Abstract
A wide range of electronic cigarette (EC) devices, from small cigarette-like (first-generation) to new-generation high-capacity batteries with electronic circuits that provide high energy to a refillable atomizer, are available for smokers to substitute smoking. Nicotine delivery to the bloodstream is important in determining the addictiveness of ECs, but also their efficacy as smoking substitutes. In this study, plasma nicotine levels were measured in experienced users using a first- vs. new-generation EC device for 1 hour with an 18 mg/ml nicotine-containing liquid. Plasma nicotine levels were higher by 35–72% when using the new- compared to the first-generation device. Compared to smoking one tobacco cigarette, the EC devices and liquid used in this study delivered one-third to one-fourth the amount of nicotine after 5 minutes of use. New-generation EC devices were more efficient in nicotine delivery, but still delivered nicotine much slower compared to tobacco cigarettes. The use of 18 mg/ml nicotine-concentration liquid probably compromises ECs' effectiveness as smoking substitutes; this study supports the need for higher levels of nicotine-containing liquids (approximately 50 mg/ml) in order to deliver nicotine more effectively and approach the nicotine-delivery profile of tobacco cigarettes.
Electronic cigarettes (ECs) have been introduced to the market in recent years as alternatives to smoking. They are considered part of tobacco harm reduction, a strategy of reducing adverse health effects by providing low-risk nicotine products to substitute smoking1. They deal with both the psycho-behavioral (through motor simulation and sensory stimulation) and the chemical (through delivery of nicotine) aspects of smoking addiction2. ECs mainly consist of a lithium battery and a part called atomizer, where the liquid is stored and evaporated by applying electrical current to a resistance and wick setup. There is a substantial variability of devices; small devices, looking similar to tobacco cigarettes (commonly referred as first-generation), consist of a low-capacity batteries and polyfil-filled atomizers, while new-generation devices consist of larger-capacity batteries, larger atomizers and electronic circuits providing the ability to set the power delivery to the atomizer.
The growing popularity of ECs3,4 has raised significant controversy in public health authorities. Organizations such as the World Health Organization and Food and Drug Administration have expressed concerns about the safety of e-cigarettes and the effects of nicotine intake. Recently, European Union has developed a new regulation which implements an upper limit of 20 mg/ml nicotine concentration in liquids that are used with ECs5. The decision was based on a study from our group, in which nicotine consumption and delivery to the user was evaluated6,7. However, the route, speed and amount of nicotine absorption (and subsequent nicotine levels in plasma) are important determinants of the efficacy of ECs to serve as smoking substitutes and of any concerns about nicotine overdose or intoxication. Data on nicotine absorption are scarce. Initially, EC use (commonly called vaping) was found to deliver minimal amounts of nicotine to the user as measured by plasma nicotine levels8,9. However, there has been a fast evolution of new, more efficient devices, and devices used at the time of those experiments are currently outdated and off the market. Surveys have shown that new-generation devices are more popular in dedicated EC users and a significant proportion of these users report complete smoking cessation10,11. However, no study has evaluated nicotine absorption from such devices. Therefore, the purpose of this study was to compare the nicotine absorption from a first- vs. a new-generation device in experienced vapers.
Results
Characteristics of the participants
Healthy experienced EC users (n = 23, all former smokers) were recruited for this study. They used a first-generation (V2 standard Cig with cartomizer) and a new-generation device (EVIC set at 9 watts with EVOD atomizer) on two separate days in a randomized cross-over design. They were requested to abstain from EC use, caffeine and alcohol intake for at least 8 hours before each experimental setting. The characteristics of the study population are shown in Table 1. Participants were former heavy smokers. They were using the EC for 19 months, while 20 of them reported that they had quit smoking within less than 1 month initiation of EC use. Twelve of them reported at least one unsuccessful attempt to quit smoking before trying ECs. Participants had lower dependence to EC use compared to smoking according to their responses to the Fagerström Test for Cigarette Dependence (FTCD)12 and the Cigarette Dependence Scale (CDS)13.
Table 1. Characteristics of the study population.
| Characteristic | EC users (n = 23) |
|---|---|
| Males, n (%) | 17 (74) |
| Age, years | 40.0 (1.9) |
| Smoking duration, years | 21.5 (1.9) |
| Smoking consumption, cigarettes per day | 33.6 (2.5) |
| EC use duration, months | 18.9 (2.3) |
| Smoking cessation duration, months | 18.2 (2.3) |
| FTCDsmoking | 6.96 (0.40) |
| FTCDsmoking-modified1 | 4.70 (0.30) |
| CDSsmoking | 53.26 (1.13) |
| CDSsmoking-modified1 | 49.00 (1.02) |
| FTCD-EC | 6.09 (0.32)2 |
| FTCD-EC-modified1 | 3.91 (0.27)2 |
| CDS-EC | 45.13 (1.51)2 |
| CDS-EC-modified1 | 41.27 (1.46)2 |
Data are presented as mean (SEM) and number (percentage).
Abbreviations. FTCD, Fagerström Test for Cigarette Dependence; CDS, Cigarette Dependence Scale; EC, electronic cigarette.
1Score by subtracting the question about cigarette and EC consumption.
2P < 0.05 compared to the respective scores for smoking (paired-samples t-test).
EC liquid used
For both experimental settings, the same liquid (Flavourart MaxBlend) was used. The nicotine concentration as mentioned in the label was 18 mg/ml. The liquid was analyzed for the presence of impurities, with results displayed in Table 2. Nicotine concentration was very close to the declared value, while impurities and contaminants were present in trace quantities.
Table 2. Analysis of the electronic cigarette liquid used in the study.
| Analysis | Quantity |
|---|---|
| Nicotine | 17.7 mg/ml |
| Propylene glycol | 389 mg/ml |
| Glycerol | 751 mg/ml |
| Diethylene glycol | ND |
| Aldehydes (total) | 18.17 μg/ml |
| Acetaldehyde | 8.51 μg/ml |
| Crotonaldehyde | 6.33 μg/ml |
| Formaldehyde | 3.33 μg/ml |
| Diacetyl | ND |
| Tobacco-Specific Nitrosamines (total) | 2.08 ng/ml |
| NNN | ND |
| NNK | 2.08 ng/ml |
| Heavy metals (total) | 35 ng/ml |
| Arsenic | 35 ng/ml |
| Chromium | ND |
| Lead | ND |
| Nickel | ND |
| pH | 8.55 |
| Water | 4.3% |
Abbreviations. ND, not detected; NNN, N'-nitrosonornicotine; NNK, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone.
Nicotine absorption
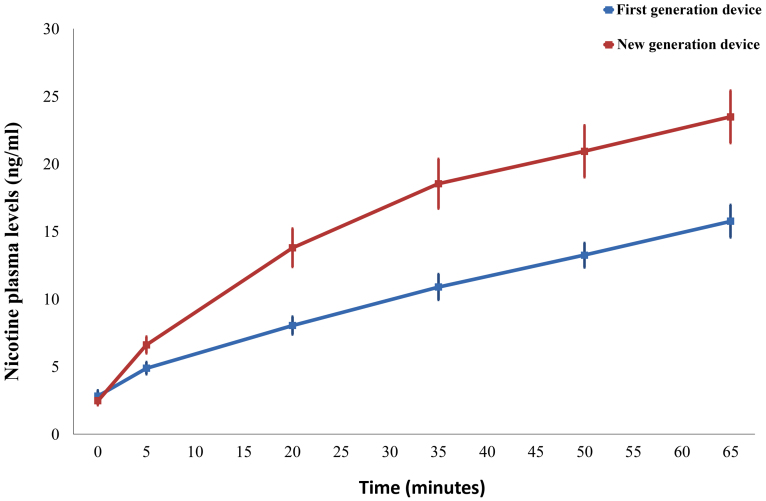
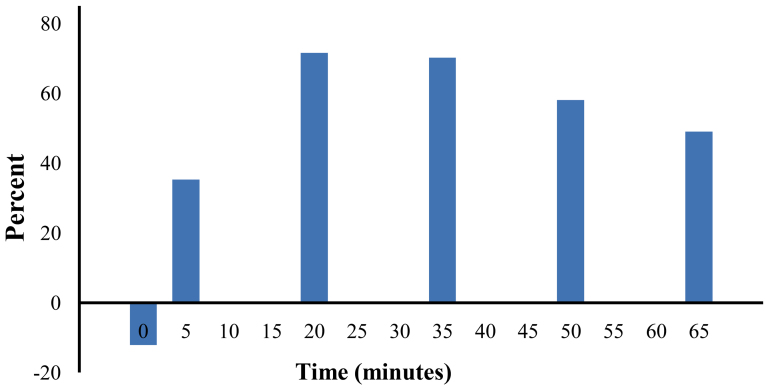
Venous blood samples were drawn at baseline, after abstinence from EC use for at least 8 hours. Additional blood samples were drawn at 5 minutes (taking 10 puffs) and every 15 minutes during 1 hour of ad lib EC use (Figure 1). Plasma levels of nicotine from the first-and the new-generation device are displayed in Figure 2. Significant differences were observed between time points (F = 113.0 P < 0.001). Additionally, significant differences were observed between sessions (F = 18.1, P < 0.001). At baseline, no differences were observed between the two sessions (P = 0.355). However, subsequent plasma nicotine levels were significantly higher when using the new-generation device (P = 0.003 at 5 minutes, P ≤ 0.001 for all other time points). At 5 minutes (10 puffs), mean plasma nicotine levels rose from 2.80 ng/ml (SEM: 0.42) to 4.87 ng/ml (SEM: 0.45) with the first-generation device, while levels went from 2.46 ng/ml (SEM: 0.33) to 6.59 ng/ml (SEM: 0.62) with the new-generation device. Maximal levels were obtained at the end of the ad lib use period, with levels reaching to 15.75 ng/ml (SEM: 1.2) and 23.47 ng/ml (SEM: 1.94) respectively. The mean percent differences between sessions at each time point are displayed in Figure 3. Nicotine plasma levels were approximately 70% higher using the new- compared to the first-generation device at 20 minutes, with the difference subsequently decreasing but still remaining at almost 50% at the end of EC use period.
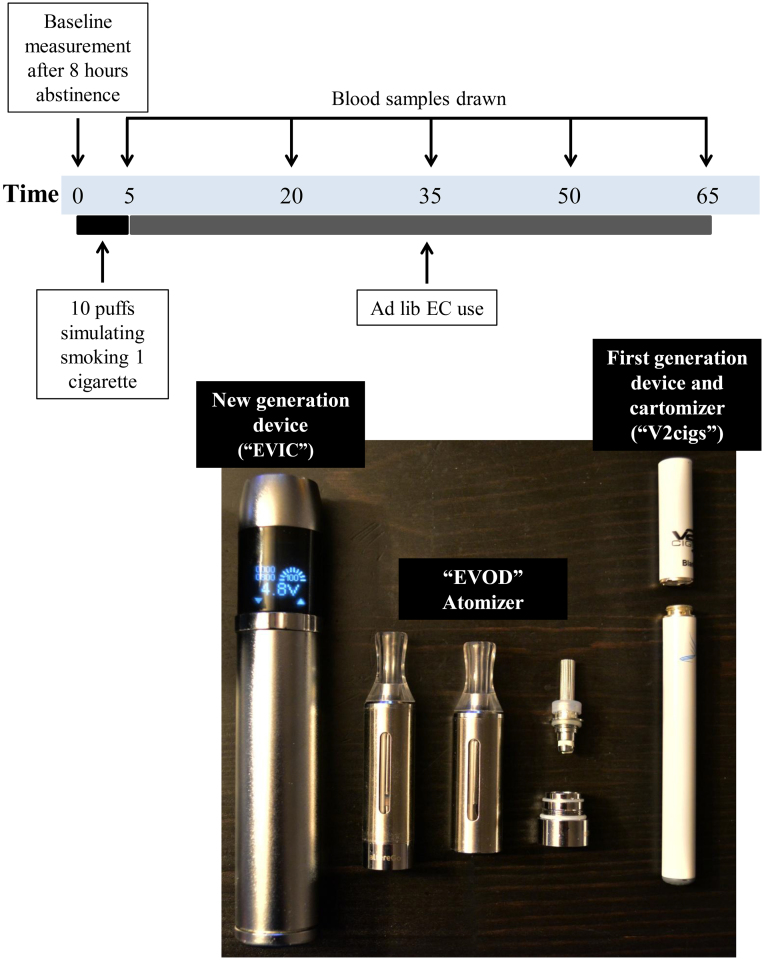
Figure 1. Protocol and materials used in this study.
Figure 2. Plasma nicotine levels at baseline and at 5, 20, 35, 50 and 65 minutes after using the first- and the new-generation device.
Error bars represent 1 SEM. There was a statistically significant difference between devices at all timing points except from baseline.
Figure 3. Percent difference in plasma nicotine levels between first- and new-generation devices at each time point.
At 20 minutes, use of the new-generation device resulted in 71.6% higher plasma nicotine levels compared to the first-generation device. The difference was reduced in subsequent timing points but was still 49.0% at 65 minutes.
Reduction in craving and perceived satisfaction
The Cigarette Withdrawal Scale (CWS), a questionnaire commonly used to assess smoking craving14,15, was used to assess the effects of EC use on craving to vape. There was a significant difference between timings (F = 48.1, P < 0.001) and a significant timing by session interaction (F = 9.2, P = 0.040). At baseline and at 5 minutes there was no statistically significant difference in the score between the two sessions, while at 65 minutes the score was significantly lower after using the new- compared with the first-generation device (P = 0.004).
Significant differences between timings (F = 258.7, P < 0.001) and between sessions (F = 39.6, P < 0.001) as well as significant timing by session interaction (F = 40.6, P < 0.001) were observed for a simple craving rating (score from 0 to 100). At baseline, craving was similar in new- and first-generation sessions [72.3 (1.9) and 71.1 (2.1) respectively, P = 0.382]. At 5 minutes, craving was significantly reduced in both sessions and was significantly lower in new- compared to first-generation session [47.9 (1.9) and 60.2 (2.11) respectively, P < 0.001 compared to baseline and between sessions]. The score was much lower at 65 minutes for both groups, with similar differences between new- and first-generation session [25.3 (2.5) and 32.0 (2.7) respectively, P < 0.001 compared to previous timings and between sessions].
The results for the direct effects of EC use and effects of nicotine, derived from 100-mm visual analogue scale questions answered at the end of the 65-minute period, are displayed in Table 3. For the effects experienced from using the ECs, “satisfying” and “throat hit” was significantly higher for the new-generation device, while “feels like a tobacco cigarette” and “looks like a tobacco cigarette” were higher for the first-generation device. From assessing the effects of nicotine, only “burning throat” was significantly higher for new-generation device; however, this is not necessarily a negative experience since it may be a response to the throat hit sensation which is favorable for smokers to feel. In general, negative effects had a low score.
Table 3. Assessment of electronic cigarette direct effects and nicotine effects after using the electronic cigarette devices.
| Questions | First-generation device | New-generation device | P1 |
|---|---|---|---|
| Effects of electronic cigarette use | |||
| Satisfying | 62.8 (2.8) | 74.7 (2.8) | 0.003 |
| Throat hit | 61.6 (3.9) | 75.1 (3.8) | < 0.001 |
| Calm | 72.5 (2.7) | 75.5 (2.3) | NS |
| Concentrate | 76.1 (2.2) | 76.0 (2.5) | NS |
| Feel sick | 10.6 (1.1) | 11.9 (1.4) | NS |
| Tastes good | 68.0 (3.3) | 71.7 (3.7) | NS |
| Tastes like tobacco cigarette | 51.5 (4.7) | 52.1 (4.6) | NS |
| Feels like tobacco cigarette | 69.8 (4.0) | 56.9 (3.7) | 0.004 |
| Effects of nicotine | |||
| Nausea | 10.5 (2.1) | 10.1 (1.8) | NS |
| Clammy skin | 8.7 (1.8) | 9.9 (1.8) | NS |
| Dizziness | 16.9 (1.8) | 18.1 (2.4) | NS |
| Lightheadedness | 26.6 (2.3) | 29.2 (3.3) | NS |
| Burning throat | 27.4 (1.9) | 40.7 (2.8) | < 0.001 |
| Tingling sensations | 6.5 (1.3) | 7.1 (1.2) | NS |
| Heart racing | 5.3 (1.1) | 6.4 (1.3) | NS |
Data are presented as mean (SEM).
1Paired-samples t-test.
Carbon monoxide levels
Exhaled carbon monoxide levels (ppm) were measured at baseline and 10 minutes after the 65-minute period of using the EC devices. There was no significant difference between timings and between devices, and no significant timing by session interaction (F ≤ 1.0, P = NS for all). Carbon monoxide levels went from 6.04 (0.43) before to 5.96 (0.32) ppm after using the first-generation device. For the second-generation device, the respective values were 6.39 (0.49) and 6.04 (0.46).
Comparison with nicotine absorption from a tobacco cigarette
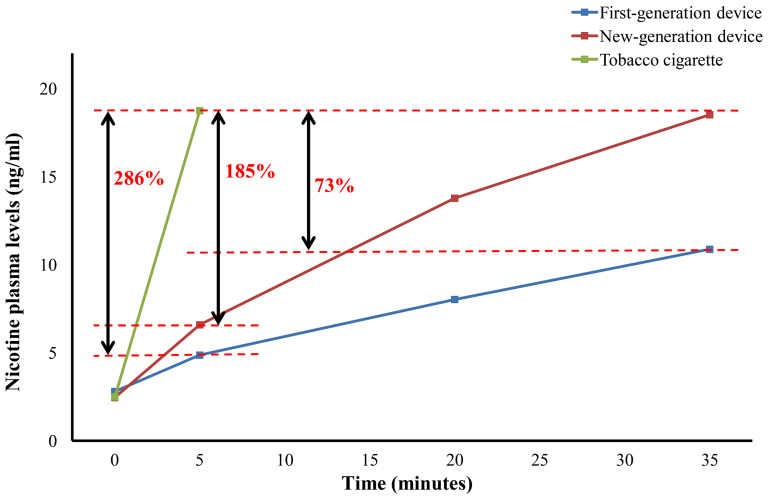
To compare nicotine absorption between tobacco cigarette and EC devices, we used the results from a study by Vansickel et al8. In that study, minimal nicotine absorption from the ECs was observed, while smoking one tobacco cigarette in 5 minutes led to an increase in plasma nicotine levels from 2.10 ng/ml to 18.80 ng/ml. Projection of the findings by Vansickel et al to our data is displayed in Figure 4. After 5 minutes of EC use, plasma nicotine levels were substantially lower compared to smoking one tobacco cigarette (almost 3-fold lower compared to new- and 4-fold lower compared to first-generation device). Plasma nicotine levels were equal between tobacco cigarette use at 5 minutes and new generation EC device at 35 minutes (18.52 ng/ml); however, tobacco cigarette showed 73% and 19% higher levels of nicotine compared to 35 and 65 minutes ad lib use of the first-generation device (10.88 ng/ml and 15.75 ng/ml respectively).
Figure 4. Comparison between tobacco cigarette and electronic cigarette devices in plasma nicotine levels.
Data for tobacco cigarette was derived from Vansickel et al7. Nicotine levels after smoking a tobacco cigarette in 5 minutes (18.8 ng/ml) are 185% and 286% higher compared to using the first and new-generation electronic cigarette device respectively. Additionally, plasma nicotine levels after smoking one tobacco cigarette are almost equal to the values after using the new-generation device for 35 minutes (18.52 ng/ml), while they are 73% higher compared to the values after using the first-generation device for 35 minutes (10.88 ng/ml).
Discussion
This is the first study that has compared the effect of using first- vs. new-generation EC devices on nicotine absorption. An 18 mg/ml nicotine-containing liquid was used, which is a popular “strength” consumed by experienced vapers and is close to the maximum level set by the European Commission regulatory proposal. Experienced vapers were recruited, who use the EC devices more intensively compared to novice users7. The results of the study showed that new-generation devices with high wattage output to the atomizer resulted in higher plasma nicotine levels compared to first-generation devices. However, both devices delivered to the bloodstream far lower nicotine compared to a tobacco cigarette.
ECs are the only nicotine delivery devices that resemble the motion and behavioral patterns of cigarette smoking. Although the psycho-behavioral part of smoking is an important part of the overall addiction to smoking16, nicotine delivery still plays a major role. Smoking characteristically delivers significant amounts of nicotine to the blood stream at a fast rate17. This is probably the main reason for making it the most reinforcing and dependence-producing form of nicotine administration17,18, although recent data suggest that other substances in tobacco cigarette may reinforce the addictive properties of nicotine19. Studies have shown that the vast majority of EC users continue to use nicotine-containing liquids despite having quit smoking for several months2,10,11. Therefore, it seems that nicotine is important in ECs' success as smoking substitutes. In this study a liquid with 18 mg/ml nicotine concentration was chosen, based on previous findings from our group showing that this is approximately the level of nicotine concentration needed for experienced vapers to consume 1 mg of nicotine in 5 minutes (which is similar to the level of nicotine in the smoke of one cigarette when smoked according to ISO 3308)7. Despite that, the main findings herein showed that such a liquid is insufficient to deliver nicotine to the blood stream as rapidly as smoking. In fact, it took about 35 minutes of vaping with the new-generation device at high wattage in order to obtain plasma levels similar to smoking one cigarette in 5 minutes. The first-generation device was even less efficient in nicotine delivery; even 65 minutes of ad lib vaping was insufficient to deliver to the bloodstream nicotine at levels similar to smoking. This was reflected in participants' answers to questionnaires, showing that satisfaction and craving reduction was higher after using the new- compared with the first-generation device. Moreover, better nicotine delivery may be the reason why new-generation devices are more popular in dedicated users, most of which have quit smoking by using ECs10,11. Considering that it is reasonable to expect EC users to self-titrate nicotine intake in a way similar to smoking20, this study indicates that there is an inherent inability of the EC to deliver nicotine to the blood stream at levels similar to tobacco cigarettes within the same time-period of use, although a previous study from our group showed that an 18 mg/ml liquid would theoreticaly be adequate for that in terms of liquid consumption7. Possible reasons for these findings may be that nicotine delivered to the EC aerosol is not absorbed from the lungs but from the oral mucosa. Therefore, nicotine absorption would be expected to occur at a similar rate to nicotine-replacement therapies (NRTs). Moreover, a significant part of nicotine deposited to the oral mucosa is expected to be swallowed, with subsequent first-pass metabolism to the liver which reduces bio-availability21. Another possibility is that the vehicle of nicotine delivery (liquid droplets of propylene glycol and glycerol) may negatively interact with nicotine absorption from the lungs compared to the particulate matter, which is the delivery-vehicle of nicotine in smoking. More studies are needed to define the reason for this lower rate of absorption from ECs. The new-generation device was more effective in this study because the higher amount of energy delivered to the atomizer results in higher amount of liquid aerosolized per puff, while first-generation devices deliver far lower energy and do not have an internal current stabilizer which maintains constant energy delivery until the battery is discharged. Due to this, it is reasonable to expect that new-generation devices may be more effective as smoking substitutes compared to first-generation devices. Two randomised studies evaluating the efficacy of EC use in smoking cessation have used first-generation devices22,23. New-generation devices seem more promising because they deliver nicotine more effectively, and this should be evaluated in future studies. In any case, findings from this study indicate that ECs may also have less addictive properties and lower abuse liability, similar to what has been observed with NRTs24, due to the slow rate of nicotine absorption. This has been specifically observed in a study by Vansickel et al25. Herein, participants reported lower dependence to ECs compared to smoking, however the retrospective nature of the answers for smoking could have biased the results.
The European Commission has recently released a proposal that the highest permissible levels of nicotine concentration in liquids used for ECs will be 20 mg/ml5. The decision was based on the level of liquid consumption from 5 minutes of use6,7, but did not take into consideration that consumption is different from absorption; the main factors associated with the effects of nicotine are the levels in the bloodstream and the speed of absorption. Results of this study show that such nicotine concentration would be insufficient to deliver nicotine at levels similar to tobacco cigarettes unless ECs are used continuously for a long time. Moreover, the incidence of nicotine overdose or intoxication from EC use can be virtually excluded. Adopting the 20 mg/ml as the highest level of nicotine content in EC liquids might significantly reduce the efficacy of ECs to substitute smoking and will especially affect first-generation devices which are more popular, more attractive to first-time users (smokers) due to their resemblance to cigarettes and easier to use compared to new-generation devices. Based on the results of this study, showing very low levels of nicotine absorption from using ECs in a similar way to smoking one tobacco cigarette, it seems that nicotine levels in EC liquids should be close to 50 mg/ml in order to approximate nicotine delivery from smoking. This could also result in reduced daily EC use and liquid consumption, since the amount of nicotine needed by each user could be obtained by reducing the time and intensity of EC use. Clinical studies should be performed assessing the efficacy and speed of nicotine delivery by using higher nicotine-containing liquids. There is a possibility that using higher nicotine-containing liquids may elevate the addictive potential of ECs; however, considering that there is currently minimal adoption of EC use by non-smokers adults and youngsters11, it is reasonable to expect that the current proposal will reduce the effectiveness of ECs in substituting smoking while no beneficial effect to other population groups will be observed. Obviously, careful monitoring of use by these population groups is warranted; however, considering the current situation, a strict regulation banning the sales of ECs to minors would be more appropriate rather than adopting upper limits in nicotine content that would potentially reduce the efficacy of ECs to substitute smoking.
In conclusion, new-generation EC devices delivering higher energy to the atomizer seem to be more effective than first-generation devices in nicotine delivery to the user and in reducing cravings for nicotine. However, both types of devices were significantly less effective in nicotine delivery compared to tobacco cigarettes when an 18 mg/ml nicotine containing liquid is used. It is reasonable to assume that nicotine levels in EC liquids should be considerably higher in order to improve their effectiveness in nicotine delivery, which is expected to make them more successful as smoking substitutes.
Methods
Study participants
Healthy experienced EC users (vapers) where recruited for this study. To participate to the study they had to be: (1) former daily smokers of at least 5 years; (2) daily EC users for at least 1 month; (3) between the ages of 18 and 60; (4) clinically healthy, with no history of cardiovascular and lung disease or hematological problems; (5) able to remain abstinent from EC use for at least 8 hours; and (6) willing to provide blood samples. Exclusion criteria were: (1) pregnant or lactating females; (2) history of fainting or feeling faint associated with providing blood samples; and (3) being unwilling to provide written informed consent to participate to the study. The protocol was approved by the ethics committee of our institution and written informed consent was signed by all subjects before participating to the study.
Materials and clinical procedure
Two types of EC devices with the same liquid were used by the participants on two separate days, in a randomized cross-over design (Figure 1). The first-generation device was a typical cigarette-like EC (V2cigs, Miami, Florida, USA). The lithium battery has a capacity of 250 mAh and was fully charged before use. Empty cartomizers were bought by the same company and were filled according to company's instructions with approximately 1 ml of an 18 mg/ml nicotine containing liquid (Max Blend, Flavourart SRL, Oleggio, Italy). Users were provided with more fully-charged batteries if discharged and new cartomizers if emptied during the 65 minute period. The new-generation device consisted of a large lithium battery part (capacity of 2600 mAh) with an internal electronic circuit which includes a current stabilizer and allows the user to manually adjust the energy applied to the atomizer (EVIC, Joyetech, ShenZhen, China). A new-generation atomizer (EVOD, KangerTech, ShenZhen, China) was used and was filled with approximately 2 ml of liquid. The energy delivery to the atomizer was set to 9 watts.
Participants visited the laboratory after abstaining from EC use, caffeine, alcohol and food intake for at least 8 hours. Carbon monoxide in exhaled breath was measured by a calibrated Bedfont Micro Smokerlyzer. Subsequently, a venous catheter was introduced in an antecubital vein and 8 ml of venous blood was collected in lithium-heparinized vacutainers. Participants were asked to take 10 puffs in 5 minutes, simulating tobacco cigarette use26. After this period, they were asked to use the ECs ad lib for 60 more minutes (total duration of use: 65 minutes). Ten minutes after the end of the 65 minute period, carbon monoxide levels were measured again.
Blood samples and nicotine measurements
Blood samples were taken after the 5 minute period and every 15 minutes during the additional 60 minute period. The samples were stored in ice and were centrifuged within 1 hour. Plasma was separated and stored at −70°C until analyzed. Measurements of nicotine levels were performed in a specialized laboratory by Gas Chromatography with an NPD-80 Specific Detector. The lowest limit of quantification (LOQ) for this method was 0.5 ng/ml. For samples with nicotine levels below the LOQ, a value of LOQ/2 was assigned for statistical analysis.
Questionnaires on smoking and EC dependence, craving assessment and EC use effects
To define their past dependence to tobacco cigarettes, two tests were performed; the Fagerström Test for Cigarette Dependence (FTCD)12 and the Cigarette Dependence Scale (CDS)13. To evaluate their current EC dependence, the previously mentioned tests were also applied for EC use. Since both questionnaires include a question for cigarette consumption, the question was adjusted for EC use based on the results of a survey of 19,441 EC users performed by our group (unpublished data). The consumption was classified according to percentiles (quartiles for FTCD and quintiles for CDS). The results of the two tests after excluding the question on cigarette and EC consumption were also reported. To avoid any interaction between the answers, the questionnaires for smoking and EC use were administered on separate days to the participants.
To assess craving for nicotine before and after EC use, a shortened version of the Cigarette Withdrawal Scale (CWS) was used14,15. It consists of rating the extend of agreement with the following statements, adopted for EC use: (1) “The only thing I can think about is using (vaping) an EC”; (2) “I miss the EC terribly”; and (3) “I feel an irresistible need to vape”. For each question, answers were scored as: 0 = “totally disagree”, 1 = “mostly disagree”, 2 = “more or less agree”, 3 = “mostly agree”, 4 = “totally agree”. The sum of the scores from each question was calculated and reported. Additionally, a simple craving rating was used, by using a 100 mm visual analogue scale and asking: “How much do you crave an EC right now?”. Participants were asked to draw a cross through the horizontal line and the score was calculated by measuring the distance between the cross and the left anchor. Both CWS and simple craving rating were asked at baseline, after 5 minutes and after 65 minutes of use.
To assess the effects of nicotine and EC use, participants responded to 100 mm visual analogue scale questions after the end of the 65 minute period of EC use. Questions were adopted from Vansickel et al8. and from Hutsmuller and Stitzer27. Each word or phrase was centered above the horizontal line that represented a scale from 0 to 100 points; the left anchor was “Not at all” and the right anchor was “Extremely”. The score was assessed in a similar way as described above for the simple craving rating.
Statistical analysis
Categorical variables were expressed as number (percentage) while continuous variables as mean (SEM). To compare CWS and simple nicotine craving scale, repeated measures analysis of variance (ANOVA) was used, with two within-subjects factors: timing (3 levels) and session (2 levels). To compare exhaled carbon monoxide levels, repeated measures ANOVA was also used but timing had 2 levels (baseline and post-65 minute); for nicotine levels, timing had 6 levels. To assess perceived effects of nicotine and EC use, paired student's t-test was used. A two-tailed P value of ≤ 0.05 was considered statistically significant. All analyses were performed with commercially available statistical software (SPSS v.18, Chicago, Illinois, USA).
Author Contributions
K.F. was responsible for the protocol design. K.F., A.S., K.T. and C.S. were responsible for participants' recruitment, blood samples collection and exhaled carbon monoxide measurements. C.S., K.F. and G.R. were responsible for data collection and analyzing answers to the questionnaires. K.F. and G.R. was responsible for statistical analysis and interpretation of the data. K.F. and V.V. were responsible for manuscript preparation. All authors reviewed and approved the manuscript before being submitted for publication.
Additional Information
Funding: The study was funded by American E-Liquid Manufacturing Standards Association (AEMSA). This is an officially non-profit association [501(c)(6) status by the IRS] that was founded and is run by electronic cigarette consumers. AEMSA has provided advocacy to public health authorities, including the Food and Drug Administration (FDA) Center for Tobacco Products, about electronic cigarettes. The study was investigator-initiated and investigator-driven. The funding body had no involvement in the study design, data collection, analysis and interpretation, writing or approving the manuscript and decision to submit themanuscript for publication. The study was presented in part during a meeting with the FDA Center for Tobacco Products by Konstantinos Farsalinos.
References
- Phillips C. V. Debunking the claim that abstinence is usually healthier for smokers than switching to a low-risk alternative, and other observations about anti-tobacco-harm-reduction arguments. Harm Reduct. J. 6, 29 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos K. E., Romagna G., Tsiapras D., Kyrzopoulos S. & Voudris V. Evaluating nicotine levels selection and patterns of electronic cigarette use in a group of “vapers” who had achieved complete substitution of smoking. Subst. Abuse 7, 139–146 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkison S. E. et al. Electronic nicotine delivery systems: international tobacco control four-country survey. Am. J. Prev. Med. 44, 207–215 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockrell M., Morison R., Bauld L. & McNeill A. E-Cigarettes: prevalence and attitudes in Great Britain. Nicotine Tob. Res. 15, 1737–1744 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission. Revision of the tobacco products directive-Press release (European Parliament). (2013) Available at: http://www.europarl.europa.eu/pdfs/news/expert/infopress/20131216IPR31001/20131216IPR31001_en.pdf (Accessed on December 28, 2013). [Google Scholar]
- European Commission. Fact sheets for information on specific policy areas of the revision of the Tobacco Products Directive: e-cigarettes. (2013). Available at: http://ec.europa.eu/health/tobacco/docs/fs_ecigarettes_en.pdf (Accessed on December 28, 2013). [Google Scholar]
- Farsalinos K. E., Romagna G., Tsiapras D., Kyrzopoulos S. & Voudris V. Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: implications for research protocol standards definition and for public health authorities' regulation. Int. J. Environ. Res. Public Health 18, 2500–2514 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansickel A. R., Cobb C. O., Weaver M. F. & Eissenberg T. E. A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiol. Biomarkers Prev. 19, 1945–1953 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen C. et al. Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: randomised cross-over trial. Tob. Control 19, 98–103 (2010). [DOI] [PubMed] [Google Scholar]
- Dawkins L., Turner J., Roberts A. & Soar K. ‘Vaping’ profiles and preferences: an online survey of electronic cigarette users. Addiction 108, 1115–1125 (2013). [DOI] [PubMed] [Google Scholar]
- Farsalinos K. E. et al. Impact of flavour variability on electronic cigarette use experience: an internet survey. Int. J. Environ. Res. Public Health 10, 7272–7282 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerström K. Determinants of tobacco use and renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nicotine Tob. Res. 14, 75–78 (2012). [DOI] [PubMed] [Google Scholar]
- Etter J. F., Le Houezec J. & Perneger T. V. A self-administered questionnaire to measure dependence on cigarettes: the cigarette dependence scale. Neuropsychopharmacology 28, 359–370 (2003). [DOI] [PubMed] [Google Scholar]
- Etter J. F. A self-administered questionnaire to measure cigarette withdrawal symptoms: the Cigarette Withdrawal Scale. Nicotine Tob. Res. 7, 47–57 (2005). [DOI] [PubMed] [Google Scholar]
- West R. & Ussher M. Is the ten-item Questionnaire of Smoking Urges (QSU-brief) more sensitive to abstinence than shorter craving measures? Psychopharmacology (Berl). 208, 427–432 (2010). [DOI] [PubMed] [Google Scholar]
- Buchhalter A. R., Acosta M. C., Evans S. E., Breland A. B. & Eissenberg T. Tobacco abstinence symptom suppression: the role played by the smoking-related stimuli that are delivered by denicotinized cigarettes. Addiction 100, 550–559 (2005). [DOI] [PubMed] [Google Scholar]
- Benowitz N. L., Hukkanen J. & Jacob P. 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb. Exp. Pharmacol. 29–60 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield J. E. & Keenan R. M. Nicotine delivery kinetics and abuse liability. J. Consult. Clin. Psychol. 61, 743–750 (1993). [DOI] [PubMed] [Google Scholar]
- Guillem K. et al. Monoamine oxidase inhibition dramatically increases the motivation to self-administer nicotine in rats. J. Neurosci. 25, 8593–8600 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz N. L., Zevin S. & Jacob P. 3rd. Suppression of nicotine intake during ad libitum cigarette smoking by high-dose transdermal nicotine. J. Pharmacol. Exp. Ther. 287, 958–962 (1998). [PubMed] [Google Scholar]
- Benowitz N. L., Jacob P. 3rd & Savanapridi, C. Determinants of nicotine intake while chewing nicotine polacrilex gum. Clin. Pharmacol. Ther. 41, 467–473 (1987). [DOI] [PubMed] [Google Scholar]
- Caponnetto P. et al. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: A prospective 12-month randomized control design study. PLoS One 8, e66317 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen C. et al. Electronic cigarettes for smoking cessation: A randomised controlled trial. Lancet 382, 1629–1637 (2013). [DOI] [PubMed] [Google Scholar]
- West R. et al. A comparison of the abuse liability and dependence potential of nicotine patch, gum, spray and inhaler. Psychopharmacology (Berl). 149, 198–202 (2000). [DOI] [PubMed] [Google Scholar]
- Vansickel A. R., Weaver M. F. & Eissenberg T. Clinical laboratory assessment of the abuse liability of an electronic cigarette. Addiction 107, 1493–1500 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breland A. B., Kleykamp B. A. & Eissenberg T. Clinical laboratory evaluation of potential reduced exposure products for smokers. Nicotine Tob. Res. 8, 727–738 (2006). [DOI] [PubMed] [Google Scholar]
- Hutsmuller E. J. & Stitzer M. L. Manipulation of cigarette craving through rapid smoking: Efficacy and effects on smoking behavior. Psychopharmacology (Berl). 142, 149–157 (1999). [DOI] [PubMed] [Google Scholar]