Abstract
We analyzed MBL2 gene variants in two cohorts of centenarians, octo-and nonagenarians and in the general population, one from Sardinia island (Italy), recruited in the frame of the AKea study, and another from Campania (southern Italy), to search for haplotypes related to longevity. We also assessed in vitro the effect of mannose-binding lectin (MBL) on various human cells at different stage of senescence. The frequency of high and null activity haplotypes was significantly lower and the frequency of intermediate activity haplotype significantly higher in centenarians and in subjects between 80 and 99 years from both the cohorts as compared each to the general population from the same geographic area. Furthermore, serum MBL concentration (also after normalization to serum albumin) was significantly lower in centenarians and in octo- and nonagenarians as compared to the general population suggesting that intermediate MBL haplotype/activity may be protective. We also demonstrated that in vitro MBL protein bound to senescent IMR90 fibroblasts thereby causing cell lysis, but not to other types of cycle-arrested cells not in senescence. This implicates a novel role of MBL in the clearance of senescent cells.
Keywords: aging, senescence, innate immunity, MBL, haplotypes
Introduction
Mannose binding lectin (MBL) is an acute phase protein produced by the liver and encoded by the MBL2 gene. In liver cells, the protein forms triple helices that once released in serum polymerize thereby forming dimers to octamers (Lipscombe et al. 1995), and the biological activity of MBL depends on the degree of serum polymerization (Dommett et al. 2006). The protein interacts with surface sugars of specific microorganisms and plays a role in innate immunity by functioning as a pattern-recognition molecule. Indeed, it: i) activates the lectin-complement pathway; ii) promotes opsonophagocytosis; and iii) modulates inflammation (Dommett et al. 2006).
Three variants in non-coding regions of MBL2 have been identified: H/L (nucleotide -550), X/Y (nucleotide -221) and P/Q (at the +4 in the 5′ UTR). These variants reduce synthesis and serum levels of the protein. Three other variants involve codons 52, 54 and 57 and are called D, B and C alleles, respectively. They impair serum polymerization and thus reduce the biological activity of MBL (Garred et al. 2006). The six MBL2 variants are in linkage disequilibrium, and their combination results in seven common haplotypes that correspond to high (HYPA and LYQA haplotypes), intermediate (LYPA haplotype), low (LXPA haplotype) and null (HYPD, LYPB, LYQC haplotypes) biological activity of the protein (Verdu et al. 2006, Boldt et al. 2010).
Subjects bearing low and null haplotypes experience severe infections particularly in early childhood before the onset of specific protection by adaptive immunity (Dommett et al. 2006). Moreover, MBL null haplotypes are a negative modifier factors in cystic fibrosis (CF) because they cause a more severe liver (Tomaiuolo et al 2009) and pulmonary (Garred et al. 1999) disease. In addition, the HYPD-null haplotype is a risk factor for gastric cancer in subjects bearing H. pylori (Scudiero et al. 2006). Finally, null MBL haplotypes are associated to more severe autoimmune diseases (Maury et al. 2007, Jacobsen et al. 2009, Gergely et al. 2009).
MBL2 variants are frequent worldwide (Dommett et al. 2006, Garred 2008). For example, we identified MBL variant haplotypes corresponding to intermediate, low or null activity in about 50% of 550 subjects from the general population of southern Italy (Scudiero et al. 2006). It has been postulated that MBL plays a redundant role in human immunity and that MBL2 variants do not affect population fitness, and thus, their frequency can increase (Verdu et al. 2006). The high heterozygosity of MBL2 may be due to a series of advantages coming from the lower activity of the protein, namely, a less severe expression of rheumatoid arthritis (Jacobsen et al. 2009, Garred et al. 2000), Sjögren disease (Ramos-Casals et al. 2009), vasculitis (Xing et al. 2009), and Crohn’s disease (Velavan et al. 2010), whereas high MBL activity causes more severe infections by intracellular pathogens that use C3 receptor to enter the host (Dommett et al. 2006), e.g., visceral leishmaniasis (Peres Alonso et al. 2007) and malaria (Boldt et al 2006).
We used two approaches to evaluate the effect exerted by MBL variants: i) we analyzed MBL2 variants in centenarians, octo-nonagenarians, and in control groups from two different italian regions to search for MBL2 haplotypes related to longevity; and ii) we assessed the effect of MBL on several human cell models at different senescence stages.
Results
MBL haplotypes
Table 1A shows the results obtained in Sardinian population. The frequency of high activity MBL haplotypes was significantly lower in centenarians than in the general population (23.2% versus 42.9%; p<0.001). The frequency of these haplotypes was also significantly lower (p<0.01), in octo-nonagenarians than in the general population. Furthermore, null haplotypes were significantly less frequent in centenarians than in the general population (p=0.01). Finally, the intermediate activity haplotype (LYPA) was significantly more frequent in centenarians than in the general population (38.7% versus 11.5%; p<0.001). This haplotype was also significantly more frequent in octo-nonagenarians than in the general population (25.3% versus 11.5%; p<0.001). Table 1B reports the data obtained from the population of Campania. The results replicated those obtained in Sardinian population. Infact, the frequency of the high activity MBL haplotypes was significantly lower either in centenarians (35.6%, p<0.001) and in octo-nonagenarians (38.1%, p<0.001), each compared to the general population (53.2%). Furthermore, the frequency of null activity haplotypes obtained in centenarians (7.6%) was significantly lower as compared to that obtained in the general population (19.0%, p=0.01). Finally, the frequency of the intermediate activity haplotype (LYPA) was significantly higher in either centenarians (27.1, p<0.001) and in octo-nonagenarians (18.3, p<0.001) each compared to the general population (4.7%).
Table 1.
Allele frequency n and (%) of MBL2 haplotypes in subjects of different age groups from Sardinia (A) and from Campania (B).
| MBL activity | High | Intermediate | Low | Null | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| groups | n. of alleles | HYPA | LYQA | all high (HYPA+LYQA) | LYPA | LXPA | HYPD | LYPB | LYQC | all null (HYPD+LYPB+LYQC) |
| A | ||||||||||
| > 100 yrs | 142 | 22 (15.5) | 11 (7.8)** | 33 (23.2)** | 55 (38.7)** | 32 (22.5) | 1 (0.7) | 20 (14.1) | 1 (0.7) | 22 (15.5)* |
| 80–99 yrs | 300 | 68 (22.7) | 28 (9.3)** | 96 (32.0)* | 76 (25.3)** | 48 (16.0) | 10 (3.3) | 53 (17.7) | 17 (5.7) | 80 (26.7) |
| general population | 366 | 88 (24.0) | 69 (18.9) | 157 (42.9) | 42 (11.5) | 69 (18.9) | 14 (3.8) | 69 (18.9) | 15 (4.1) | 98 (26.8) |
| B | ||||||||||
| > 100 yrs | 118 | 32 (27.1) | 10 (8.5)* | 42 (35.6)** | 32 (27.1)** | 35 (29.7) | 1 (0.9) | 7 (5.9) | 1 (0.9) | 9 (7.6)* |
| 80–99 yrs | 252 | 71 (28.2) | 28 (11.1)* | 96 (38.1)** | 46 (18.3)** | 64 (25.4) | 8 (3.2) | 30 (11.9) | 3 (1.2) | 47 (18.7) |
| general population | 1106 | 390 (35.3) | 198 (17.9) | 588 (53.2) | 52 (4.7) | 255 (23.1) | 49 (4.4) | 144 (13.0) | 18 (1.6) | 212 (19.0) |
Significantly different *(p<0.01) and **(p<0.001) versus the general population.
Table 2A.
Number and (frequency) of mannose binding lectin (MBL2) genotypes subjects of different age groups from Sardinia
| > 100 yrs (n = 71) | ||||
|---|---|---|---|---|
| High | Intermediate | Low | Null | |
| High | 4 (5.6) | |||
| Intermediate | 13 (18.3) | 13 (18.3) | ||
| Low | 6 (8.5) | 10 (14.1) | 4 (5.6) | |
| Null | 6 (8.5) | 6 (8.5) | 8 (11.3) | 1 (1.4) |
| 80–99 yrs (n = 150) | ||||
|---|---|---|---|---|
| High | Intermediate | Low | Null | |
| High | 18 (12.0) | |||
| Intermediate | 15 (10.0) | 18 (12.0) | ||
| Low | 17 (11.3) | 7 (4.7) | 4 (2.7) | |
| Null | 28 (18.7) | 18 (12.0) | 16 (10.7) | 9 (6.0) |
| general population (n = 183) | ||||
|---|---|---|---|---|
| High | Intermediate | Low | Null | |
| High | 37 (20.2) | |||
| Intermediate | 8 (4.4) | 11 (6.0) | ||
| Low | 27 (14.8) | 9 (4.9) | 7 (3.9) | |
| Null | 40 (21.9) | 11 (6.0) | 19 (10.4) | 14 (7.7) |
Table 2 reports the genotypes identified in the three groups of subjects. Although statistical analysis was precluded because of the low number of cases, a lower percent of centenarians was homozygous for high (4 cases from Sardinia and 7 from Campania) or homozygous for null haplotypes (1 case from Sardinia and none from Campania) compared with the general population.
Serum MBL
Either in the population from Sardinia and in that from Campania, the values of serum MBL, albumin and the MBL/albumin ratio were significantly lower either in centenarians and in octo-nonagenarians (Table 3) as compared to the general population from the same geographic area.
Table 3.
Serum MBL, albumin and MBL/albumin ratio, mean and (SD) in the three groups of subjects under study from Sardinia (A) and from Campania (B).
| groups | N | serum MBL (ng/mL) | serum albumin (mmol/L) | MBL/albumin ratio |
|---|---|---|---|---|
| A | ||||
| > 100 yrs | 71 | 879.6 (388.9)** | 37.0 (2.4)** | 23.7 (7.3)** |
| 80–99 yrs | 150 | 1126.5 (529.0)** | 38.2 (2.9)** | 33.0 (6.4)** |
| general population | 80 | 1365.4 (568.1) | 41.2 (3.3) | 37.4 (14.4) |
| B | ||||
| > 100 yrs | 59 | 1001.8 (393.7)** | 37.0 (2.5)** | 27.1 (10.6)** |
| 80–99 yrs | 126 | 939.3(461.5)** | 38.1 (3.0)** | 24.3 (12.3)** |
| general population | 160 | 1300.4 (623.8) | 40.3 (3.0) | 32.6 (15.6) |
significantly different *(p<0.01), ** (p<0.001) as compared to the general population
MBL activity toward cells
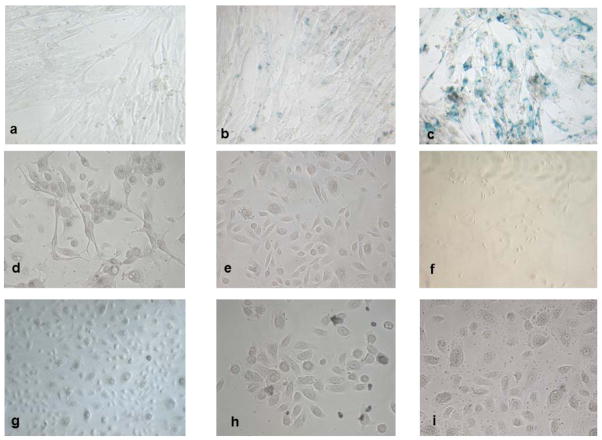
To define the number of senescent cells, we measured beta-galactosidase activity of IMR-90, NHBE cells and primary nasal epithelial cells at increasing population doubling (from PD 39, young, to 54, senescent, for IMR-90 cells; from PD 7 to 15 for NHBE cells; and from day 7 to 25 for primary nasal epithelial cells). To quantify cells positive to beta-galactosidase staining, we randomly selected for each group of cells at different PD five areas of observation and calculated the mean number. The percentage of IMR-90-stained cells significantly (p<0.001) increased from early passages (PD 39) to late ones (PD 54, see Table 4A and Figure 1A). No NHBE cells or primary epithelial cells were positive (Table 4B, C and Figure 1B, C), at any doubling stage.
Table 4.
beta-galactosidase and MBL binding assay of IMR-90, NHBE and primary nasal epithelial cells at different ages.
| A) IMR 90 | ||
|---|---|---|
| PD cells | % beta gal cells (mean of 5 assays) | % MBL stained cells (mean of 5 assays) |
| 39 | 0% | 0% |
| 46 | 34.6% (±12.1) | 3.6% (±1.3) |
| p<0.001 | p<0.001 | |
| 54 | 82.6% (±14.0) | 10.3% (±4.8) |
| B) NHBE | ||
|---|---|---|
| PD cells | % beta gal cells (mean of 3 assays) | % MBL stained cells (mean of 5 assays) |
| 7 | 0% | 0% |
| 10 | 0% | 0% |
| 15 | 0% | 0% |
| C) Primary nasal epithelial cells | ||
|---|---|---|
| Days in culture | % beta gal cells (mean of 3 assays) | % MBL stained cells (mean of 5 assays) |
| 7 | 0% | 0% |
| 14 | 0% | 0% |
| 25 | 0% | 0% |
Figure 1.
Beta-galactosidase staining (x 20) of IMR-90 (a–c), NHBE (d–f) and primary human epithelial cells (g–i) at different ages. IMR-90 cells at early-passage culture (PD 39) (a), at middle-passage culture (PD 46) (b), and at late-passage culture (PD 54) (c) undergo growth arrest and extensively express beta-galactosidase. NHBE cells at early-passage culture (PD 7) (d), at middle-passage culture (PD 10) (e), and at late-passage culture (PD 15). Primary nasal epithelial cells at early-passage culture 7 days (f), at middle-passage culture 14 days (h), and at late-passage culture 25 days (i). No beta-galactosidase activity is present in Neither NHBE nor nasal primary epithelial cells express beta-galactosidase although at late passages both cell types undergo growth arrest.
To assess the MBL binding to senescent cells, we set up an immunofluorescence assay using cells of different ages, incubated with human MBL protein followed by anti-human MBL mAb and FITC-conjugated anti-mouse IgG. Each experiment was conducted five times and the results are the mean of the five experiments. As shown in Table 4A, IMR-90 cells at PD 39 were negative to staining. At PD 46, 3.6% cells were fluorescent and staining significantly (p<0.01) increased to 10.3% of cells at 54 PD (Table 4A)
To verify that staining was due to MBL binding (and thus Ca++ dependent), we repeated the experiment: i) adding 10 mM EDTA with no calcium and ii) adding mannose. Both EDTA and mannose blocked the binding of MBL protein to senescent cells. Indeed, no cell was positive to FITC antibody (data not shown).
The same immunofluorescence assay was done for NHBE and primary nasal epithelial cells even if they were negative to beta-galactosidase staining. As expected, no fluorescent signal was detected in these cells at any age (Table 4B, C). Finally, we assessed lactate dehydrogenase (LD) values in the culture medium of IMR-90 cells before and after incubation with MBL. All experiments were performed five times and the data reported in Table 5 are the mean of five assays. The addition of MBL caused a significant (p<0.01) increase of LD in the culture medium (from 74 to 120 U/L). There was a further (not statistically significant) increase after the addition of fresh human serum (120 to 144 U/L after the subtraction of the LD value obtained in serum alone). The same experiments were performed on medium of NHBE and primary nasal epithelial cells, and the addition of MBL and MBL plus serum did not affect LD values in the medium (data not shown).
Table 5.
Lactate dehydrogenase (LD) activity in culture medium of IMR-90 cells before and after the addiction of MBL. The results are the mean of five experiments.
| Time | LD activity(U/L) |
|---|---|
| 0 | 74 (±12.4) |
| p<0.01 | |
| after MBL addiction | 120 (±20.9) |
| n.s. | |
| after serum addiction | 144 (±27.7) |
Finally, we looked for mannosyl residues on young and senescent IMR-90 cells (PD 39 and PD 54) using the staining for Concanavalin A, a lectin that recognizes specific sugar residues, in particular mannosyl residues. Old IMR-90 cells (PD 54) bound about 40% more concanavalin A than the youngest ones (PD 39).
Discussion
Our study revealed a peculiar MBL2 haplotype associated to longevity. In fact, in centenarians we observed: i) as expected, a low frequency of haplotypes associated to null protein activity (i.e., LYPB, LYQC and HYPD) and only 1 case of a homozygous genotype for null haplotypes; ii) a surprisingly low frequency of haplotypes associated to high protein activity (i.e., HYPA and LYQA) and only a few cases homozygotes for high activity haplotypes; iii) a high frequency of the LYPA haplotype, which is associated to intermediate MBL activity and a high percentage of compound genotypes for this haplotype. Furthermore, MBL serum concentration was significantly lower in centenarians than in the general population, even after normalization to serum albumin (which neutralizes the effect of the reduced liver prothydosynthesis due to age). A similar trend was observed in octo-nonagenarians versus the general population.
Such MBL2 haplotypes may confer some advantages and thus may contribute to healthy aging. In fact, MBL2-null haplotypes (that are very rare in our centenarians) are related to a wide spectrum of infections particularly in early childhood (Dommett et al. 2006), and to non infectious diseases in adults (Scudiero et al. 2006, Maury et al. 2007, Jacobsen et al. 2009). Therefore, it is not surprising that these haplotypes are rarely found in centenarians. It is more difficult to explain the low allelic frequency of haplotypes with high activity observed in our long-lived individuals. Various disadvantages have been associated to high MBL activity. For example, a high frequency of MBL variants was found in subjects from southern Africa (Lipscombe et al. 1992) and southern America (Madsen et al. 1998), and the resulting low MBL activity has been related to less severe malaria (Boldt et al. 2006) and to a lower frequency and severity of leishmaniasis (Peres Alonso et al. 2007). But unlike southern Africa and southern America, Sardinia and Campania are not areas under a high parasitic burden. Indeed, the occurrence of antibodies against such parasites in our subjects is comparable to that of other European areas (data not shown). A second disadvantage of a high MBL activity is the excessive complement activation that results in immunopathologically mediated host damage (Lipscombe et al. 1992), which may lead to more severe forms of autoimmune diseases (Maury et al. 2007, Jacobsen et al. 2009, Gergely et al. 2009). In this context, it is noteworthy that none of our centenarians has clinically evident autoimmune diseases and a review of their clinical record exclude previous relevant autoimmune disorders.
In addition, we demonstrate that MBL binds the surface of senescent fibroblasts, a cell system longly used to study cell senescence (Hayflick 1980, Killilea & Ames 2008). The addition of human serum increased the percentage of cells that were bound by MBL. This may reflect the fact that MBL binds MASPs in serum and this interaction increases the affinity for the target (Garred et al. 2006), as described in neoplastic cells (Ma Y al. 1999). The binding of MBL to senescent fibroblasts caused lysis, as indicated both by MBL fluorescence in the cytoplasm and by the increased release of LD in the medium. The interaction of MBL with membrane is peculiar to senescent cells; in fact, MBL binds only IMR-90 cells, at the end of PD, that express high levels of beta-galactosidase, a well known marker of cellular senescence (Dimri et al. 1995). On the contrary, MBL does not interact with NHBE cells and primary nasal epithelial cells even at the end of population doubling, because these cells do not express the senescent phenotype (no expression of beta-galactosidase) and thus are quiescent or terminally differentiated cells (Dimri et al. 1995). We speculate that also in vivo MBL might interact with senescent cells thereby contributing to their lysis and consequently, an excessively high activity of MBL may result in stronger activation of the complement that promotes the lysis of senescent cells. Reduced activity of serum MBL (as observed in our long-lived subjects that bear the intermediate MBL2 haplotype and lower serum levels of the protein) may be associated to a less intense activity of MBL toward senescent cells, with the advantage of reduced autoimmune triggering in such subjects. This hypothesis goes to add to the relevant literature that reports that less active inflammatory genotypes are an advantage for longevity (Licastro et al. 2005).
The activity of MBL toward senescent cells may depend on the remodulation of the surface sugar pattern observed in senescent cells (Blondal et al. 1985) and these changes trig the binding to senescent fibroblast of Concanavalin A (as observed also in the present study), a plant lectin that shows a high affinity for the same carbohydrate moieties, mainly mannose (Rüdiger & Gabius 2001). Our data suggest that these changes may render a percentage of senescent fibroblasts also the target of MBL binding, and a similar situation could involve various types of human senescent cells. On the other hand, it is known that MBL binds some types of human cells. In particular, it is involved in late apoptosis binding apoptotic cells that express altered patterns of membrane carbohydrates (Rüdiger & Gabius 2001, Nauta et al. 2003); furthermore, changes in the surface sugar distribution during neoplastic transformation promote MBL binding to cancer cells (Garred et al. 2006, Roos et al. 2004) and the protein mediates cytotoxic effects (Blondal et al. 1985, Hakomori 2001).
To conclude, centenarians from Sardinia and Campania tell us that the most advantageous MBL2 haplotype for successful aging is the intermediate haplotype, which corresponds to intermediate levels of MBL serum activity. Furthermore, our data show that MBL is active -at least in vitro- toward senescent cells.
Materials and methods
Subjects
We studied three groups of subjects from Sardinia (Italy), unrelated up to the third generation: i) 71 centenarians (27 males, mean age 100.9 ±1.3 years); ii) 150 octo- nonagenarians, i.e., subjects between 80 and 99 years (52 males, mean age: 89.1 ±6.0 years); and iii) 183 subjects from the general population (94 males) whose sex and age distribution matchs the anagraphic distribution of Sardinia. We have a complete computerized clinical record for each subject, within the frame of the AKea study (Deiana L et al. 1999, Poulain M et al. 2004) chaired by Prof. L. Deiana and approved by the Ethic committee of University of Sassari, Italy. Furthermore, we studied three groups of subjects from Campania (southern Italy) unrelated up to the third generation: 59 centenarians (30 males, man age:101.6 ±1.7); ii) 126 octo- nonagenarians, i.e., subjects between 80 and 99 years (59 males, man age: 91.0 ±4.7 years); iii) 553 subjects from the general population previously described (Scudiero O et al. 2006). All long-lived subjects are free from major diseases (i.e., cardiovascular, neoplastic, autoimmune or neurodegenerative diseases). All patients are mobile: they practice regular activity as suggested by their physicians). Each subject provided the informed consent to be included in the study, and written permission to use a biological sample for anonymous biological research. A DNA sample (extracted from peripheral blood with a commercial procedure) and a serum sample (stored at +4 °C and 80°C respectively, up to use) were available for each subject included in the study.
MBL gene variants
We analyzed the six MBL2 gene variants (Garred P et al. 2006), using a PCR-reverse dot-blot procedure (Innolipa MBL2, Innogenetics, Ghent, Belgium). In line with current nomenclature, the three polymorphisms in the 5′ non-coding regions of the gene are designated H/L (nucleotide -550), X/Y (nucleotide -221) and P/Q (at the +4 in the 5′ UTR); the three MBL2 variants at codons 52, 54 and 57 are designated D, B and C alleles, respectively. The wild-type allele at the three codons is defined allele A. Seven MBL2 haplotypes result from the combination of the seven variants and are named HYPA, LYQA (high activity haplotypes), LYPA (intermediate activity haplotype), LXPA (low activity haplotype) and HYPD, LYPB, LYQC (null activity haplotypes, Verdu P et al. 2006, Boldt AB et al. 2010). The MBL haplotype distribution is control subjects is in Hardy–Weinberg equilibrium (Scudiero et al. 2006). Furthermore it is not influenced by sex (unpublished results). So we analyzed together male and female subjects of each subgroup.
Serum MBL and albumin
Serum MBL was analyzed using a commercial ELISA procedure (Sanquin, Amsterdam, NL); serum albumin was analyzed by nephelometry (Roche, Milan, Italy). The data were expressed also as MBL/albumin ratio.
Cell culture
IMR-90 human embryonic lung fibroblasts were purchased from ATCC-LGC Standards (CCL-186, Middlesex, U.K.). Culture medium components were purchased from Sigma-Aldrich Inc. (St Louis, MO, USA) or Lonza Inc. (Walkersville, MD, USA). IMR-90 fibroblasts were cultured at 37°C with 5% CO2 in Eagle’s minimal essential medium containing 10% heat-inactivated fetal bovine serum supplemented with 1% penicillin/streptomycin solution (Sigma) and 2 mM L-glutamine. Cells were harvested by 0.5% (w/v) trypsin-EDTA solution (Gibco, Invitrogen, Carlsbad, CA, USA). All the experiments involved cultures from the population doubling (PD) 39 to 54. Normal human bronchial epithelial cells (NHBE) were obtained from Clonetics (Walkersville, MD, USA). Cells were cultured at 37°C in humidified 5% CO2 and grown in bronchial epithelial growth medium (Lonza Inc.), that consists of bronchial epithelial basal medium (BEBM) supplemented with rhEGF, bovine pituitary extract (BPE), insulin, hydrocortisone, transferrin, retinoic acid, epinephrine, triiodothyronine and gentamicin sulfate/amphothericin-B (GA-1000) (purchased from Lonza Inc.). NHBE cells were harvested and split according to the NHBE technical sheet and by using Lonza ReagentPack (Lonza Inc.) containing 100 mL trypsin/EDTA, 100 ml Trypsin Neutralizing Solution and 100 mL HEPES Buffered Saline Solution. All the experiments were carried out with NHBE cultures between the PD 7 and 15 (maximum expected lifespan). Primary nasal epithelial cells were obtained from Laboratory donors by gently brushing the inferior turbinates using an endobrush (Aiesi Hospital Services, Naples, Italy). Cells were transported at room temperature in 2 mL of RPMI1640 medium (Lonza Inc.), complemented with 3% gentamicin. Cells were placed on an Eppendorf Thermomixer, in agitation at 300 rpm for 1 h. Once the brush was removed from each sample, cells were centrifuged at 2000 rpm for 20′, the supernatant was discarded and cell pellet was treated with 150 mL of Trypsin-Versene EDTA solution (Lonza Inc.) for 4′ at 37°C, to disaggregate cell clusters. Trypsin was inactivated by adding 3 mL of serum-free bronchial epithelial cell growth medium BEGM (Lonza Inc.). After centrifugation at 2000 rpm for 10′, cells were placed in CELL+ T 25 flasks (Sarstedt Ltd, Leicester, UK). Medium was changed daily. At confluence of 60%, cells were transferred in new flasks (about 3000 viable cells/cm2 per flask). Cells were maintained in culture for 25 days and a confluence of 60–80% was reached every 6–7 days. Each cell line was plated at different seeding densities, and grown for the periods and under the conditions specified for each experiment. Cells were counted with an automated vision based counter (Countess™ Automated Cell Counter, Gibco, Invitrogen). Population doubling was calculated at each passage until growth arrest by the following formula: PD = (log10Y–log10X)/log2. Y indicates the number of cells counted at the end of the passage; X is the number of cells seeded. Cumulative population doubling (CPD) was calculated as the sum of all the changes in PD.
Cellular Senescence Assay
Cells undergoing senescence typically express beta-galactosidase activity at pH 6. Beta-galactosidase-positive cells were detected with the Chemicon Cellular Senescence Assay kit (Millipore, Temecula, CA, USA). The day before the experiment, 300,000 cells were seeded in 35-mm petri dishes. They were washed twice with 2 mL 1X Ca/Mg-free phosphate-buffered saline (PBS), and fixed with 1 mL 1X fixing solution at room temperature for 15 mins. After removing the fixing solution, the cells were washed twice with 2 mL 1X PBS and incubated overnight with 2 mL of freshly prepared 1X SA-beta-gal detection solution at 37°C, without CO2 and protected from light. Stained cells were washed twice with 2 mL 1X PBS to remove SA-beta-gal detection solution. Images were recorded using a light phase contrast microscope (Leica DM IL, Leitz, Wetzlar, Germany) linked to a Leica DFC320 camera.
Concanavalin A staining
IMR-90 cells, cultured on glass coverslips, were washed twice with cold PBS. Cells were then fixed with 4% paraformaldehyde-PBS buffer for 10 min and incubated with concanavalin A, Alexa FluorR 594 conjugate (Molecular Probes Inc., Invitrogen, Eugene, Oregon, USA) 20 mg/mL for 20 min. After incubation, cells were washed 3–4 times with cold buffer and mounted, inverted, in ProLong Gold antifade reagent with DAPI (Molecular Probes Inc., Invitrogen, Eugene, Oregon, USA) and observed with a fluorescence-inverted microscope (Leica Microsystems). Finally, fluorescence intensity was evaluated on the pictures with ImageJ (online free) software.
Assay for MBL binding to young and senescent cells
Lyophilized mannose binding protein (MBP) was purchased from USBiological (Swampscott, MA, USA). Before use, we verified by electrophoresis that MBL correctly polymerized. Binding of human MBL protein to IMR-90, NHBE and primary nasal epithelial cells was evaluated using immunofluorescence. IMR-90 cells with different PDs (39, 46 and 54) were seeded on a 96-well clear bottom white polystyrene plate (Corning Incorporated, NY, USA) at 9×103 cells per well with 200 μL of medium 24 h before the experiment. The same procedure was done with NHBE cells (PD: 7, 10 and 15) and primary nasal epithelial cells (7, 14 and 25 days), but in these cases, the seeding density was 6 ×103 cells per well. The three cell lines were washed twice with 100 μL of 1X Hank’s Balanced Salt Solution with Ca/Mg (Lonza Inc.) and were then treated with 50 μL of 5 μg/mL human MBL for 1 h at room temperature. After incubation with MBP, cells were washed twice with 100 μL of 1X HBSS and then incubated with 50 μL of 4 μg/mL anti-MBL mAb, D8.18 (Santa Cruz Biotechnology Inc., CA, USA) for 90 min at room temperature. Finally, cells were washed twice with 100 μL of 1X HBSS and incubated with 50 μL of 2μg/mL fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG for an additional 90 min at room temperature. Images of cells were acquired with a Leica DFC350FX monochrome digital camera connected to a Leica DMI4000B inverted fluorescence microscope.
Cytotoxicity assay
Cytotoxicity induced by MBL protein on senescent cells was assessed measuring lactate dehydrogenese in the culture medium using a commercial kit (Roche, Milan, Italy) with an automated analyzer.
Statistics
The Chi square test with Yates correction was used to compare in the allelic frequency of each MBL2 haplotype and MBL2 genotypes in the three study groups. The unpaired t test was used to compare serum albumin, MBL and MBL/albumin ratio in the three study groups once verified that these parameters had a Gaussian distribution.
Table 2B.
Number and (frequency) of mannose binding lectin (MBL2) genotypes in subjects of different age groups from Campania
| > 100 yrs (n = 59) | ||||
|---|---|---|---|---|
| High | Intermediate | Low | Null | |
| High | 7 (11.9) | |||
| Intermediate | 14 (23.7) | 4 (6.8) | ||
| Low | 13 (22.0) | 8 (13.6) | 4 (6.8) | |
| Null | 1 (1.7) | 2 (3.4) | 6 (10.2) | 0 |
| 80–99 yrs (n = 126) | ||||
|---|---|---|---|---|
| High | Intermediate | Low | Null | |
| High | 28 (22.2) | |||
| Intermediate | 10 (7.9) | 8 (6.4) | ||
| Low | 23 (17.8) | 11 (8.7) | 7 (5.6) | |
| Null | 10 (7.9) | 9 (7.1) | 18 (14.3) | 2 (1.6) |
| general population (n = 553) | ||||
|---|---|---|---|---|
| High | Intermediate | Low | Null | |
| High | 153 (27.7) | |||
| Intermediate | 21 (3.8) | 2 (0.4) | ||
| Low | 149 (26.9) | 12 (2.2) | 32 (5.8) | |
| Null | 112 (20.3) | 15 (2.7) | 30 (5.4) | 27 (4.9) |
Acknowledgments
We gratefully acknowledge grants from Regione Autonoma della Sardegna, Fondazione Banco di Sardegna; National Institute of Aging - Grant R01AG020549, Demographic Analysis of Sardinian Longevity; Regione Campania (DGRC 1901/2009). We thank Jean Ann Gilder (Scientific Communication srl) for text editing.
Non-standard abbreviation
- MBL
mannose binding lectin
- LD
lactate dehydrogenase
Footnotes
Author contributions
All authors contributed equally to this work. R. Tomaiuolo administered the experiments and wrote the manuscript; C. Bellia, B. Lo Sasso, A. Rocco and C. Salapete designed and performed experiments; C. Franceschi and G. Baggio recorded clinical data from all subjects. C. Carru and A. Zinellu selected the population; M. Ciaccio, G. Castaldo, L. Deiana designed experiments, analysed data and edited the paper. J Vaupel and L. Deiana promoted the AKea study. All authors discussed the results and implications and commented on the manuscript at all stages.
References
- Blondal JA, Dick JE, Wright JA. Membrane glycoprotein changes during the senescence of normal human diploid fibroblasts in culture. Mech Ageing Dev. 1985;30:273–283. doi: 10.1016/0047-6374(85)90117-4. [DOI] [PubMed] [Google Scholar]
- Boldt AB, Luty A, Grobusch MP, Dietz K, Dzeing A, Kombila M, Kremsner PG, Kun JF. Association of a new mannose binding lectin variant with severe malaria in Gabonese children. Genes Immun. 2006;7:393–400. doi: 10.1038/sj.gene.6364312. [DOI] [PubMed] [Google Scholar]
- Boldt AB, Messias-Reason IJ, Meyer D, Schrago CG, Lang F, Lell B, Dietz K, Kremsner PG, Petzl-Erler ML, Kun JF. Phylogenetic nomenclature and evolution of mannose-binding lectin (MBL2) haplotypes. BMC Genet. 2010;14:1–12. doi: 10.1186/1471-2156-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiana L, Ferrucci L, Pes GM, Carru C, Delitala G, Ganau A, Mariotti S, Nieddu A, Pettinato S, Putzu P, et al. AKentAnnos. The Sardinia Study of Extreme Longevity. Aging. 1999;11:142–149. [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dommett RM, Klein N, Turner MW. Mannose-binding lectin in innate immunity: past, present and future. Tissue Antigens. 2006;68:193–209. doi: 10.1111/j.1399-0039.2006.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garred P, Larsen F, Seyfarth J, Fujita R, Madsen HO. Mannose–binding lectin and its genetic variants. Genes Immun. 2006;7:85, 94. doi: 10.1038/sj.gene.6364283. [DOI] [PubMed] [Google Scholar]
- Garred P, Madsen HO, Marquart H, Hansen TM, Sørensen SF, Petersen J, Volck B, Svejgaard A, Graudal NA, Rudd PM, et al. Two edged role of mannose binding lectin in rheumatoid arthritis: a cross sectional study. J Rheumatol. 2000;27:26–34. [PubMed] [Google Scholar]
- Garred P, Pressler T, Madsen HO, Frederiksen B, Svejgaard A, Høiby N, Schwartz M, Koch C. Association of mannose-binding lectin gene heterogeneity with severity of lung disease and survival in cystic fibrosis. J Clin Invest. 1999;104:431–437. doi: 10.1172/JCI6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garred P. Mannose-binding lectin genetics: from A to Z. Biochem Soc Trans. 2008;36:1461–1466. doi: 10.1042/BST0361461. [DOI] [PubMed] [Google Scholar]
- Gergely P, Jr, Pazár B, Nagy ZB, Gombos T, Rajczy K, Balogh Z, Orbán I, Sevcic K, Poór G. Structural polymorphisms in the mannose-binding lectin gene are associated with juvenile idiopathic arthritis. J Rheumatol. 2009;36:843–847. doi: 10.3899/jrheum.080681. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Tumor-associated carbohydrate antigens defining tumor malignancy: basis for development of anti-cancer vaccines. Adv Exp Med Biol. 2001;491:369–402. doi: 10.1007/978-1-4615-1267-7_24. [DOI] [PubMed] [Google Scholar]
- Hayflick L. The cell biology of human aging. Sci Am. 1980;242:58–65. doi: 10.1038/scientificamerican0180-58. [DOI] [PubMed] [Google Scholar]
- Jacobsen S, Garred P, Madsen HO, Heegaard NH, Hetland ML, Stengaard-Pedersen K, Junker P, Lottenburger T, Ellingsen T, Smedegaard Andersen L, et al. Mannose-binding lectin gene polymorphisms are associated with disease activity and physical disability in untreated, anti-cyclic citrullinated peptide-positive patients with early rheumatoid arthritis. J Rheumatol. 2009;36:731–735. doi: 10.3899/jrheum.080846. [DOI] [PubMed] [Google Scholar]
- Killilea DW, Ames BN. Magnesium deficiency accelerates cellular senescence in cultured human fibroblasts. Proc Natl Acad Sci USA. 2008;105:5768–5773. doi: 10.1073/pnas.0712401105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licastro F, Candore G, Lio D, Porcellini E, Colonna-Romano G, Franceschi C, Caruso C. Innate immunity and inflammation in ageing: a key for understanding age-related diseases. Immun Ageing. 2005;18:8. doi: 10.1186/1742-4933-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscombe RJ, Sumiya M, Hill AV, Lau YL, Levinsky RJ, Summerfield JA, Turner MW. High frequencies in African and non-African populations of independent mutations in the mannose binding protein gene. Hum Mol Genet. 1992;1:709–715. doi: 10.1093/hmg/1.9.709. [DOI] [PubMed] [Google Scholar]
- Lipscombe RJ, Sumiya M, Summerfield JA, Turner MW. Distinct physicochemical characteristics of human mannose binding protein expressed by individuals of differing genotype. Immunol. 1995;85:660–667. [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Uemura K, Oka S, Kozutsumi Y, Kawasaki N, Kawasaki T. Antitumor activity of mannan-binding protein in vivo as revealed by a virus expression system: mannan-binding protein dependent cell-mediated cytotoxicity. Proc Natl Acad Sci USA. 1999;96:371–375. doi: 10.1073/pnas.96.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen HO, Satz ML, Hogh B, Svejgaard A, Garred P. Different molecular events result in low protein levels of mannan-binding lectin in populations from southeast Africa and South America. J Immunol. 1998;161:3169–3175. [PubMed] [Google Scholar]
- Maury CP, Aittoniemi J, Tiitinen S, Laiho K, Kaarela K, Hurme M. Variant mannose-binding lectin 2 genotype is a risk factor for reactive systemic amyloidosis in rheumatoid arthritis. J Intern Med. 2007;262:466–469. doi: 10.1111/j.1365-2796.2007.01838.x. [DOI] [PubMed] [Google Scholar]
- Nauta AJ, Raaschou-Jensen N, Roos A, Daha MR, Madsen HO, Borrias-Essers MC, Ryder LP, Koch C, Garred P. Mannose-binding lectin engagement with late apoptotic and necrotic cells. Eur J Immunol. 2003;33:2853–2863. doi: 10.1002/eji.200323888. [DOI] [PubMed] [Google Scholar]
- Peres AD, Ferreira AF, Ribolla PE, de Miranda Santos IK, do Socorro Pires e Cruz M, Aécio de Carvalho F, Abatepaulo AR, Lamounier Costa D, Werneck GL, Farias TJ, et al. Genotypes of the mannan-binding lectin gene and susceptibility to visceral leishmaniasis and clinical complications. J Infect Dis. 2007;195:1212–1217. doi: 10.1086/512683. [DOI] [PubMed] [Google Scholar]
- Poulain M, Pes GM, Grasland C, Carru C, Ferrucci L, Baggio G, Franceschi C, Deiana L. Identification of a geographic area characterized by extreme longevity in the Sardinia island: the AKEA study. Exp Gerontol. 2004;39:1423–1429. doi: 10.1016/j.exger.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Ramos-Casals M, Brito-Zerón P, Soria N, Nardi N, Vargas A, Muñoz S, Bové A, Suárez B, Lozano F. Mannose-binding lectin-low genotypes are associated with milder systemic and immunological disease expression in primary Sjögren’s syndrome. Rheumatology. 2009;48:65–69. doi: 10.1093/rheumatology/ken411. [DOI] [PubMed] [Google Scholar]
- Roos A, Garred P, Wildenberg ME, Lynch NJ, Munoz JR, Zuiverloon TC, Bouwman LH, Schlagwein N, Fallaux van den Houten FC, Faber-Krol MC, et al. Antibody-mediated activation of the classical pathway of complement may compensate for mannose-binding lectin deficiency. Eur J Immunol. 2004;34:2589–2598. doi: 10.1002/eji.200324401. [DOI] [PubMed] [Google Scholar]
- Rüdiger H, Gabius HJ. Plant lectins: occurrence, biochemistry, functions and applications. Glycoconj J. 2001;18:589–613. doi: 10.1023/a:1020687518999. [DOI] [PubMed] [Google Scholar]
- Scudiero O, Nardone G, Omodei D, Tatangelo F, Vitale DF, Salvatore F, Castaldo G. A mannose-binding lectin-defective haplotype is a risk factor for gastric cancer. Clin Chem. 2006;52:1625–1627. doi: 10.1373/clinchem.2006.071696. [DOI] [PubMed] [Google Scholar]
- Tomaiuolo R, Degiorgio D, Coviello DA, Baccarelli A, Elce A, Raia V, Motta V, Seia M, Castaldo G, Colombo C. An MBL2 haplotype and ABCB4 variants modulate the risk of liver disease in cystic fibrosis patients: a multicentre study. Dig Liver Dis. 2009;41:817–822. doi: 10.1016/j.dld.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Velavan TP, Boldt AB, Tomiuk J, Seibold F, Schoepfer AM, Flogerzi B, Müller S, del Abad-Grau MM, Kremsner PG, Kun JF. Variant alleles of the mannose binding lectin 2 gene (MBL2) confer heterozygote advantage within Crohn’s families. Scand J Gastroenterol. 2010;45:1129–1130. doi: 10.3109/00365521.2010.485324. [DOI] [PubMed] [Google Scholar]
- Verdu P, Barreiro LB, Patin E, Gessain A, Cassar O, Kidd JR, Kidd KK, Behar DM, Froment A, Heyer E, et al. Evolutionary insights into the high worldwide prevalence of MBL2 deficiency alleles. Hum Mol Gen. 2006;15:2650–2658. doi: 10.1093/hmg/ddl193. [DOI] [PubMed] [Google Scholar]
- Xing GQ, Chen M, Liu G, Heeringa P, Zhang JJ, Zheng XEJ, Kallenberg CG, Zhao MH. Complement activation is involved in renal damage in human antineutrophil cytoplasmic autoantibody associated pauci-immune vasculitis. J Clin Immunol. 2009;29:282–291. doi: 10.1007/s10875-008-9268-2. [DOI] [PubMed] [Google Scholar]