuronal Cells
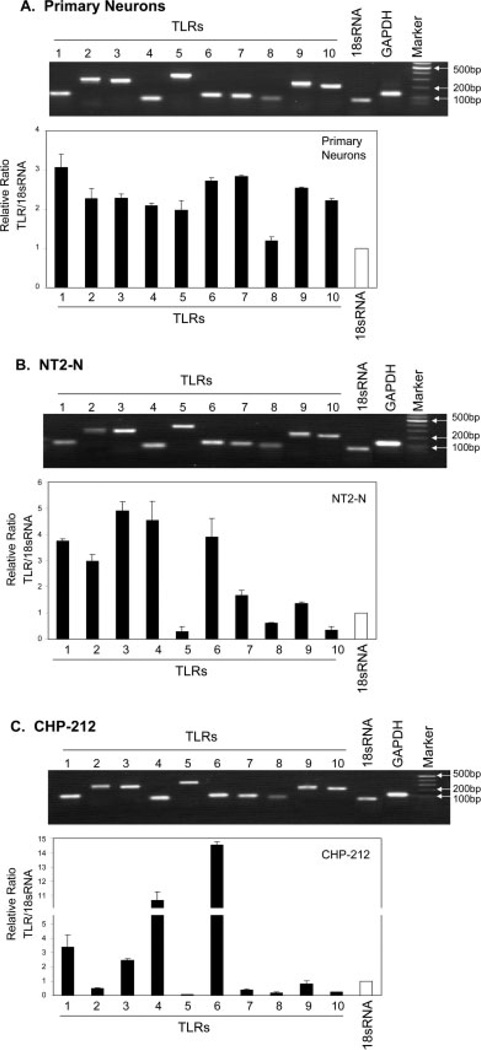
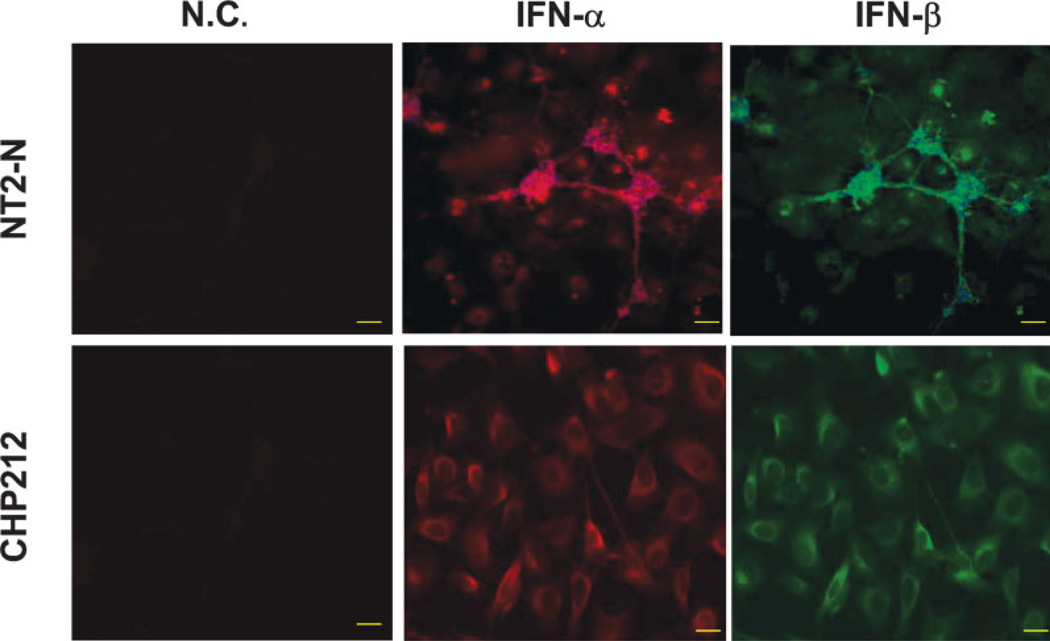
To investigate whether human neuronal cells possess TLR-mediated antiviral innate immunity, we first examined the expression of 10 currently known human TLRs in primary human neurons and two human neuronal cell lines (NT2-N and CHP-212) using the real-time RT-PCR. We found that, in addition to the expression of TLRs 1, 2, 3, and 4 (Fig. 1), which has been demonstrated previously (Prehaud et al., 2005), the transcripts of TLRs 5–10 were also detected in primary human neurons and two human neuronal cell lines, although the expression levels of TLRs varied in these cells (Fig. 1). In addition, the human neuronal cells (NT2-N, CHP-212) expressed IFN-α/β proteins (Fig. 2).
Fig. 1.
Expression of TLRs in primary neurons (A), NT2-N (B), and CHP-212 (C) cells. Total cellular RNA extracted from human neuronal cells (primary neurons, NT2-N cells, and CHP-212 cells) was subjected to the real-time RT-PCR with the primers specific for toll-like receptors (TLRs) 1–10. Amplified PCR products were displayed on 2% agarose gel. The relative ratio of the TLR mRNA to 18s mRNA level is given to semiquantify TLR expression in human neurons. The results are expressed as mean ± SD of triplicate cultures, representative of three experiments. For primary human neurons (A), three different samples were used for analysis.
Fig. 2.
Expression of type I IFNs in human neuronal cells. NT2-N cells (upper panel) and CHP-212 cells (bottom panel) were immunostained with antibodies against IFN-α or IFN-β. Mouse/goat IgG1 staining cells were included as negative controls (N.C.). The expression of IFN-α and IFN-β was visualized under microscopy (magnification ×200). Scale bars = 50 µm.
Activation of TLR3 or TLR8 Induces the Expression of IFN-α/β and IRFs
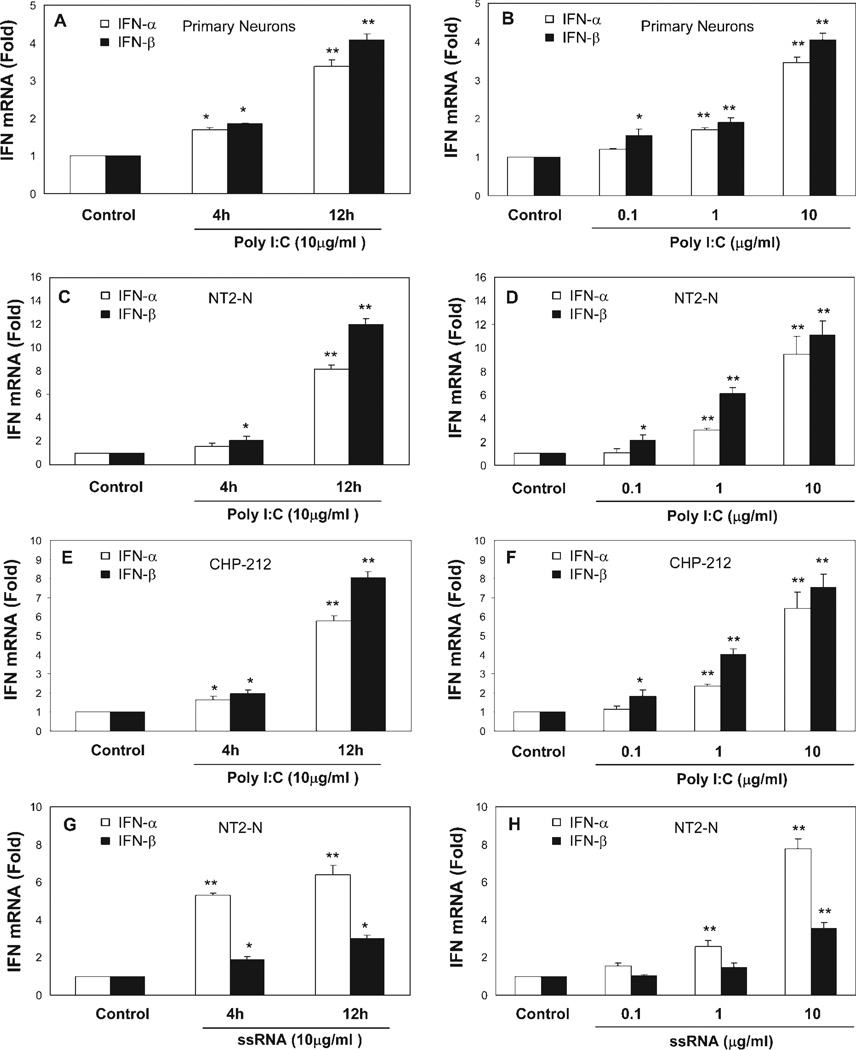
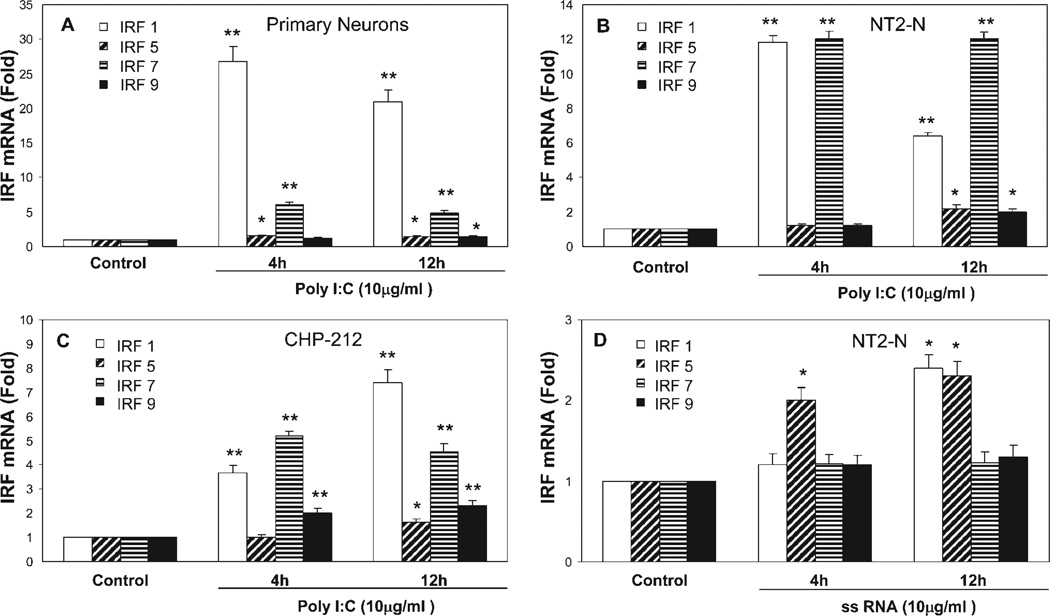
To determine whether TLRs expressed by neuronal cells are biologically functional, we treated human neuronal cells with the ligands to TLR3 or TLR8 that recognize dsRNA or ssRNA, the common intermediate products of viral replication (Alexopoulou et al., 2001; Heil et al., 2004). When treated with poly-I:C or ssRNA, primary neurons, NT2-N cells, and CHP-212 cells expressed significantly increased levels of IFN-α/β, and this induction of IFN-α/β by poly-I:C or ssRNA is time and dose dependent (Fig. 3). To investigate further whether TLR3- and TLR8-mediated induction of IFN-α/β is through the activation of IRFs, the key regulators of the IFN-α/β pathway, we examined expression of IRFs in response to TLR ligand treatment in the neuronal cells (primary human neurons, NT2-N cells, and CHP-212 cells) and found that poly-I:C-treated neuronal cells had increased levels of IRFs 1, 5, 7, and 9 (Fig. 4A–C), whereas ssRNA treatment induced the expression of IRFs 1 and 5 (Fig. 4D).
Fig. 3.
Effect of TLR3/8 activation on type I IFN expression. Neuronal cells (primary neurons, NT2-N cells, and CHP-212 cells) were treated with or without poly-I:C (10 µg/ml; A,C,E) or ssRNA (10 µg/ml; G) for 4 hr and 12 hr or were treated with or without poly-I:C (B,D,F) or ssRNA (H) at different concentrations as shown for 12 hr. Total cellular RNA extracted from poly-I:C- or ssRNA-treated or untreated neuronal cells was subjected to real-time RT-PCR for IFN-α and IFN-β RNA quantification. The data are expressed as IFN-α/β RNA levels relative (-fold) to control (without treatment, which is defined as 1). The results are mean ± SD of triplicate cultures, representative of three experiments (⋆P < 0.05, ⋆⋆P < 0.01).
Fig. 4.
Effect of TLR3/8 activation on IRF expression. Human neuronal cells (primary neurons, NT2-N cells, and CHP-212 cells) were treated with or without poly-I:C (10 µg/ml; A–C) or ssRNA (10 µg/ml; D) for 4 hr and 12 hr. Total cellular RNA extracted from poly-I:C- or ssRNA-treated or untreated NT2-N cells was subjected to real-time RT-PCR for IRF RNA quantification. The data are expressed as IRF RNA levels relative (-fold) to control (without treatment, which is defined as 1). The results are mean ± SD of triplicate cultures, representative of three experiments (⋆P < 0.05, ⋆⋆P < 0.01).
HSV-1 Infection Induces the Expression of IFN-α
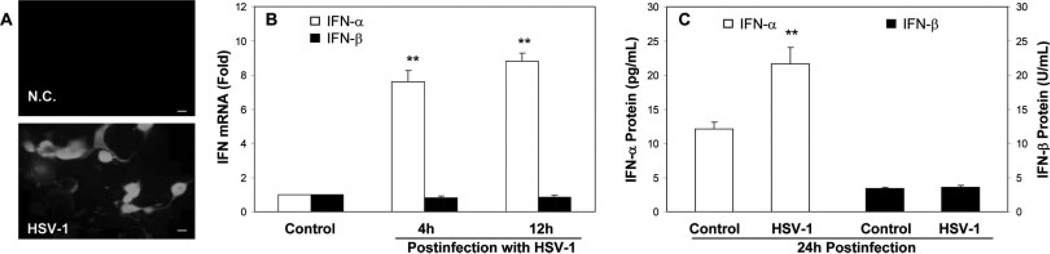
Because viral RNA molecules are recognized by receptors such as TLRs 3, 7, and 8 (Alexopoulou et al., 2001; Heil et al., 2004), we investigated whether HSV-1 infection is able to activate the TLRs and thus induce intracellular IFN-α/β expression in the neuronal cells. As expected, NT2-N cells became infected by HSV-1 as shown by the positive immunostaining with antibody to HSV-1 antigens (Fig. 5A). HSV-1 infection resulted in remarkably increased IFN-α mRNA and protein expression (Fig. 5B,C), but had little effect on IFN-β mRNA and protein expression in NT-2N cells (Fig. 5B,C).
Fig. 5.
Effect of HSV-1 infection on IFN-α/β expression in NT2-N cells. NT2-N cells were infected with HSV-1 at a multiplicity of infection (MOI) of 0.01. A: HSV-1 infection of NT2-N cells. Uninfected (negative control; NC) or infected (HSV-1) NT2-N cells were fixed at 48 hr postinfection and stained with the antibody against early/late antigens of HSV-1. The HSV-1 antigen expression was visualized under microscopy (magnification ×200). B: HSV-1 infection induces IFN-α/β expression. Total cellular RNA extracted from HSV-1-infected or uninfected NT2-N cells at indicated time points was subjected to real-time RT-PCR for IFN-α/β mRNA quantification. The data are expressed as IFN-α/β RNA levels relative (-fold) to control (without treatment, which is defined as 1). The results are mean ± SD of triplicate cultures, representative of three experiments (⋆P < 0.05, ⋆⋆P < 0.01). C: Cell lysates from NT2-N cells infected with or without HSV-1 for 24 hr were subjected to ELISA assay for IFN-α and IFN-β proteins. The results are mean ± SD of three independent experiments (⋆⋆P < 0.01). Scale bars = 50 µm.
Activation of TLR3 or TLR8 Protects Neuronal Cells From HSV-1 Infection
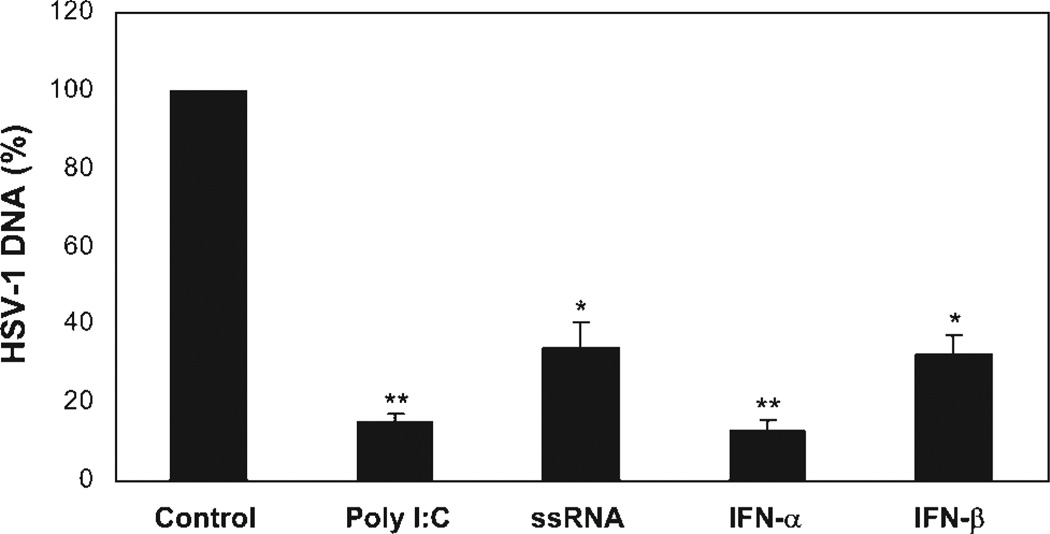
A key question to be addressed in this study was whether TLR-mediated innate immunity can protect the neuronal cells from HSV-1 infection. Therefore, we examined whether the activation of TLR3 by poly-I:C or TLR8 by ssRNA could inhibit HSV-1 infection of the neuronal cells. As shown in Figure 6, NT2-N cells pretreated with poly-I:C or ssRNA had significantly lower levels of HSV-1 DNA than untreated cells. The poly-I:C-mediated inhibition of HSV-1 was as potent (>80%) as recombinant IFN-α and IFN-β (Fig. 6), whereas ssRNA had an anti-HSV-1 effect similar to that of IFN-β (Fig. 6).
Fig. 6.
Effect of TLR3/8 activation on HSV-1 infection of NT2-N cells. NT2-N cells were treated with or without poly-I:C (10 µg/ml), ssRNA (10 µg/ml), IFN-α (20 U/ml), or IFN-β (20 U/ml) 12 hr prior to HSV-1 infection (MOI of 0.01). Total cellular DNA extracted from the cell cultures at 24 hr postinfection was subjected to real-time PCR for HSV-1 quantification. The data are expressed as HSV-1 DNA levels relative (%) to control (without treatment, which is defined as 100). The results are mean ± SD of triplicate cultures, representative of three experiments (⋆P < 0.05, ⋆⋆P < 0.01).
DISCUSSION
The TLR-mediated innate immune system and its roles in antiviral infection have been intensively studied, especially in the immune cells. Recent studies have also shown that TLRs are present in the brain, where their expression is believed to be limited to glial cells (microglia, astrocytes, and oligodentrocytes; Bsibsi et al., 2002; Olson and Miller, 2004; Jack et al., 2005). However, limited information is available on whether human neuronal cells have functional TLR-mediated innate immunity against viral infection. Different investigators studying TLR expression in the neuronal cells have obtained conflicting data. It was reported that neurons do not express TLRs (Lehnardt et al., 2003). However, several recent studies have shown the presence of TLRs in neurons (Prehaud et al., 2005; Lafon et al., 2006; Ma et al., 2006; Tang et al., 2007). One study showed that murine neurons express TLRs 1–9 and found that TLRs 5 and 9 are expressed at high levels; TLRs 2 and 4 at intermediate levels; and TLRs 1, 3, 6, and 7 at low levels (Tang et al., 2007). In this study, we demonstrate that human neuronal cells express all 10 members of human TLR family, although the expression levels varied in the neuronal cells (Fig. 1). These findings provide direct and experimental evidence to support the notion that human neuronal cells possess TLR-mediated innate immune machinery. To determine whether TLRs expressed by the neuronal cells are biologically functional, we examined the effect of the activation of TLRs on intracellular IFN-α/β expression. It is known that TLR binding by ligands triggers the activation of transcriptional factors that lead to induction of antiviral cytokines such as type I IFNs (Akira and Takeda, 2004). We focused on TLR3 and TLR8, because these TLRs play a key role in the activation of type I IFNs (Yang et al., 2005; Zhang et al., 2007). TLR3 that recognizes dsRNA (Alexopoulou et al., 2001) is a major mediator of the cellular response to viral replication (Kumar et al., 2006; Starace et al., 2008; West and Damania, 2008). After the recognition of dsRNA, some early signaling events are initiated by TLR3, which then activates transcriptional factors NF-κB and IRF, resulting in the primary response of antiviral cytokines, primarily type I IFNs (Kawano et al., 1998; Akira et al., 2001; Sharma et al., 2003; Yoneyama et al., 2004). After this primary response, the secreted IFN-β induces secondary response by binding to cellular IFN-α/β receptor and activating transcription of IFN-stimulated response elements, leading to more robust production type I IFN (Le Bon and Tough, 2002; Taniguchi and Takaoka, 2002). Our observation that both poly-I:C and ssRNA could induce IFN-α/β expression in the neuronal cells (Fig. 3) suggests that TLRs expressed by human neuronal cells are biologically functional.
Our further investigation of the mechanisms indicated that there was an up-regulation of IRFs 1, 5, 7, and 9 in neuronal cells treated with poly-I:C. Interestingly, IRF3, which is traditionally believed to be the key factor involved in TLR3 activation of type I IFN pathway (Doyle et al., 2002; Rivieccio et al., 2006), was not up-regulated in human neurons by poly-I:C stimulation. Nevertheless, the up-regulation of other IRFs, especially IRFs 1 and 7 that have been shown to be the strong activators of the transcription of type I IFN genes (Honda et al., 2006; Negishi et al., 2006; Ozato et al., 2007), provides a mechanism for poly-I:C-mediated IFN-α/β induction. In addition, ssRNA treated-NT2-N cells expressed higher levels of IRF5 than untreated cells (Fig. 4D). Insofar as both IRF1 and IRF5 are involved in the activation of IFN-α/β (Honda et al., 2006; Negishi et al., 2006; Ozato et al., 2007), these data provide a sound mechanism for the ssRNA action on intracellular IFN-α/β expression in the neuronal cells.
Human neuronal cells are the primary target for some viral infections, including HSV-1 (Prehaud et al., 2005). In agreement with studies by others (Fath et al., 2000; Prehaud et al., 2005; Megret et al., 2007), we showed that NT2-N cells could be infected by HSV-1 (Fig. 5A). Because HSV-1 infection can generate both dsRNA and ssRNA intermediates (Jacquemont and Roizman, 1975), we investigate whether HSV-1 infection can trigger TLR-mediated innate immune system in the neuronal cells. As the result of such activation, HSV-1-infected neuronal cells expressed higher levels of IFN-α than uninfected cells, although HSV-1 infection had little effect on IFN-β expression (Fig. 5B,C). These findings are partially in line with reports (Olson and Miller, 2004; Rivieccio et al., 2006) that viral infection induced the expression of TLRs and IFN-α/β in microglia and astrocytes, other key cell types in the CNS. However, our data disagree with a recent report that HSV-1 infection of neuronal cells induced neither IFN-α nor IFN-β (Prehaud et al., 2005). This discrepancy between our observation and that from others could be due to the difference in the neuronal cell types used in the studies. It is possible that the NT2-N cells that we used are more susceptible to HSV-1 infection than the neuronal cells used by others (Prehaud et al., 2005). To provide direct evidence that TLR-mediated innate immunity contributes to the protection of human neurons from HSV-1 infection, we examined whether the activation of human neuronal cells with TLR3 or TLR8 ligands prior to HSV-1 infection could provide neuronal protection. We showed that both poly-I:C and ssRNA pretreatment could significantly reduce HSV-1 infectivity in NT2-N cells (Fig. 6). This finding is in agreement with the report (Rivieccio et al., 2006) showing that poly-I:C treatment contributes to inhibition of pseudo-typed HIV-1 infection of human astrocytes.
In summary, we have provided experimental evidence showing that, similarly to immune cells and glial cells, human neurons possess a functional TLR/IFN system that is able to mount an effective immunity to HSV-1 infection. Further ex vivo and in vivo studies are necessary to support the notion that human neuronal cells are protected by TLR- and type I IFN-mediated intracellular innate immunity. TLR ligands have been suggested for multiple clinical uses, including cancer therapy, improvement of responses to vaccines, and induction of protective innate immune response, so an understanding of TLR-mediated intracellular innate antiviral responses in human neuronal cells should provide new insights into the development of an attractive and neuronal innate immunity-based therapeutic approach for treatment of viral infections in the CNS.
Acknowledgments
Contract grant sponsor: National Institutes of Health; Contract grant number: NIDA012815 (to W.H.); Contract grant number: NIDA022177 (to W.H.); Contract grant sponsor: Foerderer Fund from the Children’s Hospital of Philadelphia.
REFERENCES
- Akira S. Toll-like receptor signaling. J Biol Chem. 2003;278:38105–38108. doi: 10.1074/jbc.R300028200. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signaling. Nat Rev Immunol. 2004;4:499. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Argyris EG, Acheampong E, Wang F, Huang J, Chen K, Mukhtar M, Zhang H. The interferon-induced expression of APOBEC3G in human blood-brain barrier exerts a potent intrinsic immunity to block HIV-1 entry to central nervous system. Virology. 2007;367:440–451. doi: 10.1016/j.virol.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin G, Coulombe Z, Rivest S. Intranasal herpes simplex virus type 2 inoculation causes a profound thymidine kinase dependent cerebral inflammatory response in the mouse hindbrain. Eur J Neurosci. 2002;16:29–43. doi: 10.1046/j.1460-9568.2002.02057.x. [DOI] [PubMed] [Google Scholar]
- Bottcher T, von Mering M, Ebert S, Meyding-Lamade U, Kuhnt U, Gerber J, Nau R. Differential regulation of Toll-like receptor mRNAs in experimental murine central nervous system infections. Neurosci Lett. 2003;344:17–20. doi: 10.1016/s0304-3940(03)00404-x. [DOI] [PubMed] [Google Scholar]
- Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002;61:1013–1021. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- Doyle S, Vaidya S, O’Connell R, Dadgostar H, Dempsey P, Wu T, Rao G, Sun R, Haberland M, Modlin R, Cheng G. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity. 2002;17:251–263. doi: 10.1016/s1074-7613(02)00390-4. [DOI] [PubMed] [Google Scholar]
- Farina C, Krumbholz M, Giese T, Hartmann G, Aloisi F, Meinl E. Preferential expression and function of Toll-like receptor 3 in human astrocytes. J Neuroimmunol. 2005;159:12–19. doi: 10.1016/j.jneuroim.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Fath T, Eidenmuller J, Maas T, Brandt R. Herpes simplex virus-mediated expression of the axonal protein tau in human model neurons (NT2-N cells) Microsc Res Techniq. 2000;48:85–96. doi: 10.1002/(SICI)1097-0029(20000115)48:2<85::AID-JEMT4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Ferrari A, Ehler E, Nitsch RM, Gotz J. Immature human NT2 cells grafted into mouse brain differentiate into neuronal and glial cell types. FEBS Lett. 2000;486:121–125. doi: 10.1016/s0014-5793(00)02251-1. [DOI] [PubMed] [Google Scholar]
- Gordon S. Pattern recognition receptors: doubling up for the innate immune response. Cell. 2002;111:927–930. doi: 10.1016/s0092-8674(02)01201-1. [DOI] [PubMed] [Google Scholar]
- Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- Honda K, Yanai H, Mizutani T, Negishi H, Shimada N, Suzuki N, Ohba Y, Takaoka A, Yeh WC, Taniguchi T. Role of a transductional-transcriptional processor complex involving MyD88 and IRF-7 in Toll-like receptor signaling. Proc Natl Acad Sci USA. 2004;101:15416–15421. doi: 10.1073/pnas.0406933101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Takaoka A, Taniguchi T. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25:349–360. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Hu S, Sheng WS, Lokensgard JR, Peterson PK. Morphine induces apoptosis of human microglia and neurons. Neuropharmacology. 2002;42:829–836. doi: 10.1016/s0028-3908(02)00030-8. [DOI] [PubMed] [Google Scholar]
- Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, Shapiro A, Antel JP. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol. 2005;175:4320–4330. doi: 10.4049/jimmunol.175.7.4320. [DOI] [PubMed] [Google Scholar]
- Jacquemont B, Roizman B. RNA synthesis in cells infected with herpes simplex virus. X. Properties of viral symmetric transcripts and of double-stranded RNA prepared from them. J Virol. 1975;15:707–713. doi: 10.1128/jvi.15.4.707-713.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Sato H, Kondo E, Harada M, Koseki H, Nakayama T, Tanaka Y, Taniguchi M. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Valpha14 NKT cells. Proc Natl Acad Sci USA. 1998;95:5690–5693. doi: 10.1073/pnas.95.10.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koedel U, Angele B, Rupprecht T, Wagner H, Roggenkamp A, Pfister HW, Kirschning CJ. Toll-like receptor 2 participates in mediation of immune response in experimental pneumococcal meningitis. J Immunol. 2003;170:438–444. doi: 10.4049/jimmunol.170.1.438. [DOI] [PubMed] [Google Scholar]
- Kumar A, Zhang J, Yu FS. Toll-like receptor 3 agonist poly(I:C)-induced antiviral response in human corneal epithelial cells. Immunology. 2006;117:11–21. doi: 10.1111/j.1365-2567.2005.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafon M, Megret F, Lafage M, Prehaud C. The innate immune facet of brain: human neurons express TLR-3 and sense viral dsRNA. J Mol Neurosci. 2006;29:185–194. doi: 10.1385/JMN:29:3:185. [DOI] [PubMed] [Google Scholar]
- Le Bon A, Tough DF. Links between innate and adaptive immunity via type I interferon. Curr Opin Immunol. 2002;14:432–436. doi: 10.1016/s0952-7915(02)00354-0. [DOI] [PubMed] [Google Scholar]
- Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci USA. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Douglas SD, Pleasure DE, Lai J, Guo C, Bannerman P, Williams M, Ho W. Human neuronal cells (NT2-N) express functional substance P and neurokinin-1 receptor coupled to MIP-1 beta expression. J Neurosci Res. 2003;71:559–566. doi: 10.1002/jnr.10504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokensgard JR, Cheeran MCJ, Hu SX, Gekker G, Peterson PK. Glial cell responses to herpesvirus infections: role in defense and immunopathogenesis. J Infect Dis. 2002;186:S171–S179. doi: 10.1086/344272. [DOI] [PubMed] [Google Scholar]
- Ma Y, Li J, Chiu I, Wang Y, Sloane JA, Lu J, Kosaras B, Sidman RL, Volpe JJ, Vartanian T. Toll-like receptor 8 functions as a negative regulator of neurite outgrowth and inducer of neuronal apoptosis. J Cell Biol. 2006;175:209–215. doi: 10.1083/jcb.200606016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, Janeway CA., Jr Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- Megret F, Prehaud C, Lafage M, Moreau P, Rouas-Freiss N, Carosella ED, Lafon M. Modulation of HLA-G and HLA-E expression in human neuronal cells after rabies virus or herpes virus simplex type 1 infections. Hum Immunol. 2007;68:294–302. doi: 10.1016/j.humimm.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Munir M, Lu L, McGonigle P. Excitotoxic cell death and delayed rescue in human neurons derived from NT2 cells. J Neurosci. 1995;15:7847–7860. doi: 10.1523/JNEUROSCI.15-12-07847.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi H, Fujita Y, Yanai H, Sakaguchi S, Ouyang X, Shinohara M, Takayanagi H, Ohba Y, Taniguchi T, Honda K. Evidence for licensing of IFN-gamma-induced IFN regulatory factor 1 transcription factor by MyD88 in Toll-like receptor-dependent gene induction program. Proc Natl Acad Sci USA. 2006;103:15136–15141. doi: 10.1073/pnas.0607181103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- Ozato K, Tailor P, Kubota T. The interferon regulatory factor family in host defense: mechanism of action. J Biol Chem. 2007;282:20065–20069. doi: 10.1074/jbc.R700003200. [DOI] [PubMed] [Google Scholar]
- Pekosz A, Phillips J, Pleasure D, Merry D, Gonzalez-Scarano F. Induction of apoptosis by La Crosse virus infection and role of neuronal differentiation and human bcl-2 expression in its prevention. J Virol. 1996;70:5329–5335. doi: 10.1128/jvi.70.8.5329-5335.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleasure SJ, Page C, Lee VM. Pure, postmitotic, polarized human neurons derived from NTera 2 cells provide a system for expressing exogenous proteins in terminally differentiated neurons. J Neurosci. 1992;12:1802–1815. doi: 10.1523/JNEUROSCI.12-05-01802.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehaud C, Megret F, Lafage M, Lafon M. Virus infection switches TLR-3-positive human neurons to become strong producers of beta interferon. J Virol. 2005;79:12893–12904. doi: 10.1128/JVI.79.20.12893-12904.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivieccio MA, Suh HS, Zhao Y, Zhao ML, Chin KC, Lee SC, Brosnan CF. TLR3 ligation activates an antiviral response in human fetal astrocytes: a role for viperin/cig5. J Immunol. 2006;177:4735–4741. doi: 10.4049/jimmunol.177.7.4735. [DOI] [PubMed] [Google Scholar]
- Rootwelt T, Dunn M, Yudkoff M, Itoh T, Almaas R, Pleasure D. Hypoxic cell death in human NT2-N neurons: involvement of NMDA and non-NMDA glutamate receptors. J Neurochem. 1998;71:1544–1553. doi: 10.1046/j.1471-4159.1998.71041544.x. [DOI] [PubMed] [Google Scholar]
- Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- Starace D, Galli R, Paone A, Cesaris PD, Filippini A, Ziparo E, Riccioli A. Toll-like receptor 3 activation induces antiviral immune responses in mouse sertoli cells. Biol Reprod. 2008;79:766–775. doi: 10.1095/biolreprod.108.068619. [DOI] [PubMed] [Google Scholar]
- Stuart LM, Ezekowitz RA. Phagocytosis: elegant complexity. Immunity. 2005;22:539–550. doi: 10.1016/j.immuni.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, Lathia JD, Siler DA, Chigurupati S, Ouyang X, Magnus T, Camandola S, Mattson MP. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci USA. 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T, Takaoka A. The interferon-alpha/beta system in antiviral responses: a multimodal machinery of gene regulation by the IRF family of transcription factors. Curr Opin Immunol. 2002;14:111–116. doi: 10.1016/s0952-7915(01)00305-3. [DOI] [PubMed] [Google Scholar]
- Wang YJ, Wang X, Zhang H, Zhou L, Liu S, Kolson DL, Song L, Ye L, Ho WZ. Expression and regulation of antiviral protein APO-BEC3G in human neuronal cells. J Neuroimmunol. 2008 doi: 10.1016/j.jneuroim.2008.10.003. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J, Damania B. Upregulation of the TLR3 pathway by Kaposi’s sarcoma-associated herpesvirus during primary infection. J Virol. 2008;82:5440–5449. doi: 10.1128/JVI.02590-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- Yang K, Puel A, Zhang S, Eidenschenk C, Ku CL, Casrouge A, Picard C, von Bernuth H, Senechal B, Plancoulaine S, Al-Hajjar S, Al-Ghonaium A, Marodi L, Davidson D, Speert D, Roifman C, Garty BZ, Ozinsky A, Barrat FJ, Coffman RL, Miller RL, Li X, Lebon P, Rodriguez-Gallego C, Chapel H, Geissmann F, Jouanguy E, Casanova JL. Human TLR-7-, -8-, and -9-mediated induction of IFN-alpha/-beta and -lambda Is IRAK-4 dependent and redundant for protective immunity to viruses. Immunity. 2005;23:465–478. doi: 10.1016/j.immuni.2005.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- Zhang SY, Jouanguy E, Sancho-Shimizu V, von Bernuth H, Yang K, Abel L, Picard C, Puel A, Casanova JL. Human Toll-like receptor-dependent induction of interferons in protective immunity to viruses. Immunol Rev. 2007;220:225–236. doi: 10.1111/j.1600-065X.2007.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]